Biochar Amendment in Remediation of Heavy Metals in Paddy Soil: A Case Study in Nobewam, Ghana
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Preparation of Biochar and Application
2.3. Sampling and Sample Preparation
2.4. Physicochemical Analysis
2.5. Determination of Effective Cation Exchange Capacity (ECEC)
2.6. Soil Available Phosphorus (P) Analysis
2.7. Soil Texture Determination
2.8. Elemental Analysis of Soil and Rice Samples
2.9. Statistical Analysis and Data Evaluation
2.10. Geoaccumulation Index (Igeo)
2.11. Potential Ecological Risk Index (PERI)
2.12. Hazard Quotient and Hazard Index
3. Results and Discussion
3.1. Effects of Biochar on Soil Physicochemical Properties
3.2. Correlation Between Soil Physicochemical Properties
3.3. Effect of Biochar Application on Metal Concentrations in Soils
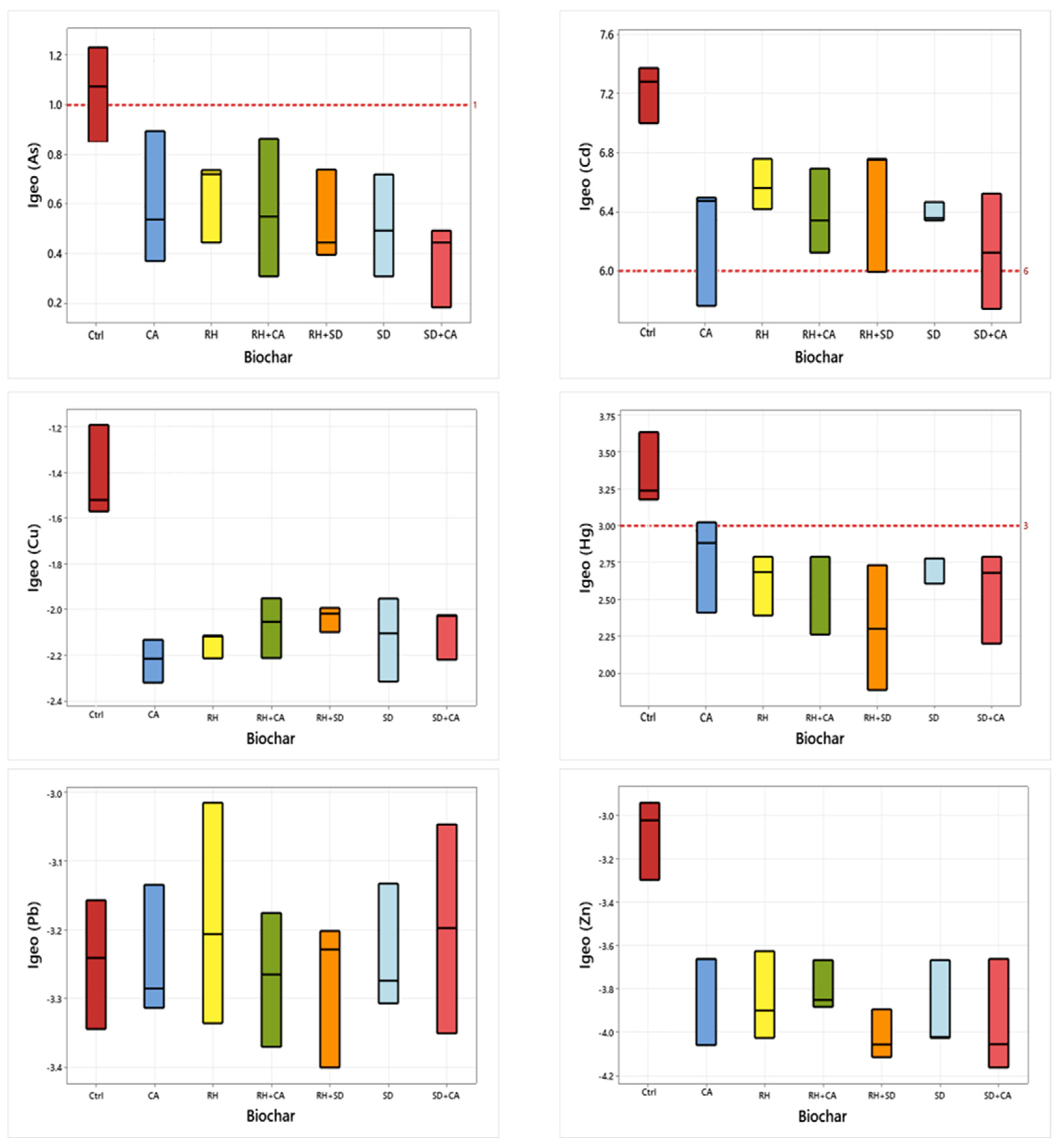
3.4. Potential Ecological Risk Index (PERI) and Geoaccumulation Indices (Igeo)
3.5. Alteration of Heavy Metal Uptake and Bioaccumulation in Rice
3.6. Bioaccumulation of Metals in Rice
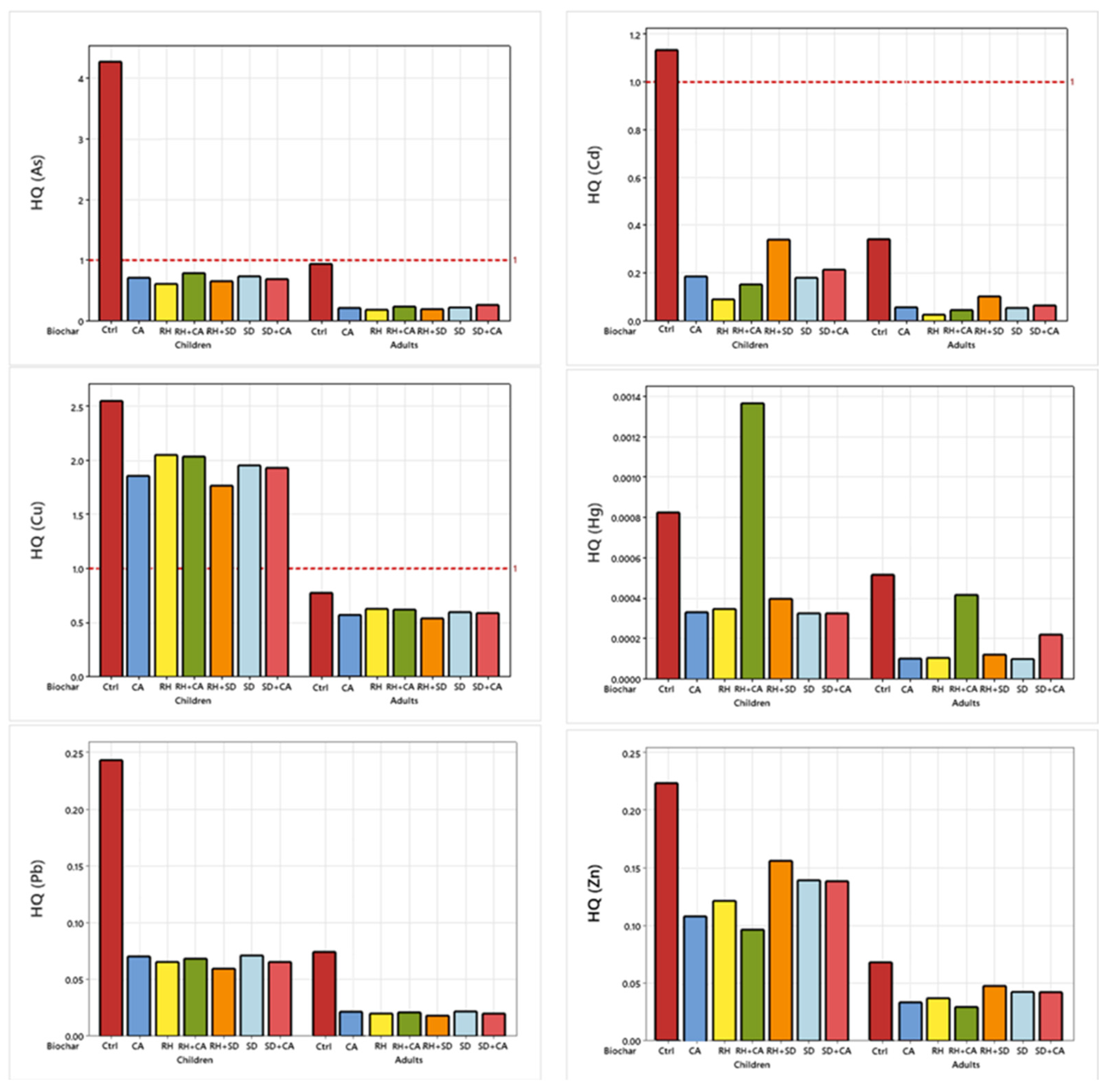
3.7. Human Health Risks Assessment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ali, W.; Mao, K.; Zhang, H.; Junaid, M.; Xu, N.; Rasool, A.; Feng, X.; Yang, Z. Comprehensive Review of the Basic Chemical Behaviours, Sources, Processes, and Endpoints of Trace Element Contamination in Paddy Soil-Rice Systems in Rice-Growing Countries. J. Hazard. Mater. 2020, 397, 122720. [Google Scholar] [CrossRef] [PubMed]
- Fahad, S.; Adnan, M.; Noor, M.; Arif, M.; Alam, M.; Khan, I.A.; Ullah, H.; Wahid, F.; Mian, I.A.; Jamal, Y.; et al. Major Constraints for Global Rice Production. In Advances in Rice Research for Abiotic Stress Tolerance; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–22. [Google Scholar] [CrossRef]
- Sarkar, M.I.U.; Shahriar, S.; Naidu, R.; Rahman, M.M. Concentrations of Potentially Toxic and Essential Trace Elements in Marketed Rice of Bangladesh: Exposure and Health Risks. J. Food Compos. Anal. 2023, 117, 105109. [Google Scholar] [CrossRef]
- MoFA. Planting for for Food and Jobs Programme; Ministry of Food and Agriculture: Accra, Ghana, 2023. Available online: https://mofa.gov.gh/site/media-centre/latest-news/item/625-pfj-2-0-objectives-and-interventions-for-agriculture (accessed on 17 April 2025).
- Coffie, I.S.; Tweneboah-Koduah, E.Y.; Ocloo, E.C.; Nkukpornu, A.; Kastner, A.N.A. Improving Local Rice Consumption in Sub-Saharan Africa through Social Marketing: Evidence from Ghana. Int. Rev. Public Nonprofit Mark. 2024, 21, 177–196. [Google Scholar] [CrossRef]
- Sebiawu, G.E.; Napolean, M.J.; Amankwah, E. Bioaccumulation of Heavy Metals Concentration in Some Selected Cereals Grown Near Illegal Mine Sites at Poyentanga in Wa of the Upper West Region, Ghana. Eur. J. Environ. Earth Sci. 2020, 1, 1–15. [Google Scholar] [CrossRef]
- Khatun, J.; Intekhab, A.; Dhak, D. Effect of Uncontrolled Fertilization and Heavy Metal Toxicity Associated with Arsenic(As), Lead(Pb) and Cadmium (Cd), and Possible Remediation. Toxicology 2022, 477, 153274. [Google Scholar] [CrossRef]
- Sharma, S.; Kaur, I.; Nagpal, A.K. Contamination of Rice Crop with Potentially Toxic Elements and Associated Human Health Risks—A Review. Environ. Sci. Pollut. Res. 2021, 28, 12282–12299. [Google Scholar] [CrossRef]
- Issaka, E.; Fapohunda, F.O.; Amu-Darko, J.N.O.; Yeboah, L.; Yakubu, S.; Varjani, S.; Ali, N.; Bilal, M. Biochar-Based Composites for Remediation of Polluted Wastewater and Soil Environments: Challenges and Prospects. Chemosphere 2022, 297, 134163. [Google Scholar] [CrossRef]
- Rehman, S.u.; De Castro, F.; Marini, P.; Aprile, A.; Benedetti, M.; Fanizzi, F.P. Vermibiochar: A Novel Approach for Reducing the Environmental Impact of Heavy Metals Contamination in Agricultural Land. Sustainability 2023, 15, 9380. [Google Scholar] [CrossRef]
- Duan, Z.; Chen, C.; Ni, C.; Xiong, J.; Wang, Z.; Cai, J.; Tan, W. How Different Is the Remediation Effect of Biochar for Cadmium Contaminated Soil in Various Cropping Systems? A Global Meta-Analysis. J. Hazard. Mater. 2023, 448, 130939. [Google Scholar] [CrossRef]
- Xia, S.; Song, Z.; Jeyakumar, P.; Bolan, N.; Wang, H. Characteristics and Applications of Biochar for Remediating Cr(VI)-Contaminated Soils and Wastewater. Environ. Geochem. Health 2020, 42, 1543–1567. [Google Scholar] [CrossRef]
- Nkoh, J.N.; Ajibade, F.O.; Atakpa, E.O.; Baquy, M.A.-A.; Mia, S.; Odii, E.C.; Xu, R. Reduction of Heavy Metal Uptake from Polluted Soils and Associated Health Risks through Biochar Amendment: A Critical Synthesis. J. Hazard. Mater. Adv. 2022, 6, 100086. [Google Scholar] [CrossRef]
- Weber, K.; Quicker, P. Properties of Biochar. Fuel 2018, 217, 240–261. [Google Scholar] [CrossRef]
- Li, S.; Harris, S.; Anandhi, A.; Chen, G. Predicting Biochar Properties and Functions Based on Feedstock and Pyrolysis Temperature: A Review and Data Syntheses. J. Clean. Prod. 2019, 215, 890–902. [Google Scholar] [CrossRef]
- Joseph, S.; Cowie, A.L.; Van Zwieten, L.; Bolan, N.; Budai, A.; Buss, W.; Cayuela, M.L.; Graber, E.R.; Ippolito, J.A.; Kuzyakov, Y.; et al. How Biochar Works, and When It Doesn’t: A Review of Mechanisms Controlling Soil and Plant Responses to Biochar. GCB Bioenergy 2021, 13, 1731–1764. [Google Scholar] [CrossRef]
- Gautam, R.K.; Goswami, M.; Mishra, R.K.; Chaturvedi, P.; Awashthi, M.K.; Singh, R.S.; Giri, B.S.; Pandey, A. Biochar for Remediation of Agrochemicals and Synthetic Organic Dyes from Environmental Samples: A Review. Chemosphere 2021, 272, 129917. [Google Scholar] [CrossRef] [PubMed]
- Pinzon-Nuñez, D.A.; Adarme-Durán, C.A.; Vargas-Fiallo, L.Y.; Rodriguez-Lopez, N.; Rios-Reyes, C.A. Biochar as a Waste Management Strategy for Cadmium Contaminated Cocoa Pod Husk Residues. Int. J. Recycl. Org. Waste Agric. 2022, 11, 101–115. [Google Scholar] [CrossRef]
- Lu, K.; Yang, X.; Gielen, G.; Bolan, N.; Ok, Y.S.; Niazi, N.K.; Xu, S.; Yuan, G.; Chen, X.; Zhang, X.; et al. Effect of Bamboo and Rice Straw Biochars on the Mobility and Redistribution of Heavy Metals (Cd, Cu, Pb and Zn) in Contaminated Soil. J. Environ. Manag. 2017, 186, 285–292. [Google Scholar] [CrossRef]
- Okoro, H.K.; Alao, S.M.; Pandey, S.; Jimoh, I.; Basheeru, K.A.; Caliphs, Z.; Ngila, J.C. Recent Potential Application of Rice Husk as an Eco-Friendly Adsorbent for Removal of Heavy Metals. Appl. Water Sci. 2022, 12, 259. [Google Scholar] [CrossRef]
- Beesley, L.; Moreno-Jiménez, E.; Gomez-Eyles, J.L.; Harris, E.; Robinson, B.; Sizmur, T. A Review of Biochars’ Potential Role in the Remediation, Revegetation and Restoration of Contaminated Soils. Environ. Pollut. 2011, 159, 3269–3282. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, J.; Tang, L.; Su, M.; Tian, D.; Zhang, L.; Li, Z.; Hu, S. Enhanced Pb Immobilization via the Combination of Biochar and Phosphate Solubilizing Bacteria. Environ. Int. 2019, 127, 395–401. [Google Scholar] [CrossRef]
- Ghana Statistical Service (GSS). Ejisu-Juaben Municipal. In District Analytical Report (Ejisu-Juabeng Municipal); 2014; p. 81. Available online: https://www2.statsghana.gov.gh/docfiles/2010_District_Report/Ashanti/Ejisu-Juaben%20Municipality.pdf (accessed on 17 April 2025).
- Bashagaluke, J.B.; Logah, V.; Opoku, A.; Sarkodie-Addo, J.; Quansah, C. Soil Nutrient Loss through Erosion: Impact of Different Cropping Systems and Soil Amendments in Ghana. PLoS ONE 2018, 13, e0208250. [Google Scholar] [CrossRef] [PubMed]
- Ouattara; Kouadio, E.; Kouassi, E.; Doudjo, S.; Soro, Y.; Yao, B.; Adouby, K.; Drogui, A.; Tyagi, D.; Aina, P. Cocoa pod husk: A review. Bioresources 2021, 16, 1988–2020. Available online: https://www.researchgate.net/publication/357157254 (accessed on 17 April 2025). [CrossRef]
- Khosrowshahi, M.S.; Mashhadimoslem, H.; Shayesteh, H.; Singh, G.; Khakpour, E.; Guan, X.; Rahimi, M.; Maleki, F.; Kumar, P.; Vinu, A. Natural Products Derived Porous Carbons for CO2 Capture. Adv. Sci. 2023, 10, 2304289. [Google Scholar] [CrossRef] [PubMed]
- Mesele, S.A.; Ocansey, C.M.; Bougma, A.; Azeez, J.O.; Ajiboye, G.A.; Logah, V.; Compaore, H.; Veenendaal, E.M.; Lloyd, J. Emerging Ecological Trends in West Africa: Implications on Soil Organic Matter and Other Soil Quality Indicators. Plant Soil 2024, 502, 605–622. [Google Scholar] [CrossRef]
- Razzaghi, F.; Arthur, E.; Moosavi, A.A. Evaluating Models to Estimate Cation Exchange Capacity of Calcareous Soils. Geoderma 2021, 400, 115221. [Google Scholar] [CrossRef]
- Solly, E.F.; Weber, V.; Zimmermann, S.; Walthert, L.; Hagedorn, F.; Schmidt, M.W.I. A Critical Evaluation of the Relationship Between the Effective Cation Exchange Capacity and Soil Organic Carbon Content in Swiss Forest Soils. Front. For. Glob. Change 2020, 3, 566869. [Google Scholar] [CrossRef]
- Asigbaase, M.; Annan, M.; Adusu, D.; Abugre, S.; Nsor, C.A.; Kumi, S.; Acheamfour, S.A. Teak-soil Interaction: Teak (Tectona Grandis) Plantations Impact and Are Impacted by Soil Properties and Fertility in Southwestern Ghana. Appl. Environ. Soil Sci. 2024, 2024, 7931830. [Google Scholar] [CrossRef]
- Mausbach, M.J.; Lytle, D.J.; Spivey, L.D. Application of Soil Survey Information to Soil Specific Farming; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2015; pp. 57–68. [Google Scholar] [CrossRef]
- Yeboah, S.; Dogbatse, J.A.; Asiedu Kumi, M.; Supe Tulcan, R.X.; Addae-Wireko, L. Heavy Metal Status in Cocoa (Theobroma cacao L.) Soils and Beans: The Case of Abuakwa North Municipality of Eastern Region, Ghana. Environ. Monit. Assess. 2024, 196, 156. [Google Scholar] [CrossRef]
- CCME. Canadian Soil Quality Guidelines for the Protection of Environmental and Human Health. 2002. Available online: https://ccme.ca/en/res/nonylphenol-and-its-ethoxylates-canadian-soil-quality-guidelines-for-the-protection-of-environmental-and-human-health-en.pdf (accessed on 17 April 2025).
- VROM. Dutch Target and Intervention Values; 2000 (the New Dutch List). Neth. Gov. Gaz. 2000, 2000, 1–12. [Google Scholar]
- Dodd, M.; Amponsah, L.O.; Grundy, S.; Darko, G. Human Health Risk Associated with Metal Exposure at Agbogbloshie E-Waste Site and the Surrounding Neighbourhood in Accra, Ghana. Environ. Geochem. Health 2023, 45, 4515–4531. [Google Scholar] [CrossRef]
- Kamani, H.; Mahvi, A.H.; Seyedsalehi, M.; Jaafari, J.; Hoseini, M.; Safari, G.H.; Dalvand, A.; Aslani, H.; Mirzaei, N.; Ashrafi, S.D. Contamination and Ecological Risk Assessment of Heavy Metals in Street Dust of Tehran, Iran. Int. J. Environ. Sci. Technol. 2017, 14, 2675–2682. [Google Scholar] [CrossRef]
- Akanchise, T.; Boakye, S.; Borquaye, L.S.; Dodd, M.; Darko, G. Distribution of Heavy Metals in Soils from Abandoned Dump Sites in Kumasi, Ghana. Sci. Afr. 2020, 10, e00614. [Google Scholar] [CrossRef]
- Hakanson, L. An Ecological Risk Index for Aquatic Pollution Control.a Sedimentological Approach. Water Res. 1980, 14, 975–1001. [Google Scholar] [CrossRef]
- Asare, P.; Sarpong, K.; Gyamfi, O.; Ankapong, E.; Agyei, V.; Amissah-Reynolds, P.K.; Dartey, E. Contamination and Health Risk Assessment of Potentially Toxic Elements in Rice (Oryza sativa) and Soil from Ashanti Region. Environ. Monit. Assess. 2024, 196, 1139. [Google Scholar] [CrossRef] [PubMed]
- Sharafi, K.; Nodehi, R.N.; Mahvi, A.H.; Pirsaheb, M.; Nazmara, S.; Mahmoudi, B.; Yunesian, M. Bioaccessibility Analysis of Toxic Metals in Consumed Rice through an in Vitro Human Digestion Model—Comparison of Calculated Human Health Risk from Raw, Cooked and Digested Rice. Food Chem. 2019, 299, 125126. [Google Scholar] [CrossRef]
- Huang, K.; Li, M.; Li, R.; Rasul, F.; Shahzad, S.; Wu, C.; Shao, J.; Huang, G.; Li, R.; Almari, S.; et al. Soil Acidification and Salinity: The Importance of Biochar Application to Agricultural Soils. Front. Plant Sci. 2023, 14, 1206820. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhang, Y.; Tao, W.; Zhang, X.; Xu, Z.; Xu, C. The Biological Effects of Biochar on Soil’s Physical and Chemical Characteristics: A Review. Sustainability 2025, 17, 2214. [Google Scholar] [CrossRef]
- Kumar, A.; Bhattacharya, T.; Shaikh, W.A.; Roy, A. Sustainable Soil Management under Drought Stress through Biochar Application: Immobilizing Arsenic, Ameliorating Soil Quality, and Augmenting Plant Growth. Environ. Res. 2024, 259, 119531. [Google Scholar] [CrossRef]
- Chen, L.; Guo, L.; Liao, P.; Xiong, Q.; Deng, X.; Gao, H.; Wei, H.; Dai, Q.; Pan, X.; Zeng, Y.; et al. Effects of Biochar on the Dynamic Immobilization of Cd and Cu and Rice Accumulation in Soils with Different Acidity Levels. J. Clean. Prod. 2022, 372, 133730. [Google Scholar] [CrossRef]
- Luo, D.; Wang, L.; Nan, H.; Cao, Y.; Wang, H.; Kumar, T.V.; Wang, C. Phosphorus Adsorption by Functionalized Biochar: A Review. Environ. Chem. Lett. 2023, 21, 497–524. [Google Scholar] [CrossRef]
- Tan, S.; Narayanan, M.; Thu Huong, D.T.; Ito, N.; Unpaprom, Y.; Pugazhendhi, A.; Lan Chi, N.T.; Liu, J. A Perspective on the Interaction between Biochar and Soil Microbes: A Way to Regain Soil Eminence. Environ. Res. 2022, 214, 113832. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Northup, B.K.; Rice, C.W.; Prasad, P.V.V. Biochar Applications Influence Soil Physical and Chemical Properties, Microbial Diversity, and Crop Productivity: A Meta-Analysis. Biochar 2022, 4, 8. [Google Scholar] [CrossRef]
- Delgado, A.N. (Ed.) Planet Earth: Scientific Proposals to Solve Urgent Issues; Springer International Publishing: Cham, Switzerland, 2024. [Google Scholar] [CrossRef]
- Rizwan, M.S.; Imtiaz, M.; Huang, G.; Chhajro, M.A.; Liu, Y.; Fu, Q.; Zhu, J.; Ashraf, M.; Zafar, M.; Bashir, S.; et al. Immobilization of Pb and Cu in Polluted Soil by Superphosphate, Multi-Walled Carbon Nanotube, Rice Straw and Its Derived Biochar. Environ. Sci. Pollut. Res. 2016, 23, 15532–15543. [Google Scholar] [CrossRef]
- Gao, Y.; Wu, P.; Jeyakumar, P.; Bolan, N.; Wang, H.; Gao, B.; Wang, S.; Wang, B. Biochar as a Potential Strategy for Remediation of Contaminated Mining Soils: Mechanisms, Applications, and Future Perspectives. J. Environ. Manag. 2022, 313, 114973. [Google Scholar] [CrossRef] [PubMed]
- Cairns, S.; Chaudhuri, S.; Sigmund, G.; Robertson, I.; Hawkins, N.; Dunlop, T.; Hofmann, T. Wood Ash Amended Biochar for the Removal of Lead, Copper, Zinc and Cadmium from Aqueous Solution. Environ. Technol. Innov. 2021, 24, 101961. [Google Scholar] [CrossRef]
- Lima, J.Z.; Ogura, A.P.; Espíndola, E.L.G.; Ferreira da Silva, E.; Rodrigues, V.G.S. Post-Sorption of Cd, Pb, and Zn onto Peat, Compost, and Biochar: Short-Term Effects of Ecotoxicity and Bioaccessibility. Chemosphere 2024, 352, 141521. [Google Scholar] [CrossRef]
- Melo, L.C.A.; Puga, A.P.; Coscione, A.R.; Beesley, L.; Abreu, C.A.; Camargo, O.A. Sorption and Desorption of Cadmium and Zinc in Two Tropical Soils Amended with Sugarcane-Straw-Derived Biochar. J. Soils Sediments 2016, 16, 226–234. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, L.; Chen, Y.; Xue, Y.; Liu, G.; Zheng, X.; Zhou, L.; Zhong, H. Effects of Varying Amounts of Different Biochars on Mercury Methylation in Paddy Soils and Methylmercury Accumulation in Rice (Oryza sativa L.). Sci. Total Environ. 2023, 874, 162459. [Google Scholar] [CrossRef]
- Jomova, K.; Makova, M.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Rhodes, C.J.; Valko, M. Essential Metals in Health and Disease. Chem. Biol. Interact. 2022, 367, 110173. [Google Scholar] [CrossRef]
- Xing, Y.; Wang, J.; Kinder, C.E.S.; Yang, X.; Slaný, M.; Wang, B.; Song, H.; Shaheen, S.M.; Leinweber, P.; Rinklebe, J. Rice Hull Biochar Enhances the Mobilization and Methylation of Mercury in a Soil under Changing Redox Conditions: Implication for Hg Risks Management in Paddy Fields. Environ. Int. 2022, 168, 107484. [Google Scholar] [CrossRef]
- Qiu, M.; Liu, L.; Ling, Q.; Cai, Y.; Yu, S.; Wang, S.; Fu, D.; Hu, B.; Wang, X. Biochar for the Removal of Contaminants from Soil and Water: A Review. Biochar 2022, 4, 19. [Google Scholar] [CrossRef]
- Ghuge, S.A.; Nikalje, G.C.; Kadam, U.S.; Suprasanna, P.; Hong, J.C. Comprehensive Mechanisms of Heavy Metal Toxicity in Plants, Detoxification, and Remediation. J. Hazard. Mater. 2023, 450, 131039. [Google Scholar] [CrossRef] [PubMed]
- Cecire, R.; Diana, A.; Giacomino, A.; Abollino, O.; Inaudi, P.; Favilli, L.; Bertinetti, S.; Cavalera, S.; Celi, L.; Malandrino, M. Rice Husk as a Sustainable Amendment for Heavy Metal Immobilization in Contaminated Soils: A Pathway to Environmental Remediation. Toxics 2024, 12, 790. [Google Scholar] [CrossRef]
- Hartley, W.; Edwards, R.; Lepp, N.W. Arsenic and Heavy Metal Mobility in Iron Oxide-Amended Contaminated Soils as Evaluated by Short- and Long-Term Leaching Tests. Environ. Pollut. 2004, 131, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Suda, A.; Makino, T. Functional Effects of Manganese and Iron Oxides on the Dynamics of Trace Elements in Soils with a Special Focus on Arsenic and Cadmium: A Review. Geoderma 2016, 270, 68–75. [Google Scholar] [CrossRef]
- Yang, Y.; Piao, Y.; Wang, R.; Su, Y.; Liu, N.; Lei, Y. Nonmetal Function Groups of Biochar for Pollutants Removal: A Review. J. Hazard. Mater. Adv. 2022, 8, 100171. [Google Scholar] [CrossRef]
- Khan, Z.; Xianting, F.; Khan, M.N.; Khan, M.A.; Zhang, K.; Fu, Y.; Shen, H. The Toxicity of Heavy Metals and Plant Signaling Facilitated by Biochar Application: Implications for Stress Mitigation and Crop Production. Chemosphere 2022, 308, 136466. [Google Scholar] [CrossRef]




| Parameter | Biochar/Amendment | Mean | Standard Deviation | Minimum | Maximum |
|---|---|---|---|---|---|
| EC (µS/cm) | Ctrl | 67.33 b | 4.73 | 62.00 | 71.00 |
| CA | 109.30 a | 28.20 | 87.00 | 141.00 | |
| RH | 75.33 b | 5.03 | 70.00 | 80.00 | |
| RH + CA | 62.33 b | 8.08 | 55.00 | 71.00 | |
| RH + SD | 55.00 b | 7.81 | 50.00 | 64.00 | |
| SD | 77.00 b | 28.50 | 49.00 | 106.00 | |
| SD + CA | 57.67 b | 14.15 | 49.00 | 74.00 | |
| F.pr. | <0.001 *** | ||||
| pH | Ctrl | 6.03 b | 0.17 | 5.89 | 6.22 |
| CA | 7.05 a | 0.10 | 6.95 | 7.15 | |
| RH | 7.08 a | 0.12 | 6.97 | 7.21 | |
| RH + CA | 7.04 a | 0.08 | 6.95 | 7.11 | |
| RH + SD | 7.20 a | 0.05 | 7.15 | 7.24 | |
| SD | 7.04 a | 0.19 | 6.89 | 7.25 | |
| SD + CA | 7.12 a | 0.15 | 6.95 | 7.25 | |
| F.pr. | <0.001 *** | ||||
| Available P (mg/kg) | Ctrl | 18.12 b | 0.03 | 18.10 | 18.15 |
| CA | 32.73 a | 14.70 | 21.34 | 49.33 | |
| RH | 28.02 ab | 0.44 | 27.74 | 28.52 | |
| RH + CA | 30.09 a | 2.21 | 28.09 | 32.46 | |
| RH + SD | 24.88 ab | 2.79 | 22.01 | 27.59 | |
| SD | 25.57 ab | 3.57 | 22.28 | 29.37 | |
| SD + CA | 31.17 a | 6.85 | 26.16 | 38.97 | |
| F.pr. | 0.003 ** | ||||
| Soil total N (%) | Ctrl | 0.08 b | 0.01 | 0.07 | 0.09 |
| CA | 0.10 a | 0.02 | 0.09 | 0.12 | |
| RH | 0.11 a | 0.02 | 0.09 | 0.13 | |
| RH + CA | 0.11 a | 0.01 | 0.10 | 0.12 | |
| RH + SD | 0.10 a | 0.02 | 0.08 | 0.12 | |
| SD | 0.10 a | 0.01 | 0.09 | 0.11 | |
| SD + CA | 0.11 a | 0.02 | 0.09 | 0.13 | |
| F.pr. | <0.001 *** | ||||
| Organic Carbon (%) | Ctrl | 0.96 a | 0.10 | 0.90 | 1.07 |
| CA | 0.98 a | 0.11 | 0.86 | 1.07 | |
| RH | 1.03 a | 0.05 | 0.98 | 1.07 | |
| RH + CA | 1.02 a | 0.09 | 0.92 | 1.09 | |
| RH + SD | 1.06 a | 0.08 | 0.98 | 1.13 | |
| SD | 1.08 a | 0.11 | 0.97 | 1.19 | |
| SD + CA | 1.07 a | 0.11 | 0.98 | 1.19 | |
| F.pr. | 0.41 | ||||
| ECEC | Ctrl | 5.21 ab | 0.37 | 4.83 | 5.57 |
| CA | 5.67 a | 0.53 | 5.19 | 6.24 | |
| RH | 5.30 ab | 0.34 | 5.07 | 5.69 | |
| RH + CA | 5.06 ab | 0.25 | 4.78 | 5.23 | |
| RH + SD | 5.08 b | 0.43 | 4.73 | 5.55 | |
| SD | 5.50 ab | 0.54 | 4.89 | 5.91 | |
| SD + CA | 5.05 ab | 0.42 | 4.73 | 5.52 | |
| F.pr. | 0.01 ** |
| Parameter | EC (µS/cm) | pH | P (mg/kg) | % N | % OC | % OM |
|---|---|---|---|---|---|---|
| pH | −0.045 | |||||
| p-value | 0.847 | |||||
| P (mg/kg) | 0.195 | 0.528 * | ||||
| p-value | 0.397 | 0.014 | ||||
| % N | −0.316 | 0.634 ** | 0.373 | |||
| p-value | 0.163 | 0.002 | 0.096 | |||
| % OC | −0.169 | 0.313 | −0.214 | 0.275 | ||
| p-value | 0.464 | 0.167 | 0.353 | 0.228 | ||
| % OM | −0.17 | 0.314 | −0.212 | 0.276 | 1.000 *** | |
| p-value | 0.462 | 0.166 | 0.355 | 0.226 | <0.001 | |
| ECEC | 0.364 | 0.055 | 0.12 | 0.208 | −0.112 | −0.112 |
| p-value | 0.105 | 0.813 | 0.605 | 0.367 | 0.629 | 0.629 |
| Metal | Biochar/Treatment | Mean | StDev | Minimum | Maximum |
|---|---|---|---|---|---|
| As | Ctrl | 0.34 a | 0.05 | 0.30 | 0.40 |
| CA | 0.06 b | 0.01 | 0.04 | 0.07 | |
| RH | 0.05 b | 0.02 | 0.04 | 0.07 | |
| RH + CA | 0.06 b | 0.02 | 0.04 | 0.08 | |
| RH + SD | 0.05 b | 0.01 | 0.04 | 0.06 | |
| SD | 0.06 b | 0.03 | 0.03 | 0.09 | |
| SD + CA | 0.06 b | 0.02 | 0.04 | 0.07 | |
| F.pr. | <0.001 *** | ||||
| Cd | Ctrl | 0.30 a | 0.05 | 0.25 | 0.35 |
| CA | 0.05 b | 0.06 | 0.02 | 0.12 | |
| RH | 0.02 b | 0.01 | 0.02 | 0.04 | |
| RH + CA | 0.04 b | 0.02 | 0.01 | 0.06 | |
| RH + SD | 0.09 b | 0.09 | 0.03 | 0.19 | |
| SD | 0.05 b | 0.01 | 0.04 | 0.06 | |
| SD + CA | 0.06 b | 0.03 | 0.03 | 0.09 | |
| F.pr. | <0.001 *** | ||||
| Cu | Ctrl | 27.06 a | 0.99 | 26.14 | 28.11 |
| CA | 19.76 b | 2.45 | 16.94 | 21.42 | |
| RH | 21.77 b | 1.57 | 20.42 | 23.49 | |
| RH + CA | 21.60 b | 1.87 | 20.14 | 23.71 | |
| RH + SD | 18.76 b | 5.36 | 15.42 | 24.95 | |
| SD | 20.74 b | 4.52 | 15.82 | 24.70 | |
| SD + CA | 20.50 b | 1.68 | 18.65 | 21.92 | |
| F.pr. | <0.001 *** | ||||
| Hg | Ctrl | 0.07 a | 0.01 | 0.05 | 0.08 |
| CA | 0.03 b | 0.00 | 0.03 | 0.03 | |
| RH | 0.03 b | 0.00 | 0.03 | 0.03 | |
| RH + CA | 0.03 b | 0.14 | 0.02 | 0.27 | |
| RH + SD | 0.03 b | 0.01 | 0.02 | 0.04 | |
| SD | 0.03 b | 0.00 | 0.03 | 0.03 | |
| SD + CA | 0.03 b | 0.00 | 0.02 | 0.03 | |
| F.pr. | <0.001 *** | ||||
| Pb | Ctrl | 0.23 a | 0.03 | 0.20 | 0.26 |
| CA | 0.07 b | 0.02 | 0.04 | 0.09 | |
| RH | 0.06 b | 0.01 | 0.05 | 0.07 | |
| RH + CA | 0.07 b | 0.01 | 0.06 | 0.07 | |
| RH + SD | 0.06 b | 0.02 | 0.04 | 0.08 | |
| SD | 0.07 b | 0.01 | 0.06 | 0.07 | |
| SD + CA | 0.06 b | 0.01 | 0.05 | 0.08 | |
| F.pr. | <0.001 *** | ||||
| Zn | Ctrl | 17.73 a | 3.10 | 14.60 | 20.80 |
| CA | 8.70 c | 3.40 | 6.40 | 12.60 | |
| RH | 9.63 ab | 2.46 | 6.80 | 11.20 | |
| RH + CA | 7.68 c | 1.94 | 6.10 | 9.85 | |
| RH + SD | 12.40 b | 2.04 | 10.10 | 14.00 | |
| SD | 11.07 ab | 1.59 | 10.10 | 12.90 | |
| SD + CA | 11.00 ab | 3.38 | 7.90 | 14.60 | |
| F.pr. | <0.001 |
| Metal | Treatment | Mean | StDev | Minimum | Maximum |
|---|---|---|---|---|---|
| As | Ctrl | 0.0084 | 0.0010 | 0.0069 | 0.0097 |
| CA | 0.0019 | 0.0004 | 0.0015 | 0.0025 | |
| RH | 0.0016 | 0.0004 | 0.0012 | 0.0021 | |
| RH + CA | 0.0018 | 0.0009 | 0.0011 | 0.0031 | |
| RH + SD | 0.0020 | 0.0004 | 0.0013 | 0.0023 | |
| SD | 0.0019 | 0.0010 | 0.0009 | 0.0035 | |
| SD + CA | 0.0022 | 0.0005 | 0.0017 | 0.0028 | |
| Cd | Ctrl | 0.0053 | 0.0013 | 0.0036 | 0.0067 |
| CA | 0.0015 | 0.0010 | 0.0004 | 0.0028 | |
| RH | 0.0005 | 0.0002 | 0.0004 | 0.0008 | |
| RH + CA | 0.0008 | 0.0005 | 0.0004 | 0.0014 | |
| RH + SD | 0.0020 | 0.0012 | 0.0006 | 0.0039 | |
| SD | 0.0014 | 0.0003 | 0.0010 | 0.0018 | |
| SD + CA | 0.0026 | 0.0014 | 0.0008 | 0.0039 | |
| Cu | Ctrl | 1.0966 | 0.1182 | 0.9120 | 1.2383 |
| CA | 1.6160 | 0.3710 | 1.1640 | 2.0330 | |
| RH | 1.5321 | 0.1787 | 1.3107 | 1.7290 | |
| RH + CA | 1.3239 | 0.1047 | 1.2000 | 1.4591 | |
| RH + SD | 1.1130 | 0.2730 | 0.9100 | 1.5850 | |
| SD | 1.2787 | 0.1680 | 1.1684 | 1.5743 | |
| SD + CA | 1.3741 | 0.1712 | 1.1276 | 1.5149 | |
| Hg | Ctrl | 0.0118 | 0.0019 | 0.0098 | 0.0147 |
| CA | 0.0090 | 0.0036 | 0.0052 | 0.0134 | |
| RH | 0.0108 | 0.0049 | 0.0065 | 0.0182 | |
| RH + CA | 0.0079 | 0.0011 | 0.0060 | 0.0089 | |
| RH + SD | 0.0184 | 0.0117 | 0.0060 | 0.0370 | |
| SD | 0.0101 | 0.0049 | 0.0066 | 0.0178 | |
| SD + CA | 0.0071 | 0.0030 | 0.0033 | 0.0109 | |
| Pb | Ctrl | 0.0146 | 0.0018 | 0.0127 | 0.0175 |
| CA | 0.0063 | 0.0025 | 0.0041 | 0.0106 | |
| RH | 0.0068 | 0.0012 | 0.0049 | 0.0080 | |
| RH + CA | 0.0071 | 0.0019 | 0.0053 | 0.0097 | |
| RH + SD | 0.0055 | 0.0036 | 0.0020 | 0.0115 | |
| SD | 0.0069 | 0.0012 | 0.0053 | 0.0086 | |
| SD + CA | 0.0073 | 0.0017 | 0.0054 | 0.0098 | |
| Zn | Ctrl | 1.2070 | 0.2950 | 0.7870 | 1.4620 |
| CA | 0.8220 | 0.3940 | 0.5270 | 1.4750 | |
| RH | 0.9664 | 0.1290 | 0.7780 | 1.1426 | |
| RH + CA | 0.7140 | 0.1007 | 0.6180 | 0.8779 | |
| RH + SD | 1.3500 | 0.2300 | 1.1120 | 1.7030 | |
| SD | 1.1565 | 0.2197 | 0.9091 | 1.4760 | |
| SD + CA | 1.1620 | 0.4230 | 0.7020 | 1.8360 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boakye, K.O.; Dodd, M.; Asante, M.D.; Logah, V.; Darko, G. Biochar Amendment in Remediation of Heavy Metals in Paddy Soil: A Case Study in Nobewam, Ghana. Soil Syst. 2025, 9, 38. https://doi.org/10.3390/soilsystems9020038
Boakye KO, Dodd M, Asante MD, Logah V, Darko G. Biochar Amendment in Remediation of Heavy Metals in Paddy Soil: A Case Study in Nobewam, Ghana. Soil Systems. 2025; 9(2):38. https://doi.org/10.3390/soilsystems9020038
Chicago/Turabian StyleBoakye, Kwadwo Owusu, Matt Dodd, Maxwell Darko Asante, Vincent Logah, and Godfred Darko. 2025. "Biochar Amendment in Remediation of Heavy Metals in Paddy Soil: A Case Study in Nobewam, Ghana" Soil Systems 9, no. 2: 38. https://doi.org/10.3390/soilsystems9020038
APA StyleBoakye, K. O., Dodd, M., Asante, M. D., Logah, V., & Darko, G. (2025). Biochar Amendment in Remediation of Heavy Metals in Paddy Soil: A Case Study in Nobewam, Ghana. Soil Systems, 9(2), 38. https://doi.org/10.3390/soilsystems9020038