Production and Characterization of Biochar from Almond Shells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection and Characterization of Almond Hulls
2.2. Production of Almond Shells Biochar
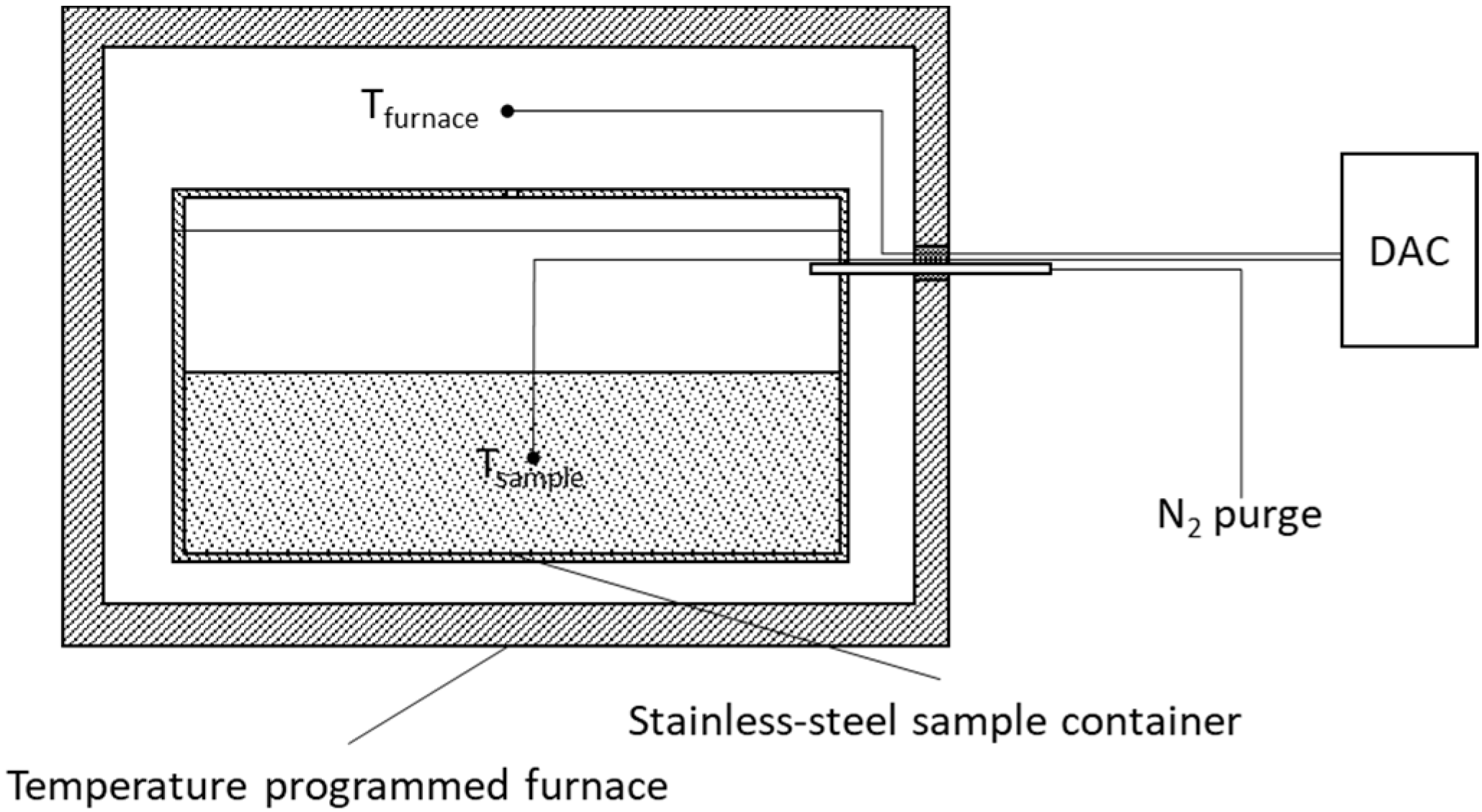
2.2.1. Laboratory Production of Biochar
2.2.2. Pilot-Scale Biochar Production
2.3. Experimental Design and Data Analysis of the Biochar Production in the Furnace
2.4. Biochar Yield Calculation and Characterization
3. Results and Discussion
3.1. Laboratory Production of Biochar
3.2. Pilot-Scale Production of Biochar
3.3. Biochar Characteristics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Almond Board of California. Almond Almanac. 2020. Available online: https://www.almonds.com/sites/default/files/2020-12/2020%20Almond%20Almanac.pdf (accessed on 1 July 2022).
- Aktas, T.; Thy, B.; Williams, R.B.; McCaffrey, Z.; Khatami, R.; Jenkins, B.M. Characterization of almond processing residues from the Central Valley of California for thermal conversion. Fuel Process. Technol. 2015, 140, 132–147. [Google Scholar] [CrossRef]
- McCaffrey, Z. Tar Management and Recycling in Biomass Gasification and Syngas Purification. Ph.D. Thesis, University of California Davis, Davis, CA, USA, 2015. [Google Scholar]
- Chen, P.; Cheng, Y.; Deng, S.; Lin, X.; Huang, G.; Ruan, R. Utilization of almond residues. Int. J. Agric. Biol. Eng. 2010, 3, 1–18. [Google Scholar]
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.E.; Zhang, M.; Bolan, N.; Mohan, D.; Ok, Y.S. Biochar as a sorbent for contaminant management in soil and water: A review. Chemosphere 2014, 99, 19–33. [Google Scholar] [CrossRef]
- González, J.F.; Ramiro, A.; González-García, C.M.; Gañán, J.; Encinar, J.M.; Sabio, E.; Jesús Rubiales, J. Pyrolysis of Almond Shells. Energy Applications of Fractions. Ind. Eng. Chem. Res. 2005, 44, 3003–3012. [Google Scholar] [CrossRef]
- Lehmann, J.; Rillig, M.C.; Thies, J.; Masiello, C.A.; Hockaday, W.C.; Crowley, D. Biochar effects on soil biota—A review. Soil Biol Biochem. 2011, 43, 1812–1836. [Google Scholar] [CrossRef]
- Matin, N.H.; Jalali, M.; Antoniadis, V.; Shaheen, S.M.; Wang, J.; Zhang, T.; Wang, H.; Rinklebe, J. Almond and walnut shell-derived biochars affect sorption-desorption, fractionation, and release of phosphorus in two different soils. Chemosphere 2020, 241, 124888. [Google Scholar] [CrossRef] [PubMed]
- Netherway, P.; Gascó, G.; Méndez, A.; Surapaneni, A.; Reichman, S.; Shah, K.; Paz-Ferreiro, J. Using Phosphorus-Rich Biochars to Remediate Lead-Contaminated Soil: Influence on Soil Enzymes and Extractable P. Agronomy 2020, 10, 454. [Google Scholar] [CrossRef]
- Lehmann, J.; Skjemstad, J.; Sohi, S.; Carter, J.; Barson, M.; Falloon, P.; Coleman, K.; Woodbury, P.; Krull, A.E. Australian climate–carbon cycle feedback reduced by soil black carbon. Nat. Geosci. 2008, 1, 832–835. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Subbotina, I.; Chen, H.; Bogomolova, I.; Xu, X. Black carbon decomposition and incorporation into soil microbial biomass estimated by 14C labeling. Soil Biol. Biochem. 2009, 41, 210–219. [Google Scholar] [CrossRef]
- Glaser, B.; Lehmann, J.; Zech, W. Ameliorating physical and chemical properties of highly weathered soils in the tropics with charcoal—A review. Biol. Fertil. Soils 2002, 35, 219–230. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association: Washington, DC, USA, 2017; ISBN 9780875532356. [Google Scholar]
- ASAE/ASABE S269.5 (R2021); Densified Products for Bulk Handling—Definitions and Method. American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2021.
- McCaffrey, Z.; Thy, P.; Long, M.; Oliveira, M.; Wang, L.; Torres, L.; Aktas, T.; Chiou, B.S.; Orts, W.; Jenkins, B.M. Air and steam gasification of almond biomass. Front. Energy Res. 2019, 7, 84. [Google Scholar] [CrossRef]
- Montgomery, D.C.; Runger, G.C. Applied Statistics and Probability for Engineers; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011. [Google Scholar]
- Brown, R.A.; Kercher, A.K.; Nguyen, T.H.; Nagle, D.C.; Ball, W.P. Production and characterization of synthetic wood chars for use as surrogates for natural sorbents. Org. Geochem. 2006, 37, 321–333. [Google Scholar] [CrossRef]
- Akçay, H.; Anagun, A.S. Multi response optimization application on a manufacturing factory. Comput. Math. Appl. 2013, 18, 531–538. [Google Scholar] [CrossRef]
- Bryden, K.M.; Ragland, K.W.; Rutland, C.J. Modeling thermally thick pyrolysis of wood. Biomass Bioenergy 2002, 22, 41–53. [Google Scholar] [CrossRef]
- Gezahegn, S.; Sain, M.; Thomas, S.C. Variation in feedstock wood chemistry strongly influences biochar liming potential. Soil Syst. 2019, 3, 26. [Google Scholar] [CrossRef]
- Ruz, P.; Banerjee, S.; Pandey, M.; Sudarsan, V.; Sastry, P.U.; Kshirsagar, R.J. Structural evolution of turbostratic carbon: Implications in H2 storage. Solid State Sci. 2016, 62, 105–111. [Google Scholar] [CrossRef]
- Brewer, C.E.; Chuang, V.J.; Masiello, C.A.; Gonnermann, H.; Gao, X.; Dugan, B.; Driver, L.E.; Panzacchi, P.; Zygourakis, K.; Davies, C.A. New approaches to measuring biochar density and porosity. Biomass Bioenergy 2014, 66, 176–185. [Google Scholar] [CrossRef]
- Brewer, C.E.; Schmidt-Rohr, K.; Satrio, J.A.; Brown, R.C. Characterization of biochar from fast pyrolysis and gasification systems. Environ. Prog. Sustain. Energy 2009, 28, 386–396. [Google Scholar] [CrossRef]
- Emmett, P.H. Adsorption and pore-size measurements on charcoal and whetlerites. Chem. Rev. 1948, 43, 69–148. [Google Scholar] [CrossRef] [PubMed]



| Parameters | Almond Shells Used in Experiments | Biochar | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kiln | Furnace | Kiln | Commercial Biochar | Furnace Temperature | |||||||||||||||
| 300 °C | 525 °C | 750 °C | |||||||||||||||||
| Residence Time (min) | |||||||||||||||||||
| 30 | 30 | 60 | 90 | 90 | 30 | 60 | 60 | 60 | 90 | 30 | 30 | 60 | 90 | 90 | |||||
| Moisture Content of Shell | |||||||||||||||||||
| 5% | 15% | 10% | 5% | 15% | 10% | 5% | 10% | 15% | 10% | 5% | 15% | 10% | 5% | 15% | |||||
| MC (%) | 8.30 | 5.40 | 18.70 | 4.50 | 1.40 | 2.40 | 2.50 | 1.40 | 1.00 | 0.60 | 2.00 | 1.17 | 3.10 | 0.70 | 0.90 | 0.50 | 2.00 | 1.70 | 1.70 |
| TS (%) | 91.70 | 94.60 | 81.30 | 95.50 | 98.60 | 97.60 | 97.50 | 98.60 | 99.00 | 99.40 | 98.00 | 98.83 | 96.90 | 99.30 | 99.10 | 99.50 | 98.00 | 98.30 | 98.30 |
| VS/TS (%) | 92.82 | ND * | 67.30 | 28.09 | 75.97 | N.D. | 71.18 | 68.95 | N.D. | 73.68 | 82.04 | 75.88 | N.D. | 75.0 | 80.8 | 93.62 | 69.65 | 85.01 | N.D. |
| pH | 4.80 | 4.90 | 9.50 | 10.50 | 7.50 | 6.90 | 7.90 | 8.00 | 8.00 | 9.90 | 9.90 | 9.83 | 9.80 | 9.90 | 9.90 | 9.90 | 10.00 | 10.00 | 10.00 |
| EC (mmhos/cm) | 11.30 | 11.30 | 23.60 | 76.40 | 11.70 | 9.88 | 13.90 | 13.70 | 8.81 | 26.40 | 30.70 | 30.70 | 18.10 | 29.30 | 30.70 | 30.80 | 45.50 | 36.90 | 33.40 |
| Sol Salts (mg/L) | 7232 | 7232 | 15,104 | 48,896 | 7488 | 6323 | 8896 | 8768 | 5638 | 16,896 | 19,648 | 19,648 | 11,584 | 18,752 | 19,648 | 19,712 | 29,120 | 23,616 | 21,376 |
| C:N Ratio | 4:1 | 5:1 | 27:1 | 36:1 | 6:1 | 6:1 | 7:1 | 7:1 | 9:1 | 13:1 | 13:1 | 14:1 | 13:1 | 15:1 | 17:1 | 17:1 | 13:1 | 17:1 | 19:1 |
| Total N (%) | 0.664 | 0.601 | 0.670 | 0.700 | 0.830 | 0.719 | 0.959 | 0.740 | 0.828 | 0.894 | 0.758 | 0.750 | 0.707 | 0.820 | 0.781 | 0.750 | 0.871 | 0.820 | 0.737 |
| Total P (%) | 0.125 | 0.138 | 0.170 | 0.700 | 0.143 | 0.120 | 0.140 | 0.127 | 0.121 | 0.188 | 0.168 | 0.170 | 0.184 | 0.187 | 0.195 | 0.176 | 0.210 | 0.205 | 0.190 |
| K (%) | 2.07 | 1.89 | 3.77 | 6.58 | 2.77 | 2.01 | 3.02 | 2.52 | 2.92 | 4.75 | 2.41 | 3.90 | 4.52 | 3.44 | 3.62 | 4.40 | 4.89 | 4.57 | 2.92 |
| Na (%) | 0.061 | 0.093 | 0.070 | 0.090 | 0.056 | 0.044 | 0.077 | 0.045 | 0.044 | 0.049 | 0.047 | 0.050 | 0.050 | 0.050 | 0.061 | 0.076 | 0.078 | 0.080 | 0.090 |
| S (%) | 0.060 | 0.060 | 0.060 | 0.110 | 0.040 | 0.030 | 0.030 | 0.020 | 0.030 | 0.030 | 0.020 | 0.020 | 0.030 | 0.030 | 0.070 | 0.050 | 0.060 | 0.090 | 0.050 |
| Ca (%) | 0.357 | 0.383 | 0.820 | 8.850 | 0.517 | 0.366 | 0.538 | 0.424 | 0.501 | 0.785 | 0.695 | 0.670 | 0.735 | 0.766 | 0.550 | 0.801 | 0.768 | 0.867 | 0.650 |
| Mg (%) | 0.155 | 0.154 | 0.480 | 0.980 | 0.199 | 0.169 | 0.212 | 0.184 | 0.198 | 0.280 | 0.246 | 0.250 | 0.245 | 0.269 | 0.217 | 0.273 | 0.275 | 0.296 | 0.257 |
| B (mg/L) | 56.80 | 50.20 | 103.00 | 407.00 | 85.90 | 80.40 | 92.10 | 84.40 | 88.30 | 116.00 | 110.00 | 107.33 | 111.00 | 118.00 | 123.00 | 105.00 | 137.00 | 138.00 | 112 |
| Zn (mg/L) | 27.60 | 21.00 | 5880.00 | 119.00 | 14.90 | 7.23 | 13.10 | 8.81 | 12.00 | 18.90 | 17.10 | 14.07 | 15.80 | 17.20 | 8.80 | 9.78 | 12.00 | 9.44 | 8.36 |
| Mn (mg/L) | 23.40 | 30.20 | 162.00 | 241.00 | 28.60 | 22.80 | 34.80 | 23.00 | 32.10 | 53.80 | 41.80 | 41.87 | 41.30 | 49.20 | 38.70 | 51.60 | 53.10 | 56.00 | 46.10 |
| Fe (mg/L) | 438 | 409 | 14,600 | 1580 | 672 | 680 | 1160 | 487 | 1160 | 1700 | 1040 | 1075 | 903 | 1430 | 775 | 1240 | 1250 | 1040 | 1230 |
| Cu (mg/L) | 10.60 | 11.10 | 26.20 | 111.00 | 17.20 | 14.10 | 20.40 | 12.20 | 19.20 | 19.70 | 21.20 | 18.40 | 21.20 | 22.00 | 16.90 | 22.00 | 22.70 | 25.20 | 18.80 |
| Bulk density (g/cm3) | 0.248 | 0.230 | 0.356 | N.D. | 0.171 | 0.168 | 0.180 | 0.169 | 0.148 | 0.159 | 0.138 | 0.170 | 0.165 | 0.154 | 0.170 | 0.162 | 0.167 | 0.169 | 0.161 |
| Experiment | Set Point Temperature (X1, °C) | Residence Time (X2, min) | Moisture Content (X3, % w.b.) | Coded Values | Biochar Yield (% of Almond Shell Weight) | |||
|---|---|---|---|---|---|---|---|---|
| X1 | X2 | X3 | Measured | Predicted | ||||
| 1 | 300 | 30 | 5 | −1 | −1 | −1 | 50.0 | 55.7 |
| 2 | 300 | 90 | 5 | −1 | 1 | −1 | 44.4 | 42.5 |
| 3 | 750 | 30 | 5 | 1 | −1 | −1 | 28.9 | 26.7 |
| 4 | 750 | 90 | 5 | 1 | 1 | −1 | 28.0 | 27.6 |
| 5 | 525 | 60 | 5 | 0 | 0 | −1 | 31.9 | 30.9 |
| 6 | 300 | 60 | 10 | −1 | 0 | 0 | 44.3 | 49.1 |
| 7 | 525 | 90 | 10 | 0 | 1 | 0 | 29.9 | 27.8 |
| 8 | 750 | 60 | 10 | 1 | 0 | 0 | 26.6 | 27.2 |
| 9 | 525 | 60 | 10 | 0 | 0 | 0 | 30.4 | 30.9 |
| 10 | 525 | 30 | 10 | 0 | −1 | 0 | 30.9 | 34.0 |
| 11 | 525 | 60 | 10 | 0 | 0 | 0 | 30.6 | 30.9 |
| 12 | 525 | 60 | 10 | 0 | 0 | 0 | 30.9 | 30.9 |
| 13 | 750 | 30 | 15 | 1 | −1 | 1 | 26.0 | 26.7 |
| 14 | 750 | 90 | 15 | 1 | 1 | 1 | 26.2 | 27.6 |
| 15 | 300 | 90 | 15 | −1 | 1 | 1 | 41.6 | 42.5 |
| 16 | 300 | 30 | 15 | −1 | −1 | 1 | 65.1 | 55.7 |
| 17 | 525 | 60 | 15 | 0 | 0 | 1 | 31.5 | 30.9 |
| Parameter | SumSq | Df | MeanSq | F | p Value |
|---|---|---|---|---|---|
| X1 | 1202.80 | 1 | 1202.80 | 107.22 | 1.670 × 10−5 |
| X2 | 94.96 | 1 | 94.96 | 8.47 | 0.023 |
| X3 | 5.27 | 1 | 5.27 | 0.47 | 0.515 |
| X1:X2 | 100.40 | 1 | 100.40 | 8.95 | 0.02 |
| X1:X3 | 36.36 | 1 | 36.36 | 3.24 | 0.114 |
| X2:X3 | 34.74 | 1 | 34.74 | 3.10 | 0.122 |
| X12 | 89.97 | 1 | 89.97 | 8.02 | 0.025 |
| X22 | 1.59 | 1 | 1.59 | 0.14 | 0.718 |
| X32 | 11.17 | 1 | 11.17 | 1.00 | 0.351 |
| Error | 78.53 | 7 | 11.22 | ||
| Total | 1786.60 | 16 | 111.66 | ||
| Model | 1708.00 | 9 | 189.78 | 16.92 | 0.0006 |
| Linear | 1303.00 | 3 | 434.34 | 38.72 | 9.962 × 10−5 |
| Nonlinear | 405.02 | 6 | 67.50 | 6.02 | 0.016 |
| Residual | 78.53 | 7 | 11.22 | ||
| Lack of fit | 78.38 | 5 | 15.68 | 219.81 | 0.005 |
| Pure error | 0.143 | 2 | 0.071 |
| Parameter | SumSq | Df | MeanSq | F | p Value |
|---|---|---|---|---|---|
| X1 | 1202.80 | 1 | 1202.80 | 83.65 | 9.31 × 10−7 |
| X2 | 94.96 | 1 | 94.96 | 6.60 | 0.0245 |
| X1:X2 | 100.40 | 1 | 100.40 | 6.98 | 0.0215 |
| X12 | 215.88 | 1 | 215.88 | 15.01 | 0.002 |
| Error | 172.54 | 12 | 14.38 | ||
| Total | 1786.60 | 16 | 111.66 | ||
| Model | 1614.00 | 4 | 403.51 | 28.06 | 5.21 × 10−6 |
| Linear | 1297.80 | 2 | 648.88 | 45.13 | 2.61 × 10−6 |
| Nonlinear | 316.28 | 2 | 158.14 | 11.00 | 0.002 |
| Residual | 172.54 | 12 | 14.38 | ||
| Lack of fit | 46.76 | 4 | 11.69 | 0.74 | 0.589 |
| Pure error | 125.79 | 8 | 15.723 |
| Experiment | Test Conditions | Particle Density (g/cm3) | ||
|---|---|---|---|---|
| Temperature (X1, °C) | Time (X2, min) | Moisture Content (X3, % w.b.) | ||
| 1 | 300 | 30 | 5 | 1.21 ± 0.02 |
| 2 | 300 | 90 | 5 | 1.27 ± 0.01 |
| 3 | 750 | 30 | 5 | 2.06 ± 0.01 |
| 4 | 750 | 90 | 5 | 2.64 ± 0.17 |
| 5 | 525 | 60 | 5 | 1.99 ± 0.13 |
| 6 | 300 | 60 | 10 | 1.19 ± 0.04 |
| 7 | 525 | 90 | 10 | 1.84 ± 0.03 |
| 8 | 750 | 60 | 10 | 1.88 ± 0.21 |
| 9 | 525 | 60 | 10 | 1.30 ± 0.02 |
| 10 | 525 | 30 | 10 | 1.62 ± 0.01 |
| 11 | 525 | 60 | 10 | 1.41 ± 0.02 |
| 13 | 750 | 30 | 15 | 2.77 ± 0.42 |
| 14 | 750 | 90 | 15 | 2.76 ± 0.06 |
| 15 | 300 | 90 | 15 | 1.11 ± 0.05 |
| 16 | 300 | 30 | 15 | 1.05 ± 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Mashad, H.M.; Edalati, A.; Zhang, R.; Jenkins, B.M. Production and Characterization of Biochar from Almond Shells. Clean Technol. 2022, 4, 854-864. https://doi.org/10.3390/cleantechnol4030053
El Mashad HM, Edalati A, Zhang R, Jenkins BM. Production and Characterization of Biochar from Almond Shells. Clean Technologies. 2022; 4(3):854-864. https://doi.org/10.3390/cleantechnol4030053
Chicago/Turabian StyleEl Mashad, Hamed M., Abdolhossein Edalati, Ruihong Zhang, and Bryan M. Jenkins. 2022. "Production and Characterization of Biochar from Almond Shells" Clean Technologies 4, no. 3: 854-864. https://doi.org/10.3390/cleantechnol4030053




