Abstract
The presence of eriochrome black T (EBT) dye in waste water causes a significant hazard to human health and ecology. In the current study, biosorption was employed to eliminate EBT from water. Thus, we utilized endophytic fungi strain Exserohilum rostratum NMS1.5 mycelia biomass as biosorbent agent. The process was carried out at room temperature by magnetic stirring. The results indicated that an increase in pH would decrease adsorption capacity and removal percentage. In addition, an increased EBT concentration would decrease the removal percentage and increase biosorption capacity. The equilibrium time indicated that after 300 min of mixing, the percentage removal and biosorption capacity were 80.5% and 100.61 mg/g, respectively. The biosorption isotherms and kinetics were compatible with the Freundlich model and the pseudo-second-order. This research indicates that E. rostratum NMS1.5 may be utilized as an environmentally friendly and affordable alternative biosorbent material for EBT removal.
1. Introduction
Textile industry effluent is a considerable problem when dyes that are present in aquatic environments are toxic, carcinogenic and mutagenic [1,2]. Even at a low concentration (<1 mg/L), the colors are visible in water [3]. Eriochrome black T (EBT) is an azo dye containing sulphonate groups and aromatic rings discovered in wastewater [4]. Conventional methods such as coagulation, ion exchange, membrane filtration, adsorption and advanced oxidation processes have been investigated for dye removal [5,6,7]. Among these, adsorption is considered one of the famous and attractive technologies that use an adsorbent [8,9].
Numerous sorbent materials such as zeolite [8] and activated carbon [10] have been studied. However, these sorbents are still a little expensive. As a result of the above reasons, researchers have reported using biological materials such as algae, fungi and bacteria for dye removal from water, which are practical and economical [11]. On the other hand, these materials are not toxic since they do not require a supply of nutrients, which are referred to as biosorbents, and the process is called biosorption [12,13]. In this study, we used the endophytic fungi strain Exserohilum rostratum NMS1.5 mycelia biomass to remove eriochrome black T (EBT) from water.
2. Materials and Methods
2.1. Materials
Eriochrome black T (EBT), sodium hydroxide (NaOH), hydrochloric acid (HCl), sulfuric acid (H2SO4), and sodium chloride (NaCl) were purchased from Kanto Chemical Co., Inc. (Tokyo, Japan). A pH meter and magnetic stirrer were used LAQUA twin Horiba and REXIM RSH-4AN, respectively. The biosorbent of Exserohilum rostratum NMS1.5 mycelia biomass was derived from prior studies [14]. The fungal endophytes were cultivated in a PDB medium and incubated at 28 °C for seven days. Then, the medium was filtered, and mycelia were oven-dried at 60 °C for three days for further experiments.
2.2. Biosorption Experiments
All experiments were conducted on a magnetic stirrer at room temperature. After treatment process, the sorbent was filtered and supernatant put in cuvette (Quartz) to check absorbance (556 nm). We analyzed the effect of pH (2–10), initial EBT concentration (10–25 mg/L), and contact time (15–300 min). The biosorption removal rate and capacity were calculated using Equations (1) and (2), respectively.
where %R: removal rate (%), q: adsorption capacity (mg/g) (t: time), Co: initial concentration (mg/L), Ce: concentration at time (mg/L), W: biosorbent mass (g), V: volume (L).
2.3. Characterization
EBT concentration was measured using a UV–Vis spectrophotometer (Jasco V-530, Tokyo, Japan). The functional group of the biosorbent was determined using ATR-FTIR (Thermo Scientific Nicolet iS10, Thermo Fisher Scientific, Waltham, MA, USA).
3. Results and Discussion
3.1. FTIR Data before and after Adsorption
Figure 1 illustrates the FTIR spectra before and after biosorption. After the biosorption process, the peak shifted from 3273 to 3333 cm−1, suggesting the formation of -OH. A peak between 2840 and 3000 cm−1 represents C-H stretching. After biosorption, an increased band peak from 1635 to 1647 cm−1 was assigned to N-H, which corresponded to the O-H groups. The bands at 1549 and 1532 cm−1 suggested that the N-O and C-H groups corresponded. A new band that formed at 1337 cm−1 after biosorption was probably dye to C-H bending [15].
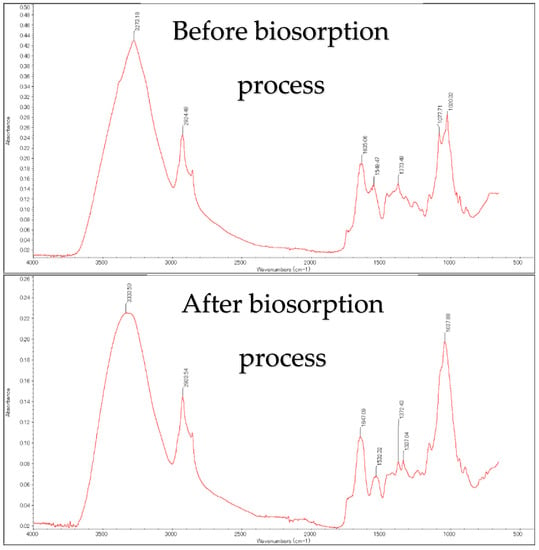
Figure 1.
FTIR data before and after EBT biosorption.
3.2. The pH Effects
The pH is the most important in biosorption experiments, since it influences both the biosorption capacity and the color of the medium. Figure 2 illustrates the pH dependency of EBT biosorption effectiveness on the biosorbent. The findings suggested that pH 2 might be suitable for EBT biosorption, similar to Rezaee et al. and Chettri et al. [16,17]. This is owing due to the strong electrostatic interaction between the positively charged surface of Exserohilum rostratum NMS1.5 and EBT, which is created by absorbing H+ ions.
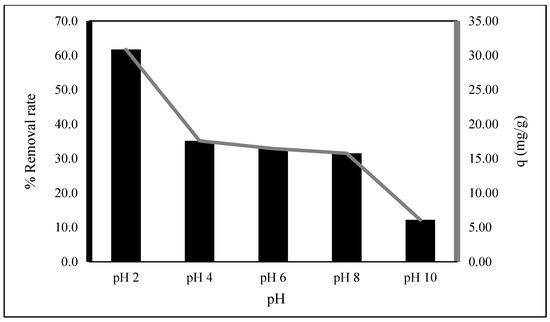
Figure 2.
pH effects (0.01 g, 50 mL (EBT: 10 mg/L), time: 15 min).
3.3. Effects of Initial EBT Concentration
The initial EBT concentration ranged from 10 to 25 mg/L and a biosorbent dose of 0.01 g/50 mL of dye solution was used to evaluate EBT biosorption (Figure 3). The biosorption capacity increased as the concentration increased from 30.86 to 66.80 mg/g, but the percentage removal decreased from 61.7 to 12.2%. This is because increased EBT concentration would be an increased number of molecules. Then, an increase in the biosorption capacity. However, it would decrease the mass transfer resistance of the adsorbate. Consequently, the removal percentage decreases [8,18,19].
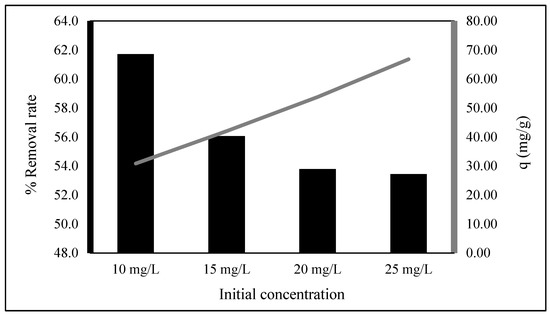
Figure 3.
Initial EBT concentration effects on adsorption capacity and removal percentage (time: 15 min).
3.4. Isotherm Studies
The adsorption isotherm models depict the biosorbent with the biosorbate and describe the interaction between the biosorption capacity and the liquid phase concentration of biosorbate under equilibrium conditions at a constant temperature [20]. In this investigation, the Langmuir and Freundlich models were used [21,22,23,24]. Langmuir occurs on a homogeneous biosorbent surface, while Freundlich on the heterogeneous biosorbent surface [18,24]. The Langmuir, Langmuir separation factor and Freundlich are presented in Equations (3)–(5), respectively.
where q is the adsorbent amount at time (mg/g), Kl is the interaction constant between adsorbate and adsorbent in Langmuir model (L/mg), qmax is the maximum biosorption capacity (mg/g), Ce is the adsorbate equilibrium concentration (mg/L). Kf is the biosorption capacity in the Freundlich model (mg/g), 1/n is the intensity of biosorption. RL is the adsorbate and biosorbent interaction which it can be classified as favorable (RL < 1), linear (RL = 1), unfavorable (RL > 1), and irreversible (RL = 0) [25].
The experimental isotherm studies and linear correlation are shown in Figure 4 and Table 1, respectively. The biosorption process was found to be favorable (RL = 0.001) and fitted the Freundlich isotherm model (R2 = 0.988).
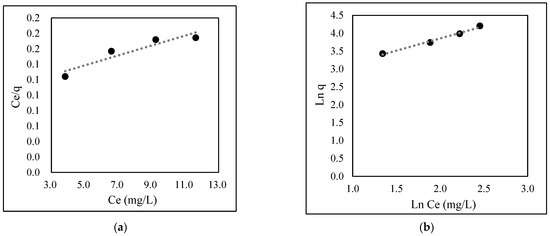
Figure 4.
Adsorption isotherm model for EBT: (a) Langmuir; (b) Freundlich.

Table 1.
Langmuir and Freundlich isotherm models of EBT adsorption.
3.5. Adsorption Kinetics
The biosorption with the interaction time was evaluated in the range of 15–300 min with 25 mg/L of EBT concentration, and 0.01 g/50 mL of EBT solution was used. Figure 5 shows that in the first 15 min, a rapid increase in the biosorption capacity and percentage removal occurred. Then, they slowed at 120 min and gradually increased to 300 min. In the early phase of biosorption, the EBT dye particle to be absorbed was almost fully present in the solution with a high possibility of contacting the biosorbents’ surface, and the active sites were unoccupied [26].
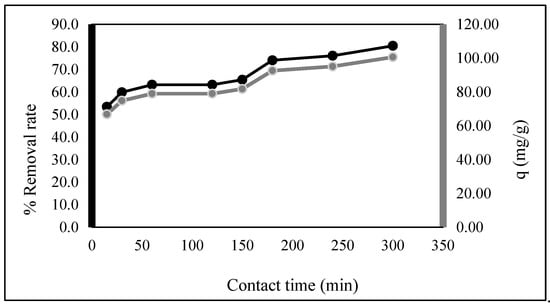
Figure 5.
Contact time effects on EBT biosorption capacity and removal percentage (0.01 g, 50 mL (EBT: 25 mg/L), time: 15–300 min).
A significant number of linear kinetic models are utilized in the literature to examine the controlling mechanism of the biosorption process. Most researchers used pseudo-first-order and pseudo-second-order models [8,27]. The equations for pseudo-first Equation (6) and second Equation (7) models are shown below.
The linear correlation and experimental data of EBT biosorption onto Exserohilum rostratum NMS1.5 are shown in Table 2 and Figure 6, respectively. We can see that the R2 value of pseudo-second-order models was better than pseudo-first-order models. This indicated the pseudo-second-order kinetic model was fit for the biosorption process.

Table 2.
Comparison between pseudo-first-order and pseudo-second order kinetics models for the biosorption of EBT.
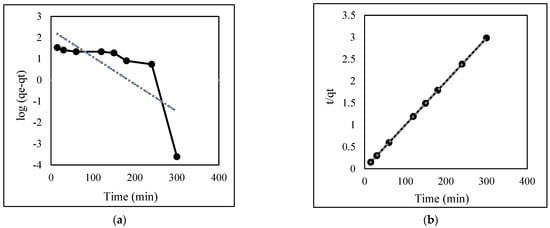
Figure 6.
Adsorption kinetics model of EBT: (a) pseudo-first order; (b) pseudo-second order.
4. Conclusions
In the present study, we made the first report for the use of affordable and non-toxic mycelia biomass of Exserohilum rostratum NMS1.5 to remove EBT dye from water. We studied the effect of pH, initial EBT concentration and contact time. The results obtained showed that the optimum conditions were at pH 2 with a removal percentage and sorption capacity of 80.5% and 100.61 mg/g, respectively, for 300 min of equilibrium time. Increases in the initial EBT concentration would decrease the removal percentage and increase sorption capacity. The isotherm and kinetic model were fitted to the Freundlich and pseudo-second-order, respectively. The results indicated that Exserohilum rostratum NMS1.5 mycelia biomass could effectively remove EBT dye from water.
Author Contributions
Conceptualization, E.H. and H.H.; methodology, E.H., H.H. and S.K.; resources, E.H., H.H. and S.K.; data curation, E.H., H.H., S.Y. and Y.M.; writing—original draft preparation, E.H.; writing—review and editing, E.H., H.H., S.Y. and Y.M.; visualization, E.H., H.H., S.Y. and Y.M.; supervision, H.H., S.Y. and Y.M.; project administration, E.H., H.H., S.Y. and Y.M.; funding acquisition, E.H. and H.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The biosorbent was obtained from https://doi.org/10.1016/j.rhisph.2021.100379 (accessed on 13 September 2022).
Acknowledgments
The author (E.H.) thanks to the MEXT scholarship for sponsoring of study at the Prefectural University of Hiroshima, Japan.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhu, Y.; Yi, B.; Yuan, Q.; Wu, Y.; Wang, M.; Yan, S. Removal of methylene blue from aqueous solution by cattle manure-derived low temperature biochar. RSC Adv. 2018, 8, 19917–19929. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Pu, S.; Hou, Y.; Zhu, R.; Zinchenko, A.; Chu, W. A highly efficient magnetic chitosan “fluid” adsorbent with a high capacity and fast adsorption kinetics for dyeing wastewater purification. J. Chem. Eng. 2018, 345, 556–565. [Google Scholar] [CrossRef]
- Pereira, L.; Alves, M. Dyes-Environmental Impact and Remediation. In Environmental Protection Strategies for Sustainable Development; Strategies for Sustainability; Malik, A., Grohmann, E., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 111–162. [Google Scholar]
- Barka, N.; Abdennouri, M.; El Makhfouk, M. Removal of methylene blue and eriochrome black T from aqueous solution by biosorption on Scolymus hispanicus L.: Kinetics, equilibrium and thermodynamics. J. Taiwan Inst. Chem. Eng. 2011, 42, 320. [Google Scholar] [CrossRef]
- San, N.O.; Celebioglu, A.; Tümtaş, Y.; Uyar, T.; Tekinay, T. Reusable bacteria immobilized electrospun nanofibrous webs for decolorization of methylene blue dye in wastewater treatment. RSC Adv. 2014, 4, 32249–32255. [Google Scholar] [CrossRef]
- Moussa, D.T.; El-Naas, M.H.; Nasser, M.; Al-Marri, M.J. A comprehensive review of electrocoagulation for water treatment: Potentials and challenges. J. Environ. Manag. 2017, 186, 24–41. [Google Scholar] [CrossRef]
- Arikan, E.B.; Isik, Z.; Bouras, H.D.; Dizge, N. Investigation of immobilized filamentous fungi for treatment of real textile industry wastewater using up flow packed bed bioreactor. Bioresour. Technol. 2019, 7, 100197. [Google Scholar] [CrossRef]
- Hidayat, E.; Harada, H.; Mitoma, Y.; Yonemura, S.; Halem, H.I.A. Rapid removal of acid red 88 by zeolite/chitosan hydrogel in aqueous solution. Polymers 2022, 14, 893. [Google Scholar] [CrossRef]
- Raval, N.P.; Shah, P.U.; Shah, N.K. Malachite green ‘a cationic dye’ and its removal from aqueous solution by adsorption. Appl. Water Sci. 2017, 7, 3407–3445. [Google Scholar] [CrossRef]
- Gusmão, G.K.A.; Gurgel, A.L.V.; Melo, S.T.M.; Gil, L.F. Application of succinylated sugarcane bagasse as adsorbent to remove methylene blue and gentian violet from aqueous solutions-Kinetic and equilibrium studies. Dyes Pigm. 2012, 92, 967–974. [Google Scholar] [CrossRef]
- Khadijah, O.; Lee, K.K.; Abdullah, M.F.F. Isolation, screening and development of local bacterial consortia with azo dyes decolourising capability. Malays. J. Microbiol. 2009, 5, 25–32. [Google Scholar] [CrossRef]
- Boudechiche, N.; Mokaddem, H.; Sadaoui, Z.; Trari, M. Biosorption of cationic dye from aqueous solutions onto lignocellulosic biomass (Luffa cylindrica): Characterization, equilibrium, kinetic and thermodynamic studies. Int. J. Ind. Chem. 2016, 7, 167–180. [Google Scholar] [CrossRef]
- Rafatullah, M.; Sulaiman, O.; Hashim, R.; Ahmad, A. Adsorption of methylene blue on low-cost adsorbents: A review. J. Hazard Mater. 2010, 177, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Khaekhum, S.; Ekspraset, J.; Suebrasri, T.; Mongkolthanaruk, W.; Riddech, N.; Jogloy, S.; Boonlue, S. The first member of Exserohilum rostratum beneficial for promoting growth and yield of sunchoke (Helianthus tuberosus L.). Rhizosphere 2021, 19, 100379. [Google Scholar] [CrossRef]
- Sripiya, R.; Kumar, R. A novel enzymatic method for preparation and characterization of collagen film from swim bladder of fish rohu (Labeo rohita). Food Sci. Nutr. 2015, 6, 1468–1478. [Google Scholar] [CrossRef]
- Rezaee, J.; Salimi, F.; Karami, C. Removal of eriochrome black T dye from water solution using modified nano-boehmite by organic compounds. Desalination Water Treat 2019, 139, 342–351. [Google Scholar] [CrossRef]
- Chettri, P.; Singh, M.K.; Tripathi, A.; Pathak, A.P.; Mandal, R.K.; Tiwari, A. Eriochrome Black T sensing using silver nanoparticle-reduced graphene oxide composite via luminescent “turn-of” mechanism and its biosorption on guava (Psidium guajava) leaf powder. Graphene Technol. 2019, 4, 41–51. [Google Scholar] [CrossRef]
- Miraboutalebi, S.M.; Nikouzad, S.K.; Peydayesh, M.; Allahgholi, N.; Vafajoo, L.; McKay, G. Methylene blue adsorption via maize silk powder: Kinetic, equilibrium, thermodynamic studies and residual error analysis. Process Saf. Environ. Prot. 2017, 106, 191–202. [Google Scholar] [CrossRef]
- Leal, P.V.B.; Gregório, A.M.; Otoni, E.; da Silva, P.R.; de Krauser, M.O.; Holzbach, J.C. Study of adsorption of methylene blue dye on babassu. J. Biotec. Biodiv. 2012, 3, 166–171. [Google Scholar] [CrossRef]
- Nascimento, R.F.; Lima, A.C.A.; Vidal, C.B.; Melo, D.Q.; Raulino, G.S.C. Adsorption: Theoretical Aspects and Environmental Applications, 1st ed.; UFC: Fortaleza, Brazil, 2014; p. 256. [Google Scholar]
- Rangabhashiyam, S.; Lata, S.; Balasubramanian, P. Biosorption characteristics of methylene blue and malachite green from simulated wastewater onto Carica papaya wood biosorbent. Surf. Interfaces 2018, 10, 197–215. [Google Scholar] [CrossRef]
- Almeida, C.A.P.; Debacher, N.A.; Downs, A.J.; Cottet, L.; Mello, C.A.D. Removal of methylene blue from colored effluents by adsorption on montmorillonite clay. J. Colloid Interf. Sci. 2009, 332, 46–53. [Google Scholar] [CrossRef]
- Vadivelan, V.K.; Kumar, V. Equilibrium, kinetics, mechanism, and process design for the sorption of methylene blue onto rice husk. J. Colloid Interf. Sci. 2005, 286, 90–100. [Google Scholar] [CrossRef]
- Tang, Y.; Zeng, Y.; Hu, T.; Zhou, Q.; Peng, Y. Preparation of lignin sulfonate-based mesoporous materials for adsorbing malachite green from aqueous solution. J. Environ. Chem. Eng. 2016, 4, 2900–2910. [Google Scholar] [CrossRef]
- Pal, S.; Ghorai, S.; Das, C.; Samrat, S.; Ghosh, A.; Panda, A.B. Carboxymethyl tamarind-g-poly (acrylamide)/silica: A high performance hybrid nanocomposite for adsorption of methylene blue dye. Ind. Eng. Chem. Res. 2012, 51, 15546–15556. [Google Scholar] [CrossRef]
- Silva, F.; Nascimento, L.; Brito, M.; da Silva, K.; Paschoal, W., Jr.; Fujiyama, R. Biosorption of methylene blue dye using natural biosorbents made from weeds. Materials 2019, 12, 2486. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Khan, A.A.; Chowdhury, A.; Bhakta, A.K.; Mekhalif, Z.; Hussain, S. Efficient and highly selective adsorption of cationic dyes and removal of ciprofloxacin antibiotic by surface modified nickel sulfide nanomaterials: Kinetics, isotherm and adsorption mechanism. Colloids Surf. 2020, 586, 124264. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).