Portable X-ray Fluorescence (p-XRF) Uncertainty Estimation for Glazed Ceramic Analysis: Case of Iznik Tiles
Abstract
1. Introduction
2. Experimental
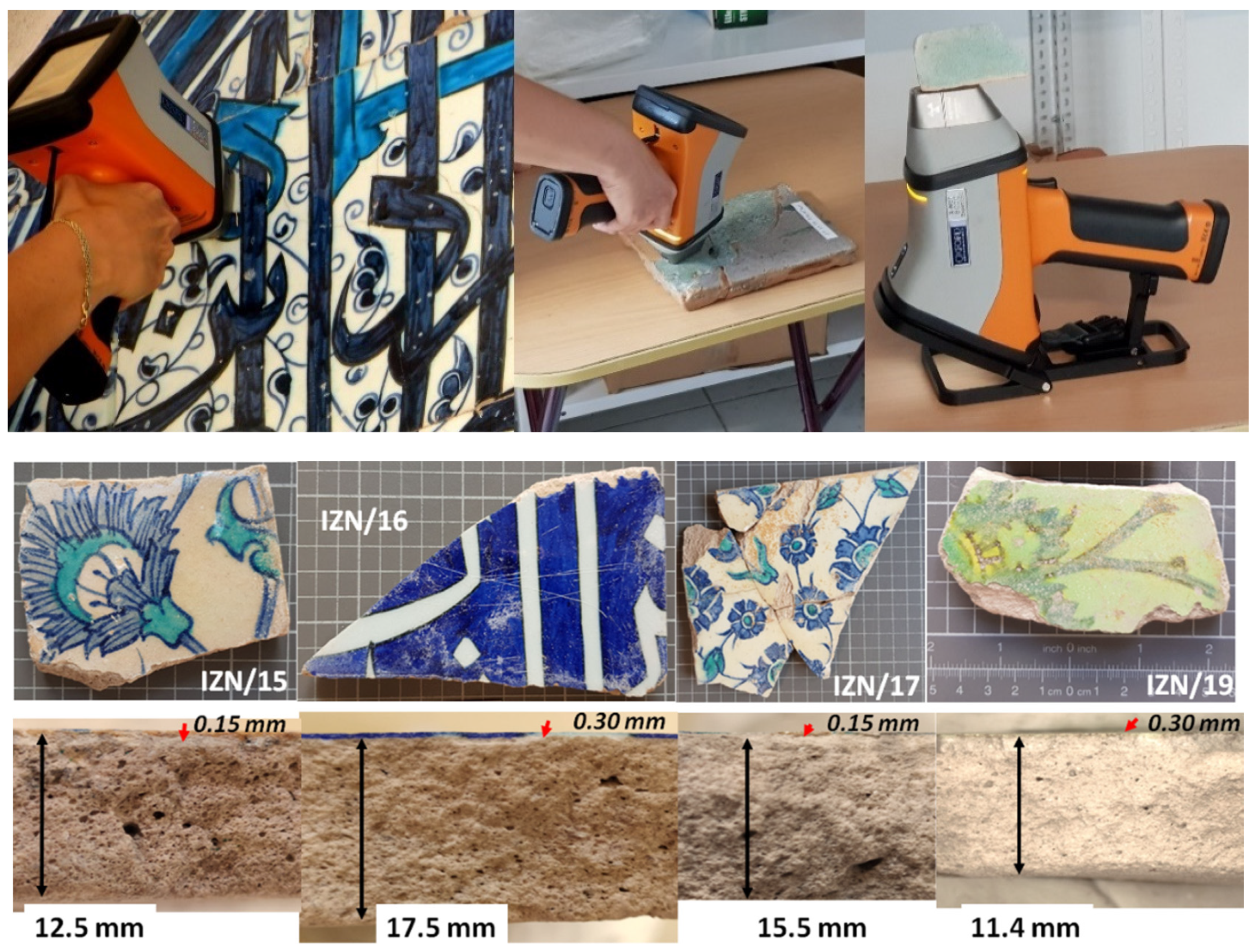
2.1. Artefacts
2.2. XRF Procedure
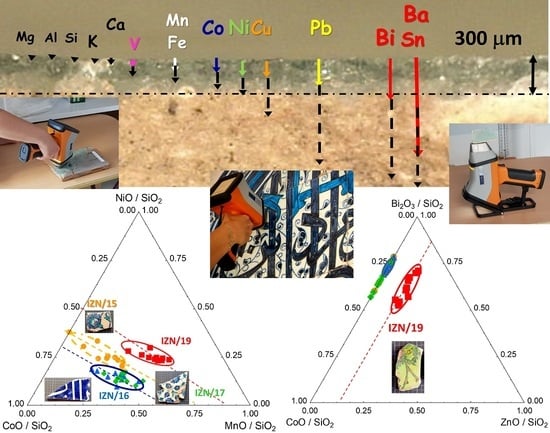
2.3. Errors and Representativeness of the Measurements
3. pXRF Methodology
4. Results—Materials: Microstructure and Elemental Composition
4.1. Body
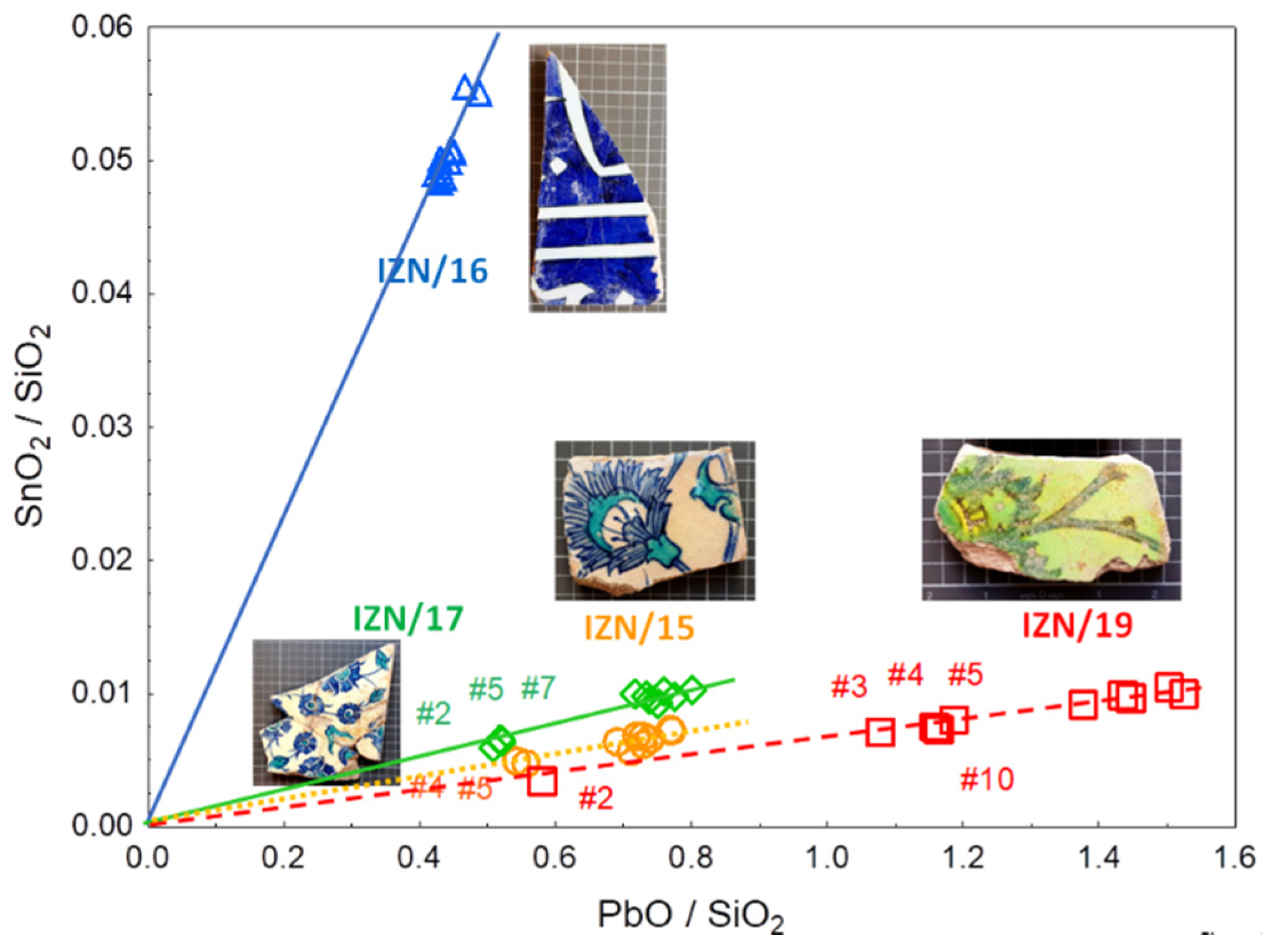
4.2. Glaze
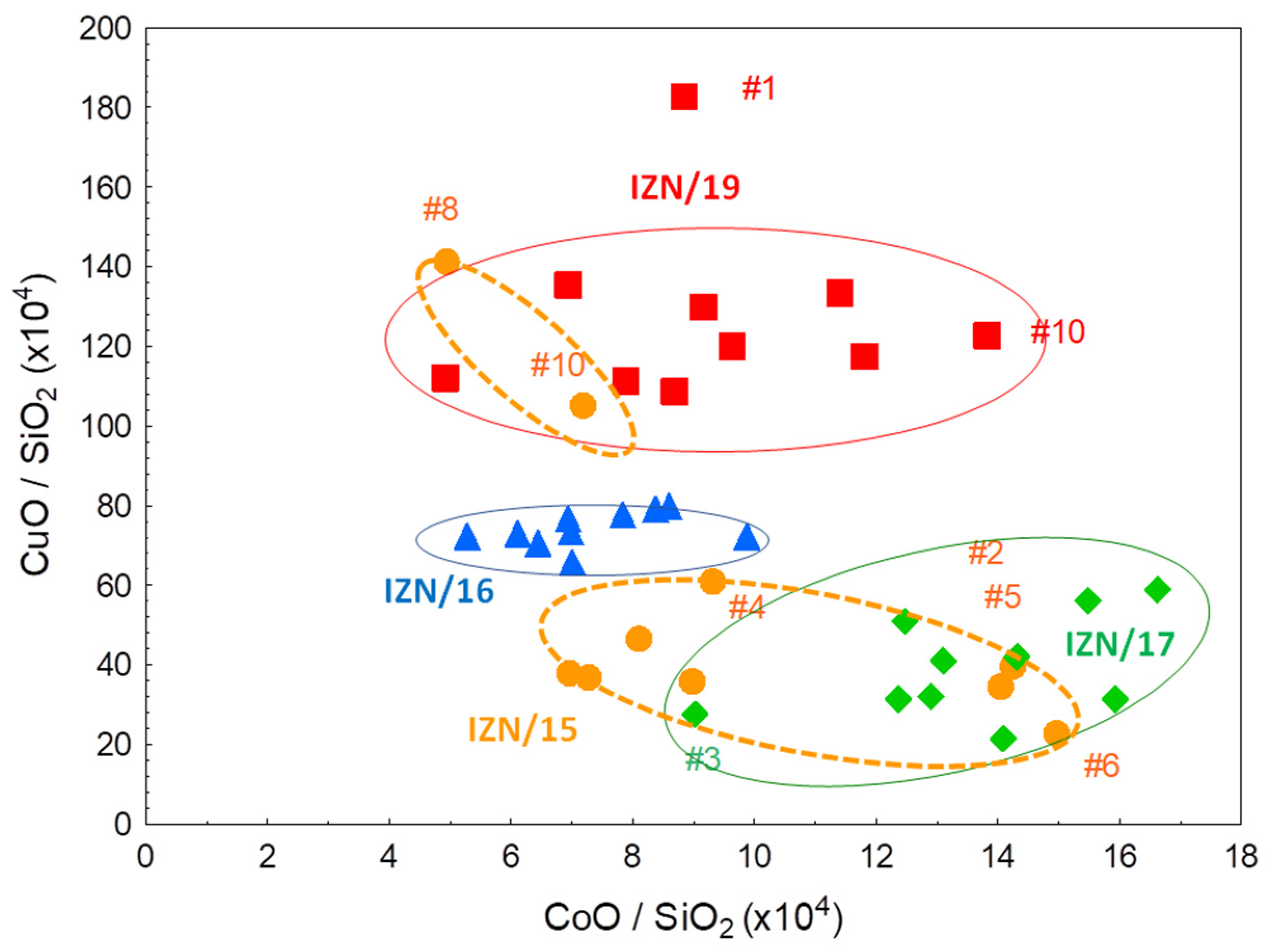
4.3. Blue Colouring Agent
5. Discussion: Comparison of the Results with the Previous Measurements
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1. Analyzed Spots




Appendix A.2. Glaze Thickness

| Label | Acquisition Time | MgO | Al2O3 | SiO2 | K2O | CaO | Fe2O3 | Rb2O | SrO | ZrO2 | PbO |
|---|---|---|---|---|---|---|---|---|---|---|---|
| SRM610 | 360 s | 0.65 | 2.34 | 77.39 | 0.26 | 0.00 | 11.00 | 0.00 | 0.07 | 0.13 | 0.65 |
| 180 s | 0.71 | 2.37 | 77.49 | 0.25 | 0.00 | 10.89 | 0.00 | 0.06 | 0.11 | 0.71 | |
| 120 s | 0.66 | 2.36 | 77.54 | 0.26 | 0.00 | 10.90 | 0.00 | 0.06 | 0.12 | 0.66 | |
| 60 s | 0.68 | 2.36 | 77.44 | 0.25 | 0.00 | 10.97 | 0.00 | 0.06 | 0.13 | 0.68 | |
| 30 s | 0.66 | 2.32 | 77.03 | 0.25 | 0.00 | 11.32 | 0.00 | 0.06 | 0.12 | 0.66 | |
| 20 s | 0.41 | 2.36 | 77.55 | 0.24 | 0.00 | 11.07 | 0.00 | 0.08 | 0.12 | 0.41 | |
| 15 s | 0.31 | 2.40 | 77.62 | 0.24 | 0.00 | 11.02 | 0.00 | 0.07 | 0.12 | 0.31 | |
| 10 s | 1.33 | 2.43 | 76.30 | 0.28 | 0.00 | 11.22 | 0.00 | 0.05 | 0.12 | 1.33 | |
| 5 s | 0.00 | 2.18 | 77.44 | 0.25 | 0.00 | 11.62 | 0.00 | 0.06 | 0.13 | 0.00 | |
| Ref [55] | 0.07 | 2.11 | 72.28 | 0.12 | 0.06 | 11.83 | 0.08 | 0.05 | 0.06 | 0.07 | |
| SRM612 | 360 s | 0.35 | 2.14 | 78.46 | 0.12 | 0.00 | 12.20 | 0.00 | 0.01 | 0.00 | 0.35 |
| 180 s | 0.34 | 2.16 | 78.50 | 0.12 | 0.00 | 12.16 | 0.00 | 0.01 | 0.00 | 0.34 | |
| 120 s | 0.39 | 2.10 | 78.24 | 0.11 | 0.00 | 12.36 | 0.00 | 0.01 | 0.00 | 0.39 | |
| 60 s | 0.00 | 2.10 | 78.60 | 0.12 | 0.00 | 12.41 | 0.00 | 0.01 | 0.00 | 0.00 | |
| 30 s | 0.00 | 2.06 | 78.48 | 0.12 | 0.00 | 12.52 | 0.00 | 0.02 | 0.00 | 0.00 | |
| 20 s | 0.30 | 2.12 | 78.30 | 0.15 | 0.00 | 12.31 | 0.00 | 0.01 | 0.00 | 0.30 | |
| 15 s | 0.00 | 2.17 | 78.33 | 0.15 | 0.00 | 12.49 | 0.00 | 0.01 | 0.00 | 0.00 | |
| 10 s | 0.59 | 2.03 | 78.16 | 0.09 | 0.00 | 12.29 | 0.00 | 0.01 | 0.00 | 0.59 | |
| 5 s | 0.00 | 1.95 | 78.45 | 0.12 | 0.00 | 12.54 | 0.00 | 0.01 | 0.00 | 0.00 | |
| Ref [55] | 0.00 | 2.10 | 71.79 | 0.01 | 0.01 | 11.91 | 0.01 | 0.01 | 0.02 | 0.00 | |
| Corning A | 360 s | 4.77 | 1.14 | 72.00 | 0.21 | 2.96 | 5.27 | 1.01 | 0.99 | 1.26 | 4.77 |
| 180 s | 4.99 | 1.20 | 71.67 | 0.20 | 2.83 | 5.03 | 0.99 | 1.01 | 1.27 | 4.99 | |
| 120 s | 4.87 | 1.25 | 71.78 | 0.21 | 2.83 | 5.03 | 0.99 | 1.02 | 1.27 | 4.87 | |
| 60 s | 4.50 | 1.12 | 71.81 | 0.18 | 2.94 | 5.28 | 1.08 | 1.05 | 1.28 | 4.50 | |
| 30 s | 4.67 | 1.22 | 71.30 | 0.17 | 2.93 | 5.25 | 1.10 | 1.09 | 1.36 | 4.67 | |
| 20 s | 4.59 | 1.24 | 71.76 | 0.20 | 2.85 | 5.10 | 0.97 | 1.01 | 1.33 | 4.59 | |
| 15 s | 4.48 | 1.25 | 71.96 | 0.20 | 2.85 | 5.15 | 0.99 | 1.03 | 1.27 | 4.48 | |
| 10 s | 5.15 | 1.14 | 71.19 | 0.20 | 2.92 | 5.13 | 1.00 | 1.08 | 1.32 | 5.15 | |
| 5 s | 5.86 | 1.21 | 70.88 | 0.21 | 2.78 | 4.96 | 1.04 | 1.05 | 1.22 | 5.86 | |
| Ref [56] | 2.66 | 1.00 | 66.56 | 0.08 | 2.87 | 5.03 | 0.79 | 1.00 | 1.09 | 2.66 |
| Label | Spot | MgO | Al2O3 | SiO2 | K2O | CaO | Fe2O3 | Rb2O | SrO | ZrO2 | PbO |
|---|---|---|---|---|---|---|---|---|---|---|---|
| IZN/15 | #1 | 2.11 | 5.19 | 80.21 | 1.16 | 2.22 | 1.28 | 0.00 | 0.01 | 0.00 | 0.63 |
| #2 | 1.45 | 4.62 | 81.53 | 0.97 | 2.12 | 1.27 | 0.00 | 0.01 | 0.01 | 0.88 | |
| #3 | 2.03 | 4.78 | 78.80 | 1.07 | 2.68 | 1.44 | 0.00 | 0.01 | 0.01 | 0.89 | |
| #4 | 2.35 | 5.93 | 74.90 | 1.35 | 3.30 | 2.07 | 0.00 | 0.01 | 0.01 | 1.23 | |
| #5 | 1.88 | 5.17 | 80.26 | 1.22 | 2.19 | 1.40 | 0.00 | 0.01 | 0.00 | 0.70 | |
| #6 | 2.14 | 5.90 | 78.08 | 1.26 | 2.64 | 1.64 | 0.00 | 0.01 | 0.01 | 0.95 | |
| #7 | 2.02 | 5.33 | 77.49 | 1.17 | 2.77 | 1.81 | 0.01 | 0.01 | 0.01 | 1.12 | |
| #8 | 2.13 | 5.63 | 76.31 | 1.19 | 3.08 | 1.66 | 0.00 | 0.01 | 0.01 | 1.20 | |
| #9 | 2.40 | 5.69 | 76.21 | 1.12 | 3.02 | 1.30 | 0.00 | 0.01 | 0.01 | 1.58 | |
| #10 | 1.85 | 4.73 | 80.78 | 1.03 | 2.17 | 1.34 | 0.00 | 0.01 | 0.01 | 0.87 | |
| IZN/16 | #1 | 2.97 | 4.67 | 75.23 | 1.06 | 5.64 | 1.20 | 0.01 | 0.02 | 0.01 | 1.85 |
| #2 | 3.00 | 5.19 | 75.16 | 1.13 | 5.83 | 1.23 | 0.00 | 0.02 | 0.01 | 1.23 | |
| #3 | 2.38 | 3.54 | 77.18 | 1.04 | 6.00 | 1.19 | 0.01 | 0.02 | 0.01 | 1.39 | |
| #4 | 2.84 | 4.85 | 74.86 | 1.14 | 5.27 | 1.35 | 0.01 | 0.02 | 0.01 | 2.15 | |
| #5 | 2.43 | 4.67 | 72.90 | 1.18 | 7.83 | 1.46 | 0.01 | 0.02 | 0.01 | 2.07 | |
| #6 | 2.81 | 4.59 | 73.33 | 1.06 | 7.43 | 1.20 | 0.01 | 0.02 | 0.01 | 1.99 | |
| #7 | 2.81 | 4.77 | 76.81 | 1.20 | 4.61 | 1.31 | 0.00 | 0.01 | 0.01 | 1.24 | |
| #8 | 2.76 | 3.84 | 77.76 | 0.99 | 4.68 | 1.15 | 0.00 | 0.02 | 0.01 | 1.46 | |
| #9 | 1.66 | 3.51 | 77.04 | 0.99 | 6.37 | 1.23 | 0.01 | 0.02 | 0.01 | 1.71 | |
| #10 | 2.04 | 3.63 | 77.44 | 1.01 | 5.17 | 1.30 | 0.00 | 0.02 | 0.01 | 1.82 | |
| IZN/17 | #1 | 2.29 | 5.18 | 76.00 | 1.08 | 3.92 | 1.52 | 0.01 | 0.01 | 0.01 | 1.40 |
| #2 | 2.73 | 5.21 | 72.81 | 1.12 | 3.99 | 1.51 | 0.01 | 0.01 | 0.01 | 1.54 | |
| #3 | 2.25 | 6.68 | 72.27 | 1.43 | 3.92 | 1.81 | 0.01 | 0.01 | 0.01 | 1.56 | |
| #4 | 2.29 | 5.59 | 72.37 | 1.17 | 4.11 | 1.77 | 0.01 | 0.01 | 0.01 | 1.92 | |
| #5 | 2.49 | 7.09 | 72.08 | 1.57 | 3.71 | 2.04 | 0.01 | 0.01 | 0.01 | 1.69 | |
| #6 | 2.20 | 5.37 | 78.13 | 1.18 | 2.75 | 1.43 | 0.00 | 0.01 | 0.01 | 1.23 | |
| #7 | 2.14 | 5.19 | 74.34 | 1.11 | 4.41 | 1.63 | 0.00 | 0.01 | 0.01 | 1.44 | |
| #8 | 1.72 | 5.11 | 78.62 | 1.05 | 2.46 | 1.47 | 0.01 | 0.01 | 0.01 | 1.29 | |
| #9 | 2.19 | 5.48 | 74.60 | 1.16 | 4.29 | 1.58 | 0.01 | 0.01 | 0.01 | 1.46 | |
| #10 | 2.44 | 6.04 | 72.80 | 1.31 | 4.03 | 1.73 | 0.01 | 0.01 | 0.01 | 1.84 | |
| IZN/19 | #1 | 2.83 | 6.48 | 73.10 | 1.44 | 5.49 | 1.94 | 0.01 | 0.02 | 0.01 | 1.04 |
| #2 | 3.02 | 5.88 | 73.67 | 1.31 | 6.04 | 1.83 | 0.00 | 0.02 | 0.01 | 0.95 | |
| #3 | 2.78 | 4.32 | 76.89 | 1.07 | 5.26 | 1.62 | 0.00 | 0.02 | 0.01 | 1.02 | |
| #4 | 2.62 | 5.34 | 76.04 | 1.19 | 4.84 | 1.67 | 0.00 | 0.02 | 0.01 | 0.95 | |
| #5 | 2.69 | 5.45 | 76.34 | 1.19 | 4.43 | 1.57 | 0.00 | 0.02 | 0.01 | 1.25 | |
| #6 | 2.55 | 5.31 | 76.69 | 1.18 | 4.22 | 1.58 | 0.00 | 0.02 | 0.01 | 1.26 | |
| #7 | 3.01 | 5.22 | 76.38 | 1.23 | 4.56 | 1.81 | 0.00 | 0.02 | 0.01 | 0.78 | |
| #8 | 3.25 | 4.74 | 76.44 | 1.10 | 4.97 | 1.54 | 0.00 | 0.02 | 0.01 | 0.83 | |
| #9 | 2.94 | 4.33 | 77.87 | 1.14 | 3.91 | 1.80 | 0.00 | 0.02 | 0.01 | 0.94 | |
| #10 | 2.56 | 5.89 | 73.36 | 1.41 | 5.23 | 2.10 | 0.01 | 0.02 | 0.01 | 2.20 |
| Label | Spot | MgO | Al2O3 | SiO2 | K2O | CaO | TiO2 | SnO2 | PbO |
|---|---|---|---|---|---|---|---|---|---|
| IZN/15 | #1 | 1.29 | 1.06 | 51.77 | 0.68 | 1.47 | 0.25 | 0.32 | 37.75 |
| #2 | 1.45 | 1.40 | 52.76 | 0.76 | 1.36 | 0.22 | 0.34 | 36.54 | |
| #3 | 0.91 | 1.12 | 50.84 | 0.70 | 1.39 | 0.26 | 0.37 | 39.26 | |
| #4 | 1.32 | 1.82 | 55.91 | 0.98 | 2.32 | 0.26 | 0.26 | 31.20 | |
| #5 | 1.09 | 1.98 | 55.66 | 1.12 | 2.61 | 0.79 | 0.28 | 30.20 | |
| #6 | 1.75 | 1.30 | 50.15 | 0.77 | 1.47 | 0.29 | 0.36 | 38.63 | |
| #7 | 1.21 | 1.25 | 51.39 | 0.76 | 1.48 | 0.25 | 0.33 | 37.99 | |
| #8 | 1.31 | 1.31 | 51.60 | 0.78 | 1.47 | 0.26 | 0.35 | 37.64 | |
| #9 | 0.64 | 1.17 | 52.46 | 0.70 | 1.41 | 0.24 | 0.36 | 37.80 | |
| #10 | 1.70 | 1.37 | 52.04 | 0.75 | 1.37 | 0.23 | 0.29 | 37.05 | |
| IZN/16 | #1 | 0.34 | 0.78 | 61.53 | 1.06 | 0.98 | 0.14 | 3.05 | 26.50 |
| #2 | 0.52 | 0.59 | 61.45 | 1.02 | 0.94 | 0.14 | 3.02 | 26.66 | |
| #3 | 0.28 | 0.70 | 61.32 | 1.20 | 1.11 | 0.19 | 3.07 | 26.46 | |
| #4 | 0.75 | 0.80 | 61.34 | 1.24 | 1.03 | 0.17 | 3.00 | 25.98 | |
| #5 | 0.85 | 0.48 | 60.66 | 1.02 | 0.93 | 0.17 | 3.08 | 27.14 | |
| #6 | 0.86 | 0.76 | 58.73 | 1.66 | 1.27 | 0.24 | 3.26 | 27.43 | |
| #7 | 0.00 | 0.52 | 59.25 | 1.17 | 1.04 | 0.18 | 3.26 | 28.99 | |
| #8 | 0.74 | 0.64 | 60.88 | 1.06 | 1.18 | 0.17 | 2.97 | 26.63 | |
| #9 | 0.91 | 0.62 | 60.22 | 1.11 | 1.00 | 0.19 | 3.05 | 27.20 | |
| #10 | 0.86 | 0.73 | 60.64 | 1.04 | 0.96 | 0.15 | 3.03 | 26.97 | |
| IZN/17 | #1 | 1.26 | 1.45 | 49.41 | 0.88 | 2.03 | 0.31 | 0.48 | 38.33 |
| #2 | 1.33 | 3.50 | 57.36 | 1.52 | 1.20 | 0.13 | 0.34 | 29.17 | |
| #3 | 1.21 | 1.45 | 51.23 | 0.88 | 1.49 | 0.24 | 0.50 | 37.58 | |
| #4 | 1.18 | 1.85 | 50.76 | 1.00 | 1.85 | 0.30 | 0.50 | 36.46 | |
| #5 | 0.86 | 3.44 | 57.28 | 1.52 | 1.16 | 0.14 | 0.36 | 29.85 | |
| #6 | 1.43 | 1.61 | 50.69 | 0.90 | 1.24 | 0.23 | 0.47 | 38.14 | |
| #7 | 1.30 | 3.38 | 57.00 | 1.48 | 1.19 | 0.20 | 0.37 | 29.62 | |
| #8 | 1.38 | 1.45 | 51.15 | 0.83 | 1.30 | 0.29 | 0.49 | 37.85 | |
| #9 | 1.33 | 1.28 | 50.74 | 0.79 | 1.23 | 0.28 | 0.51 | 38.54 | |
| #10 | 1.56 | 1.58 | 48.61 | 0.94 | 1.69 | 0.34 | 0.50 | 38.96 | |
| IZN/19 | #1 | 0.99 | 0.96 | 36.42 | 0.80 | 2.64 | 0.50 | 0.33 | 50.19 |
| #2 | 1.74 | 2.13 | 52.75 | 0.92 | 3.41 | 0.66 | 0.18 | 30.64 | |
| #3 | 2.63 | 1.60 | 39.96 | 0.83 | 3.18 | 0.67 | 0.28 | 43.07 | |
| #4 | 1.50 | 1.17 | 39.53 | 0.67 | 2.66 | 0.43 | 0.29 | 46.07 | |
| #5 | 1.65 | 1.20 | 37.89 | 0.84 | 3.06 | 3.52 | 0.28 | 43.88 | |
| #6 | 2.40 | 1.01 | 32.23 | 0.70 | 4.19 | 0.63 | 0.34 | 48.45 | |
| #7 | 1.61 | 1.12 | 31.91 | 0.71 | 4.38 | 0.63 | 0.32 | 48.70 | |
| #8 | 1.32 | 1.13 | 33.93 | 0.70 | 3.83 | 0.66 | 0.33 | 48.74 | |
| #9 | 2.80 | 1.22 | 32.65 | 0.69 | 4.23 | 0.70 | 0.31 | 47.29 | |
| #10 | 1.61 | 1.22 | 37.67 | 0.76 | 3.92 | 0.60 | 0.30 | 44.69 |
| Label | Spot | SiO2 | TiO2 | V2O5 | Cr2O3 | MnO | Fe2O3 | CoO | NiO | CuO | ZnO | SnO2 | PbO | Bi2O3 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IZN/15 | #1 | 51.23 | 0.21 | 0.49 | 0.07 | 0.02 | 0.35 | 0.04 | 0.02 | 0.19 | 0.01 | 0.36 | 38.62 | 0.10 |
| #2 | 49.40 | 0.32 | 0.54 | 0.05 | 0.03 | 0.36 | 0.07 | 0.03 | 0.19 | 0.01 | 0.35 | 39.61 | 0.11 | |
| #3 | 50.65 | 0.24 | 0.51 | 0.07 | 0.03 | 0.36 | 0.05 | 0.02 | 0.18 | 0.01 | 0.36 | 39.11 | 0.13 | |
| #4 | 50.05 | 0.27 | 0.51 | 0.06 | 0.00 | 0.36 | 0.05 | 0.03 | 0.30 | 0.01 | 0.35 | 39.26 | 0.11 | |
| #5 | 51.21 | 0.26 | 0.48 | 0.07 | 0.03 | 0.46 | 0.07 | 0.03 | 0.18 | 0.01 | 0.31 | 37.82 | 0.13 | |
| #6 | 50.60 | 0.27 | 0.49 | 0.07 | 0.01 | 0.39 | 0.08 | 0.04 | 0.11 | 0.01 | 0.35 | 38.07 | 0.14 | |
| #7 | 51.23 | 0.22 | 0.50 | 0.06 | 0.03 | 0.36 | 0.04 | 0.02 | 0.24 | 0.01 | 0.36 | 38.45 | 0.10 | |
| #8 | 50.20 | 0.25 | 0.51 | 0.07 | 0.02 | 0.37 | 0.02 | 0.01 | 0.71 | 0.01 | 0.38 | 38.89 | 0.09 | |
| #9 | 49.91 | 0.25 | 0.52 | 0.05 | 0.01 | 0.36 | 0.03 | 0.02 | 0.19 | 0.01 | 0.36 | 39.47 | 0.10 | |
| #10 | 50.41 | 0.26 | 0.51 | 0.07 | 0.02 | 0.36 | 0.04 | 0.02 | 0.53 | 0.01 | 0.35 | 38.66 | 0.09 | |
| IZN/16 | #1 | 59.82 | 0.21 | 0.38 | 0.06 | 0.03 | 0.44 | 0.05 | 0.02 | 0.47 | 0.01 | 3.04 | 27.13 | 0.11 |
| #2 | 60.15 | 0.22 | 0.37 | 0.07 | 0.02 | 0.43 | 0.06 | 0.02 | 0.43 | 0.01 | 3.00 | 26.96 | 0.14 | |
| #3 | 57.37 | 0.30 | 0.00 | 0.05 | 0.02 | 0.49 | 0.04 | 0.01 | 0.38 | 0.01 | 3.33 | 28.45 | 0.14 | |
| #4 | 61.16 | 0.19 | 0.36 | 0.09 | 0.02 | 0.41 | 0.04 | 0.01 | 0.43 | 0.01 | 3.04 | 26.88 | 0.11 | |
| #5 | 60.94 | 0.15 | 0.00 | 0.05 | 0.03 | 0.44 | 0.03 | 0.01 | 0.44 | 0.01 | 2.99 | 26.77 | 0.10 | |
| #6 | 59.94 | 0.19 | 0.37 | 0.00 | 0.04 | 0.40 | 0.05 | 0.02 | 0.48 | 0.01 | 3.15 | 27.72 | 0.12 | |
| #7 | 60.82 | 0.16 | 0.36 | 0.06 | 0.03 | 0.40 | 0.04 | 0.01 | 0.44 | 0.01 | 3.03 | 27.19 | 0.11 | |
| #8 | 61.41 | 0.14 | 0.00 | 0.07 | 0.03 | 0.41 | 0.04 | 0.02 | 0.47 | 0.01 | 3.03 | 27.02 | 0.11 | |
| #9 | 60.49 | 0.17 | 0.00 | 0.07 | 0.03 | 0.42 | 0.04 | 0.01 | 0.44 | 0.01 | 3.03 | 26.77 | 0.14 | |
| #10 | 60.47 | 0.16 | 0.00 | 0.07 | 0.02 | 0.43 | 0.05 | 0.01 | 0.47 | 0.01 | 3.05 | 27.08 | 0.12 | |
| IZN/17 | #1 | 51.69 | 0.27 | 0.51 | 0.06 | 0.05 | 0.41 | 0.07 | 0.02 | 0.17 | 0.02 | 0.49 | 37.28 | 0.12 |
| #2 | 47.84 | 0.32 | 0.49 | 0.08 | 0.05 | 0.45 | 0.08 | 0.02 | 0.28 | 0.02 | 0.51 | 39.15 | 0.12 | |
| #3 | 50.26 | 0.29 | 0.48 | 0.07 | 0.05 | 0.43 | 0.05 | 0.01 | 0.14 | 0.02 | 0.47 | 37.06 | 0.14 | |
| #4 | 50.66 | 0.24 | 0.51 | 0.06 | 0.05 | 0.42 | 0.06 | 0.02 | 0.16 | 0.01 | 0.49 | 37.88 | 0.12 | |
| #5 | 56.05 | 0.13 | 0.00 | 0.06 | 0.05 | 0.38 | 0.08 | 0.02 | 0.12 | 0.02 | 0.37 | 29.80 | 0.10 | |
| #6 | 51.10 | 0.22 | 0.48 | 0.07 | 0.05 | 0.42 | 0.07 | 0.02 | 0.21 | 0.02 | 0.48 | 37.35 | 0.14 | |
| #7 | 51.01 | 0.23 | 0.47 | 0.06 | 0.04 | 0.44 | 0.06 | 0.02 | 0.26 | 0.02 | 0.47 | 36.99 | 0.12 | |
| #8 | 48.38 | 0.30 | 0.53 | 0.05 | 0.05 | 0.47 | 0.07 | 0.02 | 0.20 | 0.02 | 0.52 | 40.67 | 0.14 | |
| #9 | 49.57 | 0.30 | 0.48 | 0.05 | 0.07 | 0.44 | 0.08 | 0.02 | 0.28 | 0.02 | 0.52 | 38.48 | 0.14 | |
| #10 | 51.87 | 0.26 | 0.48 | 0.05 | 0.04 | 0.42 | 0.08 | 0.02 | 0.16 | 0.02 | 0.46 | 35.92 | 0.14 | |
| IZN/19 | #1 | 31.90 | 0.80 | 0.53 | 0.12 | 0.05 | 0.59 | 0.03 | 0.02 | 0.58 | 0.02 | 0.34 | 52.94 | 0.10 |
| #2 | 39.24 | 0.55 | 0.52 | 0.19 | 0.06 | 0.76 | 0.04 | 0.03 | 0.51 | 0.02 | 0.28 | 45.99 | 0.09 | |
| #3 | 39.47 | 1.71 | 0.45 | 0.06 | 0.05 | 0.61 | 0.03 | 0.03 | 0.43 | 0.02 | 0.26 | 42.60 | 0.08 | |
| #4 | 38.20 | 0.56 | 0.54 | 0.23 | 0.03 | 0.85 | 0.02 | 0.02 | 0.43 | 0.02 | 0.27 | 44.01 | 0.10 | |
| #5 | 39.06 | 0.44 | 0.51 | 0.08 | 0.07 | 0.48 | 0.05 | 0.04 | 0.46 | 0.02 | 0.29 | 46.06 | 0.08 | |
| #6 | 37.87 | 0.49 | 0.49 | 0.08 | 0.05 | 0.60 | 0.04 | 0.03 | 0.50 | 0.02 | 0.30 | 48.44 | 0.08 | |
| #7 | 39.90 | 0.50 | 0.53 | 0.23 | 0.04 | 0.86 | 0.03 | 0.02 | 0.44 | 0.02 | 0.27 | 44.78 | 0.10 | |
| #8 | 36.78 | 0.52 | 0.53 | 0.00 | 0.05 | 0.58 | 0.04 | 0.03 | 0.44 | 0.02 | 0.30 | 46.77 | 0.09 | |
| #9 | 31.46 | 0.60 | 0.57 | 0.00 | 0.05 | 0.52 | 0.02 | 0.02 | 0.43 | 0.02 | 0.35 | 47.89 | 0.10 | |
| #10 | 33.11 | 0.59 | 0.55 | 0.05 | 0.04 | 0.50 | 0.05 | 0.03 | 0.41 | 0.02 | 0.31 | 46.63 | 0.07 |
References
- Craig, N.; Speakman, R.J.; Popelka-Filcoff, R.S.; Aldenderfer, M.; Blanco, L.F.; Vega, M.B.; Glascock, M.D.; Stanish, C. Macusani obsidian from southern Peru: A characterization of its elemental composition with a demonstration of its ancient use. J. Archaeol. Sci. 2010, 37, 569–576. [Google Scholar] [CrossRef]
- Frahm, E.; Goldstein, S.T.; Tryon, C.A. Late Holocene forager-fisher and pastoralist interactions along the Lake Victoria shores, Kenya: Perspectives from portable XRF of obsidian artifacts. J. Archaeol. Sci. Rep. 2017, 11, 717–742. [Google Scholar] [CrossRef]
- Liu, S.; Li, Q.H.; Fu, Q.; Gan, F.X.; Xiong, Z.M. Application of a portable XRF spectrometer for classification of potash glass beads unearthed from tombs of Han Dynasty in Guangxi, China. X-ray Spectrom. 2013, 42, 470–479. [Google Scholar] [CrossRef]
- Fermo, P.; Andreoli, M.; Bonizzoni, L.; Fantauzzi, M.; Giubertoni, G.; Ludwig, N.; Rossi, A. Characterisation of Roman and Byzantine glasses from the surroundings of Thugga (Tunisia): Raw materials and colours. Microchem. J. 2016, 129, 5–15. [Google Scholar] [CrossRef]
- Licenziati, F.; Calligaro, T. Study of mosaic glass tesserae from Delos, Greece using a combination of portable μ-Raman and X-ray fluorescence spectrometry. J. Archaeol. Sci. Rep. 2016, 7, 640–648. [Google Scholar] [CrossRef]
- Zhao, H.X.; Li, Q.H. Combined spectroscopic analysis of stratified glass eye beads from China dated to the Warring States Period. J. Raman Spectrosc. 2017, 48, 1103–1110. [Google Scholar] [CrossRef]
- Forster, N.; Grave, P. Effects of elevated levels of lead in ceramics on provenancing studies using non-destructive PXRF: A case study in Byzantine Cypriot glazed ceramics. X-ray Spectrom. 2013, 42, 480–486. [Google Scholar] [CrossRef]
- Xu, W.; Niziolek, L.C.; Feinman, G.M. Sourcing qingbai porcelains from the Java Sea Shipwreck: Compositional analysis using portable XRF. J. Archaeol. Sci. 2019, 103, 57–71. [Google Scholar] [CrossRef]
- Frankel, D.; Webb, J.M. Pottery production and distribution in prehistoric Bronze Age Cyprus. An application of pXRF analysis. J. Archaeol. Sci. 2012, 39, 1380–1387. [Google Scholar] [CrossRef]
- Padilla, J.T.; Hormes, J.; Selim, H.M. Use of portable XRF: Effect of thickness and antecedent moisture of soils on measured concentration of trace elements. Geoderma 2019, 337, 143–149. [Google Scholar] [CrossRef]
- Hunt, A.M.W.; Speakman, R.J. Portable XRF analysis of archaeological sediments and ceramics. J. Archaeol. Sci. 2015, 53, 626–638. [Google Scholar] [CrossRef]
- Laskaris, N.; Varalis, I.; Tsodoulos, C.; Dolmas, C. Evidence of Au-Hg gilding process in post Byzantine ecclesiastical silverwares (chalices) of eastern Thessaly by pXRF. Mediterranean 2020, 20, 189–203. [Google Scholar]
- Roxburgh, M.A.; Heeren, S.; Huisman, D.J.; Van Os, B.J.H. Non-Destructive Survey of Early Roman Copper-Alloy Brooches using Portable X-ray Fluorescence Spectrometry. Archaeometry 2018, 61, 55–69. [Google Scholar] [CrossRef]
- Ganetsos, T.; Regkli, A.; Laskaris, N.; Liritzis, I. Spectroscopic study of colour traces in marble sculptures and architectural parts of monuments of archaic period in Delphi, Greece. Mediterranean 2019, 19, 51–61. [Google Scholar]
- Garrido, F.; Li, T. A handheld XRF study of Late Horizon metal artifacts: Implications for technological choices and political intervention in Copiap, northern Chile. Archaeol. Anthrop. Sci. 2017, 9, 935–942. [Google Scholar] [CrossRef]
- Brand, N.W.; Brand, C.J. Performance comparison of portable XRF instruments. Geochem. Explor. Environ. Anal. 2014, 14, 125–138. [Google Scholar] [CrossRef]
- Frahm, E. Validity of “off-the-shelf” handheld portable XRF for sourcing Near Eastern obsidian chip debris. J. Archaeol. Sci. 2013, 40, 1080–1092. [Google Scholar] [CrossRef]
- Goodale, N.; Bailey, D.G.; Jones, G.T.; Prescott, C.; Scholz, E.; Stagliano, N.; Lewis, C. pXRF: A study of inter-instrument performance. J. Archaeol. Sci. 2012, 39, 875–883. [Google Scholar] [CrossRef]
- Liritzis, I.; Zacharias, N. Portable XRF of Archaeological Artifacts: Current Research, Potentials and Limitations. In X-ray Fluorescence Spectrometry (XRF) in Geoarchaeology, 1st ed.; Schackley, M.S., Ed.; Springer: New York, NY, USA, 2011; pp. 109–142. [Google Scholar]
- Shackley, M.S. Is there reliability and validity in portable X-ray fluorescence spectrometry (PXRF)? SAA Archaeol. Rec. 2010, 10, 17–20. [Google Scholar]
- Speakman, R.J.; Shackley, M.S. Silo science and portable XRF in archaeology: A response to Frahm. J. Archaeol. Sci. 2013, 40, 1435–1443. [Google Scholar] [CrossRef]
- Stroth, L.; Otto, R.; Daniels, J.T.; Braswell, G.A. Statistical artifacts: Critical approaches to the analysis of obsidian artifacts by portable X-ray fluorescence. J. Archaeol. Sci. Rep. 2019, 24, 738–747. [Google Scholar] [CrossRef]
- NIST X-ray Mass Attenuation Coefficients. Available online: https://www.nist.gov/pml/x-ray-mass-attenuation-coefficients (accessed on 12 September 2020).
- Koleini, F.; Colomban, P.; Pikirayi, I. Post-15th century European glass beads in southern Africa: Composition and classification using pXRF and Raman spectroscopy. J. Archaeol. Sci. Rep. 2020, 29, 102183. [Google Scholar] [CrossRef]
- Bonizzoni, L.; Galli, A.; Gondola, M.; Martini, M. Comparison between XRF, TXRF, and PXRF analyses for provenance classification of archaeological bricks. X-ray Spectrom. 2013, 42, 262–267. [Google Scholar] [CrossRef]
- Mitchell, D.; Grave, P.; Maccheroni, M.; Gelman, E. Geochemical characterisation of north Asian glazed stonewares: A comparative analysis of NAA, ICP-OES and non-destructive pXRF. J. Archaeol. Sci. 2012, 39, 2921–2933. [Google Scholar] [CrossRef]
- Adlington, L.; Gratuze, B.; Schibille, N. Comparison of pXRF and LA-ICP-MS analysis of lead-rich glass mosaic tesserae. J. Archaeol. Sci. Rep. 2020, 34, 102603. [Google Scholar] [CrossRef]
- Aydemir, D.; Karabulut, G.; Şimşek, G.; Gok, M.; Barlas, N.; Ulusu, N.N. Impact of the Di(2-Ethylhexyl) Phthalate Administration on Trace Element and Mineral Levels in Relation of Kidney and Liver Damage in Rats. Biol. Trace Element Res. 2018, 186, 474–488. [Google Scholar] [CrossRef]
- Franci, G.S. Handheld X-ray fluorescence (XRF) versus wavelength dispersive XRF: Characterization of Chinese blue-and-white porcelain sherds using handheld and laboratoy-type XRF instruments. Appl. Spectrosc. 2020, 74, 314–322. [Google Scholar] [CrossRef]
- Franci, G.S.; Akkas, T.; Yildirim, S.; Yilmaz, S.; Birdevrim, A.N. Characterization of a Jian-like sherd with the optical microscope, confocal Raman, wavelength-dispersive X-ray fluorescence, and portable XRF spectrometers. J. Raman Spectrosc. 2020, 51, 1343–1352. [Google Scholar] [CrossRef]
- Simsek, G.; Colomban, P.; Casadio, F.; Bellot-Gurlet, L.; Zelleke, G.; Faber, K.T.; Milande, V.; Tilliard, L. On-Site Identification of Early Böttger Red Stoneware Using Portable XRF/Raman Instruments: 2, Glaze & Gilding Analysis. J. Am. Ceram. Soc. 2015, 98, 3006–3013. [Google Scholar] [CrossRef]
- Simsek, G.; Arli, B.D.; Kaya, S.; Colomban, P. On-site pXRF analysis of body, glaze and colouring agents of the tiles at the excavation site of Iznik kilns. J. Eur. Ceram. Soc. 2019, 39, 2199–2209. [Google Scholar] [CrossRef]
- Simsek, G.; Unsalan, O.; Bayraktar, K.; Colomban, P. On-site pXRF analysis of glaze composition and colouring agents of « Iznik » tiles at Edirne mosques (15th and 16th-centuries). Ceram. Int. 2019, 45, 595–605. [Google Scholar] [CrossRef]
- Demirsar Arli, V.B.; Kaya, S.; Simsek Franci, G. Assessment of impressed/moulded ceramic wares excavated during the 2018–2019 seasons at the Iznik Tile Kilns Excavation and analysis results with a pXRF instrument on selected samples. Sanat Tarihi Yilligi 2020, 29, 1–19. [Google Scholar]
- Atasoy, N.; Raby, J. Iznik: The Pottery of Ottoman Turkey, 1st ed.; Alexandria Press: London, UK, 1989; pp. 1–384. [Google Scholar]
- Carswell, J. Iznik Pottery (Eastern Art), 1st ed.; British Museum Press: London, UK, 1998; pp. 1–128. [Google Scholar]
- Denny, W.B. Iznik La Céramique Turque et l’Art Ottoman, 1st ed.; Citadelles and Mazenod: Paris, France, 2004; pp. 1–239. [Google Scholar]
- Soustiel, J. La Céramique Islamique—Le Guide du Connaisseur, Office du Livre, 1st ed.; Editions Vilo: Paris, France, 1985. [Google Scholar]
- Valenstein, S.G. A Handbook of Chinese Ceramics, 2nd ed.; Metropolitan Museum of Art: New York, NY, USA, 1989; pp. 1–331. [Google Scholar]
- Trubner, H. Ceramic Art of Japan: One Hundred Masterpieces from Japanese Collections, 1st ed.; Seattle Art Museum: Reno, NV, USA, 1973; pp. 1–172. [Google Scholar]
- Penkala, M. European Porcelain; A Handbook for the Collector, 2nd ed.; C.E. Tuttle Co.: Rutland, VT, USA, 1968; pp. 1–256. [Google Scholar]
- Migeon, G.; Sakisian, A. La céramique d’Asie-Mineure et de Constantinople du XIVe au XVIIIe siècle, 1st ed.; Extrait de La Revue de l’Art Ancien et Moderne, Tomes XLIII-XLIV, Librairie Orientaliste Paul Geuthner: Paris, France, 1923; pp. 1–46. [Google Scholar]
- Koechlin, R.; Migeon, G. 100 planches en couleurs d’Art musulman (céramique, tissus, tapis). Syr. Archéologie Art Hist. 1929, 10, 173–174. [Google Scholar]
- Lane, A. The Ottoman pottery of Isnik. Ars Orient. 1957, 2, 247–281. [Google Scholar]
- Riefstahl, R.M. Early Turkish tile-revetments in Edirne. Ars Islam. 1937, 4, 249–281. [Google Scholar]
- Kiefer, C. Les céramiques musulmanes d’Anatolie. Cah. Céram. Arts Feu. 1956, 4, 15–30. [Google Scholar]
- Kiefer, C. Les céramiques siliceuses d’Anatolie et du Moyen-Orient. Bull. Soc. Fr. Céram. 1956, 30, 3–24, reprinted in 1956, 31, 17–34. [Google Scholar]
- Rackham, B. Turkish pottery. Trans. Orient. Ceram. Soc. 1934, 12, 35–48. [Google Scholar]
- Henderson, J.; Raby, J. The technology of fifteenth century Turkish tiles: An interim statement on the origins of the Iznik industry. World Archaeol. 1989, 21, 115–132. [Google Scholar] [CrossRef]
- Arli, B.D.; Altun, A. Anadolu Toprağının Hazinesi Çini, Osmanlı Dönemi, 1st ed.; Kale Grubu Kültür Yayınları: İstanbul, Turkey, 2008; pp. 1–373. [Google Scholar]
- Arli, B.D.; Adıgüzel, H. The Connection Between the Tile Decoration of the 16th and 17th Centuries in Istanbul with the Tile Fragments Found in Iznik Excavations. In The Art of the Islamic World and the Artistic Relationships between Polland and Islamic Countries, Proceedings of 11th Conference of the Polish Institute of World Art Studies (Former Polish Society of Oriental Art), 1st Conference of Islamic Art in Poland, Krakow, Poland, 5–7 October 2009; Slota, B.B., Ginter-Frolow, M., Malinowski, J., Eds.; Manggha, Polish Institute of World Art Studies: Krakow, Poland, 2011; pp. 235–245. [Google Scholar]
- Arlı, B.D. İznik Çini Fırınları Kazı Buluntularından Çini Örneklerin Değerlendirilmesi/Evaluation of Iznik Tiles Examples from Iznik Tile Excavation. J. Hist. Cult. Art Res. 2018, 7, 578–594. [Google Scholar] [CrossRef]
- Özer, M.; Dündar, M.; Güner, Y.; Uçar, H. Edirne Yeni Saray Kazısı (Saray-ı Cedîd-i Âmire) 2011 Yılı Çalışmaları. Sanat Tarihi Derg. 2016, 24, 73–106. [Google Scholar] [CrossRef][Green Version]
- Arlı, B.D.; Kaya, S. İznik Çini Fırınları Kazısı Çini Buluntularının Değerlendirilmesi. In Proceedings of the 16th International Congress of Turkish Art (ICTA), Ankara, Turkey, 3–5 October 2015. [Google Scholar]
- Adlington, L.W. The Corning Archaeological Reference Glasses: New Values for “Old” Compositions. Pap. Inst. Archaeol. 2017, 27, 1–8. [Google Scholar] [CrossRef]
- Pearce, N.J.; Perkins, W.T.; Westgate, J.A.; Gorton, M.P.; Jackson, S.E.; Neal, C.R.; Chenery, S.P. A Compilation of New and Published Major and Trace Element Data for NIST SRM 610 and NIST SRM 612 Glass Reference Materials. Geostand. Geoanalytical Res. 1997, 21, 115–144. [Google Scholar] [CrossRef]
- Conrey, R.M.; Goodman-Elgar, M.; Bettencourt, N.; Seyfarth, A.; Van Hoose, A.; Wolff, J.A. Calibration of a portable X-ray fluorescence spectrometer in the analysis of archaeological samples using influence coefficients. Geochem. Explor. Environ. Anal. 2014, 14, 291–301. [Google Scholar] [CrossRef]
- Tournie, A.; Ricciardi, P.; Colomban, P. Glass corrosion mechanisms: A multiscale analysis. Solid State Ion. 2008, 179, 2142–2154. [Google Scholar] [CrossRef]
- Pradell, T.; Molera, J. Ceramic technology. How to characterise ceramic glazes. Archaeol. Anthr. Sci. 2020, 12, 1–28. [Google Scholar] [CrossRef]
- Paynter, S.; Okyar, F.; Wolf, S.; Tite, M.S. The production technology of Iznik pottery—A reassessment. Archaeometry 2004, 46, 421–437. [Google Scholar] [CrossRef]
- Colomban, P.; Kırmızı, B.; Gougeon, C.; Gironda, M.; Cardinal, C. Pigments and glassy matrix of the 17th–18th century enamelled French watches: A non-invasive on-site Raman and pXRF study. J. Cult. Herit. 2020, 44, 1–14. [Google Scholar] [CrossRef]
- Tite, M. Iznik pottery: An investigation of the methods of production. Archaeometry 1989, 31, 115–132. [Google Scholar] [CrossRef]
- Matin, M. Tin-based opacifiers in archaeological glass and ceramic glazes: A review and new perspectives. Archaeol. Anthr. Sci. 2018, 11, 1155–1167. [Google Scholar] [CrossRef]
- Vane-Tempest, S.; Kronberg, T.; Fröberg, L.; Hupa, L. Chemical Resistance of Fast-Fired Raw Glazes in Solutions Containing Cleaning Agent, Acids or Bases. Qualicer 2004. Available online: http://www.qualicer.org/recopilatorio/ponencias/pdfs/0413121e.pdf (accessed on 29 September 2020).
- Colomban, P. Rocks as blue, green and black pigments/dyes of glazed pottery and enamelled glass artefacts? A review. Eur. J. Miner. 2014, 25, 863–879. [Google Scholar] [CrossRef]
- Colomban, P. Routes du lapis lazuli, lajvardina et échanges entre arts du verre, de la céramique et du livre. Taıci 2005, 4, 145–152. [Google Scholar]
- Kissin, S.A. Five element (Ni-Co-As-Ag-Bi) veins. Geosci. Can. 1992, 19, 113–124. [Google Scholar]
- Wen, R.; Pollard, A.M. The Pigments Applied to Islamic Minai Wares and the Correlation with Chinese Blue-and-White Porcelain. Archaeometry 2014, 58, 1–16. [Google Scholar] [CrossRef]
- Colomban, P.; Sagon, G.; Huy, L.Q.; Liem, N.Q.; Mazerolles, L. Vietnamese (15th Century) Blue-And-White, Tam Thai and Lustre Porcelains/Stonewares: Glaze Composition and Decoration Techniques. Archaeometry 2004, 46, 125–136. [Google Scholar] [CrossRef]
- Simsek, G.; Colomban, P.; Wong, S.; Zhao, B.; Rougeulle, A.; Liem, N.Q. Toward a fast non-destructive identification of pottery: The sourcing of 14th–16th century Vietnamese and Chinese ceramic shards. J. Cult. Herit. 2015, 16, 159–172. [Google Scholar] [CrossRef]
- Fischer, C.; Hsieh, E. Export Chinese blue-and-white porcelain: Compositional analysis and sourcing using non-invasive portable XRF and reflectance spectroscopy. J. Archaeol. Sci. 2017, 80, 14–26. [Google Scholar] [CrossRef]
- Du, F.; Su, B. Further study of sources of the imported cobalt-blue pigment used on Jingdezhen porcelain from late 13 to early 15 centuries. Sci. China Ser. E Technol. Sci. 2008, 51, 249–259. [Google Scholar] [CrossRef]
- Figueiredo, M.; Silva, T.P.; Veiga, J.P. A XANES study of cobalt speciation state in blue-and-white glazes from 16th to 17th century Chinese porcelains. J. Electron Spectrosc. 2012, 185, 97–102. [Google Scholar] [CrossRef]
- Zhu, T.Q.; Zhang, Y.C.; Xiong, H.; Feng, Z.Y.; Li, Q.; Cao, B.L. Comparison of the different types of Qinghua porcelain from Jingdezhen in the Yuan Dynasty of China (AD 1271–1368) by micro X-ray fluorescence spectroscopy (μ-XRF) and microscopy. Archaeometry 2016, 58, 966–978. [Google Scholar] [CrossRef]
- Pappalardo, G.; Costa, E.; Marchetta, C.; Pappalardo, L.; Romano, F.P.; Zucchiatti, A.; Prati, P.; Mando, P.A.; Migliori, A.; Palombo, L.; et al. Non-destructive characterization of Della Robia sculptures at the Bargello museum in Florence by the combined use of PIXE and XRF portable systems. J. Cult. Herit. 2004, 5, 183–188. [Google Scholar] [CrossRef]
- Zucchiatti, A.; Bouquillon, A.; Katona, I.; D’Alessandro, A. The ‘Della Robia Blue’: A case study for the use of cobalt pigments in ceramics during the Italian renaissance. Archaeometry 2006, 48, 131–152. [Google Scholar] [CrossRef]
- Barilaro, D.; Crupi, V.; Interdonato, S.; Majolino, D.; Venuti, V.; Barone, G.; La Russa, M.F.; Bardelli, F. Characterization of blue decorated Renaissance pottery fragments from Caltagirone (Siciliy, Italy). Appl. Phys. A 2008, 92, 91–96. [Google Scholar] [CrossRef]
- Porter, Y. Le cobalt dans le monde iranien (IXe-XVIe siecles): Notes sur son utilization en céramique et son commerce. Taoci 2000, 1, 5–14. [Google Scholar]
- Matin, M.; Pollard, A.M. Historical Accounts of Cobalt Ore Processing from the Kashan Mine, Iran. Iran 2015, 53, 171–183. [Google Scholar] [CrossRef]







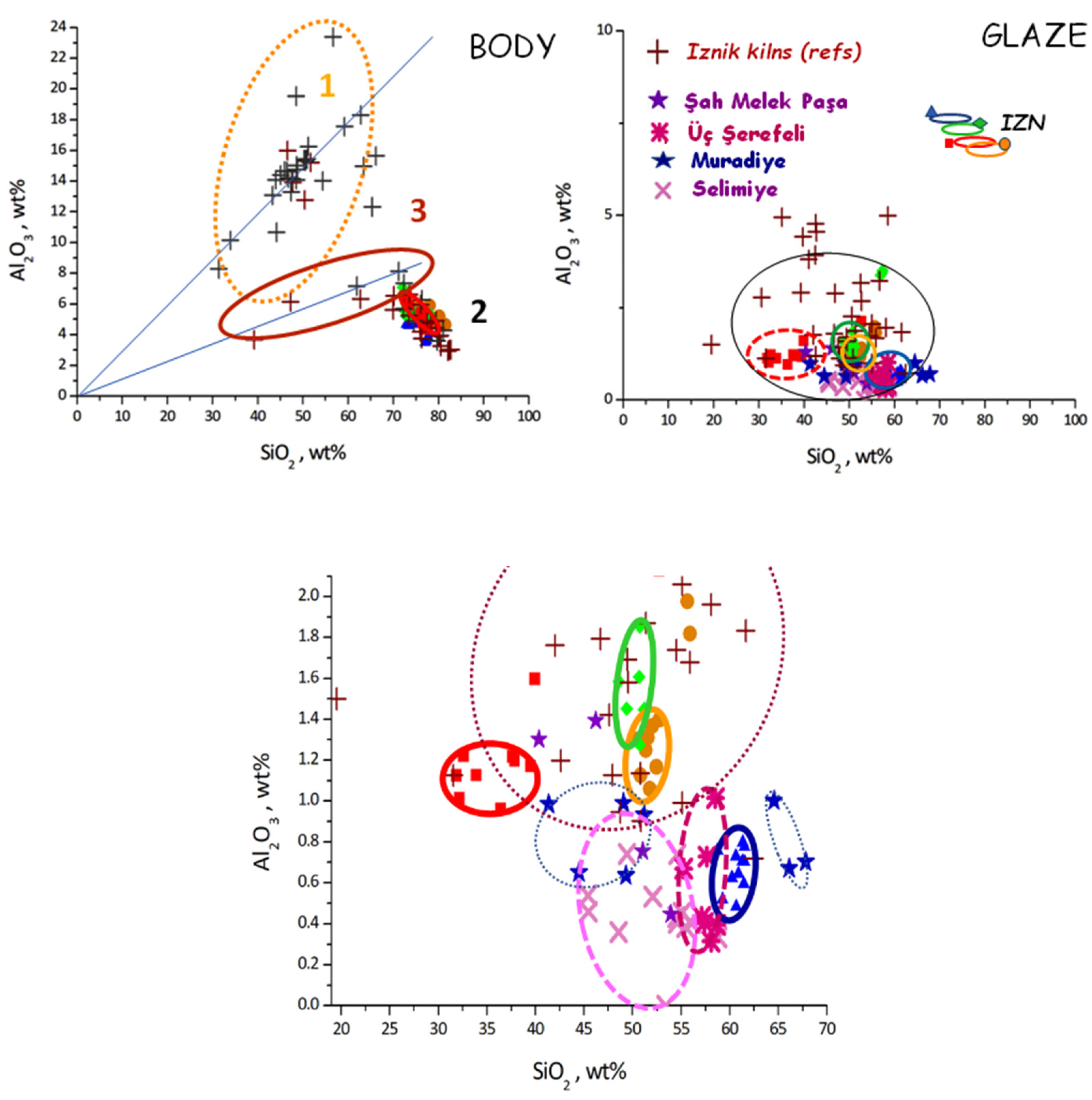


| Body | Data | Mg | Al | Si | K | Ca | Fe | Rb * | Sr * | Zr * | Ba * | Pb | Bi * |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IZN/15 | Mean | 1.23 | 2.80 | 36.67 | 0.96 | 1.87 | 1.06 | 40 | 90 | 40 | 230 | 0.93 | 30 |
| SD | 0.15 | 0.24 | 0.99 | 0.09 | 0.29 | 0.18 | 0.000 | 0.001 | 0.001 | 0.002 | 0.25 | 0.001 | |
| RSD | 12.58 | 8.66 | 2.69 | 9.29 | 15.49 | 16.64 | 12.52 | 8.48 | 15.78 | 10.26 | 26.65 | 24.43 | |
| IZN/16 | Mean | 1.55 | 2.29 | 35.42 | 0.90 | 4.21 | 0.88 | 50 | 140 | 90 | 300 | 1.57 | 80 |
| SD | 0.25 | 0.31 | 0.77 | 0.06 | 0.73 | 0.06 | 0.001 | 0.001 | 0.001 | 0.005 | 0.30 | 0.002 | |
| RSD | 16.27 | 13.72 | 2.17 | 6.65 | 17.37 | 7.04 | 14.06 | 8.15 | 10.89 | 17.91 | 19.09 | 23.08 | |
| IZN/17 | Mean | 1.37 | 3.01 | 34.78 | 1.01 | 2.69 | 1.15 | 50 | 110 | 50 | 350 | 1.43 | 50 |
| SD | 0.15 | 0.35 | 1.08 | 0.13 | 0.44 | 0.12 | 0.001 | 0.001 | 0.000 | 0.012 | 0.2 | 0.001 | |
| RSD | 10.89 | 11.51 | 3.10 | 13.10 | 16.25 | 10.83 | 12.66 | 11.01 | 9.79 | 35.21 | 13.79 | 21.25 | |
| IZN/19 | Mean | 1.70 | 2.80 | 35.37 | 1.02 | 3.50 | 1.22 | 40 | 170 | 50 | 270 | 1.04 | 40 |
| SD | 0.13 | 0.35 | 0.74 | 0.10 | 0.43 | 0.12 | 0.001 | 0.001 | 0.001 | 0.004 | 0.36 | 0.001 | |
| RSD | 7.76 | 12.40 | 2.09 | 9.60 | 12.39 | 9.89 | 16.02 | 6.79 | 17.33 | 14.24 | 34.54 | 33.87 |
| Glaze | Data | Mg | Al | Si | K | Ca | Ti | V | Sn | Sb | Ba * | Pb | Bi * |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IZN/15 | Mean | 0.76 | 0.73 | 24.52 | 0.66 | 1.17 | 0.18 | 0.24 | 0.26 | 0.19 | 600 | 33.80 | 690 |
| SD | 0.19 | 0.15 | 0.85 | 0.11 | 0.30 | 0.10 | 0.08 | 0.03 | 0.02 | 0.02 | 2.74 | 0.008 | |
| RSD | 25.16 | 20.42 | 3.45 | 16.60 | 25.74 | 53.31 | 34.42 | 10.58 | 11.76 | 35.51 | 8.10 | 12.16 | |
| IZN/16 | Mean | 0.35 | 0.35 | 28.37 | 0.95 | 0.74 | 0.10 | 0.11 | 2.42 | 0.05 | 500 | 25.02 | 710 |
| SD | 0.18 | 0.05 | 0.42 | 0.15 | 0.07 | 0.02 | 0.10 | 0.08 | 0.01 | 0.01 | 0.70 | 0.012 | |
| RSD | 49.59 | 14.78 | 1.49 | 15.43 | 10.08 | 15.22 | 91.31 | 3.16 | 9.99 | 24.72 | 2.79 | 16.64 | |
| IZN/17 | Mean | 0.77 | 1.11 | 24.51 | 0.89 | 1.03 | 0.15 | 0.24 | 0.36 | 0.10 | 500 | 32.91 | 670 |
| SD | 0.11 | 0.47 | 1.51 | 0.24 | 0.21 | 0.04 | 0.09 | 0.05 | 0.01 | 0.01 | 3.64 | 0.012 | |
| RSD | 13.60 | 42.40 | 6.16 | 26.79 | 20.65 | 27.35 | 36.01 | 13.99 | 12.81 | 23.85 | 11.05 | 17.52 | |
| IZN/19 | Mean | 1.10 | 0.67 | 17.53 | 0.63 | 2.54 | 0.54 | 0.29 | 0.23 | 0.40 | 600 | 41.93 | 840 |
| SD | 0.34 | 0.17 | 2.72 | 0.07 | 0.44 | 0.52 | 0.03 | 0.03 | 0.04 | 0.03 | 4.95 | 0.012 | |
| RSD | 30.68 | 25.61 | 15.54 | 10.37 | 17.39 | 97.11 | 11.02 | 14.90 | 10.74 | 45.23 | 11.80 | 14.16 |
| Blue | Data | Si | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Sn | Pb | Bi |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IZN/15 | Mean | 23.60 | 0.28 | 0.04 | 0.01 | 0.26 | 0.04 | 0.02 | 0.23 | 0.01 | 0.28 | 36.02 | 0.10 |
| SD | 0.27 | 0.01 | 0.01 | 0.01 | 0.02 | 0.01 | 0.01 | 0.14 | 0.00 | 0.01 | 0.52 | 0.01 | |
| RSD | 1.16 | 3.10 | 12.48 | 47.42 | 8.13 | 32.55 | 32.55 | 63.29 | 14.37 | 4.53 | 1.43 | 14.93 | |
| IZN/16 | Mean | 28.17 | 0.10 | 0.04 | 0.02 | 0.30 | 0.03 | 0.01 | 0.36 | 0.01 | 2.42 | 25.25 | 0.11 |
| SD | 0.50 | 0.10 | 0.02 | 0.01 | 0.02 | 0.01 | 0.00 | 0.02 | 0.00 | 0.07 | 0.46 | 0.01 | |
| RSD | 1.79 | 100 | 38.04 | 24.19 | 5.93 | 17.01 | 25.85 | 6.38 | 18.88 | 3.08 | 1.81 | 12.65 | |
| IZN/17 | Mean | 23.77 | 0.25 | 0.04 | 0.04 | 0.30 | 0.05 | 0.02 | 0.16 | 0.01 | 0.38 | 34.40 | 0.11 |
| SD | 1.00 | 0.08 | 0.01 | 0.01 | 0.02 | 0.01 | 0.00 | 0.04 | 0.00 | 0.03 | 2.53 | 0.01 | |
| RSD | 4.22 | 33.58 | 15.02 | 16.62 | 5.50 | 15.10 | 19.16 | 28.17 | 13.60 | 8.61 | 7.35 | 10.23 | |
| IZN/19 | Mean | 17.15 | 0.29 | 0.09 | 0.04 | 0.44 | 0.03 | 0.02 | 0.36 | 0.02 | 0.23 | 43.27 | 0.08 |
| SD | 1.46 | 0.02 | 0.05 | 0.01 | 0.09 | 0.01 | 0.00 | 0.04 | 0.00 | 0.02 | 2.49 | 0.01 | |
| RSD | 8.49 | 6.12 | 54.21 | 20.28 | 20.74 | 26.11 | 20.84 | 11.03 | 7.79 | 9.31 | 5.75 | 10.65 | |
| IZN | RSDmax | 8.5 | 100 | 54 | 47 | 21 | 33 | 33 | 65 | 19 | 9 | 7 | 15 |
| Ref [3] | Error * | <15 | - | - | <40 | <70 | <65 | - | <80 | 150 | <25 | >50 | - |
| Err/RSD | 6 | - | - | 10 | 18 | - | - | 8 | 5 | 3 | 28 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demirsar Arli, B.; Simsek Franci, G.; Kaya, S.; Arli, H.; Colomban, P. Portable X-ray Fluorescence (p-XRF) Uncertainty Estimation for Glazed Ceramic Analysis: Case of Iznik Tiles. Heritage 2020, 3, 1302-1329. https://doi.org/10.3390/heritage3040072
Demirsar Arli B, Simsek Franci G, Kaya S, Arli H, Colomban P. Portable X-ray Fluorescence (p-XRF) Uncertainty Estimation for Glazed Ceramic Analysis: Case of Iznik Tiles. Heritage. 2020; 3(4):1302-1329. https://doi.org/10.3390/heritage3040072
Chicago/Turabian StyleDemirsar Arli, Belgin, Gulsu Simsek Franci, Sennur Kaya, Hakan Arli, and Philippe Colomban. 2020. "Portable X-ray Fluorescence (p-XRF) Uncertainty Estimation for Glazed Ceramic Analysis: Case of Iznik Tiles" Heritage 3, no. 4: 1302-1329. https://doi.org/10.3390/heritage3040072
APA StyleDemirsar Arli, B., Simsek Franci, G., Kaya, S., Arli, H., & Colomban, P. (2020). Portable X-ray Fluorescence (p-XRF) Uncertainty Estimation for Glazed Ceramic Analysis: Case of Iznik Tiles. Heritage, 3(4), 1302-1329. https://doi.org/10.3390/heritage3040072