What You Clean Is What You Get: A Novel Chemical Cleaning Technique and the Interpretation of Corrosion Products Found in Late Roman Copper Alloy Coins Retrieved from the Sea
Abstract
:1. Introduction
1.1. Corrosion of Ancient Copper Alloy Objects Retrieved from Marine Environments
1.2. Removal of Concretion Cover and Corrosion Layers from Archaeological Copper Alloy Objects Retrieved from Marine Environments
2. The Underwater Site
3. Materials and Methods
3.1. The Coins
3.2. The Current Developed Chemical Cleaning Method
3.3. Analytical Methods
- (a)
- The coins were visually inspected throughout the cleaning process to monitor the procedure’s progress and assess its efficacy (i.e., the extent to which it achieves a clean surface while incurring minimum damage).
- (b)
- Morphometric data of the coins were collected at the beginning and end of the procedure. This included the coins’ diameter and thickness measured with a digital caliper and their weight attained with a digital balance (precision ± 0.01 g).
- (c)
- The clean coins’ surfaces, including the exposed metal and remaining concretions, were inspected with a 3D digital Hirox RH-2000 light microscope (LM) equipped with a multi-focus system, high-intensity LED lighting (5700 K colour temperature), and powerful 3D software [28]. It sought to assess the coins’ state of preservation and detect microscopic defects and discontinuities.
- (d)
- A handheld XRF (HH-XRF) Oxford X-MET8000 was employed to determine the elemental compositions of the coins’ concretion and underlying metal. The XRF was coupled with a Silicon Drift Detector equipped with a 45 kV Rh Target X-ray tube and LE operation mode. The instrument was calibrated with a standard steel calibration sample. Each measurement was collected for 30 seconds, with a measurement spot size of 5 mm in diameter. The measurements were made by comparing independent peaks. For example, the arsenic (As) Kα and Kβ peaks were compared with the lead (Pb) Mα and Lβ peaks, respectively [29]. Light elements, including carbon and oxygen, could not be detected with this HH-XRF instrument.
- (e)
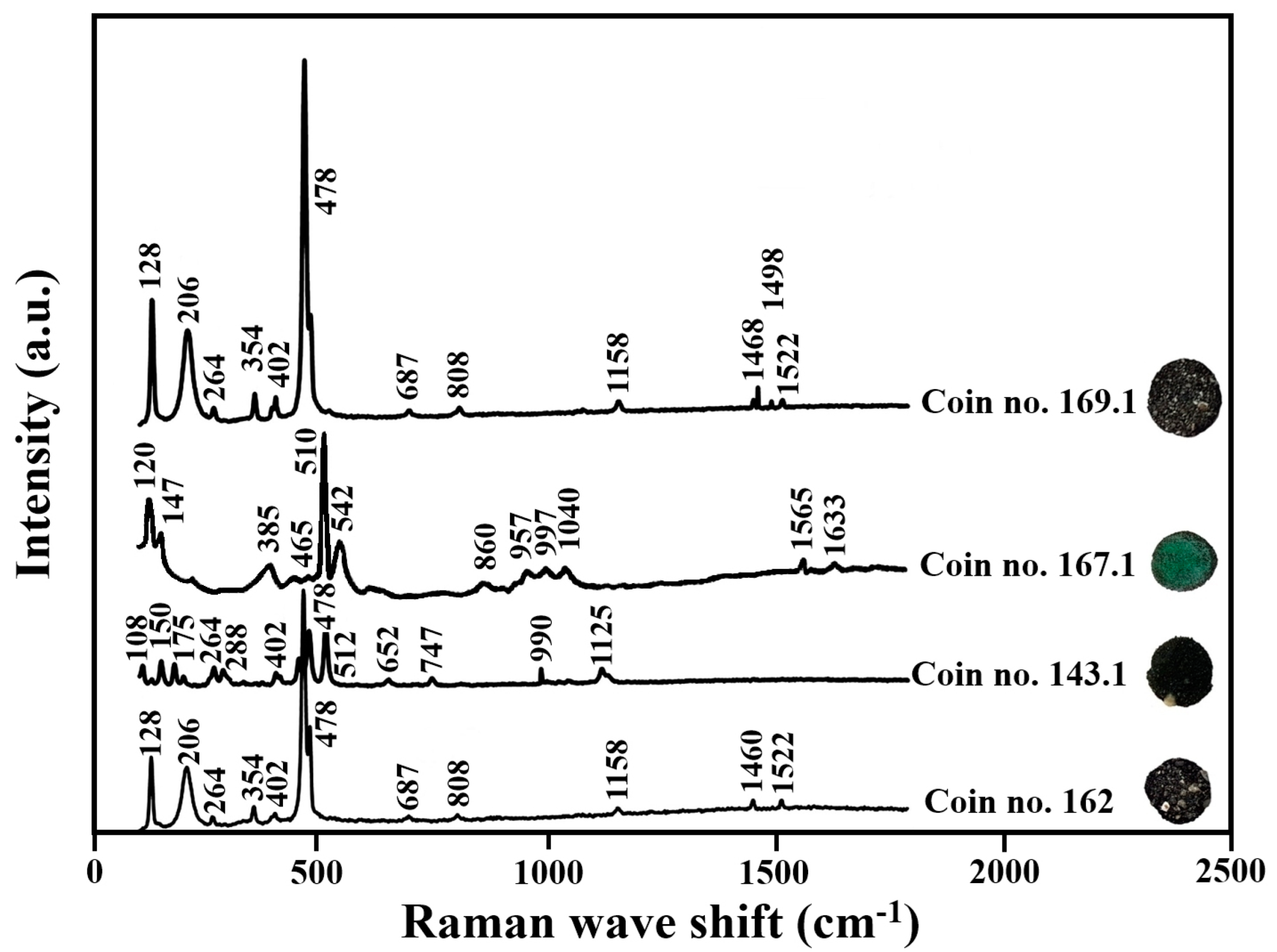
- Four coins’ concretion covers (nos. 143, 162, 167, and 169) were subjected to Raman spectroscopy, conducted with a Horiba Jobin Yvon LabRam HR spectrometer coupled to an Olympus LM. Raman spectroscopy is a powerful technique for providing information concerning the surface of metals and alloys covered with oxides and corrosion products, and its spectra can identify the different minerals. The analyses were performed using a 532 nm laser-excitation line. A 50 × objective lens was used to identify the concretion products on the coin surface. Two or three spectra were performed on each side of the external concretion. To interpret the Raman analysis results, we used the database of the Center for Nanoscience and Nanotechnology, Tel Aviv University. Additionally, the current results have been compared to the Raman studies in the literature [30].
- (f)
- The metallographic transverse cross-section (T-CS) of coin no. 120.3 was examined under a scanning electron microscope (SEM) to explore its microstructure morphologies. This analysis was done with an environmental ESEM-FEI Quanta 200FEG instrument combined with energy-dispersive spectroscopy (EDS) in high vacuum mode with an Everhart–Thornley secondary electron (SE) detector. Both secondary electron (SE) and back-scattered electron (BSE) modes were applied. The composition was detected by EDS using a Si (Li) liquid-cooled Oxford X-ray detector (calibrated with standard samples and providing measurements with a first approximation error of 1%) [18,28].
4. Results
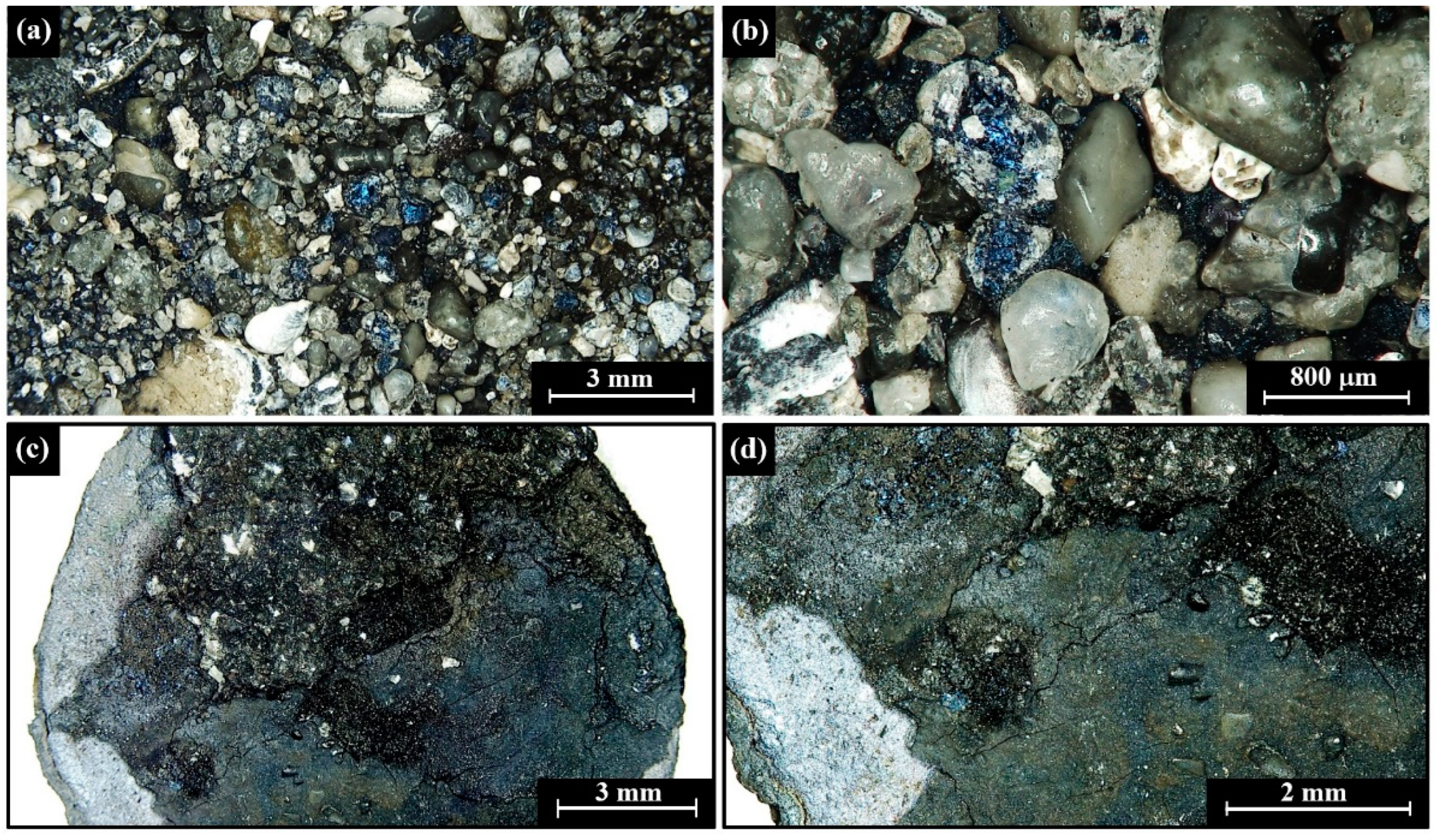
4.1. Visual Observations
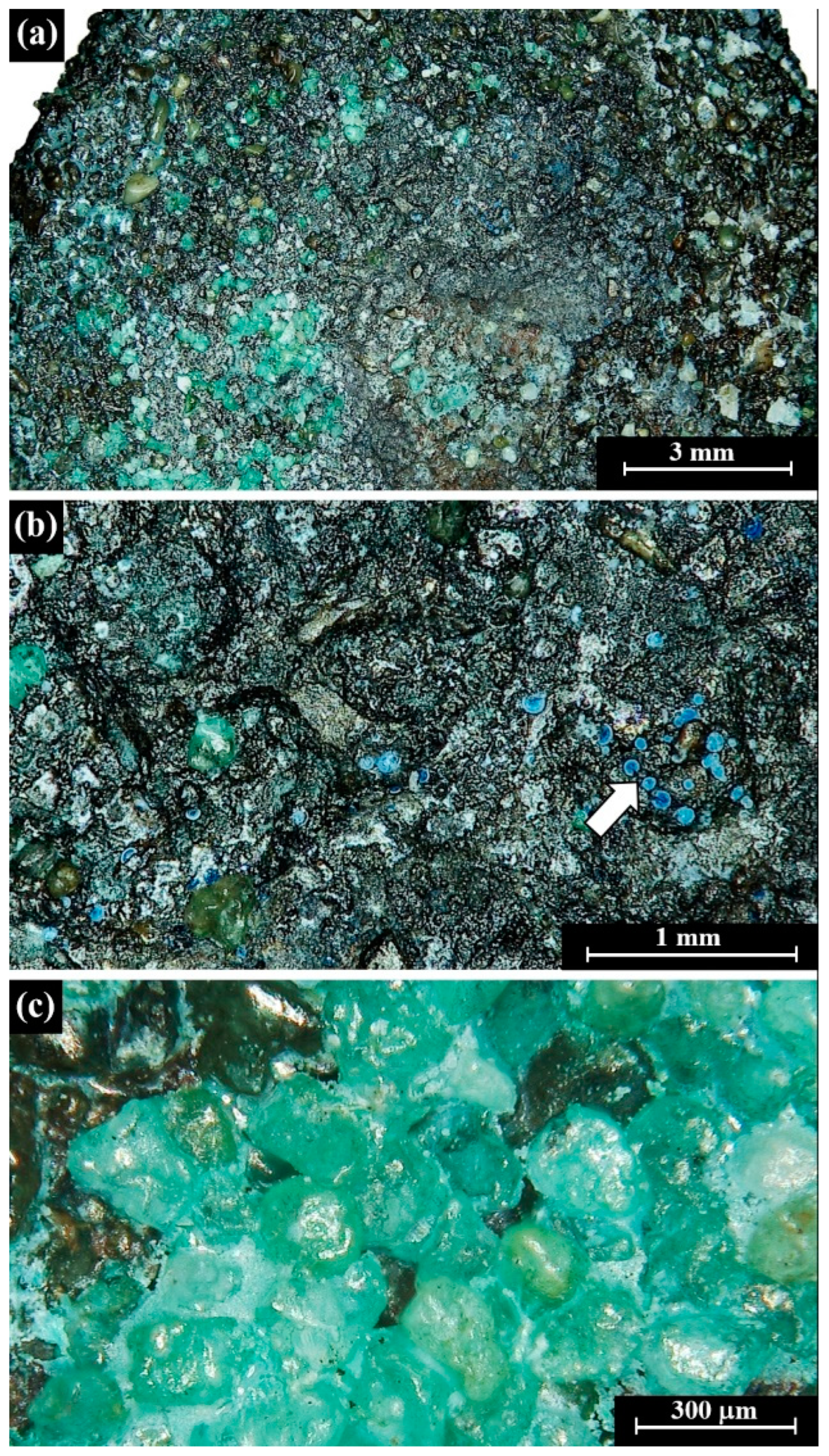


4.2. Morphometric Features
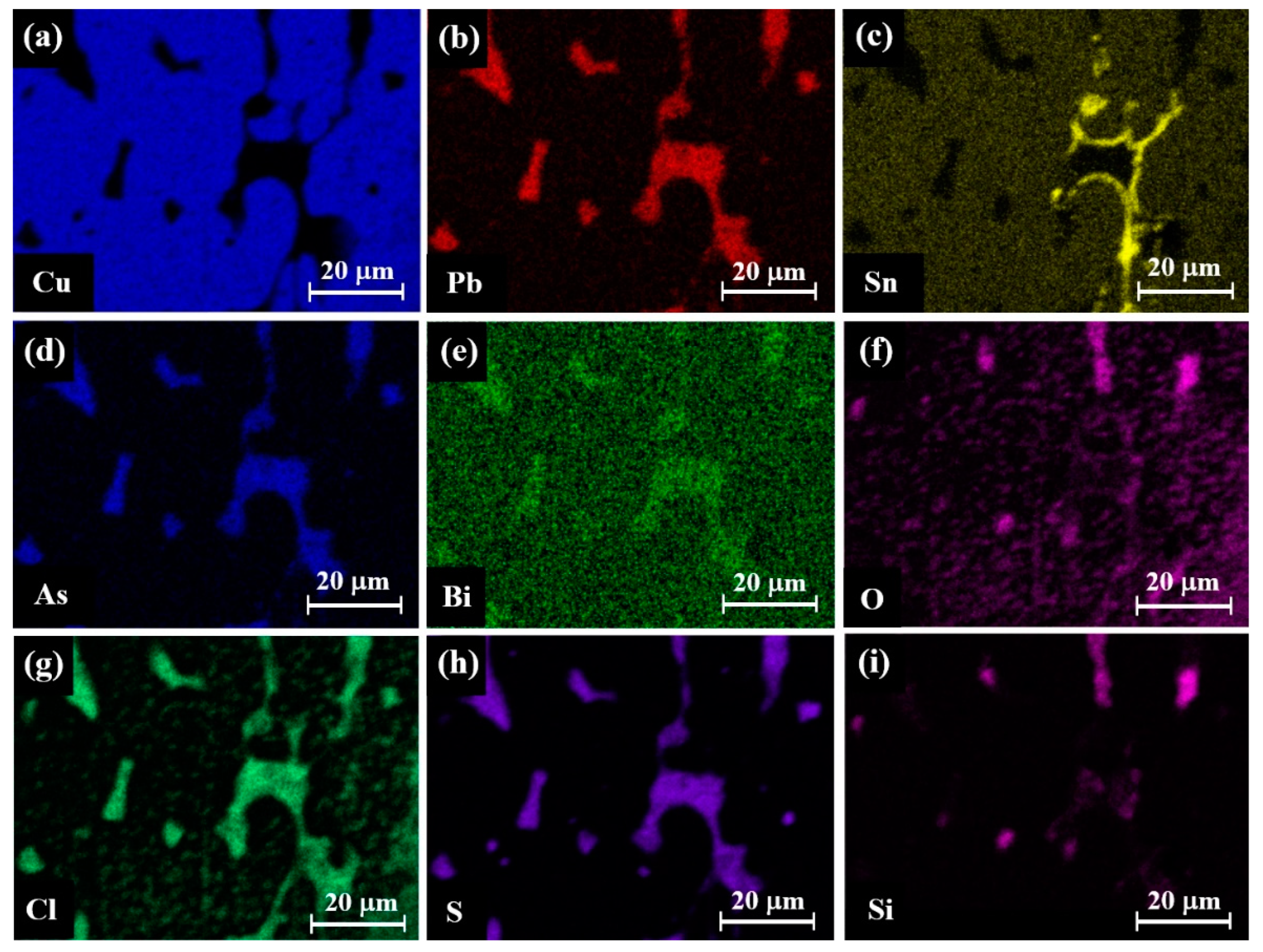
4.3. Chemical and Mineralogical Compositions
5. Discussion
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Di Turo, F. Limits and perspectives of archaeometric analysis of archaeological metals: A focus on the electrochemistry for studying ancient bronze coins. J. Cult. Herit. 2000, 43, 271–281. [Google Scholar] [CrossRef]
- Serghini-Idrissi, M.; Bernard, M.C.; Harrif, F.Z.; Joiret, S.; Rahmouni, K.; Srhiri, A.; Takenouti, H.; Vivier, V.; Ziani, M. Electrochemical and spectroscopic characterisations of patinas formed on an archaeological bronze coin. Electrochim. Acta 2005, 50, 4699–4709. [Google Scholar] [CrossRef]
- Denker, A.; Bohne, W.; Opitz-Coutureau, J.; Rauschenberg, J.; Röhrich, J.; Strub, E. Influence of corrosion layers on quantitative analysis. Nucl. Instrum. Methods. Phys. Res. B. 2005, 239, 65–70. [Google Scholar] [CrossRef]
- Di Francia, E.; Lahoz, R.; Neff, D.; de Caro, T.; Angelini, E.; Grassini, S. Laser-cleaning effects induced on different types of bronze archaeological corrosion products: Chemical-physical surface characterisation. Appl. Surf. Sci. 2022, 573, 150884. [Google Scholar] [CrossRef]
- Inberg, A.; Ashkenazi, D.; Cohen, M.; Iddan, N.; Cvikel, D. Corrosion products and microstructure of copper alloy coins from the Byzantine-period Ma‘agan Mikhael B shipwreck, Israel. Microchem. J. 2018, 143, 400–409. [Google Scholar] [CrossRef]
- Ingo, G.M.; De Caro, T.; Riccucci, C.; Angelini, E.; Grassini, S.; Balbi, S.; Bernardini, P.; Salvi, D.; Bousselmi, L.; Gener, M.; et al. Large scale investigation of chemical composition, structure and corrosion mechanism of bronze archeological artefacts from Mediterranean basin. Appl. Phys. A. 2006, 83, 513–520. [Google Scholar] [CrossRef]
- Manti, P.; Watkinson, D. Corrosion phenomena and patina on archaeological low-tin wrought bronzes: New data. J. Cul. Herit. 2022, 55, 158–170. [Google Scholar] [CrossRef]
- Robotti, S.; Rizzi, P.; Soffritti, C.; Garagnani, G.L.; Greco, C.; Facchetti, F.; Borla, M.; Operti, L.; Agostino, A. Reliability of portable X-ray Fluorescence for the chemical characterisation of ancient corroded copper-tin alloys. Spectrochim. Acta Part B At. Spectros. 2018, 146, 41–49. [Google Scholar] [CrossRef]
- Papadopoulou, O.; Vassiliou, P. The influence of archaeometallurgical copper alloy castings microstructure towards corrosion evolution in various corrosive media. Corros. Mater. Degrad. 2021, 2, 227–247. [Google Scholar] [CrossRef]
- Drakaki, E.; Karydas, A.G.; Klinkenberg, B.; Kokkoris, M.; Serafetinides, A.A.; Stavrou, E.; Vlastou, R.; Zarkadas, C. Laser cleaning on Roman coins. Appl. Phys. A. 2004, 79, 1111–1115. [Google Scholar] [CrossRef]
- Ashkenazi, D.; Inberg, A.; Langgut, D.; Hendler, N.; Cvikel, D. Brass–iron couple and brass–iron–wood ternary system of metal objects from the Akko 1 shipwreck (Israel). Corros. Sci. 2016, 110, 228–241. [Google Scholar] [CrossRef]
- MacLeod, I.D. Formation of marine concretions on copper and its alloys. Int. J. Naut. Archaeol. 1982, 11, 267–275. [Google Scholar] [CrossRef]
- Korenberg, C.; Baldwin, A. Laser cleaning tests on archaeological copper alloys using an ND: YAG Laser. Laser Chem. 2006, 2006, 75831. [Google Scholar] [CrossRef] [Green Version]
- Al-Sadoun, A.; Abdel-Kareem, O. Authentication and conservation of selected metal objects excavated from al-serein, near makkah, saudi arabia. Mediterr. Archaeol. Archaeom. 2019, 19, 23–38. [Google Scholar]
- Pini, R.; Siano, S.; Salimbeni, R.; Pasquinucci, M.; Miccio, M. Tests of laser cleaning on archeological metal artefacts. J. Cult. Herit. 2000, 1, S129–S137. [Google Scholar] [CrossRef]
- Hrnjic, M.; Angurel, L.A.; Lahoz, R.; Grassini, S.; Angelini, E.; Schiavon, N.; de la Fuente, G.F. Near-IR laser cleaning of Cu-based artefacts: A comprehensive study of the methodology standardization. In Proceedings of the 1st International Conference on Metrology for Archaeology, Benevento, Italy, 22–23 October 2015; pp. 389–394. [Google Scholar]
- Garbacz, H.; Fortuna-Zalesna, E.; Marczak, J.; Koss, A.; Zatorska, A.; Zukowska, G.Z.; Onyszczuk, T.; Kurzydlowski, K.J. Effect of laser treatment on the surface of copper alloys. Appl. Surf. Sci. 2011, 257, 7369–7374. [Google Scholar] [CrossRef]
- Cohen, M.; Ashkenazi, D.; Bijovsky, G.I.; Inberg, A.; Klein, S.; Cvikel, D. Copper alloy coins from the Byzantine-Period Ma‘agan Mikhael B Shipwreck, Israel: Metallurgical characterization. Metallogr. Microstruct. Anal. 2018, 7, 542–560. [Google Scholar] [CrossRef]
- Iddan, N.; Ashkenazi, D.; Klein, S.; Cvikel, D. Metallurgical analysis of a bronze powder chamber retrieved from an underwater excavation in Akko (Israel): An application of novel minimally destructive field multi-focal metallography. Archaeol. Anthropol. Sci. 2022, 14, 131. [Google Scholar] [CrossRef]
- Cohen, M.; Cvikel, D. Ma-agan Mikhael B, Israel: A preliminary report of a Late Byzantine–early Islamic period shipwreck. Int. J. Naut. Archaeol. 2019, 48, 189–207. [Google Scholar]
- Creisher, M.; Goren, Y.; Artzy, M.; Cvikel, D. The Amphorae of the Ma’agan Mikhael B Shipwreck: Preliminary Report. Levant 2019, 51, 105–120. [Google Scholar] [CrossRef]
- Natan, E.; Gorin-Rosen, Y.; Benzonelli, A.; Cvikel, D. Maritime trade in Early Islamic-period glass: New evidence from the Maʻagan Mikhael B shipwreck. J. Archaeol. Sci. Rep. 2021, 37, 102903. [Google Scholar] [CrossRef]
- Bijovsky, G.I. Gold Coin and Small Change: Monetary Circulation in Fifth–Seventh Century Byzantine Palestine; Università di Trieste: Trieste, Italy, 2012. [Google Scholar]
- Kahanov, Y.; Ashkenazi, D.; Cvikel, D.; Klein, S.; Navri, R.; Stern, A. Archaeometallurgical analysis of metal remains from the Dor 2006 shipwreck: A clue to the understanding of the transition in ship construction. J. Archaeol. Sci. Rep. 2015, 2, 321–332. [Google Scholar] [CrossRef]
- Turner-Walker, G. The nature of cleaning: Physical and chemical aspects of removing dirt, stains and corrosion. In Proceedings of the International Symposium on Cultural Heritage Conservation, Tainan, Taiwan, 6–8 November 2012. [Google Scholar]
- Crosera, M.; Baracchini, E.; Prenesti, E.; Giacomello, A.; Callegher, B.; Oliveri, P.; Adami, G. Elemental characterisation of surface and bulk of copper-based coins from the Byzantine-period by means of spectroscopic techniques. Microchem. J. 2019, 147, 422–428. [Google Scholar] [CrossRef]
- Di Turo, F.; Parra, R.; Piquero-Cilla, J.; Favero, G.; Doménech-Carbó, A. Crossing VIMP and EIS for studying heterogeneous sets of copper/bronze coins. J. Solid. State. Electrochem. 2019, 23, 771–781. [Google Scholar] [CrossRef]
- Ashkenazi, D.; Cvikel, D. A journey into the microstructure: Using a multifocal 3D digital light microscope to study archaeological artefacts retrieved from shipwrecks. Digit. Appl. Archaeol. Cult. Herit. 2020, 16, e00129. [Google Scholar] [CrossRef]
- Ashkenazi, D.; Bunimovitz, S.; Stern, A. Archaeometallurgical investigation of thirteenth–twelfth centuries BCE bronze objects from Tel Beth-Shemesh, Israel. J. Archaeol. Sci. Rep. 2016, 6, 170–181. [Google Scholar] [CrossRef]
- Adar, F. Introduction to interpretation of Raman spectra using database searching and functional group detection and identification. Spectroscopy 2016, 31, 16–23. [Google Scholar]
- Oancea, A.V.; Bodi, G.; Nica, V.; Ursu, L.E.; Drobota, M.; Cotofana, C.; Vasiliu, A.L.; Simionescu, B.C.; Olaru, M. Multi-analytical characterization of Cucuteni pottery. J. Eur. Ceram. Soc. 2017, 37, 5079–5098. [Google Scholar] [CrossRef]
- Bell, I.M.; Clark, R.J.; Gibbs, P.J. Raman spectroscopic library of natural and synthetic pigments (pre- ≈1850 AD). Spectrochim. Acta. A. Biomol. Spectrosc. 1997, 53, 2159–2179. [Google Scholar] [CrossRef]
- McCann, L.I.; Trentelman, K.; Possley, T.; Golding, B. Corrosion of ancient Chinese bronze money trees studied by Raman microscopy. J. Raman. Spectrosc. 1999, 30, 121–132. [Google Scholar] [CrossRef]
- Kosec, T.; Ćurković, H.O.; Legat, A. Investigation of the corrosion protection of chemically and electrochemically formed patinas on recent bronze. Electrochim. Acta 2010, 56, 722–731. [Google Scholar] [CrossRef]
- He, L.; Liang, J.; Zhao, X.; Jiang, B. Corrosion behavior and morphological features of archeological bronze coins from ancient China. Microchem. J. 2011, 99, 203–212. [Google Scholar] [CrossRef]
- Ingo, G.M.; Riccucci, C.; Guida, G.; Pascucci, M.; Giuliani, C.; Messina, E.; Fierro, G.; Di Carlo, G. Micro-chemical investigation of corrosion products naturally grown on archaeological Cu-based artefacts retrieved from the Mediterranean Sea. Appl. Surf. Sci. 2019, 470, 695–706. [Google Scholar] [CrossRef]
- Cvikel, D.; Cohen, M.; Inberg, A.; Klein, S.; Iddan, N.; Ashkenazi, D. Metallurgical characterisation of brass sheet from the 19th-century Akko Tower Wreck (Israel). Mater. Charact. 2017, 131, 175–187. [Google Scholar] [CrossRef]
- Ashkenazi, D.; Inberg, A.; Cvikel, D. Analysis of naturally etched surface of brass sheathing from a nineteenth-century shipwreck. J. Min. Metall. B. 2018, 54, 101–110. [Google Scholar] [CrossRef]
- Kossolapov, A.J.; Chugunova, K.S. Residual stress in struck and cast coins. Insight NDT Cond. Monitor. 2020, 62, 139–144. [Google Scholar] [CrossRef]
- Craddock, P.T. Early Metal Mining and Production; Edinburgh University Press: Edinburgh, Scotland, 1995. [Google Scholar]
- Ehya, F.; Lotfi, L.M.; Rasa, I. Emarat carbonate-hosted Zn–Pb deposit, Markazi Province, Iran: A geological, mineralogical and isotopic (S, Pb) study. J. Asian Earth Sci. 2010, 37, 186–194. [Google Scholar] [CrossRef]
- Ingo, G.M.; Angelini, E.; Bultrini, G.; Calliari, I.; Dabala, M.; De Caro, T. Study of long-term corrosion layers grown on high-tin leaded bronzes by means of the combined use of GDOES and SEM+ EDS. Surf. Interface Anal. 2002, 34, 337–342. [Google Scholar] [CrossRef]
- Davidde, B. Methods and strategies for the conservation and museums display in situ of underwater cultural heritage. Archaeol. Marit. Mediterr. 2004, 1, 136–150. [Google Scholar]
- Chakrabarti, D.J.; Laughlin, D.E. The Cu−Pb (copper-lead) system. Bull. Alloy Phase Diagrams. 1984, 5, 503–510. [Google Scholar] [CrossRef]
- Griesser, M.; Kockelmann, W.; Hradil, K.; Traum, R. New insights into the manufacturing technique and corrosion of high leaded antique bronze coins. Microchem. J. 2016, 126, 181–193. [Google Scholar] [CrossRef]
- Torrisi, L.; Caridi, F.; Giuffrida, L.; Torrisi, A.; Mondio, G.; Serafino, T.; Caltabiano, M.; Castrizio, E.D.; Paniz, E.; Salici, A. LAMQS analysis applied to ancient Egyptian bronze coins. Nucl. Instrum. Methods. Phys. Res. B 2010, 268, 1657–1664. [Google Scholar] [CrossRef]
- Argyropoulos, V.; Giannoulaki, M.; Michalakakos, G.P.; Siatou, A. A survey of the types of corrosion inhibitors and protective coatings used for the conservation of metal objects from museum collections in the Mediterranean basin. In Proceedings of the the International Conference on Strategies for Saving Indoor Metallic Collections with a Satellite Meeting on Legal Issues in the Conservation of Cultural Heritage, Cairo, Egypt, 25 February–1 March 2007; pp. 1–5. [Google Scholar]
- Adriaens, A.; Dowsett, M.; Leyssens, K.; van Gasse, B. Insights into electrolytic stabilization with weak polarization as treatment for archaeological copper objects. Anal. Bioanal. Chem. 2007, 387, 861–868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashkenazi, D.; Nusbaum, I.; Shacham-Diamand, Y.; Cvikel, D.; Kahanov, Y.; Inberg, A. A method of conserving ancient iron artefacts retrieved from shipwrecks using a combination of silane self-assembled monolayers and wax coating. Corr. Sci. 2017, 123, 88–102. [Google Scholar] [CrossRef]



| Coin No. | Coin Description | Diameters (mm) | Thickness (mm) | Weight (g) |
|---|---|---|---|---|
| 120.3 | Including the black concretion layer [17] | 17.10 × 17.10 | 3.00 | 2.66 |
| 143.1 | Including the black concretion layer | 15.76 × 16.30 | – | – |
| Totally corroded, no metal survived | – | – | – | |
| 154.2 | Including black concretion layer | 16.13 × 16.35 | 2.75 | 1.74 |
| After removal of concretion layer | 14.30 × 15.16 | 0.99 | 0.84 | |
| 162 | Including black concretion layer | 15.79 × 16.23 | 3.78 | 1.42 |
| 165 | Including black concretion layer | 13.65 × 16.13 | 1.44 | 0.86 |
| After removal of concretion layer | 12.51 × 14.18 | 0.96 | 0.58 | |
| 167.1 | Green-turquoise concretion | 15.01 × 16.61 | 2.10 | 1.47 |
| 167.3 | Clean, well preserved | 17.16 × 17.51 | 1.37 | 2.04 |
| 167.9 | Clean, well preserved | 14.73 × 15.10 | 1.18 | 1.31 |
| 168.2 | Including black concretion layer | 15.98 × 16.32 | 1.74 | 1.07 |
| After removal of concretion layer | 12.10 × 13.16 | 0.55 | 0.33 | |
| 168.3 | Including black concretion layer (possibly internal) | 14.37 × 15.44 | 1.92 | 1.25 |
| 169.1 | Including black concretion layer | 20.10 × 20.39 | 4.33 | 3.21 |
| 176 | Including black concretion layer | 15.11 × 16.18 | 1.44 | 1.41 |
| After removal of concretion layer | 14.88 × 14.91 | 1.16 | 1.04 | |
| 181 | Including black concretion layer | 14.14 × 15.51 | 2.25 | 0.87 |
| After removal of concretion layer | 11.17 × 12.85 | 0.73 | 0.40 | |
| 182.3 | Remains of black concretion layer | 15.23 × 16.69 | 3.42 | 1.49 |
| 186.2 | Remains of black concretion layer | 14.79 × 15.38 | 1.94 | 1.36 |
| After removal of concretion layer | 13.97 × 14.78 | 0.75 | 0.89 | |
| 186.3 | Remains of black concretion layer | 14.59 × 15.14 | 2.86 | 1.33 |
| After removal of concretion layer | 13.80 × 14.34 | 1.21 | 0.79 |
| Coin No. and Its Examined Area | Composition (wt.%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cu | Pb | Sn | Zn | As | Mg | Ag | Si | S | Fe | |
| 120.3/A, a shiny metallic surface [17] | 71.3 | 10.0 | 1.7 | 0.5 | – | – | 0.1 | 9.9 | 6.3 | 0.2 |
| 154.2/A, with black concretion | 51.6 | 4.7 | 0.7 | – | 0.5 | – | 0.6 | 23.2 | 10.3 | 8.4 |
| 154.2/B, with black concretion | 58.6 | 3.2 | 0.8 | 0.1 | 0.5 | – | 0.7 | 19.0 | 8.3 | 8.8 |
| 154.2/B, metal after partial surface chemical cleaning | 58.3 | 27.9 | 2.9 | – | 2.6 | – | 1.7 | 3.7 | – | 2.9 |
| 162/A, with black concretion | 31.6 | 0.1 | – | – | – | – | 0.3 | 59.3 | 8.2 | 0.5 |
| 162/B, with black concretion | 35.8 | 0.2 | – | – | – | – | 0.3 | 51.0 | 11.9 | 0.8 |
| 165/A, with black concretion, after partial surface chemical cleaning | 67.6 | 13.2 | 2.1 | 0.1 | – | – | 0.7 | 2.2 | 13.7 | 0.4 |
| 165/B, with black concretion, after partial surface chemical cleaning | 59.4 | 8.9 | 2.0 | 0.1 | – | – | 0.6 | 18.1 | 10.6 | 0.3 |
| 167.1/A, with green concretion | 72.3 | 3.8 | 4.7 | 0.1 | – | – | 0.4 | 18.2 | – | 0.5 |
| 167.1/A, metal after chemical cleaning and polishing treatment | 75.2 | 6.7 | 9.9 | 0.1 | – | – | 1.2 | 6.7 | – | 0.2 |
| 167.3/A, naturally polished coin (Figure 5a, centre of the coin, average of two measurements) | 90.1 | 5.6 | 2.8 | 0.1 | – | – | 1.2 | – | 0.2 | 0.2 |
| 167.3/B, naturally polished coin (Figure 5b, centre of the coin, average of two measurements) | 94.2 | 2.0 | 2.4 | 0.1 | – | – | 1.1 | – | 0.1 | 0.1 |
| 167.9/A, naturally polished coin (Figure 5c, centre of the coin, average of two measurements) | 94.2 | 1.9 | 1.8 | 0.1 | – | – | 1.9 | – | – | 0.1 |
| 167.9/B, Naturally polished coin (Figure 5d, centre of the coin, average of two measurements) | 94.2 | 1.8 | 1.9 | 0.1 | – | – | 1.9 | – | – | 0.1 |
| 168.2/A, with black concretion | 49.2 | 6.1 | 0.3 | 0.1 | – | – | 0.3 | 30.3 | 12.5 | 1.2 |
| 168.2/B, with black concretion | 39.9 | 9.9 | 0.4 | – | – | 1.6 | 0.5 | 39.4 | 6.9 | 1.4 |
| 168.3/A, with fine grey concretion | 64.4 | 0.8 | 1.4 | 0.1 | – | – | 0.3 | 11.8 | 17.0 | 4.2 |
| 168.3/B, with fine grey concretion | 65.0 | 1.5 | 1.8 | 0.1 | – | – | 0.3 | 3.5 | 20.1 | 7.7 |
| 169.1/A, with black concretion | 45.2 | 0.2 | 0.1 | – | – | – | 0.5 | 43.3 | 10.4 | 0.3 |
| 169.1/B, with black concretion | 43.4 | 0.2 | – | 0.1 | 0.1 | 0.1 | 0.8 | 44.1 | 10.5 | 0.7 |
| 176/A, with black concretion remains, after partial surface chemical cleaning | 54.6 | 25.2 | 3.2 | – | – | – | 0.5 | 3.4 | 11.9 | 1.2 |
| 176/B, with black concretion remains, after partial surface chemical cleaning | 56.0 | 19.9 | 2.6 | 0.1 | – | – | 0.5 | 7.3 | 13.1 | 0.5 |
| 181/A, with black concretion | 59.6 | 1.6 | 0.9 | 0.1 | – | 17.8 | 0.4 | 12.9 | 6.5 | 0.2 |
| 181/B, with black concretion | 50.4 | 7.5 | 1.5 | 0.1 | 0.5 | 6.2 | 0.7 | 29.4 | 3.1 | 0.6 |
| 181/B, metal covered with oxide after chemical cleaning and polishing treatment | 63.1 | 20.5 | 3.0 | 0.1 | 1.5 | – | 1.1 | 9.8 | – | 0.9 |
| 182.3/A, with black concretion | 48.6 | 1.0 | 0.5 | 0.1 | – | – | 0.4 | 35.1 | 13.8 | 0.5 |
| 182.3/B, with black concretion | 44.1 | 1.2 | 0.6 | 0.1 | – | – | 0.5 | 43.1 | 9.6 | 0.8 |
| 186.2/A, with black concretion | 60.5 | 4.1 | 1.3 | 0.1 | – | – | 0.7 | 20.7 | 12.2 | 0.4 |
| 186.2/B, with black concretion | 65.7 | 9.3 | 2.2 | 0.1 | – | – | 0.7 | 5.8 | 16.0 | 0.2 |
| 186.3/A, with black concretion | 57.5 | 8.8 | 1.3 | 0.1 | – | – | 1.5 | 23.5 | 6.9 | 0.4 |
| 186.3/B, with black concretion | 38.6 | 1.2 | 0.6 | 0.1 | – | – | 0.8 | 52.6 | 5.8 | 0.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cohen, M.; Inberg, A.; Ashkenazi, D.; Cvikel, D. What You Clean Is What You Get: A Novel Chemical Cleaning Technique and the Interpretation of Corrosion Products Found in Late Roman Copper Alloy Coins Retrieved from the Sea. Heritage 2022, 5, 3628-3647. https://doi.org/10.3390/heritage5040189
Cohen M, Inberg A, Ashkenazi D, Cvikel D. What You Clean Is What You Get: A Novel Chemical Cleaning Technique and the Interpretation of Corrosion Products Found in Late Roman Copper Alloy Coins Retrieved from the Sea. Heritage. 2022; 5(4):3628-3647. https://doi.org/10.3390/heritage5040189
Chicago/Turabian StyleCohen, Maayan, Alexandra Inberg, Dana Ashkenazi, and Deborah Cvikel. 2022. "What You Clean Is What You Get: A Novel Chemical Cleaning Technique and the Interpretation of Corrosion Products Found in Late Roman Copper Alloy Coins Retrieved from the Sea" Heritage 5, no. 4: 3628-3647. https://doi.org/10.3390/heritage5040189





