Abstract
Since the 1940s, when maritime archaeology was established, the systematic excavation of submerged wrecks began to be refined. Systematic excavations led to the recovery of a vast array of organic and inorganic artefacts, including human and non-human bones and teeth. In order to preserve the materials recovered from the sea, the discipline of maritime conservation rapidly expanded and dealt with unique issues, including, but not limited to, marine salts’ encrustation of archaeological artefacts. Bone and teeth are organic artefacts which provide crucial information about natural and social environments of the past. When recovered from marine settings, they are often friable and require conservation processes and consolidation treatments, e.g., to prevent shrinkage during drying. However, conservation processes and consolidants can interfere with pathological, traumatic and taphonomical evidence associated with bone and teeth, and can bias sample preparation and analysis through mechanical action and chemical composition. The aim of this paper, in which a few examples of interference are listed, is to highlight the need of accurately documenting any type of conservation process and/or consolidation treatment that has been performed on bone and teeth stored in archaeological collections. This becomes essential when samples are selected for study, especially if this includes destructive analysis, and will assist in clarifying any conflicting results, leading to reliable interpretations.
1. Introduction
Maritime archaeology, and therefore maritime conservation, expanded into its own discipline with the invention of a self-contained underwater breathing apparatus (SCUBA) in the 1940s [1]. The SCUBA allowed archaeologists to extend their reach into the ocean, and to begin developing a methodology to record and excavate shipwrecks and other underwater sites of archaeological interest [2]. Maritime archaeology as a discipline has been defined by its practitioners as both in opposition to terrestrial archaeology, and as a sub-discipline of archaeology [3]. For the purpose of this paper, maritime archaeology will be defined as ‘the scientific study of the material remains of [hu]man[s] and [their] activities on the sea’ [4]. As Hamilton states, the conservation of artefacts means to “document, analyse, clean, and stabilise them” [5]; therefore, maritime conservation can be defined as the minimisation of damage to, and preservation of, artefacts recovered from and around the ocean as a result of maritime archaeological activities.
2. Maritime Conservation
The discipline of maritime conservation rapidly expanded in the 1960s with the rai-sing of the 17th century warship Vasa from the Stockholm harbour, Sweden [6]. The subsequent raising of the Tudor warship Mary Rose in England, and the conservation of the Dutch East Indiaman Batavia in Australia firmly established maritime conservation as its own field of conservation [7,8,9]. Maritime conservation shares the same aims as other fields of conservation, and more specifically archaeological conservation, that is to safeguard artefacts, and to ensure their interpretability once they have been removed from their burial environment [10]. Despite these similarities, the problems faced by maritime conservators are largely unique [11]. In fact, submerged artefacts have become part of a new environment, with which they have developed physical, chemical and biological interactions. Maritime conservators often face thick encrustations on the surface of metallic objects, which are often a mix of marine life and metal corrosion. Once the encrustations are removed, the artefact underneath requires specialised attention in the removal of salts that have accumulated within it [12]. Other inorganic materials, such as ceramics and glass, also require specialised care and attention; one of the most important steps in the conservation process is desalination, or removal of chlorides from within materials [13]. If allowed to dry without desalination, crystallised salts will expand and damage the glaze of a ceramic, and cause damage to glass artefacts [14,15].
Organic artefacts pose their own unique conservation problems. Unlike the majority of terrestrial archaeological sites, organic objects can be very numerous on shipwreck sites and in maritime contexts, which are frequently anoxic [16,17]. Organic artefacts recovered from shipwreck sites can be varied, depending on the contents and cargo of the ship, but the most common ones include timber—whether that be the ship itself, or smaller wooden artefacts—rope, bone, and textiles [18]. Organic artefacts recovered from maritime settings are often friable and delicate [19], and may be encrusted with metals [20] and colonized by marine organisms [21]. As a consequence, they require not only desalination, as almost all artefacts from the ocean require, but usually also cleaning, some form of consolidation to add strength, and other treatments to prevent shrinkage during drying [22,23]. For example, waterlogged timbers are impregnated with polyethylene glycol (PEG), a synthetic polymer, to lend strength and to prevent cracking and warping during the drying and exhibition processes [24,25]. Similarly, textiles and rope may also undergo treatment with PEG, or other synthetic polymers, to prevent further deterioration once they are removed from their stable aqueous environment [26,27]. Bone, whether it be human or non-human, and whether an artefact or remains, is treated with similar techniques, requiring desalination and, in some instances, consolidation [5].
3. Bone and Teeth in Maritime Conservation
Bones and teeth are the skeletal tissues of many biological taxa, human and non-human (also defined as faunal), and are frequently recovered in both terrestrial and underwater archaeological excavation sites [28,29,30,31]. Collectively described as specialized types of connective tissue, bone is composed of cells enclosed within an extracellular matrix of framework macromolecules (mostly collagen) and hydroxyapatite, a calcium phosphate mineral, whereas teeth are mostly acellular [32,33]. The physical arrangement of the biological materials composing bone and teeth, including, but not limited to, collagen fibrils, hydroxyapatite crystals and bone lamellae, and their slightly different chemistry, are reflected by denser or looser structures, such as cortical bone, trabecular bone, dentin, enamel and cementum. Enamel, which covers the crown of teeth, is the hardest and the densest, cortical bone is chemically similar to dentin and cementum, and trabecular bone is the most porous, that is, a network of thin columns and cavities [34,35]. Thanks to their hardness and structure, bone and teeth survive long after the death of an organism and the decomposition of all the other biological tissues. In favourable environmental conditions, they can fossilize and be preserved for millions of years [36,37,38]. Their analysis provides a wealth of information about natural [39,40,41] and social [42,43,44,45,46] environments of the past.
Once safely recovered according to established guidelines and recommended professional practices both on land and underwater [47,48], archaeological bones and teeth can become very fragile [19], and may need to be consolidated using conservation processes. There has been little change in the treatment of bone and teeth from maritime settings throughout the years, though the materials used have evolved. In most cases, the typical conservation steps are desalination, the removal of stains and, if the bone has degraded and is friable, the application of consolidating treatments [5]. Desalination is crucial with all maritime archaeological artefacts. Bone is liable to be damaged if not desalinated: phy-sically, due to expansion of salt crystals upon drying, and chemically, from acid formation due to the hygroscopic nature of salts [23]. Desalination is achieved by progressive soa-king in distilled water until soluble salts have been removed [49]. In some cases, such as the human and non-human bones recovered from the Mary Rose, desalination is the only active treatment required; thereafter, bones can be slowly air-dried and stored in environmentally controlled conditions [23,50]. Once desalination has been completed, any stai-ning can be addressed. If deemed necessary, iron stains may be removed using chelating agents, including EDTA disodium salt, ammonium citrate, or oxalic acid, although these treatments will also risk demineralisation and recrystallisation of the bone mineral [5,23]. Sodium dithionite has also been investigated as a method for removing iron stains from bone, with reported results ranging from satisfactory (i.e., stains were removed with little damage to the bone) to unsatisfactory, with reports of bone softening [51,52]. A recent preliminary study investigated the use of a pulsed coaxial plasma gun in removing iron staining from bone samples, and reported a reduced visual appearance of iron staining and reduced acidity of the bones, caused by iron staining [53]. Iron sulphide stains can be removed using hydrogen peroxide, a method recommended both historically and in the present day, although prolonged treatment is likely to damage any remaining collagen [11,54].
When consolidating treatments were required, for example, in the case of flaking or splintering, early archaeologists and conservators used natural resins, such as paraffin wax or animal glue, to circumvent bone and ivory friability [55,56]. The use of animal glue as a consolidant was dismissed after the 1950s, and recent investigations into animal glue have found its potential to bias 14C dating and DNA analyses, due to their collagen base [57]. More recent materials recommended for consolidating bone include Paraloid B-72, an acrylic resin, and polyvinyl acetate (PVAc), a thermoplastic adhesive [11,58,59]. These are still largely used for bone consolidation in the present day. Paraloid B-72 is a poly (methylacrylate/ethylmethacrylate) which is regarded as a stable polymer and used in multiple conservation treatments as a consolidant when dissolved in a solvent such as acetone [58]. It can be used by itself as a consolidant for bone, or in conjunction with a stable textile material to lend strength to a fragile artefact or set of remains [60]. It is theoretically removable, if necessary, by using the solvent into which it was originally dissolved, but it is unlikely that complete removal is practicable [58]. PVA emulsions are also utilised in present-day treatments; they are chosen for their penetrative properties, allo-wing them to diffuse through the bone structure and lend strength [61]. Alternatives in the form of 20% glycerol immersion combined with slow air-drying have reportedly received satisfactory results [62]. PEG has also been investigated as an alternative consolidant for waterlogged bone, as it already has an established use in the conservation of waterlogged wood, but was found to be unsuitable. At lower molecular weights, it ‘sweats’ out of bone, obscuring features, and lends little structural support to fragile bone [63]. Ethical concerns may dictate it inappropriate to apply conservation treatments to remedy decay and consolidate human bone [64,65]. However, some skulls of the victims of the Batavia mutiny were pieced together with a polyvinyl acetate emulsion, which was planned to be dissolved and removed prior to burial, but after the acquisition of the relevant forensic information [66]. In fact, even minimal damage to bone and teeth might disguise, modify or destroy, any type of relevant evidence, overall attributable to the three broad categories of pathology, trauma and taphonomy (Figure 1 and Figure 2).

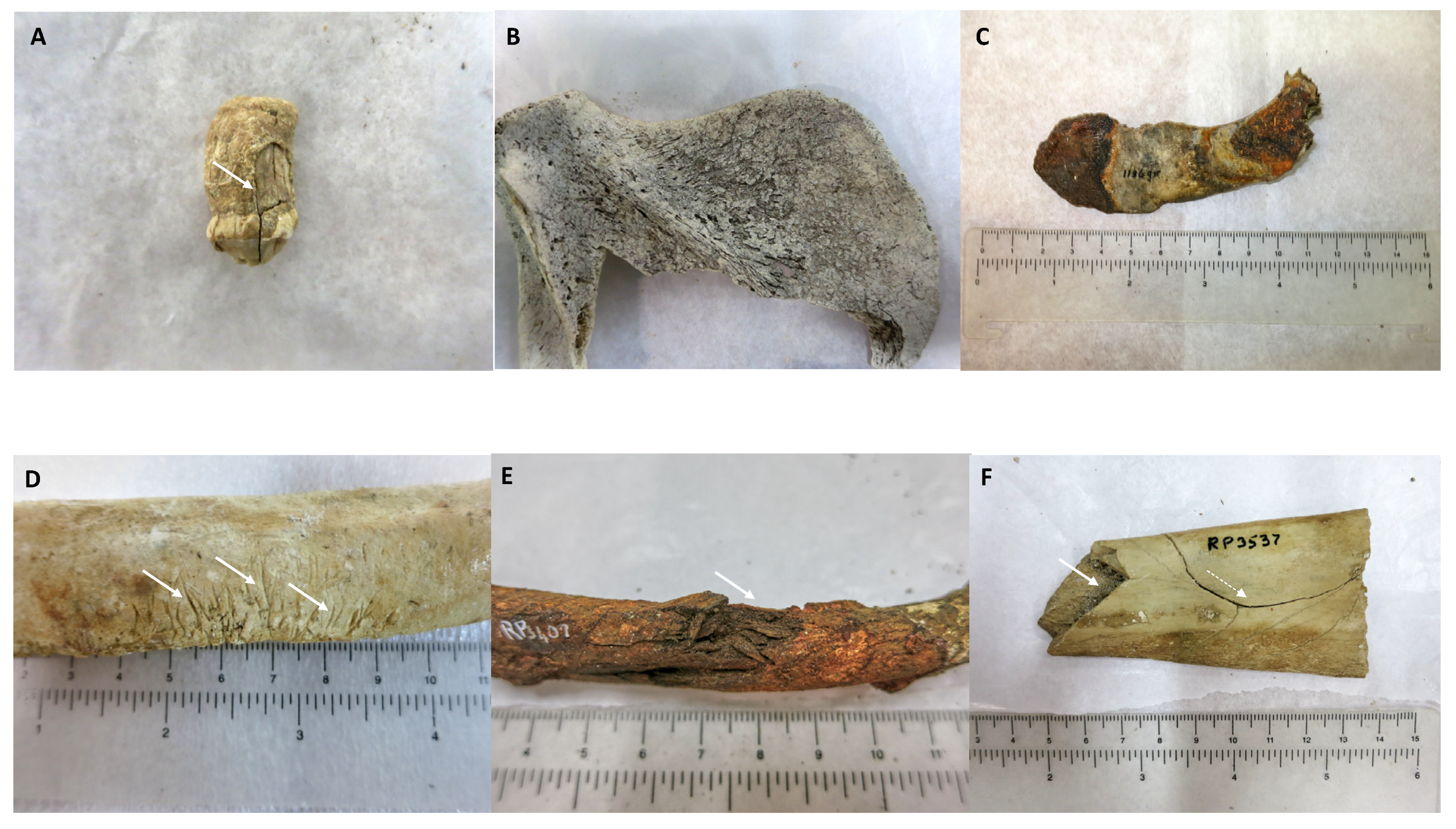
Figure 1.
Examples of evidence associated to bone and teeth recovered in maritime archaeological excavations. (A) Tooth of an Australian sea lion (Neophoca cinerea) with wear of the dental crown and a longitudinal fracture involving both the dental crown and root (solid arrow). The general morphology indicates that the fracture was produced close to either the death of the animal, or the loss of the tooth (from Batavia, 1629) (B) Scapula of juvenile Australian sea lion (N. cinerea), bleached and abraded (from Batavia, 1629) (C) Non-human bone fragment with colour changes resembling the ones produced by fire (from Vergulde Draeck, 1656) (D) Multiple knife marks (solid arrows) on the inferior margin of a non-human rib (from Rapid, 1811) (E) Non-human bone crushed as fresh (solid arrow) and heavily stained in the post-mortem period (from Rapid, 1811) (F) Butchering mark (solid arrow) produced on fresh non-human bone, with resulting curved fracture line (dashed arrow) (from Rapid, 1811).

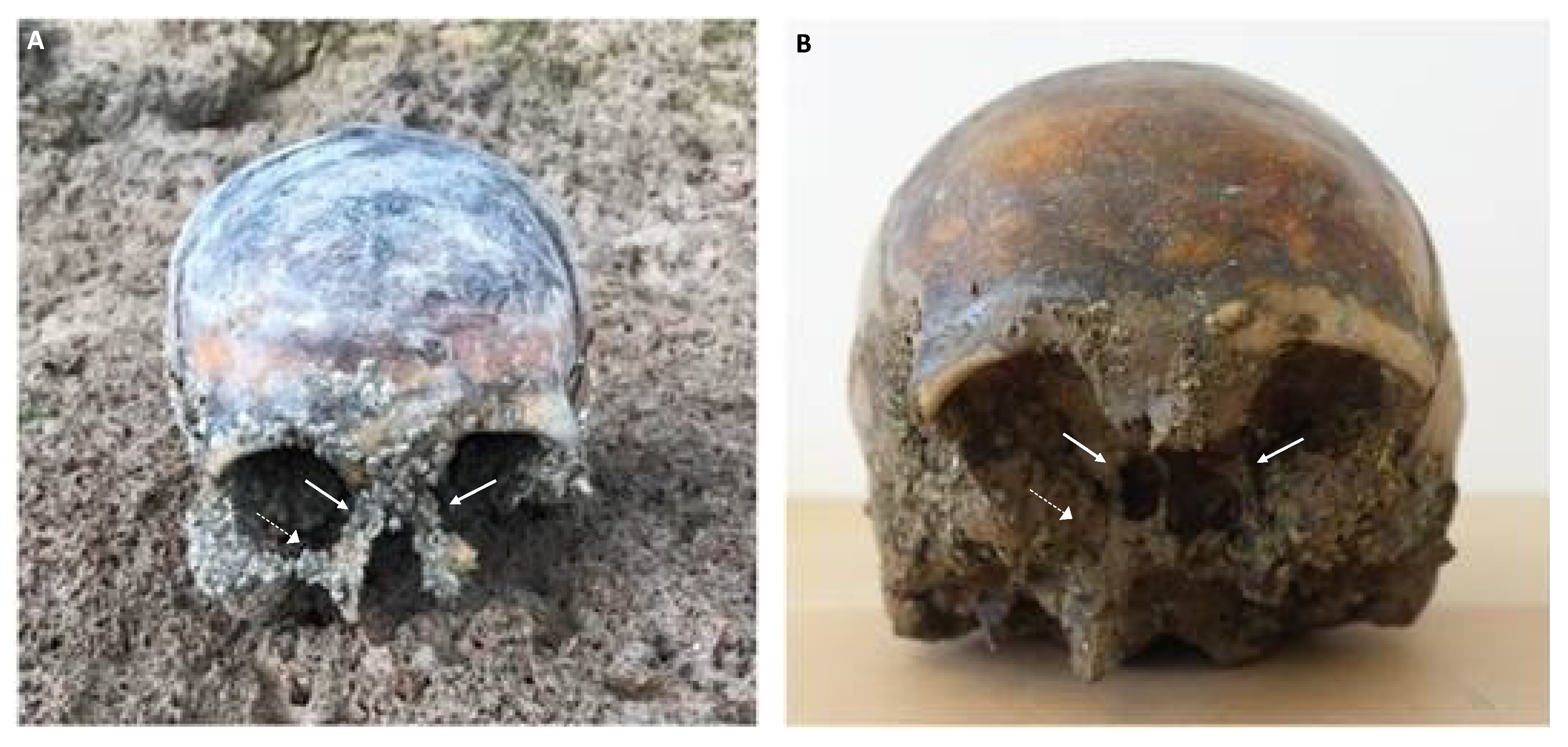
Figure 2.
Archaeological human skull recovered from an aquatic setting. (A) At the time of recovery, with both nasal bones (solid arrows) and the remnants of the facial region extensively encrusted by barnacles (dashed arrow). (B) At the time of delivery to the forensic laboratory, with the loss of both nasal bones (solid arrows) and a substantial part of the encrusting barnacles (dashed arrow), possibly caused by handling, rapid drying and cleaning attempts.
Some examples of pathological alterations potentially modifiable via conservation processes include pitting (cribra orbitalia) in the superior orbital walls in humans, smooth margins in bone remodelling, reactive periostitis and dental calculus [67,68,69]. Trauma can be represented by tool and weapon marks (e.g., knife) [70], fractures, which can be elusive when minute and limited to the bone surface like the hairline/stress type [71], or pathological, such as in Paget’s disease [72], and gunshot wounds [73]. Taphonomic alterations are the most varied, and include, although are not limited to, abrasions [74], punctures and grooves produced by predators and scavengers [75], staining [76], fire-related changes [77], cracking produced by weathering [78], tunnelling by macro- and micro-bioerosion [79] and the presence of encrustations (e.g., barnacles) [80], inclusions (e.g., fora-minifera) [81] and concretions [2]. With specific focus on bones and teeth recovered underwater, the conservation processes and the chemical components of the applied conso-lidants might affect the analytical results of physical, chemical and biological modifications of different origin.
4. Discussion
When approaching any archaeological collection of bones and teeth with the purpose of conducting anthropological (physical or forensic), paleopathological or taphonomical analyses, especially if microscopic and/or molecular/elemental, it is paramount to esta-blish which type of conservation process and/or consolidation treatment has been performed on the specimens. Any physical alteration produced through mechanical or che-mical cleaning, and/or the application of consolidants, can potentially interfere with both the preparation of the sample for analysis (e.g., demineralization, as in Privat and O’Connell [82]), and with the macroscopic, microscopic and elemental chemistry results, the interpretation of which may then be systematically biased [83]. Moreover, any substance used to remove the consolidant(s) should be acknowledged and recorded, as well as the time of any treatment. In fact, while some consolidants are chemically stable over time (e.g., Paraloid B-72) [84], others decay or become irreversible, with substantial alteration of their chemical composition (e.g., natural resins) [85,86]. Consolidants are never completely removed [87], and their composition should be noted within the treatment report, if it is identifiable. In other cases, consolidants may not need to be removed prior to retreatment, as the removal process may cause more damage to the object; in these cases, the use of a consolidant that can be easily distinguished from those used in previous treatments is recommended [61].
A few examples of the interference of conservation processes and/or consolidation treatments on bone and teeth with relevant pathological, traumatological or taphonomic evidence in maritime archaeology include vigorous desalination, brushing, air abrasion and sandblasting [53], which could displace foraminifera and remnants of other aquatic organisms potentially detectable using microscopy, such as sponges [88], as well as producing abrasions which might confound, cover or erase their traces [89] and any type of fissure [90]. Moreover, demineralization using acids (e.g., HCl) to investigate bone collagen, or to achieve histological slides, can dissolve bryozoan skeletons and alter evidence of digestion by predators, such as acid corrosion and rounding of the bone edges [91]. Cleaning through bleaching could disguise bleaching of taphonomic origin, modify any staining produced by cooking [92] or destroy potential DNA traces [93]. Coating with acrylic resins, which seep within bone pores, makes this treatment only theoretically reversible, and may interfere, through chemical cross-linking, with radiocarbon analysis [87,94,95] and stable isotope analysis [84,96], used for dating and provenancing, respectively. The treatment with EDTA and diammonium hydrogen phosphate (DAP) [97] might bias the elemental analysis of bone bioapatite and teeth enamel, essential for the study of both biogenic (e.g., linked to metabolism) and diagenetic (e.g., produced in the environment) signals [41]. Finally, destructive analyses would not allow any ex-post investigations on any conservation process or consolidation treatment.
Many studies concerning bone and teeth (e.g., [28,95,98]), including specimens stored in maritime archaeological collections, present no mention of any pre-analytic or post-analytic checks, performed with the aim of fulfilling any quality assurance requirements. In fact, conservation processes and the application and/or the removal of any consolidant/s are not described, with the phenomenon appearing to increase with the distance of the studies in the past. This may be largely due to the scarcity of reliable historical records of collections, especially in older and pre-digital times, and to the rapidly advancing analytical technology, able to detect smaller and smaller amounts of chemicals to the atomic level, that only started being broadly available in the last 30 years. Previous to that time, it is reasonable to assume that tiny amounts of consolidants in samples were not consi-dered significant in the general context of any analysis, especially if physical and macroscopic. In retrospective, this might have biased some study results, and, consequently, their interpretation. This does not apply to studies with no treatments, or with treatments reported. The expectation for future studies on bone bioapatite and organic components (e.g., collagen), and on teeth biomaterials, such as enamel, dentin and cementum, is the thorough reporting of data about any performed (or not performed) conservation process and/or consolidation treatment, along with any cleaning, processing and removal. If no data are available, a screening analysis is suggested, in the search of physical alterations and/or consolidants. This could be either achieved with a CT scan, elemental analyzer (EA) and mass spectrometry, or using the same analytical technique planned to support the study (e.g., isotope ratio mass spectrometry—IRMS; scanning electron microscopy and energy dispersive x-ray spectroscopy—SEM-EDS; Fourier-transform infrared spectroscopy—FTIR) [35,99,100], all minimally destructive, in order to detect, and correctly interpret, any unclear or conflicting results. Overall, full documentation and minimum intervention would constitute an ideal policy for future studies, and are recognized as guiding principles in all branches of conservation.
5. Conclusions
Bone and teeth are continually collected in the course of archaeological excavations, both terrestrial and maritime. Once stored in museum collections, they may require conservation processes and, on occasion, consolidation to maintain integrity, especially in the case of long-term submersion before the recovery. When selected for study, sample pre-paration is necessary for most types of analyses, some of which are destructive. In future studies on archaeological bone and teeth, especially in the maritime context, an accurate investigation and recording of any previous conservation process and/or consolidant treatment is recommended and considered essential. The aim is that of clarifying any inconsistent result possibly originating from interference with either the pre-analytical sample preparation, and/or with the principles of the selected analytical method/technique.
Author Contributions
Conceptualization, investigation, methodology, resources, writing—original draft preparation, E.E.G.; methodology, writing—review and editing, P.A.M., conceptualization, investigation, writing—original draft preparation, H.G.B. All authors have read and agreed to the published version of the manuscript.
Funding
No funds were received to write and publish this article.
Institutional Review Board Statement
The authors did not handle any human or non-human remains as part of this work. Ethics for this work were advised by Research Ethics and Integrity at Murdoch University, according to the Australian Code for the Responsible Conduct of Research (2018).
Data Availability Statement
Data sharing not applicable.
Acknowledgments
The authors wish to thank Corioli Souter, Head of Maritime Heritage Department, and Debra Shefi, Museum Curator, for the intellectual and practical assistance bestowed on the study of the faunal collections of the Shipwrecks Museum, Western Australian Museum, Fremantle, Western Australia. Four appointed anonymous reviewers also deserve sincere thanks for adding their advice and expertise.
Conflicts of Interest
The authors declare that they have no conflict of interests.
References
- Bass, G.F. The Development of Maritime Archaeology. In The Oxford Handbook of Maritime Archaeology; Catsambis, A., Ford, B., Hamilton, D.L., Eds.; Oxford University Press: Oxford, UK, 2011. [Google Scholar]
- Green, J. Maritime Archaeology: A Technical Handbook; Elsevier: San Diego, CA, USA, 2004. [Google Scholar]
- Sperry, J. More than Meets the Eyes? Archaeology Underwater, Technology, and Interpretation. Public Archaeol. 2009, 8, 20–34. [Google Scholar] [CrossRef]
- Muckelroy, K. Maritime Archaeology; Cambridge University Press: Cambridge, UK, 1978. [Google Scholar]
- Hamilton, D.L. Conservation of Cultural Materials from Underwater Sites. In Science and Technology in Historic Preservation; Williamson, R.A., Nickens, P.R., Eds.; Springer Science + Business Media: New York, NY, USA, 2000; pp. 193–227. [Google Scholar]
- Hocker, E.; Almkvist, G.; Sahlstedt, M. The Vasa experience with polyethylene glycol: A conservator’s perspective. J. Cult. Herit. 2012, 13, S175–S182. [Google Scholar] [CrossRef]
- Jones, M. Introduction. In For Future Generations: Conservation of a Tudor Maritime Collection; Jones, M., Ed.; The Mary Rose Trust Ltd.: Portsmouth, UK, 2003. [Google Scholar]
- MacLeod, I.D. Conservation of waterlogged timbers from the Batavia 1629. Bull. Aust. Inst. Marit. Archaeol. 1990, 14, 1–8. [Google Scholar]
- Pearson, C. Preface. In Conservation of Marine Archaeological Artefacts; Pearson, C., Ed.; Butterworths: London, UK, 1987. [Google Scholar]
- Caple, C. Introduction: The challenges of archaeological conservation. In Studies in Archaeological Conservation; Caple, C., Garlick, V., Eds.; Routledge: Oxon, London, UK, 2021. [Google Scholar]
- Hamilton, D.L. Methods of Conserving Archaeological Material from Underwater Sites; Nautical Archaeology Program Texas A&M University: College Station, TX, USA, 1999. [Google Scholar]
- Gherardi, F.; Stewart, H. A Multi-Analytical Protocol for Decision Making to Study Copper Alloy Artefacts from Underwater Excavations and Plan Their Conservation. Coatings 2022, 12, 1640. [Google Scholar] [CrossRef]
- Rodrigues, J.A.; Richards, V.L. Australia’s privately held historic shipwreck collections: A current overview and proposed management strategies. Bull. Aust. Inst. Marit. Archaeol. 2012, 36, 77–88. [Google Scholar]
- Buys, S.; Oakley, V. Preventive care of ceramics. In The Conservation and Restoration of Ceramics; Buys, S., Oakley, V., Eds.; Routledge: London, UK, 1996. [Google Scholar]
- Davison, S. Deterioration of glass. In Conservation and Restoration of Glass; Davison, S., Ed.; Butterworth-Heinemann: Oxford, UK, 2003; pp. 169–198. [Google Scholar]
- Broda, M.; Callum, A.S.H. Conservation of Waterlogged Wood-Past, Present and Future Perspectives. Forests 2021, 12, 1193. [Google Scholar] [CrossRef]
- Watson, J. The freeze-drying of wet and waterlogged materials from archaeological excavations. Phys. Educ. 2004, 39, 171–176. [Google Scholar] [CrossRef]
- Florian, M.-L.E. Deterioration of organic materials other than wood. In Conservation of Marine Archaeological Artefacts; Pearson, C., Ed.; Butterworths: London, UK, 1987. [Google Scholar]
- Chen, X.Q.; Xia, K.; Hu, W.; Cao, M.; Deng, K.; Fang, S. Extraction of underwater fragile artifacts: Research status and prospect. Herit. Sci. 2022, 10, 9. [Google Scholar] [CrossRef]
- Magni, P.A.; Guareschi, E.E. How centuries-old bones from Australia’s historic shipwrecks can help us solve crimes. In The Conversation; The Conversation Media Group Ltd.: Victoria, Australia, 2022. [Google Scholar]
- Dennison, K.J.; Kieser, J.A.; Buckeridge, J.S.; Bishop, P.J. Post mortem cohabitation-shell growth as a measure of elapsed time: A case report. Forensic Sci. Int. 2004, 139, 249–254. [Google Scholar] [CrossRef]
- Jones, M. Conservation of Textiles. In For Future Generations: Conservation of a Tudor Maritime Collection; Jones, M., Ed.; The Mary Rose Trust Ltd.: Portsmouth, UK, 2003; pp. 101–105. [Google Scholar]
- Jenssen, V. Conservation of wet organic artefacts excluding wood. In Conservation of Marine Archaeological Artefacts; Pearson, C., Ed.; Butterworths: London, UK, 1987. [Google Scholar]
- Fors, Y.; Sandström, M. Sulfur and iron in shipwrecks cause conservation concerns. Chem. Soc. Rev. 2005, 35, 399–415. [Google Scholar] [CrossRef]
- Grattan, D.W. A Practical Comparative Study of Several Treatments for Waterlogged Wood. Stud. Conserv. 1982, 27, 124–136. [Google Scholar]
- Godfrey, I.M.; Smith, N.K. Conservation of degraded rope from marine archaeological sites. AICCM Bull. 1990, 16, 93–107. [Google Scholar] [CrossRef]
- Bartoš, L.; Sanders, D. The Sail of the Swedish Merchantman Jeanne-Élisabeth, Wrecked off Montpellier, France, in 1755. Int. J. Naut. Archaeol. 2012, 41, 67–83. [Google Scholar] [CrossRef]
- Cartajena, I.; López, P.; Carabias, D.; Morales, C.; Vargas, G.; Ortega, C. First evidence of an underwater Final Pleistocene terrestrial extinct faunal bone assemblage from Central Chile (South America): Taxonomic and taphonomic analyses. Quat. Int. 2013, 305, 45–55. [Google Scholar] [CrossRef]
- Benjamin, J.; Bonsall, C.; Pickard, C. Submerged Prehistory; Oxbow Books: Oxford, UK, 2011; p. 336. [Google Scholar]
- González, A.G.; Sandoval, C.R.; Mata, A.T.; Sanvicente, M.B.; Acevez, E. The Arrival of Humans on the Yucatan Peninsula: Evidence from Submerged Caves in the State of Quintana Roo, Mexico. Curr. Res. Pleistocene 2008, 25, 1–24. [Google Scholar]
- Martín-Perea, D.M.; Morales, J.; Cantero, E.; Courtenay, L.A.; Fernández, M.H.; Domingo, M.S. Taphonomic analysis of Batallones-10, a Late Miocene drought-induced mammalian assemblage (Madrid basin, Spain) within the Cerro de los Batallones complex. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2021, 578, 110576. [Google Scholar] [CrossRef]
- Kono, T.; Sakae, T.; Nakada, H.; Kaneda, T.; Okada, H. Confusion between Carbonate Apatite and Biological Apatite (Carbonated Hydroxyapatite) in Bone and Teeth. Minerals 2022, 12, 170. [Google Scholar] [CrossRef]
- White, T.D.; Folkens, P.A. The Human Bone Manual; Elsevier Academic: Amsterdam, The Netherlands; Boston, MA, USA, 2005. [Google Scholar]
- Berkovitz, B.K.; Shellis, R.P. The Teeth of Mammalian Vertebrates; Academic Press, an Imprint of Elsevier: London, UK, 2018. [Google Scholar]
- Weiner, S. Microarchaeology: Beyond the Visible Archaeological Record; Cambridge University Press: New York, NY, USA, 2010. [Google Scholar]
- Noakes, S.; Garrison, E.; McFall, G. Underwater Paleontology: Recovery of a prehistoric Whale mandible offshore Georgia. In Proceedings of the American Academy of Underwater Sciences 28th Symposium, Atlanta, GA, USA, 28 September–2 October 2009. [Google Scholar]
- Snoeck, C.; Lee-Thorp, J.A. Advances in the study of diagenesis of fossil and subfossil bones and teeth. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2020, 545, 109628. [Google Scholar] [CrossRef]
- Trueman, C.N.; Benton, M.J.; Palmer, M.R. Geochemical Taphonomy of Shallow Marine Vertebrate Assemblages. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2003, 197, 151–169. [Google Scholar] [CrossRef]
- Maurer, A.F.; Barrulas, P.; Person, A.; Mirão, J.; Dias, C.B.; Boudouma, O.; Segalen, L. Testing LA-ICP-MS analysis of archaeological bones with different diagenetic histories for paleodiet prospect. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2019, 534, 109287. [Google Scholar] [CrossRef]
- Matthiesen, H.; Eriksen, A.M.H.; Hollesen, J.; Collins, M. Bone degradation at five Arctic archaeological sites: Quantifying the importance of burial environment and bone characteristics. J. Archaeol. Sci. 2021, 125, 105296. [Google Scholar] [CrossRef]
- Guareschi, E.E.; Nicholls, P.K.; Evans, N.J.; Barham, M.; McDonald, B.J.; Magni, P.A.; Tobe, S.S. Bone diagenesis in the marine environment-I: Characterization and distribution of trace elements in terrestrial mammalian bones recovered from historic shipwrecks. Int. J. Osteoarchaeol. 2021, 32, 509–523. [Google Scholar] [CrossRef]
- Orton, D.C. Taphonomy and interpretation: An analytical framework for social zooarchaeology. Int. J. Osteoarchaeol. 2012, 22, 320–337. [Google Scholar] [CrossRef]
- Papakonstantinou, N.; Booth, T.; Triantaphyllou, S. Human remains under the microscope of funerary taphonomy: Investigating the histological biography of the decaying body in the prehistoric Aegean. J. Archaeol. Sci. Rep. 2020, 34, 102654. [Google Scholar] [CrossRef]
- Guarino, F.M.; Angelini, F.; Vollono, C.; Orefice, C. Bone preservation in human remains from the Terme del Sarno at Pompeii using light microscopy and scanning electron microscopy. J. Archaeol. Sci. 2006, 33, 513–520. [Google Scholar] [CrossRef]
- Brönnimann, D.; Portmann, C.; Pichler, S.L.; Booth, T.J.; Röder, B.; Vach, W.; Schibler, J.; Rentzel, P. Contextualising the dead—Combining geoarchaeology and osteo-anthropology in a new multi-focus approach in bone histotaphonomy. J. Archaeol. Sci. 2018, 98, 45–58. [Google Scholar] [CrossRef]
- Keenleyside, A.; Song, X.; Chettle, D.R.; Webber, C.E. The Lead Content of Human Bones from the 1845 Franklin Expedition. J. Archaeol. Sci. 1996, 23, 461–465. [Google Scholar] [CrossRef]
- Carver, M. Excavation Methods in Archaeology. In Encyclopedia of Global Archaeology; Smith, C., Ed.; Springer New York: New York, NY, USA, 2014; pp. 2706–2714. [Google Scholar]
- Bowens, A. Underwater Archaeology: The NAS Guide to Principles and Practice, 2nd ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2009; pp. 1–226. [Google Scholar]
- Oxley, I. Numbering and Processing in Before the Mast: Life and Death Aboard the Mary Rose; Gardiner, J., Allen, M.J., Eds.; Oxbow Books: Oxford, UK, 2021. [Google Scholar]
- Jones, M. Conservation of Ivory, Horn, Leather and Bone. In For Future Generations: Conservation of a Tudor Maritime Collection; Jones, M., Ed.; The Mary Rose Trust Ltd.: Portsmouth, UK, 2003; pp. 101–105. [Google Scholar]
- Selwyn, L.; Tse, S. The chemistry of sodium dithionite and its use in conservation. Stud. Conserv. 2008, 53, 61–73. [Google Scholar] [CrossRef]
- Godfrey, I.M.; Kasi, K.; Schneider, S.; Williams, E. Iron removal from waterlogged ivory and bone. In Proceedings of the 8th ICOM Group on Wet Organic Archaeological Materials Conference, Stockholm, Sweden, 11–15 June 2001. [Google Scholar]
- Abdel-Maksoud, G.; Awad, H.; Rashed, U.M.; Elnagar, K. Preliminary study for the evaluation of a pulsed coaxial plasma gun for removal of iron rust stain from bone artefacts. J. Cult. Herit. 2022, 55, 128–137. [Google Scholar] [CrossRef]
- Woolley, L. Digging Up the Past; Penguin Books: London, UK, 1954. [Google Scholar]
- Museum, B. How to Observe in Archaeology: Suggestions for Travellers in the Near and Middle East; Trustees of the British Museum: London, UK, 1920. [Google Scholar]
- Hrdlička, A. Directions for Collecting Information and Speciments for Physical Anthropology; US Government Printing Office: Washington, DC, USA, 1904. [Google Scholar]
- López-Polín, L. Possible interferences of some conservation treatments with subsequent studies on fossil bones: A conservator’s overview. Quat. Int. 2012, 275, 120–127. [Google Scholar] [CrossRef]
- Horie, C.V. Materials for Conservation; Butterworth-Heinemann: Oxford, UK, 2010. [Google Scholar]
- Pagels, Z.D. Why did the Chicken cross the Ocean: An Analysis of Faunal Remains from the Emanuel Point Shipwrecks. J. Stud. Res. 2019. [Google Scholar] [CrossRef]
- Rowley-Conwy, E. Treatment of a Block-Lifted Chicken Skeleton in Studies in Archaeological Conservation; Caple, C., Garlick, V., Eds.; Routledge: London, UK, 2020. [Google Scholar]
- Ellison, J. Conservation Project: The Stengade Double Grave, Langeland Museum, Denmark in Studies in Archaeological Conservation; Caple, C., Garlick, V., Eds.; Routledge: London, UK, 2020. [Google Scholar]
- Turner-Walker, G. Degradation pathways and conservation strategies for ancient bone from wet anoxic sites. In Proceedings of the 10th Triennial Meeting of the ICOM-CC Working Group for Wet Organic Archaeological Materials, Amsterdam, The Netherlands, 10–15 September 2007. [Google Scholar]
- Stone, T.T.; Dickel, D.N.; Doran, G.H. The Preservation and Conservation of Waterlogged Bone from the Windover Site, Florida: A Comparison of Methods. J. Field Archaeol. 1990, 17, 177–186. [Google Scholar]
- Mays, S. Human remains in marine archaeology. Environ. Archaeol. 2008, 13, 123–133. [Google Scholar] [CrossRef]
- Perez-Alvaro, E. Shipwrecks and graves: Their treatment as intangible heritage. Int. J. Intang. Herit. 2022, 17, 184–195. [Google Scholar]
- MacLeod, I.D. Shipwreck graves and their conservation management. AICCM Bull. 2008, 31, 5–14. [Google Scholar] [CrossRef]
- Waldron, T. Palaeopathology, illustrat ed.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2009. [Google Scholar]
- Ortner, D.J. Identification of Pathological Conditions in Human Skeletal Remains, 2nd ed.; Academic Press: Cambridge, MA, USA, 2002; p. 645. [Google Scholar]
- Pinhasi, R.; Mays, S. Advances in Human Palaeopathology; John Wiley & Sons: Hoboken, NJ, USA; Chichester, UK, 2008. [Google Scholar]
- Willis, L.M.; Boehm, A.R. Fish bones, cut marks, and burial: Implications for taphonomy and faunal analysis. J. Archaeol. Sci. 2014, 45, 20–25. [Google Scholar] [CrossRef]
- Marshall, R.A.; Mandell, J.C.; Weaver, M.J.; Ferrone, M.; Sodickson, A.; Khurana, B. Imaging Features and Management of Stress, Atypical, and Pathologic Fractures. Radiographics 2018, 38, 2173–2192. [Google Scholar] [CrossRef]
- Kaplan, F.S. Surgical management of paget’s disease. J. Bone Miner. Res. 1999, 14, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Sorg, M.H. Differentiating trauma from taphonomic alterations. Forensic Sci. Int. 2019, 302, 109893. [Google Scholar] [CrossRef]
- Pokines, J.T.; Menschel, M.; Mills, S.; Janowiak, E.; Satish, R.; Kincer, C. Experimental Formation of Marine Abrasion on Bone and the Forensic Postmortem Submergence Interval. Forensic Anthropol. 2020, 3, 175. [Google Scholar] [CrossRef]
- Ubelaker, D.H.; DeGaglia, C.M. The impact of scavenging: Perspective from casework in forensic anthropology. Forensic Sci. Res. 2020, 5, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Shahack-Gross, R.; Bar-Yosef, O.; Weiner, S. Black-Coloured Bones in Hayonim Cave, Israel: Differentiating Between Burning and Oxide Staining. J. Archaeol. Sci. 1997, 24, 439–446. [Google Scholar] [CrossRef]
- Krap, T. Cremains, What Remains: Heat Induced Changes of Biophysical Properties of Human Bone, Introducing New Parameters and Concepts for Forensic Anthropological Analysis; Maastricht University: Maastricht, The Netherlands, 2022. [Google Scholar]
- Behrensmeyer, A.K. Taphonomic and ecologic information from bone weathering. Paleobiology 1978, 4, 150–162. [Google Scholar] [CrossRef]
- Turner-Walker, G. Light at the end of the tunnels? The origins of microbial bioerosion in mineralised collagen. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2019, 529, 24–38. [Google Scholar] [CrossRef]
- Magni, P.A.; Venn, C.; Aquila, I.; Pepe, F.; Ricci, P.; Di Nunzio, C.; Ausania, F.; Dadour, I.R. Evaluation of the floating time of a corpse found in a marine environment using the barnacle Lepas anatifera L. (Crustacea: Cirripedia: Pedunculata). Forensic Sci. Int. 2014, 247, e6–e10. [Google Scholar] [CrossRef]
- Guareschi, E.E.; Haig, D.W.; Tobe, S.S.; Nicholls, P.K.; Magni, P.A. Foraminifera–A new find in the microtaphonomical characterisation of bones from marine archaeological excavations. Int. J. Osteoarchaeol. 2021, 31, 1270–1275. [Google Scholar] [CrossRef]
- Privat, K.L.; O′Connell, T.C.; Richards, M.P. Stable Isotope Analysis of Human and Faunal Remains from the Anglo-Saxon Cemetery at Berinsfield, Oxfordshire: Dietary and Social Implications. J. Archaeol. Sci. 2002, 29, 779–790. [Google Scholar] [CrossRef]
- Chesson, L.A.; Berg, G.E. The use of stable isotopes in postconflict forensic identification. WIREs Forensic Sci. 2022, 4, e1439. [Google Scholar] [CrossRef]
- France, C.A.; Giaccai, J.A.; Doney, C.R. The effects of Paraloid B-72 and Butvar B-98 treatment and organic solvent removal on δ(13)C, δ(15)N, and δ(18)O values of collagen and hydroxyapatite in a modern bone. Am. J. Phys. Anthropol. 2015, 157, 330–338. [Google Scholar] [CrossRef]
- Williams, D.C. The past and future history of natural resins as coating materials in conservation. Mater. Sci. 1995. [Google Scholar]
- Popescu, C.-M.; Vasile, C.; Simionescu, B. Spectral characterization of natural resins used in conservation. Rev. Roum. De Chim. 2012, 57, 495–499. [Google Scholar]
- Brock, F.; Dee, M.; Hughes, A.; Snoeck, C.; Staff, R.; Ramsey, C.B. Testing the Effectiveness of Protocols for Removal of Common Conservation Treatments for Radiocarbon Dating. Radiocarbon 2018, 60, 35–50. [Google Scholar] [CrossRef]
- Schönberg, C.H.L.; Fang, J.K.-H.; Carballo, J.L. Bioeroding Sponges and the Future of Coral Reefs. In Climate Change, Ocean Acidification and Sponges: Impacts Across Multiple Levels of Organization; Carballo, J.L., Bell, J.J., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 179–372. [Google Scholar]
- Bromley, R.G.; Schönberg, C.H.L. Borings, Bodies and Ghosts: Spicules of the Endolithic Sponge Aka Akis sp. nov. Within the Boring Entobia Cretacea, Cretaceous, England, in Current Developments in Bioerosion; Wisshak, M., Tapanila, L., Eds.; Springer Berlin Heidelberg: Berlin/Heidelberg, Germany, 2008; pp. 235–248. [Google Scholar]
- Jans, M.M.E.; Kars, H.; Nielsen–Marsh, C.M.; Smith, C.I.; Nord, A.G.; Arthur, P.; Earl, N. In situ preservation of archaeological bone: A histological study within a multidisciplinary approach. Archaeometry 2002, 44, 343–352. [Google Scholar] [CrossRef]
- Butler, V.L.; Schroeder, R.A. Do Digestive Processes Leave Diagnostic Traces on Fish Bones? J. Archaeol. Sci. 1998, 25, 957–971. [Google Scholar] [CrossRef]
- Krap, T.; Ruijter, J.M.; Nota, K.; Karel, J.; Burgers, A.L.; Aalders, M.C.; Oostra, R.J.; Duijst, W. Colourimetric analysis of thermally altered human bone samples. Sci. Rep. 2019, 9, 8923. [Google Scholar] [CrossRef]
- Donoghue, H.D. Molecular Palaeopathology of Human Infectious Disease. In Advances in Human Palaeopathology; Pinhasi, R., Mays, S., Eds.; Wiley: New York, NY, USA, 2008. [Google Scholar]
- Wood, R. From revolution to convention: The past, present and future of radiocarbon dating. J. Archaeol. Sci. 2015, 56, 61–72. [Google Scholar] [CrossRef]
- Booth, T.J.; Bruck, J. Death is not the end: Radiocarbon and histo-taphonomic evidence for the curation and excarnation of human remains in Bronze Age Britain. Antiquity 2020, 94, 1186. [Google Scholar] [CrossRef]
- Bartelink, E.J.; Chesson, L.A. Recent applications of isotope analysis to forensic anthropology. Forensic Sci. Res. 2019, 4, 29–44. [Google Scholar] [CrossRef]
- Díaz-Cortés, A.; Graziani, G.; Boi, M.; López-Polín, L.; Sassoni, E. Conservation of Archaeological Bones: Assessment of Innovative Phosphate Consolidants in Comparison with Paraloid B72. Nanomaterials 2022, 12, 3163. [Google Scholar] [CrossRef]
- Sjögren, K.G.; Ahlström, T.; Blank, M.; Price, T.D.; Frei, K.M.; Hollund, H.I. Early Neolithic Human Bog Finds from Falbygden, Western Sweden: New Isotopic, Osteological and Histological Investigations. J. Neolit. Archaeol. 2017, 19, 97–126. [Google Scholar]
- Leskovar, T.; Zupanič Pajnič, I.; Jerman, I.; Črešnar, M. Separating forensic, WWII, and archaeological human skeletal remains using ATR-FTIR spectra. Int. J. Legal Med. 2020, 134, 811–821. [Google Scholar] [CrossRef] [PubMed]
- Durga, R.; Jimenez, N.; Ramanathan, S.; Suraneni, P.; Pestle, W.J. Use of thermogravimetric analysis to estimate collagen and hydroxyapatite contents in archaeological bone. J. Archaeol. Sci. 2022, 145, 105644. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).