Preserving Cultural Heritage: Enhancing Limestone Durability with Nano-TiO2 Coating
Abstract
1. Introduction
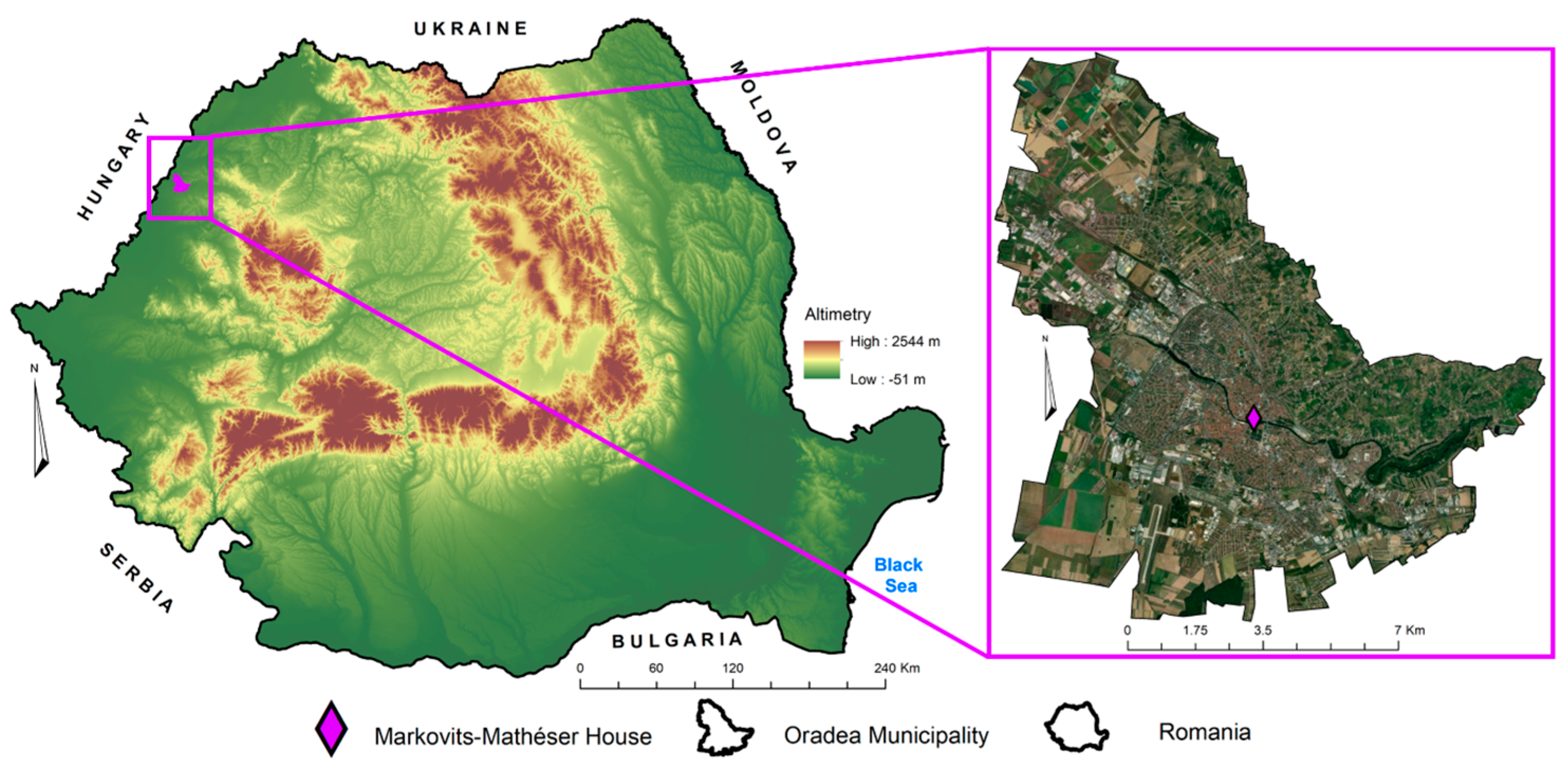
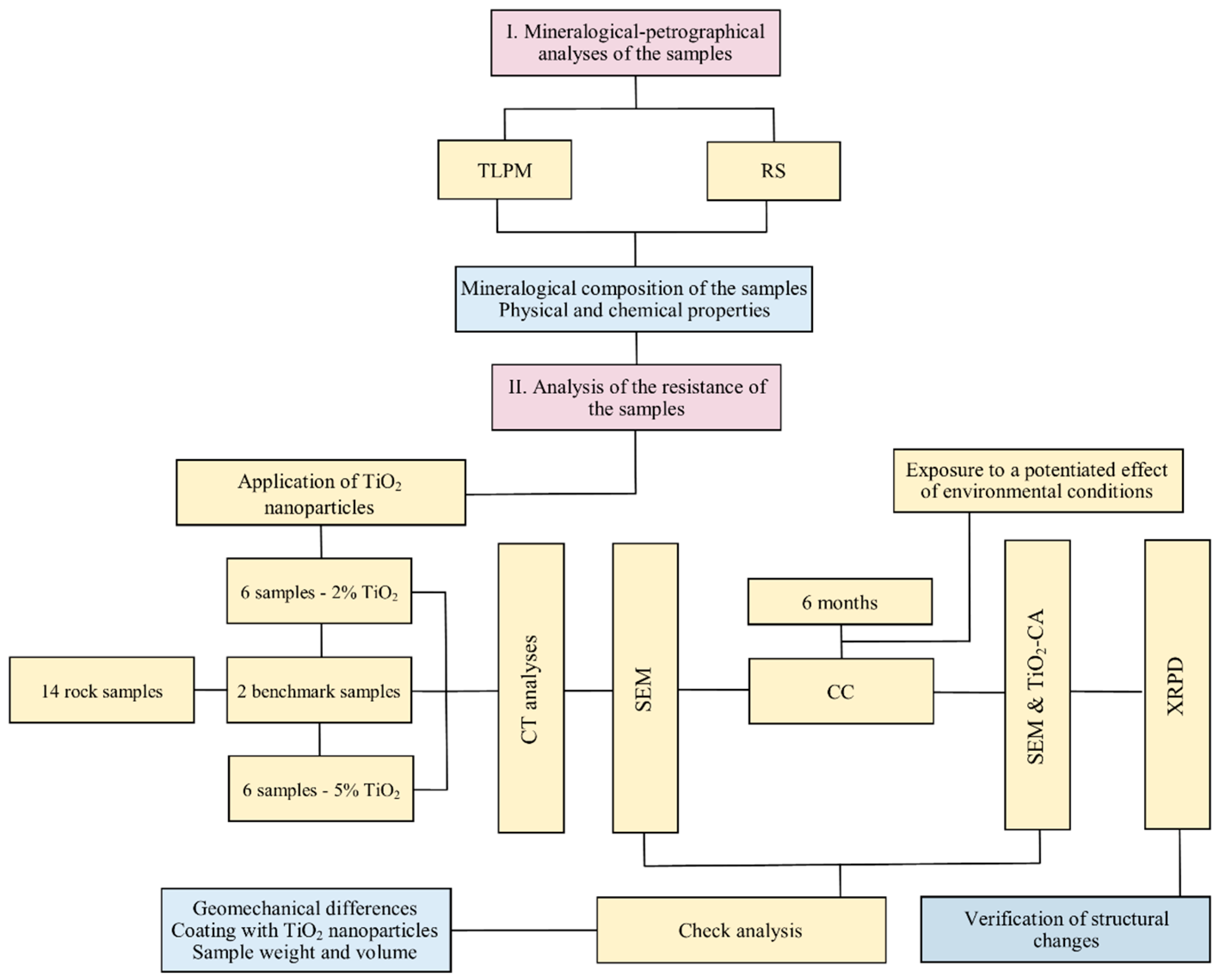
2. Materials and Methods
2.1. Mineralogical and Petrographical Analyses
2.2. Analysis of the Resistance of the Samples
3. Results
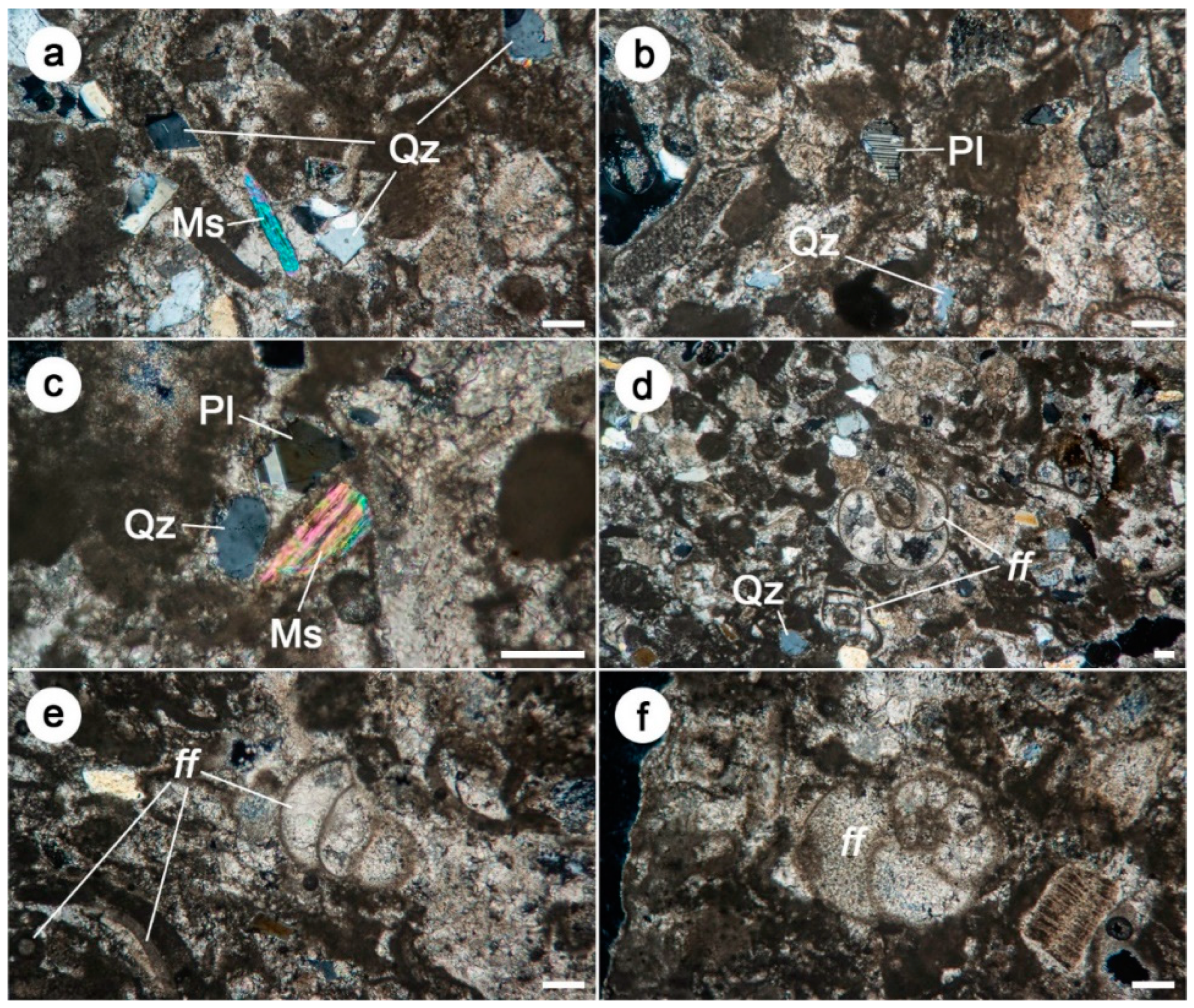
3.1. Mineralogical–Petrographical Analyses
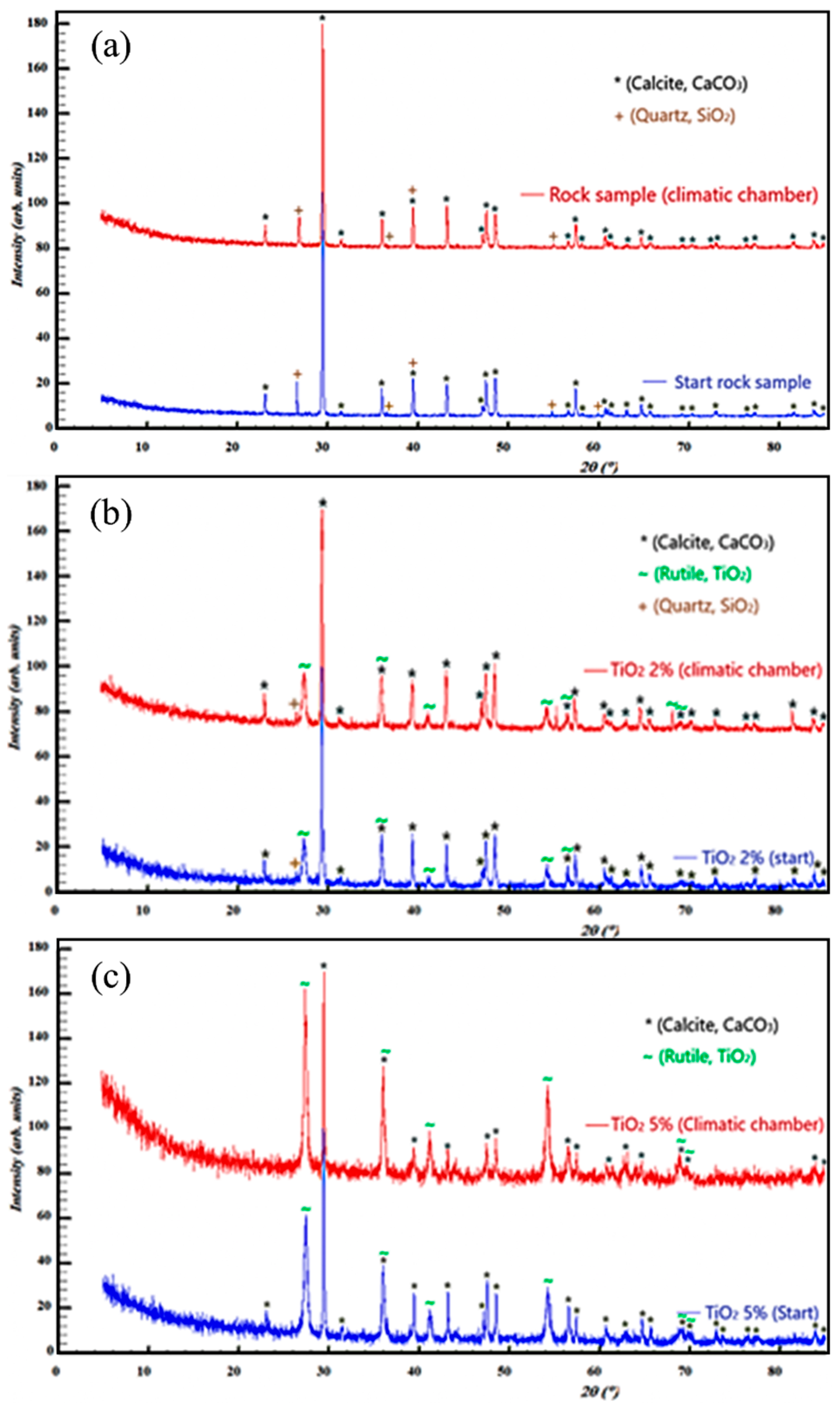
3.2. Analysis of the Resistance of the Samples
4. Conclusions
5. Limitations and Future Works
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| T | Temperatures |
| RH | Relative humidity |
| UHI | Urban heat island |
| RS | Raman Spectra |
| CT | Compression test |
| SEM | Scanning electron microscope |
| CC | Climatic chamber |
| XRPD | X-ray Powder Diffraction |
| CH | Cultural heritage |
| TiO2 | Titanium dioxide |
| TiO2-CA | Titanium dioxide coverage analysis |
| nano-TiO2 | Titanium dioxide nanoparticles (<100 nm) |
References
- Li, H.; Wang, X.; Zhao, X.; Qi, Y. Understanding systemic risk induced by climate change. Adv. Clim. Chang. Res. 2021, 12, 384–394. [Google Scholar] [CrossRef]
- Gburová, J.; Lukáč, M.; Matušíková, D. Impact of digital tools on the interest in visiting heritage objects in tourism. GeoJ. Tour. Geosites 2024, 53, 622–629. [Google Scholar] [CrossRef]
- Pérez Gálvez, J.C.; Fuentes Jiménez, P.A.; Rodríguez Gutiérrez, P.; Medina Viruel, M.J. Emotional perception and cultural motivation on loyalty to a world heritage sites destination. GeoJ. Tour. Geosites 2023, 49, 1165–1175. [Google Scholar] [CrossRef]
- Bogdan, A.; Chambre, D.; Copolovici, D.M.; Bungau, T.; Bungau, C.C.; Copolovici, L. Heritage Building Preservation in the Process of Sustainable Urban Development: The Case of Brasov Medieval City, Romania. Sustain. Sci. Pract. Policy 2022, 14, 6959. [Google Scholar] [CrossRef]
- Sabbioni, C.; Cassar, M.; Brimblecombe, P.; Tidblad, J.; Kowslowski, R.; Drdácký, M.; Saint-Jimenez, C.; Grontoft, T.; Winewright, I.; Arino, X. Global Climate Change Impact on Built Heritage and Cultural Landscapes. In Proceedings of the International Conference on Heritage, Weathering and Conservation, HWC 2006, Spain, Madrid, 21–24 June 2006; de Buergo, F.A., Heraz, G., Calvo, V., Eds.; Tailor and Francis Group: London, UK, 2006; pp. 395–401. [Google Scholar]
- Fatorić, S.; Daly, C. Towards a climate-smart cultural heritage management. Wiley Interdiscip. Reviews. Clim. Chang. 2023, 14, e855. [Google Scholar] [CrossRef]
- Hedayatnia, H.; Steeman, M.; Van Den Bossche, N. Conservation of heritage buildings in Mashhad: On the impact of climate change and the urban heat island effect. In Proceedings of the 1st International Conference on Moisture in Buildings 2021 (ICMB21), Online, 28–29 June 2021. [Google Scholar] [CrossRef]
- Zinzi, M.; Agnoli, S.; Burattini, C.; Mattoni, B. On the thermal response of buildings under the synergic effect of heat waves and urban heat island. Sol. Energy 2020, 211, 1270–1282. [Google Scholar] [CrossRef]
- Guilbert, D.; Caluwaerts, S.; Calle, K.; Bossche, N.; Cnudde, V.; Kock, T. Impact of the urban heat island on freeze-thaw risk of natural stone in the built environment, a case study in Ghent, Belgium. Sci. Total Environ. 2019, 677, 9–18. [Google Scholar] [CrossRef]
- Li, X.; Zhou, Y.; Yu, S.; Jia, G.; Li, H.; Li, W. Urban heat island impacts on building energy consumption: A review of approaches and findings. Energy 2019, 174, 407–419. [Google Scholar] [CrossRef]
- Leal Filho, W.; Wolf, F.; Castro-Díaz, R.; Li, C.; Ojeh, V.N.; Gutiérrez, N.; Nagy, G.J.; Savić, S.; Natenzon, C.E.; Quasem Al-Amin, A.; et al. Addressing the Urban Heat Islands Effect: A Cross-Country Assessment of the Role of Green Infrastructure. Sustain. Sci. Pract. Policy 2021, 13, 753. [Google Scholar] [CrossRef]
- Gomez-Villalba, L.S.; Salcines, C.; Fort, R. Application of Inorganic Nanomaterials in Cultural Heritage Conservation, Risk of Toxicity, and Preventive Measures. Nanomaterials 2023, 13, 1454. [Google Scholar] [CrossRef]
- Ilies, D.C.; Blaga, L.; Ilies, A.; Pereș, A.C.; Caciora, T.; Hassan, T.H.; Hodor, N.; Turza, A.; Taghiyari, H.R.; Barbu-Tudoran, L.; et al. Green Biocidal Nanotechnology Use for Urban Stone-Built Heritage—Case Study from Oradea, Romania. Minerals 2023, 13, 1170. [Google Scholar] [CrossRef]
- Ilieș, A.; Hodor, N.; Pantea, E.; Ilieș, D.C.; Indrie, L.; Zdrîncă, M.; Iancu, S.; Caciora, T.; Chiriac, A.; Ghergheles, C.; et al. Antibacterial Effect of Eco-Friendly Silver Nanoparticles and Traditional Techniques on Aged Heritage Textile, Investigated by Dark-Field Microscopy. Coat. World 2022, 12, 1688. [Google Scholar] [CrossRef]
- Ilies, D.C.; Zlatev, Z.; Ilies, A.; Zharas, B.; Pantea, E.; Hodor, N.; Indrie, L.; Turza, A.; Taghiyari, H.R.; Caciora, T.; et al. Interdisciplinary Research to Advance Digital Imagery and Natural Compounds for Eco-Cleaning and for Preserving Textile Cultural Heritage. Sensors 2022, 22, 4442. [Google Scholar] [CrossRef] [PubMed]
- Otero, J.; Borsoi, G.; Monasterio-Guillot, L. The Boom in Nanomaterials for Built Heritage Conservation: Why Does Size Matter? Materials 2023, 16, 3277. [Google Scholar] [CrossRef]
- Munafò, P.; Goffredo, G.B.; Quagliarini, E. TiO2-based nanocoatings for preserving architectural stone surfaces: An overview. Constr. Build. Mater. 2015, 84, 201–218. [Google Scholar] [CrossRef]
- Ruffolo, S.A.; De Leo, F.; Ricca, M.; Arcudi, A.; Silvestri, C.; Bruno, L.; Urzì, C.; La Russa, M.F. Medium-term in situ experiment by using organic biocides and titanium dioxide for the mitigation of microbial colonization on stone surfaces. Int. Biodeterior. Biodegrad. 2017, 123, 17–26. [Google Scholar] [CrossRef]
- Speziale, A.; González-Sánchez, J.F.; Taşcı, B.; Pastor, A.; Sánchez, L.; Fernández-Acevedo, C.; Oroz-Mateo, T.; Salazar, C.; Navarro-Blasco, I.; Fernández, J.M.; et al. Development of Multifunctional Coatings for Protecting Stones and Lime Mortars of the Architectural Heritage. Int. J. Archit. Herit. Conserv. Anal. Restor. 2020, 14, 1008–1029. [Google Scholar] [CrossRef]
- Mirzaei, P. Recent challenges in modeling of urban heat island. Sustain. Cities Soc. 2015, 19, 200–206. [Google Scholar] [CrossRef]
- Basu, S.; Orr, S.A.; Aktas, Y.D. A Geological Perspective on Climate Change and Building Stone Deterioration in London: Implications for Urban Stone-Built Heritage Research and Management. Atmosphere 2020, 11, 788. [Google Scholar] [CrossRef]
- Buzgar, N.; Apopei, A.I. The Raman study of certain carbonates. Geol. Tomul L 2009, 2, 97–112. [Google Scholar]
- Quagliarini, E.; Bondioli, F.; Goffredo, G.B.; Licciulli, A.; Munafò, P. Self-cleaning materials on Architectural Heritage: Compatibility of photo-induced hydrophilicity of TiO2 coatings on stone surfaces. J. Cult. Herit. 2013, 14, 1–7. [Google Scholar] [CrossRef]
- Sierra-Fernandez, A.; Gomez-Villalba, L.S.; Rabanal, M.E.; Fort, R. New nanomaterials for applications in conservation and restoration of stony materials: A review. Mater. De Construcción 2017, 67, e125. [Google Scholar] [CrossRef]
- Xie, Z.; Duan, Z.; Zhao, Z.; Li, R.; Zhou, B.; Yang, D.; Hu, Y. Nano-materials enhanced protectants for natural stone surfaces. Herit. Sci. 2021, 9, 122. [Google Scholar] [CrossRef]
- Chen, X.; Mao, S.S. Titanium dioxide nanomaterials: Synthesis, properties, modifications, and applications. Chem. Rev. 2012, 107, 2891–2959. [Google Scholar] [CrossRef] [PubMed]
- Linkous, C.; Carter, G.; Locuson, D.; Ouellette, A.; Slattery, D.; Smitha, L. Photocatalytic Inhibition of Algae Growth Using TiO2, WO3, and Cocatalyst Modifications. Environ. Sci. Technol. 2000, 34, 4754–4758. [Google Scholar] [CrossRef]
- Aldoasri, M.; Darwish, S.; Adam, M.; Elmarzugi, N.; Ahmed, S. Protecting Marble Stone Facades of Historic Buildings Using Multifunctional TiO2 Nanocoatings. Sustainability 2017, 9, 2002. [Google Scholar] [CrossRef]
- Colangiuli, D.; Lettieri, M.; Masieri, M.; Calia, A. Field study in an urban environment of simultaneous self-cleaning and hydrophobic nanosized TiO2-based coatings on stone for the protection of building surface. Sci. Total Environ. 2019, 650 Pt 2, 2919–2930. [Google Scholar] [CrossRef]
- Pozo-Antonio, J.S.; Noya, D.; Montojo, C. Aesthetic Effects on Granite of Adding Nanoparticle TiO2 to Si-Based Consolidants (Ethyl Silicate or Nano-Sized Silica). Coatings 2020, 10, 215. [Google Scholar] [CrossRef]
- Calia, A.; Lettieri, M.; Masieri, M. Durability assessment of nanostructured TiO2 coatings applied on limestones to enhance building surface with self-cleaning ability. Build. Environ. 2016, 110, 1–10. [Google Scholar] [CrossRef]
- Veltri, S.; Palermo, A.M.; De Filpo, G.; Xu, F. Subsurface treatment of TiO2 nanoparticles for limestone: Prolonged surface photocatalytic biocidal activities. Build. Environ. 2019, 149, 655–661. [Google Scholar] [CrossRef]
- Kanth, A.P.; Soni, A.K. Application of Nanocomposites for Conservation of Materials of Cultural Heritage. J. Cult. Herit. 2023, 59, 120–130. [Google Scholar] [CrossRef]
- La Russa, M.F.; Rovella, N.; Alvarez de Buergo, M.; Belfiore, C.M.; Pezzino, A.; Crisci, G.M.; Ruffolo, S.A. Nano-TiO2 coatings for cultural heritage protection: The role of the binder on hydrophobic and self-cleaning efficacy. Prog. Org. Coat. 2015, 89, 16–25. [Google Scholar] [CrossRef]
- Gherardi, F.; Colombo, A.; Goidanich, S.; Toniolo, L. Smart Hydrophobic TiO2 Nanocomposites for the Protection of Stone Cultural Heritage; Paisley University of the West of Scotland: Paisley, UK, 2016; pp. 325–332. [Google Scholar]
- Battistel, E.; Favaro, M.; Tomasin, P.; Gambirasi, A. Photocatalytic TiO2-based nanocoating for the preservation of historical limestone buildings. J. Cult. Herit. 2017, 23, 120–128. [Google Scholar]
- Ionescu, A.; Popescu, B.; Marin, C.; Dumitrescu, D.; Georgescu, E.; Vasilescu, F. Efectele tratamentului cu TiO2 pe fațadele monumentelor medievale din piatră. Rev. De Conserv. A Patrim. Cult. 2021, 8, 67–78. [Google Scholar]
- Popovici, C.; Stanciu, M.; Ionescu, R.; Mihai, L.; Dumitru, A.; Stoica, S. Impactul aplicării TiO2 pe mobilierul istoric din lemn. Rev. De Conserv. Și Restaur. 2022, 11, 78–89. [Google Scholar]
- Baglioni, P.; Carretti, E.; Dei, L. Colloid and materials science for the conservation of cultural heritage: Cleaning, consolidation, and deacidification. Langmuir 2014, 30, 2691–2703. [Google Scholar] [CrossRef] [PubMed]
- Ben Chobba, M.; Weththimuni, M.L.; Messaoud, M.; Urzi, C.; Licchelli, M. Recent Advances in the Application of Metal Oxide Nanomaterials for the Conservation of Stone Artefacts, Ecotoxicological Impact and Preventive Measures. Coat. World 2024, 14, 203. [Google Scholar] [CrossRef]
- Goffredo, G.B.; Munafò, P. Preservation of Historical Stone Surfaces by TiO2 Nanocoatings. Coat. World 2015, 5, 222–231. [Google Scholar] [CrossRef]
- Rodriguez, C.; Martinez, A.; Lopez, J.; Hernandez, P.; Sanchez, R.; Gomez, M.; Fernandez, E. Physical and Chemical Modifications Induced by TiO2 Coatings on Stone Materials: A Critical Review. J. Mater. Eng. 2020, 25, 210–225. [Google Scholar]
- Smith, D.; Johnson, L.; Brown, T.; Williams, R.; Lee, S.; Thompson, K. Recent Developments in TiO2 Application for Stone Conservation: A Review. J. Cult. Herit. Preserv. 2021, 18, 45–60. [Google Scholar]
- Costanzo, A.; Ebolese, D.; Ruffolo, S.A.; Falcone, S.; la Piana, C.; La Russa, M.F.; Musacchio, M.; Buongiorno, M.F. Detection of the TiO2 Concentration in the Protective Coatings for the Cultural Heritage by Means of Hyperspectral Data. Sustainability 2021, 13, 92. [Google Scholar] [CrossRef]
- Pinna, D.; Salvadori, B.; Galeotti, M. Monitoring the performance of innovative nanoparticles for the conservation of historical stones. J. Cult. Herit. 2013, 14, 15–21. [Google Scholar]
- Smith, J.; Davis, H.; Miller, P.; Clark, A.; Wilson, E.; Roberts, M. The Aesthetic Effects of TiO2 Application on Stone Surfaces: A Review. J. Aesthet. Conserv. 2017, 14, 78–89. [Google Scholar]
- Liu, Y.; Chen, X.; Wang, Z.; Zhang, L.; Yang, F.; Zhao, Q. Investigating the Impact of TiO2 Coatings on Stone Surface Aesthetics: A Comparative Study. J. Stone Mater. Aesthet. 2020, 18, 112–125. [Google Scholar]
- Keller, A.A.; McFerran, S.; Lazareva, A.; Suh, S. Global life cycle releases of engineered nanomaterials. J. Nanoparticle Res. 2013, 15, 1692. [Google Scholar] [CrossRef]
- Li, M.; Zhou, Y.; Wang, H.; Xu, J.; Zhang, D.; Chen, L.; Sun, J. Health Implications of TiO2 Nanoparticle Exposure: A Review. J. Nanotoxicology 2012, 16, 112–125. [Google Scholar]
- Cho, Y.; Kim, S.; Park, J.; Lee, H.; Choi, K.; Lim, D.; Shin, J. Assessing the Health Effects of TiO2 Nanoparticles: Current Understanding and Future Directions. J. Health Sci. 2018, 25, 210–223. [Google Scholar]
- Zifceac, I.; Paunescu, C.; Vais, M. The Impact of the Green Spaces On the Land Surface Temperature in Urban Areas—Analysis of the Land Surface Temperature in the City of Oradea using Landsat 8 Satellite Images. Bulletin of the Transilvania University of Brasov. Ser. I—Eng. Sci. 2022, 15, 55–62. [Google Scholar] [CrossRef]
- Pașca, M. Arhitectul Frigyes Spiegel la Oradea; Editura Arca Oradea: Oradea, Romania, 2010; ISBN 978-973-1881-49-2. [Google Scholar]
- Pașca, M. Oradea 1900: Un Ghid de Arhitectură, 3rd ed.; Editura Argonaut: Cluj-Napoca, Rumania, 2019; ISBN 978-973-109-932-3. [Google Scholar]
- Croitoru, A.-E.; Holobaca, I.-H.; Lazar, C.; Moldovan, F.; Imbroane, A. Air temperature trend and its impact on winter wheat phenology in Romania. Clim. Chang. 2012, 111, 393–410. [Google Scholar] [CrossRef]
- Herbel, I.; Croitoru, A.-E.; Rus, I.; Harpa, G.V.; Ciupertea, A.F. Detection of atmospheric urban heat island through direct measurements in Cluj-Napoca city, Romania. Hung. Geogr. Bull. 2016, 65, 117–128. [Google Scholar] [CrossRef]
- Lloyd, G.; Farmer, A.; Mainprice, D. Misorientation analysis and the formation and orientation of subgrain and grain boundaries. Tectonophysics 1997, 279, 55–78. [Google Scholar] [CrossRef]
- Foucher, F.; Guimbretière, G.; Bost, N.; Westall, F. Petrographical and mineralogical applications of Raman mapping. In Raman Spectroscopy and Applications; InTech: Penang, Malaysia, 2017. [Google Scholar] [CrossRef]
- Búrdalo-Salcedo, G.; Rodríguez, I.; Fernández-Raga, M.; Fernández-Raga, S.; Rodríguez-Fernández, C.; González-Domínguez, J.M. Adaptation of a Standard Method for Water Absorption Testing of Stone Materials: The Case of a Hydrophilic Protective Coating. Materials 2023, 16, 4228. [Google Scholar] [CrossRef] [PubMed]
- Otero, J.; Pozo-Antonio, J.S.; Montojo, C. Influence of application method and number of applications of nanolime on the effectiveness of the Doulting limestone treatments. Mater. Struct. 2021, 54, 41. [Google Scholar] [CrossRef]
- UNE EN 1926:2007; Natural Stone Test Methods—Determination of Uniaxial Compressive Strength. AENOR: Madrid, Spain, 2007.
- Banc, S.; Croitoru, A.-E.; David, N.A.; Scripcă, A.-S. Changes Detected in Five Bioclimatic Indices in Large Romanian Cities over the Period 1961–2016. Atmosphere 2020, 11, 819. [Google Scholar] [CrossRef]
- Lawrence, M. The relationship between relative humidity and the dewpoint temperature in moist air—A simple conversion and applications. Bull. Am. Meteorol. Soc. 2005, 86, 225–233. [Google Scholar] [CrossRef]
- Butler, B.M.; Hillier, S. powdR: An R package for quantitative mineralogy using full pattern summation of X-ray powder diffraction data. Comput. Geosci. 2021, 147, 104662. [Google Scholar] [CrossRef]
- Martínez-García, R.; González-Campelo, D.; Fraile-Fernández, F.J.; Castañón, A.M.; Caldevilla, P.; Giganto, S.; Ortiz-Marqués, A.; Zelli, F.; Calvo, V.; González-Domínguez, J.M.; et al. Performance study of graphene oxide as an antierosion coating for ornamental and heritage dolostone. Adv. Mater. Technol. 2023, 8, 2300486. [Google Scholar] [CrossRef]
- Dorobat, M.L.; Balta, M.M.; Dobrescu, C.M. Comparative study of some geomechanical properties of the limestones and shists in the screes of Leaota Massif (Southern Carpathians, Romania). Curr. Trends Nat. Sci. 2018, 7, 12–21. [Google Scholar]
- Lazzari, M.; Bianchi, S.; Rossi, F.; Conti, G.; Romano, L.; Greco, P. Enhancing Stone Surface Resistance to Environmental Stress Factors with TiO2 Nanoparticles. J. Stone Mater. Sci. 2018, 12, 45–58. [Google Scholar]






| Sample Group | Sample Code | Initial Weight (g) | Quantity of Nano-TiO2 Applied | Final Weight (g) | Quantity of Nano-TiO2 Retained (g) |
|---|---|---|---|---|---|
| Group A | A1 | 5.42 | 2% nano-TiO2 | 5.47 | 0.05 |
| A2 | 5.47 | 5.54 | 0.07 | ||
| A3 | 4.88 | 4.93 | 0.05 | ||
| A4 | 4.39 | 4.46 | 0.07 | ||
| A5 | 4.48 | 4.53 | 0.05 | ||
| A6 | 5.57 | 5.62 | 0.05 | ||
| Group B | B1 | 4.78 | 5% nano-TiO2 | 4.85 | 0.07 |
| B2 | 4.84 | 4.97 | 0.13 | ||
| B3 | 5.54 | 5.59 | 0.05 | ||
| B4 | 5.91 | 5.97 | 0.06 | ||
| B5 | 4.19 | 4.24 | 0.05 | ||
| B6 | 4.16 | 4.25 | 0.09 | ||
| Benchmark samples | R1 | 3.31 | 0% nano-TiO2 | - | - |
| R2 | 4.67 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ilieș, D.C.; Apopei, A.-I.; Ilieș, A.; Caciora, T.; Zharas, B.; Hodor, N.; Turza, A.; Hassan, T.H.; Barbu-Tudoran, L.; Pereș, A.C.; et al. Preserving Cultural Heritage: Enhancing Limestone Durability with Nano-TiO2 Coating. Heritage 2024, 7, 4914-4932. https://doi.org/10.3390/heritage7090232
Ilieș DC, Apopei A-I, Ilieș A, Caciora T, Zharas B, Hodor N, Turza A, Hassan TH, Barbu-Tudoran L, Pereș AC, et al. Preserving Cultural Heritage: Enhancing Limestone Durability with Nano-TiO2 Coating. Heritage. 2024; 7(9):4914-4932. https://doi.org/10.3390/heritage7090232
Chicago/Turabian StyleIlieș, Dorina Camelia, Andrei-Ionuț Apopei, Alexandru Ilieș, Tudor Caciora, Berdenov Zharas, Nicolaie Hodor, Alexandru Turza, Thowayeb H. Hassan, Lucian Barbu-Tudoran, Ana Cornelia Pereș, and et al. 2024. "Preserving Cultural Heritage: Enhancing Limestone Durability with Nano-TiO2 Coating" Heritage 7, no. 9: 4914-4932. https://doi.org/10.3390/heritage7090232
APA StyleIlieș, D. C., Apopei, A.-I., Ilieș, A., Caciora, T., Zharas, B., Hodor, N., Turza, A., Hassan, T. H., Barbu-Tudoran, L., Pereș, A. C., Ratiu, M., Safarov, B., Bilalov, B., & Gligor, E.-T. (2024). Preserving Cultural Heritage: Enhancing Limestone Durability with Nano-TiO2 Coating. Heritage, 7(9), 4914-4932. https://doi.org/10.3390/heritage7090232











