Sex Differences in Sleep and Physical Activity Patterns in Autism Spectrum Disorder
Abstract
1. Introduction
2. Results
3. Materials and Methods
3.1. Sample
3.2. Procedure
4. Data Analysis
5. Discussion
5.1. PA in ASD and Gender-Related Differences
5.2. Sleep in ASD and Gender-Related Differences
5.3. Sleep and PA in ASD and Gender-Related Differences
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sampasa-Kanyinga, H.; Colman, I.; Goldfield, G.S.; Janssen, I.; Wang, J.; Podinic, I.; Tremblay, M.S.; Saunders, T.J.; Sampson, M.; Chaput, J.P. Combinations of physical activity, sedentary time, and sleep duration and their associations with depressive symptoms and other mental health problems in children and adolescents: A systematic review. Int. J. Behav. Nutr. Phys. Act. 2020, 17, 72. [Google Scholar] [CrossRef] [PubMed]
- Friel, C.P.; Duran, A.T.; Shechter, A.; Diaz, K.M. US children meeting sleep, screen time, and physical activity guidelines. Am. J. Prev. Med. 2021, 59, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Steene-Johannessen, J.; Hansen, B.H.; Dalene, K.E.; Kolle, E.; Northstone, K.; Moller, N.C.; Grontved, A.; Wedderkopp, N.; Kriemler, S.; Page, A.S.; et al. Variations in accelerometry measured physical activity and sedentary time across Europe—Harmonized analyses of 47,497 children and adolescents. Int. J. Behav. Nutr. Phys. Act. 2020, 17, 38. [Google Scholar] [CrossRef] [PubMed]
- Kredlow, M.A.; Capozzoli, M.C.; Hearon, B.A.; Calkins, A.W.; Otto, M.W. The effects of physical activity on sleep: A meta-analytic review. J. Behav. Med. 2015, 38, 427–449. [Google Scholar] [CrossRef] [PubMed]
- Atoui, S.; Chevance, G.; Romain, A.J.; Kingsbury, C.; Lachance, J.P.; Bernard, P. Daily associations between sleep and physical activity: A systematic review and meta-analysis. Sleep Med. Rev. 2021, 57, 101426. [Google Scholar] [CrossRef] [PubMed]
- Chennaoui, M.; Arnal, P.J.; Sauvet, F.; Leger, D. Sleep and exercise: A reciprocal issue? Sleep Med. Rev. 2015, 20, 59–72. [Google Scholar] [CrossRef]
- Kline, C.E.; Krafty, R.T.; Mulukutla, S.; Hall, M.H. Associations of sedentary time and moderate-vigorous physical activity with sleep-disordered breathing and polysomnographic sleep in community-dwelling adults. Sleep Breath. 2017, 21, 427–434. [Google Scholar] [CrossRef]
- Youngstedt, S.D.; Kline, C.E. Epidemiology of exercise and sleep. Sleep Biol. Rhythm. 2006, 4, 215–221. [Google Scholar] [CrossRef]
- Chen, X.; Liu, H.; Wu, Y.; Xuan, K.; Zhao, T.; Sun, Y. Characteristics of sleep architecture in autism spectrum disorders: A meta-analysis based on polysomnographic research. Psychiatry Res. 2021, 296, 113677. [Google Scholar] [CrossRef]
- Mander, B.A.; Winer, J.R.; Walker, M.P. Sleep and Human Aging. Neuron 2017, 94, 19–36. [Google Scholar] [CrossRef]
- Santhi, N.; Lazar, A.S.; McCabe, P.J.; Lo, J.C.; Groeger, J.A.; Dijk, D.J. Sex differences in the circadian regulation of sleep and waking cognition in humans. Proc. Natl. Acad. Sci. USA 2016, 113, E2730–E2739. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Arlington, VA, USA, 2013. [Google Scholar]
- Burman, D.; Ramanujam, K.; Manzar, D.; Chattu, V.K.; Spence, D.W.; Zaki, N.F.W.; Pandi-Perumal, S.R. Sleep and autism spectrum disorder: A comprehensive review of diagnosis, markers, interventions, and treatments. Sleep Vigil. 2023, 7, 9–22. [Google Scholar] [CrossRef]
- Esposito, D.; Belli, A.; Ferri, R.; Bruni, O. Sleeping without Prescription: Management of Sleep Disorders in Children with Autism with Non-Pharmacological Interventions and Over-the-Counter Treatments. Brain Sci. 2020, 10, 441. [Google Scholar] [CrossRef] [PubMed]
- Malow, B.A.; Marzec, M.L.; McGrew, S.G.; Wang, L.; Henderson, L.M.; Stone, W.L. Characterizing sleep in children with autism spectrum disorders: A multidimensional approach. Sleep 2006, 29, 1563–1571. [Google Scholar] [CrossRef]
- Migliarese, G.; Torriero, S.; Gesi, C.; Venturi, V.; Reibman, Y.; Cerveri, G.; Vigano, V.; Decaroli, G.; Ricciardelli, P.; Mencacci, C. Sleep quality among adults with attention deficit hyperactivity disorder or autism spectrum disorder: Which is the role of gender and chronotype? Sleep Med. 2020, 76, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Schroder, C.M.; Broquere, M.A.; Claustrat, B.; Delorme, R.; Franco, P.; Lecendreux, M.; Tordjman, S. Therapeutic approaches for sleep and rhythms disorders in children with ASD. Encephale 2022, 48, 294–303. [Google Scholar] [CrossRef]
- Tse, C.Y.A.; Lee, H.P.; Chan, K.S.K.; Edgar, V.B.; Wilkinson-Smith, A.; Lai, W.H.E. Examining the impact of physical activity on sleep quality and executive functions in children with autism spectrum disorder: A randomized controlled trial. Autism 2019, 23, 1699–1710. [Google Scholar] [CrossRef]
- Gozal, D.; Kheirandish-Gozal, L. Sleep in children: A permanently evolving set of challenges. In Sleep, Health, and Society: From Aetiology to Public Health, 2nd ed.; Oxford University Press: Oxford, UK, 2018. [Google Scholar]
- American Academy of Sleep Medicine. The International Classification of Sleep Disorders, Revised; American Academy of Sleep Medicine: Chicago, IL, USA, 2005. [Google Scholar]
- Wachob, D.; Lorenzi, D. Brief Report: Influence of Physical Activity on Sleep Quality in Children with Autism. J. Autism Dev. Disord. 2015, 45, 2641–2646. [Google Scholar] [CrossRef]
- Liang, X.; Haegele, J.A.; Tse, A.C.; Li, M.; Zhang, H.; Zhao, S.; Li, S.X. The impact of the physical activity intervention on sleep in children and adolescents with autism spectrum disorder: A systematic review and meta-analysis. Sleep Med. Rev. 2024, 74, 101913. [Google Scholar] [CrossRef]
- Pan, C.-Y.; Frey, G.C. Physical activity patterns in youth with autism spectrum disorders. J. Autism Dev. Disord. 2006, 36, 597–606. [Google Scholar] [CrossRef]
- Elkhatib Smidt, S.D.; Gooneratne, N.; Brodkin, E.S.; Bucan, M.; Mitchell, J.A. Sufficient sleep duration in autistic children and the role of physical activity. Autism 2022, 26, 814–826. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.W.; Hadizadeh, M.; Cheong, J.P.G. Global Trends in Physical-Activity Research of Autism: Bibliometric Analysis Based on the Web of Science Database (1980–2021). Int. J. Environ. Res. Public Health 2022, 19, 7278. [Google Scholar] [CrossRef] [PubMed]
- Souders, M.C.; Mason, T.B.; Valladares, O.; Bucan, M.; Levy, S.E.; Mandell, D.S.; Weaver, T.E.; Pinto-Martin, J. Sleep behaviors and sleep quality in children with autism spectrum disorders. Sleep 2009, 32, 1566–1578. [Google Scholar] [CrossRef] [PubMed]
- Ablin, J.N.; Clauw, D.J.; Lyden, A.K.; Ambrose, K.; Williams, D.A.; Gracely, R.H.; Glass, J.M. Effects of sleep restriction and exercise deprivation on somatic symptoms and mood in healthy adults. Clin. Exp. Rheumatol. 2013, 31 (Suppl. S79), S53–S59. [Google Scholar] [PubMed]
- Pan, C.-Y.; Tsai, C.-L.; Hsieh, K.-W. Physical Activity Correlates for Children with Autism Spectrum Disorders in Middle School Physical Education. Res. Q. Exerc. Sport 2011, 82, 491–498. [Google Scholar] [CrossRef]
- Lai, M.C.; Lombardo, M.V.; Auyeung, B.; Chakrabarti, B.; Baron-Cohen, S. Sex/gender differences and autism: Setting the scene for future research. J. Am. Acad. Child. Adolesc. Psychiatry 2015, 54, 11–24. [Google Scholar] [CrossRef]
- Beggiato, A.; Peyre, H.; Maruani, A.; Scheid, I.; Rastam, M.; Amsellem, F.; Gillberg, C.I.; Leboyer, M.; Bourgeron, T.; Gillberg, C.; et al. Gender differences in autism spectrum disorders: Divergence among specific core symptoms. Autism Res. 2017, 10, 680–689. [Google Scholar] [CrossRef]
- Angell, A.M.; Deavenport-Saman, A.; Yin, L.; Zou, B.; Bai, C.; Varma, D.; Solomon, O. Sex Differences in Co-occurring Conditions Among Autistic Children and Youth in Florida: A Retrospective Cohort Study (2012–2019). J. Autism Dev. Disord. 2021, 51, 3759–3765. [Google Scholar] [CrossRef]
- Elkhatib Smidt, S.D.; Hitt, T.; Zemel, B.S.; Mitchell, J.A. Sex differences in childhood sleep and health implications. Ann. Hum. Biol. 2021, 48, 474–484. [Google Scholar] [CrossRef]
- Hudson, C.C.; Hall, L.; Harkness, K.L. Prevalence of Depressive Disorders in Individuals with Autism Spectrum Disorder: A Meta-Analysis. J. Abnorm. Child Psychol. 2019, 47, 165–175. [Google Scholar] [CrossRef]
- Margari, L.; Palumbi, R.; Peschechera, A.; Craig, F.; de Giambattista, C.; Ventura, P.; Margari, F. Sex-Gender Comparisons in Comorbidities of Children and Adolescents with High-Functioning Autism Spectrum Disorder. Front. Psychiatry 2019, 10, 159. [Google Scholar] [CrossRef] [PubMed]
- Mosner, M.G.; Kinard, J.L.; Shah, J.S.; McWeeny, S.; Greene, R.K.; Lowery, S.C.; Mazefsky, C.A.; Dichter, G.S. Rates of Co-occurring Psychiatric Disorders in Autism Spectrum Disorder Using the Mini International Neuropsychiatric Interview. J. Autism Dev. Disord. 2019, 49, 3819–3832. [Google Scholar] [CrossRef] [PubMed]
- Lord, C.; Risi, S.; Lambrecht, L.; Cook, E.H., Jr.; Leventhal, B.L.; DiLavore, P.C.; Pickles, A.; Rutter, M. The autism diagnostic observation schedule-generic: A standard measure of social and communication deficits associated with the spectrum of autism. J. Autism Dev. Disord. 2000, 30, 205–223. [Google Scholar] [CrossRef] [PubMed]
- Wechsler, D. Wechsler Intelligence Scale for Children; The Psychological Corporation: San Antonio, TX, USA, 2003. [Google Scholar]
- Alder, M.L.; Johnson, C.R.; Zauszniewski, J.A.; Malow, B.A.; Burant, C.J.; Scahill, L. Feasibility of Actigraphy for Evaluating Sleep and Daytime Physical Activity in Children with Autism Spectrum Disorder. J. Autism Dev. Disord. 2023, 53, 3670–3682. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, K.; Crocker, P.; Donen, R. The Physical Activity Questionnaire for Older Children (PAQ-C) and Adolescents (PAQ-A) Manual; University of Saskatchewan: Saskatoon, SK, Canada, 2004. [Google Scholar]
- Kowalski, K.C.; Crocker, P.R.; Faulkner, R.A. Validation of the physical activity questionnaire for older children. Pediatr. Exerc. Sci. 1997, 9, 174–186. [Google Scholar] [CrossRef]
- Owens, J.A.; Spirito, A.; McGuinn, M. The Children’s Sleep Habits Questionnaire (CSHQ): Psychometric properties of a survey instrument for school-aged children. Sleep 2000, 23, 1043–1052. [Google Scholar] [CrossRef]
- Zou, H.; Hastie, T. Regularization and variable selection via the elastic net. J. R. Stat. Soc. B 2005, 67, 301–320. [Google Scholar] [CrossRef]
- Thumann, B.F.; Bornhorst, C.; Michels, N.; Veidebaum, T.; Solea, A.; Reisch, L.; Moreno, L.A.; Lauria, F.; Kaprio, J.; Hunsberger, M.; et al. Cross-sectional and longitudinal associations between psychosocial well-being and sleep in European children and adolescents. J. Sleep Res. 2019, 28, e12783. [Google Scholar] [CrossRef]
- Lancioni, G.E.; O’Reilly, M.F. A review of research on physical exercise with people with severe and profound developmental disabilities. Res. Dev. Disabil. 1998, 19, 477–492. [Google Scholar] [CrossRef]
- Foti, K.E.; Eaton, D.K.; Lowry, R.; McKnight-Ely, L.R. Sufficient sleep, physical activity, and sedentary behaviors. Am. J. Prev. Med. 2011, 41, 596–602. [Google Scholar] [CrossRef]
- Jones, R.A.; Downing, K.; Rinehart, N.J.; Barnett, L.M.; May, T.; McGillivray, J.A.; Papadopoulos, N.V.; Skouteris, H.; Timperio, A.; Hinkley, T. Physical activity, sedentary behavior and their correlates in children with Autism Spectrum Disorder: A systematic review. PLoS ONE 2017, 12, e0172482. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.D.; Baillieul, S.; Guinot, M.; Doutreleau, S.; Bricout, V.A. Classification of Factors Effect on Sleep in Individuals with Down Syndrome. Brain Sci. 2021, 11, 1500. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.D.; Guinot, M.; Bricout, V.A. Effect of Daily Physical Activity on Sleep Characteristics in Children with Autism Spectrum Disorder. Sports 2021, 9, 91. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Guidelines Approved by the Guidelines Review Committee. In Guidelines on Physical Activity, Sedentary Behaviour and Sleep for Children Under 5 Years of Age; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Physical Activity Guidelines Advisory Committee. 2018 Physical Activity Guidelines Advisory Committee Scientific Report; U.S. Department of Health and Human Services: Washington, DC, USA, 2018. Available online: https://odphp.health.gov/sites/default/files/2019-09/PAG_Advisory_Committee_Report.pdf (accessed on 20 October 2024).
- Obrusnikova, I.; Cavalier, A.R. Perceived Barriers and Facilitators of Participation in After-School Physical Activity by Children with Autism Spectrum Disorders. J. Dev. Phys. Disabil. 2011, 23, 195–211. [Google Scholar] [CrossRef]
- Tyler, K.; MacDonald, M.; Menear, K. Physical activity and physical fitness of school-aged children and youth with autism spectrum disorders. Autism Res. Treat. 2014, 2014, 312163. [Google Scholar] [CrossRef]
- Sletten, T.L.; Weaver, M.D.; Foster, R.G.; Gozal, D.; Klerman, E.B.; Rajaratnam, S.M.W.; Roenneberg, T.; Takahashi, J.S.; Turek, F.W.; Vitiello, M.V.; et al. The importance of sleep regularity: A consensus statement of the National Sleep Foundation sleep timing and variability panel. Sleep Health 2023, 9, 801–820. [Google Scholar] [CrossRef]
- Reed, D.L.; Sacco, W.P. Measuring Sleep Efficiency: What Should the Denominator Be? J. Clin. Sleep Med. 2016, 12, 263–266. [Google Scholar] [CrossRef]
- Johnson, C.R.; Smith, T.; DeMand, A.; Lecavalier, L.; Evans, V.; Gurka, M.; Swiezy, N.; Bearss, K.; Scahill, L. Exploring sleep quality of young children with autism spectrum disorder and disruptive behaviors. Sleep Med. 2018, 44, 61–66. [Google Scholar] [CrossRef]
- Hodge, D.; Carollo, T.M.; Lewin, M.; Hoffman, C.D.; Sweeney, D.P. Sleep patterns in children with and without autism spectrum disorders: Developmental comparisons. Res. Dev. Disabil. 2014, 35, 1631–1638. [Google Scholar] [CrossRef]
- Cohen, S.; Conduit, R.; Lockley, S.W.; Rajaratnam, S.M.; Cornish, K.M. The relationship between sleep and behavior in autism spectrum disorder (ASD): A review. J. Neurodev. Disord. 2014, 6, 44. [Google Scholar] [CrossRef]
- Christensen, D.L.; Maenner, M.J.; Bilder, D.; Constantino, J.N.; Daniels, J.; Durkin, M.S.; Fitzgerald, R.T.; Kurzius-Spencer, M.; Pettygrove, S.D.; Robinson, C.; et al. Prevalence and Characteristics of Autism Spectrum Disorder Among Children Aged 4 Years—Early Autism and Developmental Disabilities Monitoring Network, Seven Sites, United States, 2010, 2012, and 2014. MMWR Surveill. Summ. 2019, 68, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Estes, A.; Munson, J.; St John, T.; Finlayson, R.; Pandey, J.; Gottlieb, B.; Herrington, J.; Schultz, R.T. Sleep problems in autism: Sex differences in the school-age population. Autism Res. 2023, 16, 164–173. [Google Scholar] [CrossRef]
- Jovevska, S.; Richdale, A.L.; Lawson, L.P.; Uljarevic, M.; Arnold, S.R.C.; Trollor, J.N. Sleep Quality in Autism from Adolescence to Old Age. Autism Adulthood 2020, 2, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Gotham, K.; Brunwasser, S.M.; Lord, C. Depressive and anxiety symptom trajectories from school age through young adulthood in samples with autism spectrum disorder and developmental delay. J. Am. Acad. Child. Adolesc. Psychiatry 2015, 54, 369–376.e3. [Google Scholar] [CrossRef] [PubMed]
- Uljarevic, M.; Hedley, D.; Rose-Foley, K.; Magiati, I.; Cai, R.Y.; Dissanayake, C.; Richdale, A.; Trollor, J. Anxiety and Depression from Adolescence to Old Age in Autism Spectrum Disorder. J. Autism Dev. Disord. 2020, 50, 3155–3165. [Google Scholar] [CrossRef] [PubMed]
- Master, L.; Nye, R.; Lee, S.; Nahmod, N.; Mariani, S.; Hale, L.; Buxton, O.M. Bidirectional, Daily Temporal Associations between Sleep and Physical Activity in Adolescents. Sci. Rep. 2019, 9, 7732. [Google Scholar] [CrossRef]
- Tatsumi, Y.; Mohri, I.; Shimizu, S.; Tachibana, M.; Ohno, Y.; Taniike, M. Daytime physical activity and sleep in pre-schoolers with developmental disorders. J. Paediatr. Child. Health 2015, 51, 396–402. [Google Scholar] [CrossRef]
- Stutz, J.; Eiholzer, R.; Spengler, C. Effects of evening exercise on sleep in healthy participants: A systematic review and meta-analysis. Sports Med. 2019, 49, 269–287. [Google Scholar] [CrossRef]
- Carek, P.J.; Laibstain, S.E.; Carek, S.M. Exercise for the treatment of depression and anxiety. Int. J. Psychiatry Med. 2011, 41, 15–28. [Google Scholar] [CrossRef]
- Scharoun, S.M.; Wright, K.T.; Robertson-Wilson, J.E.; Fletcher, P.C.; Bryden, P.J. Physical Activity in Individuals with Autism Spectrum Disorders (ASD): A Review; InTech: London, UK, 2017. [Google Scholar] [CrossRef]
- Bricout, V.A.; Pace, M.; Dumortier, L.; Miganeh, S.; Mahistre, Y.; Guinot, M. Motor Capacities in Boys with High Functioning Autism: Which Evaluations to Choose? J. Clin. Med. 2019, 8, 1521. [Google Scholar] [CrossRef]
- Pan, C.-Y. Age, social engagement, and physical activity in children with autism spectrum disorders. Res. Autism Spectr. Disord. 2009, 3, 22–31. [Google Scholar] [CrossRef]
- Khouzam, H.R.; El-Gabalawi, F.; Pirwani, N.; Priest, F. Asperger’s disorder: A review of its diagnosis and treatment. Compr. Psychiatry 2004, 45, 184–191. [Google Scholar] [CrossRef] [PubMed]
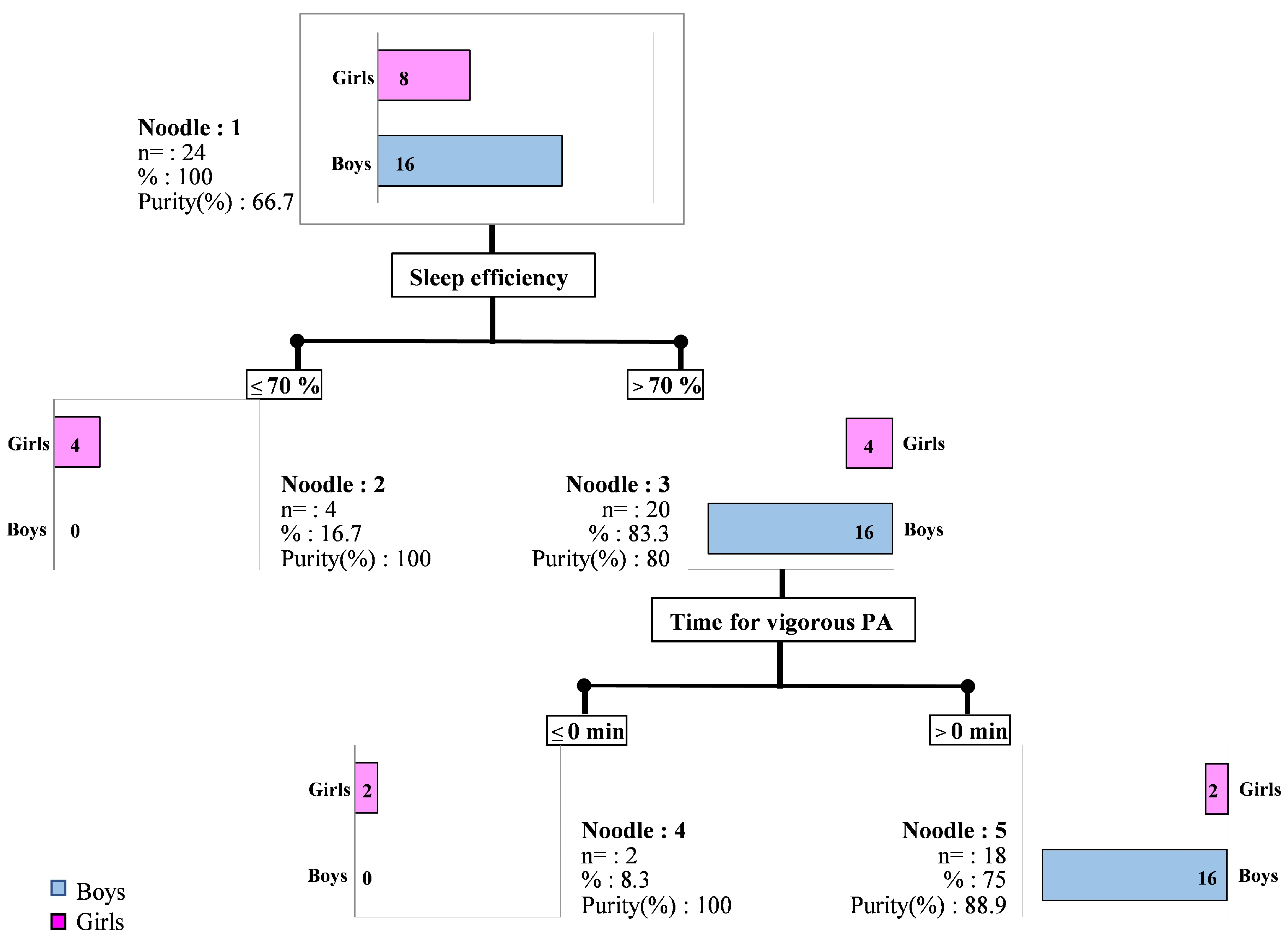
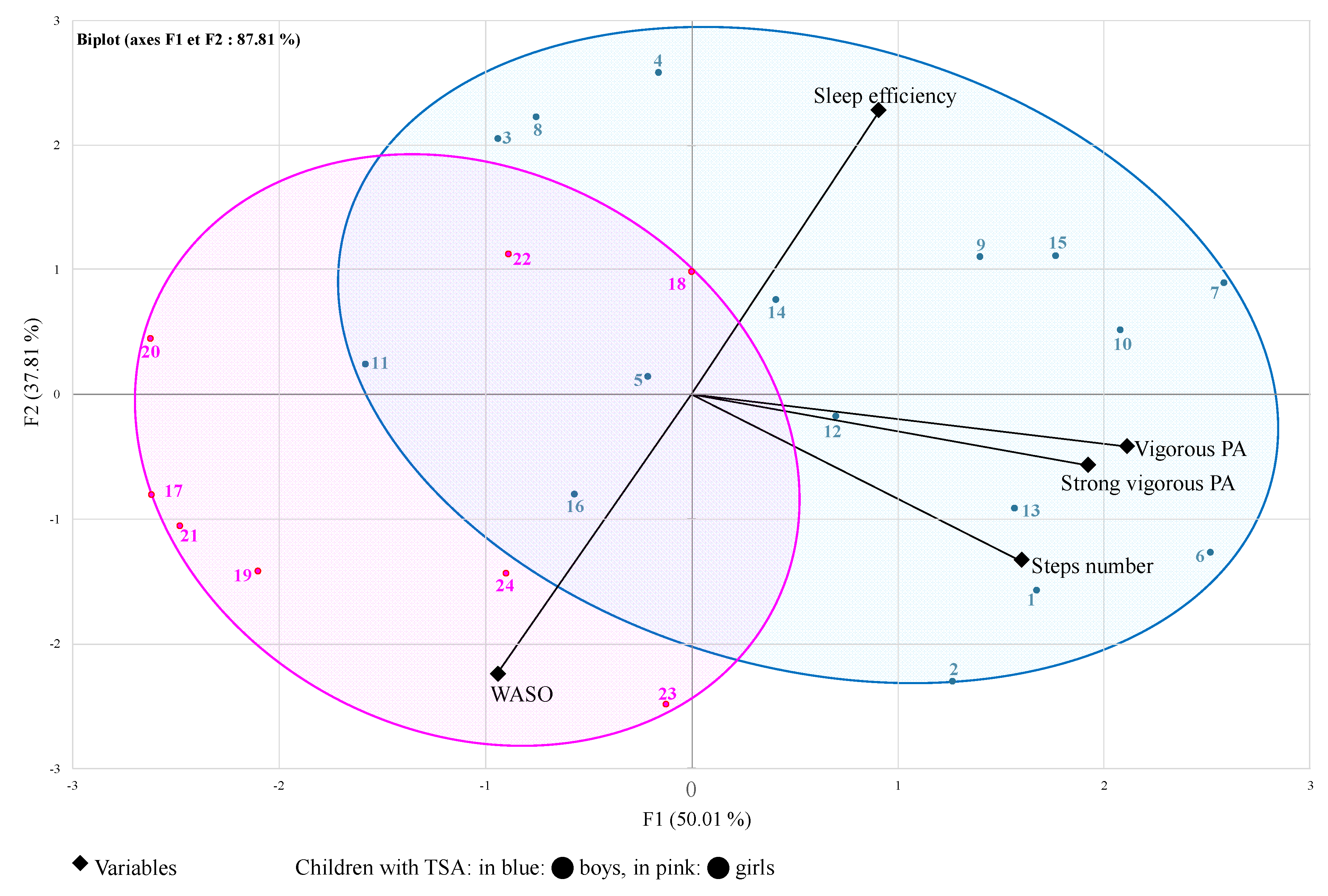
| Girls (n = 8) | Boys (n = 16) | ||
|---|---|---|---|
| Demographic characteristics and questionnaires | Age | 11.1 ± 3.9 | 10.3 ± 2.8 |
| Height (cm) | 140.6 ± 14.9 | 143.2 ± 16.0 | |
| Weight (kg) | 38.1 ± 14.7 | 35.2 ± 9.9 | |
| BMI (kg/m2) | 18.5 ± 3.8 | 16.9 ± 2.3 | |
| PAQ-C Score | 2.1 ± 0.8 | 2.6 ± 0.7 | |
| CSHQ Score | 49.9 ± 9.7 | 47.2 ± 7.2 | |
| Total time wearing of accelerometer (h) | 23.4 ± 0.5 | 23.4 ± 0.3 | |
| Sleep characteristics | Total time in bed (h) | 10.03 ± 0.96 | 9.18 ± 0.40 ** |
| Total sleep duration (h) | 7.23 ± 0.73 | 7.24 ± 0.52 | |
| Sleep efficiency (%) | 72.4 ± 6.5 | 79.0 ± 3.0 ** | |
| Bedtime resistance (min) | 11.6 ± 12.7 | 4.3 ± 3.3 | |
| Sleep latency (min) | 12.2 ± 12.3 | 14.6 ± 8.3 | |
| Wake-up time resistance (min) | 1.2 ± 2.9 | 0.8 ± 1.6 | |
| Awakening latency (min) | 15.0 ± 5.3 | 15.3 ± 6.1 | |
| WASO (min) | 128.0 ± 43.4 | 80.9 ± 20.5 ** | |
| Physical activity characteristics | Total time for PA (min) | 129.4 ± 86.5 | 338.3 ± 580.2 |
| Sedentary behavior (min) | 1276.9 ± 92.4 | 1246.5 ± 77.4 | |
| Time for MVPA (min) | 129.0 ± 86.2 | 151.8 ± 58.9 | |
| Time for moderate PA (min) | 124.0 ± 83.4 | 134.2 ± 48.2 | |
| Time for vigorous PA (min) | 4.9 ± 6.1 | 17.4 ± 15.0 ** | |
| Time for strong vigorous PA (min) | 0.4 ± 0.5 | 2.6 ± 3.3 * | |
| Daily steps number | 9363 ± 3097 | 12,369 ± 3405 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bricout, V.-A.; Covain, S.; Paterno, J.; Guinot, M. Sex Differences in Sleep and Physical Activity Patterns in Autism Spectrum Disorder. Clocks & Sleep 2024, 6, 764-776. https://doi.org/10.3390/clockssleep6040049
Bricout V-A, Covain S, Paterno J, Guinot M. Sex Differences in Sleep and Physical Activity Patterns in Autism Spectrum Disorder. Clocks & Sleep. 2024; 6(4):764-776. https://doi.org/10.3390/clockssleep6040049
Chicago/Turabian StyleBricout, Véronique-Aurélie, Sandro Covain, Jacob Paterno, and Michel Guinot. 2024. "Sex Differences in Sleep and Physical Activity Patterns in Autism Spectrum Disorder" Clocks & Sleep 6, no. 4: 764-776. https://doi.org/10.3390/clockssleep6040049
APA StyleBricout, V.-A., Covain, S., Paterno, J., & Guinot, M. (2024). Sex Differences in Sleep and Physical Activity Patterns in Autism Spectrum Disorder. Clocks & Sleep, 6(4), 764-776. https://doi.org/10.3390/clockssleep6040049






