Not All Workers Experience Equal Sleep Changes: Insights from the “WorkInCovid” Project
Abstract
:1. Introduction
- Workers who had better quality of sleep before WH could have a worsening in quality of sleep, due to the combined effect of anxiety related to the pandemic and the sudden and unorganized change in working methods.
- Workers who had poor quality sleep before the lockdown could have an improvement in sleep during teleworking, due to the reorganization of time dedicated to work and family and the avoidance of commuting.
2. Results
2.1. Sample Characteristics
2.2. Changes in Sleep Pattern in the Whole Sample
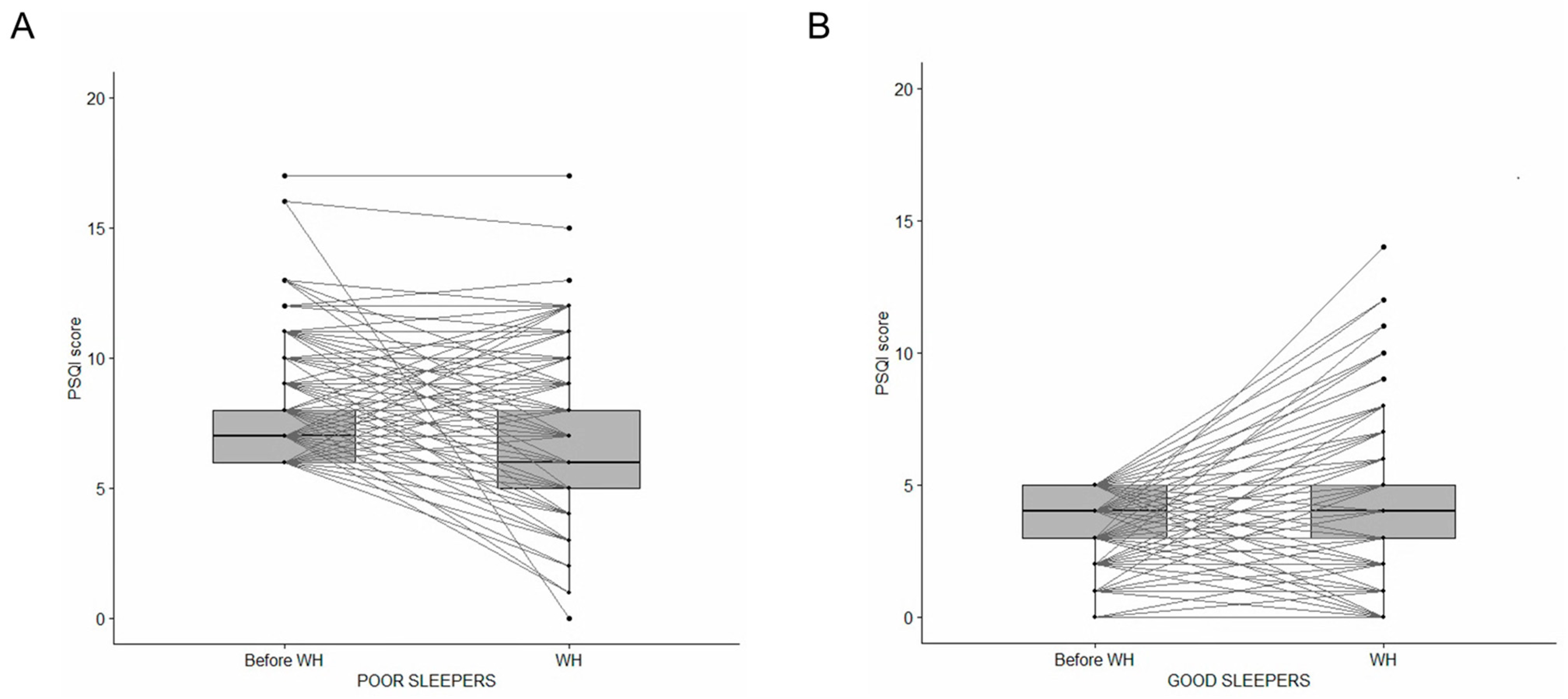
2.3. Changes in Poor and Good Sleepers
2.4. Predictors of Sleep Disturbances During WH in Poor and Good Sleepers
3. Discussion
4. Materials and Methods
4.1. Study Design and Population
4.2. Survey
4.3. Questionnaires for Outcomes Assessment
4.4. Ethical Issues
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chirico, F.; Nucera, G.; Magnavita, N. COVID-19: Protecting Healthcare Workers is a priority. Infect. Control Hosp. Epidemiol. 2020, 41, 1117. [Google Scholar] [CrossRef] [PubMed]
- Messenger, J.; Gschwind, L. Three generations of telework: New ICT and the (r)evolution from home office to virtual office. New Technol. Work Employ. 2016, 31, 195–208. [Google Scholar] [CrossRef]
- Mann, S.; Holdsworth, L. The psychological impact of teleworking: Stress, emotions and health. New Technol. Work Employ. 2003, 18, 196–211. [Google Scholar] [CrossRef]
- Tavares, A. Telework and health effects review. Int. J. Healthc. 2017, 3, 30–36. [Google Scholar] [CrossRef]
- Magnavita, N.; Tripepi, G.; Chiorri, C. Telecommuting, Off-Time Work, and Intrusive Leadership in Workers’ Well-Being. Int. J. Environ. Res. Public Health 2021, 18, 3330. [Google Scholar] [CrossRef]
- Seva, R.R.; Tejero, L.M.S.; Fadrilan-Camacho, V.F.F. Barriers and facilitators of productivity while working from home during pandemic. J. Occup. Health 2021, 63, e12242. [Google Scholar] [CrossRef]
- Moulac, M.; Pavlou, P.; Vona, L. Occupational Safety and Health: Adjusting Provisions in the Light of COVID-19; Publication for the Committee on Employment and Social Affairs, Policy Department for Economic, Scientific and Quality of Life Policies, Euro-pean Parliament: Luxembourg, 2022. [Google Scholar]
- Kim, M.; Park, I.; An, H.; Yun, B.; Yoon, J.H. Teleworking Is Significantly Associated with Anxiety Symptoms and Sleep Disturbances among Paid Workers in the COVID-19 Era. Int. J. Environ. Res. Public Health 2023, 20, 1488. [Google Scholar] [CrossRef]
- Lim, D.C.; Najafi, A.; Afifi, L.; Bassetti, C.; Buysse, D.J.; Han, F.; Hogl, B.; Melaku, Y.A.; Morin, C.M.; Pack, A.I.; et al. The need to promote sleep health in public health agendas across the globe. Lancet Public Health 2023, 8, e820–e826. [Google Scholar] [CrossRef]
- Ramar, K.; Malhotra, R.K.; Carden, K.A.; Martin, J.L.; Abbasi-Feinberg, F.; Aurora, R.N.; Kapur, V.K.; Olson, E.J.; Rosen, C.L.; Rowley, J.A.; et al. Sleep is essential to health: An American Academy of Sleep Medicine position statement. J. Clin. Sleep Med. 2021, 17, 2115–2119. [Google Scholar] [CrossRef]
- Buysse, D.J. Sleep health: Can we define it? Does it matter? Sleep 2014, 37, 9–17. [Google Scholar] [CrossRef]
- Meyer, N.; Harvey, A.G.; Lockley, S.W.; Dijk, D.J. Circadian rhythms and disorders of the timing of sleep. Lancet 2022, 400, 1061–1078. [Google Scholar] [CrossRef]
- Chattu, V.K.; Manzar, M.D.; Kumary, S.; Burman, D.; Spence, D.W.; Pandi-Perumal, S.R. The Global Problem of Insufficient Sleep and Its Serious Public Health Implications. Healthcare 2018, 7, 1. [Google Scholar] [CrossRef]
- Fang, H.; Tu, S.; Sheng, J.; Shao, A. Depression in sleep disturbance: A review on a bidirectional relationship, mechanisms and treatment. J. Cell. Mol. Med. 2019, 23, 2324–2332. [Google Scholar] [CrossRef] [PubMed]
- Sabanayagam, C.; Shankar, A. The association between active smoking, smokeless tobacco, second-hand smoke exposure and insufficient sleep. Sleep Med. 2011, 12, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Williams, N.J.; Grandner, M.A.; Wallace, D.M.; Cuffee, Y.; Airhihenbuwa, C.; Okuyemi, K.; Ogedegbe, G.; Jean-Louis, G. Social and behavioral predictors of insufficient sleep among African Americans and Caucasians. Sleep Med. 2016, 18, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Zuraikat, F.M.; Wood, R.A.; Barragan, R.; St-Onge, M.P. Sleep and Diet: Mounting Evidence of a Cyclical Relationship. Annu. Rev. Nutr. 2021, 41, 309–332. [Google Scholar] [CrossRef]
- Garbarino, S.; Lanteri, P.; Bragazzi, N.L.; Magnavita, N.; Scoditti, E. Role of sleep deprivation in immune-related disease risk and outcomes. Commun. Biol. 2021, 4, 1304. [Google Scholar] [CrossRef]
- Luyster, F.S.; Strollo, P.J., Jr.; Zee, P.C.; Walsh, J.K. Sleep: A health imperative. Sleep 2012, 35, 727–734. [Google Scholar] [CrossRef]
- Martynowicz, H.; Wichniak, A.; Wieckiewicz, M. Sleep disorders and cardiovascular risk: Focusing on sleep fragmentation. Dent. Med. Probl. 2024, 61, 475–477. [Google Scholar] [CrossRef]
- Ben Simon, E.; Rossi, A.; Harvey, A.G.; Walker, M.P. Overanxious and underslept. Nat. Hum. Behav. 2020, 4, 100–110. [Google Scholar] [CrossRef]
- Gruber, R.; Cassoff, J. The interplay between sleep and emotion regulation: Conceptual framework empirical evidence and future directions. Curr. Psychiatry Rep. 2014, 16, 500. [Google Scholar] [CrossRef] [PubMed]
- Krause, A.J.; Simon, E.B.; Mander, B.A.; Greer, S.M.; Saletin, J.M.; Goldstein-Piekarski, A.N.; Walker, M.P. The sleep-deprived human brain. Nat. Rev. Neurosci. 2017, 18, 404–418. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Liang, L.; Zheng, F.; Shi, L.; Zhong, B.; Xie, W. Association Between Sleep Duration and Cognitive Decline. JAMA Netw. Open 2020, 3, e2013573. [Google Scholar] [CrossRef] [PubMed]
- Garbarino, S.; Tripepi, G.; Magnavita, N. Sleep Health Promotion in the Workplace. Int. J. Environ. Res. Public Health 2020, 17, 7952. [Google Scholar] [CrossRef]
- LaGoy, A.D.; Kubala, A.G.; Deering, S.; Germain, A.; Markwald, R.R. Dawn of a New Dawn: Advances in Sleep Health to Optimize Performance. Sleep Med. Clin. 2023, 18, 361–371. [Google Scholar] [CrossRef]
- Davinelli, S.; Medoro, A.; Savino, R.; Scapagnini, G. Sleep and Oxidative Stress: Current Perspectives on the Role of NRF2. Cell. Mol. Neurobiol. 2024, 44, 52. [Google Scholar] [CrossRef]
- Fulek, M.; Frosztega, W.; Wieckiewicz, M.; Szymanska-Chabowska, A.; Gac, P.; Poreba, R.; Mazur, G.; Sciskalska, M.; Kepinska, M.; Martuszewski, A.; et al. The link between sleep bruxism and oxidative stress based on a polysomnographic study. Sci. Rep. 2025, 15, 3567. [Google Scholar] [CrossRef]
- Hafner, M.; Stepanek, M.; Taylor, J.; Troxel, W.M.; van Stolk, C. Why Sleep Matters-The Economic Costs of Insufficient Sleep: A Cross-Country Comparative Analysis. RAND Health Q. 2017, 6, 11. [Google Scholar]
- Gorgoni, M.; Scarpelli, S.; Mangiaruga, A.; Alfonsi, V.; Bonsignore, M.R.; Fanfulla, F.; Ferini-Strambi, L.; Nobili, L.; Plazzi, G.; De Gennaro, L.; et al. Pre-sleep arousal and sleep quality during the COVID-19 lockdown in Italy. Sleep Med. 2021, 88, 46–57. [Google Scholar] [CrossRef]
- Jahrami, H.A.; Alhaj, O.A.; Humood, A.M.; Alenezi, A.F.; Fekih-Romdhane, F.; AlRasheed, M.M.; Saif, Z.Q.; Bragazzi, N.L.; Pandi-Perumal, S.R.; BaHammam, A.S.; et al. Sleep disturbances during the COVID-19 pandemic: A systematic review, meta-analysis, and meta-regression. Sleep. Med. Rev. 2022, 62, 101591. [Google Scholar] [CrossRef]
- Marvaldi, M.; Mallet, J.; Dubertret, C.; Moro, M.R.; Guessoum, S.B. Anxiety, depression, trauma-related, and sleep disorders among healthcare workers during the COVID-19 pandemic: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2021, 126, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Magnavita, N.; Arnesano, G.; Di Prinzio, R.R.; Gasbarri, M.; Meraglia, I.; Merella, M.; Vacca, M.E. Post-COVID Symptoms in Occupational Cohorts: Effects on Health and Work Ability. Int. J. Environ. Res. Public Health 2023, 20, 5638. [Google Scholar] [CrossRef] [PubMed]
- Premraj, L.; Kannapadi, N.V.; Briggs, J.; Seal, S.M.; Battaglini, D.; Fanning, J.; Suen, J.; Robba, C.; Fraser, J.; Cho, S.M. Mid and long-term neurological and neuropsychiatric manifestations of post-COVID-19 syndrome: A meta-analysis. J. Neurol. Sci. 2022, 434, 120162. [Google Scholar] [CrossRef] [PubMed]
- Magnavita, N.; Tripepi, G.; Di Prinzio, R.R. Symptoms in Health Care Workers during the COVID-19 Epidemic. A Cross-Sectional Survey. Int. J. Environ. Res. Public Health 2020, 17, 5218. [Google Scholar] [CrossRef]
- Magnavita, N.; Soave, P.M.; Ricciardi, W.; Antonelli, M. Occupational Stress and Mental Health among Anesthetists during the COVID-19 Pandemic. Int. J. Environ. Res. Public Health 2020, 17, 8245. [Google Scholar] [CrossRef]
- Cleper, R.; Hertz-Palmor, N.; Mosheva, M.; Hasson-Ohayon, I.; Kaplan, R.; Kreiss, Y.; Afek, A.; Pessach, I.M.; Gothelf, D.; Gross, R. Sleep Difficulties Among COVID-19 Frontline Healthcare Workers. Front. Psychiatry 2022, 13, 838825. [Google Scholar] [CrossRef]
- Magnavita, N.; Soave, P.M.; Antonelli, M. A One-Year Prospective Study of Work-Related Mental Health in the Intensivists of a COVID-19 Hub Hospital. Int. J. Environ. Res. Public Health 2021, 18, 9888. [Google Scholar] [CrossRef]
- Blank, L.; Hock, E.; Cantrell, A.; Baxter, S.; Goyder, E. Exploring the relationship between working from home, mental and physical health and wellbeing: A systematic review. Public Health Res. 2023, 11, 1–100. [Google Scholar] [CrossRef]
- Bouziri, H.; Smith, D.R.M.; Descatha, A.; Dab, W.; Jean, K. Working from home in the time of COVID-19: How to best preserve occupational health? Occup. Environ. Med. 2020, 77, 509–510. [Google Scholar] [CrossRef]
- Marino, L.; Capone, V. Smart Working and Well-Being before and during the COVID-19 Pandemic: A Scoping Review. Eur. J. Investig. Health Psychol. Educ. 2021, 11, 1516–1536. [Google Scholar] [CrossRef]
- Oakman, J.; Kinsman, N.; Stuckey, R.; Graham, M.; Weale, V. A rapid review of mental and physical health effects of working at home: How do we optimise health? BMC Public Health 2020, 20, 1825. [Google Scholar] [CrossRef] [PubMed]
- Conroy, D.A.; Hadler, N.L.; Cho, E.; Moreira, A.; MacKenzie, C.; Swanson, L.M.; Burgess, H.J.; Arnedt, J.T.; Goldstein, C.A. The effects of COVID-19 stay-at-home order on sleep, health, and working patterns: A survey study of US health care workers. J. Clin. Sleep Med. 2021, 17, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Leone, M.J.; Sigman, M.; Golombek, D.A. Effects of lockdown on human sleep and chronotype during the COVID-19 pandemic. Curr. Biol. 2020, 30, R930–R931. [Google Scholar] [CrossRef] [PubMed]
- Raman, S.; Coogan, A.N. Effects of societal-level COVID-19 mitigation measures on the timing and quality of sleep in Ireland. Sleep Med. 2022, 91, 179–184. [Google Scholar] [CrossRef]
- Barrea, L.; Pugliese, G.; Framondi, L.; Di Matteo, R.; Laudisio, D.; Savastano, S.; Colao, A.; Muscogiuri, G. Does Sars-Cov-2 threaten our dreams? Effect of quarantine on sleep quality and body mass index. J. Transl. Med. 2020, 18, 318. [Google Scholar] [CrossRef]
- Jung, J.; Lim, J.; Cho, Y.H.; Park, J.B.; Jeong, I. The changing dynamics of work from home and its association with sleep disturbance through work-family conflict during the COVID-19 pandemic. J. Occup. Health 2024, 66, uiae014. [Google Scholar] [CrossRef]
- McCall, W.V.; Mensah-Bonsu, D.; Withers, A.E.; Gibson, R.W. Short-term insomnia disorder in health care workers in an academic medical center before and during COVID-19: Rates and predictive factors. J. Clin. Sleep Med. 2021, 17, 749–755. [Google Scholar] [CrossRef]
- Scoditti, E.; Bodini, A.; Sabina, S.; Leo, C.G.; Mincarone, P.; Rissotto, A.; Fusco, S.; Guarino, R.; Ponzini, G.; Tumolo, M.R.; et al. Effects of working from home on lifestyle behaviors and mental health during the COVID-19 pandemic: A survey study. PLoS ONE 2024, 19, e0300812. [Google Scholar] [CrossRef]
- Bodini, A.; Leo, C.G.; Rissotto, A.; Mincarone, P.; Fusco, S.; Garbarino, S.; Guarino, R.; Sabina, S.; Scoditti, E.; Tumolo, M.R.; et al. The medium-term perceived impact of work from home on life and work domains of knowledge workers during COVID-19 pandemic: A survey at the National Research Council of Italy. Front. Public Health 2023, 11, 1151009. [Google Scholar] [CrossRef]
- Buysse, D.J.; Reynolds, C.F., 3rd; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Curcio, G.; Tempesta, D.; Scarlata, S.; Marzano, C.; Moroni, F.; Rossini, P.M.; Ferrara, M.; De Gennaro, L. Validity of the Italian version of the Pittsburgh Sleep Quality Index (PSQI). Neurol. Sci. 2013, 34, 511–519. [Google Scholar] [CrossRef]
- Kroenke, K.; Spitzer, R.L.; Williams, J.B. The PHQ-9: Validity of a brief depression severity measure. J. Gen. Intern. Med. 2001, 16, 606–613. [Google Scholar] [CrossRef]
- Blume, C.; Schmidt, M.H.; Cajochen, C. Effects of the COVID-19 lockdown on human sleep and rest-activity rhythms. Curr. Biol. 2020, 30, R795–R797. [Google Scholar] [CrossRef]
- Salfi, F.; Lauriola, M.; D’Atri, A.; Amicucci, G.; Viselli, L.; Tempesta, D.; Ferrara, M. Demographic, psychological, chronobiological, and work-related predictors of sleep disturbances during the COVID-19 lockdown in Italy. Sci. Rep. 2021, 11, 11416. [Google Scholar] [CrossRef]
- Zeng, L.N.; Zong, Q.Q.; Yang, Y.; Zhang, L.; Xiang, Y.F.; Ng, C.H.; Chen, L.G.; Xiang, Y.T. Gender Difference in the Prevalence of Insomnia: A Meta-Analysis of Observational Studies. Front. Psychiatry 2020, 11, 577429. [Google Scholar] [CrossRef]
- Gibson, R.; Shetty, H.; Carter, M.; Munch, M. Sleeping in a bubble: Factors affecting sleep during New Zealand’s COVID-19 lockdown. Sleep Adv. 2022, 3, zpac017. [Google Scholar] [CrossRef]
- Kocevska, D.; Blanken, T.F.; Van Someren, E.J.W.; Rosler, L. Sleep quality during the COVID-19 pandemic: Not one size fits all. Sleep Med. 2020, 76, 86–88. [Google Scholar] [CrossRef]
- Gao, C.; Scullin, M.K. Sleep health early in the coronavirus disease 2019 (COVID-19) outbreak in the United States: Integrating longitudinal, cross-sectional, and retrospective recall data. Sleep Med. 2020, 73, 1–10. [Google Scholar] [CrossRef]
- Martinez-de-Quel, O.; Suarez-Iglesias, D.; Lopez-Flores, M.; Perez, C.A. Physical activity, dietary habits and sleep quality before and during COVID-19 lockdown: A longitudinal study. Appetite 2021, 158, 105019. [Google Scholar] [CrossRef]
- Taporoski, T.P.; Beijamini, F.; Gomez, L.M.; Ruiz, F.S.; Ahmed, S.S.; von Schantz, M.; Pereira, A.C.; Knutson, K.L. Subjective sleep quality before and during the COVID-19 pandemic in a Brazilian rural population. Sleep Health 2022, 8, 167–174. [Google Scholar] [CrossRef]
- Salfi, F.; Amicucci, G.; Corigliano, D.; Viselli, L.; D’Atri, A.; Tempesta, D.; Gorgoni, M.; Scarpelli, S.; Alfonsi, V.; Ferrara, M. Two years after lockdown: Longitudinal trajectories of sleep disturbances and mental health over the COVID-19 pandemic, and the effects of age, gender and chronotype. J. Sleep Res. 2022, 32, e13767. [Google Scholar] [CrossRef] [PubMed]
- Bouman, E.J.; Beulens, J.W.J.; Groeneveld, L.; de Kruijk, R.S.; Schoonmade, L.J.; Remmelzwaal, S.; Elders, P.J.M.; Rutters, F. The association between social jetlag and parameters of metabolic syndrome and type 2 diabetes: A systematic review and meta-analysis. J. Sleep Res. 2022, 32, e13770. [Google Scholar] [CrossRef] [PubMed]
- Salfi, F.; D’Atri, A.; Amicucci, G.; Viselli, L.; Gorgoni, M.; Scarpelli, S.; Alfonsi, V.; Ferrara, M. The fall of vulnerability to sleep disturbances in evening chronotypes when working from home and its implications for depression. Sci. Rep. 2022, 12, 12249. [Google Scholar] [CrossRef]
- Cheung, V.K.L. Practical Considerations of Workplace Wellbeing Management under Post-Pandemic Work-from-Home Conditions. Int. J. Environ. Res. Public Health 2024, 21, 924. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Becerik-Gerber, B.; Lucas, G.; Roll, S.C. Impacts of Working From Home During COVID-19 Pandemic on Physical and Mental Well-Being of Office Workstation Users. J. Occup. Environ. Med. 2021, 63, 181–190. [Google Scholar] [CrossRef]
- Park, S.Y.; Lee, R.; Newton, C.; Han, G. How are people coping with working from home during the COVID-19 pandemic?: Experiences from the Netherlands and South Korea. PLoS ONE 2024, 19, e0301351. [Google Scholar] [CrossRef]
- Drumheller, K.; Fan, C.W. Unprecedented times and uncertain connections: A systematic review examining sleep problems and screentime during the COVID-19 pandemic. Sleep Epidemiol. 2022, 2, 100029. [Google Scholar] [CrossRef]
- De Pasquale, C.; El Kazzi, M.; Sutherland, K.; Shriane, A.E.; Vincent, G.E.; Cistulli, P.A.; Bin, Y.S. Sleep hygiene—What do we mean? A bibliographic review. Sleep Med. Rev. 2024, 75, 101930. [Google Scholar] [CrossRef]
- Irish, L.A.; Kline, C.E.; Gunn, H.E.; Buysse, D.J.; Hall, M.H. The role of sleep hygiene in promoting public health: A review of empirical evidence. Sleep Med. Rev. 2015, 22, 23–36. [Google Scholar] [CrossRef]
- Garbarino, S.; Scoditti, E. On the role of sleep hygiene in health management during COVID-19 pandemic. Sleep Med. 2020, 77, 74. [Google Scholar] [CrossRef]
- Redeker, N.S.; Caruso, C.C.; Hashmi, S.D.; Mullington, J.M.; Grandner, M.; Morgenthaler, T.I. Workplace Interventions to Promote Sleep Health and an Alert, Healthy Workforce. J. Clin. Sleep Med. 2019, 15, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Hrehova, L.; Buskova, J.; Seifert, B.; Mezian, K. Working from home is associated with changes in sleep hygiene practice. Work 2024, 77, 1135–1142. [Google Scholar] [CrossRef] [PubMed]
- Roth, T. Effects of excessive daytime sleepiness and fatigue on overall health and cognitive function. J. Clin. Psychiatry 2015, 76, e1145. [Google Scholar] [CrossRef] [PubMed]
- Okajima, I.; Komada, Y.; Ito, W.; Inoue, Y. Sleep Debt and Social Jetlag Associated with Sleepiness, Mood, and Work Performance among Workers in Japan. Int. J. Environ. Res. Public Health 2021, 18, 2908. [Google Scholar] [CrossRef]
- Brooks, A.; Lack, L. A brief afternoon nap following nocturnal sleep restriction: Which nap duration is most recuperative? Sleep 2006, 29, 831–840. [Google Scholar] [CrossRef]
- Wichniak, A.; Wierzbicka, A.; Jernajczyk, W. Sleep as a biomarker for depression. Int. Rev. Psychiatry 2013, 25, 632–645. [Google Scholar] [CrossRef]
- Burstyn, I.; Huynh, T. Symptoms of Anxiety and Depression in Relation to Work Patterns During the First Wave of the COVID-19 Epidemic in Philadelphia PA: A Cross-Sectional Survey. J. Occup. Environ. Med. 2021, 63, e283–e293. [Google Scholar] [CrossRef]
- Somasundram, K.G.; Hackney, A.; Yung, M.; Du, B.; Oakman, J.; Nowrouzi-Kia, B.; Yazdani, A. Mental and physical health and well-being of canadian employees who were working from home during the COVID-19 pandemic. BMC Public Health 2022, 22, 1987. [Google Scholar] [CrossRef]
- Slater, G.; Steier, J. Excessive daytime sleepiness in sleep disorders. J. Thorac. Dis. 2012, 4, 608–616. [Google Scholar] [CrossRef]
- Moderie, C.; Boivin, D.B. Pathophysiological Models of Hypersomnolence Associated With Depression. Biol. Psychiatry Glob. Open Sci. 2025, 5, 100445. [Google Scholar] [CrossRef]
- Muscogiuri, G.; Barrea, L.; Annunziata, G.; Di Somma, C.; Laudisio, D.; Colao, A.; Savastano, S. Obesity and sleep disturbance: The chicken or the egg? Crit. Rev. Food Sci. Nutr. 2019, 59, 2158–2165. [Google Scholar] [CrossRef] [PubMed]
- Coskun, M.G.; Ozturk, R.I.; Tak, A.Y.; Sanlier, N. Working from Home during the COVID-19 Pandemic and Its Effects on Diet, Sedentary Lifestyle, and Stress. Nutrients 2022, 14, 4006. [Google Scholar] [CrossRef] [PubMed]
- Tudor-Locke, C.; Leonardi, C.; Johnson, W.D.; Katzmarzyk, P.T. Time spent in physical activity and sedentary behaviors on the working day: The American time use survey. J. Occup. Environ. Med. 2011, 53, 1382–1387. [Google Scholar] [CrossRef] [PubMed]
- Merikanto, I.; Kortesoja, L.; Benedict, C.; Chung, F.; Cedernaes, J.; Espie, C.A.; Morin, C.M.; Dauvilliers, Y.; Partinen, M.; De Gennaro, L.; et al. Evening-types show highest increase of sleep and mental health problems during the COVID-19 pandemic-multinational study on 19 267 adults. Sleep 2022, 45, zsab216. [Google Scholar] [CrossRef]
- Selvi, Y.; Kandeger, A.; Boysan, M.; Akbaba, N.; Sayin, A.A.; Tekinarslan, E.; Koc, B.O.; Uygur, O.F.; Sar, V. The effects of individual biological rhythm differences on sleep quality, daytime sleepiness, and dissociative experiences. Psychiatry Res. 2017, 256, 243–248. [Google Scholar] [CrossRef]
- Bergefurt, L.; Appel-Meulenbroek, R.; Arentze, T. How physical home workspace characteristics affect mental health: A systematic scoping review. Work 2023, 76, 489–506. [Google Scholar] [CrossRef]
- Manzar, M.D.; BaHammam, A.S.; Hameed, U.A.; Spence, D.W.; Pandi-Perumal, S.R.; Moscovitch, A.; Streiner, D.L. Dimensionality of the Pittsburgh Sleep Quality Index: A systematic review. Health Qual. Life Outcomes 2018, 16, 89. [Google Scholar] [CrossRef]
- Jia, Y.; Chen, S.; Deutz, N.E.P.; Bukkapatnam, S.T.S.; Woltering, S. Examining the structure validity of the Pittsburgh Sleep Quality Index. Sleep Biol. Rhythms 2019, 17, 209–221. [Google Scholar] [CrossRef]
- Helles, M.; Fletcher, R.; Munch, M.; Gibson, R. Examining the structure validity of the Pittsburgh Sleep Quality Index among female workers during New Zealand’s initial COVID-19 lockdown. Sleep Biol. Rhythms 2024, 22, 217–225. [Google Scholar] [CrossRef]
- Martynowicz, H.; Michalek-Zrabkowska, M.; Gac, P.; Blaszczyk, B.; Fulek, M.; Frosztega, W.; Wojakowska, A.; Poreba, R.; Mazur, G.; Wieckiewicz, M. Performance evaluation of portable respiratory polygraphy for assessing sleep bruxism in adults. J. Oral. Rehabil. 2024, 51, 1862–1871. [Google Scholar] [CrossRef]
- Adan, A.; Almirall, H. Horne & Ostberg morningness-eveningness questionnaire: A reduced scale. Pers. Indiv. Differ. 1991, 12, 241–253. [Google Scholar]
- Natale, V.; Esposito, M.J.; Martoni, M.; Fabbri, M. Validity of the reduced version of the morningness-eveningness questionnaire. Sleep Biol. Rhythms. 2006, 4, 72–74. [Google Scholar] [CrossRef]
- Johns, M.W. A new method for measuring daytime sleepiness: The Epworth sleepiness scale. Sleep 1991, 14, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Vignatelli, L.; Plazzi, G.; Barbato, A.; Ferini-Strambi, L.; Manni, R.; Pompei, F.; D’Alessandro, R.; Ginsen. Italian version of the Epworth sleepiness scale: External validity. Neurol. Sci. 2003, 23, 295–300. [Google Scholar] [CrossRef]
- Lumley, T.; Diehr, P.; Emerson, S.; Chen, L. The importance of the normality assumption in large public health data sets. Annu. Rev. Public Health 2002, 23, 151–169. [Google Scholar] [CrossRef]
- Hosmer, D.W.; Lemeshow, S.; Cook, E. Applied Logistic Regression, 2nd ed.; John Wiley and Sons Inc.: New York, NY, USA, 2000. [Google Scholar]
- Team, R.C. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
| Sample (n = 748) | ||
|---|---|---|
| n | % | |
| Gender | ||
| Men | 317 | 42.4 |
| Women | 431 | 57.6 |
| Age (years) | ||
| ≤39 | 90 | 12.0 |
| 40–49 | 275 | 36.8 |
| 50–59 | 285 | 38.1 |
| ≥60 | 98 | 13.1 |
| Cohabitation | ||
| Living alone | 108 | 14.4 |
| Not living alone, no children | 282 | 37.7 |
| Not living alone, with children | 358 | 47.9 |
| Place of residence a | ||
| North | 244 | 32.6 |
| Center | 261 | 34.9 |
| South | 168 | 22.5 |
| Islands | 75 | 10.0 |
| Education | ||
| Graduation | 614 | 82.1 |
| No graduation | 134 | 17.9 |
| Professional profile | ||
| Administrative and technical staff | 238 | 31.8 |
| Researcher and technologist | 510 | 68.2 |
| Pre-WH | During WH | Difference | p-Value 1 | |
|---|---|---|---|---|
| PSQI score | ||||
| Mean ± SD | 5.25 ± 2.39 | 5.15 ± 2.57 | −0.10 ± 2.19 | n.a. 2 |
| Median, IQR | 5, 4–6 | 5, 3–7 | 0, (−1)–1 | 0.04 |
| Poor sleepers (PSQI > 5) (n [%]) | 285 [38.7] | 272 [36.9] | -- | 0.37 |
| Good sleepers (PSQI ≤ 5) (n [%]) | 452 [61.3] | 465 [63.1] | -- | 0.37 |
| Sleep duration | ||||
| Min (mean ± SD) | 403 ± 56 | 416 ± 59 | 13.37 ± 51.7 | n.a. |
| Min (median, IQR) | 420, 360–420 | 420, 360–480 | 0, 0–60 | <0.001 |
| ≤6 h (n [%]) | 249 [33.8] | 201 [27.3] | -- | <0.001 |
| 7–8 h (n [%]) | 484 [65.7] | 519 [70.4] | -- | |
| ≥9 h (n [%]) | 4 [0.5] | 17 [2.3] | -- | |
| Sleep efficiency | ||||
| Mean% ± SD | 89.2 ± 10.1 | 88.0 ± 10.9 | −1.2 ± 9.23 | n.a. |
| Median%, IQR | 91.7, 83.5–100.0 | 88.9, 82.3–96.6 | 0, (−5.2)–3.3 | 0.004 |
| ESS score | ||||
| Mean ± SD | 4.46 ± 3.20 | 4.30 ± 2.85 | −0.16 ± 2.03 | n.a. |
| Median, IQR | 4, 2–6 | 4, 2–6 | 0, 0–1 | 0.16 |
| EDS (ESS > 10) (n [%]) | 40 [5.4] | 23 [3.1] | -- | 0.007 |
| PSQI Components | Stable (%) | Improved (%) | Worsened (%) |
|---|---|---|---|
| Sleep quality | 72.0 | 16.6 | 11.4 |
| Sleep latency | 75.6 | 8.4 | 16.0 |
| Sleep duration | 59.7 | 27.7 | 12.6 |
| Sleep efficiency | 70.7 | 13.4 | 15.9 |
| Sleep disturbances | 83.4 | 5.3 | 11.3 |
| Use of sleep medication | 91.0 | 3.7 | 5.3 |
| Daytime dysfunction | 75.3 | 14.4 | 10.3 |
| PSQI Component | No Meaningful Change | Meaningful Change 1 | p-Value | |
|---|---|---|---|---|
| (n) | (n) | (%) | ||
| Sleep quality | ||||
| Poor sleepers | 228 | 57 | 20.0 | <0.001 |
| Good sleepers | 415 | 37 | 8.2 | |
| Sleep latency | ||||
| Poor sleepers | 267 | 18 | 6.3 | 0.59 |
| Good sleepers | 429 | 23 | 5.1 | |
| Sleep duration | ||||
| Poor sleepers | 216 | 69 | 24.2 | <0.001 |
| Good sleepers | 410 | 42 | 9.3 | |
| Sleep efficiency | ||||
| Poor sleepers | 263 | 19 | 7.7 | 0.10 |
| Good sleepers | 435 | 17 | 3.8 | |
| Sleep disturbances | ||||
| Poor sleepers | 260 | 25 | 8.8 | 0.55 |
| Good sleepers | 405 | 47 | 10.4 | |
| Use of sleep medication | ||||
| Poor sleepers | 274 | 11 | 3.9 | 0.38 |
| Good sleepers | 441 | 11 | 2.4 | |
| Daytime dysfunction | ||||
| Poor sleepers | 236 | 49 | 17.2 | <0.001 |
| Good sleepers | 416 | 36 | 8.0 | |
| Variable | OR | LB—95% CI | UB—95%CI |
|---|---|---|---|
| Worsening in PHQ-9 score | 3.64 * | 2.04 | 6.60 |
| Circadian type | |||
| Evening type | 1.00 | -- | -- |
| Intermediate type | 0.62 | 0.26 | 1.51 |
| Morning type | 0.34 * | 0.12 | 0.95 |
| Variable | OR | LB—95% CI | UB—95%CI |
|---|---|---|---|
| Worsening in PHQ-9 score | 4.26 * | 2.50 | 7.32 |
| Worsening in ESS score | 2.47 * | 1.35 | 4.49 |
| Weight variation | |||
| Unchanged weight | 1.00 | -- | -- |
| Decreased weight | 1.88 | 0.97 | 3.58 |
| Increased weight | 2.55 * | 1.62 | 4.06 |
| Having a dedicated room available for work | 0.66 | 0.41 | 1.05 |
| Worsening in ESS score 1 | 0.961 | 0.49 | 1.86 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garbarino, S.; Bodini, A.; Sabina, S.; Leo, C.G.; Mincarone, P.; Rissotto, A.; Fusco, S.; Guarino, R.; Tumolo, M.R.; Tripepi, G.L.; et al. Not All Workers Experience Equal Sleep Changes: Insights from the “WorkInCovid” Project. Clocks & Sleep 2025, 7, 13. https://doi.org/10.3390/clockssleep7010013
Garbarino S, Bodini A, Sabina S, Leo CG, Mincarone P, Rissotto A, Fusco S, Guarino R, Tumolo MR, Tripepi GL, et al. Not All Workers Experience Equal Sleep Changes: Insights from the “WorkInCovid” Project. Clocks & Sleep. 2025; 7(1):13. https://doi.org/10.3390/clockssleep7010013
Chicago/Turabian StyleGarbarino, Sergio, Antonella Bodini, Saverio Sabina, Carlo Giacomo Leo, Pierpaolo Mincarone, Antonella Rissotto, Stanislao Fusco, Roberto Guarino, Maria Rosaria Tumolo, Giovanni Luigi Tripepi, and et al. 2025. "Not All Workers Experience Equal Sleep Changes: Insights from the “WorkInCovid” Project" Clocks & Sleep 7, no. 1: 13. https://doi.org/10.3390/clockssleep7010013
APA StyleGarbarino, S., Bodini, A., Sabina, S., Leo, C. G., Mincarone, P., Rissotto, A., Fusco, S., Guarino, R., Tumolo, M. R., Tripepi, G. L., Scoditti, E., & Magnavita, N. (2025). Not All Workers Experience Equal Sleep Changes: Insights from the “WorkInCovid” Project. Clocks & Sleep, 7(1), 13. https://doi.org/10.3390/clockssleep7010013










