A Bibliometric Analysis and a Citation Mapping Process for the Role of Soil Recycled Organic Matter and Microbe Interaction due to Climate Change Using Scopus Database
Abstract
:1. Introduction
1.1. Climate Change
1.2. Bibliometric Mapping
2. Materials and Methods
2.1. Using Scopus to Thoroughly Search Scientific Literature
2.2. Data Analysis and Bibliometric Mapping
3. Results
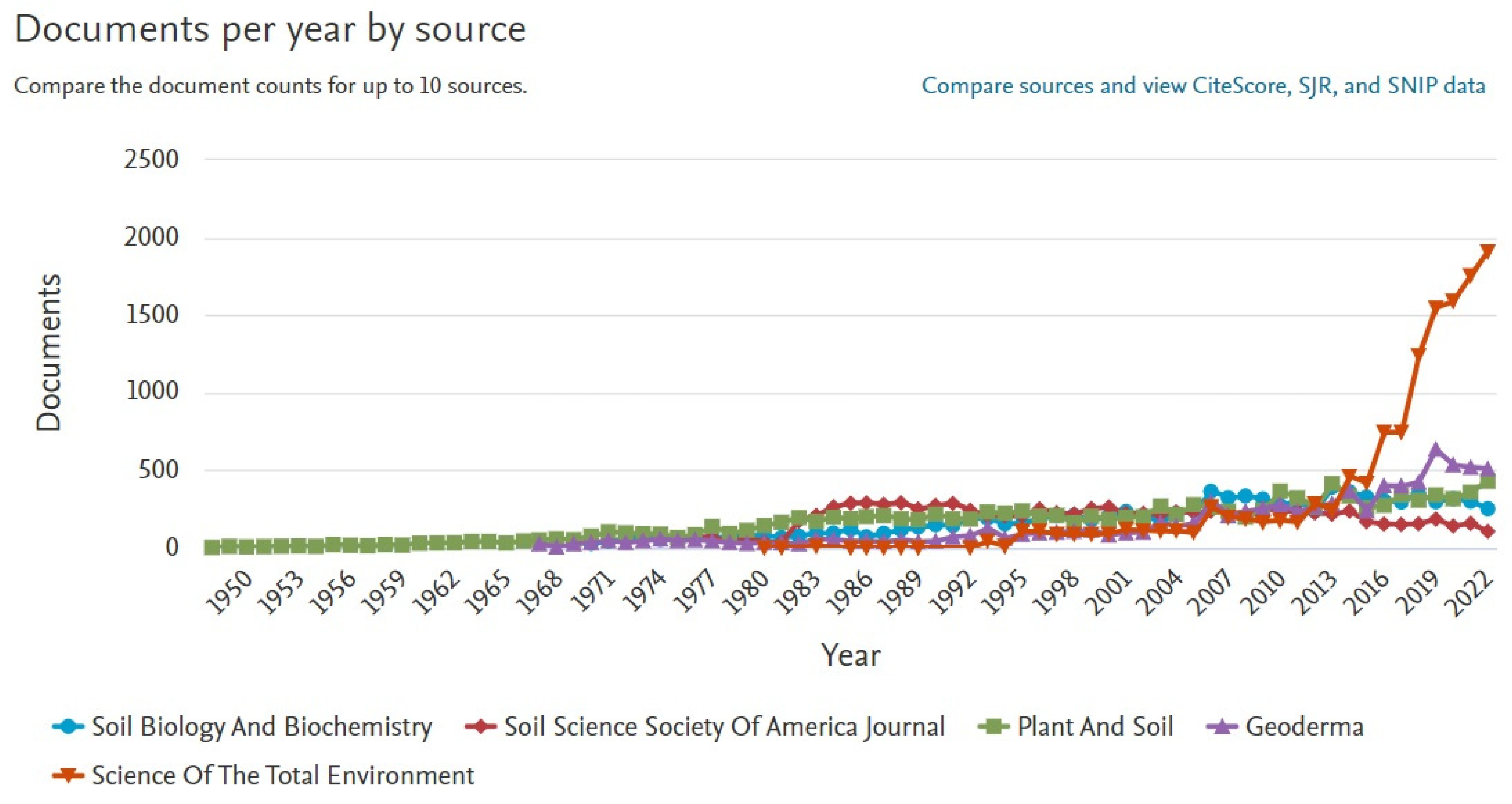
3.1. Using Scopus to Thoroughly Search Scientific Literature
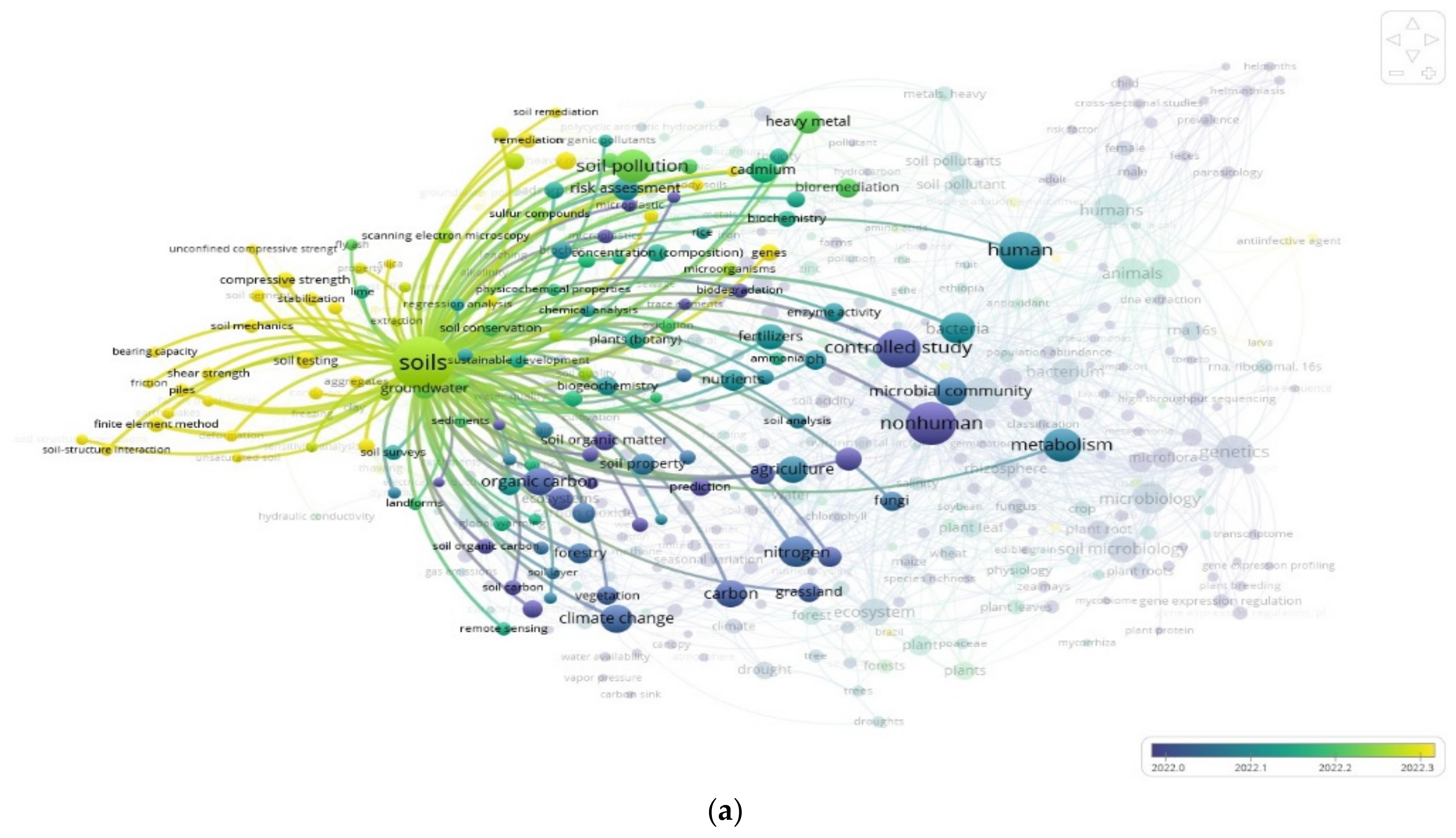
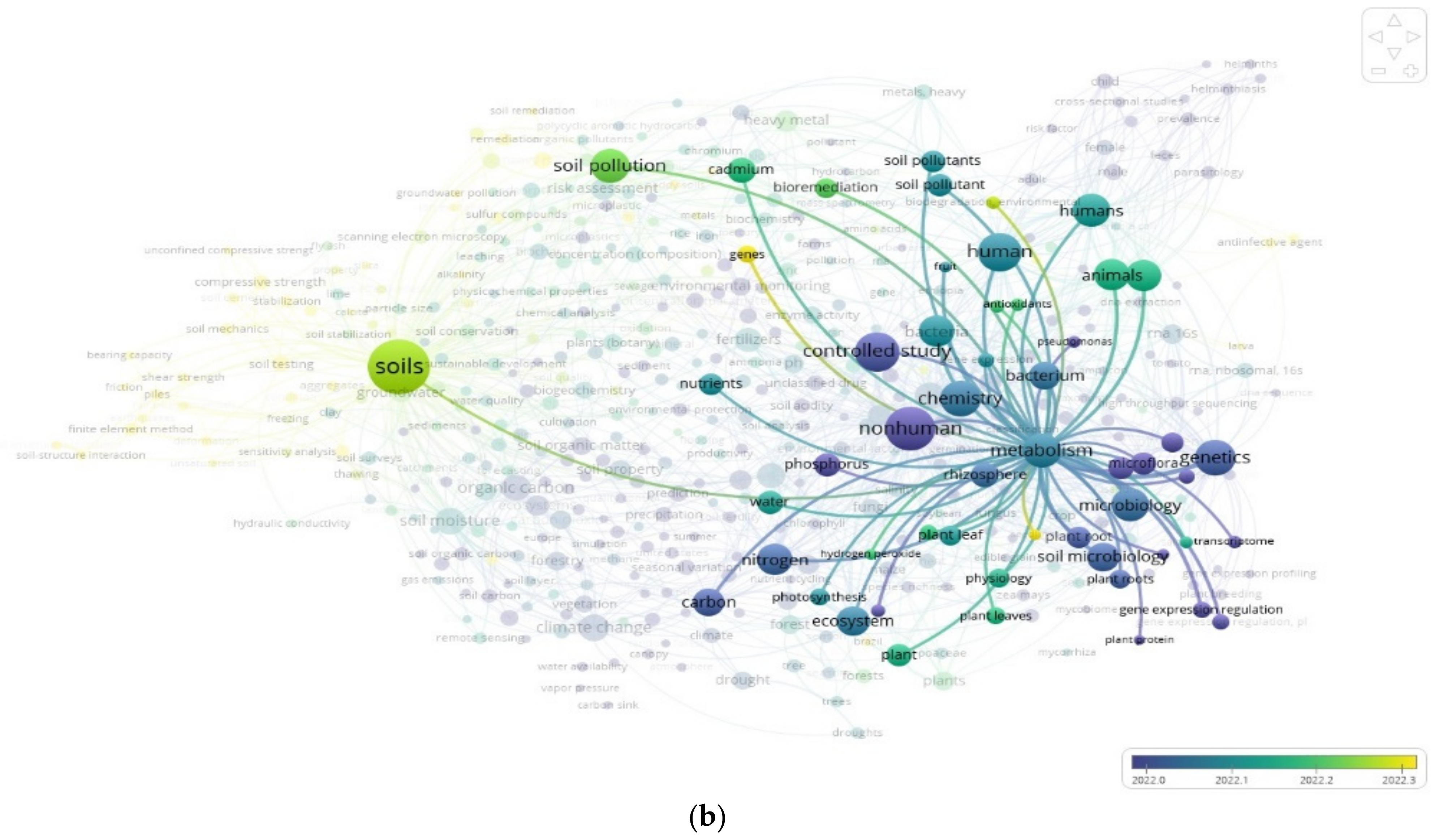
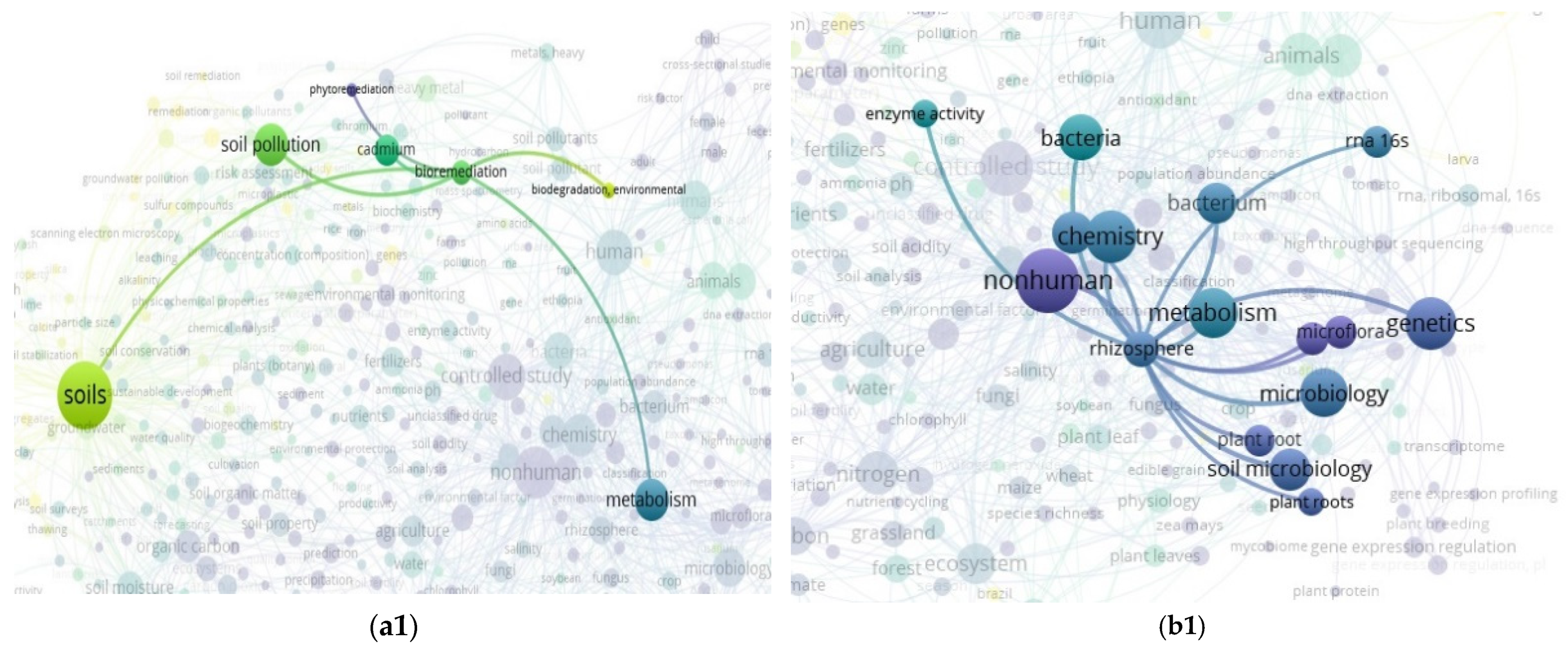
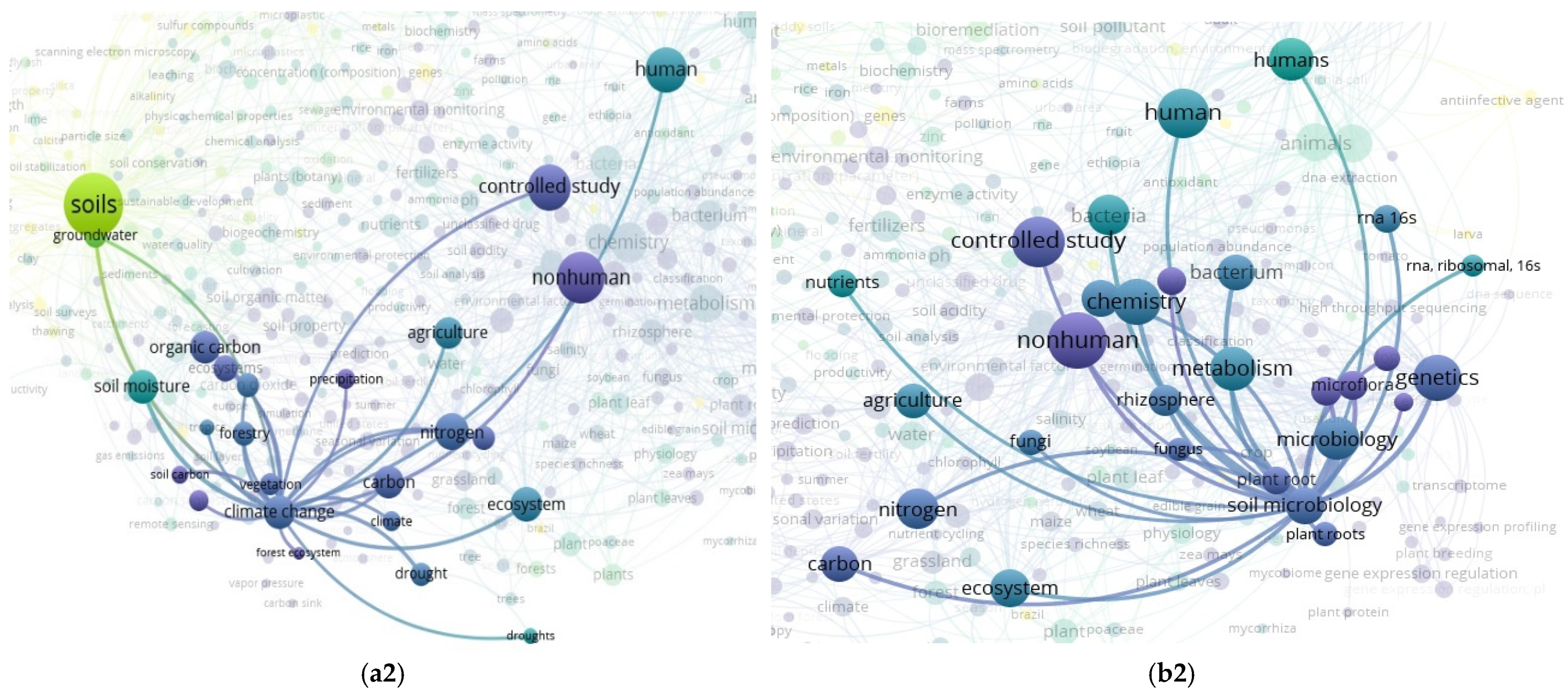
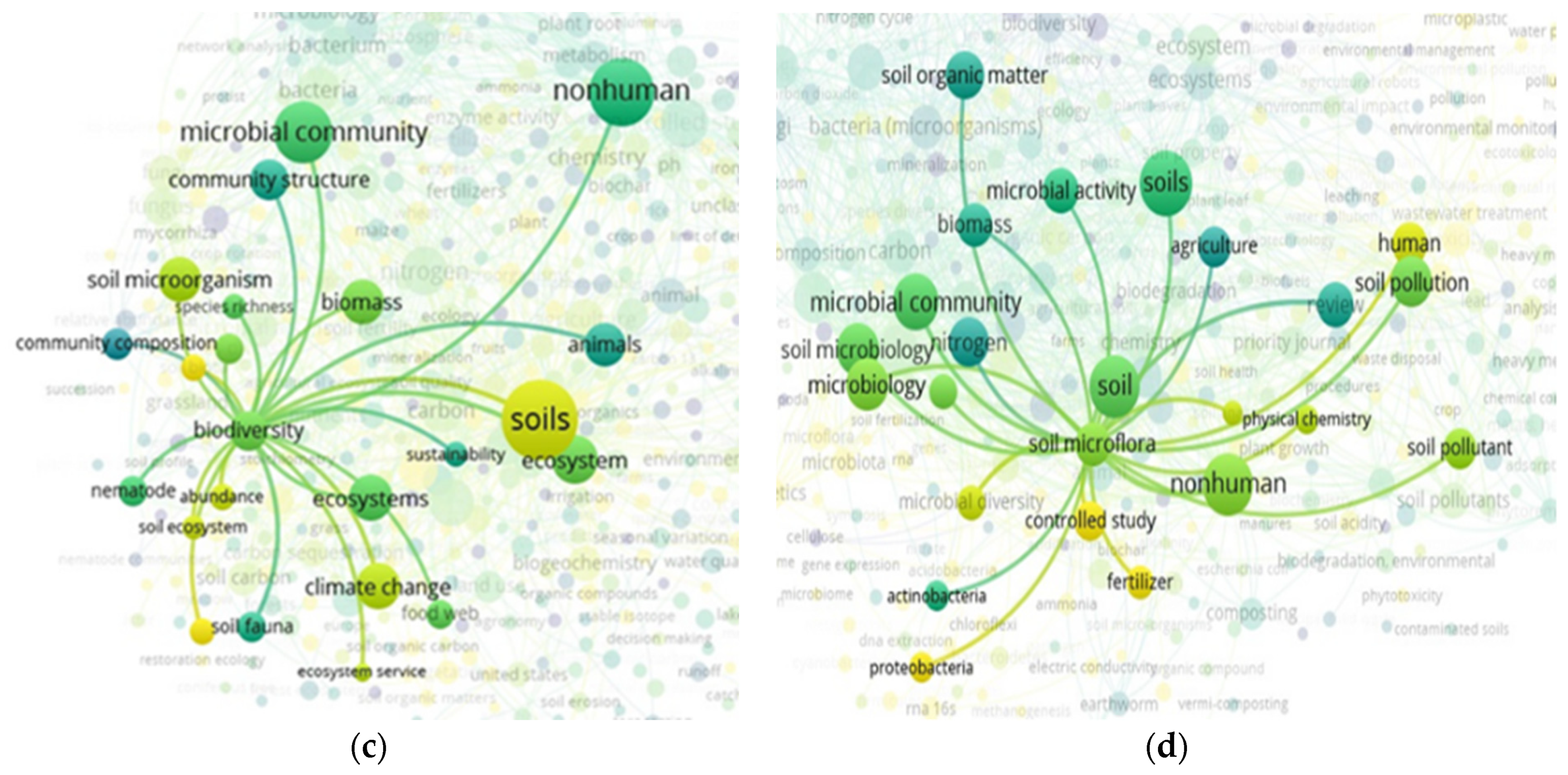
3.2. The “Soil” Paradigm
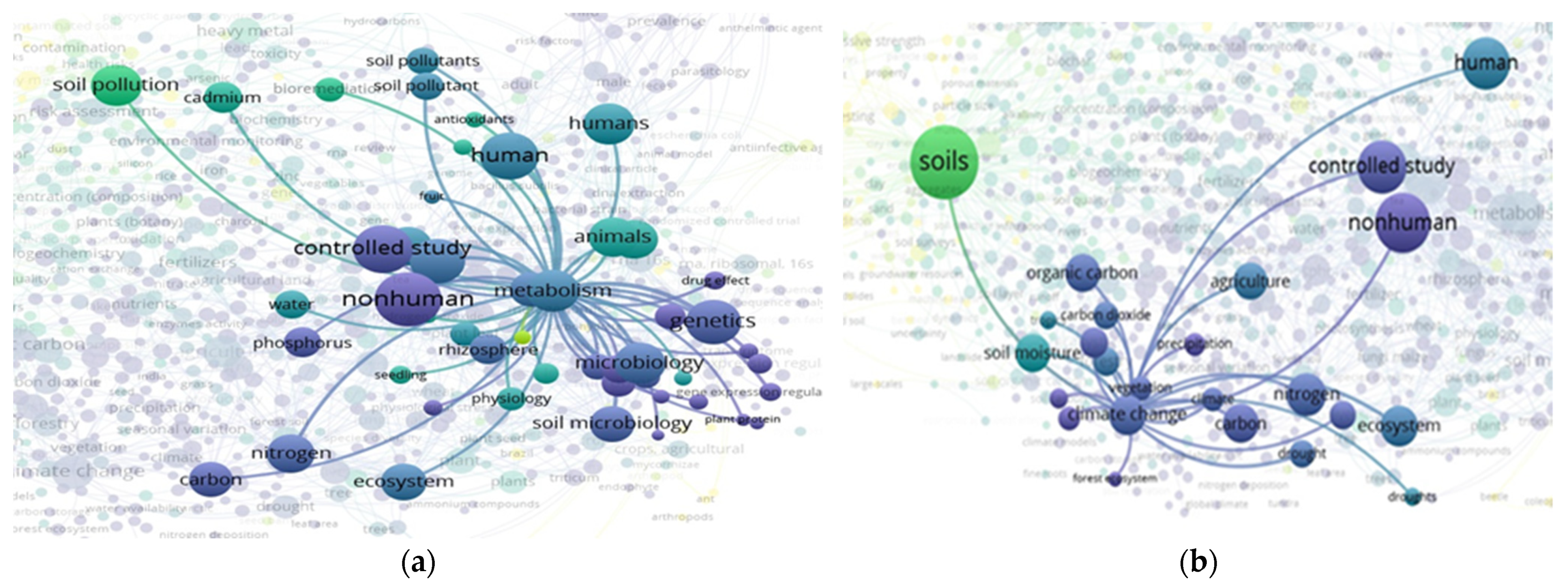
3.3. “Soil” and “Food Web” Interactions Based on the Scientific Literature Search Query on SCOPUS
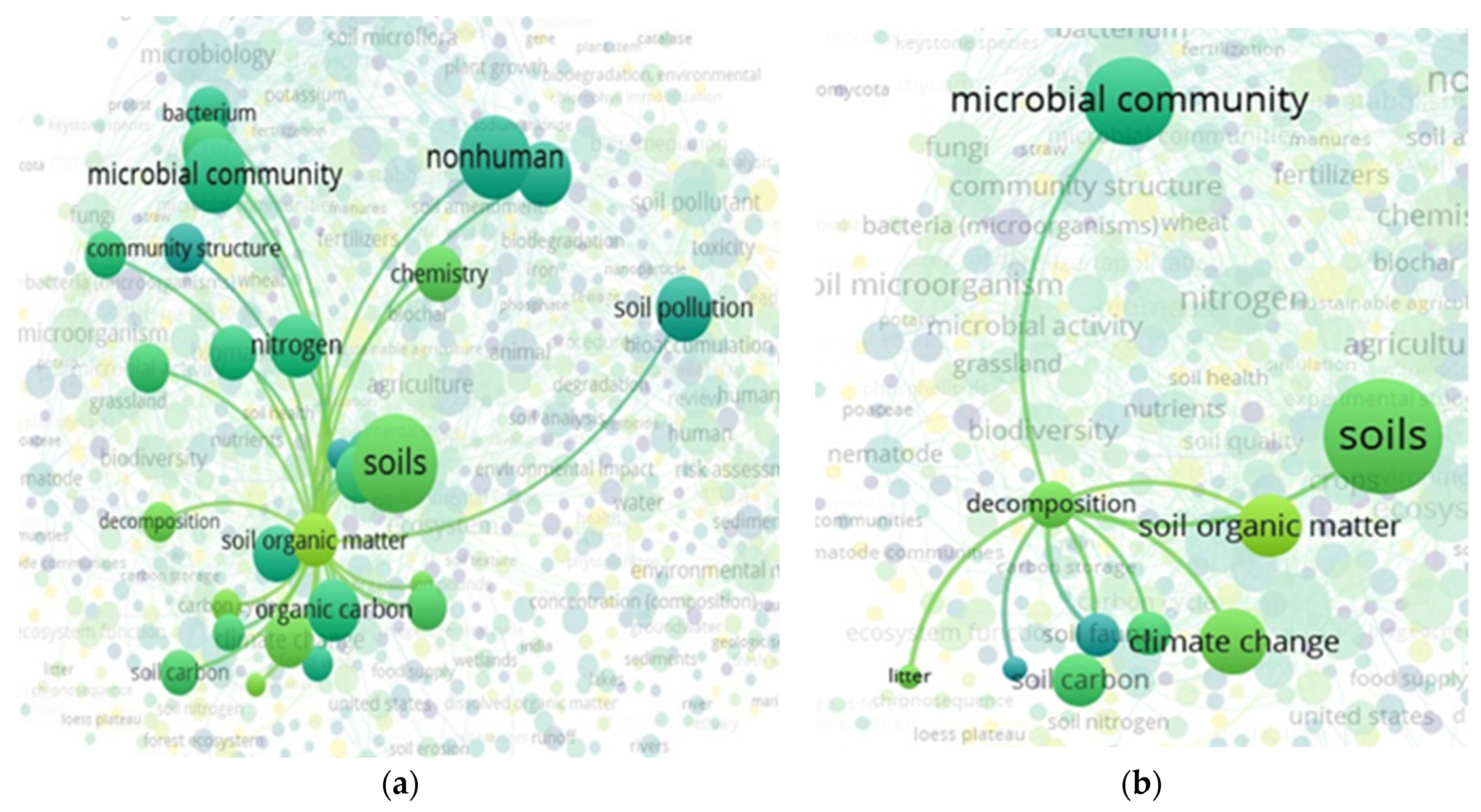
3.4. “Soil” and “Food Web” and “Organic Matter” Interactions Based on the Scientific Literature Search Query on Scopus
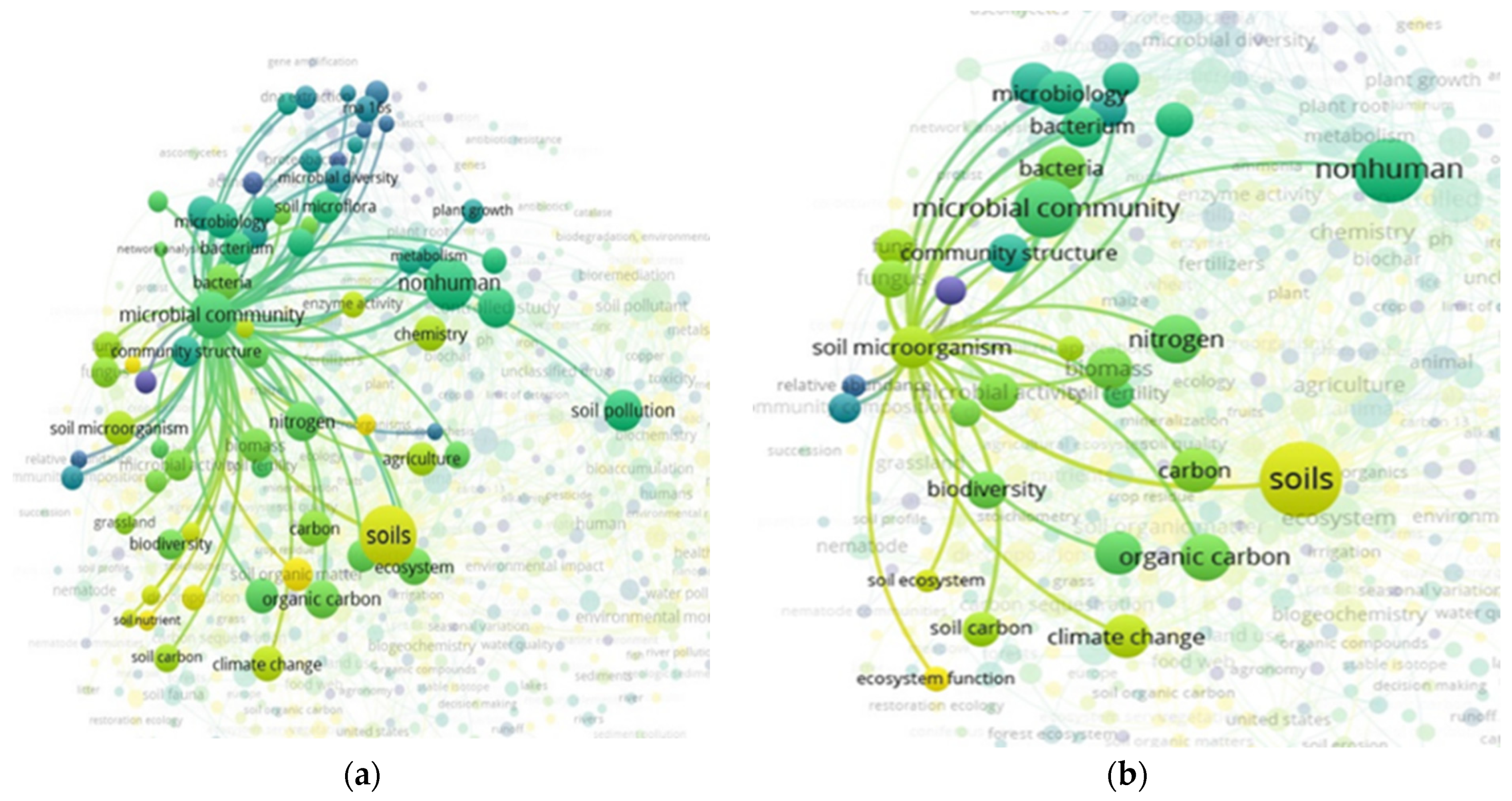
3.5. “Soil” and “Food Web” and “Organic Matter Recycling” Interactions Based on the Scientific Literature Search Query in Scopus
3.6. “Soil” and “Food Web” and “Organic Matter Recycling” and “Microbes” Interactions Based on the Scientific Literature Search Query on Scopus
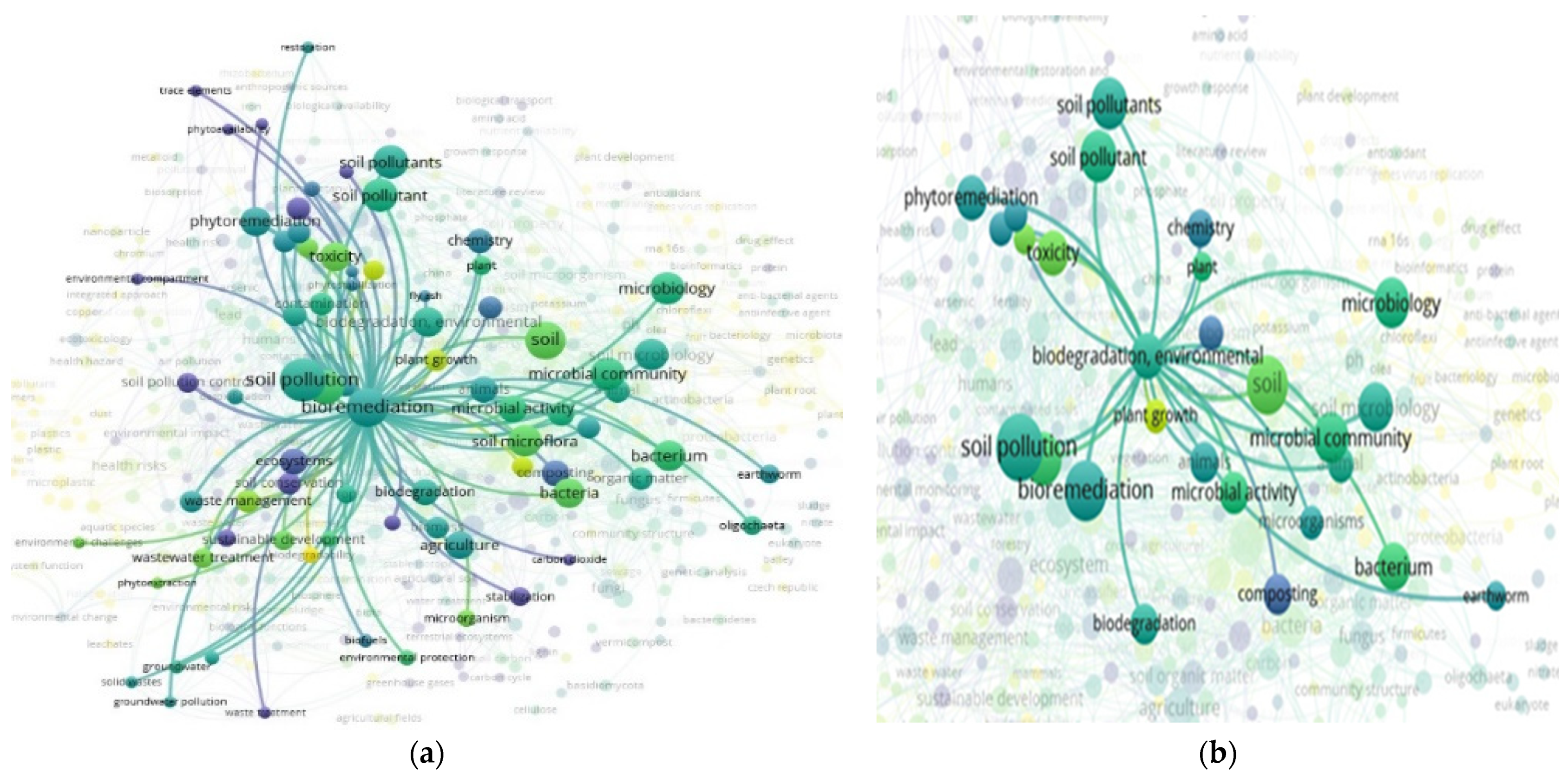
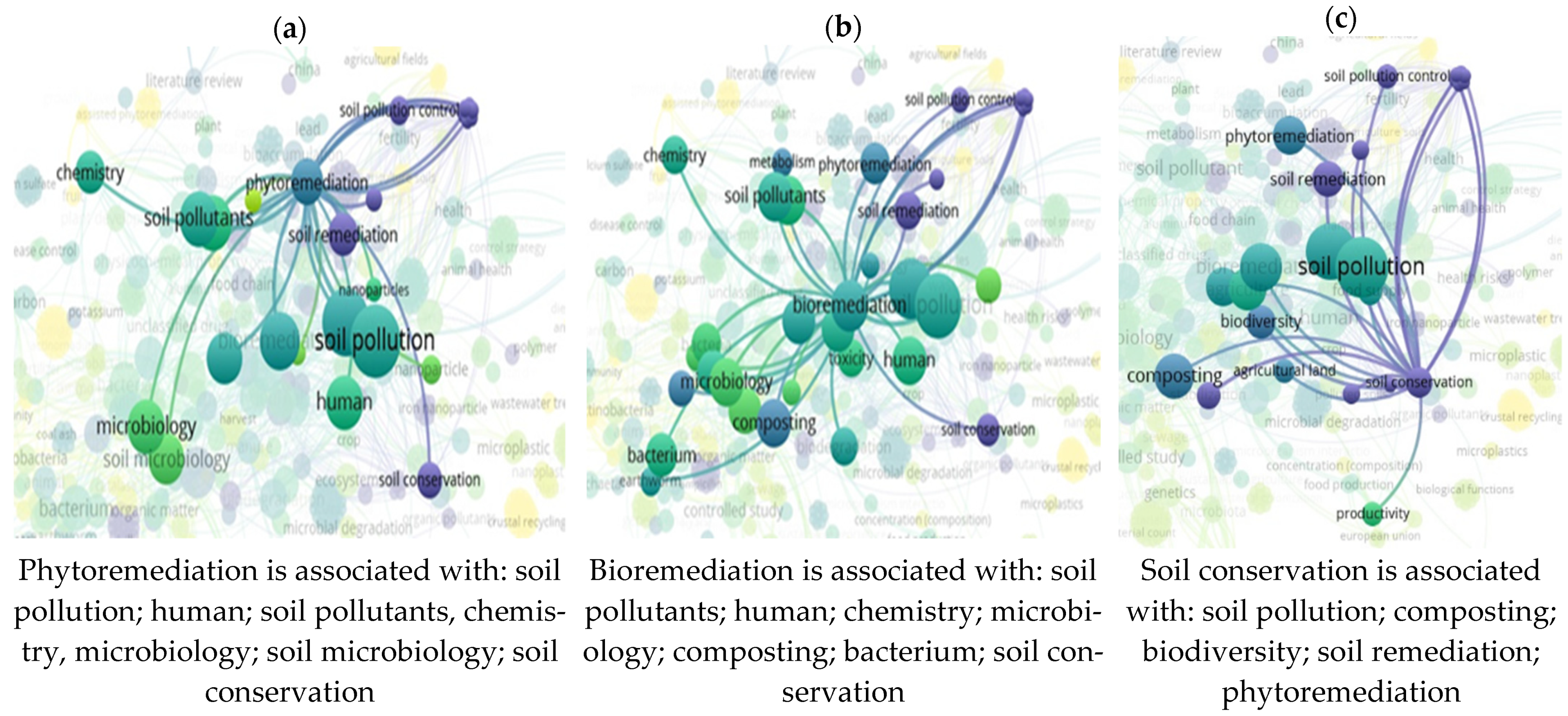
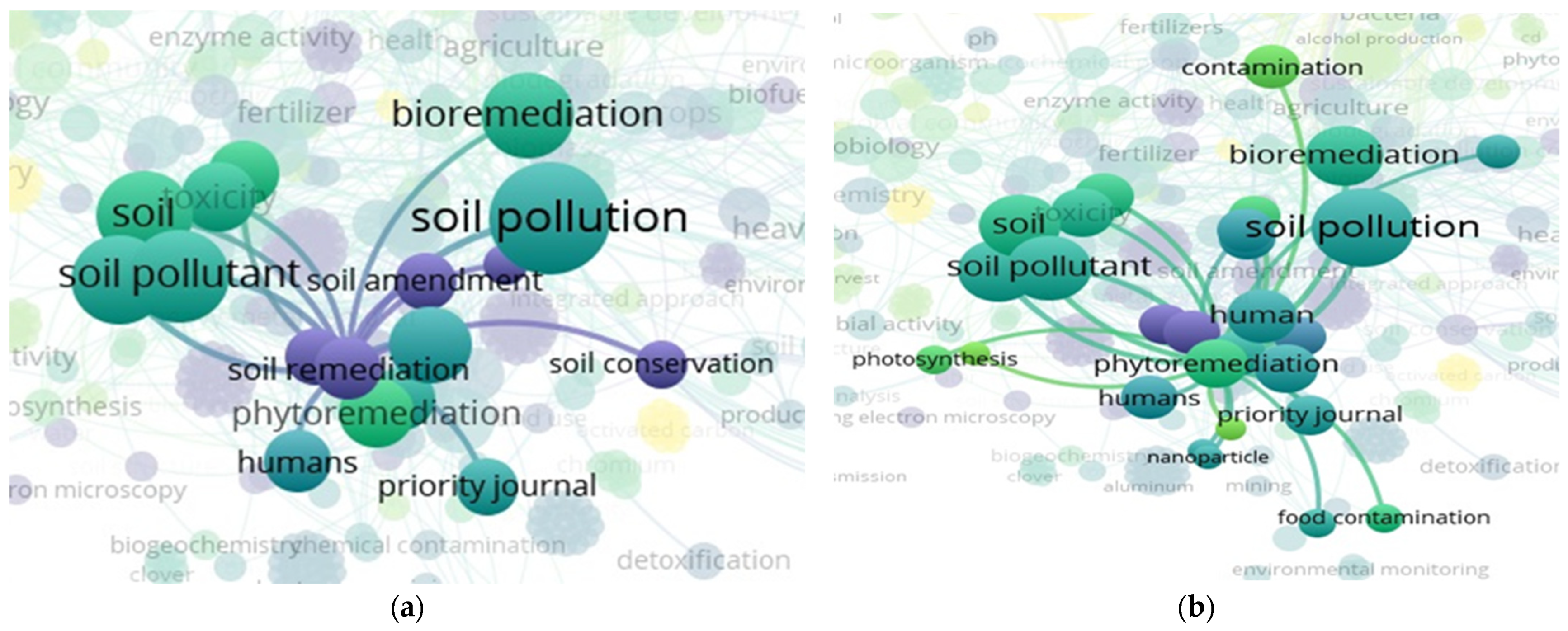
3.7. “Soil” and “Food Web” and “Organic Matter Recycling” and “Microbes” and “Bioremediation” Interactions Based on the Scientific Literature Search Query on Scopus
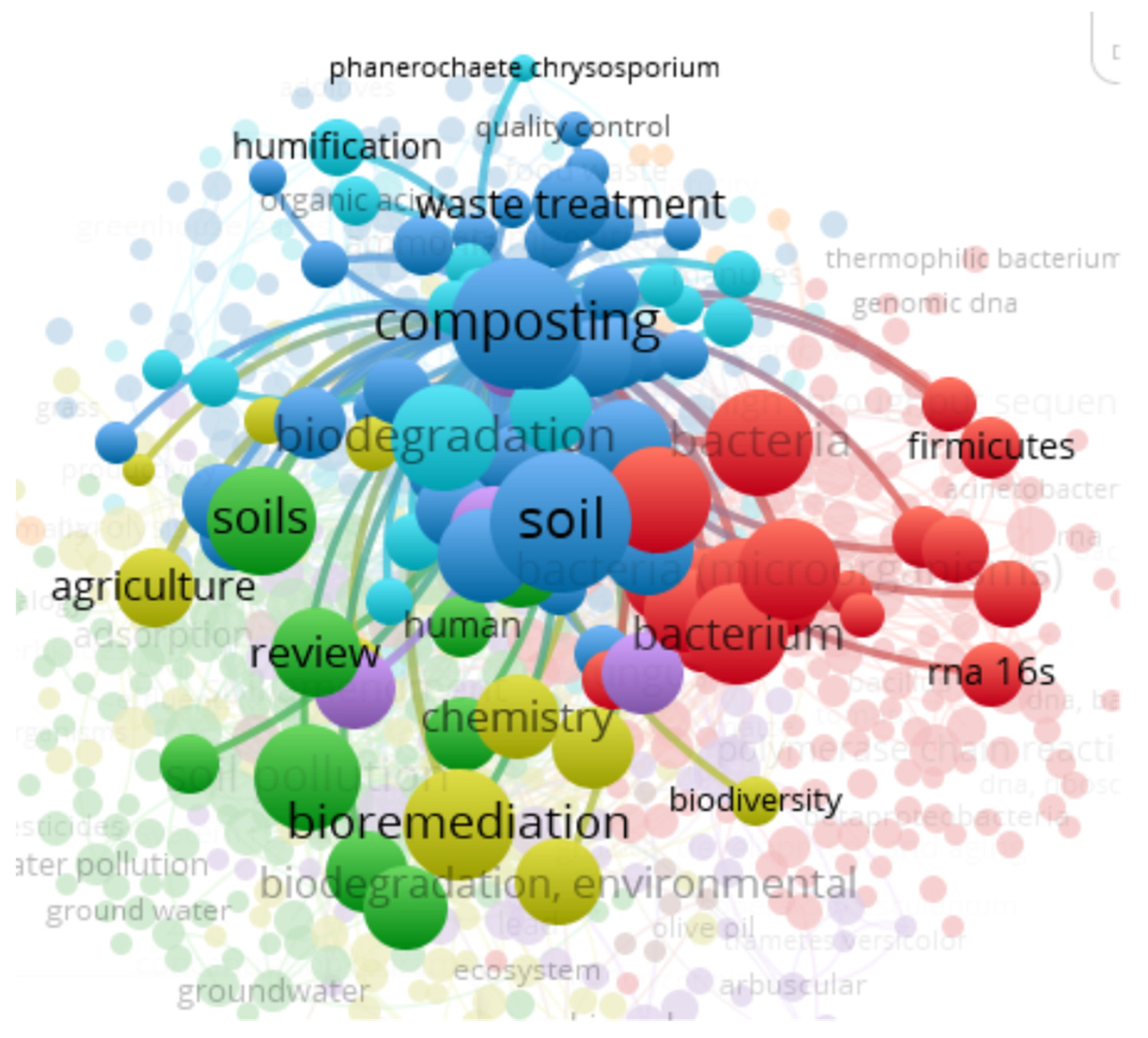
3.8. “Soil” and “Food Web” and “Organic Matter Recycling” and “Microbes” and “Bioremediation” and “Compost” Interactions Based on the Scientific Literature Search Query on Scopus
3.9. “Soil” and “Food Web” and “Organic Matter Recycling” and “Microbes” and “Bioremediation” and “Biochar” Interactions Based on the Scientific Literature Search Query on Scopus
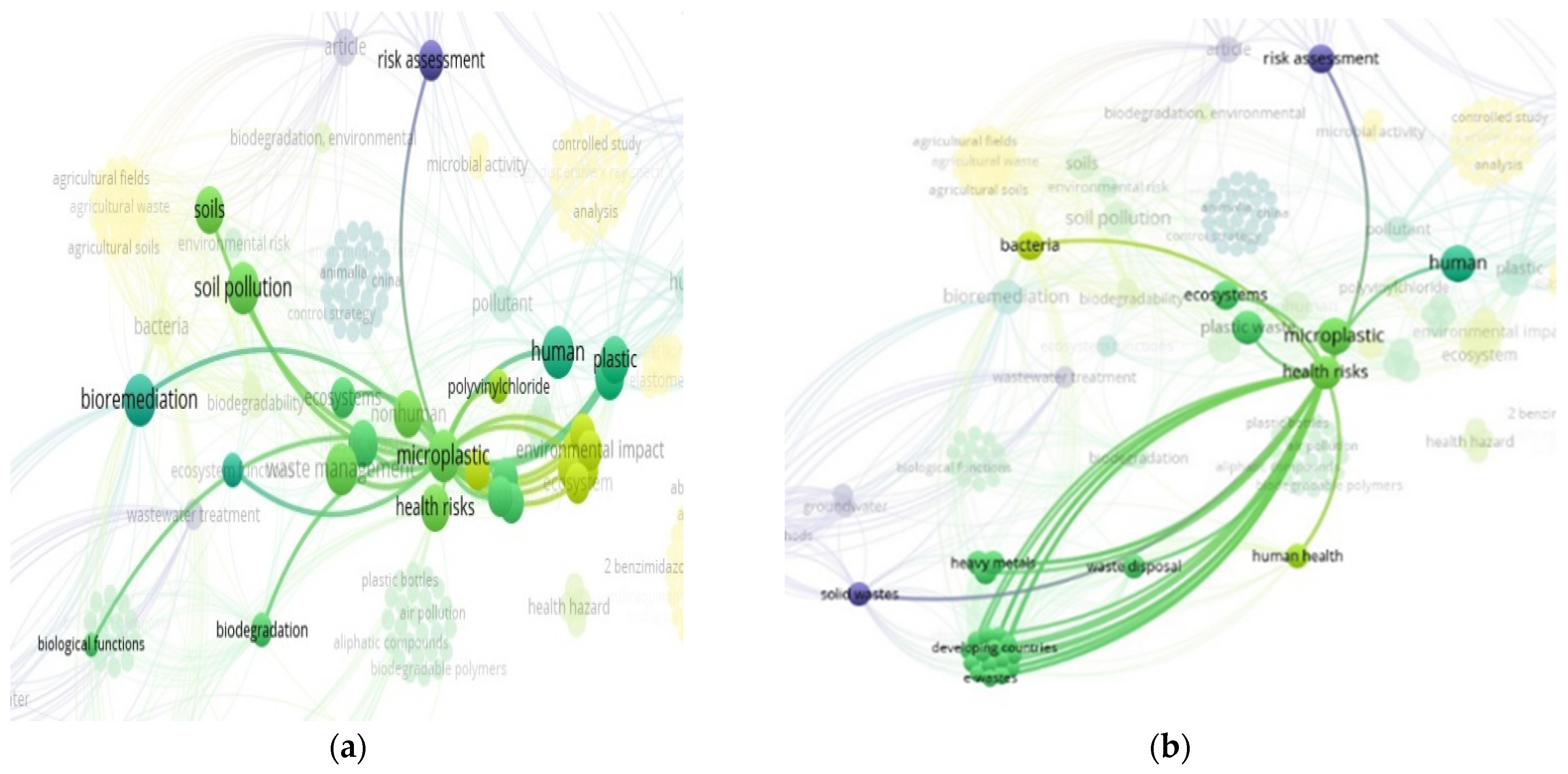
3.10. “Soil” and “Food Web” and “Organic Matter Recycling” and “Microbes” and “Bioremediation” and “Plastics” Interactions Based on the Scientific Literature Search Query on Scopus
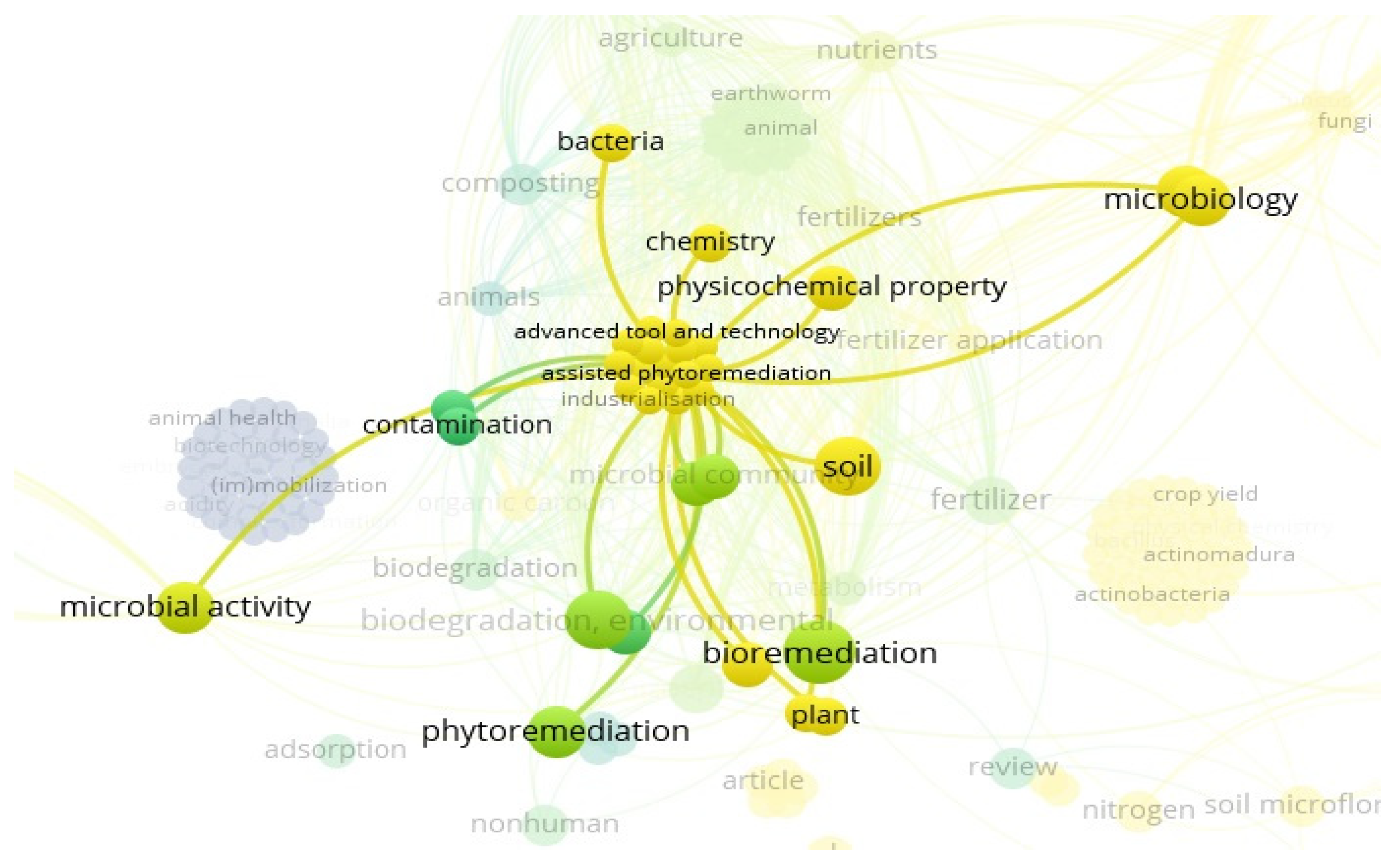
3.11. “Soil” and “Food Web” and “Organic Matter Recycling” and “Microbes” and “Bioremediation” and “Biofertilizer” Interactions Based on the Scientific Literature Search Query on Scopus
3.12. “Soil” and “Food Web” and “Organic Matter Recycling” and “Microbes” and “Bioremediation” and “Coal” Interactions Based on the Scientific Literature Search Query on Scopus
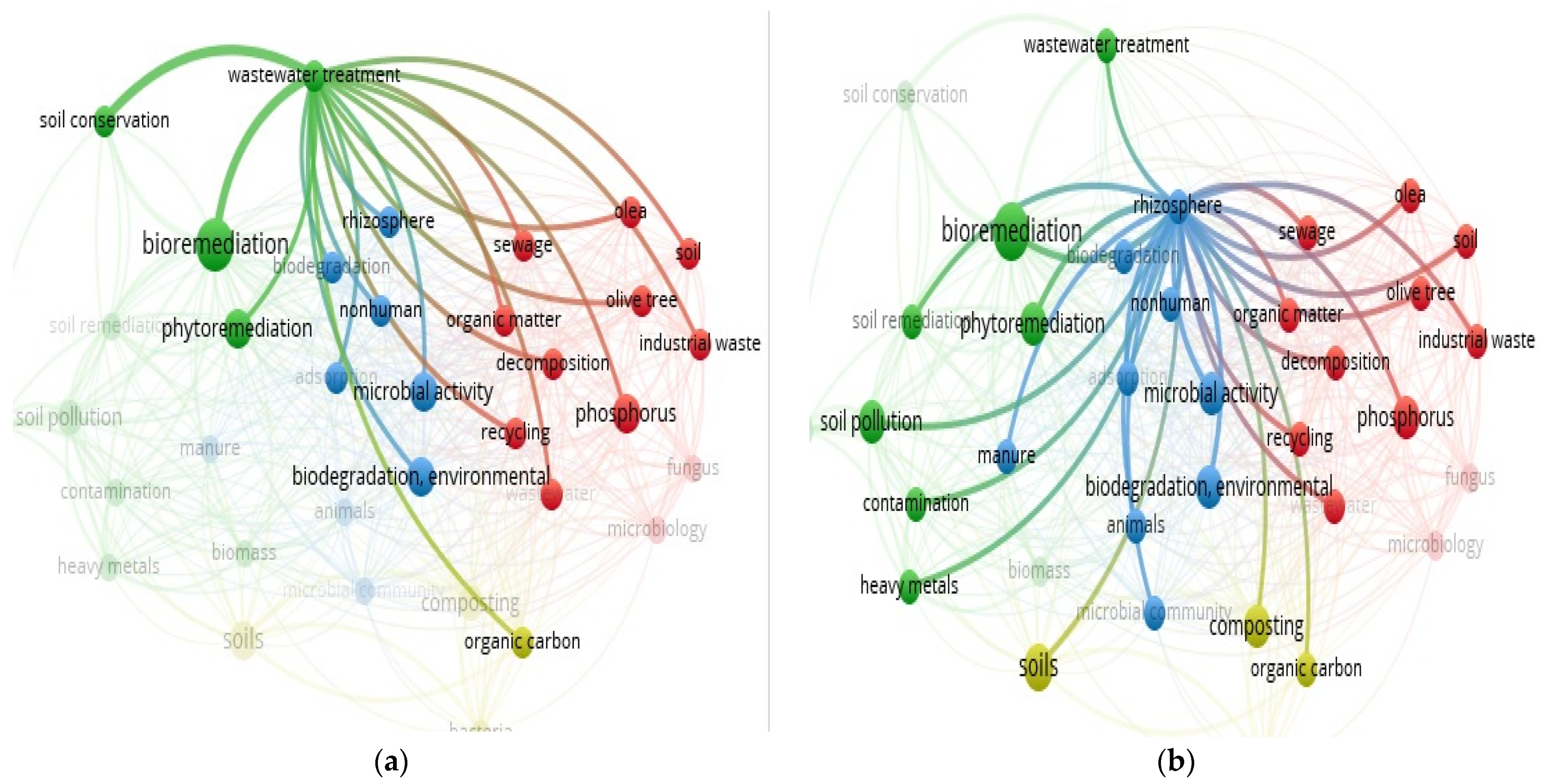
3.13. “Soil” and “Food Web” and “Organic Matter Recycling” and “Microbes” and “Bioremediation” and “Olive Mill Waste Water” Interactions Based on the Scientific Literature Search Query on Scopus
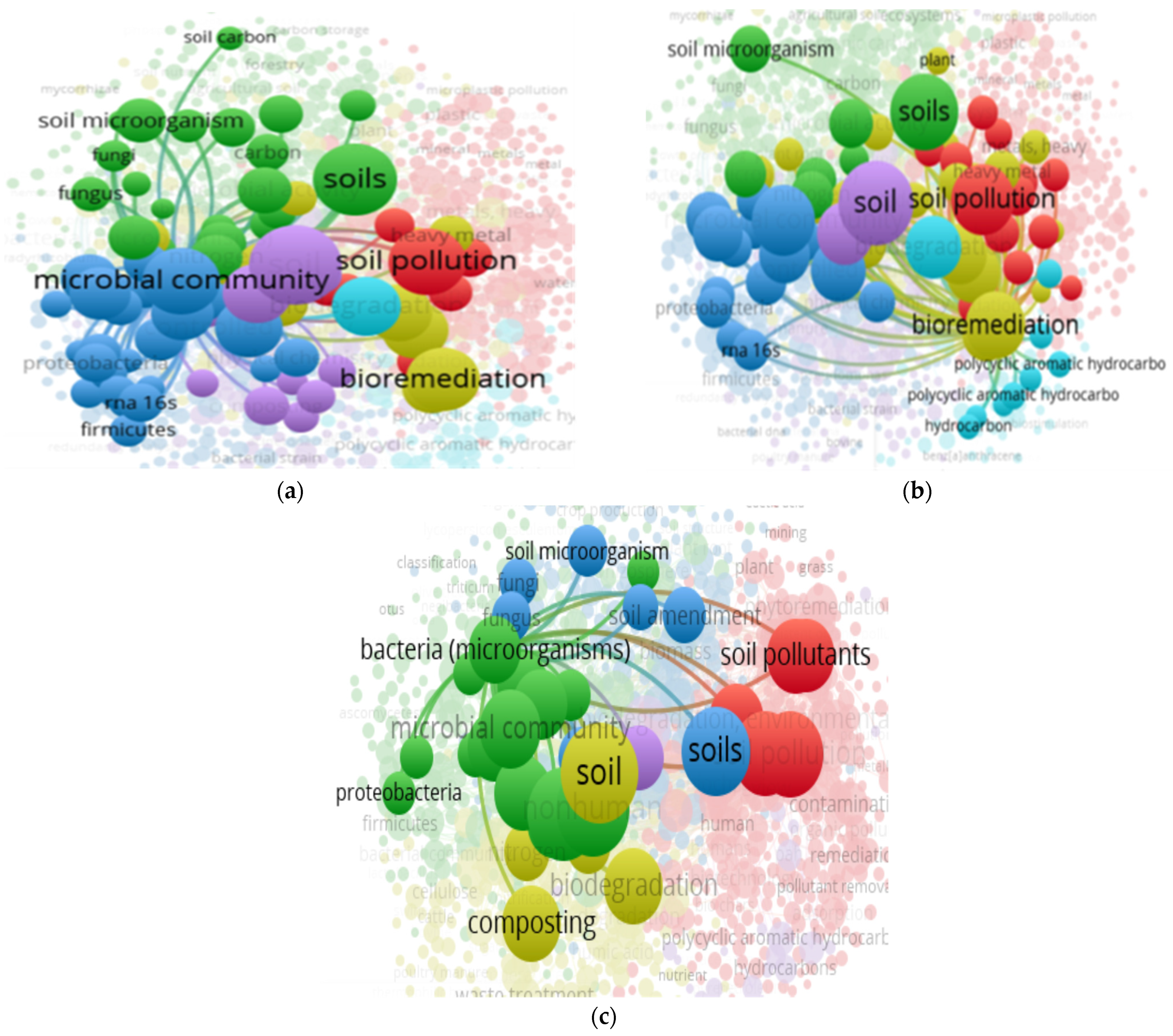
3.14. “Soil” and “Food Web” and “Organic Matter Recycling” and “Microbes” and “Biodegradation” Interactions Based on the Scientific Literature Search Query on Scopus
3.15. “Soil” and “Food Web” and “Organic Matter Recycling” and “Microbes” and “Biodegradation” and “Compost” Interactions Based on the Scientific Literature Search Query on Scopus
3.16. “Soil” and “Food Web” and “Organic Matter Recycling” and “Microbes” and “Biodegradiation” and “Olive Mill Waste Water” Interactions Based on the Scientific Literature Search Query on Scopus
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ebi, K.L.; Mearns, L.O.; Nyenzi, B. Weather and climate: Changing human exposures. In Climate Change and Human Health. Risks and Responses; McMichael, A.J., Campbell-Lendrum, D.H., Corvalán, C.F., Ebi, K.L., Githeko, A.K., Scheraga, J.D., Woodward, A., Eds.; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- Riedy, C. Climate change. In Blackwell Encyclopedia of Sociology; Ritzer, G., Ed.; Blackwell: Hoboken, NJ, USA, 2016. [Google Scholar]
- Royal Society. Climate Change. Evidence and Causes. An Overview from the Royal Society and the US National Academy of Sciences; National Academy of Sciences and Royal Society: Washington, DC, USA, 2020. [Google Scholar]
- Ghosh, P. Climate change education. Curr. Sci. 2016, 110, 1887. [Google Scholar]
- Thuiller, W. Climate change and the ecologist. Nature 2007, 448, 550–552. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, S.; Zougmoré, R.; Wollenberg, E.; Thornton, P.; Nelson, G.; Kristjanson, P.; Kinyangi, J.; Jarvis, A.; Hansen, J.; Challinor, A.; et al. Climate change, agriculture and food security: A global partnership to link research and action for low-income agricultural producers and consumers. Curr. Opin. Environ. Sustain. 2012, 4, 128–133. [Google Scholar] [CrossRef]
- Frieler, K.; Levermann, A.; Elliott, J.; Heinke, J.; Arneth, A.; Bierkens, M.F.P.; Ciais, P.; Clark, D.B.; Deryng, D.; Döll, P.; et al. A framework for the cross-sectoral integration of multi-model impact projections: Land use decisions under climate impacts uncertainties. Earth Syst. Dyn. 2015, 6, 447–460. [Google Scholar] [CrossRef]
- Rosenzweig, C.; Elliott, J.; Deryng, D.; Ruane, A.C.; Müller, C.; Arneth, A.; Boote, K.J.; Folberth, C.; Glotter, M.; Khabarov, N.; et al. Assessing agricultural risks of climate change in the 21st century in a global gridded crop model intercomparison. Proc. Natl. Acad. Sci. USA 2014, 111, 3268–3273. [Google Scholar] [CrossRef]
- Falco, C.; Donzelli, F.; Olper, A. Climate change, agriculture and migration: A Survey. Sustainability 2018, 10, 1405. [Google Scholar] [CrossRef]
- Gowdy, J. Our hunter-gatherer future: Climate change, agriculture and uncivilization. Futures 2020, 15, 102488. [Google Scholar] [CrossRef]
- McMichael, A.J.; Campbell-Lendrum, D.H.; Corvalán, C.F.; Ebi, K.L.; Githeko, A.K.; Scheraga, J.D.; Woodward, A. Climate Change and Human Health. Risks and Responses; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- Inoue, Y. Satellite and drone-based remote sensing of crops and soils for smart farming—A review. Soil Sci. Plant Nutr. 2020, 66, 798–810. [Google Scholar] [CrossRef]
- Friha, O.; Ferrag, M.A.; Shu, L.; Maglaras, L.A.; Wang, X. Internet of things for the future of smart agriculture: A comprehensive survey of emerging technologies. IEEE CAA J. Autom. Sin. 2021, 8, 718–752. [Google Scholar] [CrossRef]
- FAO. Climate Change and Food Security: Risks and Responses; Food and Agriculture Organization of the United Nations: Rome, Italy, 2015; Available online: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwif5tS48sn9AhVVRPEDHa1gBGYQFnoECAsQAQ&url=https%3A%2F%2Fwww.fao.org%2F3%2Fi5188e%2FI5188E.pdf&usg=AOvVaw1_SU62-xkdqLasBc8LmOGx (accessed on 15 December 2022).
- National Research Council. Climate Change, Evidence, Impacts and Choices; National Academy of Sciences: Washington, DC, USA, 2012. [Google Scholar]
- Anatolioti, V.; Leontopoulos, S.; Skoufogianni, G.; Skenderidis, P. A study on the potential use of energy crops as alternative cultivation in Greece. Issues of farmer’s attitudes. In Proceedings of the 4th International Conference on Food and Biosystems Engineering, Crete Island, Greece, 30 May–19 June 2019; pp. 410–445. [Google Scholar]
- Leontopoulos, S.; Arabatzis, G. The contribution of energy crops to biomass production. In Low Carbon Energy Technologies in Sustainable Energy Systems; Kyriakopoulos, D., Ed.; Elsevier: London, UK, 2020; pp. 47–94. [Google Scholar]
- IPCC. Climate change 2014: Synthesis Report. In Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Core Writing Team, Pachauri, R.K., Meyer, L.A., Eds.; IPCC: Geneva, Switzerland, 2014; p. 151. [Google Scholar]
- Uleberg, E.; Hanssen-Bauer, I.; van Oort, B.; Dalmannsdottir, S. Impact of climate change on agriculture in Northern Norway and potential strategies for adaptation. Clim. Chang. 2014, 122, 27–39. [Google Scholar] [CrossRef]
- Svobodová, E.; Trnka, M.; Dubrovský, M.; Semerádová, D.; Eitzinger, J.; Stěpánek, P.; Zalud, Z. Determination of areas with the most significant shift in persistence of pests in Europe under climate change. Pest Manag. Sci. 2014, 70, 708–715. [Google Scholar] [CrossRef] [PubMed]
- Cressman, K. Climate change and locusts in the WANA Region. In Climate Change and Food Security in West Asia and North Africa; Sivakumar, M.V.K., Lal, R., Selvaraju, M.R., Hamdan, I., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 131–143. [Google Scholar]
- Hannukkala, A.O.; Kaukoranta, T.; Lehtinen, A.; Rahkonen, A. Late-blight epidemics on potato in Finland, 1933-2002; increased and earlier occurrence of epidemics associated with climate change and lack of rotation. Plant Pathol. 2007, 56, 167–176. [Google Scholar] [CrossRef]
- Leontopoulos, S.V.; Vagelas, I.K.; Gravanis, F.T. Estimate the emergence of Pectinophora gossypiella Saunders. (Lepidoptera: Gelechiidae) with degree days in the region of Thessaly, Greece. J. Agric. Sci. Technol. 2011, 1, 182–186. [Google Scholar]
- Baker, M.B.; Venugopal, P.D.; Lamp, W.O. Climate change and phenology: Empoasca fabae (Hemiptera: Cicadellidae) migration and severity of impact. PLoS ONE 2015, 10, e0124915. [Google Scholar] [CrossRef]
- Luedeling, E.; Steinmann, K.P.; Zhang, M.; Brown, P.H.; Grant, J.; Girvetz, E.H. Climate change effects on walnut pests in California. Glob. Change Biol. 2011, 17, 228–238. [Google Scholar] [CrossRef]
- Ghini, R.; Hamada, E.; Pedro, M.L.; Marengo, J.A.; Gonçalves, R.R.V. Risk analysis of climate change on coffee nematodes and leaf miner in Brazil. Pesqui. Agropecuária Bras. 2008, 43, 187–195. [Google Scholar] [CrossRef]
- Leontopoulos, S.V.; Petrotos, K.; Anatolioti, V.; Skenderidis, P.; Tsilfoglou, S.; Vagelas, I. Chemotactic responses of Pseudomonas oryzihabitans and second stage juveniles of Meloidogyne javanica on tomato root tip exudates. Int. J. Food Biosyst. Eng. 2017, 5, 75–100. [Google Scholar]
- Leontopoulos, S.; Petrotos, K.; Anatolioti, V.; Skenderidis, P.; Tsilfoglou, S.; Papaioannou, C.; Kokkora, M.; Vagelas, I. Preliminary studies on mobility and root colonization ability of Pseudomonas oryzihabitans. Int. J. Food Biosyst. Eng. 2017, 3, 73–89. [Google Scholar]
- Luck, J.; Spackman, M.; Freeman, A.; Trebicki, P.; Griffiths, W.; Finlay, K.; Chakraborty, S. Climate change and diseases of food crops. Plant Pathol. 2011, 60, 113–121. [Google Scholar] [CrossRef]
- Pautasso, M.; Döring, T.F.; Garbelotto, M.; Pellis, L.; Jeger, M.J. Impacts of climate change on plant diseases-opinions and trends. Eur. J. Plant Pathol. 2012, 133, 295–313. [Google Scholar] [CrossRef]
- Porter, J.R.; Xie, L.; Challinor, A.J.; Cochrane, K.; Howden, S.M.; Iqbal, M.M.; Lobell, D.B.; Travasso, M.I. Food security and food production systems. In Climate Change 2014: Impacts, Adaptation, and Vulnerability. Part A: Global and Sectoral Aspects; Field, C.B., Barros, V.R., Dokken, D.J., Mach, K.J., Mastrandrea, M.D., Bilir, T.E., Chatterjee, M., Ebi, K.L., Estrada, Y.O., Genova, R.C., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2014; pp. 485–533. [Google Scholar]
- Lobell, D.B.; Schlenker, W.; Costa-Roberts, J. Climate trends and global crop production since 1980. Science 2011, 333, 616–620. [Google Scholar] [PubMed]
- Pearce, D.W.; Cline, W.R.; Achanta, A.N.; Fankhauser, S.; Pachauri, R.K.; Tol, R.S.J.; Vellinga, P. The Social costs of climate change: Greenhouse damage and the benefits of control. In Climate Change 1995: Economic and Social Dimension; Bruce, J.P., Lee, H., Haites, E.F., Eds.; Cambridge University Press: Cambridge, UK, 1996; pp. 179–224. [Google Scholar]
- Fankhauser, S.; Tol, R.S.J. Climate change costs—Recent advancements in the economic assessment. Energy Policy 1996, 24, 665–673. [Google Scholar] [CrossRef]
- Nordhaus, W.D.; Boyer, J.G. Warming the World: Economic Models of Global Warming; MIT Press: Cambridge, UK, 1999. [Google Scholar]
- Zirnov, S.; Mardon, A.; Johnson, P. Climate Change. 2021. Available online: https://www.academia.edu/81278489/Climate_Change (accessed on 10 October 2022).
- Tol, R.S.J. On the difference in impact of two almost identical climate change scenarios. Energy Policy 1998, 26, 13–20. [Google Scholar] [CrossRef]
- Mendelsohn, R.O.; Morrison, W.N.; Schlesinger, M.E.; Andronova, N.G. Country-specific market impacts of climate change. Clim. Change 1998, 45, 553–569. [Google Scholar] [CrossRef]
- Tol, R.S.J. Estimates of the damage costs of climate change—Part 1: Benchmark Estimates. Environ. Resour. Econ. 2002, 21, 47–73. [Google Scholar] [CrossRef]
- Schneider, S.H. Abrupt Non-Linear Climate Change, Irreversibility and Surprise; (ENV/EPOC/GSP(2003)13/FINAL); OECD: Paris, France, 2003. [Google Scholar]
- Mahlman, J.D. Uncertainties in projections of human-caused climate warming. Science 1997, 278, 1416–1417. [Google Scholar] [CrossRef]
- Wigley, T. Modelling Climate Change under No-Policy and Policy Emissions Pathways; (ENV/EPOC/GSP(2003)7/FINAL); OECD: Paris, France, 2003. [Google Scholar]
- Mendelsohn, R.O.; Schlesinger, M.E. Climate-response functions. Ambio 1999, 28, 362–366. [Google Scholar]
- Smith, J.; Hitz, S. Background Paper: Estimating Global Impacts from Climate Change; (ENV/EPOC/GSP (2002)12/FINAL); OECD: Paris, France, 2003. [Google Scholar]
- Tol, R.S.J.; Downing, T.E.; Kuik, O.J.; Smith, J.B. Improving Information for Policy Makers Distributional Aspects of Climate Change Impacts. Climate Change. Working Party on Global and Structural Policies OECD. Workshop on the Benefits of Climate Policy. 2003. Available online: https://www.oecd.org/env/cc/2483223.pdf (accessed on 22 February 2023).
- Filho, W.L.; Nagy, G.J.; Setti, A.F.F.; Sharifi, A.; Donkor, F.K.; Batista, K.; Djekic, I. Handling the impacts of climate change on soil biodiversity. Sci. Total Environ. 2023, 869, 161671. [Google Scholar] [CrossRef]
- FAO. Global Forest Resources Assessment 2020: Main Report; Food and Agriculture Organization of the United Nations: Rome, Italy. [CrossRef]
- Makipaa, R.; Abramoff, R.; Adamczyk, B.; Baldy, V.; Biryol, C.; Bosela, M.; Casals, P.; Yuste, J.C.; Dondini, M.; Filipek, S.; et al. How does management affect soil C sequestration and greenhouse gas fluxes in boreal and temperate forests?—A review. For. Ecol. Manag. 2023, 529, 120637. [Google Scholar]
- Lal, R. Soil carbon sequestration impacts on global climate change and food security. Science 2004, 304, 1623–1627. [Google Scholar] [CrossRef]
- Rovai, A.S.; Twilley, R.R.; Castaneda-Moya, E.; Riul, P.; Cifuentes-Jara, M.; Manrow-Villalobos, M.; Horta, P.A.; Simonassi, J.C.; Fonseca, A.; Pagliosa, P.R. Global controls on carbon storage in mangrove soils. Nat. Clim. Chang. 2018, 8, 534–538. [Google Scholar] [CrossRef]
- Wang, Q.; Le Noe, J.; Li, Q.; Lan, T.; Gao, X.; Deng, O.; Li, Y. Incorporating agricultural practices in digital mapping improves prediction of cropland soil organic carbon content: The case of the Tuojiang River Basin. J. Environ. Manag. 2023, 330, 117203. [Google Scholar] [CrossRef] [PubMed]
- Soong, J.L.; Fuchslueger, L.; Marañon-Jimenez, S.; Torn, M.S.; Janssens, I.A.; Penuelas, J.; Richter, A. Microbial carbon limitation: The need for integrating microorganisms into our understanding of ecosystem carbon cycling. Glob. Change Biol. 2020, 26, 1953–1961. [Google Scholar] [CrossRef]
- Rosinger, C.; Rousk, J.; Sanden, H. Can enzymatic stoichiometry be used to determine growth-limiting nutrients for microorganisms?—A critical assessment in two subtropical soils. Soil Biol. Biochem. 2019, 128, 115–126. [Google Scholar] [CrossRef]
- Lu, W.; Ren, H.; Ding, W.; Li, H.; Yao, X.; Jiang, X. The effects of climate warming on microbe-mediated mechanisms of sediment carbon emission. J. Environ. Sci. 2023, 129, 16–29. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Chen, L.; Zhang, B.; Liu, L. Linking temperature sensitivity of soil CO2 release to substrate, environmental, and microbial properties across alpine ecosystems. Glob. Biogeochem. Cycles 2016, 30, 1310–1323. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, Y.; Guo, X.; Ning, D.; Zhou, X.; Feng, J. Reduction of microbial diversity in grassland soil is driven by long-term climate warming. Nat. Microbiol. 2022, 7, 1054–1062. [Google Scholar]
- Yang, Y.; Cheng, S.; Fang, H.; Guo, Y.; Li, Y.; Zhou, Y. Interactions between soil organic matter chemical structure and microbial communities determine the spatial variation of soil basal respiration in boreal forests. Appl. Soil Ecol. 2023, 183, 10474. [Google Scholar]
- Bond-Lamberty, B.; Bailey, V.L.; Chen, M.; Gough, C.M.; Vargas, R. Globally rising soil heterotrophic respiration over recent decades. Nature 2018, 560, 80–83. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, S.; Tian, P. Carbon quality and soil microbial property control the latitudinal pattern in temperature sensitivity of soil microbial respiration across Chinese forest ecosystems. Glob. Chang. Biol. 2018, 24, 2841–2849. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Giaramida, L.; Reich, P.B.; Khachane, A.N.; Hamonts, K.; Edwards, C.; Lawton, L.A.; Singh, B.K. Lack of functional redundancy in the relationship between microbial diversity and ecosystem functioning. J. Ecol. 2016, 104, 936–946. [Google Scholar] [CrossRef]
- Liu, Y.R.; Delgado-Baquerizo, M.; Wang, J.T.; Hu, H.W.; Yang, Z.; He, J.Z. New insights into the role of microbial community composition in driving soil respiration rates. Soil Biol. Biochem. 2018, 118, 35–45. [Google Scholar] [CrossRef]
- Neurauter, M.; Yuan, M.; Hicks, L.C.; Rousk, J. Soil microbial resource limitation along a subarctic ecotone from birch forest to tundra heath. Soil Biol. Biochem. 2023, 177, 108919. [Google Scholar] [CrossRef]
- Ziman, J.M. An Introduction to Science Studies: The Philosophical and Social Aspects of Science and Technology; Cambridge University Press: Cambridge, UK, 1984; ISBN 0-521-34680-0. [Google Scholar]
- van Raan, A.F.J. On the growth, aging and fractal differentiation of science. Scientometrics 2000, 47, 347–362. [Google Scholar] [CrossRef]
- Price, D.J.D. Little Science, Big Science; Columbia University Press: New York, NY, USA, 1963. [Google Scholar]
- Noyons, E.C.M. Bibliometric Mapping as a Science Policy and Research Management Tool; DSWO Press: Leiden, The Netherlands, 1999; Available online: https://hdl.handle.net/1887/38308 (accessed on 10 December 2022).
- Fellnhofer, K. Toward a taxonomy of entrepreneurship education research literature: A bibliometric mapping and visualization. Educ. Res. Rev. 2019, 27, 28–55. [Google Scholar] [CrossRef]
- Braun, T. Handbook of Quantitative Science and Technology Research. The Use of Publication and Patent Statistics in Studies of S & T Systems; Springer: Dordrecht, The Netherlands, 2005. [Google Scholar]
- van Leeuwen, T. Descriptive Versus Evaluative Bibliometrics, Handbook of Quantitative Science and Technology Research; Springer: Dordrecht, The Netherlands, 2004. [Google Scholar]
- Börner, K.; Chen, C.; Boyack, K.W. Visualizing knowledge domains. Annu. Rev. Inf. Sci. Technol. 2003, 37, 179–255. [Google Scholar] [CrossRef]
- Cronin, B. Bibliometrics and beyond: Some thoughts on web-based citation analysis. J. Inf. Sci. 2001, 27, 1–7. [Google Scholar] [CrossRef]
- Ding, Y. Scientific collaboration and endorsement: Network analysis of co-authorship and citation networks. J. Informetr. 2011, 5, 187–203. [Google Scholar] [CrossRef]
- Moya-Anegón, F.; Vargas-Quesada, B.; Herrero-Solana, V.; Chinchilla-Rodríguez, Z.; Corera-Álvarez, E.; Munoz-Fernández, F.J. A new technique for building maps of large scientific domains based on the cocitation of classes and categories. Scientometrics 2004, 61, 129–145. [Google Scholar] [CrossRef]
- Velasco-Muñoz, J.F.; Aznar-Sánchez, J.A.; Belmonte-Ureña, L.J.; López-Serrano, M.J. Advances in water use efficiency in agriculture: A bibliometric analysis. Water 2018, 10, 377. [Google Scholar] [CrossRef]
- Sweileh, W.M. Bibliometric analysis of peer-reviewed literature on food security in the context of climate change from 1980 to 2019. Agric. Food Secur. 2020, 9, 11. [Google Scholar] [CrossRef]
- Fusco, G. Twenty years of common agricultural policy in Europe: A bibliometric analysis. Sustainability 2021, 13, 10650. [Google Scholar]
- Pan, X.; Lv, J.; Dyck, M.; He, H. Bibliometric analysis of soil nutrient research between 1992 and 2020. Agriculture 2021, 11, 223. [Google Scholar] [CrossRef]
- Papadopoulou, C.I.; Loizou, E.; Melfou, K.; Chatzitheodoridis, F. The knowledge based agricultural bioeconomy: A bibliometric network analysis. Energies 2021, 14, 6823. [Google Scholar] [CrossRef]
- Rejeb, A.; Abdollahi, A.; Rejeb, K.; Treiblmaier, H. Drones in agriculture: A review and bibliometric analysis. Comput. Electron. Agric. 2022, 198, 107017. [Google Scholar] [CrossRef]
- Mühl, D.D.; de Oliveira, L. A bibliometric and thematic approach to agriculture 4.0. Heliyon 2022, 8, 09369. [Google Scholar] [CrossRef] [PubMed]
- Quastel, J.H. Soil metabolism. Proc. Royal Soc. Lond. Ser. B Biol. Sci. 1955, 911, 159–178. [Google Scholar] [CrossRef]
- Xie, Z.; Yu, Z.; Li, Y.; Wang, G.; Liu, X.; Tang, C.; Lian, T.; Adams, J.; Liu, J.; Liu, J.; et al. Soil microbial metabolism on carbon and nitrogen transformation links the crop-residue contribution to soil organic carbon. NPJ Biofilms Microbiomes 2022, 8, 14. [Google Scholar] [CrossRef]
- García-Orenes, F.; Morugán-Coronado, A.; Zornoza, R.; Scow, K.M. Changes in soil microbial community structure influenced by agricultural management practices in a Mediterranean agro-ecosystem. PLoS ONE 2013, 8, e80522. [Google Scholar] [CrossRef]
- Jansson, J.K.; Hofmockel, K.S. Soil microbiomes and climate change. Nat. Rev. Microbiol. 2019, 18, 35–46. [Google Scholar] [CrossRef]
- Ibrahim, A.D.; Uhuami, A.O.; Abdulkadir, N.; Uzoh, I.M. Climate change alters microbial communities. In Soil Biology, Climate Change and the Microbiome; Choudhary, D.K., Mishra, A., Varma, A., Eds.; Springer: Cham, Switzerland, 2021; p. 63. [Google Scholar]
- Wahdan, S.F.; Hossen, S.; Tanunchai, B.; Sansupa, C.; Schädler, M.; Noll, M.; Dawoud, T.M.; Wu, Y.; Buscot, F.; Purahong, W. Life in the wheat litter: Effects of future climate on microbiome and function during the early phase of decomposition. Microb. Ecol. 2021, 84, 90–105. [Google Scholar] [CrossRef] [PubMed]
- Cotrufo, M.; Galdo, I.; Piermatteo, D. Litter decomposition: Concepts, methods and future perspectives. In Soil Carbon Dynamics: An Integrated Methodology; Kutsch, W., Bahn, M., Heinemeyer, A., Eds.; Cambridge University Press: Cambridge, UK, 2010; pp. 76–90. [Google Scholar]
- Wu, X.; Niklas, K.; Sun, S. Climate change affects detritus decomposition rates by modifying arthropod performance and species interactions. Curr. Opin. Insect Sci. 2021, 47, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Stuble, K.L.; Ma, S.; Liang, J.; Luo, Y.; Classen, A.T.; Souza, L. Long-term impacts of warming drive decomposition and accelerate the turnover of labile, not recalcitrant, carbon. Ecosphere 2019, 10, 2715. [Google Scholar] [CrossRef]
- Sharma, B.; Singh, B.N.; Dwivedi, P.; Rajawat, M.V. Interference of climate change on plant-microbe interaction: Present and future prospects. Front. Agron. 2022, 3, 725804. [Google Scholar] [CrossRef]
- Cavicchioli, R.; Ripple, W.J.; Timmis, K.N.; Azam, F.; Bakken, L.R.; Baylis, M.; Behrenfeld, M.J.; Boetius, A.; Boyd, P.W.; Classen, A.T.; et al. Scientists’ warning to humanity: Microorganisms and climate change. Nat. Rev. Microbiol. 2019, 17, 569–586. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, G.; Chen, Q.S.; Han, X. Soil bacterial communities respond to climate changes in a temperate steppe. PLoS ONE 2013, 8, 78616. [Google Scholar] [CrossRef]
- Trumbore, S.; Brando, P.; Hartmann, H. Forest health and global change. Science 2015, 349, 814–818. [Google Scholar] [CrossRef]
- Lesk, C.; Rowhani, P.; Ramankutty, N. Influence of extreme weather disasters on global crop production. Nature 2016, 529, 84. [Google Scholar] [CrossRef]
- Glassman, S.I.; Weihe, C.; Li, J.; Albright, M.B.; Looby, C.I.; Martiny, A.C.; Treseder, K.K.; Allison, S.D.; Martiny, J.B. Decomposition responses to climate depend on microbial community composition. Proc. Natl. Acad. Sci. USA 2018, 115, 11994–11999. [Google Scholar] [CrossRef]
- Bouwer, E.J.; Zehnder, A.J. Bioremediation of organic compounds--putting microbial metabolism to work. Trends Biotechnol. 1993, 11, 360–367. [Google Scholar] [CrossRef]
- Bala, S.; Garg, D.; Thirumalesh, B.V.; Sharma, M.; Sridhar, K.; Inbaraj, B.S.; Tripathi, M. Recent strategies for bioremediation of emerging pollutants: A review for a green and sustainable environment. Toxics 2022, 10, 484. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, S.; Poudel, D.K.; Marahatha, R.; Dawadi, S.; Khadayat, K.; Phuyal, S.R.; Shrestha, S.; Gaire, S.; Basnet, K.; Khadka, U.; et al. Microbial enzymes used in bioremediation. J. Chem. 2021, 2021, 8849512. [Google Scholar] [CrossRef]
- Alkorta, I.; Epelde, L.; Garbisu, C. Environmental parameters altered by climate change affect the activity of soil microorganisms involved in bioremediation. FEMS Microbiol. Lett. 2017, 364, 19. [Google Scholar] [CrossRef] [PubMed]
- Abatenh, E.; Gizaw, B.; Tsegaye, Z.; Wassie, M. The role of microorganisms in bioremediation. A review. Open J. Environ. Biol. 2017, 2, 38–46. [Google Scholar] [CrossRef]
- Boughattas, I.; Hattab, S.; Alphonse, V.; Livet, A.; Giusti-Miller, S.; Boussetta, H.; Banni, M.; Bousserrhine, N. Use of earthworms Eisenia andrei on the bioremediation of contaminated area in north of Tunisia and microbial soil enzymes as bioindicator of change on heavy metals speciation. J. Soils Sediments 2018, 19, 296–309. [Google Scholar] [CrossRef]
- Pandey, J.; Sarkar, S.; Pandey, V.C. Compost-assisted phytoremediation. In Assisted Phytoremediation; Elsevier: Amsterdam, The Netherlands, 2022; pp. 243–264. [Google Scholar]
- Berti, W.R.; Cunningham, S.D. Phytostabilization of metals. In Phytoremediation of Toxic Metals: Using Plants to Clean-Up the Environment; Raskin, I., Ensley, B.D., Eds.; John Wiley & Sons, Inc.: New York, NY, USA, 2010; pp. 71–88. [Google Scholar]
- Yan, A.; Wang, Y.; Tan, S.N.; Mohd Yusof, M.L.; Ghosh, S.; Chen, Z. Phytoremediation: A promising approach for revegetation of heavy metal-polluted land. Front. Plant Sci. 2020, 11, 359. [Google Scholar] [CrossRef] [PubMed]
- Poonam, A.; Bhardwaj, R.; Sharma, R.C.; Handa, N.; Kaur, H.; Kaur, R.; Sirhindi, G.; Thukral, A.K. Prospects of field crops for phytoremediation of contaminants. Emerg. Technol. Manag. Crop Stress Toler. 2014, 2, 449–470. [Google Scholar]
- Guo, M.; Song, W.; Tian, J. Biochar-facilitated soil remediation: Mechanisms and efficacy variations. Front. Environ. Sci. 2020, 8, 521512. [Google Scholar] [CrossRef]
- Alsafran, M.; Usman, K.; Ahmed, B.; Rizwan, M.; Saleem, M.H.; Al jabri, H. Understanding the phytoremediation mechanisms of potentially toxic elements: A proteomic overview of recent advances. Front. Plant Sci. 2022, 13, 881242. [Google Scholar] [CrossRef]
- Joseph, S.; Cowie, A.L.; van Zwieten, L.; Bolan, N.S.; Budai, A.; Buss, W.; Cayuela, M.; Graber, E.R.; Ippolito, J.A.; Kuzyakov, Y.; et al. How biochar works, and when it doesn’t: A review of mechanisms controlling soil and plant responses to biochar. GCB Bioenergy 2021, 13, 1731–1764. [Google Scholar] [CrossRef]
- He, D.; Luo, Y.; Lu, S.; Liu, M.; Song, Y.; Lei, L. Microplastics in soils: Analytical methods, pollution characteristics and ecological risks. TrAC Trends Anal. Chem. 2018, 109, 163–172. [Google Scholar] [CrossRef]
- Rabani, M.S.; Hameed, I.; Mir, T.A.; Gupta, M.K.; Habib, A.; Jan, M.; Hussain, H.; Tripathi, S.; Pathak, A.; Ahad, M.B.; et al. Microbial-assisted phytoremediation. In Phytoremediation; Elsevier: Amsterdam, The Netherlands, 2022; pp. 91–114. [Google Scholar]
- Coninx, L.; Martinová, V.; Rineau, F. Mycorrhiza-assisted phytoremediation. Adv. Bot. Res. 2017, 83, 127–188. [Google Scholar]
- Lebrun, M.; Nandillon, R.; Miard, F.; Bourgerie, S.; Morabito, D. Biochar assisted phytoremediation for metal(loid) contaminated soils. In Assisted Phytoremediation; Elsevier: Amsterdam, The Netherlands, 2022; pp. 101–130. [Google Scholar]
- Ranđelović, D.; Jakovljević, K.; Zeremski, T. Chelate-assisted phytoremediation. In Assisted Phytoremediation; Elsevier: Amsterdam, The Netherlands, 2022; pp. 131–154. [Google Scholar]
- Ali, A.; Guo, D.; Mahar, A.; Ma, F.; Li, R.; Shen, F.; Wang, P.; Zhang, Z. Streptomyces pactum assisted phytoremediation in Zn/Pb smelter contaminated soil of Feng County and its impact on enzymatic activities. Sci. Rep. 2017, 7, 46087. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.; Dumat, C.; Khalid, S.; Niazi, N.K.; Antunes, P.M. Cadmium bioavailability, uptake, toxicity and detoxification in soil-plant system. Rev. Environ. Contam. Toxicol. 2017, 241, 73–137. [Google Scholar] [PubMed]
- O’Connor, D.; Zheng, X.; Hou, D.; Shen, Z.; Li, G.; Miao, G.; O’Connell, S.; Guo, M. Phytoremediation: Climate change resilience and sustainability assessment at a coastal brownfield redevelopment. Environ. Int. 2019, 130, 104945. [Google Scholar] [CrossRef] [PubMed]
- Sharma, I. Bioremediation Techniques for Polluted Environment: Concept, Advantages, Limitations, and Prospects. In Trace Metals in the Environment—New Approaches and Recent Advances; IntechOpen: London, UK, 2021. [Google Scholar]
- Cassano, A.; Conidi, C.; Galanakis, C.M.; Castro-Muñoz, R. Recovery of polyphenols from olive mill wastewaters by membrane operations. In Membrane Technologies for Biorefining; Elsevier: Amsterdam, The Netherlands, 2016; pp. 163–187. [Google Scholar]
- Sakarika, M.; Koutra, E.; Tsafrakidou, P.; Terpou, A.; Kornaros, M.E. Microalgae-based remediation of wastewaters. In Microalgae Cultivation for Biofuels Production; Elsevier: Amsterdam, The Netherlands, 2020; pp. 317–335. [Google Scholar]
- Leontopoulos, S.; Skenderidis, P.; Vagelas, I.K. Potential use of polyphenolic compounds obtained from Olive Mill Waste Waters on plant pathogens and plant parasitic nematodes. In Plant Defence: Biological Control; Ramawat, K.G., Ed.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 137–177. [Google Scholar]
- Skenderidis, P.; Leontopoulos, S.; Petrotos, K.; Mitsagga, C.; Giavasis, I. The in vitro and in vivo synergistic antimicrobial activity assessment of Vacuum Microwave Assisted aqueous extracts from pomegranate and avocado fruit peels and avocado seeds based on a mixtures design model. Plants 2021, 10, 1757. [Google Scholar] [CrossRef] [PubMed]
- Skenderidis, P.; Leontopoulos, S.; Petrotos, K.; Giavasis, I. Vacuum Microwave-Assisted aqueous extraction of polyphenolic compounds from avocado (Persea americana) solid waste. Sustainability 2021, 13, 2166. [Google Scholar] [CrossRef]
- Skenderidis, P.; Leontopoulos, S. Goji berry: Important bioactive ingredients: A mini review. Biomed. J. Sci. Tech. Res. 2022, 41, 34125–34128. [Google Scholar]
- Michalopoulos, G.K.; Kasapi, A.; Koubouris, G.; Psarras, G.; Arampatzis, G.; Hatzigiannakis, E.; Kavvadias, V.; Xiloyannis, C.; Montanaro, G.; Malliaraki, S.; et al. Adaptation of Mediterranean olive groves to climate change through sustainable cultivation practices. Climate 2020, 8, 54. [Google Scholar] [CrossRef]
- Kavvadias, V.; Papadopoulou, M.; Vavoulidou, E.; Theocharopoulos, S.; Koubouris, G.; Psarras, G.; Manolaraki, C.; Giakoumaki, G.; Vasiliadis, A. Effect of sustainable management of olive tree residues on soil fertility in irrigated and rain-fed olive orchards. J. Water Clim. Chang. 2018, 9, 764–774. [Google Scholar] [CrossRef]
- Morianou, G.; Kourgialas, N.; Psarras, G.; Koubouris, G. Mapping sensitivity to desertification in Crete (Greece), the risk for agricultural areas. J. Water Clim. Change 2018, 9, 691–702. [Google Scholar] [CrossRef]
- Meftah, O.; Guergue, Z.; Braham, M.; Sayadi, S.; Mekki, A. Long term effects of olive mill wastewaters application on soil properties and phenolic compounds migration under arid climate. Agric. Water Manag. 2019, 212, 119–125. [Google Scholar] [CrossRef]
- Sierra, J.; Marti, E.; Garau, M.A.; Cruanas, R. Effects of the agronomic use of olive oil mill wastewater: Field experiment. Sci. Total Environ. 2007, 378, 90–94. [Google Scholar] [CrossRef]
- Leontopoulos, S.V.; Giavasis, I.; Petrotos, K.; Kokkora, M.; Makridis, C. Effect of different formulations of polyphenolic compounds obtained from OMWW on the growth of several fungal plant and food borne pathogens. Studies in vitro and in vivo. Agric. Agric. Sci. Procedia 2015, 4, 327–337. [Google Scholar] [CrossRef]
- Leontopoulos, S.V.; Kokkora, M.I.; Petrotos, K.B. In vivo evaluation of liquid polyphenols obtained from OMWW as natural bio-chemicals against several fungal pathogens on tomato plants. Desalination Water Treat. 2016, 57, 20646–20660. [Google Scholar]
- Leontopoulos, S.; Mitsagga, C.; Giavasis, I.; Papaioannou, C.; Vasilakoglou, I.; Petrotos, K. Potential synergistic action of liquid olive Fruit polyphenol extract with aqueous extracts of solid wastes of pomegranate or/and orange juice industry as organic phyto-protective agents against important plant pathogens—Part 1 (in vitro Studies). Univ. J. Agric. Res. 2020, 8, 202–222. [Google Scholar] [CrossRef]
- Leontopoulos, S.; Petrotos, K.; Papaioannou, C.; Vasilakoglou, I. Effectiveness of olive fruit polyphenol extract combined with aqueous extracts of solid wastes of pomegranate or/and orange juice against important plant pathogens—Part 2 (in vivo studies). Univ. J. Agric. Res. 2020, 9, 23–38. [Google Scholar] [CrossRef]
- Leontopoulos, S.; Skenderidis, P.; Skoufogianni, G. Potential use of medicinal plants as biological crop protection agents. Biomed. J. Sci. Tech. Res. 2020, 25, 19320–19324. [Google Scholar] [CrossRef]
- Lambakis, D.; Skenderidis, P.; Leontopoulos, S. Technologies and extraction methods of polyphenolic compounds derived from pomegranate (Punica granatum) peels. A mini review. Processes 2021, 9, 236. [Google Scholar] [CrossRef]
- Leontopoulos, S.; Skenderidis, P.; Petrotos, K.; Mitsagga, C.; Giavasis, I. Preliminary studies on suppression of important plant pathogens by using pomegranate and avocado residual peel and seed extracts. Horticulturae 2022, 8, 283. [Google Scholar] [CrossRef]
- Sbaoui, Y.; Ezaouine, A.; Toumi, M.; Farkas, R.; Kbaich, M.A.; Habbane, M.; El Mouttaqui, S.; Kadiri, F.Z.; El Messal, M.; Tóth, E.; et al. Effect of climate on bacterial and archaeal diversity of Moroccan marine microbiota. Microorganisms 2022, 10, 1622. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Xu, P.; Zeng, G.; Yang, C.; Huang, D.; Zhang, J. Bioremediation of soils contaminated with polycyclic aromatic hydrocarbons, petroleum, pesticides, chlorophenols and heavy metals by composting: Applications, microbes and future research needs. Biotechnol. Adv. 2015, 33, 745–755. [Google Scholar] [CrossRef]
- Ahmed, P.M.; Fernandez, P.M.; de Figuero, L.I.C.; Pajot, H.F. Exploitation alternatives of olive mill wastewater: Production of value-added compounds useful for industry and agriculture. Biofuel Res. J. 2019, 22, 980–994. [Google Scholar] [CrossRef]
- Kefalogianni, I.; Skiada, V.; Tsagou, V.; Efthymiou, A.; Xexakis, K.; Chatzipavlidis, I. Co-composting of cotton residues with olive mill wastewater: Process monitoring and evaluation of the diversity of culturable microbial populations. Environ. Monit. Assess. 2021, 193, 641. [Google Scholar] [CrossRef]
- Elmansour, T.E.; Mandi, L.; Hejjaj, A.; Ouazzani, N. Nutrients’ behavior and removal in an activated sludge system receiving Olive Mill Wastewater. J. Environ. Manag. 2022, 305, 114254. [Google Scholar] [CrossRef]
- Papadimitriou, C.A.; Rouse, J.D.; Karapanagioti, H.K. Treatment efficiency and sludge characteristics in conventional and suspended PVA gel beads activated sludge treating phenol containing wastewater. Glob. NEST J. 2018, 20, 42–48. [Google Scholar]
- Uygur, A.; Kargi, F. Phenol inhibition of biological nutrient removal in a four-step sequencing batch reactor. Process Biochem. 2004, 39, 2123–2128. [Google Scholar] [CrossRef]
- Munir, N.; Hanif, M.; Abideen, Z.; Sohail, M.; El-Keblawy, A.A.; Radicetti, E.; Mancinelli, R.; Haider, G. Mechanisms and strategies of plant microbiome interactions to mitigate abiotic stresses. Agronomy 2022, 12, 2069. [Google Scholar] [CrossRef]
- Kopittke, P.M.; Menzies, N.W.; Wang, P.; McKenna, B.A.; Lombi, E. Soil and the intensification of agriculture for global food security. Environ. Int. 2019, 132, 105078. [Google Scholar] [CrossRef]
- Harrison, S.P. Plant community diversity will decline more than increase under climatic warming. Philos. Trans. R. Soc. B 2020, 375, 20190106. [Google Scholar] [CrossRef] [PubMed]
- Balser, T.C.; Gutknecht, J.L.M.; Liang, C. How will climate change impact soil microbial communities? In Soil Microbiology and Sustainable Crop Production; Dixon, G., Tilston, E., Eds.; Springer: Dordrecht, The Netherlands, 2010. [Google Scholar]
- Montealegre, C.M.; van Kessel, C.; Russelle, M.P.; Sadowsky, M.J. Changes in microbial activity and composition in a pasture ecosystem exposed to elevated atmospheric carbon dioxide. Plant Soil 2004, 243, 197–207. [Google Scholar] [CrossRef]
- Lützow, M.V.; Kögel-Knabner, I. Temperature sensitivity of soil organic matter decomposition-what do we know? Biol. Fertil. Soils 2009, 46, 1–15. [Google Scholar] [CrossRef]
- Pendall, E.; Bridgham, S.D.; Hanson, P.J.; Hungate, B.A.; Kicklighter, D.W.; Johnson, D.W.; Law, B.E.; Luo, Y.; Megonigal, J.P.; Olsrud, M.; et al. Below-ground process responses to elevated CO2 and temperature: A discussion of observations, measurement methods, and models. New Phytol. 2004, 162, 311–322. [Google Scholar] [CrossRef]
- Zogg, G.P.; Zak, D.R.; Ringelberg, D.B.; White, D.C.; MacDonald, N.W.; Pregitzer, K.S. Compositional and functional shifts in microbial communities due to soil warming. Soil Sci. Soc. Am. J. 1997, 61, 475–481. [Google Scholar] [CrossRef]
- Gray, S.B.; Classen, A.T.; Kardol, P.; Yermakov, Z.; Mille, R.M. Multiple climate change factors interact to alter soil microbial community structure in an old-field ecosystem. Soil Sci. Soc. Am. J. 2011, 75, 2217–2226. [Google Scholar] [CrossRef]
- Jensen, K.D.; Beier, C.; Michelsen, A.; Emmett, B.A. Effects of experimental drought on microbial processes in two temperate heathlands at contrasting water conditions. Appl. Soil Ecol. 2003, 24, 165–176. [Google Scholar] [CrossRef]
- Drenovsky, R.E.; Vo, D.H.; Graham, K.J.; Scow, K.M. Soil water content and organic carbon availability are major determinants of soil microbial community composition. Microb. Ecol. 2003, 48, 424–430. [Google Scholar] [CrossRef]
- Bastida, F.; Eldridge, D.J.; García, C.; Kenny Png, G.; Bardgett, R.D.; Delgado-Baquerizo, M. Soil microbial diversity–biomass relationships are driven by soil carbon content across global biomes. ISME J. 2021, 15, 2081–2091. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vagelas, I.; Leontopoulos, S. A Bibliometric Analysis and a Citation Mapping Process for the Role of Soil Recycled Organic Matter and Microbe Interaction due to Climate Change Using Scopus Database. AgriEngineering 2023, 5, 581-610. https://doi.org/10.3390/agriengineering5010037
Vagelas I, Leontopoulos S. A Bibliometric Analysis and a Citation Mapping Process for the Role of Soil Recycled Organic Matter and Microbe Interaction due to Climate Change Using Scopus Database. AgriEngineering. 2023; 5(1):581-610. https://doi.org/10.3390/agriengineering5010037
Chicago/Turabian StyleVagelas, Ioannis, and Stefanos Leontopoulos. 2023. "A Bibliometric Analysis and a Citation Mapping Process for the Role of Soil Recycled Organic Matter and Microbe Interaction due to Climate Change Using Scopus Database" AgriEngineering 5, no. 1: 581-610. https://doi.org/10.3390/agriengineering5010037






