Drying of Gymnema sylvestre Using Far-Infrared Radiation: Antioxidant Activity and Optimization of Drying Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material Preparation
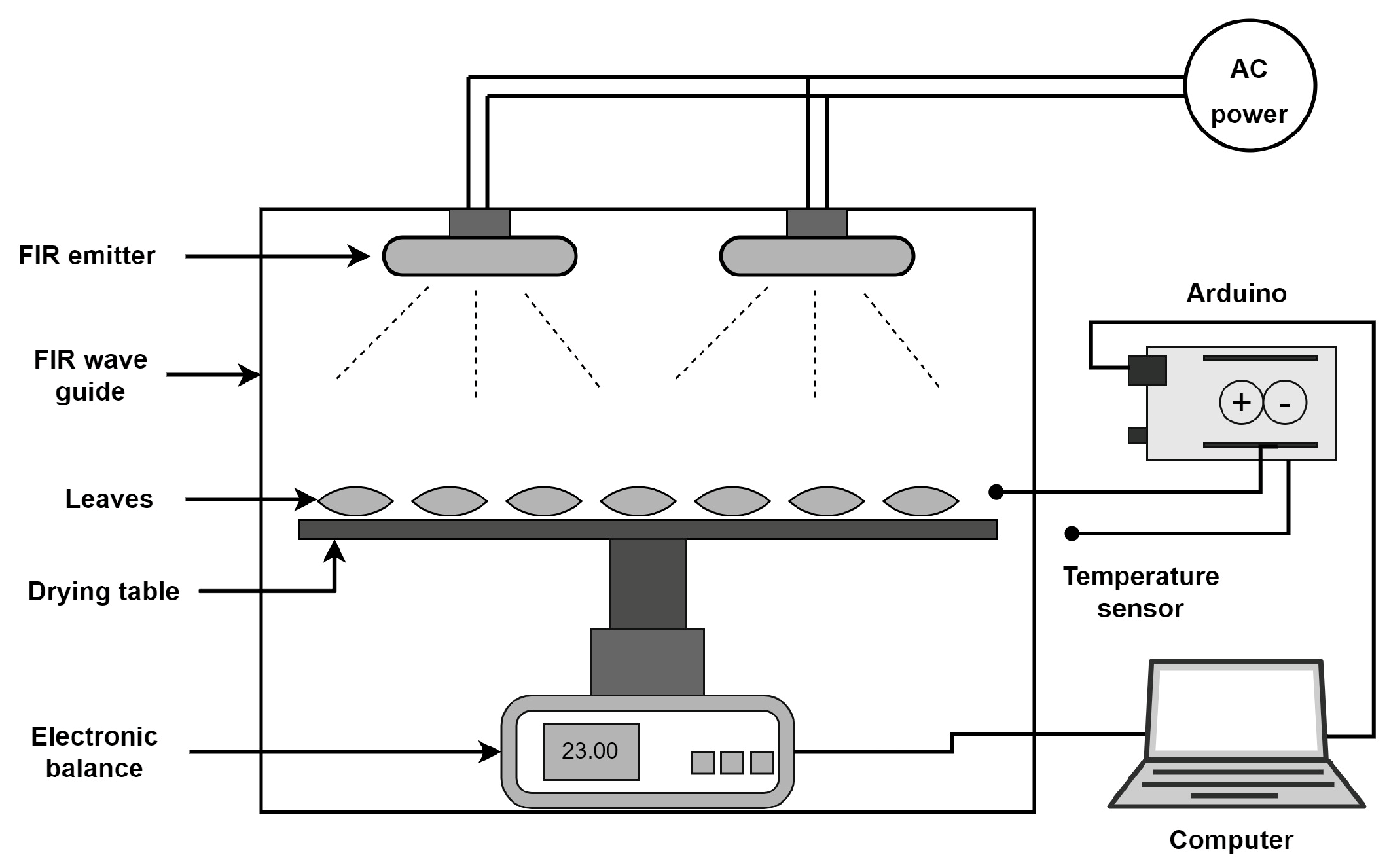
2.2. Drying Setup and Treatments
2.3. Drying Kinetics
2.4. Specific Energy Consumption
2.5. Color Measurement
2.6. Water-Ethanol Extract of the Leaves
2.7. Total Phenolic Content
2.8. DPPH Radical Scavenging Assay
2.9. FRAP (Ferric Reducing Antioxidant Power) Assay
2.10. Optimization of Drying Conditions
2.11. Data Analysis
3. Results and Discussion
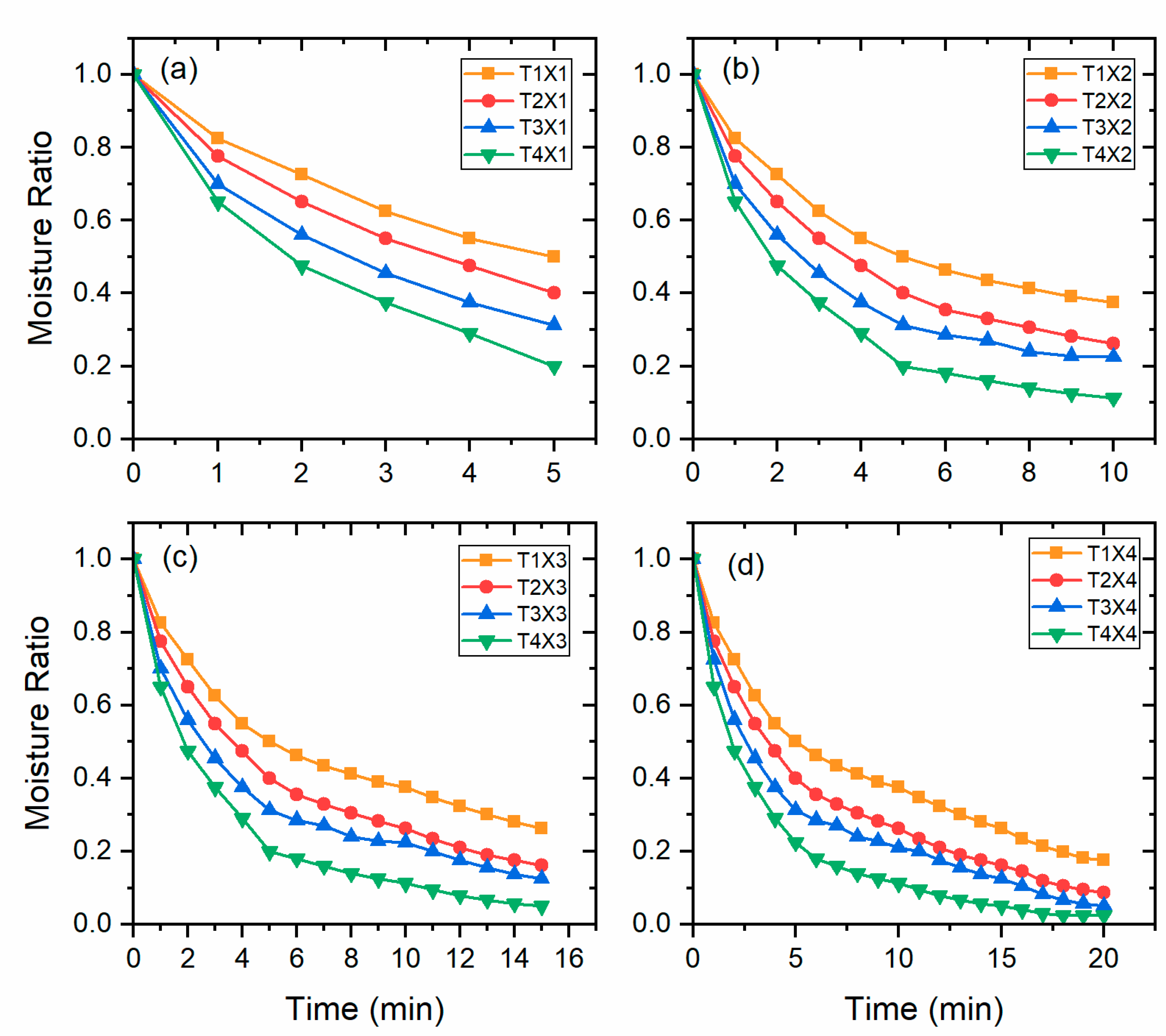
3.1. Drying Kinetics
3.2. Specific Energy Consumption (SEC)
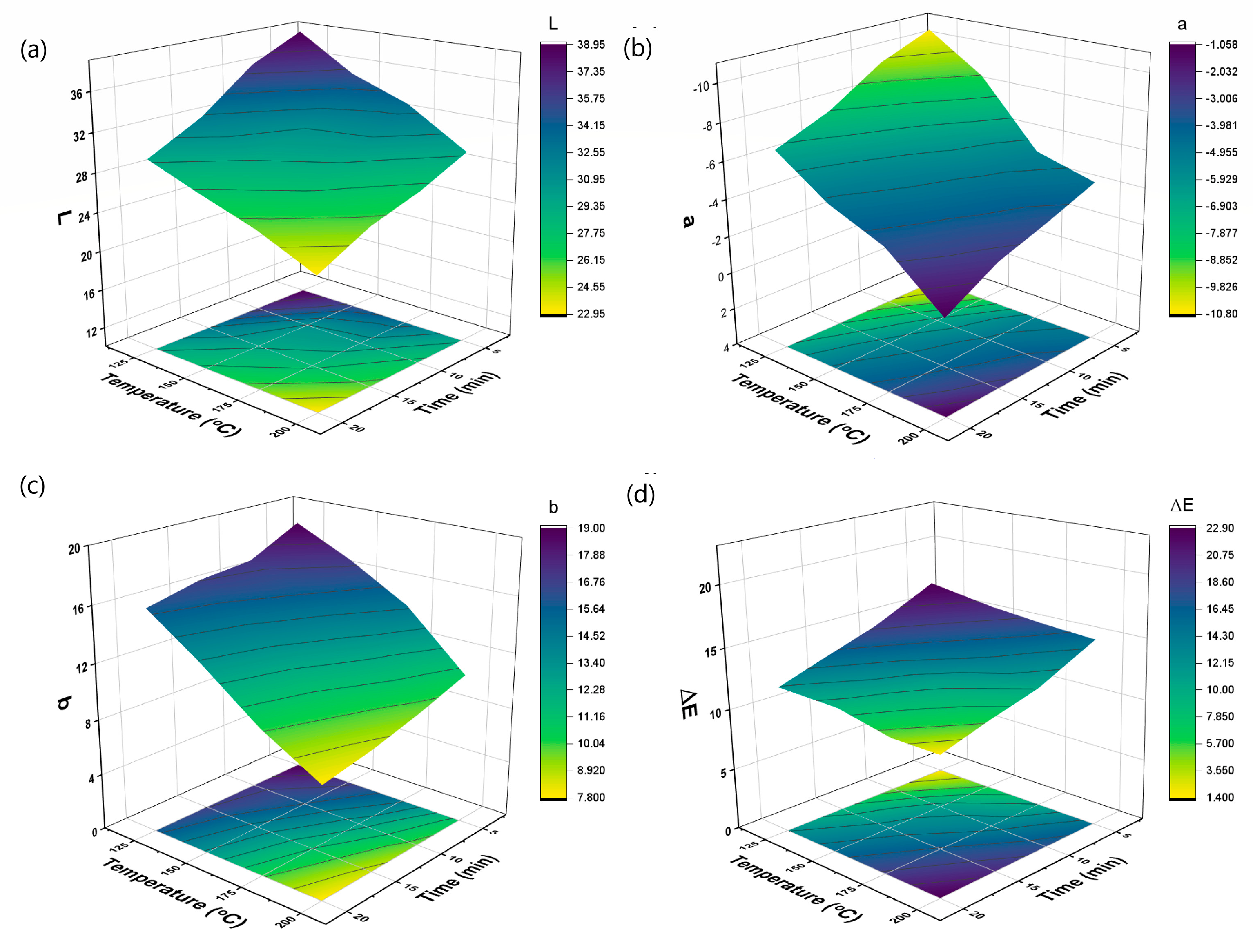
3.3. Color Changes
3.4. Total Phenolic Content (TPC)
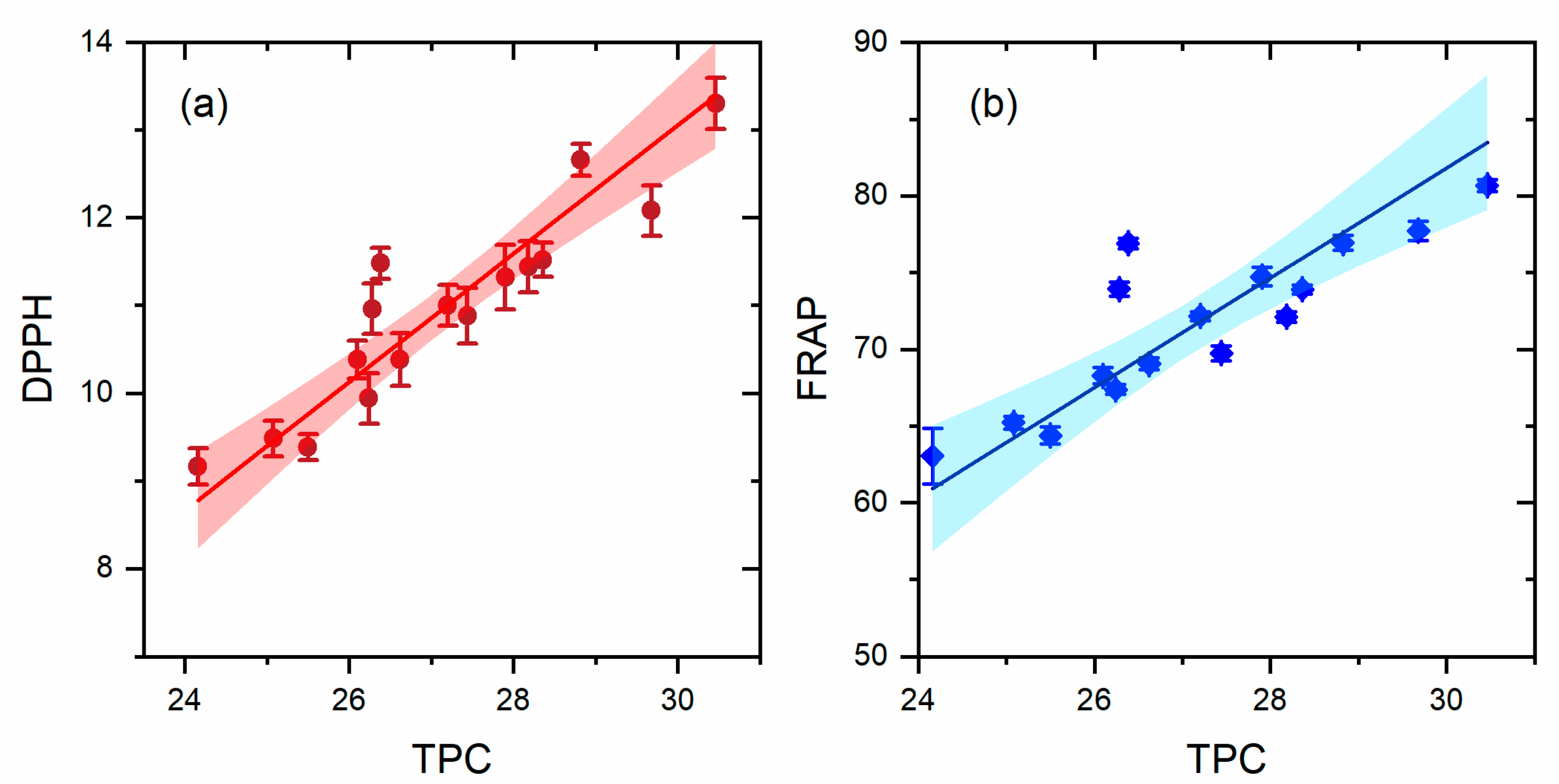
3.5. Antioxidant Activity
3.6. Optimization of Drying Conditions
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Saneja, A.; Sharma, C.; Aneja, K.; Pahwa, R. Gymnema sylvestre (Gurmar): A review. Der Pharm. Lett. 2010, 2, 275–284. [Google Scholar]
- Tiwari, P.; Mishra, B.; Sangwan, N.S. Phytochemical and pharmacological properties of Gymnema sylvestre: An important medicinal plant. Biomed Res. Int. 2014, 2014, 830285. [Google Scholar] [CrossRef] [Green Version]
- Chung, I.-M.; Rajakumar, G.; Subramanian, U.; Venkidasamy, B.; Thiruvengadam, M. Impact of copper oxide nanoparticles on enhancement of bioactive compounds using cell suspension cultures of Gymnema sylvestre (Retz.) R. Br. Appl. Sci. 2019, 9, 2165. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, A.R.; Alsayari, A.; Habib, A.H.; Wahab, S.; Nadig, A.P.; Rafeeq, M.M.; Binothman, N.; Aljadani, M.; Al-Dhuayan, I.S.; Alaqeel, N.K. Anti-Tumor Potential of Gymnema sylvestre Saponin Rich Fraction on In Vitro Breast Cancer Cell Lines and In Vivo Tumor-Bearing Mouse Models. Antioxidants 2023, 12, 134. [Google Scholar] [CrossRef]
- Parke, A.; Parke, D. The pathogenesis of inflammatory disease: Surgical shock and multiple system organ failure. Inflammopharmacology 1995, 3, 149–168. [Google Scholar] [CrossRef] [Green Version]
- Pham-Huy, L.A.; He, H.; Pham-Huy, C. Free radicals, antioxidants in disease and health. Int. J. Biomed. Sci. 2008, 4, 89. [Google Scholar] [PubMed]
- Batiha, G.E.-S.; Beshbishy, A.M.; Ikram, M.; Mulla, Z.S.; El-Hack, M.E.A.; Taha, A.E.; Algammal, A.M.; Elewa, Y.H.A. The Pharmacological Activity, Biochemical Properties, and Pharmacokinetics of the Major Natural Polyphenolic Flavonoid: Quercetin. Foods 2020, 9, 374. [Google Scholar] [CrossRef] [Green Version]
- Meghana, K.; Sanjeev, G.; Ramesh, B. Curcumin prevents streptozotocin-induced islet damage by scavenging free radicals: A prophylactic and protective role. Eur. J. Pharmacol. 2007, 577, 183–191. [Google Scholar] [CrossRef]
- Sakar, E.H.; Khtira, A.; Aalam, Z.; Zeroual, A.; Gagour, J.; Gharby, S. Variations in physicochemical characteristics of olive oil (cv ‘Moroccan Picholine’) according to extraction technology as revealed by multivariate analysis. Agric. Eng. 2022, 4, 922–938. [Google Scholar] [CrossRef]
- Čakar, U.; Čolović, M.; Milenković, D.; Medić, B.; Krstić, D.; Petrović, A.; Đorđević, B. Protective Effects of Fruit Wines against Hydrogen Peroxide—Induced Oxidative Stress in Rat Synaptosomes. Agronomy 2021, 11, 1414. [Google Scholar] [CrossRef]
- Rachh, P.; Patel, S.; Hirpara, H.; Rupareliya, M.; Rachh, M.; Bhargava, A.; Patel, N.; Modi, D. In vitro evaluation of antioxidant activity of Gymnema sylvestre r. br. leaf extract. Rom. J. Biol. Plant Biol. 2009, 54, 141–148. [Google Scholar]
- Singh, K.; Deo, B. Phytochemical evaluation and in vitro antioxidant activity of Gymnema sylvestre R. Br. Br. J. Med. Plants Stud. 2014, 2, 19–23. [Google Scholar]
- Rahman, M.M.; Habib, M.R.; Hasan, M.A.; Al Amin, M.; Saha, A.; Mannan, A. Comparative assessment on in vitro antioxidant activities of ethanol extracts of Averrhoa bilimbi, Gymnema sylvestre and Capsicum frutescens. Pharmacogn. Res. 2014, 6, 36. [Google Scholar]
- Spasov, A.; Samokhina, M.; Bulanov, A. Antidiabetic properties of Gymnema sylvestre (a review). Pharm. Chem. J. 2008, 42, 626–629. [Google Scholar] [CrossRef]
- Song, Y.; Tao, Y.; Zhu, X.; Han, Y.; Show, P.L.; Song, C.; Zaid, H.F.M. Ultrasound-enhanced hot air drying of germinated highland barley seeds: Drying characteristics, microstructure, and bioactive profile. Agric. Eng. 2019, 1, 496–510. [Google Scholar] [CrossRef] [Green Version]
- Pääkkönen, K.; Havento, J.; Galambosi, B. Infrared drying of herbs (Research Note). Agric. Food Sci. 1999, 8, 19–27. [Google Scholar] [CrossRef]
- Kumara, H.; Abhiram, G.; Raveendran, K.; Prematilake, K. Pre-Drying of Fermented Black Tea Leaves Using Far-Infrared. In Proceedings of the 4th International Conference of Agricultural Sciences, Belihuloya, Shri Lanka, 26-27 January 2022. [Google Scholar]
- Qiu, L.; Zhang, M.; Mujumdar, A.S.; Liu, Y. Recent developments in key processing techniques for oriental spices/herbs and condiments: A review. Food Rev. Int. 2022, 38, 1791–1811. [Google Scholar] [CrossRef]
- Abhiram, G.; Amaratunga, K.; Galahitiyawa, D.; Karunasinhe, K. Colour development and changes of the gelatinization percentage of rice flour gelatinizes by far-infrared radiation. In Proceedings of the Peradeniya University Research Sessions, Peradeniya, Sri Lanka, 11–12 November 2012; p. 88. [Google Scholar]
- Abhiram, G. The Correlation of Colour and Viscosity Changes of Rice Flour with Gelatinization Percentage under Infrared Heating. Int. J. Res. Rev. 2018, 5, 36–40. [Google Scholar]
- Sakai, N.; Hanzawa, T. Applications and advances in far-infrared heating in Japan. Trends Food Sci. Technol. 1994, 5, 357–362. [Google Scholar] [CrossRef]
- Fernando, A.J.; Gunathunga, C.; Brumm, T.; Amaratunga, S. Drying turmeric (Curcuma longa L.) using far-Infrared radiation: Drying characteristics and process optimization. J. Food Process Eng. 2021, 44, e13780. [Google Scholar] [CrossRef]
- Abhiram, G.; Manathunga, M.; Raveendran, K.; Withanage, N. Performance Analysis of High-Efficiency Motor and Variable Speed Drive in Black Tea Processing Machinery. J. Biosyst. Eng. 2020, 45, 310–317. [Google Scholar] [CrossRef]
- Sledz, M.; Wiktor, A.; Nowacka, M.; Witrowa-Rajchert, D. Drying kinetics, microstructure and antioxidant properties of basil treated by ultrasound. J. Food Process Eng. 2017, 40, e12271. [Google Scholar] [CrossRef] [Green Version]
- Khramov, V.; Spasov, A.; Samokhina, M. Chemical composition of dry extracts of Gymnema sylvestre leaves. Pharm. Chem. J. 2008, 42, 29–31. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J. [2] Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1999; Volume 299, pp. 15–27. [Google Scholar]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salarikia, A.; Miraei Ashtiani, S.H.; Golzarian, M.R. Comparison of drying characteristics and quality of peppermint leaves using different drying methods. J. Food Process. Preserv. 2017, 41, e12930. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, K.; Chen, C. Artificial Neural Network Assisted Multiobjective Optimization of Postharvest Blanching and Drying of Blueberries. Foods 2022, 11, 3347. [Google Scholar] [CrossRef]
- Therdthai, N.; Zhou, W. Characterization of microwave vacuum drying and hot air drying of mint leaves (Mentha cordifolia Opiz ex Fresen). J. Food Eng. 2009, 91, 482–489. [Google Scholar] [CrossRef]
- Hervert-Hernández, D.; Goñi, I. Dietary polyphenols and human gut microbiota: A review. Food Rev. Int. 2011, 27, 154–169. [Google Scholar] [CrossRef]
- Lee, S.-C.; Kim, J.-H.; Jeong, S.-M.; Kim, D.-R.; Ha, J.-U.; Nam, K.; Ahn, D. Effect of far-infrared radiation on the antioxidant activity of rice hulls. J. Agric. Food Chem. 2003, 51, 4400–4403. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-C.; Jeong, S.-M.; Kim, S.-Y.; Nam, K.; Ahn, D. Effect of far-infrared irradiation on the antioxidant activity of defatted sesame meal extracts. J. Agric. Food Chem. 2005, 53, 1495–1498. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-C.; Jeong, S.-M.; Kim, S.-Y.; Park, H.-R.; Nam, K.; Ahn, D. Effect of far-infrared radiation and heat treatment on the antioxidant activity of water extracts from peanut hulls. Food Chem. 2006, 94, 489–493. [Google Scholar] [CrossRef]
- Niwa, Y.; Miyachi, Y. Antioxidant action of natural health products and Chinese herbs. Inflammation 1986, 10, 79–91. [Google Scholar] [CrossRef]
- Vega-Gálvez, A.; Ah-Hen, K.; Chacana, M.; Vergara, J.; Martínez-Monzó, J.; García-Segovia, P.; Lemus-Mondaca, R.; Di Scala, K. Effect of temperature and air velocity on drying kinetics, antioxidant capacity, total phenolic content, colour, texture and microstructure of apple (var. Granny Smith) slices. Food Chem. 2012, 132, 51–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, K.; Ko, E.Y.; Assefa, A.D.; Ha, S.; Nile, S.H.; Lee, E.T.; Park, S.W. Temperature-dependent studies on the total phenolics, flavonoids, antioxidant activities, and sugar content in six onion varieties. J. Food Drug Anal. 2015, 23, 243–252. [Google Scholar] [CrossRef] [Green Version]
- Manzocco, L.; Calligaris, S.; Mastrocola, D.; Nicoli, M.C.; Lerici, C.R. Review of non-enzymatic browning and antioxidant capacity in processed foods. Trends Food Sci. Technol. 2000, 11, 340–346. [Google Scholar] [CrossRef]

| Temperature | Time | SEC | TPC | DPPH (IC50) | FRAP |
|---|---|---|---|---|---|
| (°C) | (min) | (MJ kg−1 H2O) | mg TAE/g Leaf Weight (w.b) | (mg mL−1) | μmol g−1 Leaf Weight (w.b) |
| 125 (T1) | 5 (X1) | 38.8 ± 0.8 h | 24.2 ± 0.3 j | 9.2 ± 0.2 i | 63.0 ± 1.8 i |
| 10 (X2) | 60.6 ± 2.1 e | 25.5 ± 0.4 hi | 9.4 ± 0.1 hi | 64.4 ± 0.5 hi | |
| 15 (X3) | 79.4 ± 1.1 c | 26.2 ± 0.2 g | 9.9 ± 0.3 gh | 67.4 ± 0.3 g | |
| 20 (X4) | 93.9 ± 1.1 a | 26.6 ± 0.4 fg | 10.4 ± 0.3 fg | 69.1 ± 0.4 ef | |
| 150 (T2) | 5 (X1) | 32.4 ± 1.7 i | 25.1 ± 0.3 i | 9.5 ± 0.2 hi | 65.2 ± 0.4 h |
| 10 (X2) | 52.5 ± 1.5 f | 26.1 ± 0.3 gh | 10.4 ± 0.2 fg | 68.3 ± 0.5 fg | |
| 15 (X3) | 70.2 ± 1.6 d | 27.2 ± 0.2 ef | 11 ± 0.2 de | 72.2 ± 0.3 d | |
| 20 (X4) | 85.8 ± 2.1 b | 27.9 ± 0.2 de | 11.3 ± 0.4 de | 74.7 ± 0.6 c | |
| 175 (T3) | 5 (X1) | 29.1 ± 2.1 i | 27.4 ± 0.2 e | 10.9 ± 0.3 ef | 69.7 ± 0.5 e |
| 10 (X2) | 49.8 ± 2.9 f | 28.4 ± 0.2 cd | 11.5 ± 0.2 cd | 73.9 ± 0.3 c | |
| 15 (X3) | 66.5 ± 1.6 d | 29.7 ± 0.4 b | 12.1 ± 0.3 c | 77.7 ± 0.6 b | |
| 20 (X4) | 82.1 ± 2.6 bc | 26.3 ± 0.4 g | 10.9 ± 0.3 de | 73.9 ± 0.5 c | |
| 200 (T4) | 5 (X1) | 24.5 ± 1.5 j | 28.2 ± 0.3 cd | 11.4 ± 0.3 de | 72.1 ± 0.3 d |
| 10 (X2) | 44.3 ± 2.6 g | 28.8 ± 0.3 c | 12.7 ± 0.2 b | 76.9 ± 0.5 b | |
| 15 (X3) | 61.0 ± 2.5 e | 30.5 ± 0.2 a | 13.3 ± 0.3 a | 80.7 ± 0.4 a | |
| 20 (X4) | 80.6 ± 2.2 c | 26.4 ± 0.4 g | 11.5 ± 0.2 d | 76.9 ± 0.3 b |
| Response Variable | Model | R2 |
|---|---|---|
| Specific energy consumption (SEC) | SEC = 79.7 − 0.684 Temperature + 4.682 Time + 0.001488 Temperature × Temperature − 0.0430 Time × Time + 0.00005 Temperature × Time | 99.74 |
| Total Color Change (ΔE) | ΔE = −30.89 + 0.2402 Temperature + 1.243 Time − 0.000138 Temperature × Temperature—0.00823 Time × Time − 0.002653 Temperature × Time | 99.37 |
| Total Phenolic Content (TPC) | TPC = 4.8 + 0.141 Temperature + 1.319 Time − 0.000168 Temperature × Temperature − 0.02580 Time × Time − 0.00379 Temperature × Time | 78.48 |
| 2,2-diphenyl-1-picrylhydrazyl (DPPH) | DPPH = 0.94 + 0.0451 Temperature + 0.586 Time + 0.000012 Temperature × Temperature − 0.01290 Time × Time − 0.001258 Temperature × Time | 88.74 |
| Ferric-reducing power (FRAP) | FRAP = 20.2 + 0.338 Temperature + 1.806 Time − 0.000522 Temperature × Temperature − 0.0418 Time × Time − 0.00198 Temperature × Time | 92.89 |
| Moisture Content (MC) | MC = 134.5 − 0.565 Temperature − 4.031 Time + 0.000600 Temperature × Temperature + 0.0350 Time × Time + 0.01056 Temperature × Time | 99.07 |
| p Value | ||||||
|---|---|---|---|---|---|---|
| Source | SEC | ΔE | TPC | DPPH | FRAP | MC |
| Model | <0.001 * | <0.001 * | 0.004 * | <0.001 * | <0.001 * | <0.001 * |
| Temperature (T) | <0.001 * | <0.001 * | 0.003 * | <0.001 * | <0.001 * | <0.001 * |
| Time (X) | <0.001 * | <0.001 * | 0.201 | 0.019 * | <0.001 * | <0.001 * |
| T × T | 0.021 * | 0.559 | 0.671 | 0.951 | 0.458 | 0.275 |
| X × X | 0.010 * | 0.181 | 0.023 * | 0.022 * | 0.033 * | 0.022 * |
| T × X | 0.983 | 0.016 * | 0.033 * | 0.130 | 0.481 | <0.001 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abhiram, G.; Briyangari, A.; Eeswaran, R. Drying of Gymnema sylvestre Using Far-Infrared Radiation: Antioxidant Activity and Optimization of Drying Conditions. AgriEngineering 2023, 5, 611-622. https://doi.org/10.3390/agriengineering5010038
Abhiram G, Briyangari A, Eeswaran R. Drying of Gymnema sylvestre Using Far-Infrared Radiation: Antioxidant Activity and Optimization of Drying Conditions. AgriEngineering. 2023; 5(1):611-622. https://doi.org/10.3390/agriengineering5010038
Chicago/Turabian StyleAbhiram, Gunaratnam, Abhiram Briyangari, and Rasu Eeswaran. 2023. "Drying of Gymnema sylvestre Using Far-Infrared Radiation: Antioxidant Activity and Optimization of Drying Conditions" AgriEngineering 5, no. 1: 611-622. https://doi.org/10.3390/agriengineering5010038
APA StyleAbhiram, G., Briyangari, A., & Eeswaran, R. (2023). Drying of Gymnema sylvestre Using Far-Infrared Radiation: Antioxidant Activity and Optimization of Drying Conditions. AgriEngineering, 5(1), 611-622. https://doi.org/10.3390/agriengineering5010038






