Development of a Greenhouse Wastewater Stream Utilization System for On-Site Microalgae-Based Biostimulant Production
Abstract
:1. Introduction
2. Materials and Methods
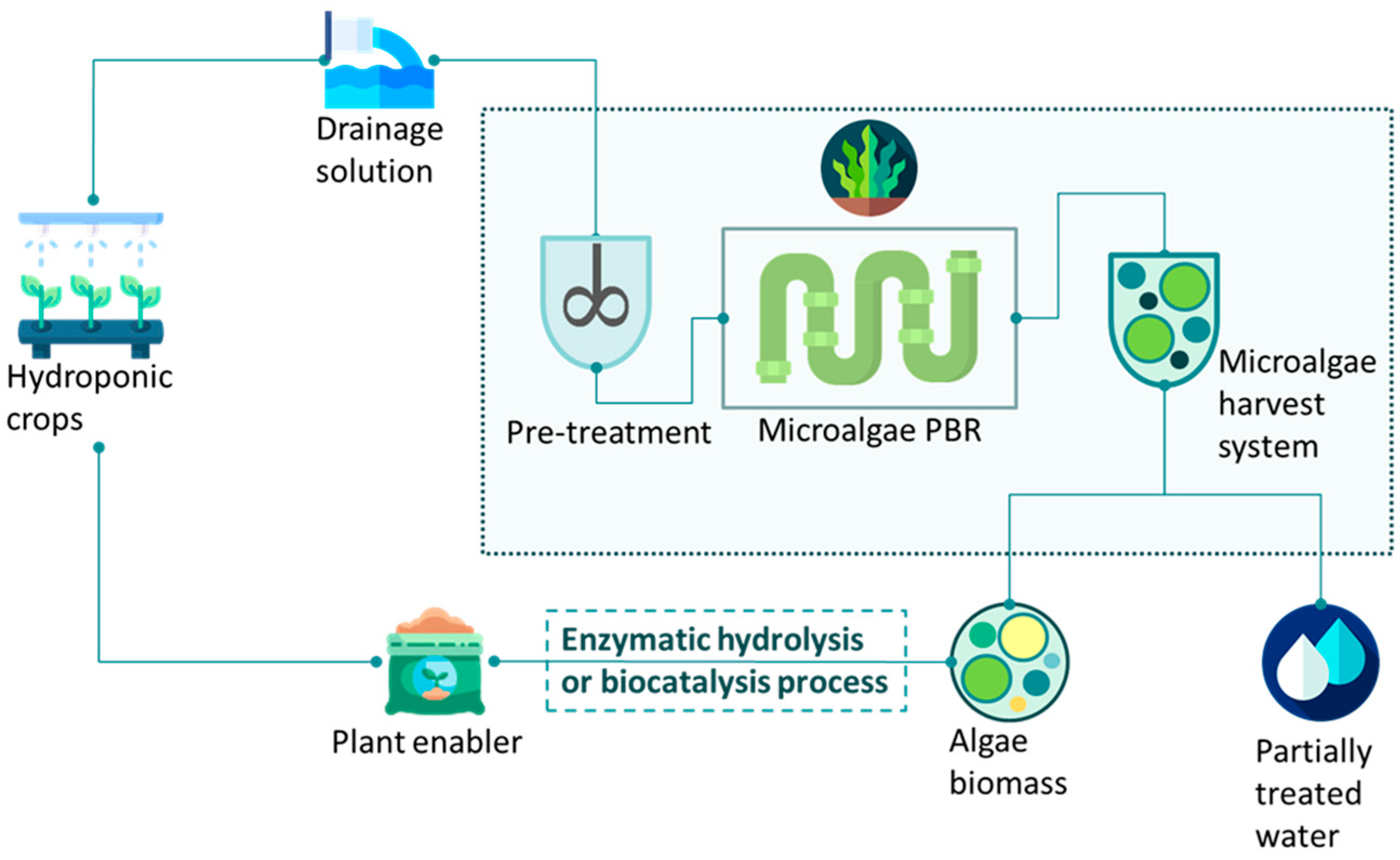
2.1. Development of an Automated Agro-Wastewater Treatment Plant
Working Phases and Algae Growth Cycles
2.2. Optimizing Sedimentation Practices and Enzyme Complexes for Biostimulant Production
2.2.1. Microalgae Cultivation and Pre-Concentration of Microalgae Biomass for Lab Biocatalysis
2.2.2. Biocatalysis Process under Laboratory Conditions
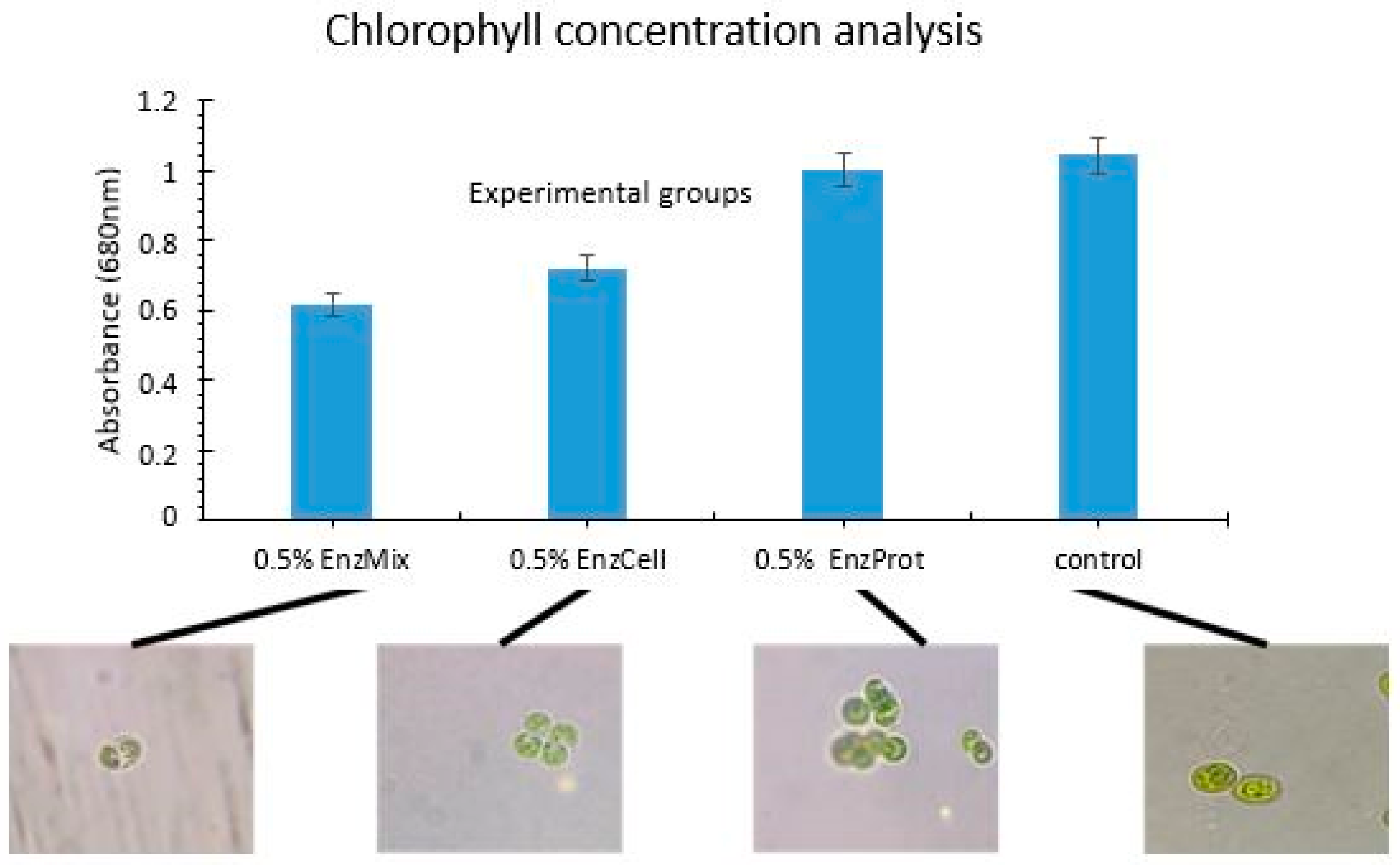
Selection of the Optimum Enzyme Complex for the Biocatalysis Process
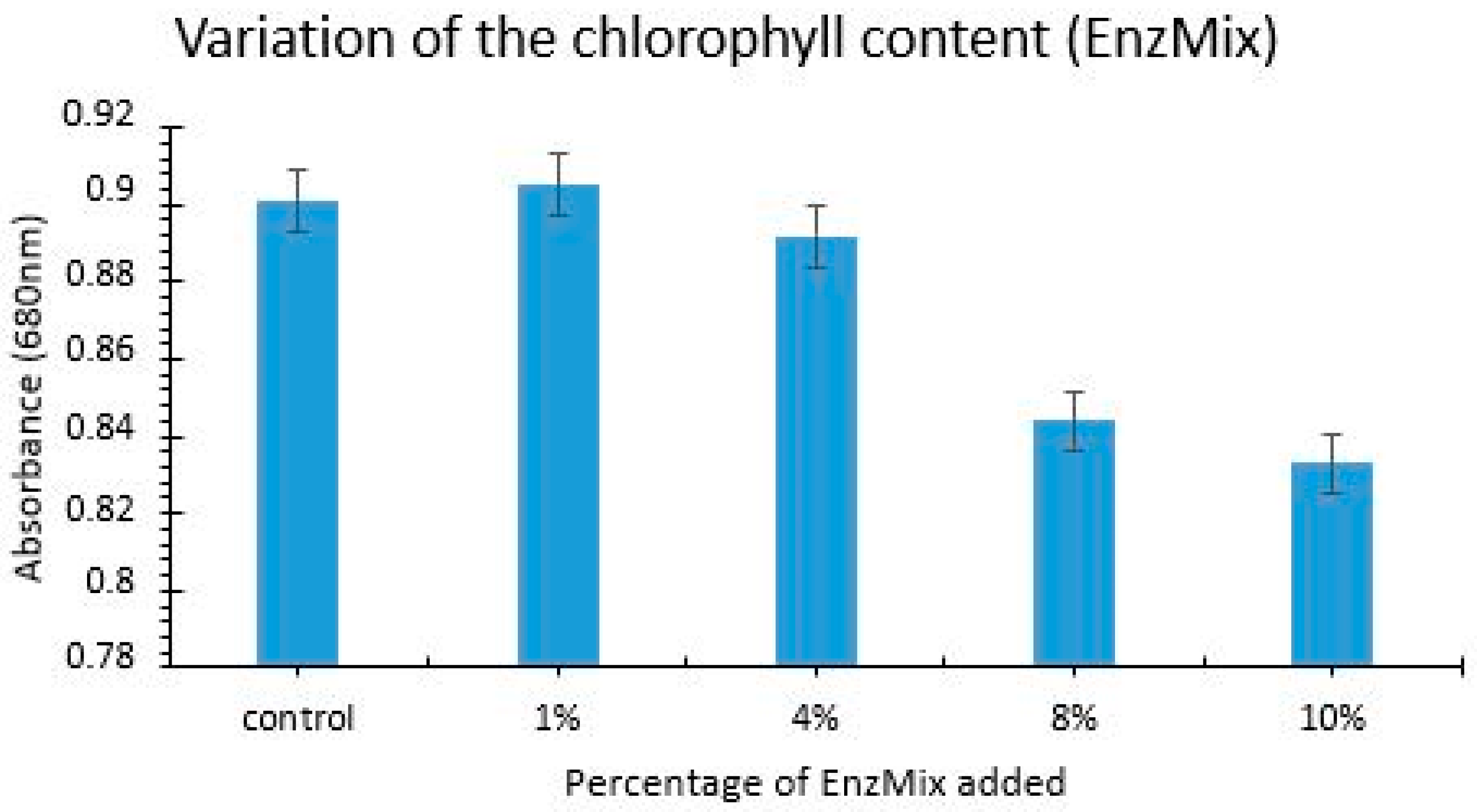
Selection of the Optimum Dose and Conditions of the Enzymatic Complex for the Biocatalysis Process
2.3. Desmodesmus sp. Cultivation for Field Biostimulant Production
2.3.1. Desmodesmus sp. Cultivation at Laboratory Scale for On-Site Biostimulant Production
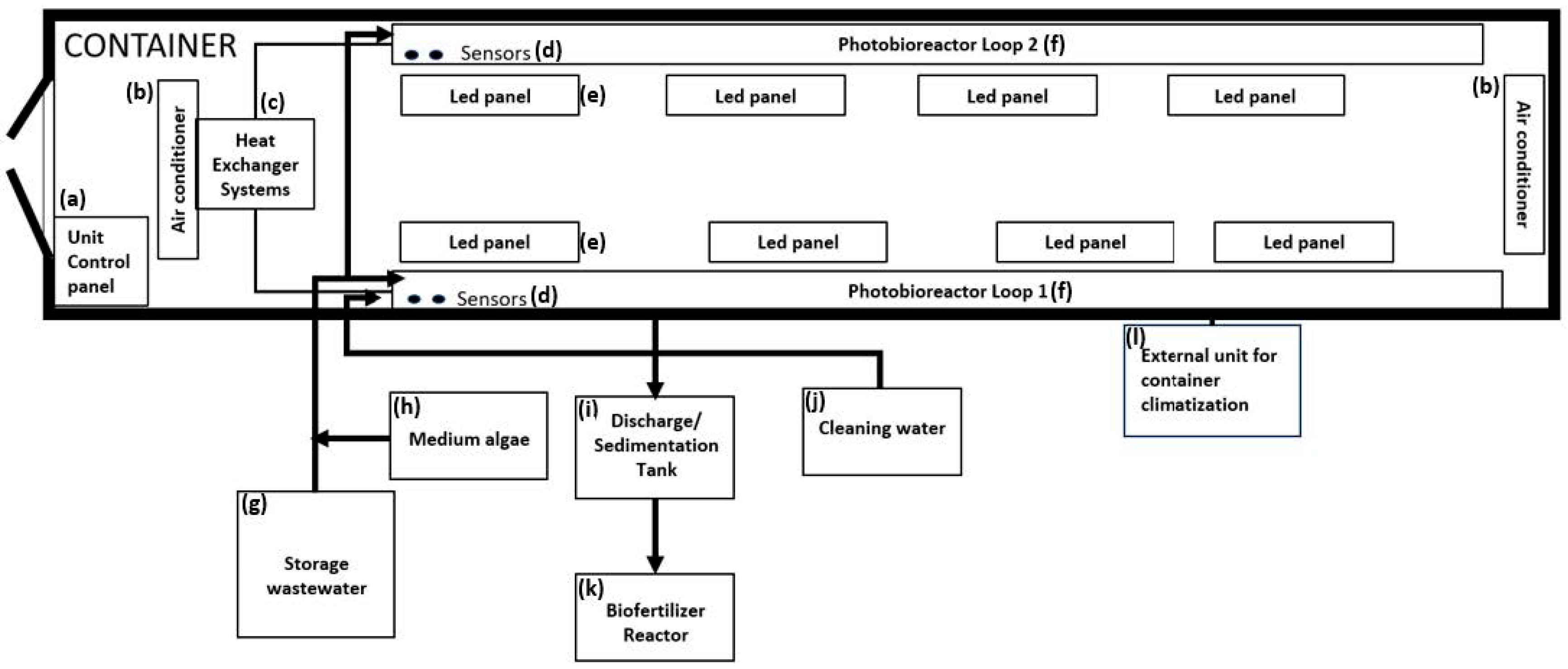
2.3.2. Desmodesmus sp. Cultivation at Container Scale for On-Site Biostimulant Production
2.3.3. Desmodesmus sp. Production at Laboratory Scale for On-Site Biostimulant Production
2.3.4. Desmodesmus sp. Production at Container Scale through CO2 Infusion and Increased Lighting for On-Site Biostimulant Production
2.3.5. Measurements
2.3.6. Biocatalysis Process
3. Results
3.1. Determining the Best Sedimentation Method and Enzyme Complex for Biostimulant Production
3.1.1. Pre-Concentration of Microalgae Biomass
3.1.2. Biocatalysis Process under Laboratory Conditions
Selection of the Optimum Enzyme Complex for the Biocatalysis Process
Selection of the Optimum Dose and Conditions of the Enzymatic Complex for the Biocatalysis Process
3.2. Desmodesmus sp. Production Using Agro-WasteWaters for Field Biostimulant Production
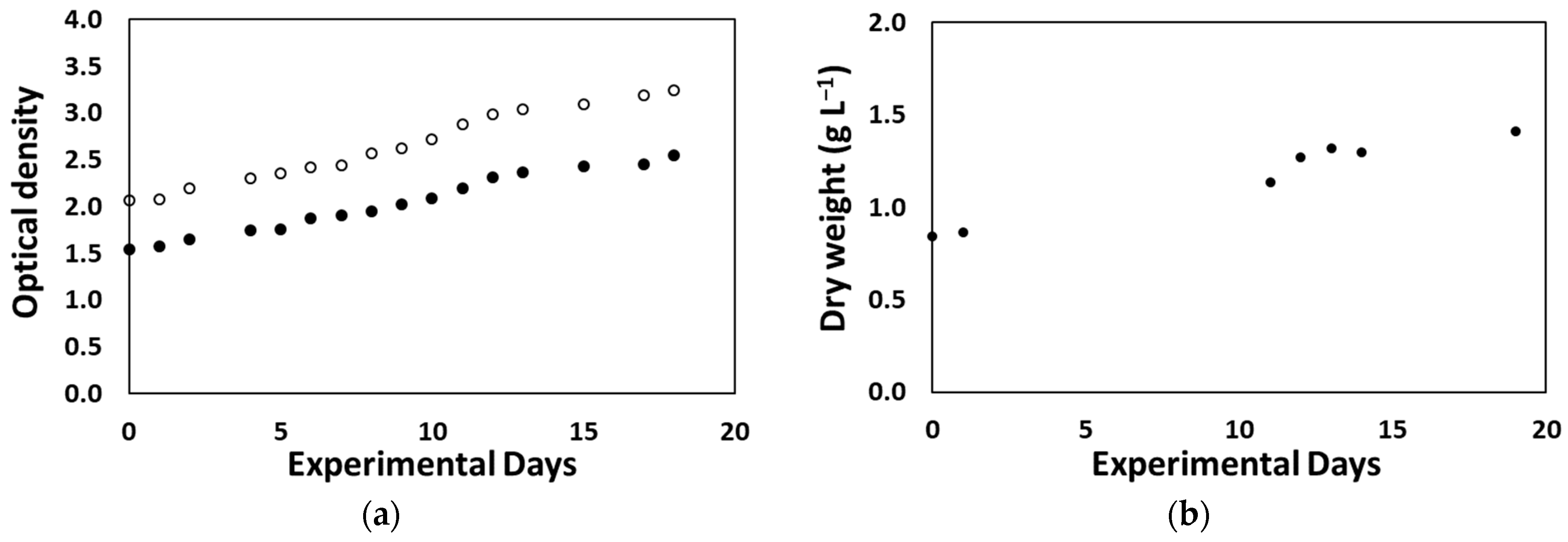
3.2.1. Desmodesmus sp. Production at Lab Scale for On-Site Biostimulant Production
3.2.2. Desmodesmus sp. Production at Container Scale for On-Site Biostimulant Production
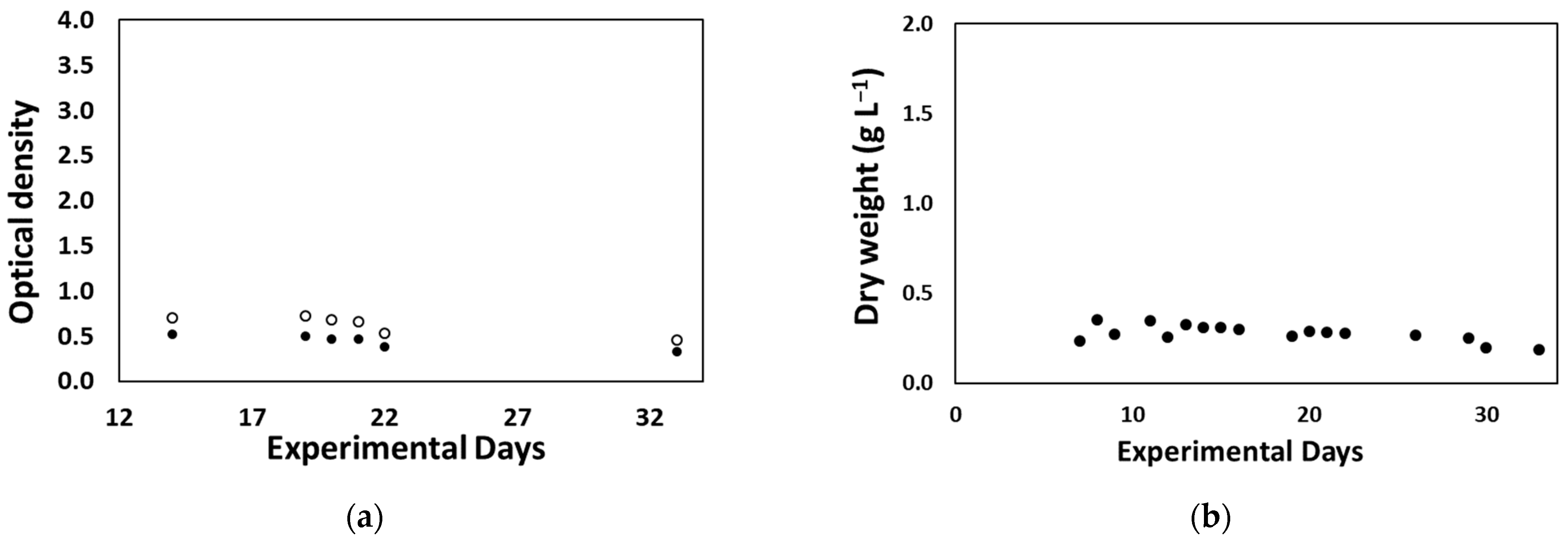
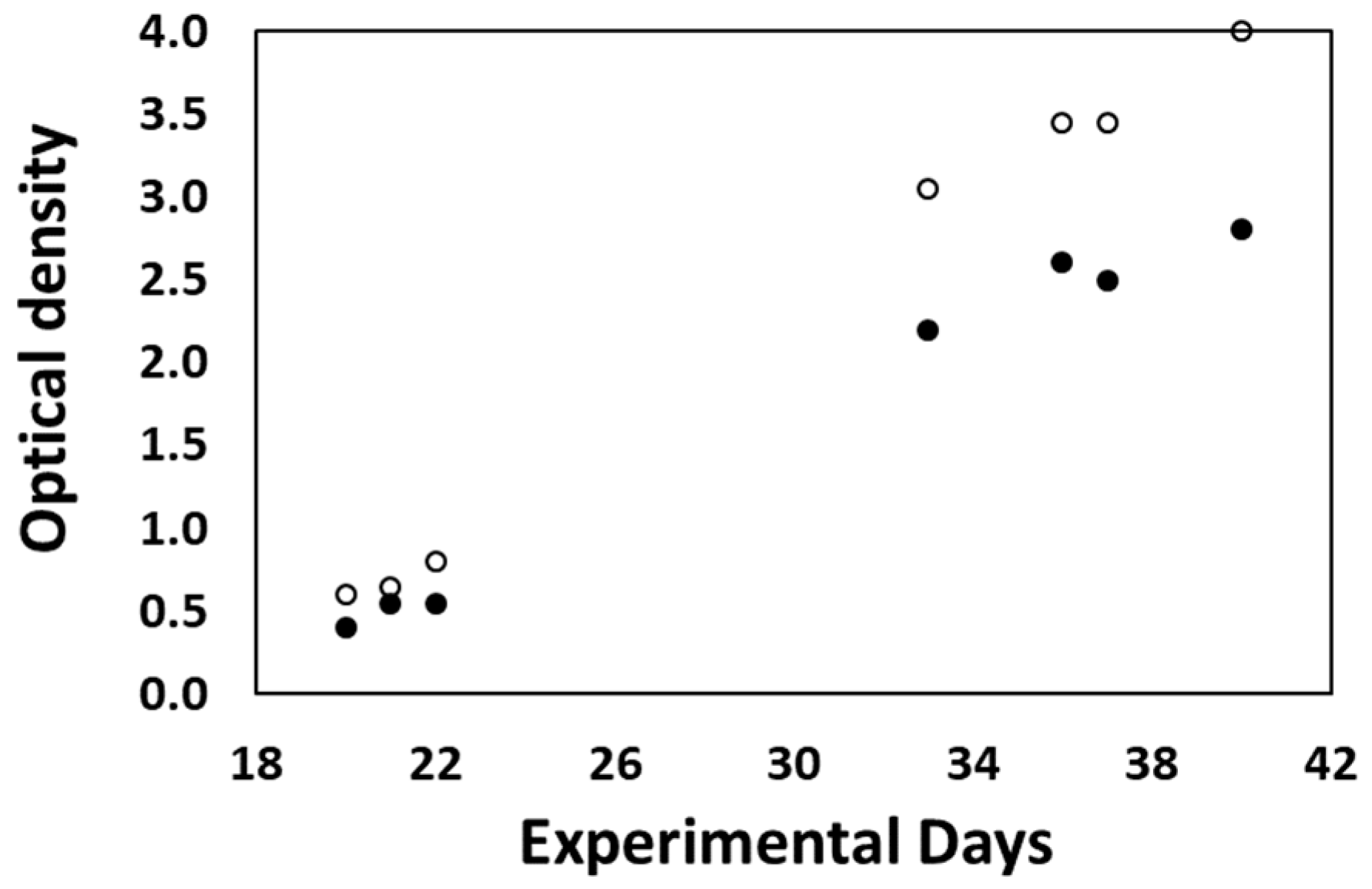
3.2.3. Desmodesmus sp. Production at Lab Scale for On-Site Biostimulant Production
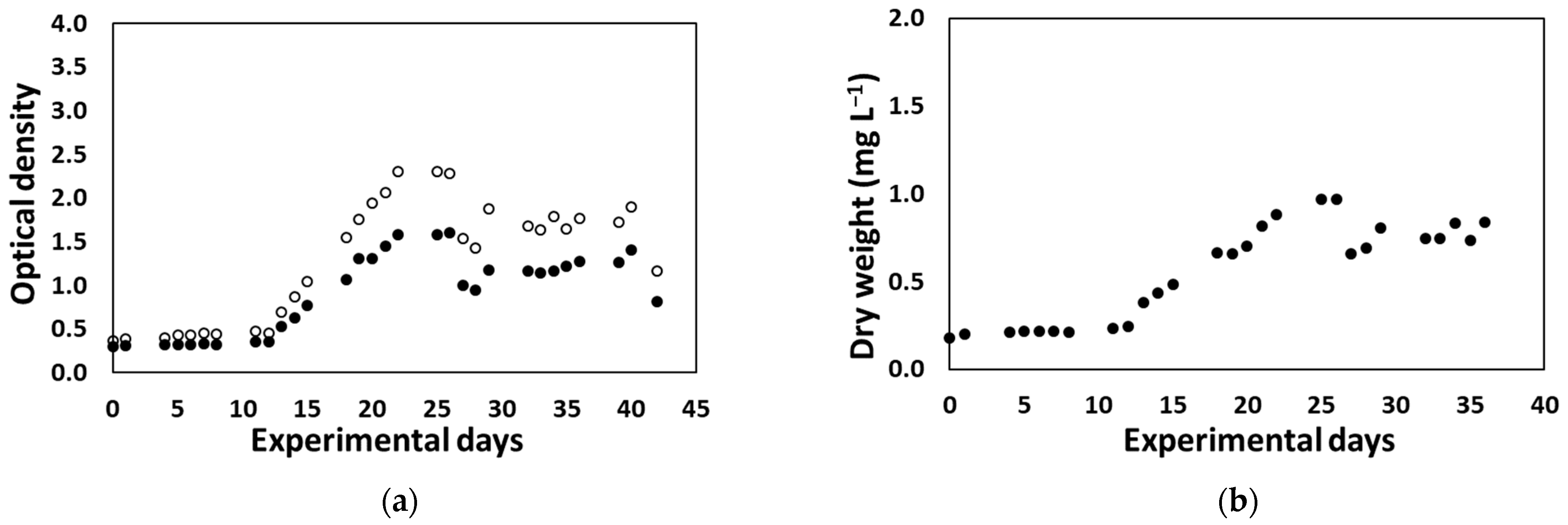
3.2.4. Desmodesmus sp. Production at Container Scale through CO2 Infusion and Increased Lighting for On-Site Biostimulant Production
3.2.5. On-Site Biostimulant Production
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Silva, J.A. Wastewater treatment and reuse for sustainable water resources management: A systematic literature review. Sustainability 2023, 15, 10940. [Google Scholar] [CrossRef]
- Lin, L.; Yang, H.; Xu, X. Effects of water pollution on human health and disease heterogeneity: A Review. Front. Environ. Sci. 2022, 10, 880246. [Google Scholar] [CrossRef]
- Englande, A.J., Jr.; Krenkel, P.; Shamas, J. Wastewater treatment & water reclamation. In Reference Module in Earth Systems and Environmental Sciences; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar] [CrossRef]
- Silva, J.A. Water supply and wastewater treatment and reuse in future cities: A systematic literature review. Water 2023, 15, 3064. [Google Scholar] [CrossRef]
- Kapoore, R.V.; Wood, E.E.; Llewellyn, C.A. Algae biostimulants: A critical look at microalgal biostimulants for sustainable agricultural practices. Biotechnol. Adv. 2021, 49, 107754. [Google Scholar] [CrossRef]
- Mógor, Á.F.; Ördög, V.; Lima, G.P.P.; Molnár, Z.; Mógor, G. Biostimulant properties of cyanobacterial hydrolysate related to polyamines. J. Appl. Phycol. 2018, 30, 453–460. [Google Scholar] [CrossRef]
- Olabi, A.G.; Shehata, N.; Sayed, E.T.; Rodriguez, C.; Anyanwu, R.C.; Russell, C.; Abdelkareem, M.A. Role of microalgae in achieving sustainable development goals and circular economy. Sci. Total Environ. 2023, 854, 158689. [Google Scholar] [CrossRef]
- Navarro-López, E.; Cerón-García, M.d.C.; López-Rodríguez, M.; Acién-Fernández, F.G.; Molina-Grima, E. Biostimulants obtained after pilot-scale high-pressure homogenization of Scenedesmus sp. grown in pig manure. Algal Res. 2020, 52, 102123. [Google Scholar] [CrossRef]
- Veerabadhran, M.; Natesan, S.; MubarakAli, D.; Xu, S.; Yang, F. Using different cultivation strategies and methods for the production of microalgal biomass as a raw material for the generation of bioproducts. Chemosphere 2021, 285, 131436. [Google Scholar] [CrossRef]
- Wang, S.-K.; Stiles, A.R.; Guo, C.; Liu, C.-Z. Microalgae cultivation in photobioreactors: An overview of light characteristics. Eng. Life Sci. 2014, 14, 550–559. [Google Scholar] [CrossRef]
- Osorio-Reyes, J.G.; Valenzuela-Amaro, H.M.; Pizaña-Aranda, J.J.P.; Ramírez-Gamboa, D.; Meléndez-Sánchez, E.R.; López-Arellanes, M.E.; Castañeda-Antonio, M.D.; Coronado-Apodaca, K.G.; Gomes Araújo, R.; Sosa-Hernández, J.E.; et al. Microalgae-based biotechnology as alternative biofertilizers for soil enhancement and carbon footprint reduction: Advantages and implications. Mar. Drugs 2023, 21, 93. [Google Scholar] [CrossRef]
- Krujatz, F.; Fehse, K.; Jahnel, M.; Gommel, C.; Schurig, C.; Lindner, F.; Bley, T.; Weber, J.; Steingroewer, J. MicrOLED-photobioreactor: Design and characterization of a milliliter-scale Flat-Panel-Airlift-photobioreactor with optical process monitoring. Algal Res. 2016, 18, 225–234. [Google Scholar] [CrossRef]
- Molina Grima, E.; Belarbi, E.-H.; Acién Fernández, F.G.; Robles Medina, A.; Chisti, Y. Recovery of microalgal biomass and metabolites: Process options and economics. Biotechnol. Adv. 2003, 20, 491–515. [Google Scholar] [CrossRef]
- Nwoba, E.G.; Parlevliet, D.A.; Laird, D.W.; Alameh, K.; Moheimani, N.R. Light management technologies for increasing algal photobioreactor efficiency. Algal Res. 2019, 39, 101433. [Google Scholar] [CrossRef]
- Akach, J.; Kabuba, J.; Ochieng, A. Simulation of the Light Distribution in a Solar Photocatalytic Bubble Column Reactor Using the Monte Carlo Method. Ind. Eng. Chem. Res. 2020, 59, 17708–17719. [Google Scholar] [CrossRef]
- Liyanaarachchi, V.C.; Premaratne, M.; Ariyadasa, T.U.; Nimarshana, P.H.V.; Malik, A. Two-stage cultivation of microalgae for production of high-value compounds and biofuels: A review. Algal Res. 2021, 57, 102353. [Google Scholar] [CrossRef]
- Álvarez-Gil, M.; Blanco-Vieites, M.; Suárez-Montes, D.; Casado-Bañares, V.; Delgado-Ramallo, J.F.; Rodríguez, E. Revolutionizing agriculture: Leveraging hydroponic greenhouse wastewater for sustainable microalgae-based biostimulant production. Sustainability 2023, 15, 14398. [Google Scholar] [CrossRef]
- Kyriazopoulos, V.; Gioti, M.; Varlamis, C.; Mekeridis, E.D.; Pechlivani, E.M.; Logothetidis, S. High efficiency solution processable polymer OLEDs: Manufacturing and characterization. Mater. Today Proc. 2021, 37, A21–A31. [Google Scholar] [CrossRef]
- Bani, A.; Fernandez, F.G.A.; D’Imporzano, G.; Parati, K.; Adani, F. Influence of photobioreactor set-up on the survival of microalgae inoculum. Bioresour. Technol. 2021, 320, 124408. [Google Scholar] [CrossRef]
- Ji, F.; Hao, R.; Liu, Y.; Li, G.; Zhou, Y.; Dong, R. Isolation of a novel microalgae strain Desmodesmus sp. and optimization of environmental factors for its biomass production. Bioresour. Technol. 2013, 148, 249–254. [Google Scholar] [CrossRef]
- González-Pérez, B.K.; Rivas-Castillo, A.M.; Valdez-Calderón, A.; Gayosso-Morales, M.A. Microalgae as biostimulants: A new approach in agriculture. World J. Microbiol. Biotechnol. 2021, 38, 4. [Google Scholar] [CrossRef]
- Okoro, V.; Azimov, U.; Munoz, J.; Hernandez, H.H.; Phan, A.N. Microalgae cultivation and harvesting: Growth performance and use of flocculants—A review. Renew. Sustain. Energy Rev. 2019, 115, 109364. [Google Scholar] [CrossRef]
- Subhadra, B.G. Water management policies for the algal biofuel sector in the Southwestern United States. Appl. Energy 2011, 88, 3492–3498. [Google Scholar] [CrossRef]
- Miranda, A.M.; Hernandez-Tenorio, F.; Villalta, F.; Vargas, G.J.; Sáez, A.A. Advances in the development of biofertilizers and biostimulants from microalgae. Biology 2024, 13, 199. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, M.M.R.; Sanchez, J.L.G.; Sevilla, J.M.F.; Fernandez, F.G.A.; Perez, J.A.S.; Grima, E.M. Outdoor continuous culture of Porphyridium cruentum in a tubular photobioreactor: Quantitative analysis of the daily cyclic variation of culture parameters. J. Biotechnol. 1999, 70, 271–288. [Google Scholar] [CrossRef]
- Harun, R.; Singh, M.; Forde, G.M.; Danquah, M.K. Bioprocess engineering of microalgae to produce a variety of consumer products. Renew. Sustain. Energ. Rev. 2010, 14, 1037–1047. [Google Scholar] [CrossRef]
- Tan, C.H.; Show, P.L.; Chang, J.-S.; Ling, T.C.; Lan, J.C.-W. Novel approaches of producing bioenergies from microalgae: A recent review. Biotechnol. Adv. 2015, 33, 1219–1227. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhang, J.; Li, H.; Hu, R.; Yao, X.; Liu, Y.; Zhou, Y.; Lyu, T. Towards high-quality biodiesel production from microalgae using original and anaerobically-digested livestock wastewater. Chemosphere 2021, 273, 128578. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Yang, H.; He, Q.; Hu, C. Physiological responses of freshwater oleaginous microalgae Desmodesmus sp. NMX451 under nitrogen deficiency and alkaline pH-induced lipid accumulation. J. Appl. Phycol. 2015, 27, 649–659. [Google Scholar] [CrossRef]
- Hernández-García, A.; Velásquez-Orta, S.B.; Novelo, E.; Yáñez-Noguez, I.; Monje-Ramírez, I.; Orta Ledesma, M.T. Wastewater-leachate treatment by microalgae: Biomass, carbohydrate and lipid production. Ecotoxicol. Environ. Saf. 2019, 174, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Molina-Grima, E.; García-Camacho, F.; Acién-Fernández, F.G.; Sánchez-Mirón, A.; Plouviez, M.; Shene, C.; Chisti, Y. Pathogens and predators impacting commercial production of microalgae and cyanobacteria. Biotechnol. Adv. 2021, 53, 107884. [Google Scholar] [CrossRef]
- Van Den Hende, S.; Vervaeren, H.; Boon, N. Flue gas compounds and microalgae: (Bio)chemical interactions leading to bio-technological opportunities. Biotechnol. Adv. 2012, 30, 1405–1424. [Google Scholar] [CrossRef] [PubMed]
- Chisti, Y. Biodiesel from microalgae beats bioethanol. Trends Biotechnol. 2008, 26, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Lardon, L.; Helias, A.; Sialve, B.; Steyer, J.P.; Bernard, O. Life-cycle assessment of biodiesel production from microalgae. Environ. Sci. Technol. 2009, 43, 6475–6481. [Google Scholar] [CrossRef] [PubMed]
- Samorì, G.; Samorì, C.; Guerrini, F.; Pistocchi, R. Growth and nitrogen removal capacity of Desmodesmus communis and of a natural microalgae consortium in a batch culture system in view of urban wastewater treatment: Part I. Water Res. 2013, 47, 791–801. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Lan, C.Q. Biomass production and nitrogen and phosphorus removal by the green alga Neochloris oleoabundans in simulated wastewater and secondary municipal wastewater effluent. Bioresour. Technol. 2011, 102, 5639–5644. [Google Scholar] [CrossRef] [PubMed]
- Anand, A.; Tripathi, K.; Kumar, A.; Gupta, S.; Raghuvanshi, S.; Verma, S.K. Bio-Mitigation of carbon dioxide using Desmodesmus sp. in the custom-designed pilot-scale loop photobioreactor. Sustainability 2021, 13, 9882. [Google Scholar] [CrossRef]
- Tang, D.; Han, W.; Li, P.; Miao, X.; Zhong, J. CO2 biofixation and fatty acid composition of Scenedesmus obliquus and Chlorella pyrenoidosa in response to different CO2 levels. Bioresour. Technol. 2011, 102, 3071–3076. [Google Scholar] [CrossRef] [PubMed]
- Chiarini, A.; Quadrio, M. The light/dark cycle of microalgae in a thin-layer photobioreactor. J. Appl. Phycol. 2021, 33, 183–195. [Google Scholar] [CrossRef]
- Chen, L.; Wang, C.; Wang, W.; Wei, J. Optimal conditions of different flocculation methods for harvesting Scenedesmus sp. cultivated in an open-pond system. Bioresour. Technol. 2013, 133, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-G.; La, H.-J.; Ahn, C.-Y.; Park, Y.-H.; Oh, H.-M. Harvest of Scenedesmus sp. with bioflocculant and reuse of culture medium for subsequent high-density cultures. Bioresour. Technol. 2011, 102, 3163–3168. [Google Scholar] [CrossRef]
- Guillaume, A.; Thorigné, A.; Carré, Y.; Vinh, J.; Levavasseur, L. Contribution of proteases and cellulases produced by solid-state fermentation to the improvement of corn ethanol production. Bioresour. Bioprocess. 2019, 6, 7. [Google Scholar] [CrossRef]





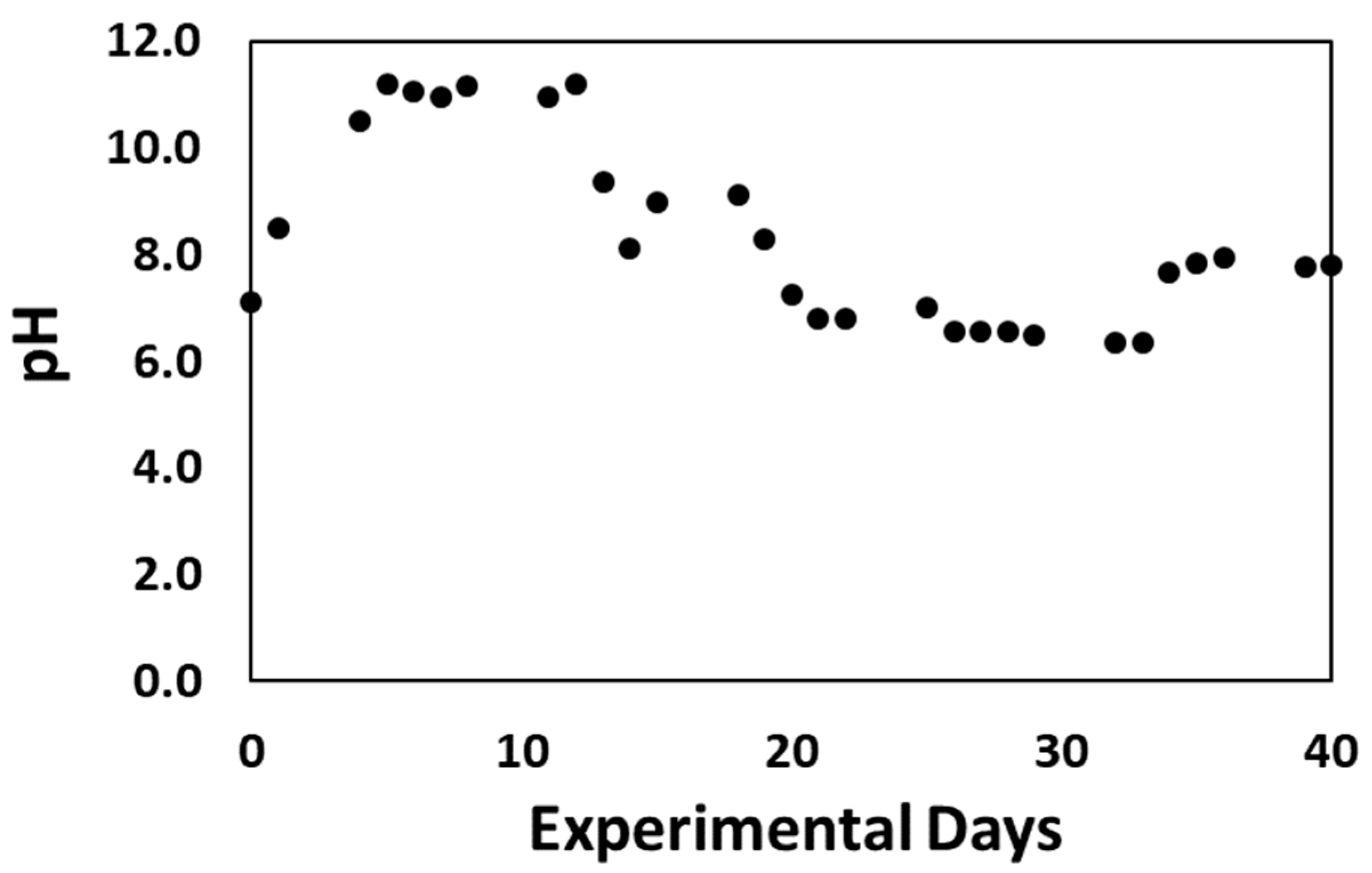
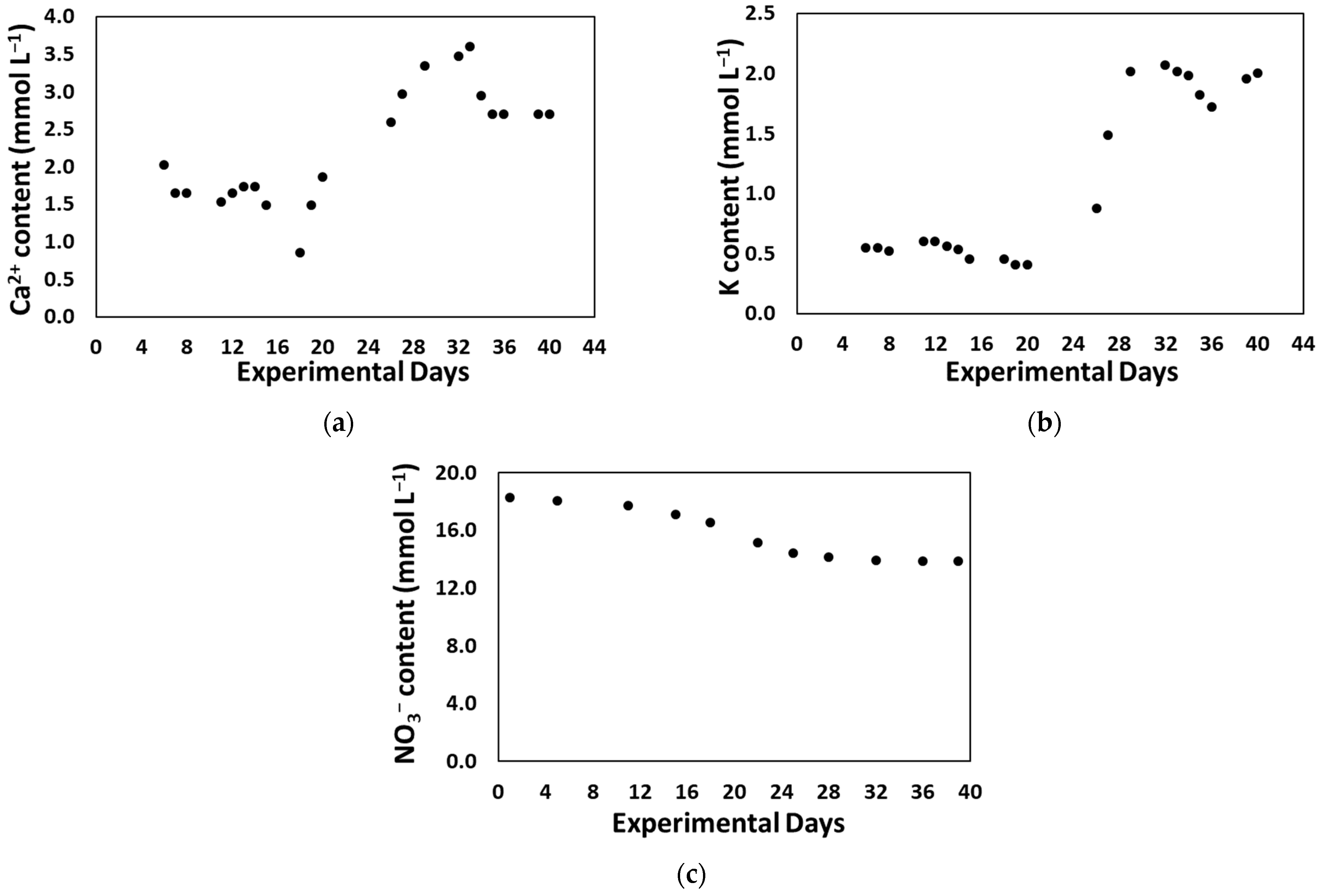
| Macronutrient Compound | Concentration (mM) |
|---|---|
| NaNO3 | 17.60 |
| K2HPO4 | 0.23 |
| MgSO4·7H2O | 0.30 |
| CaCl2·2H2O | 0.24 |
| Citric Acid·H2O | 0.03 |
| Ferric Ammonium Citrate | 0.02 |
| Na2EDTA·2H2O | 0.003 |
| Na2CO3 | 0.19 |
| Sodium Thiosulfate Pentahydrate | 1.00 |
| BG-11 Trace metals | Concentration (mM) |
| H3BO3 | 46.00 |
| MnCl2·4H2O | 9.00 |
| ZnSO4·7H2O | 0.77 |
| Na2MoO4·2H2O | 1.60 |
| CuSO4·5H2O | 0.30 |
| Co(NO3)2·6H2O | 0.17 |
| Flocculant Name | Added Dose of Flocculant (mg L−1) | Sedimentation Speed of Flocculant (cm min−1) | Biomass Recovery from Culture (g L−1) |
|---|---|---|---|
| FeCl3 | 50.0 | 8.7 | 10.1 |
| 75.0 | 9.5 | 11.8 | |
| 150.0 | 9.9 | 12.9 | |
| Chitosan | 60.0 | 4.0 | 11.5 |
| 70.0 | 5.1 | 10.1 |
| Macronutrients | BG-11 (mmol L−1) | Drainage (mmol L−1) | Nutrient Saving (%) |
|---|---|---|---|
| NO3− | 12.9 | 9.5 | 73.6 |
| K | 0.05 | 3.81 | 100.0 |
| PO43− | 0.13 | 0.76 | 100.0 |
| Ca2+ | 0.09 | 4.73 | 100.0 |
| Amino Acids | Batch A | Batch B | Batch C | Batch D | Batch E | Batch F |
|---|---|---|---|---|---|---|
| Alanine | 0.049 | 0.027 | 0.027 | 0.027 | <0.25 | <0.25 |
| Arginine | 0.010 | 0.016 | 0.015 | 0.016 | <0.25 | 0.42 |
| Asparagine | 0.008 | 0.055 | 0.056 | 0.053 | <0.25 | <0.25 |
| Aspartic Acid | 0.462 | 0.011 | 0.011 | 0.014 | <0.25 | <0.25 |
| Cysteine | 0.012 | 0.016 | 0.016 | 0.017 | 0.54 | 0.67 |
| Glutamic acid | 0.048 | 0.042 | 0.047 | 0.042 | <0.25 | 0.25 |
| Glutamine | 0.019 | 0.024 | 0.023 | 0.024 | <0.25 | <0.25 |
| Glycine | 0.015 | 0.013 | 0.013 | 0.011 | 0.31 | 0.53 |
| Histidine | 0.022 | 0.024 | 0.025 | 0.029 | <0.25 | <0.25 |
| Isoleucine | 0.010 | 0.026 | 0.028 | 0.024 | <0.25 | <0.25 |
| Leucine | 0.019 | 0.007 | 0.008 | 0.010 | <0.25 | <0.25 |
| Lysine | 0.009 | 0.017 | 0.018 | 0.020 | <0.25 | <0.25 |
| Methionine | 0.012 | 0.007 | 0.007 | 0.009 | <0.25 | <0.25 |
| Phenylalanine | 0.005 | 0.015 | 0.015 | 0.014 | <0.25 | <0.25 |
| Proline | 0.190 | 0.064 | 0.066 | 0.067 | 0.34 | 0.29 |
| Serine | 0.062 | 0.076 | 0.075 | 0.074 | <0.25 | <0.25 |
| Threonine | 0.146 | 0.154 | 0.155 | 0.153 | <0.25 | <0.25 |
| TOTAL | 1.098 | 0.594 | 0.605 | 0.604 | 1.190 | 1.910 |
| NPK (%) | Batch A | Batch B | Batch C | Batch D | Batch E | Batch F |
|---|---|---|---|---|---|---|
| N | 0.284 | 0.205 | 0.234 | 0.245 | 0.100 | 0.100 |
| P | 0.462 | 0.367 | 0.386 | 0.399 | 0.209 | 0.199 |
| K | 0.302 | 0.245 | 0.222 | 0.239 | 0.140 | 0.220 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faliagka, S.; Kountrias, G.; Dimitriou, E.; Álvarez-Gil, M.; Blanco-Vieites, M.; Magrassi, F.; Notari, M.; Pechlivani, E.M.; Katsoulas, N. Development of a Greenhouse Wastewater Stream Utilization System for On-Site Microalgae-Based Biostimulant Production. AgriEngineering 2024, 6, 1898-1923. https://doi.org/10.3390/agriengineering6030111
Faliagka S, Kountrias G, Dimitriou E, Álvarez-Gil M, Blanco-Vieites M, Magrassi F, Notari M, Pechlivani EM, Katsoulas N. Development of a Greenhouse Wastewater Stream Utilization System for On-Site Microalgae-Based Biostimulant Production. AgriEngineering. 2024; 6(3):1898-1923. https://doi.org/10.3390/agriengineering6030111
Chicago/Turabian StyleFaliagka, Sofia, Georgios Kountrias, Eleni Dimitriou, Maria Álvarez-Gil, Mario Blanco-Vieites, Fabio Magrassi, Marta Notari, Eleftheria Maria Pechlivani, and Nikolaos Katsoulas. 2024. "Development of a Greenhouse Wastewater Stream Utilization System for On-Site Microalgae-Based Biostimulant Production" AgriEngineering 6, no. 3: 1898-1923. https://doi.org/10.3390/agriengineering6030111







