Integrating Aquaponics with Macrobrachium amazonicum (Palaemonidae: Decapoda) Cultivation for the Production of Microgreens: A Sustainable Approach
Abstract
:1. Introduction
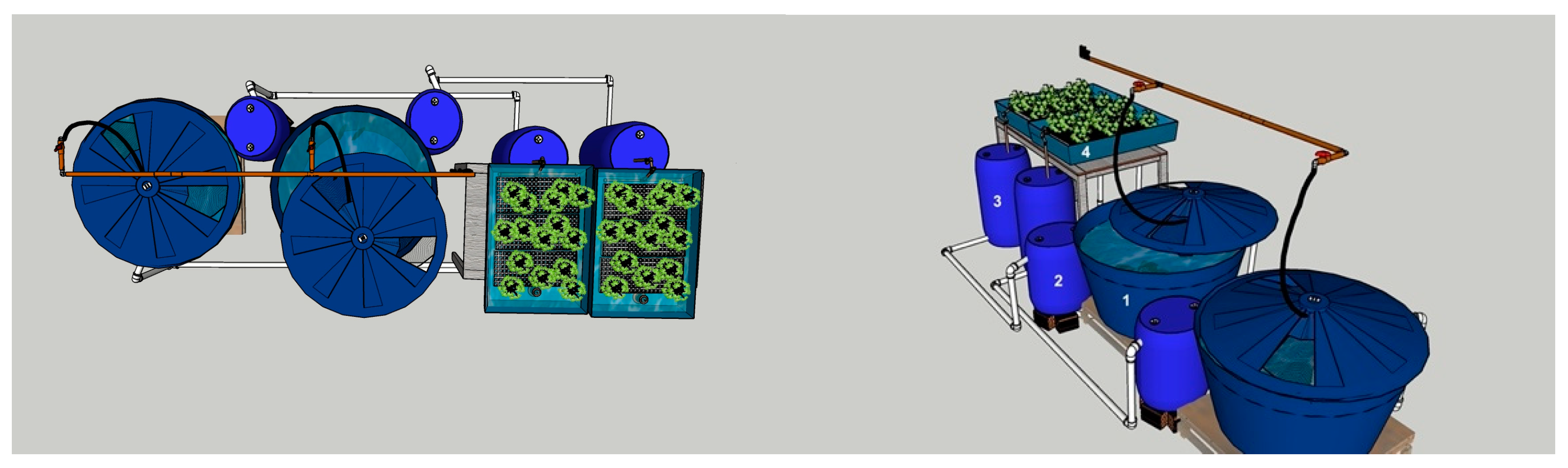
2. Materials and Methods
2.1. Plant Material
2.2. Analysis of the Water
2.3. Prawn Growth
2.4. Plant Growth
2.5. Statistical Analysis
3. Results
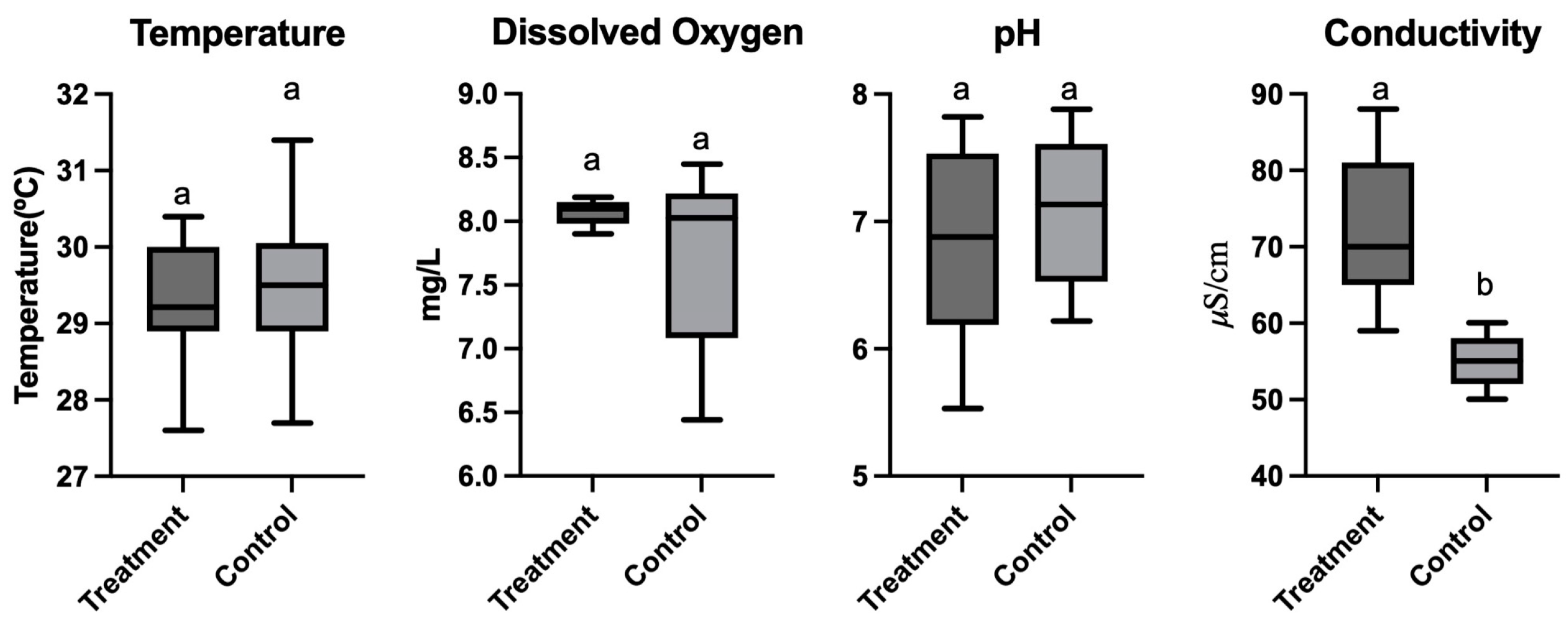
3.1. Water Quality
3.2. Prawn Growth
3.3. Plant Development
3.3.1. Brassicaceae
3.3.2. Amaranthacea
3.3.3. Productivity
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bhaswant, M.; Shanmugam, D.K.; Miyazawa, T.; Abe, C.; Miyazawa, T. Microgreens-A Comprehensive Review of Bioactive Molecules and Health Benefits. Molecules 2023, 28, 867. [Google Scholar] [CrossRef] [PubMed]
- Lone, J.K.; Pandey, R. Gayacharan, Microgreens on the rise: Expanding our horizons from farm to fork. Heliyon 2024, 10, e25870. [Google Scholar] [CrossRef]
- Mir, S.A.; Shah, M.A.; Mir, M.M. Microgreens: Production, shelf life, and bioactive components. Crit. Rev. Food Sci. Nutr. 2017, 57, 2730–2736. [Google Scholar] [CrossRef]
- Moraru, P.I.; Rusu, T.; Mintas, O.S. Trial Protocol for Evaluating Platforms for Growing Microgreens in Hydroponic Conditions. Foods 2022, 11, 1327. [Google Scholar] [CrossRef] [PubMed]
- Yep, B.; Zheng, Y. Aquaponic trends and challenges—A review. J. Clean. Prod. 2019, 228, 1586–1599. [Google Scholar] [CrossRef]
- Rakocy, J.E. Aquaponics—Integrating Fish and Plant Culture. In Aquaculture Production Systems; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; pp. 344–386. [Google Scholar] [CrossRef]
- Love, D.C.; Fry, J.P.; Genello, L.; Hill, E.S.; Frederick, J.A.; Li, X.; Semmens, K. An International Survey of Aquaponics Practitioners. PLoS ONE 2014, 9, e102662. [Google Scholar] [CrossRef]
- Liang, J.-Y.; Chien, Y.-H. Effects of feeding frequency and photoperiod on water quality and crop production in a tilapia–water spinach raft aquaponics system. Int. Biodeterior. Biodegrad. 2013, 85, 693–700. [Google Scholar] [CrossRef]
- Buzby, K.M.; Lin, L.-S. Scaling aquaponic systems: Balancing plant uptake with fish output. Aquacult. Eng. 2014, 63, 39–44. [Google Scholar] [CrossRef]
- Roy, K.; Kajgrova, L.; Mraz, J. TILAFeed: A bio-based inventory for circular nutrients management and achieving bioeconomy in future aquaponics. New Biotechnol. 2022, 70, 9–18. [Google Scholar] [CrossRef]
- Azad, K.N.; Salam, M.A.; Azad, K.N. Aquaponics in Bangladesh: Current status and future prospects. J. Biosci. Agric. Res. 2016, 7, 669–677. [Google Scholar] [CrossRef]
- Maciel, C.R.; Valenti, W.C. Effect of tank colour on larval performance of the Amazon River prawn Macrobrachium amazonicum. Aquacult. Res. 2014, 45, 1041–1050. [Google Scholar] [CrossRef]
- Jatobá, A.; Legarda, E.C.; Stockhausen, L.; Vieira, F.d.N. First report: Amazon River Prawn reared in biofloc technology. Revista de Ciências Agroveterinárias 2022, 19, 377–380. [Google Scholar] [CrossRef]
- Sterzelecki, F.C.; Santos, G.R.; de Gusmão, M.T.A.; de Carvalho, T.C.C.; dos Reis, A.R.; Guimarães, R.; Santos, M.d.L.S.; de Melo, N.F.A.C.; Luz, R.K.; Palheta, G.D.A. Effects of hydroponic supplementation on Amazon river prawn (Macrobrachium amazonicum Heller, 1862) and lettuce seedling (Lactuca sativa L.) development in aquaponic system. Aquaculture 2021, 543, 736916. [Google Scholar] [CrossRef]
- Maciel, C.; Valenti, W. Biology, Fisheries, and Aquaculture of the Amazon River Prawn Macrobrachium amazonicum: A Review. Nauplius 2009, 17, 61–79. [Google Scholar]
- Moraes-Riodades, P.M.C.; Valenti, W. Freshwater prawn farming in Brazilian Amazonia shows potential for economic and social development. Glob. Aquac. Advocate 2001, 4, 73–74. [Google Scholar]
- Moraes-Valenti, P.; Valenti, W.C. Culture of the Amazon River Prawn Macrobrachium Amazonicum. In Freshwater Prawns; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009; pp. 485–501. [Google Scholar] [CrossRef]
- Kutty, M.N.; Herman, F.; Menn, H.L. Culture of other Prawn Species. In Freshwater Prawn Culture; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2000; pp. 393–410. [Google Scholar] [CrossRef]
- Guest, W.C. Laboratory Life History of the Palaemonid Shrimp Macrobrachium Amazonicum (Heller) (Decapoda, Palaemonidae). Crustaceana 1979, 37, 141–152. [Google Scholar] [CrossRef]
- Araujo, M.C.; Valenti, W.C. The effects of light intensities on larval development of Amazon River prawn, Macrobrachium amazonicum. Boletim do Instituto de Pesca 2011, 37, 155–164. [Google Scholar]
- Bastos, A.M.; Lima, J.F.; Tavares-Dias, M. Effect of increase in temperature on the survival and growth of Macrobrachium amazonicum (Palaemonidae) in the Amazon. Aquat. Living Resour. 2018, 31, 21. [Google Scholar] [CrossRef]
- Araujo, M.C.d.; Valenti, W.C. Effects of feeding strategy on larval development of the Amazon River prawn Macrobrachium amazonicum. Revista Brasileira de Zootecnia 2017, 46, 85–90. [Google Scholar] [CrossRef]
- Danaher, J.J.; Shultz, R.C.; Rakocy, J.E.; Bailey, D.S. Alternative Solids Removal for Warm Water Recirculating Raft Aquaponic Systems. J. World Aquacult. Soc. 2013, 44, 374–383. [Google Scholar] [CrossRef]
- Tyson, R.V.; Treadwell, D.; Simonne, E.H. Opportunities and Challenges to Sustainability in Aquaponic Systems. Horttechnology 2011, 21, 6–13. [Google Scholar] [CrossRef]
- Love, D.C.; Fry, J.P.; Li, X.; Hill, E.S.; Genello, L.; Semmens, K.; Thompson, R.E. Commercial aquaponics production and profitability: Findings from an international survey. Aquaculture 2015, 435, 67–74. [Google Scholar] [CrossRef]
- Resh, H. Hydroponic Food Production: A Definitive Guidebook for the Advanced Home Gardener and the Commercial Hydroponic Grower; Taylor & Francis: London, UK, 2022. [Google Scholar]
- Tyson, R.V.; Simonne, E.H.; White, J.M.; Lamb, E. Reconciling water quality parameters impacting nitrification in aquaponics: The pH levels. Proc. Fla. State Hortic. Soc. 2004, 117, 79–83. [Google Scholar]
- Bregnballe, J. A Guide to Recirculation Aquaculture—An Introduction to the New Environmentally Friendly and Highly Productive Closed Fish Farming Systems; FAO, Eurofish International Organisation: Rome, Italy, 2022. [Google Scholar] [CrossRef]
- Katsenios, N.; Christopoulos, M.V.; Kakabouki, I.; Vlachakis, D.; Kavvadias, V.; Efthimiadou, A. Effect of Pulsed Electromagnetic Field on Growth, Physiology and Postharvest Quality of Kale (Brassica oleracea), Wheat (Triticum durum) and Spinach (Spinacia oleracea) Microgreens. Agronomy 2021, 11, 1364. [Google Scholar] [CrossRef]
- Pattillo, D.A.; Hager, J.V.; Cline, D.J.; Roy, L.A.; Hanson, T.R. System design and production practices of aquaponic stakeholders. PLoS ONE 2022, 17, e0266475. [Google Scholar] [CrossRef] [PubMed]
- Amitrano, C.; Paglialunga, G.; Battistelli, A.; De Micco, V.; Del Bianco, M.; Liuzzi, G.; Moscatello, S.; Paradiso, R.; Proietti, S.; Rouphael, Y.; et al. Defining growth requirements of microgreens in space cultivation via biomass production, morpho-anatomical and nutritional traits analysis. Front. Plant Sci. 2023, 14, 1190945. [Google Scholar] [CrossRef]
- Lennard, W.A.; Leonard, B.V. A Comparison of Three Different Hydroponic Sub-systems (gravel bed, floating and nutrient film technique) in an Aquaponic Test System. Aquacult. Int. 2006, 14, 539–550. [Google Scholar] [CrossRef]
- El-Nakhel, C.; Pannico, A.; Graziani, G.; Giordano, M.; Kyriacou, M.C.; Ritieni, A.; De Pascale, S.; Rouphael, Y. Mineral and Antioxidant Attributes of Petroselinum crispum at Different Stages of Ontogeny: Microgreens vs. Baby Greens. Agronomy 2021, 11, 857. [Google Scholar] [CrossRef]
- Brlek, T. The Effect of Growing Media on Yield and Nutritive Value of Vegetable and Sunflower Microgreens. Master’s Thesis, University of Zagreb Faculty of Agriculture, Zagreb, Croatia, 30 September 2019. [Google Scholar]
- Somerville, C.; Cohen, M.; Pantanella, E.; Stankus, A.; Lovatelli, A. Small-Scale Aquaponic Food Production: Integrated Fish and Plant Farming; Food and Agriculture Organization of the United Nations: Rome, Italy, 2014. [Google Scholar]
- Schmautz, Z.; Graber, A.; Jaenicke, S.; Goesmann, A.; Junge, R.; Smits, T.H. Microbial diversity in different compartments of an aquaponics system. Arch. Microbiol. 2017, 199, 613–620. [Google Scholar] [CrossRef]
- Fabek Uher, S.; Radman, S.; Opačić, N.; Dujmović, M.; Benko, B.; Lagundžija, D.; Mijić, V.; Prša, L.; Babac, S.; Šic Žlabur, J. Alfalfa, Cabbage, Beet and Fennel Microgreens in Floating Hydroponics—Perspective Nutritious Food? Plants 2023, 12, 2098. [Google Scholar] [CrossRef]
- de Farias Lima, J.; Duarte, S.S.; Bastos, A.M.; Carvalho, T. Performance of an aquaponics system using constructed semi-dry wetland with lettuce (Lactuca sativa L.) on treating wastewater of culture of Amazon River shrimp (Macrobrachium amazonicum). Environ. Sci. Pollut. Res. 2019, 26, 13476–13488. [Google Scholar] [CrossRef] [PubMed]
| Growth Performance | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatments | NP (cm) | TL (cm) | RL (cm) | APL (cm) | NL (cm) | SD (cm) | RW (g) | APW (g) | DAPW (g) | DRW (g) | TW (g) | TDW (g) |
| Densities (seeds/cells) | ||||||||||||
| 5 | 4.50 ± 0.60 a | 21.19 ± 3.94 a | 14.41 ± 4.46 a | 6.78 ± 2.33 a | 16.72 ± 2.33 a | 1.11 ± 0.19 a | 0.35 ± 0.10 a | 0.93 ± 0.35 a | 0.06 ± 0.02 a | 0.04 ± 0.04 a | 1.12 ± 0.55 a | 0.10 ± 0.04 a |
| 10 | 8.61 ± 1.34 b | 23.81 ± 3.24 ab | 15.81 ± 2.92 ab | 7.99 ± 1.11 b | 30.83 ± 4.69 b | 1.18 ± 0.21 a | 0.53 ± 0.14 b | 1.62 ± 0.54 b | 0.04 ± 0.02 a | 0.05 ± 0.04 a | 2.16 ± 0.60 b | 0.09 ± 0.03 a |
| 15 | 14.11 ± 2.02 c | 26.26 ± 5.55 b | 17.93 ± 5.17 b | 8.33 ± 1.60 b | 45.89 ± 5.59 c | 1.07 ± 0.15 a | 0.61 ± 0.16 b | 2.02 ± 0.68 b | 0.07 ± 0.01 b | 0.02 ± 0.00 b | 2.63 ± 0.73 b | 0.08 ± 0.02 a |
| 20 | 17.44 ± 1.95 d | 24.56 ± 4.17 b | 15.95 ± 3.88 ab | 8.62 ± 2.15 b | 59.11 ± 7.88 d | 1.08 ± 0.16 a | 1.34 ± 0.66 b | 2.50 ± 0.67 c | 0.08 ± 0.02 b | 0.02 ± 0.01 b | 3.24 ± 0.72 c | 0.10 ± 0.02 a |
| Type of water | ||||||||||||
| DA | 11.61 ± 5.36 | 23.36 ± 4.27 | 16.65 ± 3.72 | 6.71 ± 1.31 | 36.25 ± 15.80 | 1.01 ± 0.15 | 0.89 ± 0.66 | 1.39 ± 0.53 | 0.06 ± 0.02 | 0.03 ± 0.03 | 1.90 ± 0.83 | 0.09 ± 0.03 |
| WPC | 10.72 ± 5.05 | 24.55 ± 4.98 | 15.40 ± 4.86 | 9.15 ± 1.79 | 40.03 ± 17.59 | 1.21 ± 0.16 | 0.53 ± 0.18 | 2.14 ± 0.87 | 0.06 ± 0.03 | 0.03 ± 0.03 | 2.67 ± 1.03 | 0.09 ± 0.03 |
| p-value | ||||||||||||
| DA | 0.78 ns | 0.12 ns | 0.09 ns | 0.66 | 0.09 ns | 0.08 ns | 0.14 ns | 0.001 | 0.12 ns | 0.11 ns | 0.19 ns | 0.10 ns |
| WPC | <0.001 | <0.001 | 0.007 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Interaction | 0.79 ns | 0.16 ns | 0.14 ns | <0.001 | 0.008 | <0.001 | 0.021 | <0.001 | 0.17 ns | 0.56 ns | 0.66 ns | 0.22 ns |
| Growth Performance | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatments | NP (cm) | TL (cm) | RL (cm) | APL (cm) | NL (cm) | SD (cm) | RW (g) | APW (g) | DAPW (g) | DRW (g) | TW (g) | TDW (g) |
| Densities (seeds/cells) | ||||||||||||
| 5 | 3.89 ± 0.57 a | 18.34 ± 2.95 a | 8.74 ± 5.13 a | 6.22 ± 0.75 a | 12.94 ± 2.86 a | 1.14 ± 0.22 a | 0.40 ± 0.08 a | 0.75 ± 0.22 a | 0.05 ± 0.02 a | 0.01 ± 0.01 a | 1.15 ± 0.26 a | 0.06 ± 0.02 a |
| 10 | 7.33 ± 1.00 b | 22.86 ± 2.87 b | 15.54 ± 2.61 b | 7.32 ± 1.09 b | 23.39± 4.08 b | 1.17 ± 0.11 a | 0.57 ± 0.15 a | 1.42 ± 0.30 b | 0.07 ± 0.01 a | 0.02 ± 0.00 a | 1.99 ± 0.27 b | 0.09 ± 0.01 a |
| 15 | 12.06 ± 2.15 c | 23.65 ± 2.73 b | 15.31 ± 2.54 b | 8.34 ± 1.20 c | 36.00 ± 7.06 c | 1.14 ± 0.15 a | 0.69 ± 0.14 ab | 2.15 ± 0.58 bc | 0.09 ± 0.01 b | 0.03 ± 0.01 a | 2.84 ± 0.54 c | 0.12 ± 0.02 b |
| 20 | 15.33 ± 2.11 d | 22.49 ± 3.26 b | 14.34 ± 2.49 b | 8.15 ± 1.10 c | 43.33 ± 5.46 c | 0.99 ± 0.19 b | 0.81 ± 0.31 c | 2.56 ± 0.71 c | 0.11 ± 0.04 b | 0.03 ± 0.01 a | 3.37 ± 0.80 d | 0.15 ± 0.04 b |
| Type of water | ||||||||||||
| DA | 9.50 ± 4.70 | 20.48 ± 3.85 | 12.12 ± 5.50 | 6.66 ± 0.91 | 26.67 ± 11.13 | 1.06 ± 0.20 | 0.69 ± 0.25 | 1.37 ± 0.59 | 0.08 ± 0.03 | 0.02 ± 0.01 | 2.06 ± 0.80 | 0.10 ± 0.04 |
| WPC | 9.81 ± 4.62 | 23.19 ± 2.74 | 14.84 ± 2.08 | 8.35 ± 1.17 | 31.17 ± 13.79 | 1.15 ± 0.16 | 0.55 ± 0.22 | 2.07 ± 0.93 | 0.09 ± 0.04 | 0.02 ± 0.01 | 2.62 ± 1.08 | 0.11 ± 0.05 |
| p-value | ||||||||||||
| DA | 0.80 ns | 0.17 ns | 0.08 ns | 0.66 | 0.08 ns | 0.08 ns | 0.15 ns | 0.001 | 0.12 ns | 0.14 ns | 0.10 ns | 0.10 ns |
| WPC | <0.001 | <0.001 | 0.0006 | <0.001 | <0.001 | <0.0001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Interaction | 0.89 ns | 0.17 ns | 0.12 ns | <0.001 | 0.008 | <0.0001 | 0.0241 | <0.001 | 0.15 ns | 0.56 ns | 0.66 ns | 0.22 ns |
| Growth Performance | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatments | NP (cm) | TL (cm) | RL (cm) | APL (cm) | NL (cm) | SD (cm) | RW (g) | APW (g) | DAPW (g) | DRW (g) | TW (g) | TDW (g) |
| Densities (seeds/cells) | ||||||||||||
| 5 | 2.13 ± 0.93 a | 23.55 ± 9.12 a | 15.53 ± 8.41 a | 8.03 ± 1.24 a | 9.94 ± 5.25 a | 1.24 ± 0.27 a | 0.13 ± 0.06 a | 0.29 ± 0.16 a | 0.01 ± 0.01 a | 0.01 ± 0.00 a | 0.42 ± 0.20 a | 0.02 ± 0.01 a |
| 10 | 3.52 ± 1.47 a | 22.78 ± 5.91 ab | 13.93 ± 5.72 a | 8.85 ± 0.96 a | 16.78 ± 6.37 b | 1.18 ± 0.18 a | 0.17 ± 0.08 a | 0.43 ± 0.15 b | 0.02 ± 0.01 a | 0.01 ± 0.00 a | 0.60 ± 0.22 b | 0.02 ± 0.01 a |
| 15 | 5.17 ± 1.89 a | 23.05 ± 7.79 ab | 14.10 ± 7.48 a | 8.95 ± 1.11 a | 23.46 ± 8.37 c | 0.97 ± 0.14 b | 0.23 ± 0.10 b | 0.53 ± 0.23 b | 0.02 ± 0.01 a | 0.01 ± 0.00 a | 0.76 ± 0.32 b | 0.03 ± 0.01 a |
| 20 | 5.54 ± 2.43 a | 20.41 ± 5.39 b | 12.00 ± 4.99 a | 8.41 ± 1.46 a | 23.83 ± 9.58 c | 0.92 ± 0.19 c | 0.20 ± 0.09 ab | 0.45 ± 0.25 b | 0.02 ± 0.01 a | 0.01 ± 0.01 a | 0.65 ± 0.32 b | 0.03 ± 0.02 a |
| Type of water | ||||||||||||
| DA | 4.42 ± 2.18 | 26.36 ± 7.15 | 17.79 ± 6.74 | 8.58 ± 1.23 | 20.49 ± 8.99 | 1.02 ± 0.20 | 0.18 ± 0.08 | 0.46 ± 0.20 | 0.02 ± 0.01 | 0.01 ± 0.01 | 0.64 ± 0.27 | 0.03 ± 0.01 |
| WPC | 4.12 ± 2.29 | 18.03 ± 3.79 | 9.40 ± 3.01 | 8.63 ± 1.28 | 18.05 ± 9.69 | 1.11 ± 0.25 | 0.20 ± 0.10 | 0.41 ± 0.24 | 0.02 ± 0.01 | 0.01 ± 0.00 | 0.61 ± 0.32 | 0.02 ± 0.01 |
| p-value | ||||||||||||
| DA | 0.88 ns | 0.33 ns | 0.39 ns | 0.19 ns | 0.67 ns | 0.53 ns | 0.88 ns | 0.47 ns | 0.11 ns | 0.17 ns | 0.19 ns | 0.22 ns |
| WPC | <0.001 | 0.63 c | 0.61 c | 0.05 | <0.001 | <0.001 | 0.01 | 0.02 | 0.01 | 0.02 | 0.01 | 0.02 |
| Interaction | 0.04 | <0.001 | <0.001 | 0.84 c | 0.01 | 0.02 | 0.89 c | 0.11 c | 0.14 c | 0.22 c | 0.13 c | 0.11 c |
| Growth Performance | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatments | NP (cm) | TL (cm) | RL (cm) | APL (cm) | NL (cm) | SD (cm) | RW (g) | APW (g) | DAPW (g) | DRW (g) | TW (g) | TDW (g) |
| Densities (seeds/cells) | ||||||||||||
| 5 | 5.88 ± 0.83a | 29.17 ± 3.10 a | 21.72 ± 3.06 a | 7.45 ± 0.93 a | 15.50 ± 3.95 a | 1.16 ± 0.15 a | 43.68 ± 20.45 a | 0.28 ± 0.09 a | 0.03 ± 0.01 a | 0.02 ± 0.02 a | 1.86 ± 0.37 a | 0.05 ± 0.02 a |
| 10 | 11.71 ± 1.86 b | 29.78 ± 3.37 a | 21.23 ± 3.58 a | 8.55 ± 0.80 ab | 26.92 ± 5.27 b | 1.14 ± 0.14 a | 1.43 ± 0.25 b | 0.42 ± 0.11 b | 0.04 ± 0.02 a | 0.02 ± 0.01 a | 1.85 ± 0.35 a | 0.06 ± 0.02 a |
| 15 | 17.83 ± 2.44 c | 30.75 ± 2.18 a | 21.81 ± 2.17 a | 8.94 ± 0.87 ab | 38.08 ± 5.89 c | 1.05 ± 0.18 a | 2.01 ± 0.41 c | 0.58 ± 0.14 c | 0.05 ± 0.02 a | 0.02 ± 0.01 a | 2.58 ± 0.53 b | 0.07 ± 0.02 a |
| 20 | 23.04 ± 2.78 d | 30.55 ± 2.65 a | 21.40 ± 2.85 a | 9.16 ± 1.19 a | 46.54 ± 5.47 d | 1.01 ± 0.14 a | 2.30 ± 0.35 d | 0.67 ± 0.12 d | 0.08 ± 0.14 a | 0.03 ± 0.01 a | 2.97 ± 0.45 b | 0.10 ± 0.13 a |
| Type of water | ||||||||||||
| DA | 14.54 ± 6.88 | 30.66 ± 3.44 | 22.65 ± 3.09 | 8.22 ± 0.98 | 30.69 ± 13.31 | 1.07 ± 0.16 | 23.03 ± 146.74 | 0.48 ± 0.19 | 0.06 ± 0.10 | 0.02 ± 0.01 | 23.50 ± 146.72 | 0.07 ± 0.10 |
| WPC | 14.69 ± 6.68 | 29.26 ± 2.41 | 20.43 ± 2.35 | 8.83 ± 1.25 | 32.83 ± 12.15 | 1.11 ± 0.17 | 1.69 ± 0.67 | 0.49 ± 0.19 | 0.04 ± 0.02 | 0.02 ± 0.02 | 2.18 ± 0.85 | 0.07 ± 0.02 |
| p-value | ||||||||||||
| DA | 0.32 ns | 0.68 ns | 0.56 ns | 0.29 ns | 0.03 | 0.55 ns | 0.70 ns | 0.87 ns | 0.10 ns | 0.19 ns | 0.22 ns | 0.55 ns |
| WPC | <0.001 | 0.57 ns | 0.94 ns | 0.001 | <0.001 | 0.05 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Interaction | 0.48 ns | 0.01 | <0.001 | 0.10 ns | 0.24 ns | 0.08 ns | 0.53 ns | 0.98 ns | 0.62 ns | 0.52 ns | 0.99 ns | 0.12 ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guerreiro, S.L.M.; Cabral Júnior, J.F.G.; Eiras, B.J.C.F.; Miranda, B.d.S.; Lopes, P.C.A.; Melo, N.F.A.C.d.; Luz, R.K.; Sterzelecki, F.C.; Palheta, G.D.A. Integrating Aquaponics with Macrobrachium amazonicum (Palaemonidae: Decapoda) Cultivation for the Production of Microgreens: A Sustainable Approach. AgriEngineering 2024, 6, 2718-2731. https://doi.org/10.3390/agriengineering6030158
Guerreiro SLM, Cabral Júnior JFG, Eiras BJCF, Miranda BdS, Lopes PCA, Melo NFACd, Luz RK, Sterzelecki FC, Palheta GDA. Integrating Aquaponics with Macrobrachium amazonicum (Palaemonidae: Decapoda) Cultivation for the Production of Microgreens: A Sustainable Approach. AgriEngineering. 2024; 6(3):2718-2731. https://doi.org/10.3390/agriengineering6030158
Chicago/Turabian StyleGuerreiro, Sávio L. M., João Francisco Garcez Cabral Júnior, Bruno J. C. F. Eiras, Bruna dos Santos Miranda, Priscila Caroline Alves Lopes, Nuno Filipe Alves Correia de Melo, Ronald Kennedy Luz, Fábio Carneiro Sterzelecki, and Glauber David Almeida Palheta. 2024. "Integrating Aquaponics with Macrobrachium amazonicum (Palaemonidae: Decapoda) Cultivation for the Production of Microgreens: A Sustainable Approach" AgriEngineering 6, no. 3: 2718-2731. https://doi.org/10.3390/agriengineering6030158
APA StyleGuerreiro, S. L. M., Cabral Júnior, J. F. G., Eiras, B. J. C. F., Miranda, B. d. S., Lopes, P. C. A., Melo, N. F. A. C. d., Luz, R. K., Sterzelecki, F. C., & Palheta, G. D. A. (2024). Integrating Aquaponics with Macrobrachium amazonicum (Palaemonidae: Decapoda) Cultivation for the Production of Microgreens: A Sustainable Approach. AgriEngineering, 6(3), 2718-2731. https://doi.org/10.3390/agriengineering6030158







