Objective Assessment of the Damage Caused by Oulema melanopus in Winter Wheat with Intensive Cultivation Technology Under Field Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Field and Settings
2.2. Determination of Leaf Area Decay by Objective Image Analysis
Estimation of Chlorophyll Content by SPAD Index Determination
2.3. Quality Analysis of Wheat Grain Yields by NIR Spectroscopy
3. Results
3.1. Effect of Damage on Photosynthetic Surface Area and Chlorophyll Content
3.2. Yield Quality of Winter Wheat Depends on Insecticide Spraying
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Willocquet, L.; Aubertot, J.N.; Lebard, S.; Robert, C.; Lannou, C.; Savary, S. Simulating multiple pest damage in varying winter wheat production situations. Field Crops Res. 2008, 107, 12–28. [Google Scholar] [CrossRef]
- Wilson, M.C.; Shade, R.E. Survival and development of larvae of the cereal leaf beetle, Oulema melanopa (Coleoptera: Chrysomelidae), on various species of Gramineae. Ann. Entomol. Soc. Am. 1966, 59, 170–173. [Google Scholar] [CrossRef]
- Wellso, S.G.; Hoxie, R.P. Biology of Oulema. In Biology of Chrysomelidae; Jolivet, P., Petitpierre, E., Hsiao, T.H., Eds.; Series Entomologica; Springer: Dordrecht, The Netherlands, 1988; Volume 42, pp. 497–511. [Google Scholar] [CrossRef]
- Kher, S.V.; Dosdall, L.M.; Cárcamo, H.A. Biology, host preferences and fitness of Oulema melanopus (Coleoptera: Chrysomelidae), a recent invasive pest in Western Canada. Arthropod-Plant Interact. 2016, 10, 365–376. [Google Scholar] [CrossRef]
- Lampert, E.P.; Haynes, D.L. Population dynamics of the cereal leaf beetle, Oulema melanopus (Coleoptera: Chrysomelidae), at low population densities. Environ. Entomol. 1985, 14, 74–79. [Google Scholar] [CrossRef]
- Olfert, O.; Weiss, R.M.; Woods, S.; Philip, H.; Dosdall, L. Potential distribution and relative abundance of an invasive cereal crop pest, Oulema melanopus (Coleoptera: Chrysomelidae), in Canada1. Can. Entomol. 2004, 136, 277–287. [Google Scholar] [CrossRef]
- Barari, H. Investigating the damage caused by Oulema melanopus (L.) (Coleoptera: Chrysomelidae) in wheat fields. Plant Prot. Sci. J. Agric. 2019, 41, 43–55. [Google Scholar] [CrossRef]
- Lukács, H.; Jócsák, I.; Somfalvi-Tóth, K.; Keszthelyi, S. Physiological responses manifested by some conventional stress parameters and biophoton emission in winter wheat as a consequence of cereal leaf beetle infestation. Front. Plant Sci. 2022, 13, 839855. [Google Scholar] [CrossRef]
- Griffin-Nolan, R.J.; Slette, I.J.; Knapp, A.K. Deconstructing precipitation variability: Rainfall event size and timing uniquely alter ecosystem dynamics. J. Ecol. 2021, 109, 3356–3369. [Google Scholar] [CrossRef]
- McDowell, N.G.; Sapes, G.; Pivovaroff, A.; Adams, H.D.; Allen, C.D.; Anderegg, W.R.; Arend, M.; Breshears, D.D.; Brodribb, T.; Choat, B.; et al. Mechanisms of woody-plant mortality under rising drought, CO2 and vapour pressure deficit. Nat. Rev. Earth Environ. 2022, 3, 294–308. [Google Scholar] [CrossRef]
- Kauppi, K.; Rajala, A.; Huusela, E.; Kaseva, J.; Ruuttunen, P.; Jalli, H.; Alakukku, L.; Jalli, M. Impact of pests on cereal grain and nutrient yield in boreal growing conditions. Agronomy 2021, 11, 592. [Google Scholar] [CrossRef]
- Szwarc, J.; Niemann, J.; Bocianowski, J.; Jakubus, M.; Mrówczyński, M. Connection between nutrient content and resistance to selected pests analyzed in Brassicaceae hybrids. Agriculture 2021, 11, 94. [Google Scholar] [CrossRef]
- Skendžić, S.; Zovko, M.; Lešić, V.; Pajač Živković, I.; Lemić, D. Detection and evaluation of environmental stress in winter wheat using remote and proximal sensing methods and vegetation indices—A review. Diversity 2023, 14, 481. [Google Scholar] [CrossRef]
- Steinger, T.; Klötzli, F.; Ramseier, H. Experimental assessment of the economic injury level of the cereal leaf beetle (Coleoptera: Chrysomelidae) in winter wheat. J. Econ. Entomol. 2020, 113, 1823–1830. [Google Scholar] [CrossRef] [PubMed]
- Samu, F.; Szita, É.; Simon, J.; Cséplő, M.; Botos, E.; Pertics, B.; Růžičková, J.; Gerstenbrand, R.; Rakszegi, M.; Elek, Z.; et al. Cereal leaf beetle (Oulema spp.) damage reduces yield and is more severe when natural enemy action is prevented. Crop Prot. 2024, 185, 106893. [Google Scholar] [CrossRef]
- Balla, K.; Rakszegi, M.; Li ZhonGy, L.Z.; Békés, F.; Bencze, S.; Veisz, O. Quality of winter wheat in relation to heat and drought shock after anthesis. Czech J. Food Sci. 2011, 29, 117–128. [Google Scholar] [CrossRef]
- Gallun, R.L.; Everly, R.T.; Yamazaki, W.T. Yield and milling quality of monon wheat damaged by feeding of cereal leaf beetle. J. Econ. Entomol. 1967, 60, 356–359. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 14 November 2024).
- Mazurkiewicz, A.; Jakubowska, M.; Tumialis, D.; Bocianowski, J.; Roik, K. Foliar application of entomopathogenic nematodes against cereal leaf beetle Oulema melanopus L. (Coleoptera: Chrysomelidae) on wheat. Agronomy 2021, 11, 1662. [Google Scholar] [CrossRef]
- Ulrich, W.; Czarnecki, A.; Kruszynski, T. Occurrence of pest species of the genus Oulema (Coleoptera: Chrysomelidae) in cereal fields in Northern Poland. Electr. J. Pol. Agric. Univ. 2004, 7, 4. [Google Scholar]
- Sadras, V.O.; Fereres, A.; Ratcliffe, R.H. Wheat growth, yield, and quality as affected by insect herbivores. In Wheat: Ecology and Physiology of Yield Determination; Satorre, E.H., Slafer, G.A., Eds.; Food Product Press: New York, NY, USA, 1999; pp. 183–227. [Google Scholar] [CrossRef]
- Kerchev, P.I.; Fenton, B.; Foyer, C.H.; Hancock, R.D. Plant responses to insect herbivory: Interactions between photosynthesis, reactive oxygen species and hormonal signalling pathways. Plant Cell Environ. 2012, 35, 441–453. [Google Scholar] [CrossRef]
- Császár, O.; Tóth, F.; Lajos, K. Estimation of the expected maximal defoliation and yield loss caused by cereal leaf beetle (Oulema melanopus L.) larvae in winter wheat (Triticum aestivum L.). Crop Prot. 2021, 145, 105644. [Google Scholar] [CrossRef]
- Pobereżny, J.; Wszelaczyńska, E.; Lamparski, R.; Lemanowicz, J.; Bartkowiak, A.; Szczepanek, M.; Gościnna, K. The impact of spring wheat species and sowing density on soil biochemical properties, secondary plant metabolites’ content and Oulema ssp’s presence. PeerJ 2023, 11, e14916. [Google Scholar] [CrossRef] [PubMed]
- Buntin, G.D.; Flanders, K.L.; Slaughter, R.W.; Delamar, Z.D. Damage loss assessment and control of the cereal leaf beetle (Coleoptera: Chrysomelidae) in winter wheat. J. Econ. Entomol. 2004, 97, 374–382. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Császár, O.; Tóthné Bogdányi, F.; Tóth, F.; Lajos, K. Evaluation of two artificial defoliation methods to simulate damage by the cereal leaf beetle (Oulema melanopus) larvae in winter wheat. Acta Aliment. 2022, 57, 115–126. [Google Scholar] [CrossRef]
- Avila-Sakar, G.; Leist, L.L.; Stephenson, A.G. Effects of the spatial pattern of leaf damage on growth and reproduction: Nodes and branches. J. Ecol. 2003, 91, 867–879. [Google Scholar] [CrossRef]
- Bosnyákné Egri, H.; Kerepesi, I.; Keszthelyi, S. Adverse effect of two-spotted spider mite (Tetranychus urticae Koch) on soybean protein composition. Acta Aliment. 2017, 46, 355–360. [Google Scholar] [CrossRef]
- Keszthelyi, S.; Bosnyakne, E.H.; Horváth, D.; Csóka, Á.; Kovacs, G.; Donkó, T. Nutrient content restructuring and CT-measured density, volume attritions on damaged beans caused by Acanthoscelides obtectus Say (Coleoptera: Chrysomelidae). J. Plant Prot. Res. 2018, 58, 91–95. [Google Scholar] [CrossRef]
- Keszthelyi, S.; Pál-Fám, F.; Kerepesi, I. Effect of cotton bollworm (Helicoverpa armigera Hübner) caused injury on maize grain content, especially regarding to the protein alteration. Acta Biol. Hung. 2011, 62, 57–64. [Google Scholar] [CrossRef]
- Hatchett, J.H.; Starks, K.J.; Webster, J.A. Insect and mite pests of wheat. Wheat Wheat Improv. 1987, 13, 625–675. [Google Scholar] [CrossRef]
- Torres-Ruiz, J.M.; Cochard, H.; Delzon, S.; Boivin, T.; Burlett, R.; Cailleret, M.; Martin-St Paul, N.K. Plant hydraulics at the heart of plant, crops and ecosystem functions in the face of climate change. New Phytol. 2024, 241, 984–999. [Google Scholar] [CrossRef]
- Machado, B.B.; Orue, J.P.; Arruda, M.S.; Santos, C.V.; Sarath, D.S.; Goncalves, W.N.; Silva, G.G.; Pistori, H.; Roel, A.R.; Rodrigues, J.F., Jr. BioLeaf: A professional mobile application to measure foliar damage caused by insect herbivory. Comp. Electr. Agric. 2016, 129, 44–55. [Google Scholar] [CrossRef]
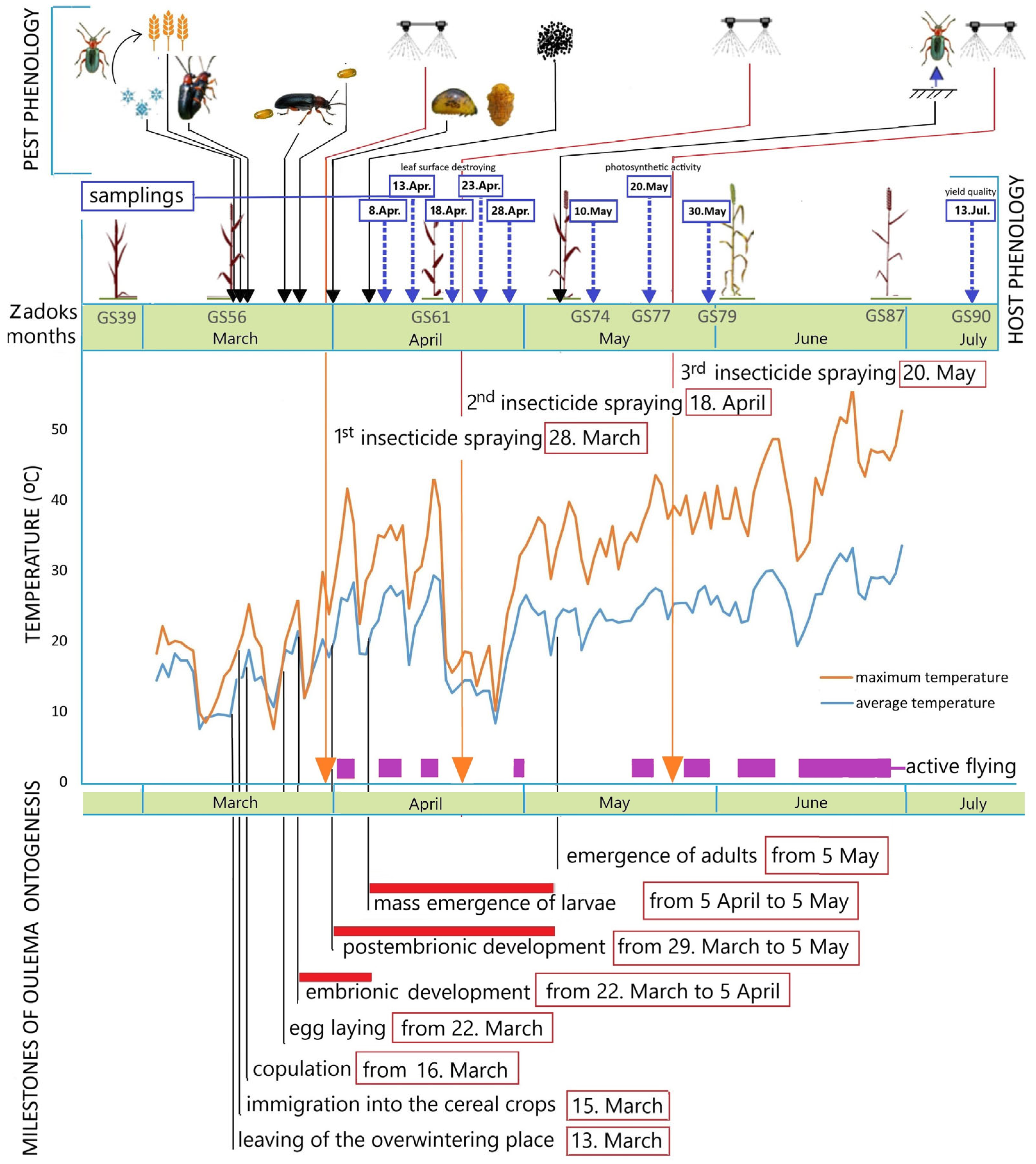


| Date | Insecticide a.i. * | Dose | Fungicide a.i. | Dose | |
|---|---|---|---|---|---|
| 1. | 28 March 2024 | esfenvalerate 50 g/L | 0.2 L/ha | mefentrifluconazole 100 g/L + pyraclostrobin 100 g/L | 0.75 L/ha |
| 2. | 18 April 2024 | tau-fluvalinate 240 g/L | 0.2 L/ha | pyraclostrobin 333 g/L + fluxapyroxad 167 g/L | 0.85 L/ha |
| 3. | 20 May 2024 | tau-fluvalinate 240 g/L | 0.2 L/ha | proquinazid 50 g/L + protiokonazole 200 g/L | 1 L/ha |
| Zadoks: GS74 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| plant height (cm) | untreated | treated | one-way ANOVA | ||||||||||||
| spike | mean | ± | SE | mean | ± | SE | df | F | p | SPAD index | |||||
| 10. | 63–70 | 53.20 | ± | 3.88 | 59.36 | ± | 0.98 | 1 | 4.44 | =0.044 | 54–60 | ||||
| 9. | 56–63 | 36.96 | ± | 6.36 | 55.88 | ± | 0.55 | 9.66 | =0.004 | 48–54 | |||||
| 8. | 49–56 | 28.06 | ± | 4.65 | 58.82 | ± | 1.48 | 42.48 | <0.001 | 42–48 | |||||
| 7. | 42–49 | 36.17 | ± | 5.96 | 53.49 | ± | 1.23 | 8.67 | =0.006 | 36–42 | |||||
| 6. | 35–42 | 30.20 | ± | 4.20 | 58.85 | ± | 0.98 | 47.11 | <0.001 | 30–36 | |||||
| 5. | 28–35 | 29.97 | ± | 5.99 | 58.21 | ± | 1.18 | 22.91 | <0.001 | 24–30 | |||||
| 4. | 21–28 | 18.96 | ± | 4.87 | 59.31 | ± | 1.15 | 69.69 | <0.001 | 18–24 | |||||
| 3. | 14–21 | 35.94 | ± | 5.08 | 57.92 | ± | 0.97 | 19.30 | <0.001 | 12–18 | |||||
| 2. | 7–14 | 23.35 | ± | 6.21 | 54.45 | ± | 1.22 | 25.84 | <0.001 | 6–12 | |||||
| 1. | 0–7 | 24.19 | ± | 4.95 | 59.15 | ± | 0.75 | 52.17 | <0.001 | 0–6 | |||||
| mean | 31.70 | 57.74 | |||||||||||||
| Zadoks: GS77 | ▼ | ▼ | |||||||||||||
| (cm) | df = 1; F = 4.82; p = 0.041 | df = 1; F = 0.35; p = 0.559 | one-way ANOVA | ||||||||||||
| spike | ▲ | ▲ | df | F | p | ||||||||||
| 10. | 63–70 | 20.69 | ± | 5.81 | 57.76 | ± | 1.16 | 1 | 33.42 | <0.001 | |||||
| 9. | 56–63 | 25.34 | ± | 4.83 | 58.01 | ± | 1.39 | 67.34 | <0.001 | ||||||
| 8. | 49–56 | 12.16 | ± | 3.67 | 56.10 | ± | 1.56 | 193.77 | <0.001 | ||||||
| 7. | 42–49 | 5.56 | ± | 2.87 | 57.37 | ± | 1.27 | 434.58 | <0.001 | ||||||
| 6. | 35–42 | 22.71 | ± | 5.47 | 56.18 | ± | 1.30 | 56.77 | <0.001 | ||||||
| 5. | 28–35 | 30.73 | ± | 4.80 | 56.94 | ± | 1.90 | 41.24 | <0.001 | ||||||
| 4. | 21–28 | 24.66 | ± | 5.12 | 57.98 | ± | 1.51 | 62.36 | <0.001 | ||||||
| 3. | 14–21 | 22.99 | ± | 4.21 | 56.90 | ± | 1.47 | 92.41 | <0.001 | ||||||
| 2. | 7–14 | 33.73 | ± | 4.86 | 55.59 | ± | 1.51 | 29.47 | <0.001 | ||||||
| 1. | 0–7 | 29.25 | ± | 3.98 | 58.21 | ± | 0.98 | 74.94 | <0.001 | ||||||
| mean | 22.78 | 57.10 | |||||||||||||
| Zadoks: GS79 | ▼ | ▼ | |||||||||||||
| (cm) | df = 1; F = 0.36; p = 0.551 | df = 1; F = 0.68; p = 0.418 | one-way ANOVA | ||||||||||||
| spike | ▲ | ▲ | df | F | p | ||||||||||
| 10. | 63–70 | 12.32 | ± | 4.31 | 57.65 | ± | 1.75 | 1 | 102.27 | <0.001 | |||||
| 9. | 56–63 | 18.27 | ± | 4.29 | 59.55 | ± | 1.04 | 103.42 | <0.001 | ||||||
| 8. | 49–56 | 27.81 | ± | 5.33 | 56.82 | ± | 1.30 | 30.13 | <0.001 | ||||||
| 7. | 42–49 | 8.84 | ± | 3.20 | 55.22 | ± | 1.73 | 175.24 | <0.001 | ||||||
| 6. | 35–42 | 14.81 | ± | 2.59 | 57.48 | ± | 1.12 | 245.82 | <0.001 | ||||||
| 5. | 28–35 | 28.68 | ± | 3.83 | 55.07 | ± | 1.44 | 44.88 | <0.001 | ||||||
| 4. | 21–28 | 19.37 | ± | 4.10 | 56.73 | ± | 1.43 | 79.63 | <0.001 | ||||||
| 3. | 14–21 | 33.66 | ± | 5.07 | 56.22 | ± | 1.27 | 20.05 | <0.001 | ||||||
| 2. | 7–14 | 14.22 | ± | 4.09 | 55.26 | ± | 1.41 | 96.85 | <0.001 | ||||||
| 1. | 0–7 | 35.22 | ± | 4.49 | 52.42 | ± | 1.55 | 14.10 | <0.001 | ||||||
| mean | 21.32 | 56.44 | |||||||||||||
| Treated (Mean ± SE) | Different (%) | Untreated (Mean ± SE) | One-Way ANOVA | |||||
|---|---|---|---|---|---|---|---|---|
| df | F | p | ||||||
| milling quality | ||||||||
| hectolitre weight | 75.80 ± 0.13 | > | 5.21 | 71.85 ± 0.09 | 1 | 8735.07 | <0.001 | |
| moisture content: | 12.77 ± 0.18 | > | 11.35 | 11.32 ± 0.16 | 933.07 | |||
| mixed content | 0.10 ± 0.00 | < | 66.66 | 0.30 ± 0.00 | 45.50 | |||
| broken seed | 0 ± 0.00 | < | 100 | 0.75 ± 0.03 | 22.60 | |||
| baking quality | ||||||||
| protein content | 12.72 ± 0.08 | > | 28.88 | 9.05 ± 0.06 | 1 | 169.30 | <0.001 | |
| wet gluten content | 26.67 ± 0.16 | > | 35.05 | 17.32 ± 0.13 | 132.67 | |||
| Hagberg falling number | 220.00 ± 0.00 | > | 10.22 | 197.50 ± 2.50 | 2207.75 | |||
| Zeleny sedimentation index | 48.45 ± 0.21 | > | 33.79 | 32.07 ± 0.11 | 156.10 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keszthelyi, S.; Hoffmann, R.; Lukács, H. Objective Assessment of the Damage Caused by Oulema melanopus in Winter Wheat with Intensive Cultivation Technology Under Field Conditions. AgriEngineering 2024, 6, 4538-4548. https://doi.org/10.3390/agriengineering6040259
Keszthelyi S, Hoffmann R, Lukács H. Objective Assessment of the Damage Caused by Oulema melanopus in Winter Wheat with Intensive Cultivation Technology Under Field Conditions. AgriEngineering. 2024; 6(4):4538-4548. https://doi.org/10.3390/agriengineering6040259
Chicago/Turabian StyleKeszthelyi, Sándor, Richárd Hoffmann, and Helga Lukács. 2024. "Objective Assessment of the Damage Caused by Oulema melanopus in Winter Wheat with Intensive Cultivation Technology Under Field Conditions" AgriEngineering 6, no. 4: 4538-4548. https://doi.org/10.3390/agriengineering6040259
APA StyleKeszthelyi, S., Hoffmann, R., & Lukács, H. (2024). Objective Assessment of the Damage Caused by Oulema melanopus in Winter Wheat with Intensive Cultivation Technology Under Field Conditions. AgriEngineering, 6(4), 4538-4548. https://doi.org/10.3390/agriengineering6040259







