Abstract
Closed hydroponics is an environmentally friendly and economical method for growing crops by circulating a nutrient solution while measuring and supplementing various ions contained in the solution. However, conventional monitoring systems in hydroponics do not measure individual ions in the nutrient solution; instead, they predict the total ion content from the pH and electrical conductivity (EC). This method cannot be used to supplement individual ions and adjusts the concentration of the circulating nutrient solution by diluting or supplying a premixed nutrient solution. A more advanced system should be able to identify the concentration of each ion in the nutrient solution and supplement any deficient ions, thus requiring individual ion monitoring systems. Therefore, we first investigated the nitrate, ammonium, phosphate, and potassium (NPK) ion concentration and pH range commonly used for nutrient solutions. Subsequently, we discuss the latest research trends in electrochemical and optical sensors for measuring NPK ions. We then compare the conventional monitoring system (pH and EC-based) and advanced monitoring systems (individual ion sensors) and discuss the respective research trends. In conclusion, we present the hurdles that researchers must overcome in developing agricultural ion sensors for advanced monitoring systems and propose the minimum specifications for agricultural NPK ion sensors.
1. Introduction
Soilless cultivation, also known as hydroponics, is an agricultural method used to grow crops without soil [1]. It is used to cultivate various crops, including leafy greens. There are various hydroponic methods, such as ebb and flow, nutrient film technique (NFT), deep-water culture (DWC), and deep-flow technique (DFT) [2]. These methods can be categorized into closed or open hydroponics based on the circulation of nutrient solutions [3]. In closed hydroponics, nutrient solutions are recirculated and reused. Adopting closed hydroponics not only conserves water but also reduces fertilizer usage by reusing the nutrient solution [4].
There are different types of nutrient solutions, such as those used in the Steiner and Hoagland methods [5]. When nutrient solutions are continuously circulated and reused, the concentrations of nutrients existing in ionic form, absorbed by plants, or released from plants undergo changes. If the ion concentration in the nutrient solution is too high, it can lead to excessive nutrient absorption, potentially causing toxicity to the crops [6]. However, if the ion concentration is too low, crops may not absorb sufficient nutrients for optimal growth. In addition, different crops have varying nutrient requirements at different growth stages [7]. Meeting these specific nutritional needs not only reduces fertilizer usage but also enhances productivity. Therefore, monitoring ion concentrations in nutrient solutions is crucial in closed hydroponic systems.
Traditionally, the assessment of optimal ion concentrations in nutrient solutions has relied on measuring electrical conductivity (EC) and pH [8]. However, these methods only provide information on the overall electrical conductivity and offer no insight into the concentrations of individual ions [9]. Consequently, in closed hydroponic systems where identifying and supplementing deficient nutrients is crucial, the need for sensing platforms capable of measuring the concentrations of individual ions in nutrient solution has emerged [6]. Methods for measuring the individual ion concentrations in nutrient solutions can be broadly categorized into electrochemical and optical approaches [10].
Electrochemical methods are widely employed for ion sensing across various fields, such as agriculture, the environment, and food, and offer real-time monitoring of ion concentrations [11]. These methods, including voltammetry [12,13], potentiometry [14,15], and amperometry [16], observe electrical signals generated from chemical changes to measure ion concentrations. Potentiometry uses ion-selective electrodes (ISEs) to measure individual ion concentrations based on the Nernst equation [17,18]. ISEs detect potential differences arising from ion movement between high and low concentrations through selective binding at specific locations within the membrane. Sensors based on current measurements involve both working and reference electrodes. The applied potential induces the electron transfer reactions of ions in solution, and the resulting current measurement allows for the determination of nutrient concentrations. Thus, the applied potential at the electrode initiates electron transfer reactions of ions in the nutrient solution, and the measured current provides information about the nutrient concentrations.
Optical methods encompass various techniques that primarily detect differences arising from interactions between the analyte, reagent, or light source to measure ion concentrations within a solution. Techniques such as fiber optics and surface plasmon resonance (SPR) detect interactions with the target analyte through optical fibers or SPR, respectively, thereby enabling the detection of ion concentrations at the surface. Colorimetry utilizes reagents that induce color changes upon interaction with target analytes. Based on the linear change in color corresponding to variations in the concentration, this method quantifies the ion concentrations in the solution. Light emitted by an LED at a specific wavelength passes through the mixture, and some light is absorbed before reaching a light-detecting sensor, such as a photodiode or a phototransistor. The transmitted light is then converted into a signal, typically a voltage or current, and using this value, the individual ion concentrations can be determined. The absorbance is calculated based on the Beer–Lambert law. Although optical methods exhibit a high sensitivity and selectivity, they often require laboratory equipment and expertise. However, recent advancements in microfluidic platforms, such as lab-on-a-chip (LOC), have led to the development of devices capable of detecting various ions in liquid samples.
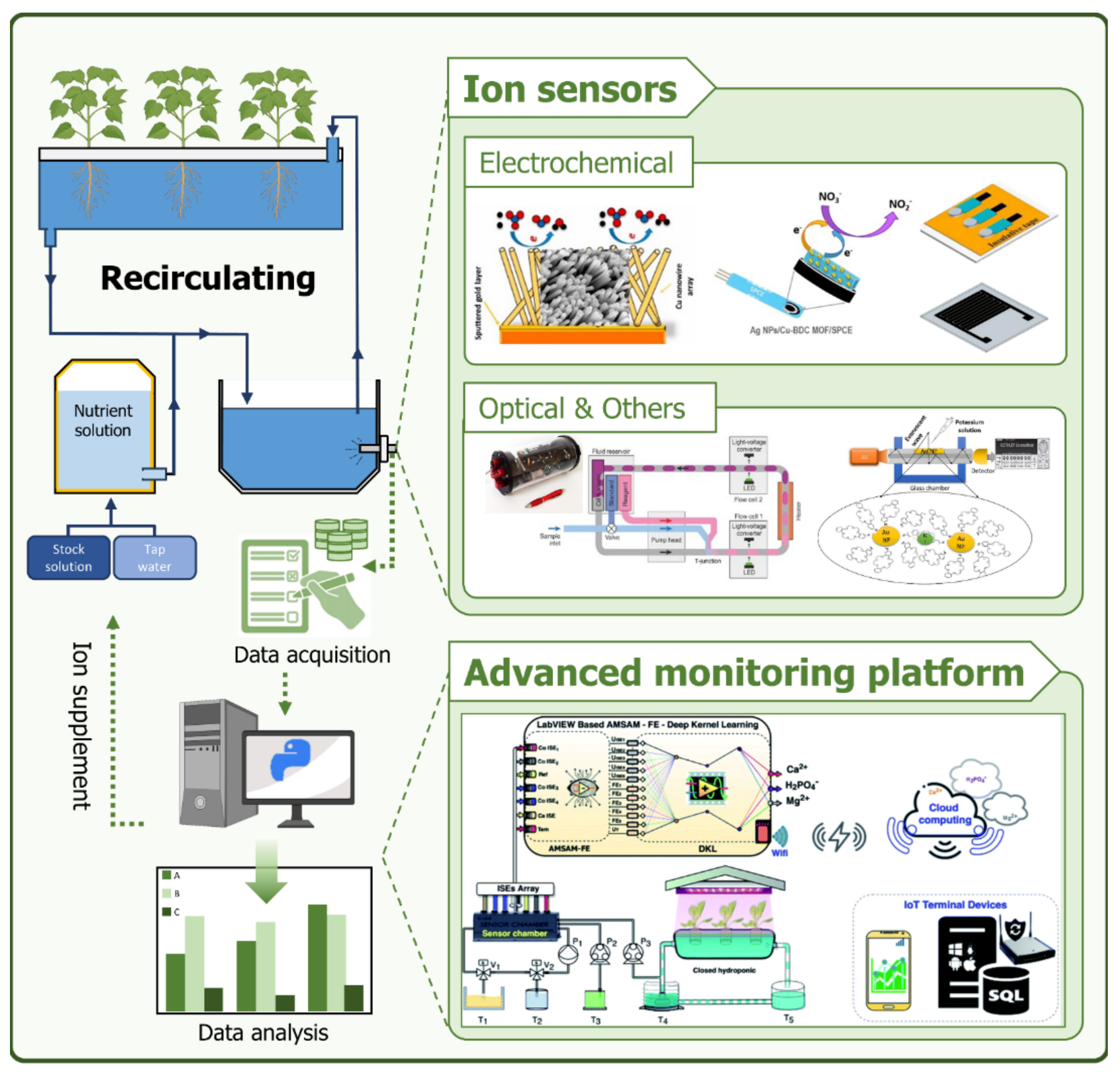
This review not only covers sensors and sensing platforms capable of measuring NPK ions but also discusses ion monitoring systems composed of these sensors (Figure 1). In Section 2, we provide an overview of the types of hydroponics and standard nutrient solution concentrations used in hydroponic systems. Section 3 focuses on sensors and sensing platforms for measuring ion concentrations related to three essential fertilizer elements: nitrogen, phosphorus, and potassium (NPK). Finally, in Section 4, we discuss the current ion concentration measurement methods applied in smart farming or hydroponic systems, as well as comprehensive ion concentration monitoring systems.
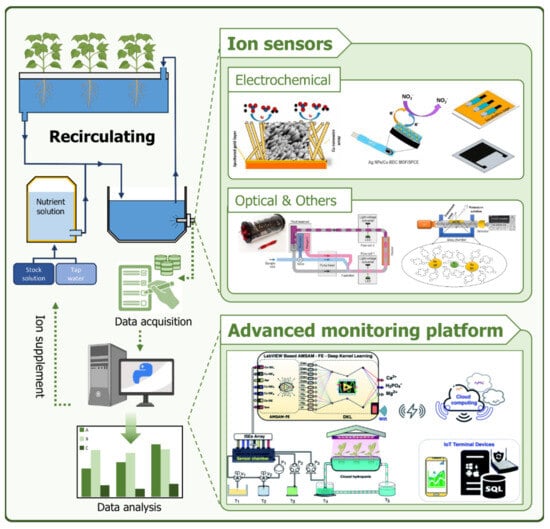
Figure 1.
Schematic diagram of an advanced ion monitoring platform applied to closed hydroponics systems.
2. Composition of Nutrient Solutions Used in Hydroponics
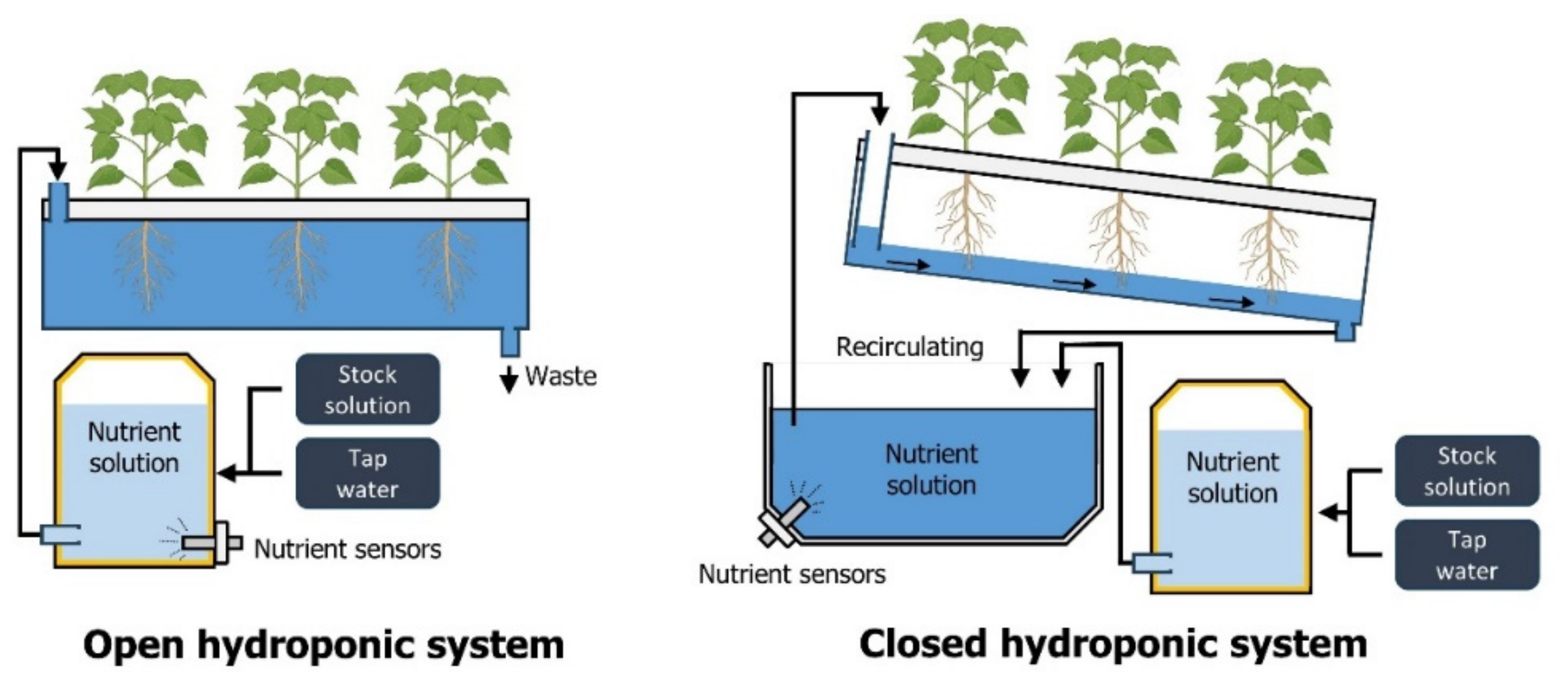
Crop growth requires not only NPK but also essential elements such as calcium, magnesium, and sulfur, and trace elements such as iron, manganese, copper, zinc, boron, and molybdenum [19,20]. In traditional soil cultivation, plants absorb nutrients from the soil or receive them from fertilizers. However, hydroponic systems involve diluting essential nutrients in a nutrient solution and supplying them directly to crops [21]. Hydroponics offers several advantages over traditional soil cultivation methods. First, as nutrients are supplied directly without the need for soil, labor costs during fertilization are reduced [22]. This approach ensures an even distribution of nutrient concentrations across individual crops, thereby facilitating a uniform nutrient supply. Consequently, the quality of the harvested produce is more consistent, and overall fertilizer usage can be minimized compared to conventional cultivation methods [22,23]. Second, hydroponics typically requires less water than traditional soil-based agriculture [24]. It can be applied in regions with water scarcity where it is less affected by climatic conditions. Supplying adequate water quantity through irrigation is crucial in conventional agriculture [25]. However, in such systems, water supplied through irrigation may not efficiently reach plant roots because of drainage or evaporation. In contrast, a closed hydroponic system prevents additional water usage through evaporation or drainage because the nutrient solution circulates (Figure 2). These advantages make closed hydroponics a sustainable and efficient alternative to traditional soil-based cultivation methods, especially for addressing water scarcity issues and achieving uniform nutrient distribution.
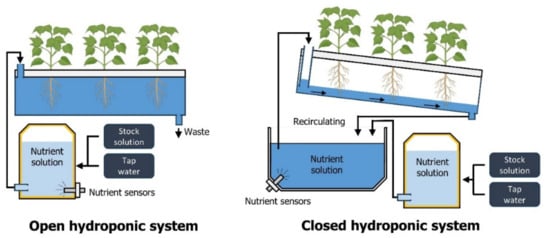
Figure 2.
Schematics of open and closed hydroponic systems. Closed hydroponic systems recycle and reuse the nutrient solution.
Various types of nutrient solutions, each with different nutrient concentrations, are employed in hydroponic systems used in agriculture. The nutrient requirements of crops vary during different growth stages and are an area of extensive research. Additionally, various nutrient solutions developed by researchers such as Arnon, Steiner, and Resh have been employed in studies exploring hydroponic cultivation. Numerous studies have emphasized that the optimal nutrient solution concentration required to achieve maximum yield and quality varies depending on the crop type and growth stage. To understand conditions that promote plant growth, we also investigated the composition of the nutrient solutions, which are summarized in Table 1. The reference value in Table 1 represents the average concentrations of NPK ions commonly found in nutrient solutions used for hydroponics [26]. According to our findings, the concentrations of nitrate, ammonium, phosphate, and potassium ions in the nutrient solution ranged from 0.758 to 9.99, 0 to 6.2091, 0.0416 to 2.9981, and 1.6625 to 15.1669 mM, respectively. Additionally, the overall pH of the nutrient solution was observed to be in the range of 5.5–7.0, indicating a slightly acidic, close-to-neutral condition. Therefore, sensors capable of monitoring the concentration of each ion should be designed to provide linear measurement results within these ranges of nutrient solution compositions and should operate efficiently under the pH conditions of the nutrient solution.

Table 1.
Contents of NPK ions in nutrient solution.
3. State-of-the-Art Sensors for NPK Ion Measurement
Nutrients exist in solutions in the ionic form, and it is crucial that they are present in a form suitable for plant absorption. This chapter introduces the various methods for measuring NPK in nutrient solutions. Considering the form in which the nutrient is absorbed by plants, methods targeting nitrate and ammonium ions for nitrogen, phosphate for phosphorous, and potassium ions for potassium are introduced. Table 2 summarizes the ions targeted by each ion sensor, the method used, and the specifications and features of the sensor.

Table 2.
Spec and feature of the advanced ion sensors.
- Electrochemical sensors consistently demonstrate superior sensitivity and flexibility across all ion targets, making them a reliable choice for precise nutrient monitoring. For instance, voltammetry-based sensors achieve LODs as low as 10−⁹ M, particularly for nitrate and potassium ions.
- Optical sensors, while less sensitive than electrochemical methods, excel in non-invasive and portable applications. Their compatibility with field monitoring and lab-on-a-chip technologies makes them suitable for on-site measurements, especially in resource-limited settings.
- Transistor-based sensors offer the best performance for long-term monitoring, with lifespans extending up to 6 months for ammonium and potassium detection. Their durability and stability make them ideal for extended deployments in agriculture or environmental monitoring.
- Linear range performance varies significantly between methods. Electrochemical sensors provide broad ranges suitable for various nutrient concentrations, whereas optical sensors focus on narrower ranges with specialized applications.
- Lifespan limitations remain a challenge, particularly for optical and certain electrochemical sensors, which may require frequent replacement or recalibration. However, recent innovations such as multi-layered coatings and nanomaterials are extending operational durability.
3.1. Nitrogen Sensors for Hydroponics
In plants, nitrogen is absorbed as nitrate and ammonium. Nitrogen plays a crucial role in the biochemical and biological aspects of plants and is directly linked to crop yield and quality of crops [65]. Numerous studies have been conducted on the nitrogen content of nutrient solutions by exploring various ratios of nitrate to ammonium to supply nitrogen efficiently. Boon et al. reported that maintaining a low NH4/NO3 ratio under poor light conditions during the initial growth stage and increasing it towards the end resulted in superior lettuce weight and quality of lettuce [66]. Nitrate and ammonium ion concentrations play crucial roles in providing plants with the necessary nitrogen during growth. To prevent the excessive accumulation of ammonium in plants and create an optimal environment for plant growth, it is essential to measure the nitrogen content in the nutrient solution according to the requirements of the plant.
3.1.1. Nitrate Sensors for Hydroponics
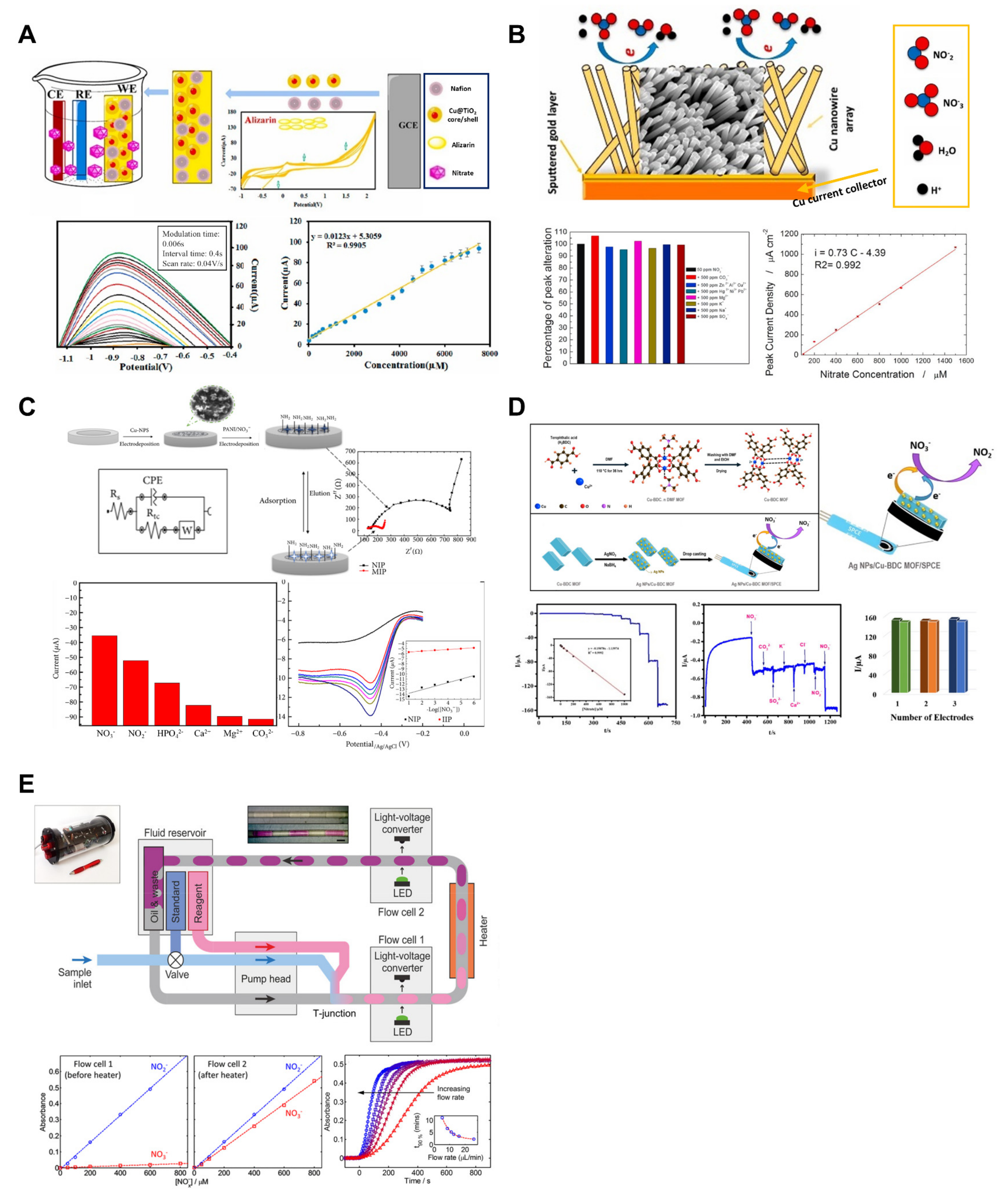
Owing to its advantages, such as low cost, high sensitivity, low detection limit, simplicity, and short response time, an electrochemical method is employed for nitrate ion detection. Proper surface treatment of the electrodes is necessary for electrochemical analysis, and numerous studies have been conducted in this regard. Among them, core–shell structures containing titanium oxide have been widely used in research because of their high chemical stability, good environmental safety, ion-exchange ability, chemical activity, and low cost. Amini et al. developed a voltammetric nitrate sensor based on Cu@TiO2 core–shell and Nafion/polyalizarin yellow R conductive polymers on a glassy carbon electrode (Figure 3A) [30]. The synthesized Cu nanoparticles and Cu@TiO2 core–shell were used for the surface modification of a glassy carbon electrode in conjunction with conductive polymers such as Nafion and Polyalizarin yellow R. The electrode’s limit of detection (LOD) was 2.1 μM, and the linear range was observed to be 5–7500 μM. In the repeatability test conducted at a nitrate concentration of 1000 μM, the coefficient of variation was found to be 2.5% across six consecutive measurements. The sensor’s lifespan was assessed, showing a retention of 98.5% and 92% of the initial current on the 1st and 21st days, respectively. Additionally, selectivity tests performed at a nitrate concentration of 800 μM in the presence of interfering ions such as Br−, Cl−, F−, Na⁺, and K⁺ (at 50-fold concentrations) demonstrated that the sensor was unaffected by these ions. Patella et al. developed a voltammetric nitrate sensor using a Cu nanowire array (Figure 3B) [31]. The copper nanowires used in the sensor were obtained via simple galvanic deposition. The nanostructured sensors have an LOD of less than 10 μM and exhibit two linear ranges of 10–50 μM (sensitivity of 0.0636 μA μM−1 cm−2) and 50–1500 μM (sensitivity of 0.73 μA μM−1 cm−2). The selectivity of the sensor was verified through interference studies. When measuring samples containing calcium, magnesium, potassium, chloride, heavy metals, and carbonate ions, the highest interference was shown at approximately 5%.
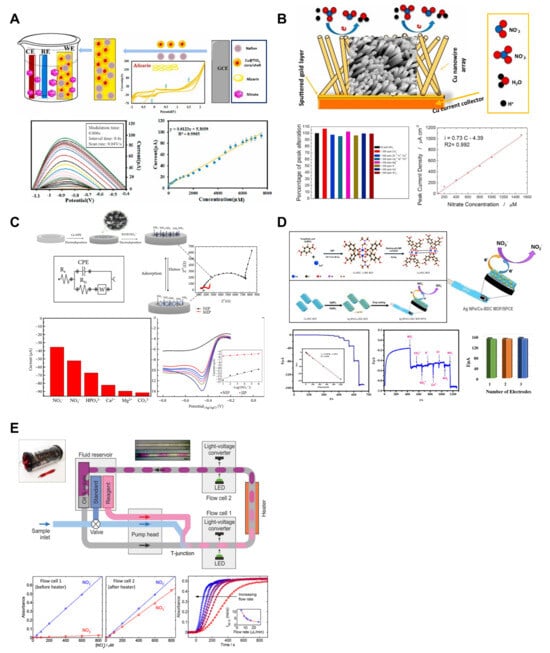
Figure 3.
Nitrate ion sensors. (A) Voltammetric nitrate sensor based on Cu@TiO2 core–shell and conductive polymers on a glassy carbon electrode. Reproduced with permission from Ref. [30]. (B) The surfaces of the electrodes were modified with a copper nanowire array by using galvanic deposition into a nanoporous membrane. Reproduced with permission from Ref. [31]. (C) Copper nanoparticles and aniline-modified glassy carbon electrodes were developed. Reproduced with permission from Ref. [67]. (D) Silver nanoparticles and copper (II)-terephthalate metal–organic frameworks hybrid were synthesized and used to modify the surface of the electrode. Reproduced with permission from Ref. [35]. (E) A droplet microfluidic chip-based colorimetric nitrate sensor was developed. Reproduced with permission from Ref. [36].
Numerous studies have reported the modification of the electrode surface using nanomaterials or nanostructures such as copper nanowires [66]. Essousi et al. developed a nitrate-sensing sensor based on an ion-imprinted polymer (IIP) matrix using a copper-nanoparticles–polyaniline nanocomposite (Cu-NPs/PANI) (Figure 3C) [67]. The aniline concentration in the film was adjusted to achieve the optimal conditions for the IIP film. The current was highest when the monomer concentration was 0.1 M, indicating that insufficient quantities of the functional monomer resulted in the formation of an inadequate number of binding sites, leading to low current. Conversely, an excessive monomer concentration resulted in the thickening of the IIP film, burying the binding sites within the polymeric matrix and rendering them nonfunctional. The sensor exhibited an LOD of 0.2 μM and a linear range of 1 μM–100 mM. To evaluate the reproducibility of the sensor, signals were measured using seven independently fabricated sensors for 1 mM nitrate, and the RSD was found to be 3%, demonstrating high reproducibility. Additionally, for durability assessment, the sensors were soaked in 0.1 M hydrochloric acid solution and tested daily. The results showed an RSD of 5% for 1.0 mM nitrate over a period of four weeks. Lastly, for 0.1 mM nitrate, tests conducted with the addition of interfering ions (NO2−, Ca+, PO43−, and Mg2+) at 100-fold concentration revealed no impact on detection. This was interpreted as the complementary cavities in the polymer matrix providing selectivity through interactions with the unique molecular structure of nitrate. Amali et al. synthesized a hybrid of silver nanoparticles (Ag NPs) and copper (II)-terephthalate metal–organic frameworks (Cu-BDC MOFs) to develop a sensor for nitrate detection (Figure 3D) [35]. This hybrid material was applied to a screen-printed carbon electrode. Cuboid-shaped Cu-BDC MOFs with numerous spherical Ag NPs deposited on them through redox reactions enhanced electrical conductivity, aligning with the characteristics of amperometry, which is a method for measuring substance concentration. The sensor demonstrated an LOD of 0.24 µM and a linear range of 0.5 to 1000 µM. Selectivity experiments were conducted with co-interfering species, such as CO32−, SO42−, K+, Ca2+, Cl−, and NO2−. The sensor exhibited excellent selectivity for nitrate measurement, which is attributed to three reasons. First, the high binding efficiency of nitrate on the Cu active centers facilitates nitrate reduction. Second, the redox process of the Cu-BDC MOFs promotes faster nitrate-to-nitrite reduction kinetics than the interference ions. Finally, the use of a negative operating potential in amperometry may restrict the oxidation or reduction in other electroactive compounds.
In addition to voltammetry, these measurement methods utilize ISEs. Solid-contact ISEs (SC-ISEs), which replace the inner filling solution commonly used in conventional commercial ISEs (liquid-contact type), offer easier miniaturization and flexible design than liquid-contact ISEs [31]. Kim et al. synthesized a reduced graphene oxide aerogel (rGOA) with a porous structure to develop SC-ISEs for measuring nitrate and calcium [16]. Carbon materials are highly hydrophobic, making them suitable as ion-to-electron transducers for SC-ISEs because they do not form a polymeric sensing membrane or a solid-contact electrode interfacial aqueous film. They designed a disposable, low-cost potentiometric sensor for measurements in complex and easily contaminated environments. The rGOA served as an ion-to-electron transducer in the intermediate film, and the sensor exhibited excellent sensitivity (for nitrate and calcium, Nernstian slopes of 59.1 and 28.4 mV/decade, respectively) because of its wide surface area arising from its porous structure and outstanding electrical conductivity. Furthermore, the electrode exhibits stable potential differences because of its large double-layer capacitance and rapid charge transfer. For nitrate measurement, the LOD was determined to be 759 nM, demonstrating high precision, and a wide linear range from 10−8 to 10−1 M was observed.
Nightingale et al. developed a droplet microfluidic-based in situ nitrate and nitrite sensor (Figure 3E) [36]. This sensor utilizes Griess reagent to measure nitrate and nitrite. Initially, nitrite reacts with the Griess reagent, causing a color change. Subsequently, nitrate is reduced to nitrite by vanadium(III) and undergoes a reaction for measurement. To promote the reduction in nitrate, localized heating is applied after the nitrite measurement, and the sample/reagent mixture containing reduced nitrate is measured again. The system employs droplet flow using oil. In an LOC system, the use of droplets has several advantages. First, laminar flow in continuous microfluidics restricts mixing between two fluids, whereas discrete droplets separated by immiscible oil promote mixing within each droplet through chaotic advection. Second, Taylor dispersion caused by friction between the channel walls and fluid in continuous flow is avoided in droplet flow. The composition of each droplet is maintained as the sample moves, allowing the examination of reactions within each droplet from formation to measurement and ensuring a uniform composition until the transported fluid reaches the measurement point. Finally, direct contact of the fluid with the channel walls is avoided, eliminating the need for a separate flushing process. The LOD of the developed sensor was determined to be 1.7 μM, with a linear range confirmed to span from 1.7 × 10−6 to 8 × 10−4 M. A particularly noteworthy aspect of the developed sensor is its ability to consume only about 2.8 mL of reagent and sample per day, allowing for measurements every 10 s for 11 months using just 1 L of reagent. This highlights its significant potential for application in nutrient solution monitoring systems for hydroponics.
3.1.2. Ammonium Sensors for Hydroponics
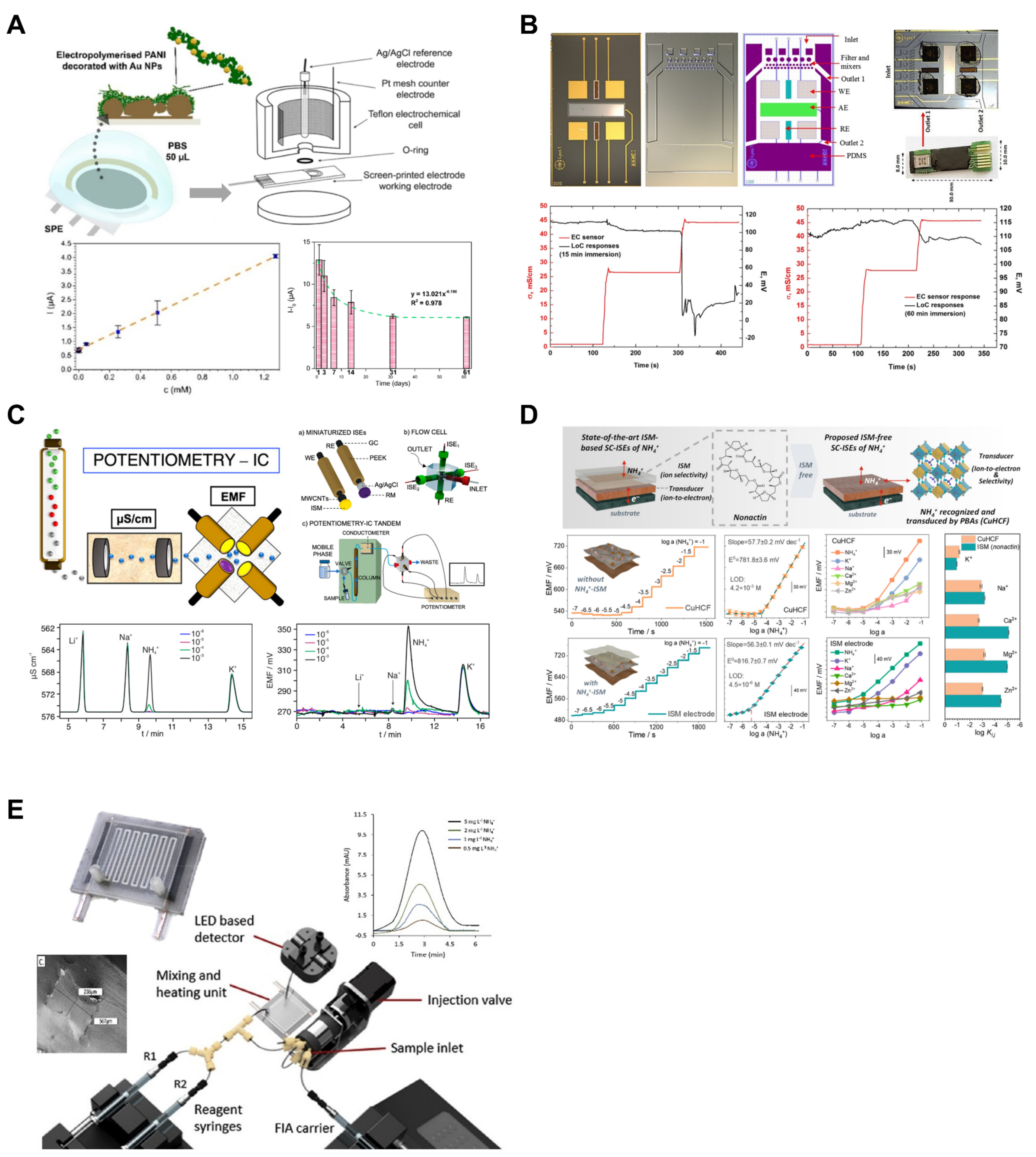
Polyaniline (PANI) is a conductive polymer with chemiresistive properties that behaves like a p-type semiconductor. It reacts with NH3, undergoes deprotonation, and loses conductivity, making it the subject of extensive research for NH3 gas detection [68,69]. Additionally, the ability of PANI to store NH4+ makes it suitable for application as an NH4+-attracting and -storing material [70]. Inspired by these properties, Korent et al. developed an Au-decorated polyaniline-based sensing platform for aqueous ammonia monitoring (Figure 4A) [44]. The sensor was operated using an amperometric method. When compared to a pure PANI sensor and a PANI sensor with 20 nm Au NPs from previous research, the developed sensor achieved a significantly lower LOD (1.44 µM) and LOQ (2.55 µM), being 17 times and 20 times lower, respectively. The sensor demonstrated a wide detection range with linear ranges of 0–5.1 mM and 5.1–510 mM. During stability testing, the sensor was stored in a desiccator for 61 days, and consistent measurements were taken on specific storage days (3, 7, 14, 31, and 61 days). The results showed a progressive decrease in the current response of the sensor, attributed to the use of a small-molecule volatile acid (HCl) as a dopant, leading to a reduction in the sensor’s conductivity. The current response was found to decrease gradually and become stable after two months of storage, and it was reported that calibration would be necessary when using the sensor thereafter.
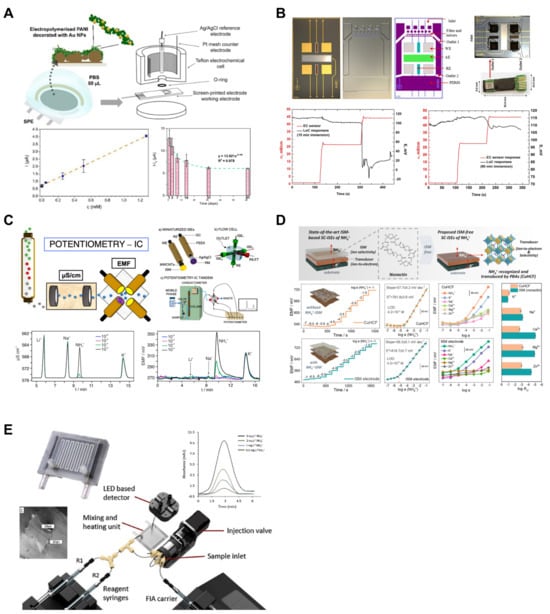
Figure 4.
Ammonium ion sensors. (A) For amperometric detection of ammonium ion, polyaniline and gold nanoparticles were applied to the commercial screen-printed electrode. Reproduced with permission from Ref. [44]. (B) Electrodes were integrated in the lab-on-a-chip to detect ammonium ions in flowing water. Reproduced with permission from Ref. [41]. (C) Ion chromatography and potentiometry were integrated. Each ion was separated after the chromatographic column and was orderly detected in ISEs. Reproduced with permission from Ref. [42]. (D) A Prussian blue analog of copper(II)-hexacyanoferrate-based ISM-free potentiometric ammonium sensor was developed. Reproduced with permission from Ref. [43]. (E) A colorimetric lab-on-a-chip ammonium ion sensor with a heating system was suggested. The heating system accelerates colorimetric reaction. Reproduced with permission from Ref. [46].
Gallardo-Gonzalez et al. developed a fully integrated LOC device for real-time ammonium measurements (Figure 4B) [41]. The developed device combines a microfluidic platform with potentiometric electrodes, including a transducer array (gold working electrode (WE), Ag/AgCl reference electrode (RE), and Pt auxiliary electrode (AE)), a polydimethylsiloxane (PDMS) microfluidic chip containing a micromixer and filter, and a detection chamber. The four AEs included in the sensor successfully detected ammonium ions in the sample flowing through the chip in a laminar flow. The passive-type sensing platform, which does not require actuators or pumps commonly used in traditional LOC devices, makes the device more compact and straightforward. In addition, as it does not require a separate power source, it can be used in inaccessible environments. The LOD of the developed device was reported as 40 μM, with a linear range of 40–100 μM. Regarding the sensor response time, experiments were conducted with stagnant samples and samples with laminar flow, showing that the LOC device had a delay of approximately 15 s compared with a standard EC sensor in experiments conducted in a beaker. However, in experiments conducted with flowing water, no delay was observed in the LOC device. This was attributed to the application of laminar flow inside the chip, which promoted diffusion and quickly reached the detection chamber within the device.
Ammonium ion-selective membranes (ISMs) play a crucial role in selectively allowing only ammonium ions to come into contact with the electrode using ionophores [71]. Nonactin is an ionophore employed as an ammonium ion receptor [72]. However, Wang et al. reported that because of the similar charge-to-radius ratios of K+ and NH4+ (0.133 nm and 0.143 nm, respectively), K+ strongly interferes with NH4 sensors based on nonactin [73]. Gil et al. overcame the limitations of nonactin ionophores by designing a potentiometric ion chromatography (IC) system (Figure 4C) [42]. The measurements obtained using the developed device allowed for the acquisition of time-series data for NH4+ and interference cations such as Na+ and K+ in a separate form. The developed system includes a multielectrode flow cell consisting of three types of electrodes (NH4+, Na+, and K+), with each ISM arranged to contact the fluid containing ammonium ions as it flows through the channels. Each ISE utilized in the system is a potentiometric sensor that employs glassy-carbon-based, all-solid-state ISEs. The ions present in the supplied sample are sequentially directed to the multi-electrode flow cell as they pass through a chromatographic column, where they are then measured. The LOD for the developed system is 0.3 μM, with a linear range observed from 1.0 to 1000 μM.
Xu et al. created a sensor capable of measuring ammonium ions without the use of ISMs using a Prussian blue analog of copper(II)-hexacyanoferrate (CuHCH) (Figure 4D) [43]. Prussian blue analogs (PBAs) possess electronic and ion-conducting characteristics, allowing them to store ions at crystal sites or perform electron transfer [74]. CuHCF-PBA was produced via a co-precipitation method using Cu(NO3)2 and K3[Fe(CN)6] as precursors. It operates based on the potentiometric response resulting from electron transfer between the Fe redox center and ammonium ions. A comparison with an ISE using the nonactin ionophore revealed that the LODs for CuHCF-PBA and ISE were 42 μM and 4.5 μM, respectively. The authors suggested that the difficulty of low concentrations of ammonium ions diffusing into solid CuHCF makes it challenging to achieve thermodynamic equilibrium. A selectivity evaluation using the separation solution method (SSM) confirmed that ions other than K+ (Na+, Ca+, Mg2+, and Zn2+) did not significantly affect the potential response. The selectivity coefficient for K+, considered the most significant interfering factor, was reported as −1.15 for the CuHCF electrode and −0.95 for the ISE, indicating the superior selectivity of the CuHCF electrode compared to the nonactin ISE. Furthermore, the estimated production cost of a single CuHCF electrode was 2.4 USD × 10−4, making it cost-effective compared to the 0.09 USD for an ISM electrode.
Electrochemical electrode-based measurement methods such as ISEs offer advantages such as low cost and ease of use. However, signal drift, which can occur during continuous measurements involving prolonged interactions with the target analyte, renders continuous monitoring challenging [75]. Fornells et al. developed a LOC sensing platform to measure ammonium in environmental water (Figure 4E) [46]. The microfluidic heater integrated into the developed platform was fabricated using a multimaterial 3D printer and was utilized to facilitate the reaction between ammonium ions and the modified Berthelot reagent. Setting the heater temperature to 60 °C enabled a significantly shorter reaction time (1.6 min) than the conventional reaction time (8 min). Moreover, linear absorbance values were confirmed for NH4+ concentrations ranging from 0.5 to 5 mgL−1, with an LOD of 0.15 mgL−1.
3.2. Phosphorus Sensors for Hydroponics
Absorption of an adequate amount of phosphorus during the early stages of crop growth plays a crucial role in the development of plant reproductive structures [76]. Additionally, it strengthens roots, enhances plant vitality, and improves disease resistance. However, phosphorus present in the soil tends to exist in an insoluble form bound to soil cations, rather than in a form readily absorbed by plants as phosphates [77]. In hydroponics, phosphorus is supplied in the form of phosphate ions [78]. Phosphate ions exist in different forms depending on the pH conditions. Specifically, phosphate predominantly exists as dihydrogen phosphate (H2PO4−) around pH 5, monohydrogen phosphate (HPO42−) at pH 7.3, and triphosphate (PO43−) at pH levels higher than 7. Since the pH of nutrient solutions in hydroponic systems typically ranges from 5.5 to 6.5, phosphate sensors used in such systems are primarily associated with the detection of H2PO4− [79]. This relationship highlights the importance of calibrating phosphate sensors to accurately measure dihydrogen phosphate concentrations within this pH range to ensure effective nutrient management in hydroponic agriculture. To ensure that phosphate ions are supplied to plants in appropriate amounts, it is essential to determine the concentration of ions in the nutrient solution.
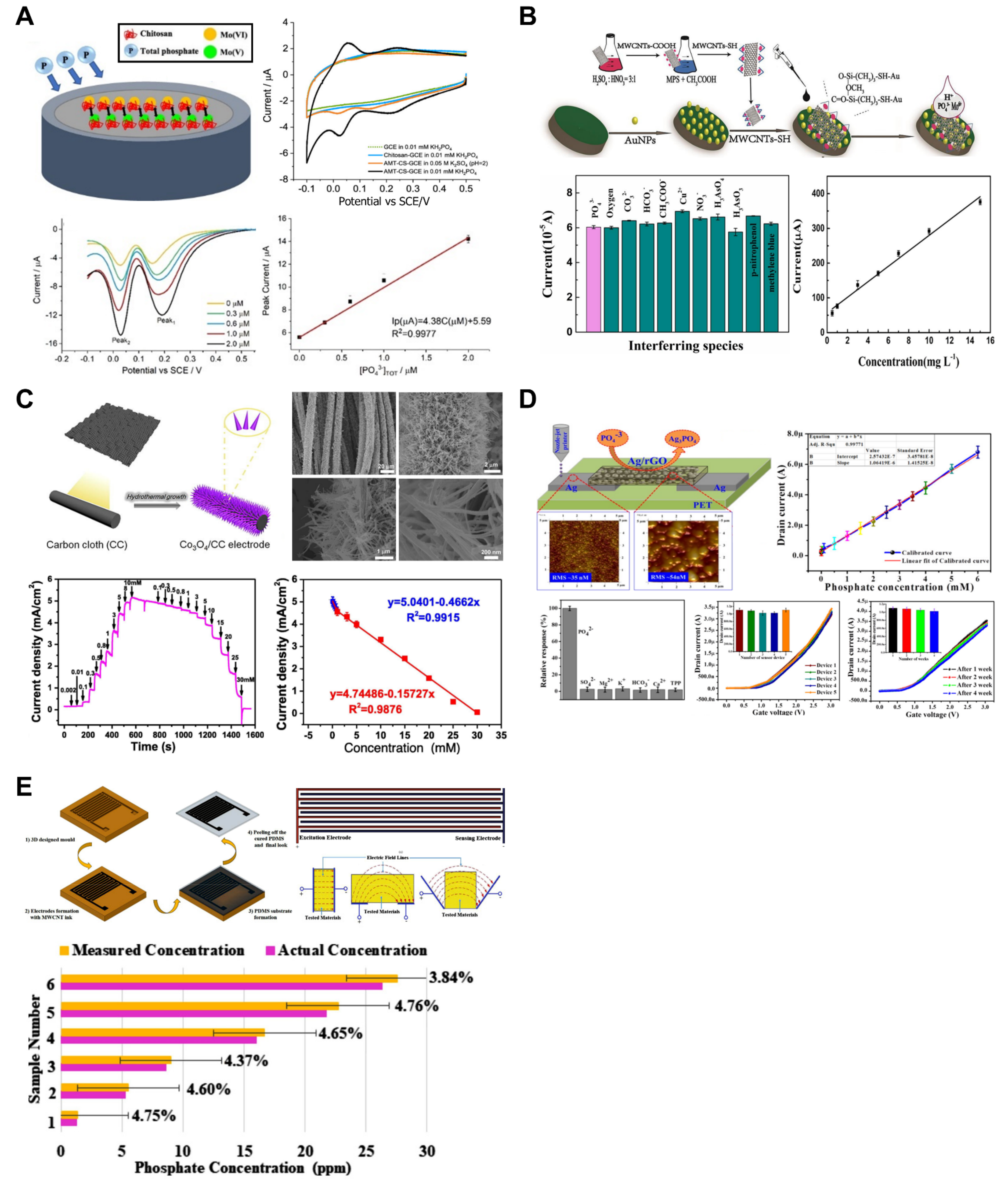
Owing to their characteristics, solid-state electrodes offer advantages such as portability and reduced maintenance requirements, as they do not require an inner filling solution [50,52]. Based on these advantages, research related to various materials (such as cobalt, nickel, molybdenum, and tungsten) [80,81] and forms [15,82,83] of solid-state electrodes has been reported. However, ISEs for phosphate measurement based on the potentiometric method exhibit Nernstian sensitivity, where triply charged anions show a sensitivity equivalent to one-third that of singly charged anions [84]. Additionally, these electrodes exhibit a significant pH dependency on the measured solution. The size of the phosphate anion further complicates the fabrication of size-exclusion phosphate-selective membranes. Furthermore, when measuring phosphate through amperometric or voltammetric detection based on redox reactions, electrochemical reduction and oxidation are challenging because of the structure of the phosphate ions [85]. In this context, the chemical derivatization of phosphate is required for its measurement, and several studies have reported electrode modifications using preloaded reagents or enzymatic systems [17,20,86,87,88,89]. Compton et al. developed an ammonium molybdate tetrahydrate (AMT)–chitosan-modified glassy carbon macroelectrode (AMT-CS-GCE) for phosphate detection (Figure 5A) [48]. The developed sensor utilized CV and square wave voltammetry (SWV). The reduction in solution-phase molybdate(VI) and phosphate was investigated through CV, and when measured using SWV, the sensitivity was improved by reducing the influence of capacitative background currents. The developed electrode exhibited an LOD of 0.15 μM (sensitivity: 4.4 ± 0.1 μA/μM) with a linear range of 0.3–2.0 μM at pH 2.0 and an LOD of 0.17 μM (sensitivity: 6.5 ± 0.3 μA/μM) with a linear range of 0.05–0.8 μM at pH 5.8. At pH 2.0, the signal became saturated when the KH2PO4 concentration exceeded 2.0 μM due to the saturation of adsorption sites on the electrode surface, while at pH 5.8, the signal saturation was observed at concentrations above 1.0 μM. Additionally, interference tests revealed that Cl− and NO3− at concentrations below 1 mM affected the detection of PO₄3− by approximately 12–13%. Recovery tests conducted using mixed samples of tap water and pond water with KH2PO4 standard solutions (0.05, 0.15, and 0.35 μM) demonstrated recovery rates close to 100%. Huang et al. developed a voltammetric sensor based on silanized multi-walled carbon nanotubes (MWCNTs-SH) combined with Au nanoparticles (AuNPs) (Figure 5B) [49]. The MWCNTs-SH used in the sensor increased the catalytic response current of the molybdophosphate complex by approximately two times compared to that of pristine MWCNTs. This enhancement was attributed to the nanotube structure with nanodefects and conical emission centers formed on the outer wall, increasing the effective surface area of the electrode and surface potential of the electric double layer. SWV was employed for the measurement of phosphate, demonstrating an LOD of 2.2 μM and a linear range of 3.6–110 μM. The sensor exhibited a rapid response time of 8 s and excellent performance in selectivity tests against interfering species, including 100-fold HCO3−, CO32−, NO3−, CH3COO−, Cu2+, 5-fold H3AsO3, methylene blue, p-nitrophenol, and 2-fold H3AsO4.
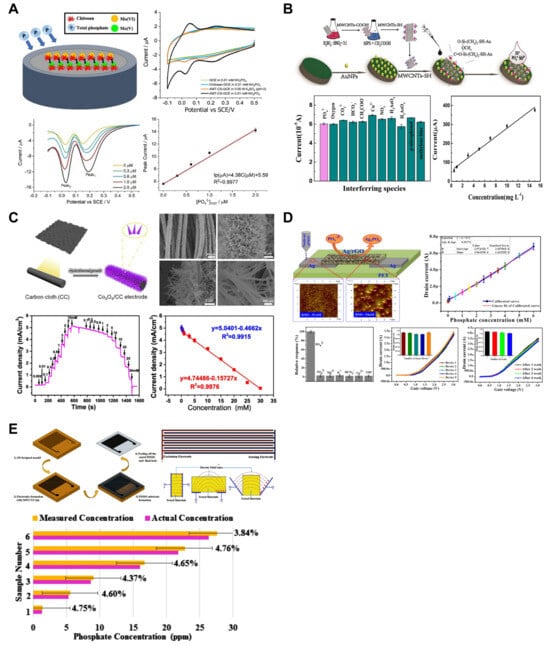
Figure 5.
Phosphate ion sensors. (A) For phosphate ion detection, the surface of the glassy carbon electrode was modified with molybdate tetrahydrate and chitosan. Reproduced with permission from Ref. [48]. (B) Silanized multi-walled carbon nanotubes and gold nanoparticles were applied for a fast and low-cost voltametric phosphate sensor. Reproduced with permission from Ref. [49]. (C) Cobalt oxide nanoneedle arrays were grown on the carbon cloth by the hydrothermal method. Dense nanoneedle arrays offer larger surface area. Reproduced with permission from Ref. [53]. (D) Using ink composed of silver and reduced graphene oxide, a field-effect transistor was nozzle-jet-printed for phosphate ion sensing. Reproduced with permission from Ref. [55]. (E) Multi-walled carbon nanotubes and polydimethylsiloxane-based phosphate sensors were fabricated by 3D printing. The K-nearest neighbor machine learning algorithm was applied in the sensing system. Reproduced with permission from Ref. [54].
Owing to their high stability, cost-effectiveness, and good catalytic activity, electrochemical sensors that use non-enzymatic catalysts (i.e., transition-metal oxides (TMOs), NiO, Cu2O, TiO2, ZnO, MnO2, and Co3O4) have been developed to detect phosphate [90]. Song et al. developed a hydrothermal method to create freestanding cobalt oxide (Co3O4) nanoneedle arrays on the surface of flexible carbon cloth (CC) and fabricated an amperometric sensor for phosphate detection (Figure 5C) [53]. The nanostructure on the surface significantly increased the contact area between the sensor and analyte, demonstrating outstanding performance in measuring phosphate ions in a glucose-containing alkaline solution. The developed Co3O4/CC electrode had an LOD of 10 μM and linear ranges of 0.1–1.0 mM and 1.0–30.0 mM, enabling the quantification of phosphate anion concentrations in the sample.
In addition to electrochemical methods employing ISEs, there is growing interest in sensors based on field-effect transistors (FETs) within the sensing community. Hahn et al. developed a nozzle-jet-printed silver/graphene composite-based enzyme-less FET sensor for phosphate ion detection (Figure 5D) [55]. Nozzle jet printing technology utilized in sensor fabrication is a straightforward and effective tool for creating electronic devices using various materials. Furthermore, the reduced graphene oxide (rGO)-based nanostructure applied to the sensor has been widely adopted in diverse sensor-related studies due to its outstanding electronic properties. Considering these aspects, the research focused on developing a facile, cost-effective, and high-performance sensor. The fabricated sensor measures phosphate concentration in the sample through I-V measurements. The operational principle involves the interaction between the phosphate ions in the sample and the Ag/rGO composite material, initiating a redox reaction and causing changes in the conductivity of the sensor surface. The research team determined that the slope of the measured drain current variation when increasing the gate voltage enabled the quantification of the phosphate concentration in the sample. Calibration plots were generated by measuring the current response at the gate voltage of 1.7 V. The developed sensor exhibited an LOD of 1.2 μM and an excellent linear range of 5–6000 μM, demonstrating superior performance.
Electrochemical Impedance Spectroscopy (EIS) is a technique used to analyze systems that exhibit electrochemical behavior, such as batteries and biochemical sensors [91]. EIS provides a near real-time response with the ability to detect low concentrations of chemicals. Additionally, the Nyquist plot obtained from the EIS measurements allows the analysis of parameters such as charge transferability and the double-layer effect at the electrode-solution interface. Akhter et al. developed a sensor using multi-walled carbon nanotubes (MWCNTs) and PDMS and applied it to a phosphate detection system (Figure 5E) [54]. The equivalent circuit for the developed sensor was obtained through EIS, enabling the analysis of the dynamic relationship between the electrode and the phosphate solution. A K-nearest neighbor (KNN) machine learning algorithm was implemented in the measurement system to predict phosphate ion concentrations. For system training, the ion concentrations of phosphate, nitrate, calcium, temperature, pH, resistance, and impedance data were utilized. The developed system demonstrated an LOD of 0.01 ppm and a linear range of 0.01–40 ppm. Moreover, the response time of the developed sensor was exceptionally short (1 s), allowing real-time monitoring.
In the context of LOC devices, numerous colorimetric sensors have been developed to detect specific substances in samples [92,93,94]. Reagents used in colorimetric detection are susceptible to interference from impurities, leading to measurement errors in complex water matrices such as wastewater [95]. In laboratory settings, ion chromatography (IC) is often employed to detect inorganic anions such as phosphate. Lace et al. developed an IC system for phosphate detection in both environmental and industrial water [96]. The optical detection cell used in the sensor was fabricated using stereolithography (SLA) three-dimensional printing with a nanocomposite material. The sensing platform was constructed based on IC using a potassium phthalate-based eluent, isocratic elution, and an IonPac CS5A analytical column. Indirect UV detection of phosphate ions was performed in a 3D-printed detection cell using a UV LED at 255 nm and a photodiode. The sensor demonstrated an LOD of 0.4 mg L−1, with a linear range of 0.5–30 mg·L−1.
3.3. Potassium Sensors for Hydroponics
Potassium is one of the primary components of plant cells and plays a crucial role in regulating cell turgor pressure, membrane electric potential, and intracellular pH to maintain appropriate physiological conditions [97]. Additionally, potassium plays a vital role in conferring resistance to diseases and is essential for physiological processes in plants, such as photosynthesis [98]. Therefore, maintaining a consistent level of potassium in the nutrient solution is crucial for ensuring optimal growth of the plant.
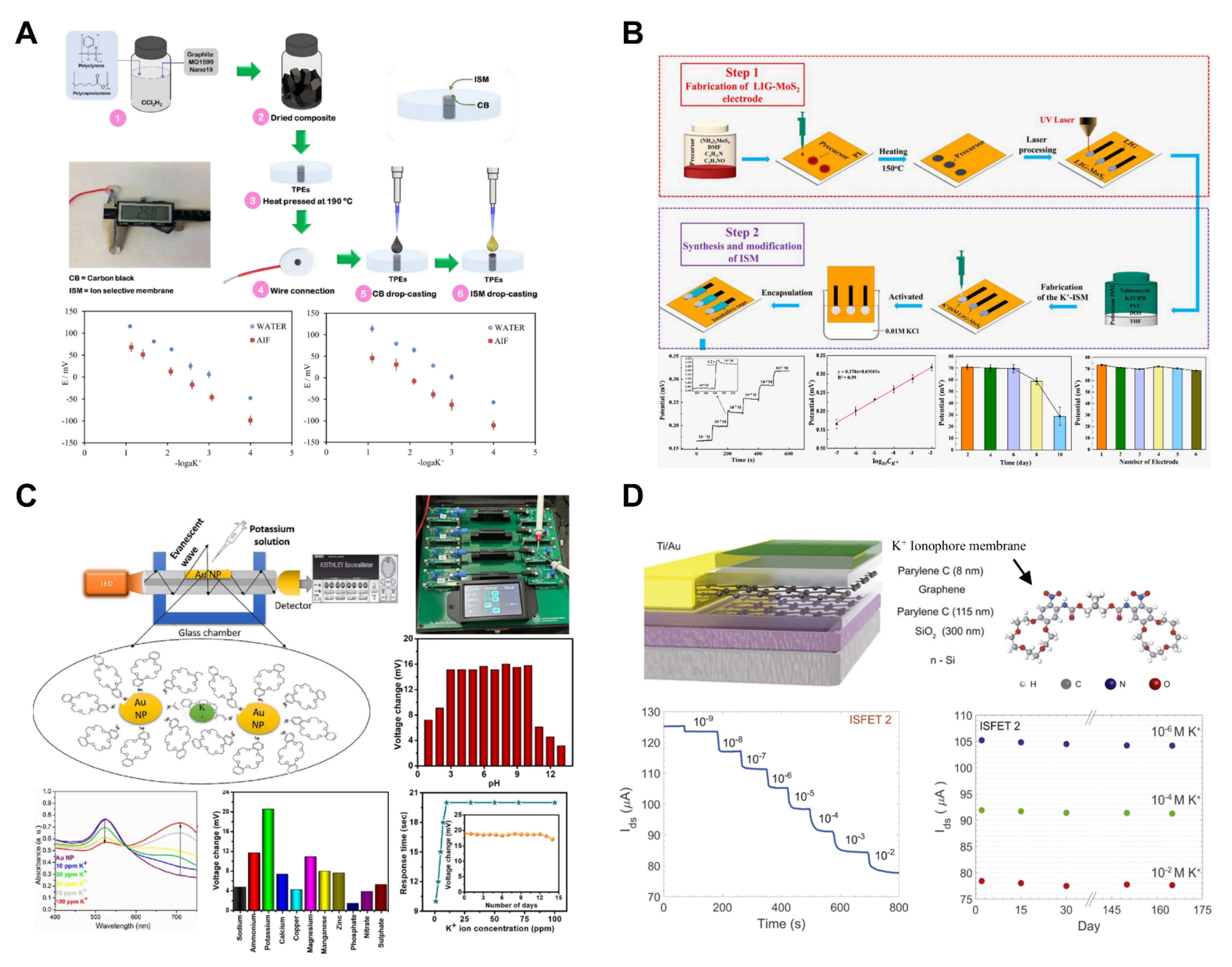
Ozer et al. developed polystyrene/polycaprolactone (PS/PCL) thermoplastic electrodes (TPEs) coated with polymer membranes based on carbon black (CB) and poly (vinyl chloride) (PVC) for potassium measurements (Figure 6A) [60]. The fabricated electrode measured the potassium concentration in a liquid using a potentiometric method, serving as an all-solid-state ion-selective sensor that did not require a separate inner reference solution. The thin water layer formed at the contact surface between the ion-selective membrane and the metal induced potential drift, interfering with the measurements [59]. To address this issue, CB, which is known for its good conductivity, large surface area, and strong hydrophobicity, was used to create the membrane. Previous electrodes fabricated by Henry’s group using a mixture of PS and PCL exhibited characteristics such as lower capacitance and faster electron transfer compared with PCL TPEs. Based on these results, the CB membranes were used. The pH stability of the sensor showed consistent values from 3.0 to 9.0, with a slight potential drift observed between 9.0 and 10.0. The sensor’s performance indicated an LOD of 100 μM, a linear range of 0.1–100 mM, and a response time of 4 s, placing it on the shorter side compared to traditional potassium-ISE [99]. Thuy et al. utilized molybdenum disulfide (MoS2) and laser-induced graphene (LIG) to develop a LIG-MoS2 solid-contact ISE for potassium measurement applicable to hydroponics (Figure 6B) [62]. The ISE was fabricated through direct laser writing, and the MoS2 precursor was treated on a polyimide film to achieve a one-step fabrication. The developed sensor exhibited high selectivity against interfering ions such as Ca2+, Mg2+, Na+, H+, and Cl− and reported a linear range of 10−7–10−2 M. Six electrodes were manufactured, and their responses to the same measurement were examined, showing a high reproducibility with a standard deviation of 1.59. Regarding the reusability of the sensor, the electrode response was monitored every two days, maintaining performance until the sixth day. However, a decrease in the signal was observed after the eighth day. Ultimately, the developed sensor was employed to measure potassium ions in a hydroponic solution, displaying a Nernstian potentiometric response of 21.4 mV/decade and an excellent R2 value of 0.98.
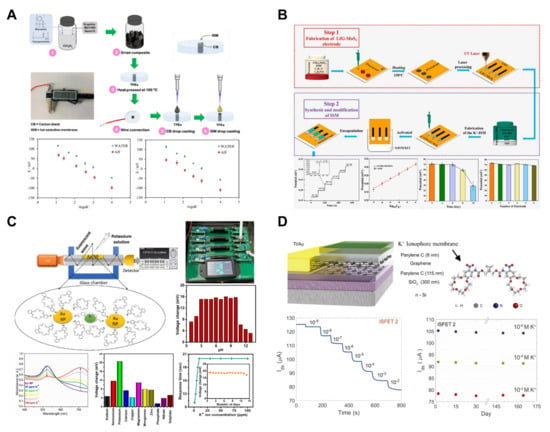
Figure 6.
Potassium ion sensors. (A) An all-solid-state potassium-selective thermoplastic electrode was fabricated. Reproduced with permission from Ref. [60]. (B) A potassium-selective electrode based on laser-induced graphene and molybdenum disulfide was developed. Reproduced with permission from Ref. [62]. (C) An optical potassium sensor utilizing evanescent wave was developed. Reproduced with permission from Ref. [64]. (D) A parylene-encapsulated graphene-based potassium-selective high-resolution field-effect transistor was fabricated. Reproduced with permission from Ref. [63].
Potdar et al. developed an evanescent wave sensor for potassium ion measurement using Au nanoparticles (NPs) and 4′-aminodibenzo-18-crown-6 ether (Figure 6C) [64]. The optical fiber consisted of a core (polymethyl methacrylate) and cladding (black polyethylene jacket). To coat the optical fiber with Au NPs, 10 mm of cladding was removed using a blade, and ultrasonic spray coating applied 13 μm of Au NPs. The Au NP-coated optical fiber ends were connected to LEDs and a detector, with each LED emitting red, green, and blue light at wavelengths of 645, 522, and 470 nm, respectively. The resulting sensor demonstrated a linear range of 1–100 ppm, with a very low LOD of 0.14 ppm, enabling the detection of potassium at extremely low concentrations. The sensor exhibited a rapid response time of 10 s. The utilization of optical fibers and colorimetric reagents in chemical sensors shows their potential for applications such as small sample volumes, low cost, and in situ detection, as evidenced by numerous studies in this field.
Fakih et al. developed ion-sensitive field-effect transistors (ISFETs) based on large-area graphene to measure potassium ions (Figure 6D) [63]. The developed ISFET included potassium-ionophore sensing layers and featured a broad active area of 0.4 cm2. This design resulted in an excellent channel area, charge carrier mobility, and capacitance, demonstrating a low LOD of 1 nM. To enhance mobility and reduce hysteresis, graphene was encapsulated in hydrophobic parylene C. This encapsulation led to a reduction in the root mean square (RMS) current noise, improving the sensor resolution to ~2 × 10−3 log[K+], which is remarkably higher than that of traditional potentiometric sensors. Moreover, the parylene encapsulation demonstrated the long-term stability of the ISFET sensor, operating reliably for over five months in stability tests.
4. Research Trends in Nutrient Solution Monitoring Systems
In this section, we discuss ion concentration measurement methods employed in various sensors and sensing platforms applied in hydroponic systems. We conducted a survey of research trends, categorizing monitoring systems based on conventional methods relying on pH and EC measurements and advanced methods focusing on quantifying individual ion concentrations [100]. Although conventional methods face challenges in quantifying individual ion concentrations, they have been widely used in research because of their ability to determine nutrient concentrations based on pH and EC measurements [101]. Chen et al. implemented an IoT-based approach in a hydroponic system by measuring the EC and pH of a nutrient solution [102]. They employed fuzzy logic to automatically control these parameters to provide an environment with optimal EC and pH for plant growth. To achieve this, the system automatically adjusts the nutrient solution supply. It utilizes user-defined values to approach the desired settings by proportionally injecting high-EC water, acid, and alkaline solutions. The system inputs were the EC and pH sensor values, whereas the outputs included pumps A (high-EC solution), B (water), C (acid solution), and D (alkaline solution). Fuzzy sets were established for each pump with ambiguously defined range sets using triangular membership functions. Vincentdo et al. developed a hydroponic monitoring and control system incorporating an adaptive neuro fuzzy inference system (ANFIS) [103]. Precise control of the actuators in the system is crucial for appropriately maintaining the pH and EC of the nutrient solution. This challenge can be addressed using fuzzy logic, but it typically requires expert knowledge to define the membership functions and rules. However, by employing ANFIS, a combination of neural networks and fuzzy logic, it is possible to control the system without relying on expert knowledge. Compared with the conventional Sugeno fuzzy method used for fuzzy controller creation, the ANFIS demonstrated a 67% higher accuracy. Based on this improvement, a web application dashboard for monitoring hydroponic systems was proposed.
The advanced method involves the monitoring of individual ions. In this regard, sensors are required to measure each ion, and research involving ISEs [104,105,106] and FET arrays [107] has been widely reported. However, issues such as potential drift due to redox reactions in Ag/AgCl-based reference electrodes, ion selectivity, and the presence of a water layer have been reported to hinder the ISE performance. While these issues can be addressed by coating protective layers on Ag/AgCl reference electrodes or applying solid-contact ISEs, recent advancements have focused on improving selectivity through software approaches such as machine learning. Tuan et al. enhanced the accuracy of a monitoring system that measures calcium, phosphate, and magnesium ions using ISEs by incorporating an automatic multivariate standard addition method (AMSAM) and deep kernel learning (DKL) [108]. Chen et al. developed a sensing platform combining ISE arrays and artificial neural networks (ANNs) for NPK measurement [109]. The NO3−, H2PO4−, and K+ concentrations obtained from the ISE array were used for the training and optimization of the ANNs. The ANN improved the sensor selectivity for target ions and contributed to reducing the cross-sensitivity among interference ions. The electrodes used in the developed system were located on a printed circuit board (PCB), coated with ion-selective membranes (ISM), and positioned opposite to the Ag/AgCl reference electrode. The data used for optimizing ANN architecture were divided into training (70%), validation (15%), and testing (15%), resulting in decreased RMSE values for NO3−, H2PO4−, and K+ ions from 0.53, 1.50, and 1.73 to 0.28, 0.31, and 0.3, respectively. The slopes of the linear fitting curves related to the predicted concentrations of NO3−, H2PO4−, and K+ ions demonstrated a high correlation between the predicted and actual ion concentrations, with values of 0.934, 0.927, and 0.943, respectively. Even when additional interference ions (Cl− and Ca+) were introduced, the RMSE between the sensor measurement values and the ANN model results decreased from 0.48, 0.55, and 0.66 to 0.35, 0.46, and 0.43, respectively. The slopes of the linear fitting curves also showed a high correlation with the target ion concentrations, with values of 0.969, 0.899, and 0.925, respectively.
Studies have reported the use of ion chromatography as an alternative to multi-ion monitoring systems for water sources, using ISEs [110]. Particularly in the context of hydroponic systems, Kozaki et al. devised a method using ion-exclusion/cation-exchange chromatography (IEC/CEC) to measure anions (SO42−, Cl−, NO3−, and HPO42−) and cations (Na+, NH4+, K+, Mg2+, and Ca2+) [111]. In conventional IEC/CEC systems, colorimetric reagents such as molybdenum yellow or molybdenum blue are used to measure phosphate, requiring additional optical detectors. However, the dual-ion-exchange groups used in this study, consisting of carboxy and sulfo groups, have been reported to quantitatively measure phosphate ions along with anions and cations. Tartaric acid and 3 mM 18-crown-6 were used as eluents in ion chromatography; increasing the concentration of tartaric acid decreased the retention time. However, using a high concentration of tartaric acid led to a slight reduction in the peak resolution between the Na+-NH4+ and NH4+-K+ ions. Therefore, the optimal concentration of tartaric acid was determined to be 5 mM. As the concentration of 18-crown-6 increased, the peak resolution between NH4+ and K+ and the retention time of K+ also increased. The final concentration of 18-crown-6 was 3 mM. The developed IEC/CEC system secured a linear range of 0.1–1 mM for all ions except Cl−, which showed a linear range of 0.3–3.0 mM.
Monteiro-Silva et al. developed a sensing system for direct UV–Vis spectroscopy-based measurement of NPK concentrations in nutrient solutions [112]. This system uses optical fibers and a spectrometer to analyze the absorption spectra of the nutrient solution and measure the concentrations of individual ions. Owing to the scattering and interference from various substances within the solution, the measurements are obtained in the form of superimposed spectra. To measure NPK, a series of tests were performed on samples containing two or more ions. For instance, tests were conducted using samples composed of nitrite and nitrate, and phosphate was added to observe how the data were transformed under the influence of phosphate. AI was used to analyze the ion concentrations within the samples. The local partial least squares (PLS) method was used as a multivariate linear model to analyze raw data. In the self-learning AI prediction model, nitrate, phosphate, and potassium exhibited correlations of 0.9997, 0.9984, and 0.9984, respectively. The mean absolute percentage error (MAPE) values were 0.48%, 2.40%, and 2.40%, respectively. Because the measurement system relies on the concentration of nitrate to assess phosphate and potassium ions, the concentrations of phosphate and potassium ions are fixed, whereas the nitrate concentration varies during the learning process. Therefore, this study suggests that accuracy can be further improved by obtaining more training data based on varying nitrate concentrations.
5. Considerations for Advanced NPK Ion Measurement in Hydroponics
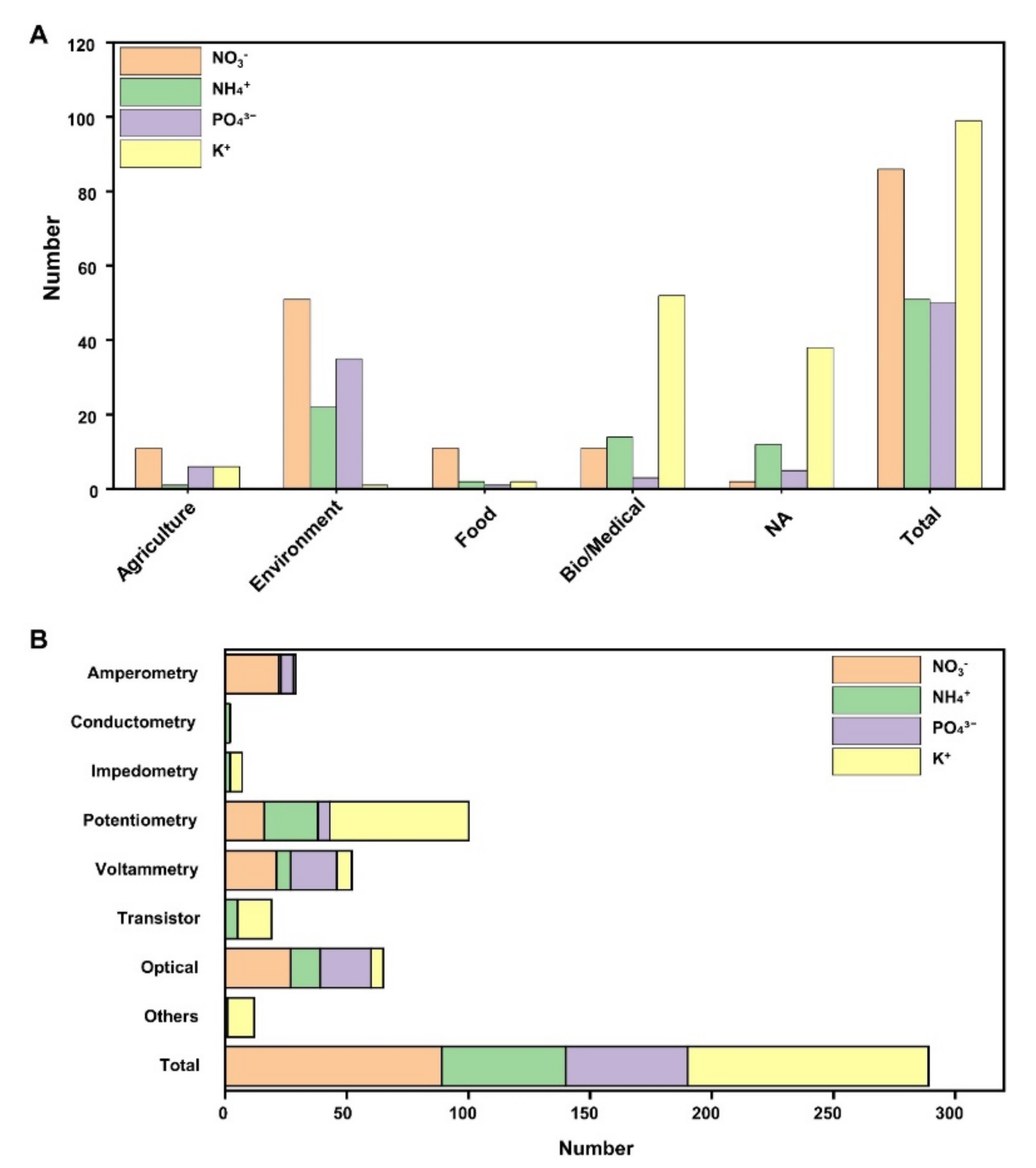
To understand the trends in the development of sensors for NPK ions, papers published between 2019 and the present were reviewed (Figure 7). Out of the 288 papers, the majority focused on electrochemical sensors, with potassium ions being the most extensively studied. This dominance can be attributed to the prevalence of research on potassium measurements in the fields of biology and medicine. Nitrate has also garnered significant attention, particularly in areas related to water quality and the environment.
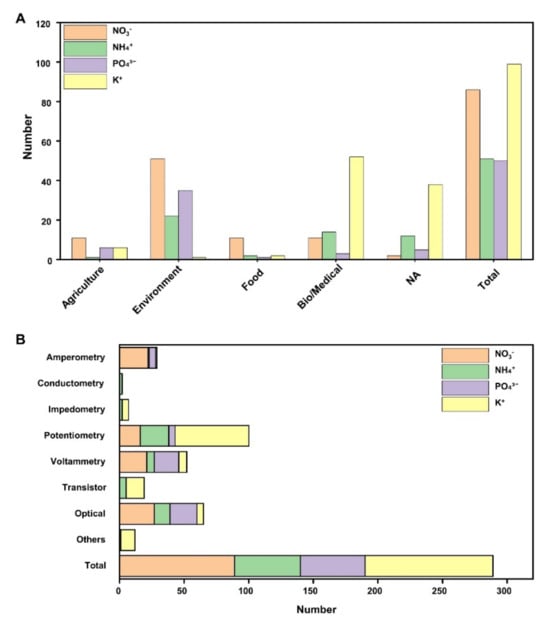
Figure 7.
Literature related to NPK ion sensors. NPK sensors used in solutions were classified. The studies related to electrochemical and optical sensors were presented from 2019 to the present. (A) NPK sensors were classified with application fields (agriculture, environment, food, bio/medical, and not applicable (NA)). (B) NPK sensors were classified by operation method. Categorized into electrochemical, optical, and other methods, respectively.
However, within the overall research landscape, studies on NPK ion sensing in agriculture, particularly in hydroponics and soilless cultivation, are notably lacking. This gap may be attributed to the traditional use of conventional ion monitoring methods to regulate ions in nutrient solutions in previous NPK monitoring systems. Addressing this research gap is crucial for advancing agricultural practices. Developing robust NPK ion sensors would enable more accurate nutrient management, leading to improved resource efficiency, reduced environmental impact, and enhanced crop productivity. Furthermore, such advancements could provide the agricultural sector with the tools needed to transition toward precision farming, where nutrient application is not only optimized but also sustainable. Closing this gap in NPK ion sensing research represents a vital step in ensuring long-term food security and sustainability in agriculture.
These findings highlight the need for more research in the agricultural sector, especially in areas related to NPK ion sensing using innovative cultivation methods such as closed hydroponics. When developing agricultural sensors to measure individual ions within a nutrient solution, several factors need to be considered, including the sensor’s lifespan, detection range, and ion form. Electrochemical methods, which are commonly used in sensors, often use electrodes with relatively short lifespans. Overcoming this limitation requires research into systems or materials that can extend the longevity of the electrodes. In particular, the lifespan of ISEs is critically important for their practical application, as the sensor’s longevity directly impacts overall costs. Recent studies have reported attempts to enhance sensor performance by tuning surface properties such as morphology and wettability of LIG substrates using CO2 lasers [113]. While extending the lifespan of the sensor itself is essential for real-time monitoring in precision agriculture, it is equally important to consider the predefined lifespan of the sensor and conduct research on optimizing sensing cycles to maximize its utility.
Optical methods using colorimetric reagents may have a longer lifespan, but their response times tend to be longer than those of electrochemical methods. Additionally, managing reagent disposal and maintaining the overall system can pose challenges for users. Moreover, as the number of target ions to be measured increases, the system becomes more complex owing to the need for individual reagents for each ion. Managing individual reagents can become challenging as the number of targets increases. One example of an optical sensor is the paper-based colorimetric sensor, which offers the capability to simultaneously measure various target substances [114,115]. However, its disposable nature presents challenges for continuous ion concentration monitoring. On the other hand, this disposable characteristic can be advantageous, as replacing the paper after each measurement avoids issues such as signal degradation that often occurs in ISEs with repeated use. Furthermore, the integration of LOC devices with colorimetric reagents provides a means to enhance the reaction speed of existing reagents. Incorporating standardized reagents within the system can further enable highly precise measurements. Additionally, studies utilizing acoustofluidics for rapid mixing, which promote efficient interactions between reagents and samples, suggest potential advancements in improving the response time of optical ion sensors.
Before designing ion sensors for hydroponic applications, it is crucial to consider the pH of the nutrient solution and accurately determine the ion form to be measured. For instance, in aqueous environments, NH4+ has a pKa of 9.2, which makes it a weak acid. At pH 7, approximately 99% of ammonia exists in the form of NH4+, and at pH > 9.2, NH4+ reacts as a proton acceptor to form NH3. The pH of the nutrient solutions used in hydroponic cultivation typically falls between 5.5 and 6.597. Therefore, agricultural sensors designed to measure the concentration of NH4+ in nutrient solutions should operate smoothly within a pH range of approximately 5.5 to 6.5. A clear identification of the ion form based on the pH range allows the development of systems with pH compensation, thereby providing more accurate ion concentrations. This ensures that users can effectively apply the desired amount of fertilizer.
As examined in Section 2 regarding the composition of nutrient solutions, monitoring ion concentrations in hydroponics requires consideration of the sensor’s linear range, tailored to the concentrations of individual ions in the solution. According to the survey, nutrient solutions commonly used in hydroponics typically contain approximately 2.6, 0.5, 0.5, and 6.2 mmol/L of NO3−, NH4⁺, H2PO4−, and K⁺ ions, respectively. Therefore, rather than focusing on highly precise measurements, it is essential to align the sensor’s linear range with the concentration ranges relevant to agricultural nutrient solutions.
In addition to NPK ions, nutrient solutions contain a variety of other ions that may act as interfering factors during measurement. This makes sensor selectivity a critical requirement, alongside the need to ensure effective operation under real-world nutrient solution conditions. Furthermore, the concentration of the target substance directly influences the sensor’s response time. Higher target ion concentrations lead to faster reactions with the sensor’s active sites, resulting in a quicker increase in output. While lowering the sensor’s LOD can enhance response time, maintaining an appropriate linear range remains a key challenge.
Constructing a platform capable of independently measuring each ion is inherently complex due to the diverse composition of nutrient solutions. As a result, conventional methods, such as monitoring EC and pH for nutrient solution replenishment, are still widely used. However, there is a growing demand for ion-sensing platforms equipped with individual ion sensors. Achieving this requires focused efforts to enhance sensor performance in terms of lifespan, measurement range, and selectivity.
For example, significant advancements have recently been made in modifying the surface of ISEs with nanomaterials to detect low-concentration substances. These nanomaterials have proven effective in improving the LOD by expanding the reactive surface area of the sensors. In summary, hydroponic cultivation requires sensors capable of achieving optimal response times and linear ranges while minimizing interference from non-target ions through features such as ion-selective membranes. This balance is essential for the accurate and reliable monitoring of ion concentrations in nutrient solutions.
6. Conclusions
Numerous studies have emphasized that the optimal nutrient solution concentration to achieve maximum yield and quality varies depending on the crop type and growth stage. Therefore, a sensing platform capable of monitoring the concentration of a nutrient solution in real time and maintaining the optimal concentration based on the measured values is crucial. However, monitoring systems based on pH and EC alone cannot accurately measure the concentrations of individual ions, which makes it challenging to maintain an optimal concentration for each ion. Therefore, there is demand for sensing platforms capable of measuring individual ions. In this review, we have presented the performance requirements of sensors for measuring each ion in relation to the NPK elements of nutrient solutions. Furthermore, we have classified the NPK-related sensors developed from 2019 to the present into electrochemical and optical methods. Finally, we have discussed the systems used for ion monitoring in hydroponics and described the limitations of currently developed ion sensors. This review provides insights into research related to nutrient solution monitoring systems capable of measuring individual ions in hydroponics and presents measurable concentration ranges and pH ranges relevant to sensors for hydroponics. Additionally, we have discussed challenges such as lifespan, selectivity, and stability that agricultural sensors must realistically address. While current systems based on pH and EC provide general insights into nutrient solution conditions, they lack the precision required to monitor and maintain optimal individual ion concentrations. To address these limitations, this review emphasizes the development of sensing platforms capable of real-time, accurate individual ion detection. These platforms should integrate multi-ion sensing capabilities with robust calibration mechanisms to ensure reliability and usability in practical agricultural settings. Moreover, future research must tackle critical challenges, including improving sensor lifespan and stability under varying environmental conditions, enhancing selectivity to reduce cross-interference, and achieving cost-effective scalability for commercial applications. By highlighting these aspects, this review underscores the necessity of transitioning from conventional monitoring methods to innovative, ion-specific sensing technologies. Such advancements are crucial for optimizing nutrient management in hydroponics, ultimately contributing to improved crop yield and quality. Through a comprehensive examination of current limitations and potential solutions, this review provides a foundation for future efforts in developing advanced ion sensing systems tailored to the demands of modern agriculture.
Author Contributions
Conceptualization, Y.H. and J.L.; investigation, Y.H. and J.L.; resources, Y.H.; writing—original draft, Y.H. and J.L.; writing—review and editing, K.-J.J., J.K. and S.P.; visualization, Y.H. and J.L.; supervision, K.-J.J., S.P. and J.K.; project administration, K.-J.J.; funding acquisition, S.P. and J.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Ministry of Education of the Republic of Korea and the National Research Foundation of Korea (NRF-2021R1G1A1005881). This work was supported by the research grant of the Gyeongsang National University in 2020. This work was supported by New Faculty Startup Fund from Seoul National University (Project No. 0525-20240062). This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2022R1I1A1A01065625).
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors, [K.-J.J. and J.-H.K.], upon reasonable request.
Conflicts of Interest
The authors report there are no competing interests to declare.
References
- Perez, S.L.; Ferro, R.B.; Corrêa, B.; Casarin, R.; Corrêa, T.Q.; Blanco, K.C.; Bagnato, V.S. Enhanced Vegetable Production in Hydroponic Systems Using Decontamination of Closed Circulating Fluid. Sci. Rep. 2024, 14, 602. [Google Scholar] [CrossRef] [PubMed]
- Fussy, A.; Papenbrock, J. An Overview of Soil and Soilless Cultivation Techniques—Chances, Challenges and the Neglected Question of Sustainability. Plants 2022, 11, 1153. [Google Scholar] [CrossRef] [PubMed]
- Maboko, M.; Du Plooy, C.; Bertling, I. Comparative Performance of Tomato Cultivars Cultivated in Two Hydroponic Production Systems. S. Afr. J. Plant Soil 2011, 28, 97–102. [Google Scholar] [CrossRef]
- Sharma, A.; Manpoong, C.; Devadas, V.; Kartha, B.D.; Pandey, H.; Wangsu, M. Crop Hydroponics, Phyto-Hydroponics, Crop Production, and Factors Affecting Soilless Culture. ACS Agric. Sci. Technol. 2022, 2, 1134–1150. [Google Scholar] [CrossRef]
- Sonneveld, C.; Voogt, W. Nutrient Solutions for Soilless Cultures. In Plant Nutrition of Greenhouse Crops; Elsevier: Amsterdam, Netherlands, 2009; pp. 257–275. [Google Scholar]
- Hosseinzadeh, S.; Verheust, Y.; Bonarrigo, G.; Van Hulle, S. Closed Hydroponic Systems: Operational Parameters, Root Exudates Occurrence and Related Water Treatment. Rev. Environ. Sci. Biotechnol. 2017, 16, 59–79. [Google Scholar] [CrossRef]
- Cho, W.-J.; Gang, M.-S.; Jung, D.-H. Decision-Tree-Based Ion-Specific Dosing Algorithm for Enhancing Closed Hydroponic Efficiency and Reducing Carbon Emissions. Front. Plant Sci. 2023, 14, 1301490. [Google Scholar] [CrossRef]
- Wang, X.; Fang, W.; Zhao, Z. Establishment of a Model and System for Secondary Fertilization of Nutrient Solution and Residual Liquid. Sustainability 2023, 15, 1851. [Google Scholar] [CrossRef]
- Cho, W.-J.; Kim, H.-J.; Jung, D.-H.; Kim, D.-W.; Ahn, T.I.; Son, J.-E. On-Site Ion Monitoring System for Precision Hydroponic Nutrient Management. Comput. Electron. Agric. 2018, 146, 51–58. [Google Scholar] [CrossRef]
- Ban, B.; Ryu, D.; Lee, M. Machine Learning Approach to Remove Ion Interference Effect in Agricultural Nutrient Solutions. In Proceedings of the 2019 International Conference on Information and Communication Technology Convergence (ICTC), Jeju, Republic of Korea, 16–18 October 2019; pp. 1156–1161. [Google Scholar]
- Sinfield, J.V.; Fagerman, D.; Colic, O. Evaluation of Sensing Technologies for On-the-Go Detection of Macro-Nutrients in Cultivated Soils. Comput. Electron. Agric. 2010, 70, 1–18. [Google Scholar] [CrossRef]
- Baranwal, J.; Barse, B.; Gatto, G.; Broncova, G.; Kumar, A. Electrochemical Sensors and Their Applications: A Review. Chemosensors 2022, 10, 363. [Google Scholar] [CrossRef]
- Kałuża, D.; Michalska, A.; Maksymiuk, K. Voltammetric Properties of All-Solid-State Ion-Selective Electrodes with Multiwalled Carbon Nanotubes-Poly(3-Octylthiophene-2,5-Diyl) Nanocomposite Transducer. Electroanalysis 2019, 31, 2379–2386. [Google Scholar] [CrossRef]
- Torrezani, L.; Saczk, A.A.; Firmino de Oliveira, M.; Stradiotto, N.R.; Okumura, L.L. Voltammetric Determination of Phosphate in Brazilian Biodiesel Using Two Different Electrodes. Electroanalysis 2011, 23, 2456–2461. [Google Scholar] [CrossRef]
- Zhu, L.; Zhou, X.; Shi, H. A Potentiometric Cobalt-Based Phosphate Sensor Based on Screen-Printing Technology. Front. Environ. Sci. Eng. 2014, 8, 945–951. [Google Scholar] [CrossRef]
- Kim, M.-Y.; Lee, J.-W.; Lee, J.-Y.; Myung, N.V.; Kwon, S.H.; Lee, K.H. Highly Stable Potentiometric Sensor with Reduced Graphene Oxide Aerogel as a Solid Contact for Detection of Nitrate and Calcium Ions. J. Electroanal. Chem. 2021, 897, 115553. [Google Scholar] [CrossRef]
- Gilbert, L.; Jenkins, A.T.A.; Browning, S.; Hart, J. Development of an Amperometric, Screen-Printed, Single-Enzyme Phosphate Ion Biosensor and Its Application to the Analysis of Biomedical and Environmental Samples. Sens. Actuators B Chem. 2011, 160, 1322–1327. [Google Scholar] [CrossRef]
- Bobacka, J.; Ivaska, A.; Lewenstam, A. Potentiometric Ion Sensors. Chem. Rev. 2008, 108, 329–351. [Google Scholar] [CrossRef]
- Lan, W.-J.; Zou, X.U.; Hamedi, M.M.; Hu, J.; Parolo, C.; Maxwell, E.J.; Bühlmann, P.; Whitesides, G.M. Potentiometric Ion Sensing. Anal. Chem. 2014, 86, 9548–9553. [Google Scholar] [CrossRef]
- Suman, M.; Sangma, P.D.; Singh, D. Role of Micronutrients (Fe, Zn, B, Cu, Mg, Mn, and Mo) in Fruit Crops. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 3240–3250. [Google Scholar] [CrossRef]
- Sidhu, M.K.; Raturi, H.C.; Kachwaya, D.S.; Sharma, A. Role of Micronutrients in Vegetable Production: A Review. J. Pharmacogn. Phytochem. 2019, 8, 332–340. [Google Scholar]
- Marr, C.W. Hydroponic Systems. In Greenhouse Vegetable Production; Kansas State University Agricultural Experiment Station and Cooperative Extension Service: Manhattan, KS, USA, 1994. [Google Scholar]
- Swain, A.; Chatterjee, S.; Vishwanath, M. Hydroponics in Vegetable Crops: A Review. Pharma Innov. J. 2021, 10, 629–634. [Google Scholar]
- Debangshi, U. Hydroponics—An Overview. Chron. Bioresour. Manag. 2021, 5, 110–114. [Google Scholar]
- Sousa, R.D.; Bragança, L.; Da Silva, M.V.; Oliveira, R.S. Challenges and Solutions for Sustainable Food Systems: The Potential of Home Hydroponics. Sustainability 2024, 16, 817. [Google Scholar] [CrossRef]
- Houston, L.L. Nutrient Uptake and Management Strategies in Recirculating Hydroponic Systems. Master’s Thesis, University of Arkansas, Fayetteville, AR, USA, 2021. [Google Scholar]
- Smith, G.; Johnston, C.; Cornforth, I. Comparison of Nutrient Solutions for Growth of Plants in Sand Culture. New Phytol. 1983, 94, 537–548. [Google Scholar] [CrossRef]
- Resh, H.M. Hydroponic Food Production: A Definitive Guidebook for the Advanced Home Gardener and the Commercial Hydroponic Grower, 7th ed.; CRC Press: Boca Raton, FL, USA, 2022. [Google Scholar]
- Mattson, N.; Lieth, J.H. Liquid Culture Hydroponic System Operation. In Soilless Culture; Elsevier: Amsterdam, Netherlands, 2019; pp. 567–585. [Google Scholar]
- Amini, N.; Maleki, A.; Maleki, P. Electrochemical Detection of Nitrate Ions via Reduction of NO2− and Oxidation of NO Reactions Based on Cu@TiO2 Core-Shell/Nafion/Polyalizarin Immobilized Electrode. Mater. Chem. Phys. 2021, 264, 124384. [Google Scholar] [CrossRef]
- Patella, B.; Russo, R.; O’Riordan, A.; Aiello, G.; Sunseri, C.; Inguanta, R. Copper Nanowire Array as Highly Selective Electrochemical Sensor of Nitrate Ions in Water. Talanta 2021, 221, 121643. [Google Scholar] [CrossRef]
- Hanane, K.; Messaoud, B.; Houcine, B.; Moncef, T. Highly Sensitive Modified Glassy Carbon Sensor Based on TDAN for Nitrate Detection in Real Water. Monatsh. Chem. 2020, 151, 153–158. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Zhu, R.; Tao, Y.; Chen, Y.; Liu, Q.; Liu, X.; Wang, D. Woven Fiber Organic Electrochemical Transistors Based on Multiwalled Carbon Nanotube Functionalized PEDOT Nanowires for Nondestructive Detection of Potassium Ions. Mater. Sci. Eng. B 2022, 278, 115657. [Google Scholar] [CrossRef]
- Fan, Y.; Huang, Y.; Linthicum, W.; Liu, F.; Beringhs, A.O.R.; Dang, Y.; Xu, Z.; Chang, S.-Y.; Ling, J.; Huey, B.D. Toward Long-Term Accurate and Continuous Monitoring of Nitrate in Wastewater Using Poly(Tetrafluoroethylene)–Solid-State Ion-Selective Electrodes (S-ISEs). ACS Sens. 2020, 5, 3182–3193. [Google Scholar] [CrossRef]
- Amali, R.; Lim, H.; Ibrahim, I.; Zainal, Z.; Ahmad, S. Silver Nanoparticles-Loaded Copper (II)-Terephthalate Framework Nanocomposite as a Screen-Printed Carbon Electrode Modifier for Amperometric Nitrate Detection. J. Electroanal. Chem. 2022, 918, 116440. [Google Scholar] [CrossRef]
- Nightingale, A.M.; Hassan, S.-U.; Warren, B.M.; Makris, K.; Evans, G.W.; Papadopoulou, E.; Coleman, S.; Niu, X. A Droplet Microfluidic-Based Sensor for Simultaneous In Situ Monitoring of Nitrate and Nitrite in Natural Waters. Environ. Sci. Technol. 2019, 53, 9677–9685. [Google Scholar] [CrossRef]
- Wang, F.; Zhu, J.; Hu, X.; Chen, L.; Zuo, Y.; Yang, Y.; Jiang, F.; Sun, C.; Zhao, W.; Han, X. Rapid Nitrate Determination with a Portable Lab-on-Chip Device Based on Double Microstructured Assisted Reactors. Lab Chip 2021, 21, 1109–1117. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ding, J.; Cui, G.; Zhao, C.; Suo, H.; He, D. A Novel Electrochemical Ammonia–Nitrogen Sensor Based on Carbon Cloth-Supported Hierarchical Pt Nanosheets-Ni(OH)2 Nanosheets Nanocomposites. Chem. Eng. Sci. 2021, 239, 116634. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, J.; Peng, X.; Cui, Q.; He, D.; Zhao, C.; Suo, H. Fabrication of a Ni Foam-Supported Platinum Nanoparticles-Silver/Polypyrrole Electrode for Aqueous Ammonia Sensing. Synth. Met. 2020, 259, 116257. [Google Scholar] [CrossRef]
- Lahari, S.A.; Amreen, K.; Dubey, S.K.; Ponnalagu, R.; Goel, S. Modified Ultra Micro-Carbon Electrode for Efficient Ammonia Sensing for Water Quality Assessment. IEEE Trans. NanoBiosci. 2022, 22, 301–307. [Google Scholar] [CrossRef]
- Gallardo-Gonzalez, J.; Baraket, A.; Boudjaoui, S.; Metzner, T.; Hauser, F.; Rößler, T.; Krause, S.; Zine, N.; Streklas, A.; Alcácer, A. A Fully Integrated Passive Microfluidic Lab-on-a-Chip for Real-Time Electrochemical Detection of Ammonium: Sewage Applications. Sci. Total Environ. 2019, 653, 1223–1230. [Google Scholar] [CrossRef]
- Gil, R.L.; Amorim, C.G.; Cuartero, M. Addressing the Detection of Ammonium Ion in Environmental Water Samples via Tandem Potentiometry–Ion Chromatography. ACS Meas. Sci. Au 2022, 2, 199–207. [Google Scholar] [CrossRef]
- Xu, L.; Zhong, L.; Tang, Y.; Han, T.; Liu, S.; Sun, Z.; Bao, Y.; Wang, H.; He, Y.; Wang, W. Beyond Nonactin: Potentiometric Ammonium Ion Sensing Based on Ion-Selective Membrane-Free Prussian Blue Analogue Transducers. Anal. Chem. 2022, 94, 10487–10496. [Google Scholar] [CrossRef]
- Korent, A.; Trafela, Š.; Soderžnik, K.Ž.; Samardžija, Z.; Šturm, S.; Rožman, K.Ž. Au-Decorated Electrochemically Synthesised Polyaniline-Based Sensory Platform for Amperometric Detection of Aqueous Ammonia in Biological Fluids. Electrochim. Acta 2022, 430, 141034. [Google Scholar] [CrossRef]
- Joly, M.; Marlet, M.; Durieu, C.; Bene, C.; Launay, J.; Temple-Boyer, P. Study of Chemical Field Effect Transistors for the Detection of Ammonium and Nitrate Ions in Liquid and Soil Phases. Sens. Actuators B Chem. 2022, 351, 130949. [Google Scholar] [CrossRef]
- Fornells, E.; Murray, E.; Waheed, S.; Morrin, A.; Diamond, D.; Paull, B.; Breadmore, M. Integrated 3D Printed Heaters for Microfluidic Applications: Ammonium Analysis within Environmental Water. Anal. Chim. Acta 2020, 1098, 94–101. [Google Scholar] [CrossRef]
- Arvas, M.B.; Gorduk, O.; Gencten, M.; Sahin, Y. Preparation of a Novel Electrochemical Sensor for Phosphate Detection Based on a Molybdenum Blue Modified Poly(Vinyl Chloride) Coated Pencil Graphite Electrode. Anal. Methods 2019, 11, 3874–3881. [Google Scholar] [CrossRef]
- Lu, Y.; Li, X.; Li, D.; Compton, R.G. Amperometric Environmental Phosphate Sensors. ACS Sens. 2021, 6, 3284–3294. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Xia, D.; Xu, J.; Ye, C.; Zhang, D.; Deng, D.; Zhang, J.; Huang, G. Sequential Injection-Square Wave Voltammetric Sensor for Phosphate Detection in Freshwater Using Silanized Multi-Walled Carbon Nanotubes and Gold Nanoparticles. Microchem. J. 2021, 167, 106311. [Google Scholar] [CrossRef]
- Sedaghat, S.; Jeong, S.; Zareei, A.; Peana, S.; Glassmaker, N.; Rahimi, R. Development of a Nickel Oxide/Oxyhydroxide-Modified Printed Carbon Electrode as an All Solid-State Sensor for Potentiometric Phosphate Detection. New J. Chem. 2019, 43, 18619–18628. [Google Scholar] [CrossRef]
- Xu, K.; Wu, B.; Wan, J.; Li, Y.; Li, M. A Potentiometric Phosphate Ion Sensor Based on Electrochemically Modified Nickel Electrode. Electrochim. Acta 2022, 412, 140065. [Google Scholar] [CrossRef]
- Xu, K.; Li, Y.; Li, M. Potentiometric Phosphate Ion Sensor Based on Electrochemical Modified Tungsten Electrode. ACS Omega 2021, 6, 13795–13801. [Google Scholar] [CrossRef]
- Xu, J.; Gao, Z.; Dou, X.; Song, Y.-Y. Needle-Like Co3O4 Nanoarrays as a Dual-Responsive Amperometric Sensor for Enzyme-Free Detection of Glucose and Phosphate Anion. J. Electroanal. Chem. 2021, 897, 115605. [Google Scholar] [CrossRef]
- Akhter, F.; Siddiquei, H.; Alahi, M.E.E.; Mukhopadhyay, S. Design and Development of an IoT-Enabled Portable Phosphate Detection System in Water for Smart Agriculture. Sens. Actuators A Phys. 2021, 330, 112861. [Google Scholar] [CrossRef]
- Bhat, K.S.; Nakate, U.T.; Yoo, J.-Y.; Wang, Y.; Mahmoudi, T.; Hahn, Y.-B. Nozzle-Jet-Printed Silver/Graphene Composite-Based Field-Effect Transistor Sensor for Phosphate Ion Detection. ACS Omega 2019, 4, 8373–8380. [Google Scholar] [CrossRef]
- Zhu, J.; Han, G.; Hu, X.; Zuo, Y.; Chen, L.; Wang, F.; Yang, Y.; Jiang, F.; Sun, C.; Zhao, W. A Portable and Accurate Phosphate Sensor Using a Gradient Fabry–Pérot Array. ACS Sens. 2020, 5, 1381–1388. [Google Scholar] [CrossRef]
- Beaton, A.D.; Schaap, A.M.; Pascal, R.; Hanz, R.; Martincic, U.; Cardwell, C.L.; Morris, A.; Clinton-Bailey, G.; Saw, K.; Hartman, S.E. Lab-On-Chip for In Situ Analysis of Nutrients in the Deep Sea. ACS Sens. 2022, 7, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, M.; Cooper, J.F.; Clarke, S.J.; Rasche, B.; Compton, R.G. Designing Selective Electrode Materials for Electroanalysis–New Tungsten Bronzes as Selective Potassium Hosts. ChemElectroChem 2020, 7, 3160–3167. [Google Scholar] [CrossRef]
- Yin, T.; Pan, D.; Qin, W. All-Solid-State Polymeric Membrane Ion-Selective Miniaturized Electrodes Based on a Nanoporous Gold Film as Solid Contact. Anal. Chem. 2014, 86, 11038–11044. [Google Scholar] [CrossRef] [PubMed]
- Ozer, T.; Henry, C.S. All-Solid-State Potassium-Selective Sensor Based on Carbon Black Modified Thermoplastic Electrode. Electrochim. Acta 2022, 404, 139762. [Google Scholar] [CrossRef]
- Węgrzyn, K.; Michalska, A.; Maksymiuk, K. Electrochemical Properties and Analytical Advantages of Potassium-Selective Sensor with Tungsten Trioxide Solid Contact. J. Electroanal. Chem. 2023, 928, 117061. [Google Scholar] [CrossRef]
- Thuy, N.T.D.; Zhao, G.; Wang, X.; Awuah, E.; Zhang, L. Potassium Ion-Selective Electrode with a Sensitive Ion-to-Electron Transducer Composed of Porous Laser-Induced Graphene and MoS2 Fabricated by One-Step Direct Laser Writing. Electroanalysis 2023, 35, e202200194. [Google Scholar] [CrossRef]
- Fakih, I.; Centeno, A.; Zurutuza, A.; Ghaddab, B.; Siaj, M.; Szkopek, T. High-Resolution Potassium Sensing with Large-Area Graphene Field-Effect Transistors. Sens. Actuators B Chem. 2019, 291, 89–95. [Google Scholar] [CrossRef]
- Potdar, R.P.; Khollam, Y.B.; Shaikh, S.F.; Raut, R.W.; Pandit, B.; More, P.S. Evanescent Wave Sensor for Potassium Ion Detection with Special Reference to Agricultural Application. J. Photochem. Photobiol. A Chem. 2023, 441, 114707. [Google Scholar] [CrossRef]
- Leghari, S.J.; Wahocho, N.A.; Laghari, G.M.; HafeezLaghari, A.; MustafaBhabhan, G.; HussainTalpur, K.; Bhutto, T.A.; Wahocho, S.A.; Lashari, A.A. Role of Nitrogen for Plant Growth and Development: A Review. Adv. Environ. Biol. 2016, 10, 209–219. [Google Scholar]
- Van der Boon, J.; Steenhuizen, J.; Steingrover, E.G. Growth and Nitrate Concentration of Lettuce as Affected by Total Nitrogen and Chloride Concentration, NH4/NO3 Ratio and Temperature of the Recirculating Nutrient Solution. J. Hortic. Sci. 1990, 65, 309–321. [Google Scholar] [CrossRef]
- Essousi, H.; Barhoumi, H.; Bibani, M.; Ktari, N.; Wendler, F.; Al-Hamry, A.; Kanoun, O. Ion-Imprinted Electrochemical Sensor Based on Copper Nanoparticles-Polyaniline Matrix for Nitrate Detection. J. Sens. 2019, 2019, 1–9. [Google Scholar] [CrossRef]
- Gautam, S.K.; Panda, S. Field Effect Characteristics and Gas Sensing Properties of Vertically Grown PANI Nanofibers. Org. Electron. 2023, 123, 106938. [Google Scholar] [CrossRef]
- Zhu, C.; Xu, Y.; Zhou, T.; Liu, L.; Chen, Q.; Gao, B.; Zhang, T. Self-Assembly Polyaniline Films for the High-Performance Ammonia Gas Sensor. Sens. Actuators B Chem. 2022, 365, 131928. [Google Scholar] [CrossRef]
- Shao, Y.; Ying, Y.; Ping, J. Recent Advances in Solid-Contact Ion-Selective Electrodes: Functional Materials, Transduction Mechanisms, and Development Trends. Chem. Soc. Rev. 2020, 49, 4405–4465. [Google Scholar] [CrossRef]
- Cuartero, M.; Colozza, N.; Fernández-Pérez, B.M.; Crespo, G.A. Why Ammonium Detection is Particularly Challenging but Insightful with Ionophore-Based Potentiometric Sensors–An Overview of the Progress in the Last 20 Years. Analyst 2020, 145, 3188–3210. [Google Scholar] [CrossRef]
- Chou, N.-H.; Chou, J.-C.; Sun, T.-P.; Hsiung, S.-K. All Solid-State Potentiometric Biosensors for Creatinine Determination Based on pH and Ammonium Electrodes. IEEE Sens. J. 2009, 9, 665–672. [Google Scholar] [CrossRef]
- Wang, T.; Xu, Z.; Huang, Y.; Dai, Z.; Wang, X.; Lee, M.; Bagtzoglou, C.; Brückner, C.; Lei, Y.; Li, B. Real-Time In Situ Auto-Correction of K+ Interference for Continuous and Long-Term NH4+ Monitoring in Wastewater Using Solid-State Ion Selective Membrane (S-ISM) Sensor Assembly. Environ. Res. 2020, 189, 109891. [Google Scholar] [CrossRef]
- Zhang, H.; Jiang, Q.; Hadden, J.H.; Xie, F.; Riley, D.J. Pd Ion-Exchange and Ammonia Etching of a Prussian Blue Analogue to Produce a High-Performance Water-Splitting Catalyst. Adv. Funct. Mater. 2021, 31, 2008989. [Google Scholar] [CrossRef]
- Jin, X.; Saha, A.; Jiang, H.; Oduncu, M.R.; Yang, Q.; Sedaghat, S.; Maize, K.; Allebach, J.P.; Shakouri, A.; Glassmaker, N. Steady-State and Transient Performance of Ion-Sensitive Electrodes Suitable for Wearable and Implantable Electrochemical Sensing. IEEE Trans. Biomed. Eng. 2021, 69, 96–107. [Google Scholar] [CrossRef]
- Matse, D.T.; Huang, C.-H.; Huang, Y.-M.; Yen, M.-Y. Effects of Coinoculation of Rhizobium with Plant Growth Promoting Rhizobacteria on the Nitrogen Fixation and Nutrient Uptake of Trifolium Repens in Low Phosphorus Soil. J. Plant Nutr. 2020, 43, 739–752. [Google Scholar] [CrossRef]
- Sharma, S.B.; Sayyed, R.Z.; Trivedi, M.H.; Gobi, T.A. Phosphate Solubilizing Microbes: Sustainable Approach for Managing Phosphorus Deficiency in Agricultural Soils. SpringerPlus 2013, 2, 1–14. [Google Scholar] [CrossRef] [PubMed]
- De Rijck, G.; Schrevens, E. pH Influenced by the Elemental Composition of Nutrient Solutions. J. Plant Nutr. 1997, 20, 911–923. [Google Scholar] [CrossRef]
- Malkawi, S.; Hagare, D.; Maheshwari, B. Phosphorus Recovery from Hydroponics Waste Nutrient Solution and Its Economic Potential. Resour. Conserv. Recycl. 2024, 209, 107710. [Google Scholar] [CrossRef]
- Chen, G.; Xiao, S.; Lorke, A.; Liu, J.; Zhang, P. Assessment of a Solid-State Phosphate Selective Electrode Based on Tungsten. J. Electrochem. Soc. 2018, 165, B787–B792. [Google Scholar] [CrossRef]
- Xu, K.; Kitazumi, Y.; Kano, K.; Sasaki, T.; Shirai, O. Fabrication of a Phosphate Ion Selective Electrode Based on Modified Molybdenum Metal. Anal. Sci. 2020, 36, 201–205. [Google Scholar] [CrossRef]
- Xu, K.; Kitazumi, Y.; Kano, K.; Shirai, O. Phosphate Ion Sensor Using a Cobalt Phosphate Coated Cobalt Electrode. Electrochim. Acta 2018, 282, 242–246. [Google Scholar] [CrossRef]
- Wang, X.; Ma, X.; Lee, W.H.; Cho, H.J. Sensor Response Mechanism and Characterization of Co-Based Phosphate Nanosensors. In Proceedings of the 2018 IEEE Sensors Applications Symposium (SAS), Seoul, Republic of Korea, 12–14 March 2018; pp. 1–5. [Google Scholar]
- Zeitoun, R.; Biswas, A. Electrochemical Mechanisms in Potentiometric Phosphate Sensing Using Pure Cobalt, Molybdenum and Their Alloy for Environmental Applications. Electroanalysis 2021, 33, 421–430. [Google Scholar] [CrossRef]
- Kim, H.-J.; Hummel, J.W.; Birrell, S.J.; Sudduth, K.A. Evaluation of Phosphate Ion-Selective Membranes for Real-Time Soil Nutrient Sensing. In Proceedings of the 2005 ASAE Annual Meeting, Tampa, FL, USA, 17–20 July 2005; p. 1. [Google Scholar]
- Barus, C.; Romanytsia, I.; Striebig, N.; Garçon, V. Toward an In Situ Phosphate Sensor in Seawater Using Square Wave Voltammetry. Talanta 2016, 160, 417–424. [Google Scholar] [CrossRef]
- Ahmad, R.; Ahn, M.-S.; Hahn, Y.-B. ZnO Nanorods Array Based Field-Effect Transistor Biosensor for Phosphate Detection. J. Colloid Interface Sci. 2017, 498, 292–297. [Google Scholar] [CrossRef]
- Talarico, D.; Arduini, F.; Amine, A.; Moscone, D.; Palleschi, G. Screen-Printed Electrode Modified with Carbon Black Nanoparticles for Phosphate Detection by Measuring the Electroactive Phosphomolybdate Complex. Talanta 2015, 141, 267–272. [Google Scholar] [CrossRef]
- Kolliopoulos, A.V.; Kampouris, D.K.; Banks, C.E. Rapid and Portable Electrochemical Quantification of Phosphorus. Anal. Chem. 2015, 87, 4269–4274. [Google Scholar] [CrossRef] [PubMed]
- Ngernpradab, P.; Insin, N.; Wongravee, K.; Srisa-Art, M. A Novel PDMS-Based Digital Magnetofluidic Platform for Lab-on-a-Chip Applications. Talanta 2024, 266, 125053. [Google Scholar] [CrossRef] [PubMed]
- Magar, H.S.; Hassan, R.Y.; Mulchandani, A. Electrochemical Impedance Spectroscopy (EIS): Principles, Construction, and Biosensing Applications. Sensors 2021, 21, 6578. [Google Scholar] [CrossRef] [PubMed]
- Pol, R.; Céspedes, F.; Gabriel, D.; Baeza, M. Microfluidic Lab-on-a-Chip Platforms for Environmental Monitoring. TrAC Trends Anal. Chem. 2017, 95, 62–68. [Google Scholar] [CrossRef]
- Wang, R.; Prabhakar, A.; Iglesias, R.A.; Xian, X.; Shan, X.; Tsow, F.; Forzani, E.S.; Tao, N. A Microfluidic-Colorimetric Sensor for Continuous Monitoring of Reactive Environmental Chemicals. IEEE Sens. J. 2011, 12, 1529–1535. [Google Scholar] [CrossRef]
- Pinto, V.; Araújo, C.; Sousa, P.; Gonçalves, L.; Minas, G. A Low-Cost Lab-on-a-Chip Device for Marine pH Quantification by Colorimetry. Sens. Actuators B Chem. 2019, 290, 285–292. [Google Scholar] [CrossRef]
- Cho, Y.B.; Jeong, S.H.; Chun, H.; Kim, Y.S. Selective Colorimetric Detection of Dissolved Ammonia in Water via Modified Berthelot’s Reaction on Porous Paper. Sens. Actuators B Chem. 2018, 256, 167–175. [Google Scholar] [CrossRef]
- Lace, A.; Byrne, A.; Bluett, S.; Malaquin, L.; Raimbault, V.; Courson, R.; Hayat, Z.; Moore, B.; Murray, E. Ion Chromatograph with Three-Dimensional Printed Absorbance Detector for Indirect Ultraviolet Absorbance Detection of Phosphate in Effluent and Natural Waters. J. Sep. Sci. 2022, 45, 1042–1050. [Google Scholar] [CrossRef]
- Saito, S.; Uozumi, N. Calcium-Regulated Phosphorylation Systems Controlling Uptake and Balance of Plant Nutrients. Front. Plant Sci. 2020, 11, 44. [Google Scholar] [CrossRef]
- Wang, M.; Zheng, Q.; Shen, Q.; Guo, S. The Critical Role of Potassium in Plant Stress Response. Int. J. Mol. Sci. 2013, 14, 7370–7390. [Google Scholar] [CrossRef]
- Pandey, P.C.; Singh, G.; Srivastava, P.K. Electrochemical Synthesis of Tetraphenylborate Doped Polypyrrole and Its Applications in Designing a Novel Zinc and Potassium Ion Sensor. Electroanalysis 2002, 14, 427–432. [Google Scholar] [CrossRef]
- Richa, A.; Fizir, M.; Touil, S. Advanced Monitoring of Hydroponic Solutions Using Ion-Selective Electrodes and the Internet of Things: A Review. Environ. Chem. Lett. 2021, 19, 3445–3463. [Google Scholar] [CrossRef]
- Chowdhury, M.E.; Khandakar, A.; Ahmed, S.; Al-Khuzaei, F.; Hamdalla, J.; Haque, F.; Reaz, M.B.I.; Al Shafei, A.; Al-Emadi, N. Design, Construction and Testing of IoT Based Automated Indoor Vertical Hydroponics Farming Test-Bed in Qatar. Sensors 2020, 20, 5637. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-H.; Jeng, S.-Y.; Lin, C.-J. Fuzzy Logic Controller for Automating Electrical Conductivity and pH in Hydroponic Cultivation. Appl. Sci. 2021, 12, 405. [Google Scholar] [CrossRef]
- Vincentdo, V.; Surantha, N. Nutrient Film Technique-Based Hydroponic Monitoring and Controlling System Using ANFIS. Electronics 2023, 12, 1446. [Google Scholar] [CrossRef]
- Cho, W.-J.; Kim, H.-J.; Jung, D.-H.; Han, H.-J.; Cho, Y.-Y. Hybrid Signal-Processing Method Based on Neural Network for Prediction of NO3, K, Ca, and Mg Ions in Hydroponic Solutions Using an Array of Ion-Selective Electrodes. Sensors 2019, 19, 5508. [Google Scholar] [CrossRef]
- Jung, D.-H.; Kim, H.-J.; Cho, W.-J.; Park, S.H.; Yang, S.-H. Validation Testing of an Ion-Specific Sensing and Control System for Precision Hydroponic Macronutrient Management. Comput. Electron. Agric. 2019, 156, 660–668. [Google Scholar] [CrossRef]
- Miras, M.; García, M.S.; Martínez, V.; Ortuño, J.Á. Inexpensive Ion-Selective Electrodes for the Simultaneous Monitoring of Potassium and Nitrate Concentrations in Nutrient Solutions. Anal. Methods 2021, 13, 3511–3520. [Google Scholar] [CrossRef]
- Bhat, K.S.; Ahmad, R.; Mahmoudi, T.; Hahn, Y.-B. High Performance Chemical Sensor with Field-Effect Transistors Array for Selective Detection of Multiple Ions. Chem. Eng. J. 2021, 417, 128064. [Google Scholar] [CrossRef]
- Tuan, V.N.; Dinh, T.D.; Zhang, W.; Khattak, A.M.; Le, A.T.; Saeed, I.A.; Gao, W.; Wang, M. A Smart Diagnostic Tool Based on Deep Kernel Learning for On-Site Determination of Phosphate, Calcium, and Magnesium Concentration in a Hydroponic System. RSC Adv. 2021, 11, 11177–11191. [Google Scholar] [CrossRef]
- Chen, Y.; Tang, Z.; Zhu, Y.; Castellano, M.J.; Dong, L. Miniature Multi-Ion Sensor Integrated with Artificial Neural Network. IEEE Sens. J. 2021, 21, 25606–25615. [Google Scholar] [CrossRef]
- Nakatani, N.; Kozaki, D.; Mori, M.; Tanaka, K. Recent Progress and Applications of Ion-Exclusion/Ion-Exchange Chromatography for Simultaneous Determination of Inorganic Anions and Cations. Anal. Sci. 2012, 28, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Kozaki, D.; Sago, Y.; Fujiwara, T.; Mori, M.; Kubono, C.; Koga, T.; Mitsui, Y.; Tachibana, T. Ion-Exclusion/Cation-Exchange Chromatography Using Dual-Ion-Exchange Groups for Simultaneous Determination of Inorganic Ionic Nutrients in Fertilizer Solution Samples for the Management of Hydroponic Culture. Agronomy 2021, 11, 1847. [Google Scholar] [CrossRef]
- Monteiro-Silva, F.; Jorge, P.A.; Martins, R.C. Optical Sensing of Nitrogen, Phosphorus and Potassium: A Spectrophotometrical Approach Toward Smart Nutrient Deployment. Chemosensors 2019, 7, 51. [Google Scholar] [CrossRef]
- Soares, R.R.; Milião, G.L.; Pola, C.C.; Jing, D.; Opare-Addo, J.; Smith, E.; Claussen, J.C.; Gomes, C.L. Insights into Solid-Contact Ion-Selective Electrodes Based on Laser-Induced Graphene: Key Performance Parameters for Long-Term and Continuous Measurements. Microchim. Acta 2024, 191, 615. [Google Scholar] [CrossRef]
- Noviana, E.; Ozer, T.; Carrell, C.S.; Link, J.S.; McMahon, C.; Jang, I.; Henry, C.S. Microfluidic Paper-Based Analytical Devices: From Design to Applications. Chem. Rev. 2021, 121, 11835–11885. [Google Scholar] [CrossRef]
- Yan-Qi, L.; Liang, F. Progress in Paper-Based Colorimetric Sensor Array. Chin. J. Anal. Chem. 2020, 48, 1448–1457. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).