The Catalytic Role of D-block Elements and Their Compounds for Improving Sorption Kinetics of Hydride Materials: A Review
Abstract
:1. Introduction
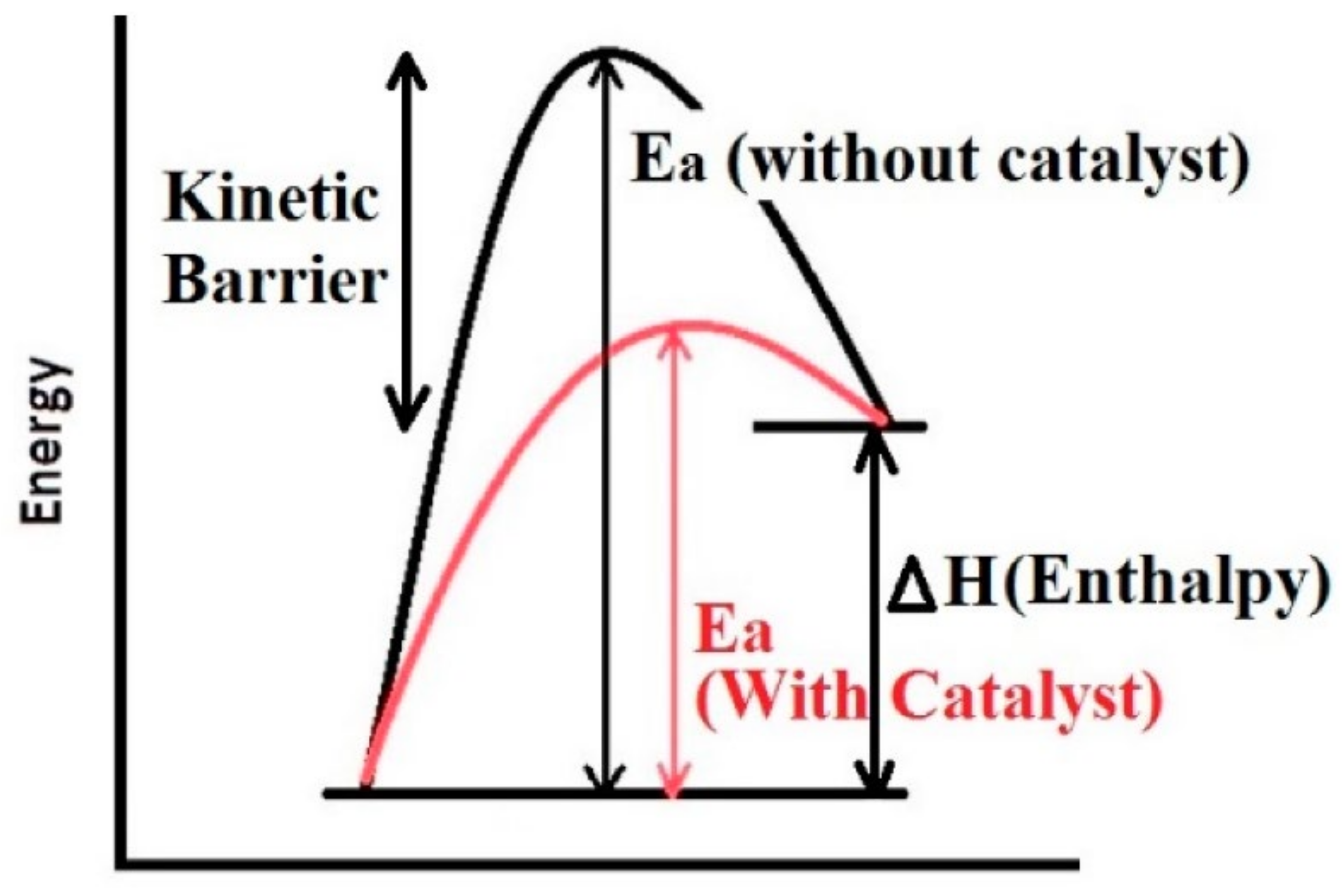
2. Basic Understanding of Kinetics and a Light on the Kinetics of Metal Hydride
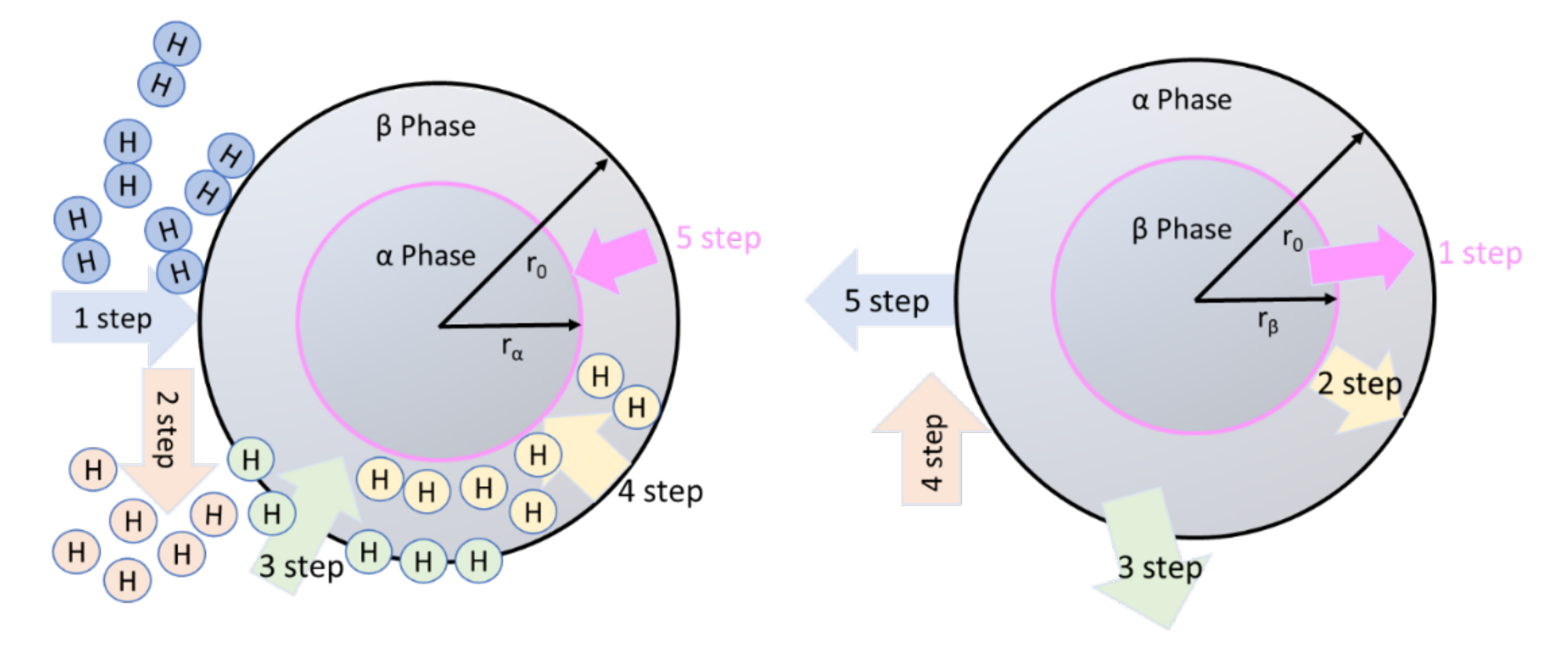
3. Mechanism of Hydrogen Absorption/Desorption and Need of Catalyst
- (1).
- Physisorption;
- (2).
- Chemisorption;
- (3).
- Surface penetration;
- (4).
- Diffusion through hydride layer to the metal/hydride interface;
- (5).
- Formation of hydride at interface of metal hydride.
- (1).
- Hydride decomposition;
- (2).
- Diffusion of hydrogen atom through metal;
- (3).
- Surface penetration;
- (4).
- Recombination of hydrogen atom into molecule;
- (5).
- Desorption to the gas phase.
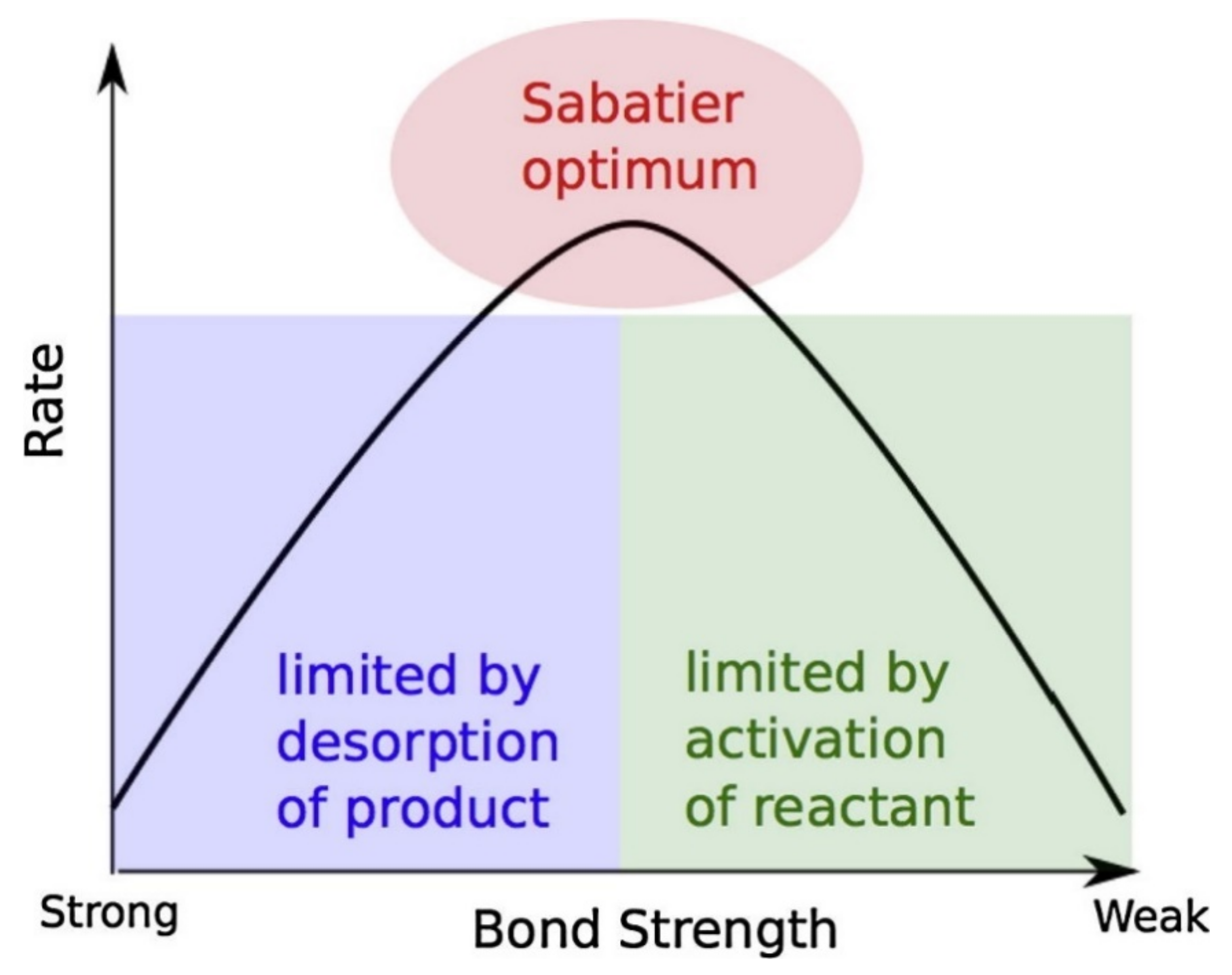
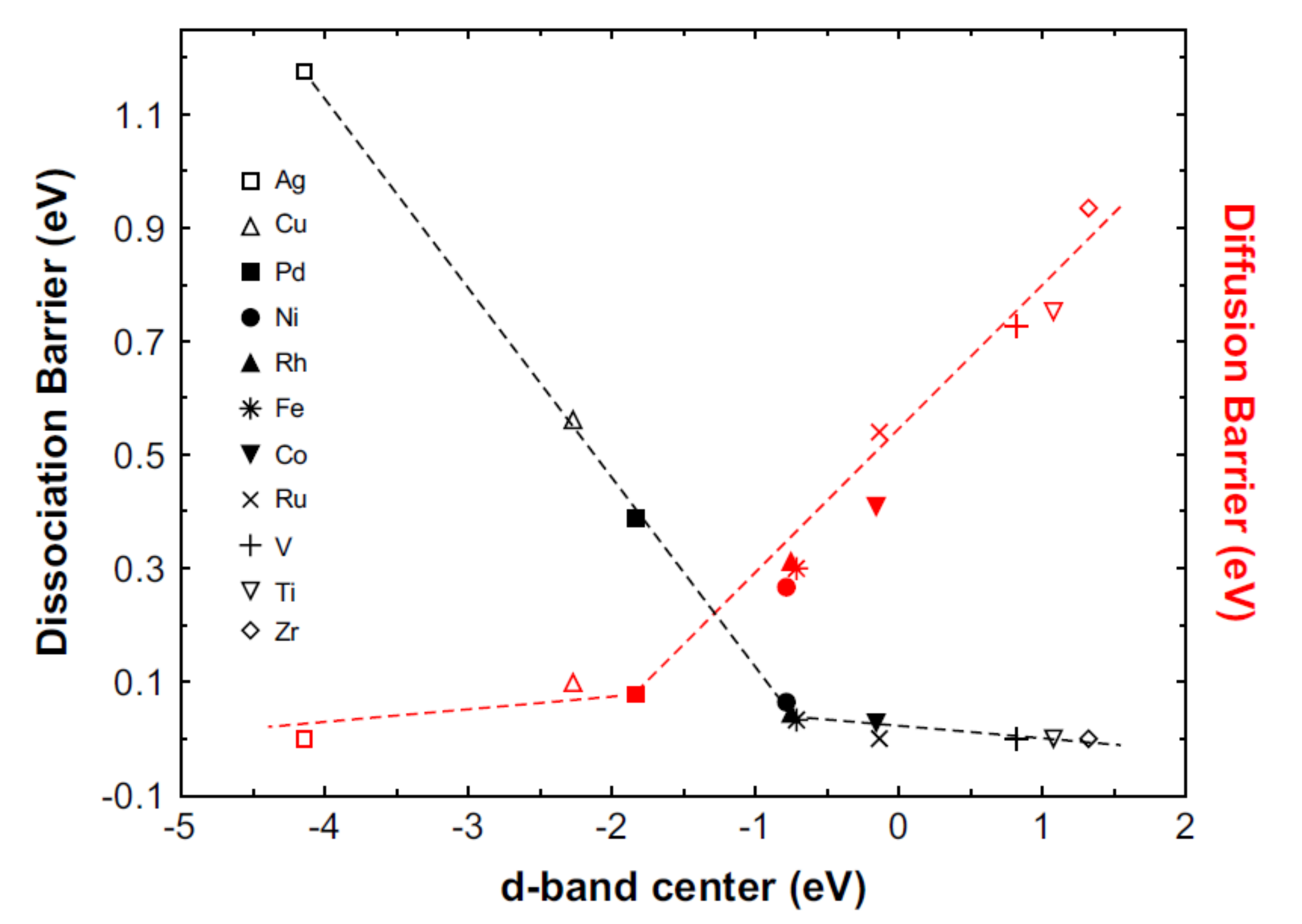
4. D-block (Transition Metal) Elements as Catalyst
4.1. Transition Unary Metals as Catalyst
4.2. D-block Binary Metal Catalyst (Metal-Metal, Metal Oxides, Metal Halides, etc.)
4.3. D-block Ternary Metal Catalysts and Miscellaneous Catalysts
5. Summary and Future Perspective
Conflicts of Interest
References
- Fcto_Targets_onboard_Hydro_Storage_Explanation. Available online: http://www.doc88.com/p-7324389963306.html (accessed on 16 August 2021).
- Schlapbach, L.; Züttel, A. Hydrogen-storage materials for mobile applications. Mater. Sustain. Energy 2010, 265–270. [Google Scholar] [CrossRef]
- Zheng, J.; Wang, C.G.; Zhou, H.; Ye, E.; Xu, J.; Li, Z.; Loh, X.J. Current Research Trends and Perspectives on Solid-State Nanomaterials in Hydrogen Storage. Research 2021, 1–39. [Google Scholar] [CrossRef]
- Mohan, M.; Sharma, V.K.; Kumar, E.A.; Gayathri, V. Hydrogen storage in carbon materials—A review. Energy Storage 2019, 1, e35. [Google Scholar] [CrossRef]
- Jia, J.; Lin, X.; Wilson, C.; Blake, A.J.; Champness, N.R.; Hubberstey, P.; Walker, G.; Cussena, E.J.; Schröder, M. Twelve-connected porous metal–organic frameworks with high H2 adsorption. Chem. Commun. 2007, 8, 840–842. [Google Scholar] [CrossRef] [Green Version]
- Van den Berg, A.W.C.; Areán, C.O. Materials for hydrogen storage: Current research trends and perspectives. Chem. Commun. 2008, 6, 668–681. [Google Scholar] [CrossRef]
- Budd, P.M.; Butler, A.; Selbie, J.; Mahmood, K.; McKeown, N.B.; Ghanem, B.; Msayib, K.; Book, D.; Waltonc, A. The potential of organic polymer-based hydrogen storage materials. Phys. Chem. Chem. Phys. 2007, 9, 1802–1808. [Google Scholar] [CrossRef]
- Tedds, S.; Walton, A.; Broom, D.P.; Book, D. Characterisation of porous hydrogen storage materials: Carbons, zeolites, MOFs and PIMs. Faraday Discuss. 2011, 151, 75–94. [Google Scholar] [CrossRef]
- Selvaraj, S.; Jain, A.; Miyaoka, H.; Kojima, Y.; Ichikawa, T. Hydrogen Sorption and Cyclic Compressor Performance of V40Ti21.5Cr33.5M5 (M= Nb, Zr, Fe) Alloys. J. Jpn. Inst. Energy 2019, 98, 157–164. [Google Scholar] [CrossRef] [Green Version]
- Guo, F.; Jain, A.; Miyaoka, H.; Kojima, Y.; Ichikawa, T. Critical Temperature and Pressure Conditions of Degradation during Thermochemical Hydrogen Compression: A Case Study of V-Based Hydrogen Storage Alloy. Energies 2020, 13, 2324. [Google Scholar] [CrossRef]
- Guo, F.; Namba, K.; Miyaoka, H.; Jain, A.; Ichikawa, T. Hydrogen storage behavior of TiFe alloy activated by different methods. Mater. Lett. X 2021, 9, 100061. [Google Scholar]
- Liang, G.; Huot, J.; Schulz, R. Hydrogen storage properties of the mechanically alloyed LaNi5-based materials. J. Alloys Compd. 2001, 320, 133–139. [Google Scholar] [CrossRef]
- Grochala, W.; Edwards, P.P. Thermal Decomposition of the Non-Interstitial Hydrides for the Storage and Production of Hy-drogen. Chem. Rev. 2004, 104, 1283–1316. [Google Scholar] [CrossRef]
- De Jongh, P.E.; Adelhelm, P. Nanosizing and Nanoconfinement: New Strategies Towards Meeting Hydrogen Storage Goals. ChemSusChem 2010, 3, 1332–1348. [Google Scholar] [CrossRef]
- Jain, A.; Agarwal, S.; Ichikawa, T. Catalytic Tuning of Sorption Kinetics of Lightweight Hydrides: A Review of the Materials and Mechanism. Catalysts 2018, 8, 651. [Google Scholar] [CrossRef] [Green Version]
- Adams, B.D.; Chen, A. The role of palladium in a hydrogen economy. Mater. Today 2011, 14, 282–289. [Google Scholar] [CrossRef]
- Konda, S.K.; Chen, A. Palladium based nanomaterials for enhanced hydrogen spillover and storage. Mater. Today 2016, 19, 100–108. [Google Scholar] [CrossRef]
- Wu, Y.; Yu, H.; Guo, Y.; Jiang, X.; Qi, Y.; Sun, B.; Li, H.; Zheng, J.; Li, X. A rare earth hydride supported ruthenium catalyst for the hydrogenation of N-heterocycles: Boosting the activity via a new hydrogen transfer path and controlling the stereoselectivity. Chem. Sci. 2019, 10, 10459–10465. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Fu, J.; Fu, K.; Xiao, R.; Wu, Y.; Zheng, X.; Liu, Z.; Zheng, J.; Li, X. Combining catalysis and hydrogen storage in direct borohydride fuel cells: Towards more efficient energy utilization. J. Mater. Chem. A 2017, 5, 14310–14318. [Google Scholar] [CrossRef]
- Yang, M.; Dong, Y.; Fei, S.; Ke, H.; Cheng, H. A comparative study of catalytic dehydrogenation of perhydro-N-ethylcarbazole over noble metal catalysts. Int. J. Hydrogen Energy 2014, 39, 18976–18983. [Google Scholar]
- Huang, Y.; An, C.; Zhang, Q.; Zhag, L.; Shao, H.; Liu, Y.; Zhang, Y.; Yuan, H.; Wang, C.; Wang, Y. Cost-effective mechanochemical synthesis of highly dispersed supported transition metal catalysts for hydrogen storage. Nano Energy 2021, 80, 105535. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, A.; Wang, W.; Huang, Y.; Liu, X.; Miao, S.; Liu, J.; Zhang, T. Co–N–C Catalyst for C–C Coupling Reactions: On the Catalytic Performance and Active Sites. ACS Catal. 2015, 5, 6563–6572. [Google Scholar]
- Zhang, J.; Yan, S.; Xia, G.; Zhou, X.; Lu, X.; Yu, L.; Yu, X.; Peng, P. Stabilization of low-valence transition metal towards advanced catalytic effects on the hydrogen storage performance of magnesium hydride. J. Magnes. Alloy. 2021, 9, 647–657. [Google Scholar] [CrossRef]
- Sharma, S.; Guo, F.; Ichikawa, T.; Kojima, Y.; Agarwal, S.; Jain, A. Iron based catalyst for the improvement of the sorption properties of KSiH3. Int. J. Hydrogen Energy 2020, 45, 33681–33686. [Google Scholar] [CrossRef]
- Agarwal, S.; Mangal, R.K.; Kumar, M.; Awasthi, K.; Kumar, S.; Jain, A. Hydrogen Sorption Characteristics of ZrCrAl Ternary Alloy as a Function of Milling Time. Macromol. Symp. 2017, 376, 1700047. [Google Scholar]
- Pal, P.; Jain, A.; Miyaoka, H.; Kojima, Y.; Ichikawa, T. Eutectic melting in x(2LiBH4-MgH2) hydrogen storage system by the addition of KH. Int. J. Hydrogen Energy 2020, 45, 17000–17005. [Google Scholar] [CrossRef]
- Kumar, S.; Jain, A.; Kojima, Y. Thermodynamics and kinetics of hydrogen absorption–desorption of vanadium synthesized by aluminothermy. J. Therm. Anal. Calorim. 2017, 130, 721–726. [Google Scholar] [CrossRef]
- Yoshino, M.; Komiya, K.; Takahashi, Y.; Shinzato, Y.; Yukawa, H.; Morinaga, M. Nature of the chemical bond in complex hydrides, NaAlH4, LiAlH4, LiBH4 and LiNH2. J. Alloys Compd. 2005, 404–406, 185–190. [Google Scholar] [CrossRef]
- Schüth, F.; Bogdanović, B.; Felderhoff, M. Light metal hydrides and complex hydrides for hydrogen storage. Chem. Commun. 2004, 20, 2249–2258. [Google Scholar] [CrossRef]
- Modi, P.; Aguey-Zinsou, K.-F. Room Temperature Metal Hydrides for Stationary and Heat Storage Applications: A Review. Front. Energy Res. 2021, 9, 128. [Google Scholar] [CrossRef]
- Jain, I.P.; Jain, P.; Jain, A. Novel hydrogen storage materials: A review of lightweight complex hydrides. J. Alloys Compd. 2010, 503, 303–339. [Google Scholar] [CrossRef]
- Sakintuna, B.; Lamari-Darkrim, F.; Hirscher, M. Metal hydride materials for solid hydrogen storage: A review. Int. J. Hydrogen Energy 2007, 32, 1121–1140. [Google Scholar] [CrossRef]
- Bouaricha, S.; Dodelet, J.P.; Guay, D.; Huot, J.; Schulz, R. Study of the activation process of Mg-based hydrogen storage materials modified by graphite and other carbonaceous compounds. J. Mater. Res. 2001, 16, 2893–2905. [Google Scholar] [CrossRef]
- Bellosta von Colbe, J.M.; Puszkiel, J.; Capurso, G.; Franz, A.; Ulrich Benz, H.; Zoz, H.; Klassen, T.; Dornheim, M. Scale-up of milling in a 100 L device for processing of TiFeMn alloy for hydrogen storage applications: Procedure and characterization. Int. J. Hydrogen Energy 2019, 44, 29282–29290. [Google Scholar] [CrossRef]
- Shinzato, K.; Hamamoto, S.; Miyaoka, H.; Ichikawa, T. Room-Temperature Hydrogen Absorption of Titanium with Surface Modification by Organic Solvents. J. Phys. Chem. C 2019, 123, 19269–19274. [Google Scholar] [CrossRef]
- Jain, I.P.; Lal, C.; Jain, A. Hydrogen storage in Mg: A most promising material. Int. J. Hydrogen Energy 2010, 35, 5133–5144. [Google Scholar] [CrossRef]
- Vajeeston, P.; Ravindran, P.; Fichtner, M.; Fjellvåg, H. Influence of Crystal Structure of Bulk Phase on the Stability of Nanoscale Phases: Investigation on MgH2 Derived Nanostructures. J. Phys. Chem. 2012, 116, 18965–18972. [Google Scholar] [CrossRef] [Green Version]
- Nogita, K.; Tran, X.Q.; Yamamoto, T.; Tanaka, E.; McDonald, S.D.; Gourlay, C.M.; Yasuda, K.; Matsumura, S. Evidence of the hydrogen release mechanism in bulk MgH2. Sci. Rep. 2015, 5, 8450. [Google Scholar]
- Wang, H.; Lin, H.J.; Cai, W.T.; Ouyang, L.Z.; Zhu, M. Tuning kinetics and thermodynamics of hydrogen storage in light metal element based systems—A review of recent progress. J. Alloys Compd. 2016, 658, 280–300. [Google Scholar] [CrossRef]
- Kitagawa, Y.; Tanabe, K. Development of a kinetic model of hydrogen absorption and desorption in magnesium and analysis of the rate-determining step. Chem. Phys. Lett. 2018, 699, 132–138. [Google Scholar] [CrossRef]
- Zhou, C.; Zhang, J.; Bowman, R.C., Jr.; Fang, Z.Z. Roles of Ti-Based Catalysts on Magnesium Hydride and Its Hydrogen Storage Properties. Inorganics 2021, 9, 36. [Google Scholar] [CrossRef]
- Ruse, E.; Pevzner, S.; Bar, I.P.; Nadiv, R.M.; Skripnyuk, V.; Rabkin, E.; Regev, O. Hydrogen storage and spillover kinetics in carbon nanotube-Mg composites. Int. J. Hydrogen Energy 2016, 41, 2814–2819. [Google Scholar] [CrossRef]
- Pevzner, S.; Pri-Bar, I.; Lutzky, I.; Ben-Yehuda, E.; Ruse, E.; Regev, O. Carbon Allotropes Accelerate Hydrogenation via Spillover Mechanism. J. Phys.Chem C 2014, 118, 27164–27169. [Google Scholar]
- Zhou, W.; Zhao, Y.; Wang, Y.; Wang, S.; Ma, X. Glycerol Hydrogenolysis to 1,3-Propanediol on Tungstate/Zirconia-Supported Platinum: Hydrogen Spillover Facilitated by Pt(1 1 1) Formation. ChemCatChem 2016, 8, 3663–3671. [Google Scholar] [CrossRef]
- Medford, J.A.; Vojvodic, A.S.; Hummelshøj, J.; Voss, J.; Abild-Pedersen, F.; Studt, F.; Bligaard, T.; Nilsson, A.; Nørskov, J.K. From the Sabatier principle to a predictive theory of transition-metal heterogeneous catalysis. J. Catal. 2015, 328, 36–42. [Google Scholar] [CrossRef] [Green Version]
- Trasatti, S. Work function, electronegativity, and electrochemical behaviour of metals: III. Electrolytic hydrogen evolution in acid solutions. J. Electroanal. Chem. Interfacial Electrochem. 1972, 39, 163–184. [Google Scholar] [CrossRef]
- Litovchenko, V.G.; Efremov, A.A. The enhanced catalytic dissociation of adsorbed hydrogen containing molecules. Condens. Matter Phys. 1999, 2, 561. [Google Scholar] [CrossRef] [Green Version]
- Zeradjanin, A.R.; Grote, J.P.; Polymeros, G.; Mayrhofer, K.J.J. A Critical Review on Hydrogen Evolution Electrocatalysis: Re-exploring the Volcano-relationship. Electroanalysis 2016, 28, 2256–2269. [Google Scholar] [CrossRef]
- Pelletier, J.F.; Huot, J.; Sutton, M.; Schulz, R.; Sandy, A.R.; Lurio, L.B.; Mochrie, S.G.J. Hydrogen desorption mechanism in MgH2−Nb nanocomposites. Phys. Rev. B 2001, 63, 052103. [Google Scholar] [CrossRef]
- Borgschulte, A.; Bösenberg, U.; Barkhordarian, G.; Dornheim, M.; Bormann, R. Enhanced hydrogen sorption kinetics of magnesium by destabilized MgH2−δ. Catal. Today 2007, 120, 262–269. [Google Scholar] [CrossRef] [Green Version]
- Charbonnier, J.; Rango, P.D.; Fruchart, D.; Miraglia, S.; Skryabina, N.; Huot, J.; Hauback, B.; Pitt, M.; Rivoirard, S. Structural analysis of activated Mg(Nb)H2. J. Alloys Compd. 2005, 404–406, 541–544. [Google Scholar] [CrossRef]
- Zhang, X.L.; Liu, Y.F.; Zhang, X.; Hu, J.J.; Gao, M.X.; Pan, H.G. Empowering hydrogen storage performance of MgH2 by nanoengineering and nanocatalysis. Mater. Today Nano 2020, 9, 100064. [Google Scholar] [CrossRef]
- Pozzo, M.; Alfè, D. Hydrogen dissociation and diffusion on transition metal (=Ti, Zr, V, Fe, Ru, Co, Rh, Ni, Pd, Cu, Ag)-doped Mg(0001) surfaces. Int. J. Hydrogen Energy 2009, 34, 1922–1930. [Google Scholar] [CrossRef] [Green Version]
- Andrés, T.B.; Zélis Luis, M.; Marcos, M. Differences in the heterogeneous nature of hydriding/dehydriding kinetics of MgH2—TiH2 nanocomposites. Int. J. Hydrogen Energy 2020, 45, 27421–27433. [Google Scholar] [CrossRef]
- Ren, C.; Fang, Z.Z.; Zhou, C.; Lu, J.; Ren, Y.; Zhang, X.; Luo, X. In situ X-ray diffraction study of dehydrogenation of MgH2 with Ti-based additives. Int. J. Hydrogen Energy 2014, 39, 5868–5873. [Google Scholar] [CrossRef]
- Ponthieu, M.; Calizzi, M.; Pasquini, L.; Fernandez, J.F.; Cuevas, F. Synthesis by reactive ball milling and cycling properties of MgH2–TiH2 nanocomposites: Kinetics and isotopic effects. Int. J. Hydrogen Energy 2014, 39, 9918–9923. [Google Scholar] [CrossRef]
- Friedrichs, O.; Sánchez-López, J.C.; López-Cartes, C.; Klassen, T.; Bormann, R.; Fernández, A. Nb2O5 “Pathway Effect” on Hydrogen Sorption in Mg. J. Phys. Chem. B 2006, 110, 7845–7850. [Google Scholar] [CrossRef]
- Crivello, J.-C.; Dam, B.; Denys, R.V.; Dornheim, M.; Grant, D.M.; Huot, J.; Jensen, T.R.; Jongh, P.D.; Milanese, C.; Milčius, D.; et al. Review of magnesium hydride-based materials: Development and optimisation. Appl. Phys. A 2016, 122, 97. [Google Scholar] [CrossRef] [Green Version]
- Yin, Y.; Qi, Y.; Li, B.; Gu, H.; Zhao, J.; Ji, L.; Zhang, B.; Yuan, Z.; Zhang, Y. A comparative study of NbF5 catalytic effects on hydrogenation/dehydrogenation kinetics of Mg-Zn-Ni and Mg-Cu-Ni systems. Mater. Charact. 2021, 174, 110993. [Google Scholar] [CrossRef]
- Lakhnik, A.M.; Kirian, I.M.; Rud, A.D. The Mg/MAX-phase composite for hydrogen storage. Int. J. Hydrogen Energy 2021, in press. [Google Scholar] [CrossRef]
- Luo, Q.; An, X.H.; Pan, Y.B.; Zhang, X.; Zhang, J.-Y.; Li, Q. The hydriding kinetics of Mg–Ni based hydrogen storage alloys: A comparative study on Chou model and Jander model. Int. J. Hydrogen Energy 2010, 35, 7842–7849. [Google Scholar] [CrossRef]
- Luo, Q.; Li, J.; Li, B.; Liu, B.; Shao, H.; Li, Q. Kinetics in Mg-based hydrogen storage materials: Enhancement and mechanism. J. Magnes. Alloy 2019, 7, 58–71. [Google Scholar] [CrossRef]
- Mooij, L.; Dam, B. Nucleation and growth mechanisms of nano magnesium hydride from the hydrogen sorption kinetics. Phys. Chem. Chem. Phys. 2013, 15, 11501–11510. [Google Scholar] [CrossRef]
- Ouyang, L.; Liu, F.; Wang, H.; Liu, J.; Yang, X.-S.; Sun, L.; Zhu, M. Magnesium-based hydrogen storage compounds: A review. J. Alloys Compd. 2020, 832, 154865. [Google Scholar] [CrossRef]
- Jain, A.; Jain, R.K.; Agarwal, S.; Jain, I.P. Structural and thermodynamical investigations of La0.23Ni0.34Co0.33Nd0.08Ti0.01Al0.01 hydrogen storage alloy. Int. J. Hydrogen Energy 2008, 33, 356–359. [Google Scholar] [CrossRef]
- Pal, P.; Kumari, P.; Wang, Y.; Isobe, S.; Kumar, M.; Ichikawa, T.; Jain, A. Destabilization of LiBH4 by the infusion of Bi2X3 (X = S, Se, Te): An in situ TEM investigation. J. Mater. Chem. A 2020, 8, 25706–25715. [Google Scholar] [CrossRef]
- Jangir, M.; Jain, A.; Yamaguchi, S.; Ichikawa, T.; Lal, C.; Jain, I.P. Catalytic effect of TiF4 in improving hydrogen storage properties of MgH2. Int. J. Hydrogen Energy 2016, 41, 14178–14183. [Google Scholar] [CrossRef]
- Jain, A.; Miyaoka, H.; Ichikawa, T. Destabilization of lithium hydride by the substitution of group 14 elements: A review. Int. J. Hydrogen Energy 2016, 41, 5969–5978. [Google Scholar] [CrossRef]
- Jain, A.; Miyaoka, H.; Ichikawa, T. Two-Peak Mystery of LiNH2–NaH Dehydrogenation Is Solved? A Study of the Analogous Sodium Amide/Lithium Hydride System. J. Phys. Chem. C 2016, 120, 27903–27909. [Google Scholar] [CrossRef]
- Kumar, S.; Jain, A.; Miyaoka, H.; Ichikawa, T.; Kojima, Y. Study on the thermal decomposition of NaBH4 catalyzed by ZrCl4. Int. J. Hydrogen Energy 2017, 42, 22432–22437. [Google Scholar] [CrossRef]
- Selvaraj, S.; Jain, A.; Kumar, S.; Zhang, T.; Isobe, S.; Miyaoka, H.; Kojima, Y.; Ichikawa, T. Study of cyclic performance of V-Ti-Cr alloys employed for hydrogen compressor. Int. J. Hydrogen Energy 2018, 43, 2881–2889. [Google Scholar] [CrossRef]
- Vyas, D.; Jain, P.; Khan, J.; Kulshrestha, V.; Jain, A.; Jain, I.P. Effect of Cu catalyst on the hydrogenation and thermodynamic properties of Mg2Ni. Int. J. Hydrogen Energy 2012, 37, 3755–3760. [Google Scholar] [CrossRef]
- Barkhordarian, G.; Klassen, T.; Bormann, R. Catalytic Mechanism of Transition-Metal Compounds on Mg Hydrogen Sorption Reaction. J. Phys. Chem. B 2006, 110, 11020–11024. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liang, Z.; Zheng, H.; Cao, R. Recent Progress on Defect-rich Transition Metal Oxides and Their Energy-Related Applications. Chem. Asian J. 2020, 15, 3717–3736. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Jain, A.; Miyaoka, H.; Ichikawa, T.; Kojima, Y. Catalytic effect of bis (cyclopentadienyl) nickel II on the improvement of the hydrogenation-dehydrogenation of Mg-MgH2 system. Int. J. Hydrogen Energy 2017, 42, 17178–17183. [Google Scholar] [CrossRef]
- Vyas, D.; Jain, P.; Agarwal, G.; Jain, A.; Jain, I.P. Hydrogen storage properties of Mg2Ni affected by Cr catalyst. Int. J. Hydrogen Energy 2012, 37, 16013–16017. [Google Scholar] [CrossRef]
- Ma, X.; Liu, S.; Huang, S. Hydrogen adsorption and dissociation on the TM-doped (TM=Ti, Nb) Mg55 nanoclusters: A DFT study. Int. J. Hydrogen Energy 2017, 42, 24797–24810. [Google Scholar] [CrossRef]
- Jain, A.; Miyaoka, H.; Ichikawa, T.; Kojima, Y. Tailoring the absorption–desorption properties of KSiH3 compound using nano-metals (Ni, Co, Nb) as catalyst. J. Alloys Compd. 2015, 645, S144–S147. [Google Scholar] [CrossRef]
- Mao, J.F.; Wu, Z.; Chen, T.J.; Weng, B.C.; Xu, N.X.; Huang, T.S.; Guo, Z.P.; Liu, H.K.; Grant, D.M.; Walker, G.S.; et al. Improved Hydrogen Storage of LiBH4 Catalyzed Magnesium. J. Phys. Chem. C 2007, 111, 12495–12498. [Google Scholar] [CrossRef] [Green Version]
- Lillo-Ródenas, M.A.; Aguey-Zinsou, K.F.; Cazorla-Amorós, D.; Linares-Solano, A.; Guo, Z.X. Effects of Carbon-Supported Nickel Catalysts on MgH2 Decomposition. J. Phys. Chem. C 2008, 112, 5984–5992. [Google Scholar] [CrossRef]
- Kaupp, G. Reactive milling with metals for environmentally benign sustainable production. CrystEngCom. 2011, 13, 3108–3121. [Google Scholar] [CrossRef]
- Hudson, M.S.L.; Takahashi, K.; Ramesh, A.; Awasthi, S.; Ghosh, A.K.; Ravindran, P.; Srivastava, O.N. Graphene decorated with Fe nanoclusters for improving the hydrogen sorption kinetics of MgH2—Experimental and theoretical evidence. Catal. Sci. Technol. 2016, 6, 261–268. [Google Scholar] [CrossRef]
- Zhang, L.; Ji, L.; Yao, Z.; Yan, N.; Sun, Z.; Yang, X.; Zhu, X.; Hu, S.; Chen, L. Facile synthesized Fe nanosheets as superior active catalyst for hydrogen storage in MgH2. Int. J. Hydrogen Energy 2019, 44, 21955–21964. [Google Scholar] [CrossRef]
- Mao, J.; Guo, Z.; Yu, X.; Liu, H.; Wu, Z.; Ni, J. Enhanced hydrogen sorption properties of Ni and Co-catalyzed MgH2. Int. J. Hydrogen Energy 2010, 35, 4569–4575. [Google Scholar] [CrossRef]
- Zaluska, A.; Zaluski, L.; Ström–Olsen, J.O. Nanocrystalline magnesium for hydrogen storage. J. Alloys Compd. 1999, 288, 217–225. [Google Scholar] [CrossRef]
- Webb, C.J. A review of catalyst-enhanced magnesium hydride as a hydrogen storage material. J. Phys. Chem. Solids 2015, 84, 96–106. [Google Scholar] [CrossRef]
- Wang, Y.; Lan, Z.; Fu, H.; Liu, H.; Guo, J. Synergistic catalytic effects of ZIF-67 and transition metals (Ni, Cu, Pd, and Nb) on hydrogen storage properties of magnesium. Int. J. Hydrogen Energy 2020, 45, 13376–13386. [Google Scholar] [CrossRef]
- Pukazhselvan, D.; Nasani, N.; Pérez, J.; Hortigüela, M.J.; Yang, T.; Bdikin, I.; Fagg, D.P. Two step mechanochemical synthesis of Nb doped MgO rock salt nanoparticles and its application for hydrogen storage in MgH2. Int. J. Hydrogen Energy 2016, 41, 11716–11722. [Google Scholar] [CrossRef]
- Hanada, N.; Ichikawa, T.; Fujii, H. Catalytic effect of Ni nano-particle and Nb oxide on H-desorption properties in MgH2 prepared by ball milling. J. Alloys Compd. 2005, 404–406, 716–719. [Google Scholar] [CrossRef]
- Yang, W.N.; Shang, C.X.; Guo, Z.X. Site density effect of Ni particles on hydrogen desorption of MgH2. Int. J. Hydrogen Energy 2010, 35, 4534–4542. [Google Scholar] [CrossRef]
- Liu, Y.; Zou, J.; Zeng, X.; Wu, X.; Li, D.; Ding, W. Hydrogen Storage Properties of a Mg–Ni Nanocomposite Coprecipitated from Solution. J. Phys. Chem. C 2014, 118, 18401–18411. [Google Scholar] [CrossRef]
- Sun, Y.; Ma, T.; Aguey-Zinsou, K.-F. Magnesium Supported on Nickel Nanobelts for Hydrogen Storage: Coupling Nanosizing and Catalysis. ACS Appl. Nano Mater. 2018, 1, 1272–1279. [Google Scholar] [CrossRef]
- Yang, X.; Hou, Q.; Yu, L.; Zhang, J. Improvement of the hydrogen storage characteristics of MgH2 with a flake Ni nano-catalyst composite. Dalton Trans. 2021, 50, 1797–1807. [Google Scholar] [CrossRef] [PubMed]
- Gasnier, A.; Amica, G.; Juan, J.; Troiani, H.; Gennari, F.C. N-Doped Graphene-Rich Aerogels Decorated with Nickel and Cobalt Nanoparticles: Effect on Hydrogen Storage Properties of Nanoconfined LiBH4. J. Phys. Chem. C 2020, 124, 115–125. [Google Scholar] [CrossRef]
- Meng, X.; Wan, C.B.; Wang, Y.T.; Ju, X. Porous Ni@C derived from bimetallic Metal–Organic Frameworks and its application for improving LiBH4 dehydrogenation. J. Alloys Compd. 2018, 735, 1637–1647. [Google Scholar] [CrossRef]
- Montone, A.; Aurora, A.; Mirabile Gattia, D.; Vittori Antisari, M. Microstructural and Kinetic Evolution of Fe Doped MgH2 during H2 Cycling. Catalysts 2012, 2, 400–411. [Google Scholar] [CrossRef] [Green Version]
- Gattia, D.M.; Jangir, M.; Jain, I.P. Study on nanostructured MgH2 with Fe and its oxides for hydrogen storage applications. J. Alloys Compd. 2019, 801, 188–191. [Google Scholar] [CrossRef]
- Antiqueira, F.J.; Leiva, D.R.; Zepon, G.; de Cunha, B.F.R.F.; Figueroa, S.J.A.; Botta, W.J. Fast hydrogen absorption/desorption kinetics in reactive milled Mg-8 mol% Fe nanocomposites. Int. J. Hydrogen Energy 2020, 45, 12408–12418. [Google Scholar] [CrossRef]
- Liu, T.; Ma, X.; Chen, C.; Xu, L.; Li, X. Catalytic Effect of Nb Nanoparticles for Improving the Hydrogen Storage Properties of Mg-Based Nanocomposite. J. Phys. Chem. C 2015, 119, 14029–14037. [Google Scholar] [CrossRef]
- Wang, P.; Jensen, C.M. Method for preparing Ti-doped NaAlH4 using Ti powder: Observation of an unusual reversible dehydrogenation behavior. J. Alloys Compd. 2004, 379, 99–102. [Google Scholar] [CrossRef]
- Chaudhuri, S.; Muckerman, J.T. First-Principles Study of Ti-Catalyzed Hydrogen Chemisorption on an Al Surface: A Critical First Step for Reversible Hydrogen Storage in NaAlH4. J. Phys. Chem. B 2005, 109, 6952–6957. [Google Scholar] [CrossRef]
- Blomqvist, A.; Araujo, C.M. Dehydrogenation from 3d-transition-metal-doped NaAlH4: Prediction of catalysts. Appl. Phys. Lett. 2007, 90, 141904. [Google Scholar] [CrossRef]
- Huang, C.; Zhao, Y.-J.; Sun, T.; Guo, J.; Sun, L.-X.; Zhu, M. Influence of Transition Metal Additives on the Hydriding/Dehydriding Critical Point of NaAlH4. J. Phys. Chem. C 2009, 113, 9936–9943. [Google Scholar] [CrossRef]
- Cui, J.; Liu, J.; Wang, H.; Ouyang, L.; Sun, D.; Zhu, M.; Yao, X. Mg–TM (TM: Ti, Nb, V, Co, Mo or Ni) core–shell like nanostructures: Synthesis, hydrogen storage performance and catalytic mechanism. J. Mater. Chem. 2014, 2, 9645–9655. [Google Scholar] [CrossRef]
- Korablov, D.; Besenbacher, F.; Jensen, T.R. Kinetics and thermodynamics of hydrogenation-dehydrogenation for Mg-25%TM (TM = Ti, Nb or V) composites synthesized by reactive ball milling in hydrogen. Int. J. Hydrogen Energy 2018, 43, 16804–16814. [Google Scholar] [CrossRef]
- Xie, W.; West, D.J.; Sun, Y.; Zhang, S. Role of nano in catalysis: Palladium catalyzed hydrogen desorption from nanosized magnesium hydride. Nano Energy 2013, 2, 742–748. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, J.; Liu, Z.; Zhu, Y.; Zhang, J.; Li, L. Magnesium Nanoparticles with Pd Decoration for Hydrogen Storage. Front. Chem. 2020, 7, 949. [Google Scholar] [CrossRef] [Green Version]
- Rosi, N.L.; Eckert, J.; Eddaoudi, M.; Vodak, D.T.; Kim, J.; O’Keeffe, M.; Yaghi, O.M. Hydrogen Storage in Microporous Metal-Organic Frameworks. Science 2003, 300, 1127–1129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Lan, Z.; Huang, X.; Liu, H.; Guo, J. Study on catalytic effect and mechanism of MOF (MOF = ZIF-8, ZIF-67, MOF-74) on hydrogen storage properties of magnesium. Int. J. Hydrogen Energy 2019, 44, 28863–28873. [Google Scholar] [CrossRef]
- Ma, Z.; Zou, J.; Hu, C.; Zhu, W.; Khan, D.; Zeng, X.; Ding, W. Effects of trimesic acid-Ni based metal organic framework on the hydrogen sorption performances of MgH2. Int. J. Hydrogen Energy 2019, 44, 29235–29248. [Google Scholar] [CrossRef]
- Ma, Z.; Zou, J.; Khan, D.; Zhu, W.; Hu, C.; Zeng, X.; Ding, W. Preparation and hydrogen storage properties of MgH2-trimesic acid-TM MOF (TM=Co, Fe) composites. J. Mater. Sci. Technol. 2019, 35, 2132–2143. [Google Scholar] [CrossRef]
- Zhang, J.; He, L.; Yao, Y.; Zhou, X.J.; Yu, L.P.; Lu, X.Z.; Zhou, D.W. Catalytic effect and mechanism of NiCu solid solutions on hydrogen storage properties of MgH2. Renew. Energy 2020, 154, 1229–1239. [Google Scholar]
- Chen, M.; Wang, Y.; Xiao, X.; Lu, Y.; Zhang, M.; Zheng, J.; Chen, L. Highly efficient ZrH2 nanocatalyst for the superior hydrogenation kinetics of magnesium hydride under moderate conditions: Investigation and mechanistic insights. Appl. Surf. Sci. 2021, 541, 148375. [Google Scholar] [CrossRef]
- Ismail, M. Effect of adding different percentages of HfCl4 on the hydrogen storage properties of MgH2. Int. J. Hydrogen Energy 2021, 46, 8621–8628. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, K.; Zhang, X.; Hu, J.; Gao, M.; Pan, H.; Liu, Y. Synthesis process and catalytic activity of Nb2O5 hollow spheres for reversible hydrogen storage of MgH2. Int. J. Energy Res. 2021, 45, 3129–3141. [Google Scholar] [CrossRef]
- Gi, H.; Shinzato, K.; Balgis, R.; Ogi, T.; Sadakane, M.; Wang, Y.; Isobe, S.; Miyaoka, H.; Ichikawa, T. Effective Factor on Catalysis of Niobium Oxide for Magnesium. ACS Omega 2020, 5, 21906–21912. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Sun, Z.; Yao, Z.; Yang, L.; Yan, N.; Lu, X.; Xiao, B.; Zhu, X.; Chen, L. Excellent catalysis of Mn3O4 nanoparticles on the hydrogen storage properties of MgH2: An experimental and theoretical study. Nanoscale Adv. 2020, 2, 1666–1675. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Ji, L.; Yan, N.; Sun, Z.; Lu, X.; Zhang, L.; Zhu, X.; Chen, L. Superior catalytic effects of FeCo nanosheets on MgH2 for hydrogen storage. Dalton Trans. 2019, 48, 12699–12706. [Google Scholar] [CrossRef] [PubMed]
- Berezovets, V.V.; Denys, R.V.; Zavaliy, I.Y.; Kosarchyn, Y.V. Effect of Ti-based nanosized additives on the hydrogen storage properties of MgH2. Int. J. Hydrogen Energy 2021, 19. [Google Scholar] [CrossRef]
- Liu, Y.; Du, H.; Zhang, X.; Yang, Y.; Gao, M.; Pan, H. Superior catalytic activity derived from a two-dimensional Ti3C2 precursor towards the hydrogen storage reaction of magnesium hydride. Chem. Commun. 2016, 52, 705–708. [Google Scholar] [CrossRef]
- Ismail, M. Influence of different amounts of FeCl3 on decomposition and hydrogen sorption kinetics of MgH2. Int. J. Hydrogen Energy 2014, 39, 2567–2574. [Google Scholar] [CrossRef]
- Ojeda, X.A.; Castro, F.J.; Pighin, S.A.; Troiani, H.E.; Moreno, M.S.; Urretavizcaya, G. Hydrogen absorption and desorption properties of Mg/MgH2 with nanometric dispersion of small amounts of Nb(V) ethoxide. Int. J. Hydrogen Energy 2021, 46, 4126–4136. [Google Scholar] [CrossRef]
- Zhang, L.; Cai, Z.; Zhu, X.; Yao, Z.; Sun, Z.; Ji, L.; Yan, N.; Xiao, B.; Chen, L. Two-dimensional ZrCo nanosheets as highly effective catalyst for hydrogen storage in MgH2. J. Alloys Compd. 2019, 805, 295–302. [Google Scholar] [CrossRef]
- Song, M.Y.; Kwak, Y.J. Hydrogen charging kinetics of Mg—10wt% Fe2O3 prepared via MgH2-forming mechanical milling. Mater. Res. Bull. 2021, 140, 111304. [Google Scholar] [CrossRef]
- Cai, W.; Wang, H.; Liu, J.; Jiao, L.; Wang, Y.; Ouyang, L.; Sun, T.; Sun, D.; Wang, H.; Yao, X.; et al. Towards easy reversible dehydrogenation of LiBH4 by catalyzing hierarchic nanostructured CoB. Nano Energy 2014, 10, 235–244. [Google Scholar] [CrossRef]
- Rafi-ud-din, Xuanhui, Q.; Ping, L.; Zhang, L.; Qi, W.; Iqbal, M.Z.; Rafique, M.Y.; Farooq, M.H.; Islam-ud-din. Superior Catalytic Effects of Nb2O5, TiO2, and Cr2O3 Nanoparticles in Improving the Hydrogen Sorption Properties of NaAlH4. J. Phys. Chem. C 2012, 116, 11924–11938. [Google Scholar] [CrossRef]
- Khan, J.; Jain, I.P. Catalytic effect of Nb2O5 on dehydrogenation kinetics of NaAlH4. Int. J. Hydrogen Energy 2016, 41, 8264–8270. [Google Scholar] [CrossRef]
- Rafi-ud-din, Xuanhui, Q.; Ping, L.; Zhang, L.; Ahmad, M. Hydrogen Sorption Improvement of LiAlH4 Catalyzed by Nb2O5 and Cr2O3 Nanoparticles. J. Phys. Chem. C 2011, 115, 13088–13099. [Google Scholar] [CrossRef]
- Ismail, M.; Zhao, Y.; Yu, X.B.; Nevirkovets, I.P.; Dou, S.X. Significantly improved dehydrogenation of LiAlH4 catalysed with TiO2 nanopowder. Int. J. Hydrogen Energy 2011, 36, 8327–8334. [Google Scholar] [CrossRef]
- Jain, A.; Agarwal, S.; Kumar, S.; Yamaguchi, S.; Miyaoka, H.; Kojimaa, Y.; Ichikawa, T. How does TiF4 affect the decomposition of MgH2 and its complex variants?—An XPS investigation. J. Mater. Chem. A. 2017, 5, 15543–15551. [Google Scholar] [CrossRef]
- Zhang, L.; Cai, Z.; Yao, Z.; Ji, L.; Sun, Z.; Yan, N.; Zhang, B.; Xiao, B.; Du, J.; Zhu, X.; et al. A striking catalytic effect of facile synthesized ZrMn2 nanoparticles on the de/rehydrogenation properties of MgH2. J. Mater. Chem. A 2019, 7, 5626–5634. [Google Scholar] [CrossRef]
- El-Eskandarany, M.S.; Saeed, M.; Al-Nasrallah, E.; Al-Ajmi, F.; Banyan, M. Effect of LaNi3 Amorphous Alloy Nanopowders on the Performance and Hydrogen Storage Properties of MgH2. Energies 2019, 12, 1005. [Google Scholar] [CrossRef] [Green Version]
- Mahmoudi, N.; Kaflou, A.; Simchi, A. Hydrogen desorption properties of MgH2–TiCr1.2Fe0.6 nanocomposite prepared by high-energy mechanical alloying. J. Power Sources 2011, 196, 4604–4608. [Google Scholar] [CrossRef]
- Zhou, C.; Fang, Z.-Z.; Ren, C.; Li, J.; Lu, J. Effect of Ti Intermetallic Catalysts on Hydrogen Storage Properties of Magnesium Hydride. J. Phys. Chem. C 2013, 117, 12973–12980. [Google Scholar] [CrossRef]
- Wang, K.; Du, H.; Wang, Z.; Gao, M.; Pan, H.; Liu, Y. Novel MAX-phase Ti3AlC2 catalyst for improving the reversible hydrogen storage properties of MgH2. Int. J. Hydrogen Energy 2017, 42, 4244–4251. [Google Scholar] [CrossRef]
- Meena, P.; Jangir, M.; Kumar, A.; Singh, R.; Sharma, V.K.; Jain, I.P. Improved dehydrogenation kinetics of MgH2 due to NiMnAl. Mater. Res. Express 2017, 4, 116520. [Google Scholar] [CrossRef]
- Motavalli, A.; Rajabi, M. Catalytic effect of melt-spun Ni3FeMn alloy on hydrogen desorption properties of nanocrystalline MgH2 synthesized by mechanical alloying. Int. J. Hydrogen Energy 2014, 39, 17047–17053. [Google Scholar] [CrossRef]
- Singh, S.; Bhatnagar, A.; Shukla, V.; Vishwakarma, A.K.; Soni, P.K.; Verma, S.K.; Shaz, M.A.; Sinha, A.S.K.; Srivastavaa, O.N. Ternary transition metal alloy FeCoNi nanoparticles on graphene as new catalyst for hydrogen sorption in MgH2. Int. J. Hydrogen Energy 2020, 45, 774–786. [Google Scholar] [CrossRef]
- Ismail, M.; Mustafa, N.S.; Ali, N.A.; Sazelee, N.A.; Yahya, M.S. The hydrogen storage properties and catalytic mechanism of the CuFe2O4-doped MgH2 composite system. Int. J. Hydrogen Energy 2019, 44, 318–324. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, H.; Liu, J.; Ouyang, L.; Zhu, M. Destabilizing the dehydriding thermodynamics of MgH2 by reversible intermetallics formation in Mg−Ag−Zn ternary alloys. J. Power Sources 2018, 396, 796–802. [Google Scholar] [CrossRef]
- Sazelee, N.A.; Idris, N.H.; Md Din, M.F.; Yahya, M.S.; Ali, N.A.; Ismaila, M. LaFeO3 synthesised by solid-state method for enhanced sorption properties of MgH2. Results Physics. 2020, 16, 102844. [Google Scholar] [CrossRef]
- Agarwal, S.; Jain, A.; Jain, P.; Jangir, M.; Jain, I.P. Kinetic Enhancement in the Sorption Properties by Forming Mg–x wt % ZrCrCu Composites. J. Phys. Chem. C 2013, 117, 11953–11959. [Google Scholar] [CrossRef]
- Agarwal, S.; Aurora, A.; Jain, A.; Montone, A. Structural and H2 sorption properties of MgH2–10 wt%ZrCrM (M = Cu, Ni) nano-composites. J. Nanoparticle Res. 2011, 13, 5719–5726. [Google Scholar] [CrossRef]
- Agarwal, S.; Aurora, A.; Jain, A.; Jain, I.P.; Montone, A. Catalytic effect of ZrCrNi alloy on hydriding properties of MgH2. Int. J. Hydrogen Energy 2009, 34, 9157–9162. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, Z.; Cai, Z.; Yan, N.; Lu, X.; Zhu, X.; Chen, L. Enhanced hydrogen storage properties of MgH2 by the synergetic catalysis of Zr0.4Ti0.6Co nanosheets and carbon nanotubes. Appl. Surf. Sci. 2020, 504, 144465. [Google Scholar] [CrossRef]
- Ali, N.A.; Idrisa, N.H.; Md Din, M.F.; Yahya, M.S.; Ismail, M. Nanoflakes MgNiO2 synthesised via a simple hydrothermal method and its catalytic roles on the hydrogen sorption performance of MgH2. J. Alloys Compd. 2019, 796, 279–286. [Google Scholar] [CrossRef]
- Yahya, M.S.; Ismail, M. Catalytic effect of SrTiO3 on the hydrogen storage behaviour of MgH2. J. Energy Chem. 2019, 28, 46–53. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Li, P.; Wan, Q.; Zhai, F.; Volinskyd, A.A.; Qu, X. Superior destabilization effects of LiBH4 with the addition of nano-sized nickel ferrite NiFe2O4. RSC Adv. 2015, 5, 81212–81219. [Google Scholar] [CrossRef]
- Mo, X.; Jiang, W.; Cao, S. First-principles study on the dehydrogenation characteristics of LiBH4 modified by Ti. Results Phys. 2017, 7, 3236–3242. [Google Scholar] [CrossRef]
- Wan, Q.; Li, P.; Li, Z.; Zhao, K.; Liu, Z.; Wang, L.; Zhai, F.; Qu, X.; Volinsky, A.A. NaAlH4 dehydrogenation properties enhanced by MnFe2O4 nanoparticles. J. Power Sources 2014, 248, 388–395. [Google Scholar] [CrossRef]
- Huang, Y.; Li, P.; Wan, Q.; Zhang, J.; Li, Y.; Li, R.; Dong, R.; Qu, X. Improved dehydrogenation performance of NaAlH4 using NiFe2O4 nanoparticles. J. Alloys Compd. 2017, 709, 850–856. [Google Scholar] [CrossRef]
- Li, Z.; Zhai, F.; Wan, Q.; Liu, Z.; Shan, J.; Li, P.; Volinskyc, A.A.; Qua, X. Enhanced hydrogen storage properties of LiAlH4 catalyzed by CoFe2O4 nanoparticles. RSC Adv. 2014, 4, 18989–18997. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Li, Z.; Zhai, F.; Wan, Q.; Li, X.; Qu, X.; Volinsky, A.A. NiFe2O4 Nanoparticles Catalytic Effects of Improving LiAlH4 Dehydrogenation Properties. J. Phys. Chem. C 2013, 117, 25917–25925. [Google Scholar] [CrossRef] [Green Version]
- Ali, N.A.; Idris, N.H.; Sazelee, N.A.; Yahya, M.S.; Halim Yap, F.A.; Ismaila, M. Catalytic effects of MgFe2O4 addition on the dehydrogenation properties of LiAlH4. Int. J. Hydrogen Energy 2019, 44, 28227–28234. [Google Scholar] [CrossRef]
- Tan, C.-Y.; Tsai, W.-T. Effects of Ni and Co-decorated MWCNTs addition on the dehydrogenation behavior and stability of LiAlH4. Int. J. Hydrogen Energy 2015, 40, 14064–14071. [Google Scholar] [CrossRef]
- Jiao, C.; Sun, L.; Xu, F.; Liu, S.-S.; Zhang, J.; Jiang, X.; Yang, L. NiCo nanoalloy encapsulated in graphene layers for improving hydrogen storage properties of LiAlH4. Sci. Rep. 2016, 6, 27429. [Google Scholar] [CrossRef]
- Xia, Y.; Wei, S.; Huang, Q.; Li, J.; Cen, X.; Zhang, H.; Chu, H.; Sun, L.; Xu, L.; Huang, P. Facile synthesis of NiCo2O4-anchored reduced graphene oxide nanocomposites as efficient additives for improving the dehydrogenation behavior of lithium alanate. Inorg. Chem. Front. 2020, 7, 1257–1272. [Google Scholar] [CrossRef]
- Wei, S.; Xue, S.; Huang, C.; Che, B.; Zhang, H.; Sun, L.; Xu, F.; Xia, Y.; Cheng, R.; Zhang, C.; et al. Multielement synergetic effect of NiFe2O4 and h-BN for improving the dehydrogenation properties of LiAlH4. Inorg. Chem. Front. 2021, 8, 3111–3126. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhu, Y.; Liu, J.; Ma, Z.; Zhang, J.; Liu, Y.; Li, Y.; Li, L. Enhancing hydrogen storage properties of MgH2 by core-shell CoNi@C. J. Alloys Compd. 2021, 862, 158004. [Google Scholar] [CrossRef]
- Meng, Z.; Zhang, X.; Bosang, L.; Meijia, L.; Man, C.; Lixin, C. Superior de/hydrogenation performances of MgH2 catalyzed by 3D flower-like TiO2@C nanostructures. J. Energy Chem. 2020, 46, 191–198. [Google Scholar]
- Liu, J.; Ma, Z.; Liu, Z.; Tang, Q.; Zhu, Y.; Lin, H.; Zhang, Y.; Zhang, J.; Liu, Y.; Li, L. Synergistic effect of rGO supported Ni3Fe on hydrogen storage performance of MgH2. Int. J. Hydrogen Energy 2020, 45, 16622–16633. [Google Scholar] [CrossRef]
- Ding, Z.; Fu, Y.; Wang, Y.; Bi, J.; Zhang, L.; Peng, D.; Li, Y.; Han, S. MgCNi3 prepared by powder metallurgy for improved hydrogen storage properties of MgH2. Int. J. Hydrogen Energy 2019, 44, 8347–8356. [Google Scholar] [CrossRef]
- Ding, Z.; Zhang, L.; Fu, Y.; Wang, W.; Wang, Y.; Bi, J.; Li, Y.; Han, S. Enhanced kinetics of MgH2 via in situ formed catalysts derived from MgCCo1.5Ni1.5. J. Alloys Compd. 2020, 822, 153621. [Google Scholar] [CrossRef]
- Meena, P.; Singh, R.; Sharma, V.K.; Jain, I.P. Role of NiMn9.3Al4.0Co14.1Fe3.6 alloy on dehydrogenation kinetics of MgH2. J. Magnes. Alloy. 2018, 6, 318–325. [Google Scholar] [CrossRef]
- Kumar, P.; Singh, S.; Hashmi, S.A.R.; Kim, K.-H. MXenes: Emerging 2D materials for hydrogen storage. Nano Energy 2021, 85, 105989. [Google Scholar] [CrossRef]
- Li, J.; Wang, S.; Du, Y.; Liao, W. Catalytic effect of Ti2C MXene on the dehydrogenation of MgH2. Int. J. Hydrogen Energy 2019, 44, 6787–6794. [Google Scholar] [CrossRef]
- Zhu, W.; Panda, S.; Lu, C.; Ma, Z.; Khan, D.; Dong, J.; Sun, F.; Xu, H.; Zhang, Q.; Zou, J. Using a Self-Assembled Two-Dimensional MXene-Based Catalyst (2D-Ni@Ti3C2) to Enhance Hydrogen Storage Properties of MgH2. ACS Appl. Mater. Interfaces 2020, 12, 50333–50343. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Lu, C.; Wang, X.; Xu, L.; Huang, X.; Wang, X.; Ning, H.; Lan, Z.; Guo, J. Combinations of V2C and Ti3C2 MXenes for Boosting the Hydrogen Storage Performances of MgH2. ACS Appl. Mater. Interfaces 2021, 13, 13235–13247. [Google Scholar] [CrossRef]
- Fan, Y.; Chen, D.; Liu, X.; Fan, G.; Liu, B. Improving the hydrogen storage performance of lithium borohydride by Ti3C2 MXene. Int. J. Hydrogen Energy 2019, 44, 29297–29303. [Google Scholar] [CrossRef]
- Fan, Y.; Yuan, Z.; Zou, G.; Zhang, Q.; Liu, B.; Peng, Q. Two-dimensional MXene/A-TiO2 composite with unprecedented catalytic activation for sodium alanate. Catal. Today 2018, 318, 167–174. [Google Scholar] [CrossRef]
- Li, Z.; Gao, M.; Gu, J.; Xian, K.; Yao, Z.; Shang, C.; Liu, Y.; Guo, Z.; Pan, H. In Situ Introduction of Li3BO3 and NbH Leads to Superior Cyclic Stability and Kinetics of a LiBH4-Based Hydrogen Storage System. ACS Appl. Mater. Interfaces 2020, 12, 893–903. [Google Scholar] [CrossRef]
- Yuan, Z.; Fan, Y.; Chen, Y.; Liu, X.; Liu, B.; Han, S. Two-dimensional C@TiO2/Ti3C2 composite with superior catalytic performance for NaAlH4. Int. J. Hydrogen Energy 2020, 45, 21666–21675. [Google Scholar] [CrossRef]
- Jiang, R.; Xiao, X.; Zheng, J.; Chen, M.; Chen, L. Remarkable hydrogen absorption/desorption behaviors and mechanism of sodium alanates in-situ doped with Ti-based 2D MXene. Mater. Chem. Phys. 2020, 242, 122529. [Google Scholar] [CrossRef]
- Li, Z.; Yu, J.Z.; Zhang, Y.; Liu, D.M.; Wang, C.Y.; Si, T.Z.; Li, Y.T.; Zhang, Q.A. Coupling of nanoconfinement with metallic catalysis in supported NaAlH4 for low-temperature hydrogen storage. J. Power Sources 2021, 491, 229611. [Google Scholar] [CrossRef]
- Chen, W.; You, L.; Xia, G.; Yu, X. A balance between catalysis and nanoconfinement towards enhanced hydrogen storage performance of NaAlH4. J. Mater. Sci. Technol. 2021, 79, 205–211. [Google Scholar] [CrossRef]






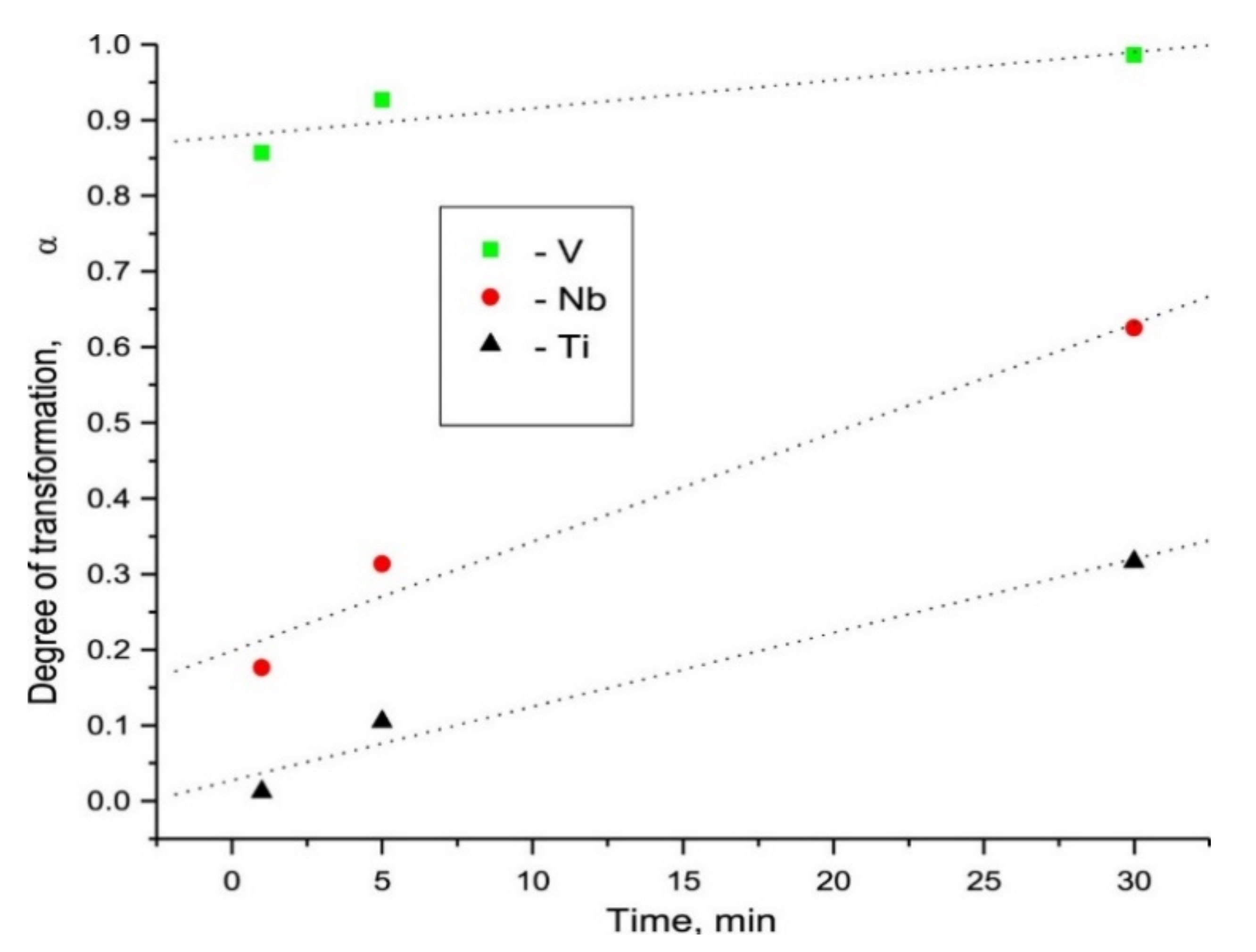
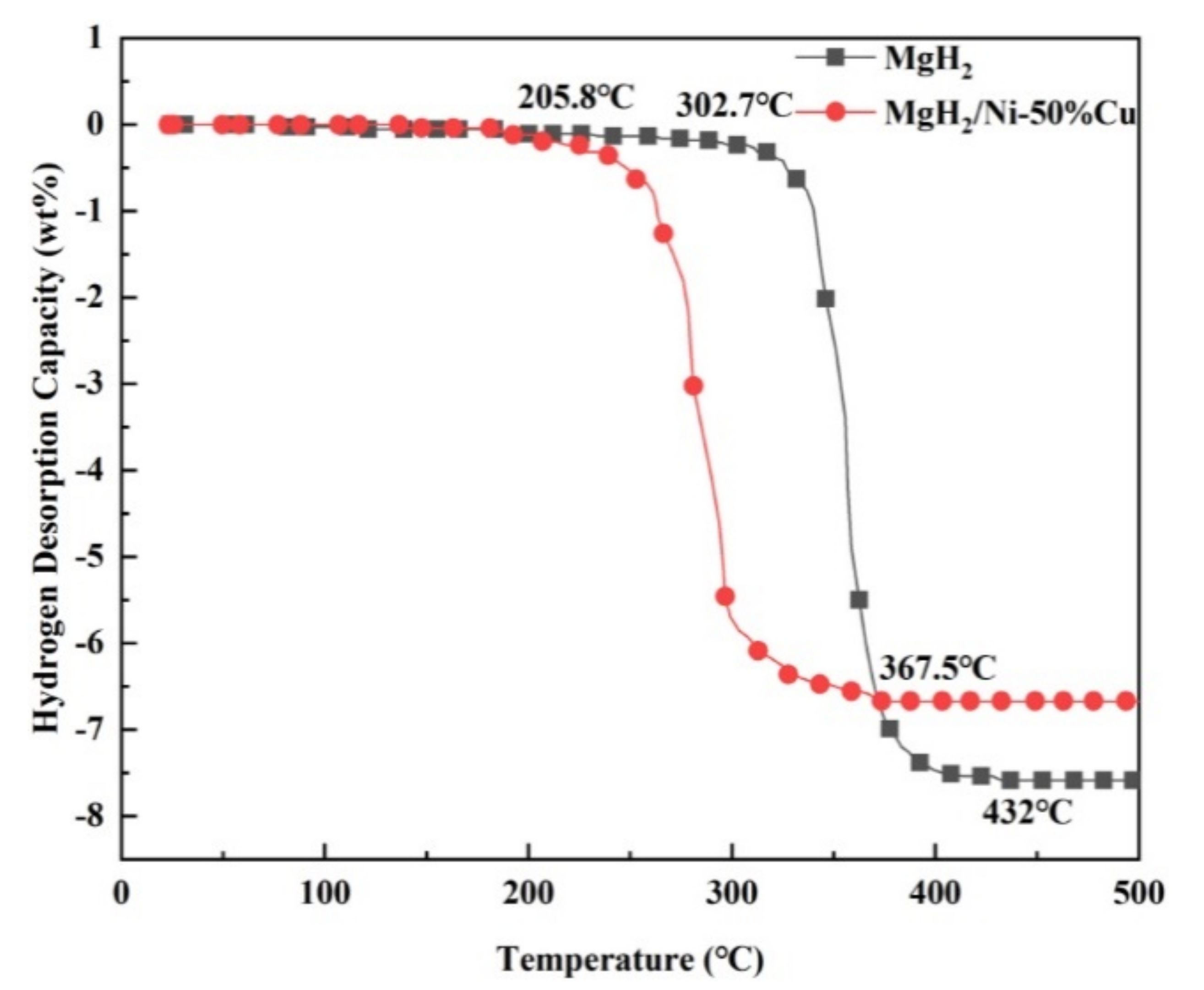
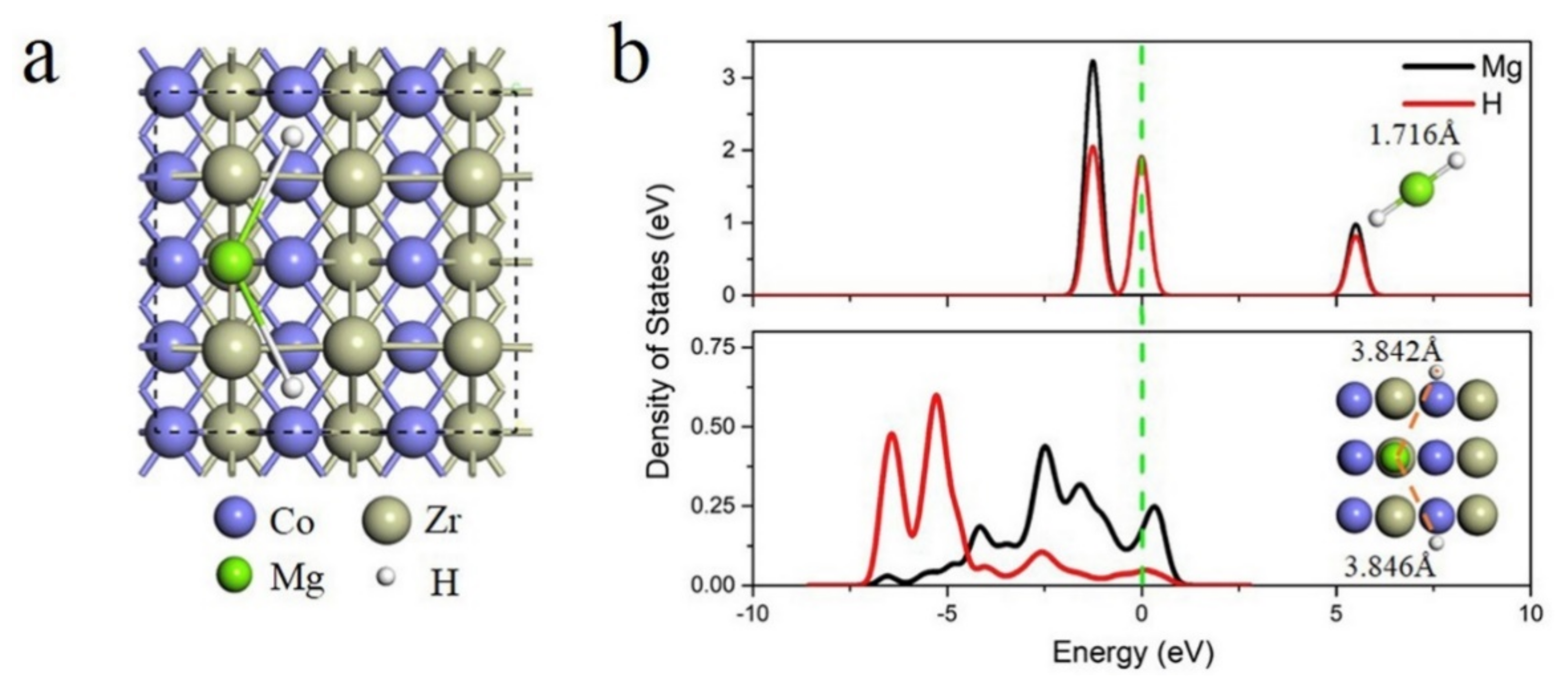
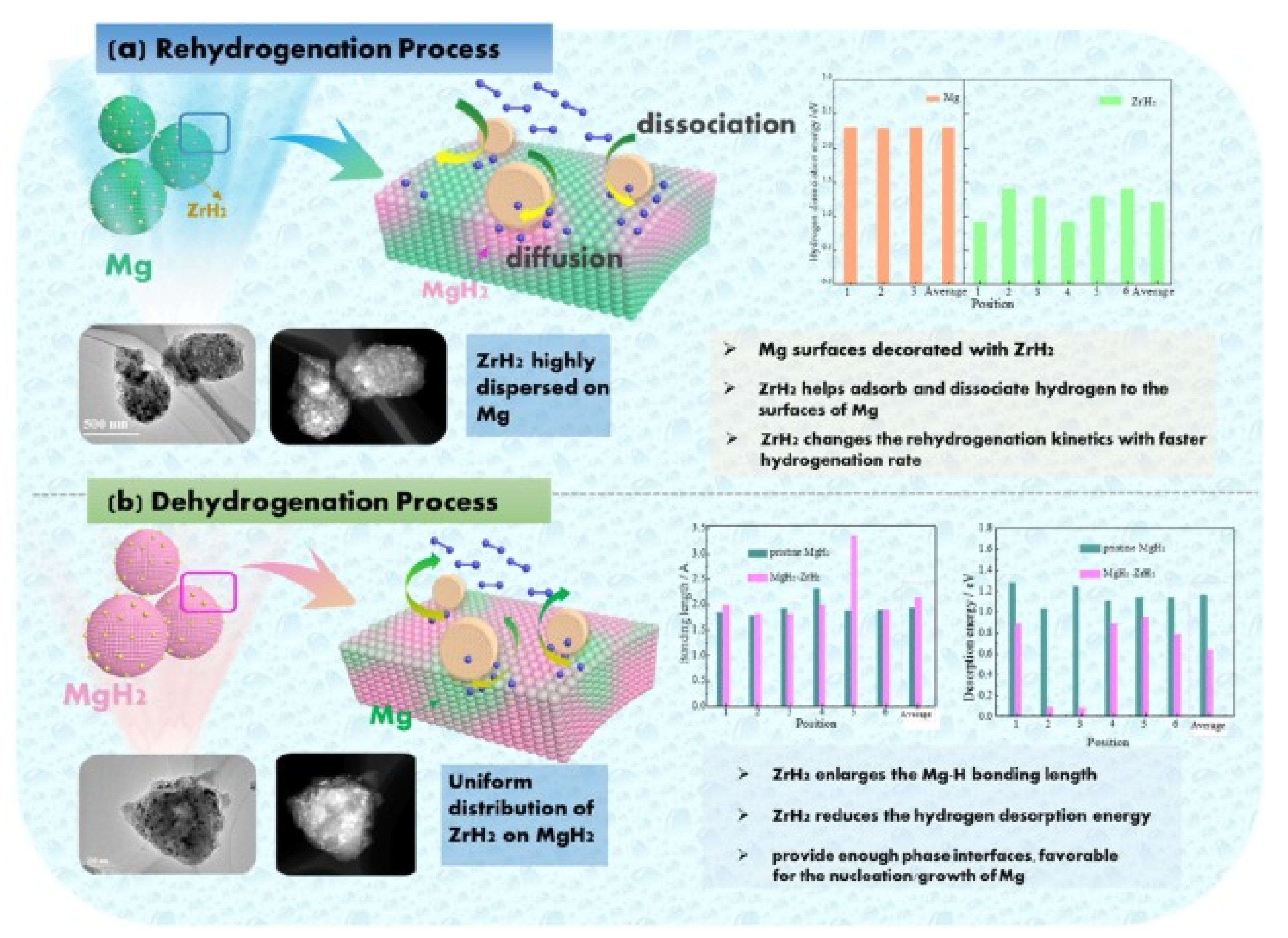



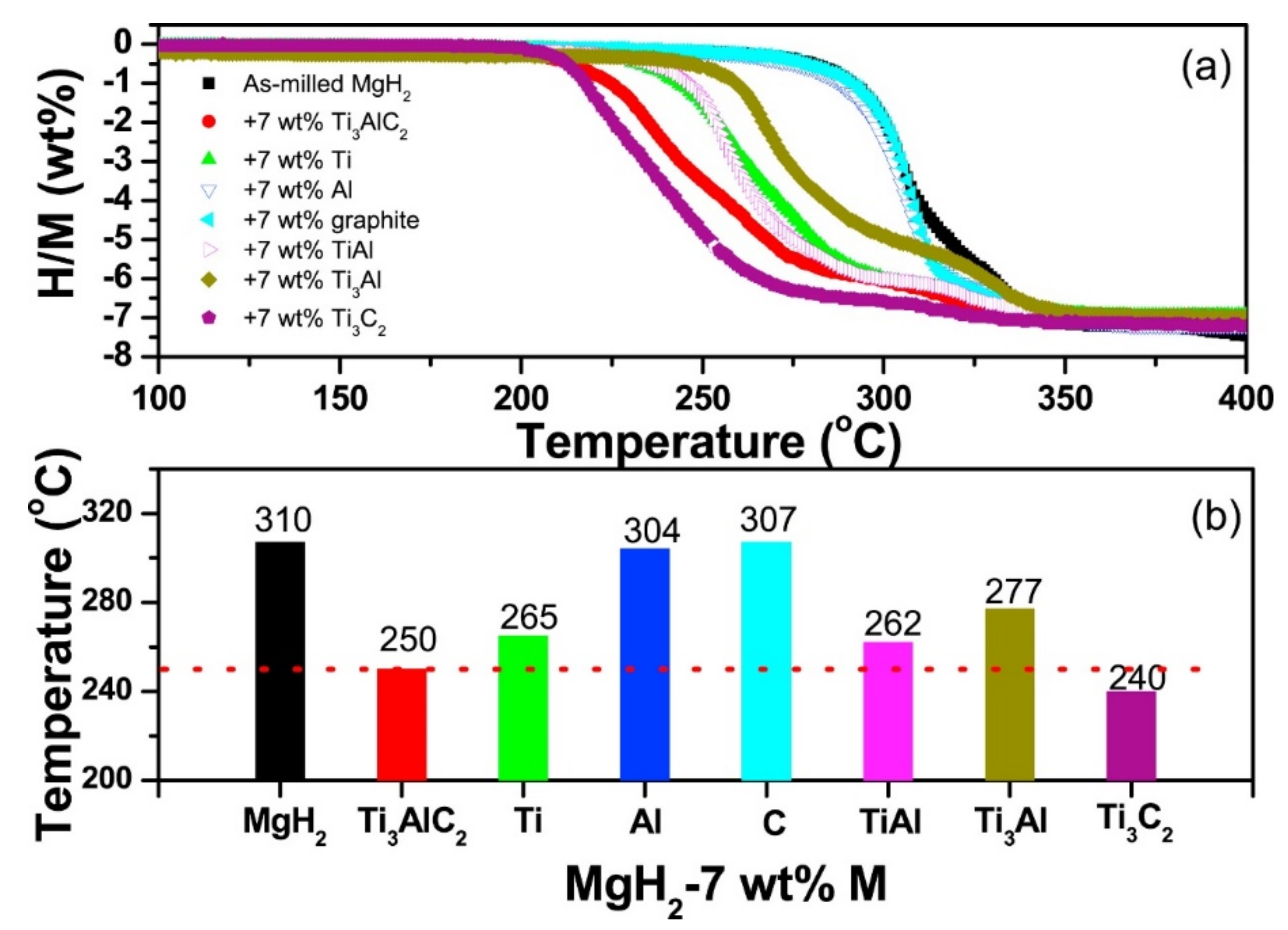
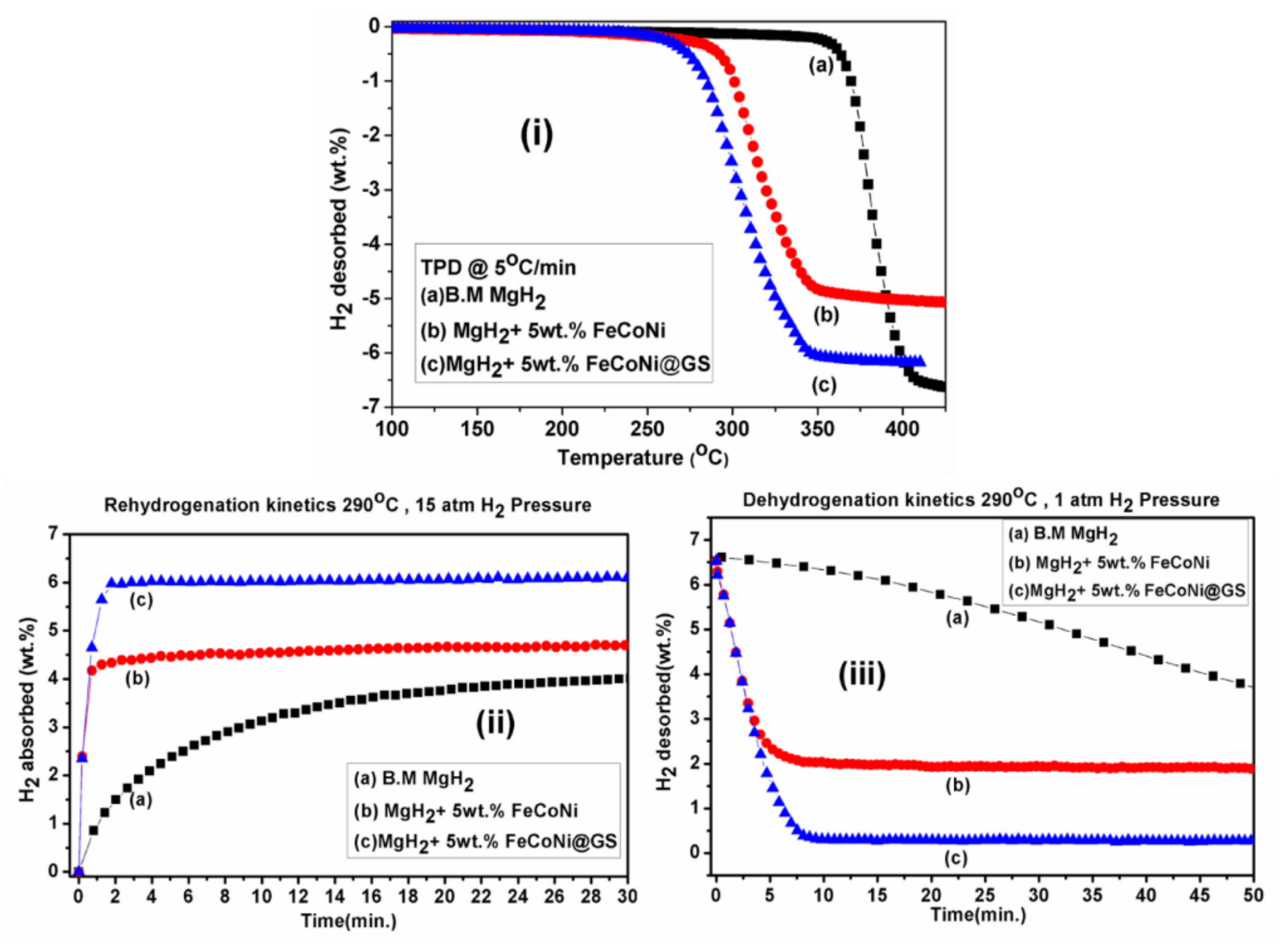


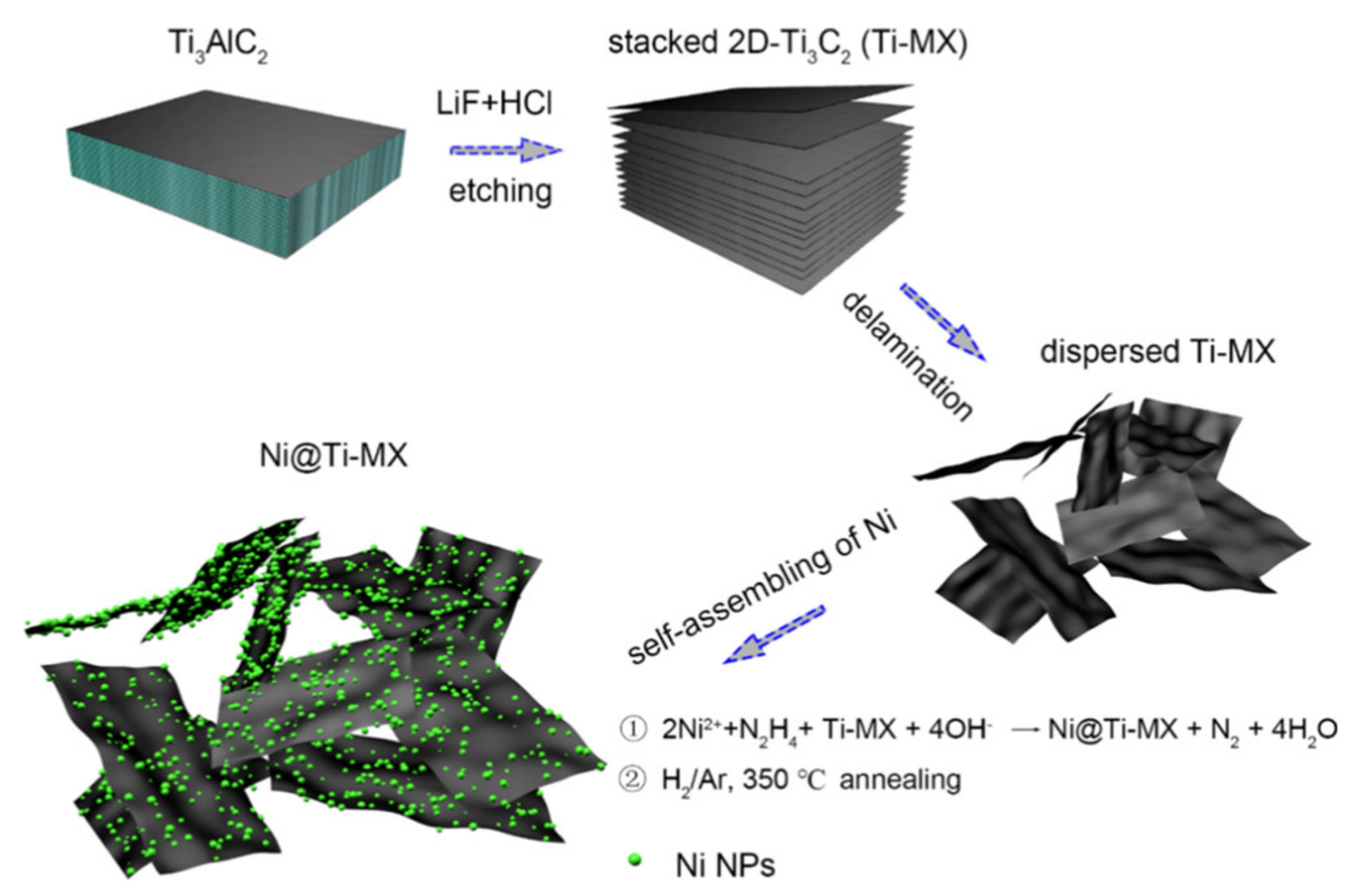

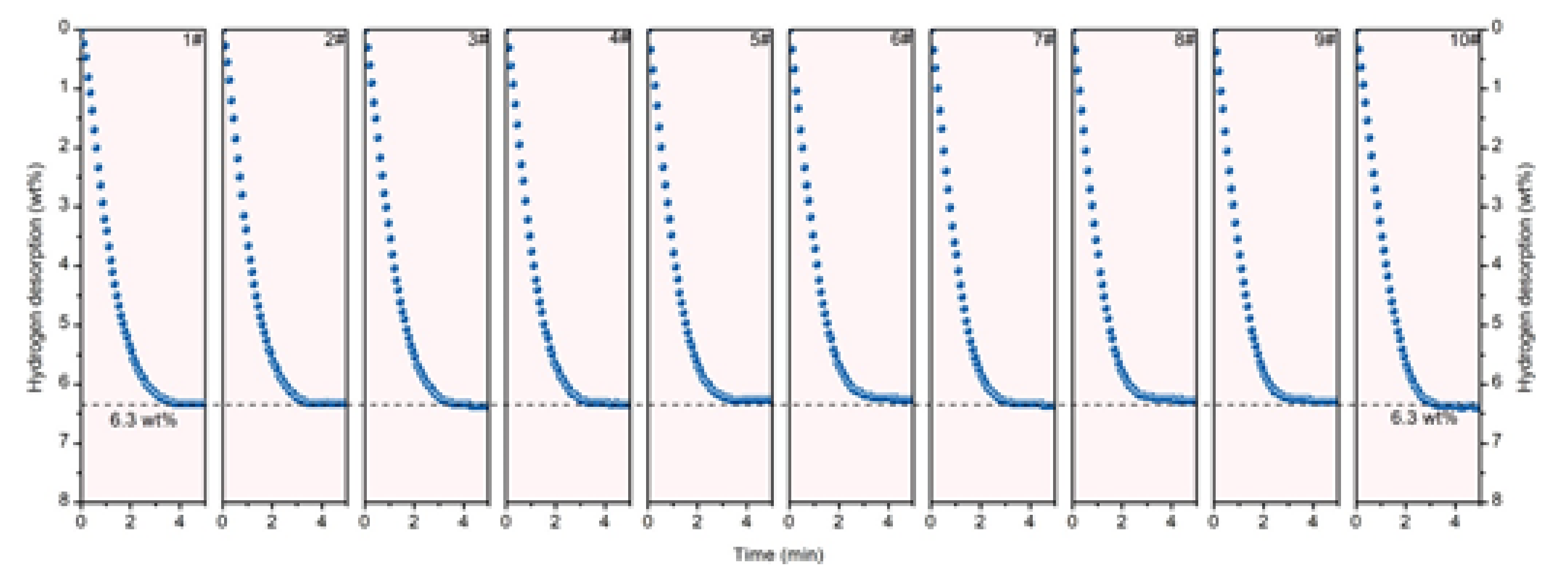
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pal, P.; Ting, J.-M.; Agarwal, S.; Ichikawa, T.; Jain, A. The Catalytic Role of D-block Elements and Their Compounds for Improving Sorption Kinetics of Hydride Materials: A Review. Reactions 2021, 2, 333-364. https://doi.org/10.3390/reactions2030022
Pal P, Ting J-M, Agarwal S, Ichikawa T, Jain A. The Catalytic Role of D-block Elements and Their Compounds for Improving Sorption Kinetics of Hydride Materials: A Review. Reactions. 2021; 2(3):333-364. https://doi.org/10.3390/reactions2030022
Chicago/Turabian StylePal, Pratibha, Jyh-Ming Ting, Shivani Agarwal, Takayuki Ichikawa, and Ankur Jain. 2021. "The Catalytic Role of D-block Elements and Their Compounds for Improving Sorption Kinetics of Hydride Materials: A Review" Reactions 2, no. 3: 333-364. https://doi.org/10.3390/reactions2030022
APA StylePal, P., Ting, J.-M., Agarwal, S., Ichikawa, T., & Jain, A. (2021). The Catalytic Role of D-block Elements and Their Compounds for Improving Sorption Kinetics of Hydride Materials: A Review. Reactions, 2(3), 333-364. https://doi.org/10.3390/reactions2030022







