Two-Dimensional Lamellar Stacked Bi2O3/CeO2 Type-II Heterojunctions Promote Carrier Separation to Enhance Ciprofloxacin Oxidation
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Photocatalysts
2.2. Characterization of Photocatalysts
2.3. Photocatalytic Activity Testing
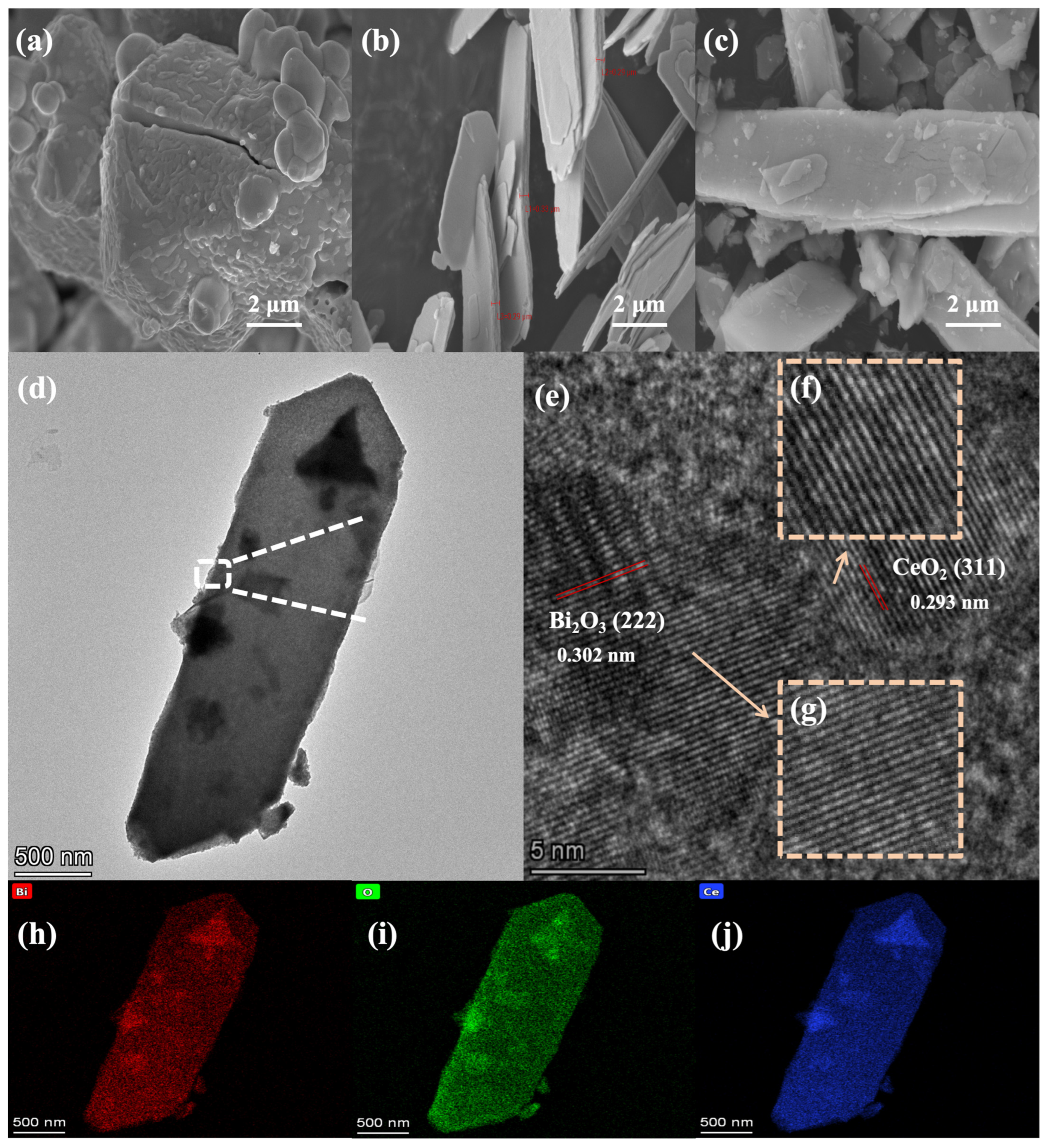
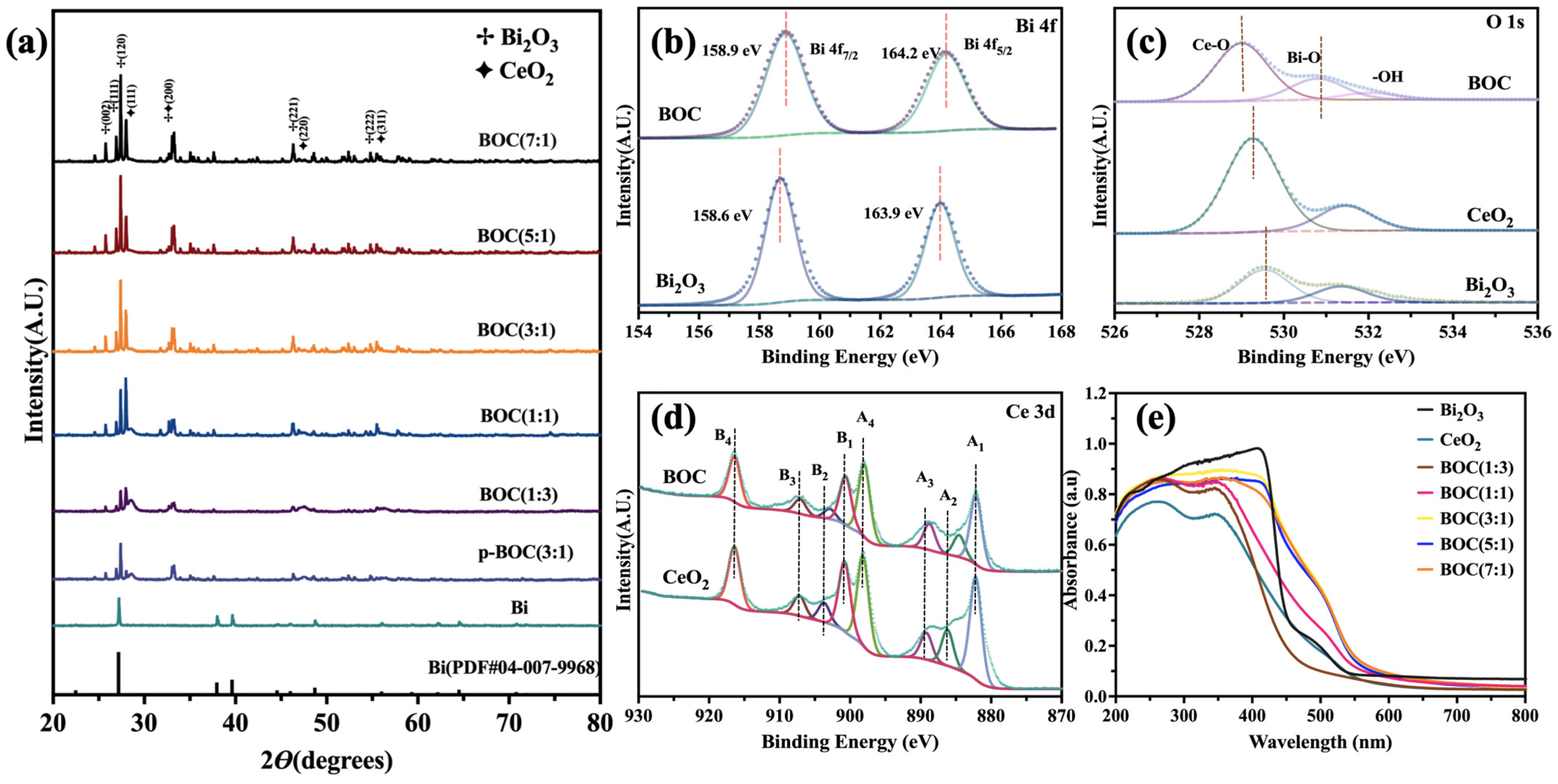
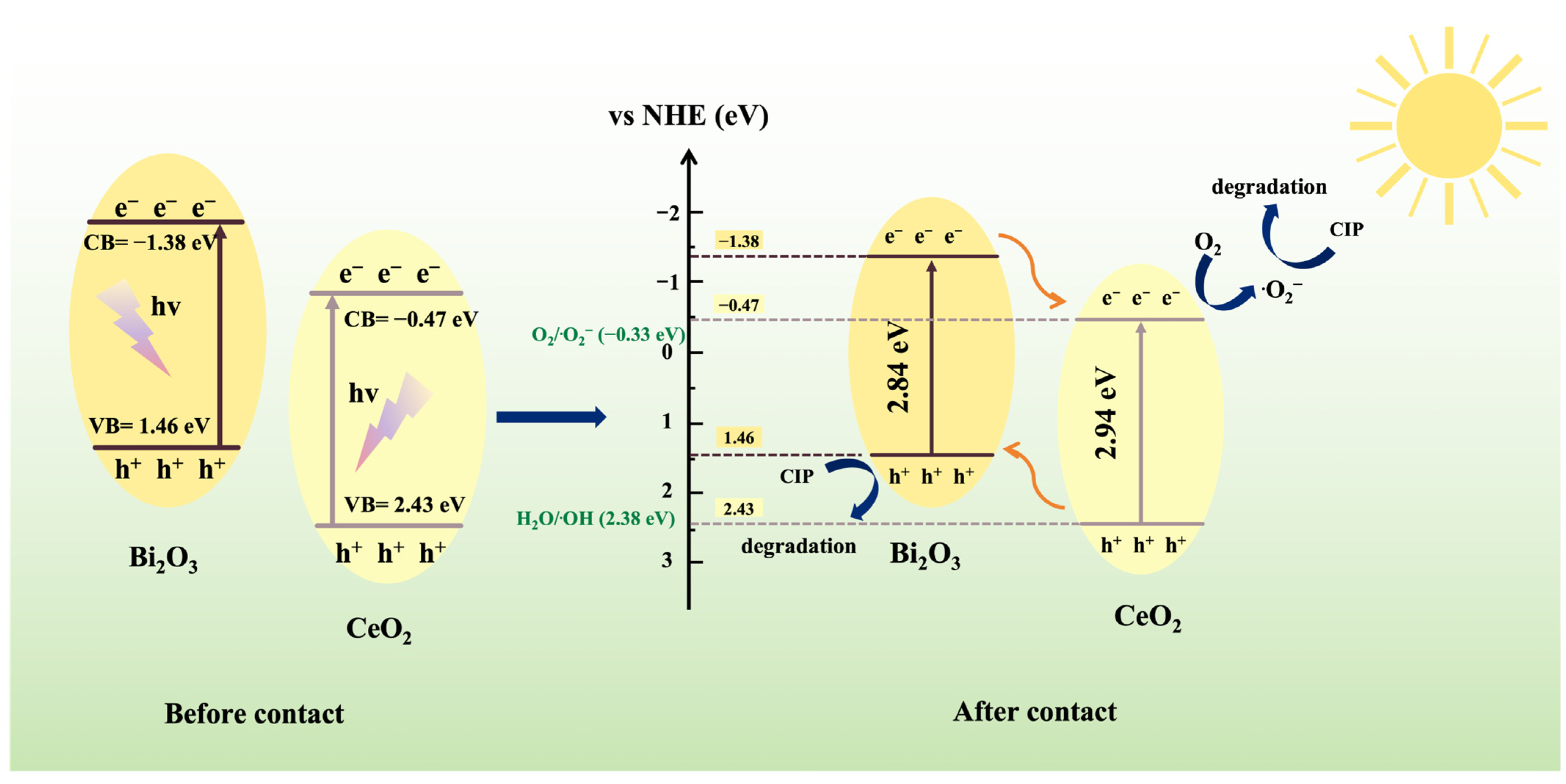
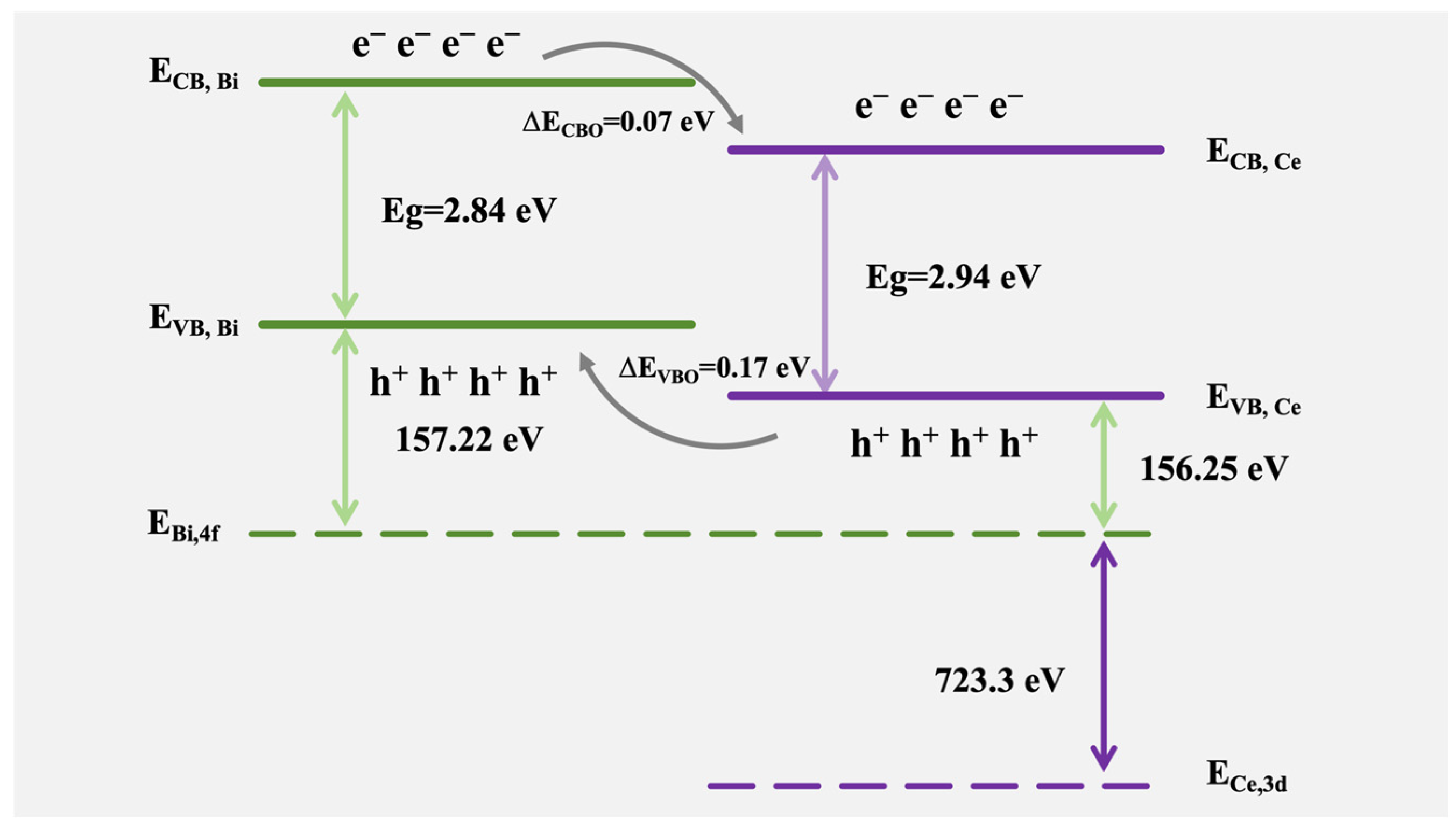
3. Results and Discussion
Characterizations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BOC | Bi2O3/CeO2 |
| MB | Methylene blue |
| CIP | Ciprofloxacin |
| XRD | X-ray diffraction |
| SEM | Scanning electron microscopy |
| EDX | Energy-dispersive X-ray spectroscopy |
| TEM | Transmission electron microscopy |
| UV-Vis DRS | Ultraviolet–visible diffuse reflectance spectroscopy |
| XPS | X-ray photoelectron spectroscopy |
| PL | Photoluminescence |
| ESR | Electron spin resonance |
| TEMPO | C9H18NO2 |
| IPA | Isopropanol |
| AO | Ammonium oxalate |
References
- Deng, F.; Zhao, L.N.; Luo, X.B.; Luo, S.L.; Dionysiou, D.D. Highly Efficient Visible-Light Photocatalytic Performance of Ag/AgIn5S8 for Degradation of Tetracycline Hydrochloride and Treatment of Real Pharmaceutical Industry Wastewater. Chem. Eng. J. 2018, 333, 423–433. [Google Scholar] [CrossRef]
- Wang, G.L.; Bi, W.X.; Zhang, Q.M.; Dong, X.L.; Zhang, X.F. Hydrothermal Carbonation Carbon-Based Photocatalysis under Visible Light: Modification for Enhanced Removal of Organic Pollutant and Novel Insight into the Photocatalytic Mechanism. J. Hazard. Mater. 2022, 426, 127821. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wu, T.; Liang, L.; Tian, Q.W.; Yang, Q.; Zhu, Y.W.; Fang, G.G. Localized Surface-Plasmon Resonance Effect as a New Driver of Z-Scheme Electron Transfer in Cerium Dioxide–Graphitic Carbon Nitride Heterojunctions. Sep. Purif. Technol. 2024, 349, 127818. [Google Scholar] [CrossRef]
- Santana, R.W.R.; Lima, A.E.B.; de Souza, L.K.; Santos, E.C.; Santos, C.C.; de Menezes, A.S.; Sharma, S.K.; Cavalcante, L.S.; da Costa, M.E.H.; Sales, T.O.; et al. BiOBr/ZnWO4 Heterostructures: An Important Key Player for Enhanced Photocatalytic Degradation of Rhodamine B Dye and Antibiotic Ciprofloxacin. J. Phys. Chem. Solids 2023, 173, 111093. [Google Scholar] [CrossRef]
- Wang, H.J.; Li, X.; Zhao, X.X.; Li, C.Y.; Song, X.H.; Zhang, P.; Huo, P.; Li, X. A Review on Heterogeneous Photocatalysis for Environmental Remediation: From Semiconductors to Modification Strategies. Chin. J. Catal. 2022, 43, 178–214. [Google Scholar] [CrossRef]
- Park, H.; Park, Y.; Kim, W.; Choi, W. Surface Modification of TiO2 Photocatalyst for Environmental Applications. J. Photochem. Photobiol. C Photochem. Rev. 2013, 15, 1–20. [Google Scholar] [CrossRef]
- Bai, X.J.; Wang, L.; Wang, Y.J.; Yao, W.Q.; Zhu, Y.F. Enhanced Oxidation Ability of G-C3N4 Photocatalyst via C60 Modification. Appl. Catal. B Environ. 2014, 152–153, 262–270. [Google Scholar] [CrossRef]
- Long, Z.Q.; Li, Q.G.; Wei, T.; Zhang, G.M.; Ren, Z.J. Historical Development and Prospects of Photocatalysts for Pollutant Removal in Water. J. Hazard. Mater. 2020, 395, 122599. [Google Scholar] [CrossRef]
- Wang, F.; Zeng, F.S.; Yu, Z.Y.; Chen, C.Y.; Huang, X.; Zhang, W.; Lan, Y.; Li, J. A Comparative Study about the Influence of Nitrogen Doping and Oxygen Vacancies on the Photocatalytic Performance of Ceria. Surf. Interfaces 2024, 46, 103889. [Google Scholar] [CrossRef]
- Wang, X.X.; Fu, K.; Wen, X.Y.; Qi, S.C.; Tong, G.X.; Wang, X.J.; Wu, W.H. Oxygen Vacancy Boosted Microwave Absorption in CeO2 Hollow Nanospheres. Appl. Surf. Sci. 2022, 598, 153826. [Google Scholar] [CrossRef]
- Li, L.; Li, P.; Wang, Y.J.; Lin, L.; Shah, A.H.; He, T. Modulation of Oxygen Vacancy in Hydrangea-like Ceria via Zr Doping for CO2 Photoreduction. Appl. Surf. Sci. 2018, 452, 498–506. [Google Scholar] [CrossRef]
- Wang, N.; Pan, Y.; Lu, T.; Li, X.Z.; Wu, S.K.; Wu, J.L. A New Ribbon-Ignition Method for Fabricating p-CuO/n-CeO2 Heterojunction with Enhanced Photocatalytic Activity. Appl. Surf. Sci. 2017, 403, 699–706. [Google Scholar] [CrossRef]
- You, D.T.; Pan, B.; Jiang, F.; Zhou, Y.G.; Su, W.Y. CdS Nanoparticles/CeO2 Nanorods Composite with High-Efficiency Visible-Light-Driven Photocatalytic Activity. Appl. Surf. Sci. 2016, 363, 154–160. [Google Scholar] [CrossRef]
- Ji, P.F.; Zhang, J.L.; Chen, F.; Anpo, M. Study of Adsorption and Degradation of Acid Orange 7 on the Surface of CeO2 under Visible Light Irradiation. Appl. Catal. B Environ. 2009, 85, 148–154. [Google Scholar] [CrossRef]
- Li, H.; Wang, G.; Zhang, F.; Cai, Y.; Wang, Y.; Djerdj, I. Surfactant-Assisted Synthesis of CeO2 Nanoparticles and Their Application in Wastewater Treatment. RSC Adv. 2012, 2, 12413–12423. [Google Scholar] [CrossRef]
- Lai, H.; Zeng, X.D.; Song, T.; Yin, S.H.; Long, B.; Ali, A.; Deng, G.-J. Fast Synthesis of Porous Iron Doped CeO2 with Oxygen Vacancy for Effective CO2 Photoreduction. J. Colloid Interface Sci. 2022, 608, 1792–1801. [Google Scholar] [CrossRef]
- Cyran, J.D.; Backus, E.H.G.; van Zadel, M.-J.; Bonn, M. Comparative Adsorption of Acetone on Water and Ice Surfaces. Angew. Chem. Int. Ed. 2019, 58, 3620–3624. [Google Scholar] [CrossRef]
- Huang, J.R.; Wang, M.Y.; Zhang, X.Z.; Tao, J.N.; Lu, L.; Qiao, G.J.; Liu, G.W. Anchoring of 2D CdS on Nb2CTX MXene Nanosheets for Boosting Photocatalytic H2 Evolution. J. Alloys Compd. 2022, 923, 166256. [Google Scholar] [CrossRef]
- Chen, C.F.; Li, M.R.; Jia, Y.S.; Chong, R.F.; Xu, L.P.; Liu, X. Surface Defect-Engineered Silver Silicate/Ceria p-n Heterojunctions with a Flower-like Structure for Boosting Visible Light Photocatalysis with Mechanistic Insight. J. Colloid Interface Sci. 2020, 564, 442–453. [Google Scholar] [CrossRef]
- Wen, X.J.; Niu, C.G.; Zhang, L.; Liang, C.; Zeng, G.M. A Novel Ag2O/CeO2 Heterojunction Photocatalysts for Photocatalytic Degradation of Enrofloxacin: Possible Degradation Pathways, Mineralization Activity and an in-Depth Mechanism Insight. Appl. Catal. B Environ. 2018, 221, 701–714. [Google Scholar] [CrossRef]
- Sun, D.; Chen, Y.J.; Yu, X.Y.; Yin, Y.J.; Tian, G.H. Novel Defect-Transit Dual Z-Scheme Heterojunction: Sulfur-Doped Carbon Nitride Nanotubes Loaded with Bismuth Oxide and Bismuth Sulfide for Efficient Photocatalytic Amine Oxidation. J. Colloid Interface Sci. 2024, 674, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.Y.; Zhang, Y.M.; Wang, Y.L.; Xin, C.L.; Zhang, P.; Liu, D.; Mamba, B.B.; Kefeni, K.K.; Kuvarega, A.T.; Gui, J. Hollow β-Bi2O3@CeO2 Heterostructure Microsphere with Controllable Crystal Phase for Efficient Photocatalysis. Chem. Eng. J. 2020, 387, 124100. [Google Scholar] [CrossRef]
- Xu, Z.; Shi, Y.X.; Li, L.L.; Sun, H.R.; Amin, M.S.; Guo, F.; Wen, H.; Shi, W. Fabrication of 2D/2D Z-Scheme Highly Crystalline Carbon Nitride/δ-Bi2O3 Heterojunction Photocatalyst with Enhanced Photocatalytic Degradation of Tetracycline. J. Alloys Compd. 2022, 895, 162667. [Google Scholar] [CrossRef]
- Bao, Y.P.; Lim, T.T.; Zhong, Z.Y.; Wang, R.; Hu, X. Acetic Acid-Assisted Fabrication of Hierarchical Flower-like Bi2O3 for Photocatalytic Degradation of Sulfamethoxazole and Rhodamine B under Solar Irradiation. J. Colloid Interface Sci. 2017, 505, 489–499. [Google Scholar] [CrossRef]
- Ke, J.; Liu, J.; Sun, H.Q.; Zhang, H.Y.; Duan, X.G.; Liang, P.; Li, X.; Tade, M.O.; Liu, S.; Wang, S. Facile Assembly of Bi2O3/Bi2S3/MoS2 n-p Heterojunction with Layered n-Bi2O3 and p-MoS2 for Enhanced Photocatalytic Water Oxidation and Pollutant Degradation. Appl. Catal. B Environ. 2017, 200, 47–55. [Google Scholar] [CrossRef]
- Liu, L.; Chen, N.; Lei, Y.; Xue, X.Y.; Li, L.; Wang, J.; Komarneni, S.; Zhu, H.; Yang, D. Micro-Nanostructured δ-Bi2O3 with Surface Oxygen Vacancies as Superior Adsorbents for SeOx2− Ions. J. Hazard. Mater. 2018, 360, 279–287. [Google Scholar] [CrossRef]
- Zhou, G.T.; Huang, Y.L.; Wei, D.L.; Bi, S.; Seo, H.J. Preparation and Optical Properties of Te4+/V5+-Stabilized δ-Bi2O3 for Visible Light-Driven Photocatalyst. Mater. Des. 2019, 181, 10806. [Google Scholar] [CrossRef]
- Li, L.Z.; Yan, B. CeO2–Bi2O3 Nanocomposite: Two Step Synthesis, Microstructure and Photocatalytic Activity. J. Non-Cryst. Solids 2009, 355, 776–779. [Google Scholar] [CrossRef]
- Mangalaraja, R.V.; Mouzon, J.; Hedström, P.; Kero, I.; Ramam, K.V.S.; Camurri, C.P.; Odén, M. Combustion Synthesis of Y2O3 and Yb–Y2O3. J. Mater. Process. Technol. 2008, 208, 415–422. [Google Scholar] [CrossRef]
- Wang, D.; Yin, F.X.; Cheng, B.; Xia, Y.; Yu, J.-G.; Ho, W.-K. Enhanced Photocatalytic Activity and Mechanism of CeO2 Hollow Spheres for Tetracycline Degradation. Rare Met. 2021, 40, 2369–2380. [Google Scholar] [CrossRef]
- Masula, K.; Bhongiri, Y.; Raghav Rao, G.; Vijay Kumar, P.; Pola, S.; Basude, M. Evolution of Photocatalytic Activity of CeO2–Bi2O3 Composite Material for Wastewater Degradation under Visible-Light Irradiation. Opt. Mater. 2022, 126, 112201. [Google Scholar] [CrossRef]
- Hsieh, S.-H.; Manivel, A.; Lee, G.-J.; Wu, J.J. Synthesis of Mesoporous Bi2O3/CeO2 Microsphere for Photocatalytic Degradation of Orange II Dye. Mater. Res. Bull. 2013, 48, 4174–4180. [Google Scholar] [CrossRef]
- Zhu, J.Y.; Li, Y.P.; Wang, X.J.; Zhao, J.; Wu, Y.S.; Li, F.-T. Simultaneous Phosphorylation and Bi Modification of BiOBr for Promoting Photocatalytic CO2 Reduction. ACS Sustain. Chem. Eng. 2019, 7, 14953–14961. [Google Scholar] [CrossRef]
- Aswini, R.; Padmanaban, A.; Acchutharaman, K.R.; Sivaraj, D.; Vigneshwaran, S.; Valdes, H.; Arunachalam, S. Influence of Fuel in the Bismuth Oxide Photocatalytic Performance for the Degradation of Acid Blue-25 under Visible Light. Surf. Interfaces 2023, 41, 103243. [Google Scholar] [CrossRef]
- Meng, S.; Ogawa, T.; Okumura, H.; Ishihara, K.N. Enhanced Photocatalytic Activity of BiVO4/Bi2S3/SnS2 Heterojunction under Visible Light. Catalysts 2020, 10, 1294. [Google Scholar] [CrossRef]
- Zheng, Y.H.; Zheng, L.; Zhan, Y.Y.; Lin, X.Y.; Zheng, Q.; Wei, K.M. Ag/ZnO Heterostructure Nanocrystals: Synthesis, Characterization, and Photocatalysis. Inorg. Chem. 2007, 46, 6980–6986. [Google Scholar] [CrossRef]
- Lu, M.J.; Liu, M.; Wei, Y.X.; Xie, H.H.; Fan, W.; Huang, J.; Hu, J.; Wei, P.; Zhang, W.; Xie, Y.; et al. Photocatalytic Activity and Mechanism of Cerium Dioxide with Different Morphologies for Tetracycline Degradation. J. Alloys Compd. 2023, 936, 168273. [Google Scholar] [CrossRef]
- Sunaja Devi, K.R.; Mathew, S.; Rajan, R.; Georgekutty, J.; Pinheiro, D.; Ananthapadmanabhan, U.; Sundararajan, M. Synthesis and Characterization of CeO2/Bi2O3/g-C3N4 Ternary Z-Scheme Nanocomposite. Int. J. Appl. Ceram. Technol. 2020, 17, 2346–2356. [Google Scholar] [CrossRef]
- Nie, J.L.; Zhu, G.Q.; Zhang, W.B.; Gao, J.Z.; Zhong, P.; Xie, X.; Huang, Y.; Hojamberdiev, M. Oxygen Vacancy Defects-Boosted Deep Oxidation of NO by β-Bi2O3/CeO2-δ p-n Heterojunction Photocatalyst in Situ Synthesized from Bi/Ce(CO3)(OH) Precursor. Chem. Eng. J. 2021, 424, 130327. [Google Scholar] [CrossRef]
- Shen, C.H.; Wen, X.J.; Fei, Z.H.; Liu, Z.T.; Mu, Q.M. Visible-Light-Driven Activation of Peroxymonosulfate for Accelerating Ciprofloxacin Degradation Using CeO2/Co3O4 p-n Heterojunction Photocatalysts. Chem. Eng. J. 2020, 391, 123612. [Google Scholar] [CrossRef]
- Sudrajat, H.; Sujaridworakun, P. Correlation between Particle Size of Bi2O3 Nanoparticles and Their Photocatalytic Activity for Degradation and Mineralization of Atrazine. J. Mol. Liq. 2017, 242, 433–440. [Google Scholar] [CrossRef]
- Ma, J.; Fan, L.; Wang, X.; Li, L.; Zhang, Y.; Zhao, G.; Chai, B.; Gao, J. Defect Engineering on Metal-Organic Frameworks for Enhanced Photocatalytic Reduction of Cr(VI). Surf. Interfaces 2024, 54, 105174. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, S.; Cui, Z.; Gao, F.; Tai, Y.; Liu, Q. Enhanced Photocatalytic Ozonation of Bisphenol a Using Ce Doped Bi-MOF Derived Oxygen-Rich Vacancies Bi2O3/CeO2. Sep. Purif. Technol. 2025, 364, 132350. [Google Scholar] [CrossRef]
- Shang, Z.C.; Wang, T.; Ren, A.; Yu, Y.; Zheng, Y.; Tao, Y.; Feng, P.; Xiao, Y.; Wang, X. Hollow Macroporous CeO2/β-Bi2O3 Heterostructure Sphere via One-Step Spray Solution Combustion Synthesis for Efficient Photocatalysis. Appl. Surf. Sci. 2023, 619, 156718. [Google Scholar] [CrossRef]
- Hezam, A.; Namratha, K.; Drmosh, Q.A.; Yamani, Z.H.; Byrappa, K. Synthesis of Heterostructured Bi2O3–CeO2–ZnO Photocatalyst with Enhanced Sunlight Photocatalytic Activity. Ceram. Int. 2017, 43, 5292–5301. [Google Scholar] [CrossRef]
- Jiang, L.S.; Wang, K.; Wu, X.Y.; Zhang, G.K.; Yin, S. Amorphous Bimetallic Cobalt Nickel Sulfide Cocatalysts for Significantly Boosting Photocatalytic Hydrogen Evolution Performance of Graphitic Carbon Nitride: Efficient Interfacial Charge Transfer. ACS Appl. Mater. Interfaces 2019, 11, 26898–26908. [Google Scholar] [CrossRef]
- Fang, X.; Chen, J.; Zhan, J. Heterojunction Photocatalyst for Organic Degradation: Superior Photocatalytic Activity through the Phase and Interface Engineering. Ceram. Int. 2020, 46, 23245–23256. [Google Scholar] [CrossRef]
- Li, X.X.; Feng, X.Y.; Meng, D.L.; Hu, X.J.; Li, L.; Zhang, Y.M.; Wang, X.S. Fabrication of TiO2/MOF Type II Heterojunction by Growth of TiO2 on Cr-Based MOF for Enhanced Photocatalytic Hydrogen Production. Cryst. Growth Des. 2025, 25, 1182–1189. [Google Scholar] [CrossRef]
- Xu, Q.L.; Zhang, L.Y.; Cheng, B.; Fan, J.J.; Yu, J.G. S-Scheme Heterojunction Photocatalyst. Chem 2020, 6, 1543–1559. [Google Scholar] [CrossRef]
- Bai, S.; Zhang, N.; Gao, C.; Xiong, Y. Defect Engineering in Photocatalytic Materials. Nano Energy 2018, 53, 296–336. [Google Scholar] [CrossRef]
- Jin, X.; Lv, C.; Zhou, X.; Zhang, C.; Meng, Q.; Liu, Y.; Chen, G. Molecular Adsorption Promotes Carrier Migration: Key Step for Molecular Oxygen Activation of Defective Bi4O5I2. Appl. Catal. B Environ. 2018, 226, 53–60. [Google Scholar] [CrossRef]
- Hou, J.; Cao, S.; Wu, Y.; Liang, F.; Sun, Y.; Lin, Z.; Sun, L. Simultaneously Efficient Light Absorption and Charge Transport of Phosphate and Oxygen-Vacancy Confined in Bismuth Tungstate Atomic Layers Triggering Robust Solar CO2 Reduction. Nano Energy 2017, 32, 359–366. [Google Scholar] [CrossRef]
- Uddin, M.T.; Nicolas, Y.; Olivier, C.; Toupance, T.; Servant, L.; Müller, M.M.; Kleebe, H.J.; Ziegler, J.; Jaegermann, W. Nanostructured SnO2–ZnO Heterojunction Photocatalysts Showing Enhanced Photocatalytic Activity for the Degradation of Organic Dyes. Inorg. Chem. 2012, 51, 7764–7773. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Zhao, X.; Zhang, K.; Wu, B.; Yang, X.; Zou, H.; Zhang, L.; Shao, H.; Ma, T.; Zhou, H.; et al. Two-Dimensional Lamellar Stacked Bi2O3/CeO2 Type-II Heterojunctions Promote Carrier Separation to Enhance Ciprofloxacin Oxidation. Reactions 2025, 6, 29. https://doi.org/10.3390/reactions6020029
Chen L, Zhao X, Zhang K, Wu B, Yang X, Zou H, Zhang L, Shao H, Ma T, Zhou H, et al. Two-Dimensional Lamellar Stacked Bi2O3/CeO2 Type-II Heterojunctions Promote Carrier Separation to Enhance Ciprofloxacin Oxidation. Reactions. 2025; 6(2):29. https://doi.org/10.3390/reactions6020029
Chicago/Turabian StyleChen, Lihong, Xiufei Zhao, Kuo Zhang, Biyu Wu, Xiao Yang, Haonan Zou, Lei Zhang, Huahao Shao, Tianyi Ma, Hu Zhou, and et al. 2025. "Two-Dimensional Lamellar Stacked Bi2O3/CeO2 Type-II Heterojunctions Promote Carrier Separation to Enhance Ciprofloxacin Oxidation" Reactions 6, no. 2: 29. https://doi.org/10.3390/reactions6020029
APA StyleChen, L., Zhao, X., Zhang, K., Wu, B., Yang, X., Zou, H., Zhang, L., Shao, H., Ma, T., Zhou, H., & Zhang, Y. (2025). Two-Dimensional Lamellar Stacked Bi2O3/CeO2 Type-II Heterojunctions Promote Carrier Separation to Enhance Ciprofloxacin Oxidation. Reactions, 6(2), 29. https://doi.org/10.3390/reactions6020029






