Highly Efficient Visible-Light-Driven Photocatalysis of Rose Bengal Dye and Hydrogen Production Using Ag@Cu/TiO2 Ternary Nanocomposites
Abstract
1. Introduction
2. Experimental Section
2.1. Materials and Methods
2.2. Synthesis of Ag@Cu/TiO2
2.3. Photocatalytic Degradation of RB and Hydrogen Production
3. Result and Discussion
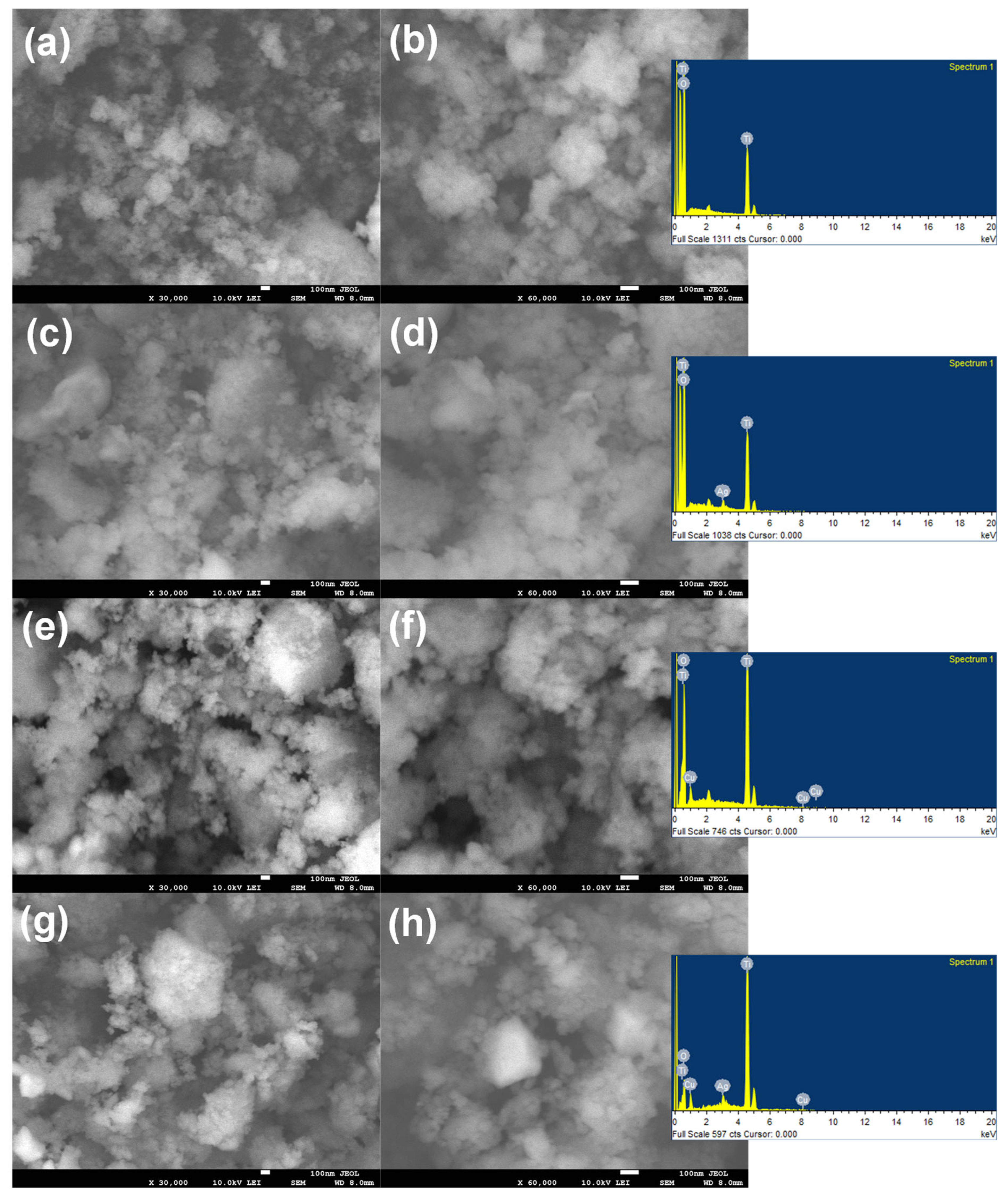
3.1. Surface Morphological Analysis
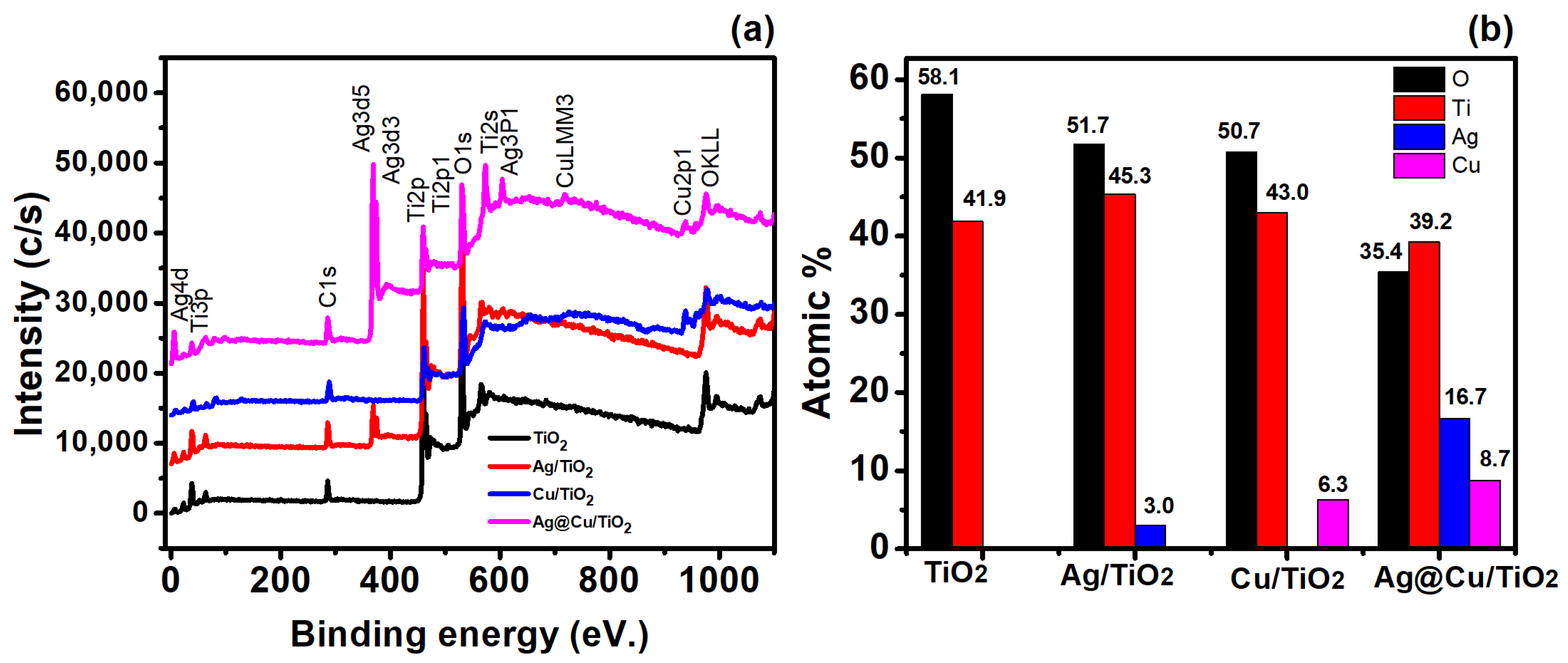
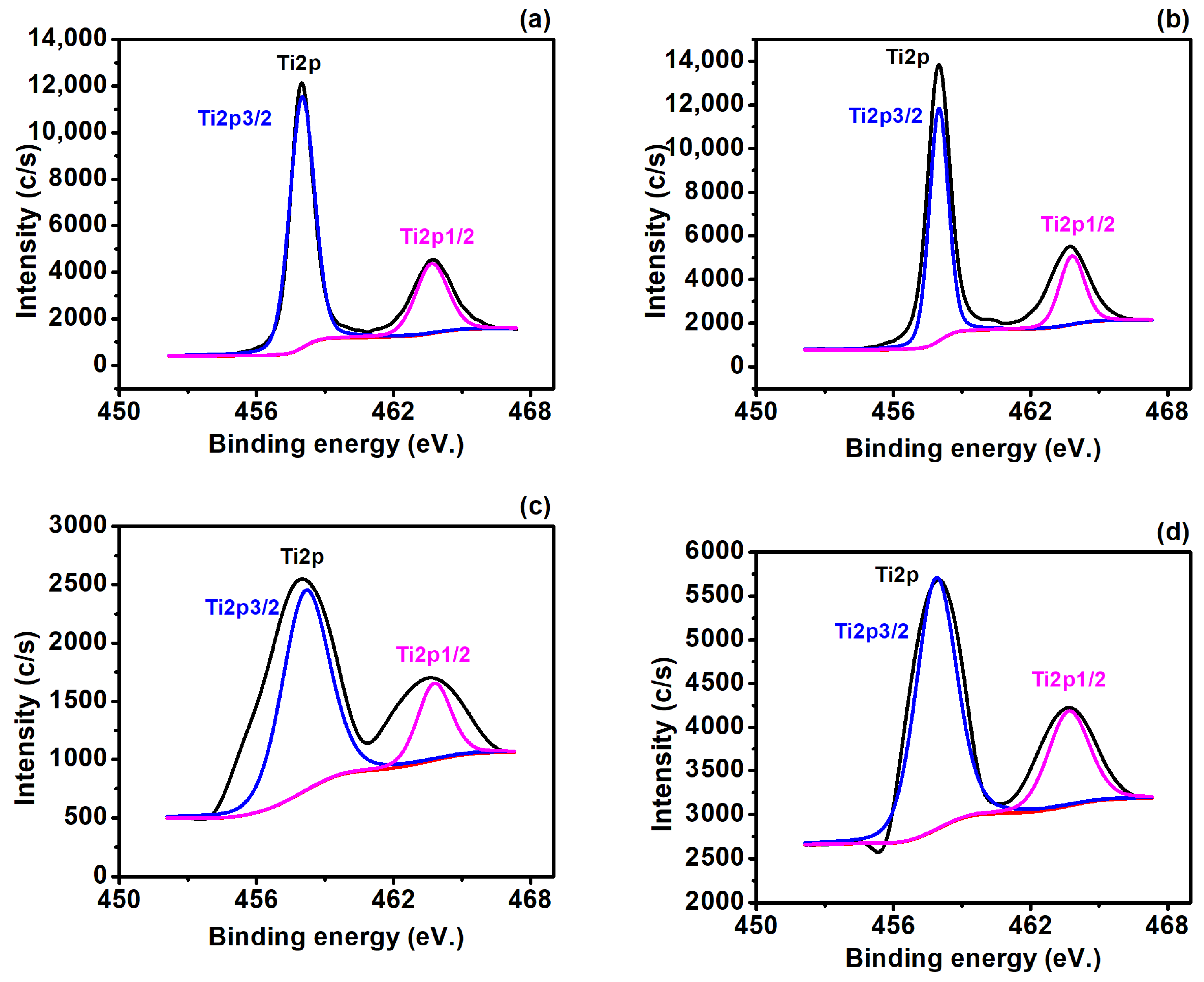
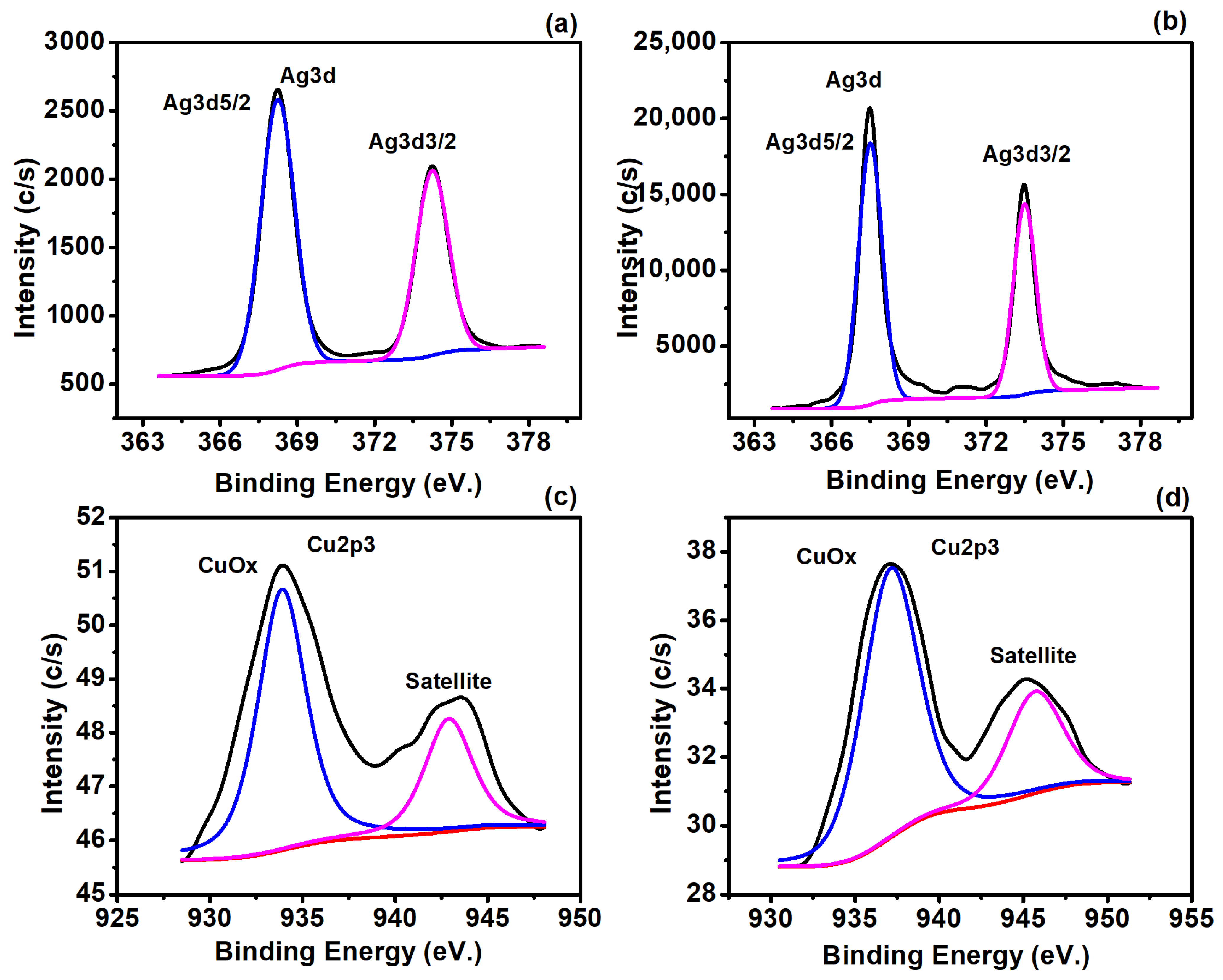
3.2. Surface Compositional and Chemical Analysis
3.3. Charge Carrier Movement
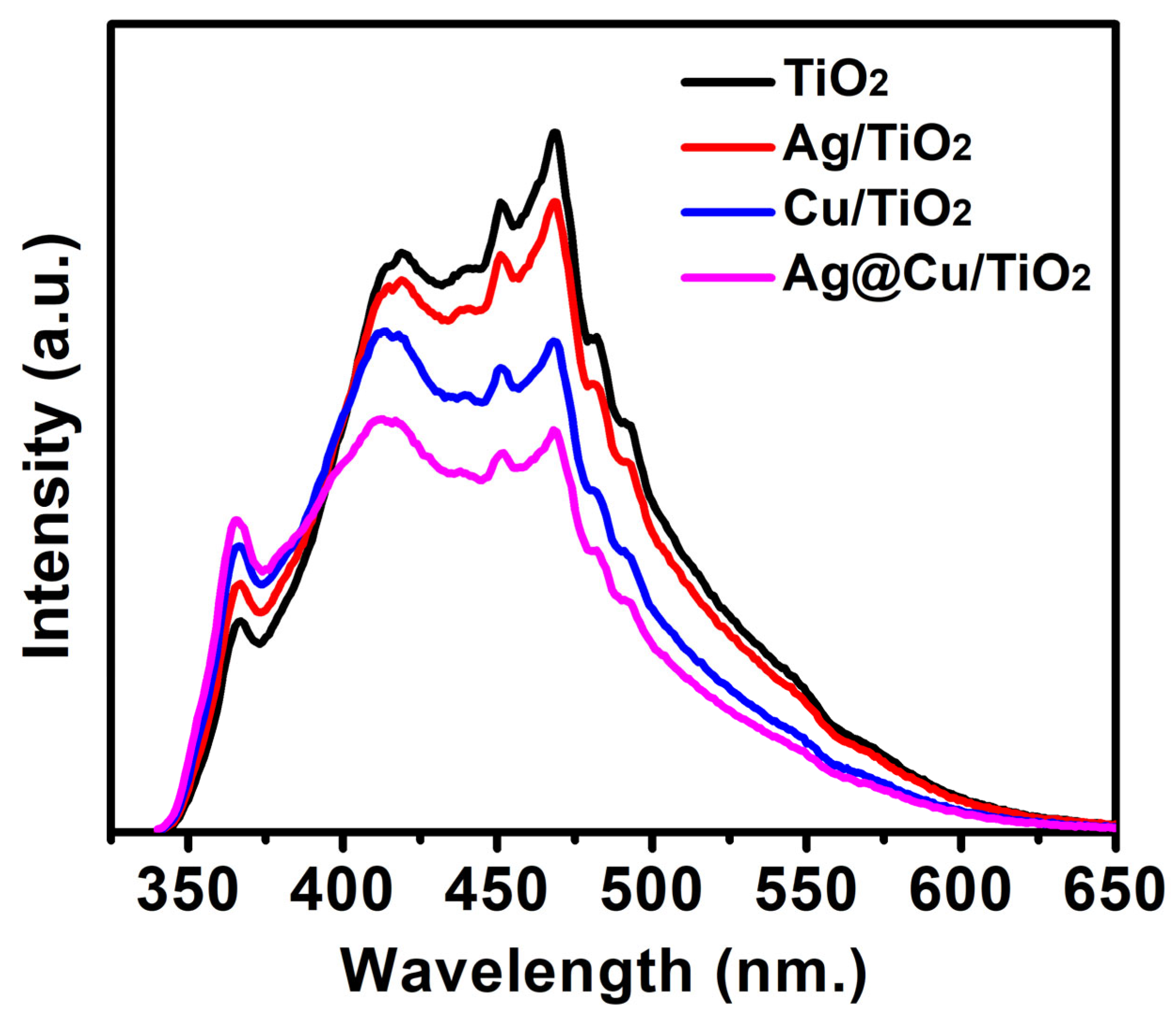
3.4. Charge Recombination Ratio
3.5. Photocatalytic Degradation of RB
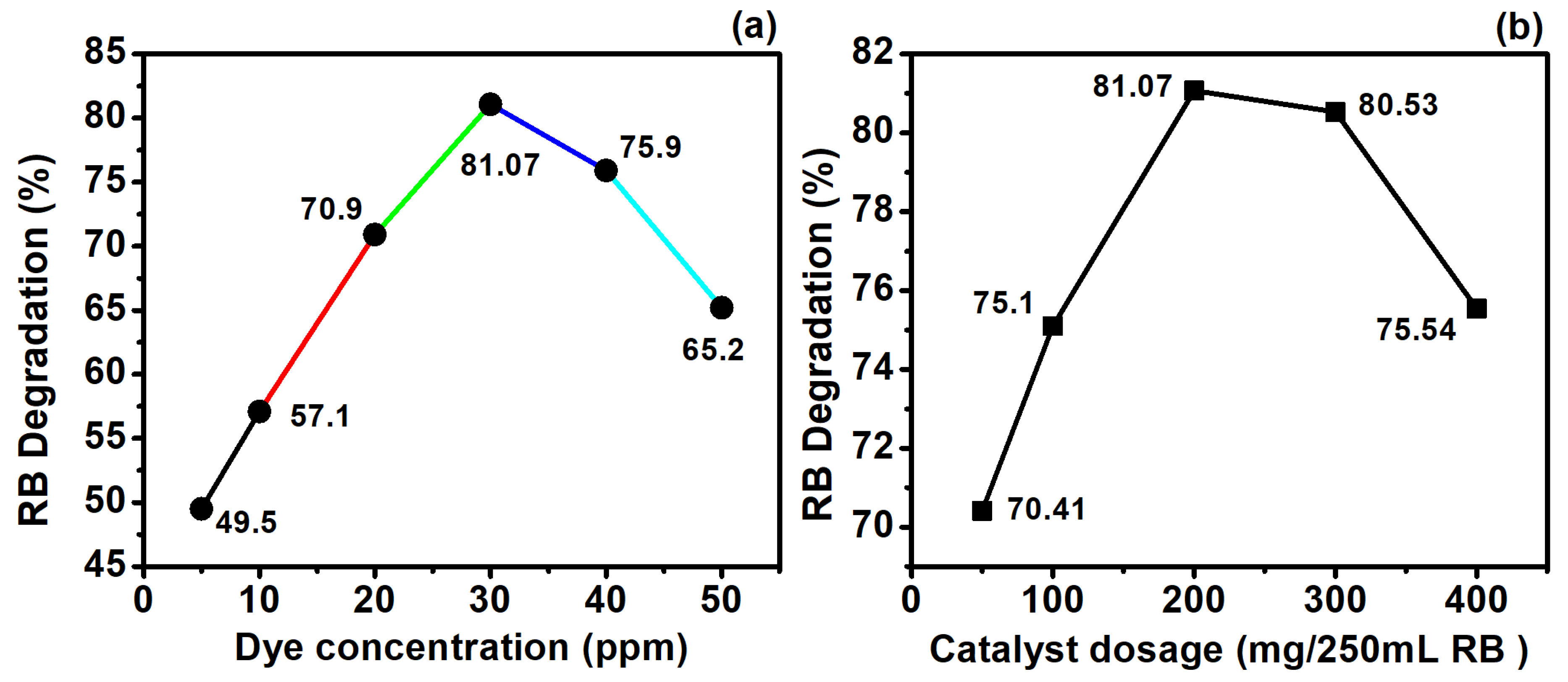
3.5.1. Effect of RB Concentration
3.5.2. Effect of Ag@Cu/TiO2 Dosage
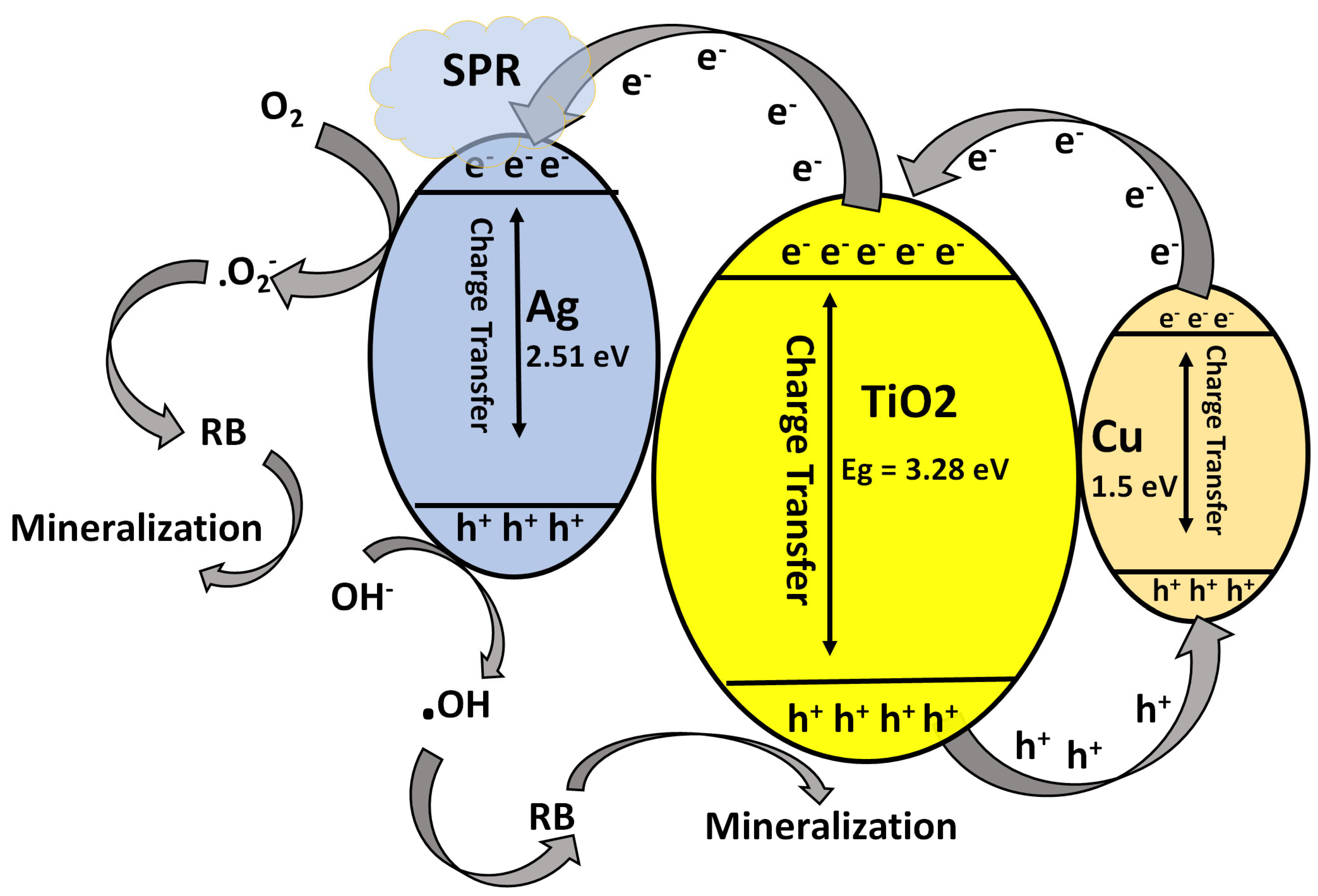
3.5.3. Proposed Photocatalytic Mechanism
3.6. Hydrogen Production
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feng, A.; Feng, J.; Xing, W.; Jiang, K.; Tang, W. Versatile Applications of Electrochemical Flow-through Systems in Water Treatment Processes. Chem. Eng. J. 2023, 473, 145400. [Google Scholar] [CrossRef]
- Huang, L.; Huang, X.; Yan, J.; Liu, Y.; Jiang, H.; Zhang, H.; Tang, J.; Liu, Q. Research Progresses on the Application of Perovskite in Adsorption and Photocatalytic Removal of Water Pollutants. J. Hazard. Mater. 2023, 442, 130024. [Google Scholar] [CrossRef] [PubMed]
- Noor, R.; Maqsood, A.; Baig, A.; Pande, C.B.; Zahra, S.M.; Saad, A.; Anwar, M.; Singh, S.K. A Comprehensive Review on Water Pollution, South Asia Region: Pakistan. Urban Clim. 2023, 48, 101413. [Google Scholar] [CrossRef]
- Huang, S.-T.; Lee, W.W.; Chang, J.-L.; Huang, W.-S.; Chou, S.-Y.; Chen, C.-C. Hydrothermal Synthesis of SrTiO3 Nanocubes: Characterization, Photocatalytic Activities, and Degradation Pathway. J. Taiwan Inst. Chem. Eng. 2014, 45, 1927–1936. [Google Scholar] [CrossRef]
- Solayman, H.M.; Hossen, M.A.; Abd Aziz, A.; Yahya, N.Y.; Hon, L.K.; Ching, S.L.; Monir, M.U.; Zoh, K.-D. Performance Evaluation of Dye Wastewater Treatment Technologies: A Review. J. Environ. Chem. Eng. 2023, 11, 109610. [Google Scholar] [CrossRef]
- Beheshti, A.; Hashemi, F.; Behvandi, F.; Mayer, P.; Atzei, D. Selective High Adsorption Capacity for Congo Red Dye of a New 3D Supramolecular Complex and Its Magnetic Hybrid. Inorg. Chem. Front. 2018, 5, 694–704. [Google Scholar] [CrossRef]
- Amiri, S.; Vatanpour, V.; He, T. Optimization of Coagulation-Flocculation Process in Efficient Arsenic Removal from Highly Contaminated Groundwater by Response Surface Methodology. Molecules 2022, 27, 7953. [Google Scholar] [CrossRef] [PubMed]
- Gan, Y.; Ding, C.; Xu, B.; Liu, Z.; Zhang, S.; Cui, Y.; Wu, B.; Huang, W.; Song, X. Antimony (Sb) Pollution Control by Coagulation and Membrane Filtration in Water/Wastewater Treatment: A Comprehensive Review. J. Hazard. Mater. 2023, 442, 130072. [Google Scholar] [CrossRef] [PubMed]
- Saeed, U.; Jilani, A.; Iqbal, J.; Al-Turaif, H. Reduced Graphene Oxide-Assisted Graphitic Carbon Nitride@ ZnO Rods for Enhanced Physical and Photocatalytic Degradation. Inorg. Chem. Commun. 2022, 142, 109623. [Google Scholar] [CrossRef]
- Blázquez-Moraleja, A.; Moya, P.; Marin, M.L.; Bosca, F. Synthesis of Novel Heterogeneous Photocatalysts Based on Rose Bengal for Effective Wastewater Disinfection and Decontamination. Catal. Today 2023, 413, 113948. [Google Scholar] [CrossRef]
- Flores, J.; Moya, P.; Bosca, F.; Marin, M.L. Photoreactivity of New Rose Bengal-SiO2 Heterogeneous Photocatalysts with and without a Magnetite Core for Drug Degradation and Disinfection. Catal. Today 2023, 413, 113994. [Google Scholar] [CrossRef]
- Shivaji, K.; Sridharan, K.; Kirubakaran, D.D.; Velusamy, J.; Emadian, S.S.; Krishnamurthy, S.; Devadoss, A.; Nagarajan, S.; Das, S.; Pitchaimuthu, S. Biofunctionalized CdS Quantum Dots: A Case Study on Nanomaterial Toxicity in the Photocatalytic Wastewater Treatment Process. ACS Omega 2023, 8, 19413–19424. [Google Scholar] [CrossRef] [PubMed]
- Lei, B.; Robertson, N. TiO2 Mesocrystals: Immobilisation, Surface Fluorination and Application in Photocatalytic Water Treatment. Appl. Surf. Sci. 2023, 616, 156487. [Google Scholar] [CrossRef]
- Arabkhani, P.; Saeedi, N.; Sadeghi, H.; Nouripour-Sisakht, S.; Gharaghani, M.; Asfaram, A. Plant Extracts-Mediated Green Synthesis of Zinc Oxide/Carbon Nanofiber Nanocomposites with Highly Efficient Photocatalytic and Antimicrobial Properties for Wastewater Treatment. J. Water Process Eng. 2023, 54, 104020. [Google Scholar] [CrossRef]
- Guo, M.; Yuan, B.; Sui, Y.; Xiao, Y.; Dong, J.; Yang, L.; Bai, L.; Yang, H.; Wei, D.; Wang, W.; et al. Rational Design of Molybdenum Sulfide/Tungsten Oxide Solar Absorber with Enhanced Photocatalytic Degradation toward Dye Wastewater Purification. J. Colloid Interface Sci. 2023, 631, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Christoforidis, K.C.; Fornasiero, P. Photocatalytic Hydrogen Production: A Rift into the Future Energy Supply. ChemCatChem 2017, 9, 1523–1544. [Google Scholar] [CrossRef]
- Dong, S.; Feng, J.; Fan, M.; Pi, Y.; Hu, L.; Han, X.; Liu, M.; Sun, J.; Sun, J. Recent Developments in Heterogeneous Photocatalytic Water Treatment Using Visible Light-Responsive Photocatalysts: A Review. RSC Adv. 2015, 5, 14610–14630. [Google Scholar] [CrossRef]
- Palanisamy, V.K.; Manoharan, K.; Raman, K.; Sundaram, R. Efficient Sunlight-Driven Photocatalytic Behavior of Zinc Sulfide Nanorods towards Rose Bengal Degradation. J. Mater. Sci. Mater. Electron. 2020, 31, 14795–14809. [Google Scholar] [CrossRef]
- Tayeb, A.M.; Hussein, D.S. Synthesis of TiO2 Nanoparticles and Their Photocatalytic Activity for Methylene Blue. Am. J. Nanomater. 2015, 3, 57–63. [Google Scholar]
- Li, Z.; Meng, X.; Zhang, Z. Recent Development on MoS2-Based Photocatalysis: A Review. J. Photochem. Photobiol. C Photochem. Rev. 2018, 35, 39–55. [Google Scholar] [CrossRef]
- Li, Z.; Wang, S.; Wu, J.; Zhou, W. Recent Progress in Defective TiO2 Photocatalysts for Energy and Environmental Applications. Renew. Sustain. Energy Rev. 2022, 156, 111980. [Google Scholar] [CrossRef]
- Chun-Te Lin, J.; Sopajaree, K.; Jitjanesuwan, T.; Lu, M.-C. Application of Visible Light on Copper-Doped Titanium Dioxide Catalyzing Degradation of Chlorophenols. Sep. Purif. Technol. 2018, 191, 233–243. [Google Scholar]
- Zhang, H.; Wang, G.; Chen, D.; Lv, X.; Li, J. Tuning Photoelectrochemical Performances of Ag−TiO2 Nanocomposites via Reduction/Oxidation of Ag. Chem. Mater. 2008, 20, 6543–6549. [Google Scholar] [CrossRef]
- Sun, M.; Wang, Y.; Fang, Y.; Sun, S.; Yu, Z. Construction of MoS2/CdS/TiO2 Ternary Composites with Enhanced Photocatalytic Activity and Stability. J. Alloys Compd. 2016, 684, 335–341. [Google Scholar] [CrossRef]
- Dustgeer, M.R.; Asma, S.T.; Jilani, A.; Raza, K.; Hussain, S.Z.; Shakoor, M.B.; Iqbal, J.; Abdel-wahab, M.S.; Darwesh, R. Synthesis and Characterization of a Novel Single-Phase Sputtered Cu2O Thin Films: Structural, Antibacterial Activity and Photocatalytic Degradation of Methylene Blue. Inorg. Chem. Commun. 2021, 128, 108606. [Google Scholar] [CrossRef]
- Jilani, A.; Iqbal, J.; Rafique, S.; Abdel-wahab, M.S.; Jamil, Y.; Al-Ghamdi, A.A. Morphological, Optical and X-ray Photoelectron Chemical State Shift Investigations of ZnO Thin Films. Optik 2016, 127, 6358–6365. [Google Scholar] [CrossRef]
- Kumari, A.; Zaman, M.; Kumar, A.; Singh, V.R.; Ghosh, A.; Sahoo, S.K.; Rahaman, A.; Mandal, S.K.; Bhunia, S. An Alternative Approach to Study Photo-Catalytic Behavior of TiO2 Using Synchrotron-Based Advanced Spectroscopic Techniques. J. Mater. Eng. Perform 2023, 32, 10391–10401. [Google Scholar] [CrossRef]
- Shi, Y.; Li, L.; Xu, Z.; Guo, F.; Li, Y.; Shi, W. Synergistic Coupling of Piezoelectric and Plasmonic Effects Regulates the Schottky Barrier in Ag Nanoparticles/Ultrathin g-C3N4 Nanosheets Heterostructure to Enhance the Photocatalytic Activity. Appl. Surf. Sci. 2023, 616, 156466. [Google Scholar] [CrossRef]
- Di, S.; Guo, S.; Wang, Y.; Wang, W.; Jung, Y.M.; Chen, L.; Wang, L. Surface Plasmon Resonance Effect on Charge Transfer in Ag@ Cu2O-rGO Composites. Mater. Chem. Phys. 2023, 301, 127621. [Google Scholar] [CrossRef]
- Hanafi, M.F.; Sapawe, N. Effect of Initial Concentration on the Photocatalytic Degradation of Remazol Brilliant Blue Dye Using Nickel Catalyst. Mater. Today Proc. 2020, 31, 318–320. [Google Scholar] [CrossRef]
- Reza, K.M.; Kurny, A.; Gulshan, F. Parameters Affecting the Photocatalytic Degradation of Dyes Using TiO2: A Review. Appl. Water Sci. 2017, 7, 1569–1578. [Google Scholar] [CrossRef]
- Mansfeldova, V.; Zlamalova, M.; Tarabkova, H.; Janda, P.; Vorokhta, M.; Piliai, L.; Kavan, L. Work Function of TiO2 (Anatase, Rutile, and Brookite) Single Crystals: Effects of the Environment. J. Phys. Chem. C 2021, 125, 1902–1912. [Google Scholar] [CrossRef]
- Huang, K.; Li, C.; Zheng, Y.; Wang, L.; Wang, W.; Meng, X. Recent Advances on Silver-Based Photocatalysis: Photocorrosion Inhibition, Visible-Light Responsivity Enhancement, and Charges Separation Acceleration. Sep. Purif. Technol. 2022, 283, 120194. [Google Scholar] [CrossRef]
- Yadav, A.A.; Hunge, Y.M.; Kang, S.-W. Spongy Ball-like Copper Oxide Nanostructure Modified by Reduced Graphene Oxide for Enhanced Photocatalytic Hydrogen Production. Mater. Res. Bull. 2021, 133, 111026. [Google Scholar] [CrossRef]
- Wu, D.; Liu, H.; Chen, J.; Liu, W.; Histand, G.; Wang, T. Cu NPs-Embedded Cross-Linked Microporous 3D Reduced Graphene Hydrogels as Photocatalyst for Hydrogen Evolution. J. Colloid Interface Sci. 2020, 577, 441–449. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yadav, S.; Jilani, A.; Sachan, S.; Kumar, P.; Ansari, S.A.; Afzal, M.; Ansari, M.O. Highly Efficient Visible-Light-Driven Photocatalysis of Rose Bengal Dye and Hydrogen Production Using Ag@Cu/TiO2 Ternary Nanocomposites. Chemistry 2024, 6, 489-505. https://doi.org/10.3390/chemistry6030028
Yadav S, Jilani A, Sachan S, Kumar P, Ansari SA, Afzal M, Ansari MO. Highly Efficient Visible-Light-Driven Photocatalysis of Rose Bengal Dye and Hydrogen Production Using Ag@Cu/TiO2 Ternary Nanocomposites. Chemistry. 2024; 6(3):489-505. https://doi.org/10.3390/chemistry6030028
Chicago/Turabian StyleYadav, Satish, Asim Jilani, Sarika Sachan, Pramod Kumar, Sajid Ali Ansari, Muhammad Afzal, and Mohammad Omaish Ansari. 2024. "Highly Efficient Visible-Light-Driven Photocatalysis of Rose Bengal Dye and Hydrogen Production Using Ag@Cu/TiO2 Ternary Nanocomposites" Chemistry 6, no. 3: 489-505. https://doi.org/10.3390/chemistry6030028
APA StyleYadav, S., Jilani, A., Sachan, S., Kumar, P., Ansari, S. A., Afzal, M., & Ansari, M. O. (2024). Highly Efficient Visible-Light-Driven Photocatalysis of Rose Bengal Dye and Hydrogen Production Using Ag@Cu/TiO2 Ternary Nanocomposites. Chemistry, 6(3), 489-505. https://doi.org/10.3390/chemistry6030028








