Abstract
This study was conducted to assess, for the first time, the survival of the pathogenic bacteria Listeria monocytogenes, Salmonella spp., Escherichia coli O157:H7, and Staphylococcus aureus during the ripening of protected designation of origin (PDO) Pecorino Romano cheese. A total of twenty-four cheese-making trials (twelve from raw milk and twelve from thermized milk) were performed under the protocol specified by PDO requirements. Sheep cheese milk was first inoculated before processing with approximately 106 colony-forming unit (CFU) mL−1 of each considered pathogen and the experiment was repeated six times for each selected pathogen. Cheese composition and pathogens count were then evaluated in inoculated raw milk, thermized milk, and cheese after 1, 90, and 150 days of ripening. pH, moisture, water activity, and salt content of cheese were within the range of the commercial PDO Pecorino Romano cheese. All the cheeses made from raw and thermized milk were microbiologically safe after 90 days and 1 day from their production, respectively. In conclusion, when Pecorino Romano cheese is produced under PDO specifications, from raw or thermized milk, a combination of factors including the speed and extent of curd acidification in the first phase of the production, together with an intense salting and a long ripening time, preclude the possibility of growth and survival of L. monocytogenes, Salmonella spp., and E. coli O157:H7. Only S. aureus can be still detectable at such low levels that it does not pose a risk to consumers.
1. Introduction
Consumer demand for unpasteurized milk cheeses is constantly increasing because of their more intense flavor and varied aroma than those of pasteurized milk cheeses [1,2,3]. However, especially when made from unpasteurized milk, cheese can hold a risk for the consumer, because of the possible presence of some pathogenic bacteria such as Listeria monocytogenes, Salmonella spp., Staphylococcus aureus, Campylobacter spp., Brucella spp., and pathogenic Escherichia coli [3,4,5]. Based on a data collection of dairy products-associated outbreaks in the United States from 1993 to 2006, Langer et al. [6] determined that outbreaks attributed to the consumption of unpasteurized dairy products were approximately 150 times more frequent, based on the unit of consumption than those related to pasteurized milk or pasteurized milk products. Likewise, Costard et al. [7] reported that in the United States, from 2009 to 2014, the consumption of unpasteurized dairy products caused 840 times more disease and 45 times more hospitalizations than that of pasteurized products.
Among the pathogenic agents, L. monocytogenes, Salmonella spp., Shiga toxin-producing E. coli (STEC) as the serotype O157:H7 and enterotoxin-producing S. aureus are the most involved in foodborne outbreaks related to the consumption of raw milk cheese in industrialized countries [3,5]. These foodborne pathogens usually cause disease with acute symptoms restricted to the gastrointestinal tract such as diarrhea, abdominal cramps, nausea, vomiting, and limited in time and severity [8]. However, in some cases, they can cause serious diseases such as hemolytic uremic syndrome and thrombotic thrombocytopenic purpura associated to E. coli O157:H7 or meningitis and septicemia caused by L. monocytogenes, with a significant mortality rate in vulnerable groups such as infants, elderly, and immunocompromised adults [9,10].
These pathogenic bacteria can originate in raw milk by direct excretion from animals infected udder, by fecal contamination or from the farm environment more generally [5,11]. The foodborne pathogens can reach cheeses via contaminated milk or through the dairy plants environment and the processing equipment [4]. A recent survey shows how L. monocytogenes was first detected in a newly established cheese-making facility just nine months after the starting of the production [12]. Moreover, some pathogens like L. monocytogenes and S. aureus can colonize abiotic surfaces by forming biofilms, that make the bacteria immune to the action of antimicrobial agents [13,14]. Thus, they can persist for a long time in the manufacturing environment, where these microorganisms could be a potential cause of cross-contamination of the dairy products. Workers can also be an important source of contamination as a result of improper handling and some of them could be asymptomatic carriers of S. aureus [15,16].
The growth and survival of microbial pathogens during cheese-making is hindered by several factors such as milk heat treatments, curd cooking, rate of curd acidification by starter cultures, final product pH, salt addition, and competition with the native microflora present in milk [17]. Despite these hurdles, foodborne pathogens can express an adaptive response to different sublethal stresses, essential for their survival in harsher environments. Moreover, pathogenic bacteria adapted to a sublethal stress may exhibit cross-protection with enhanced resistance to different stresses [18,19,20]. Several challenge studies have shown that some foodborne pathogens are able to survive during the manufacturing and ripening of different kinds of cheese made from raw milk [9,21,22,23]. Some authors especially report that L. monocytogenes, Salmonella spp., and E. coli O157:H7 remained detectable after selective enrichment even for more than 200 days of ripening [24,25,26].
However, no studies have investigated the behavior of foodborne pathogens during the production and ripening of Pecorino Romano cheese. Pecorino Romano is an Italian protected designation of origin (PDO) semi-cooked hard cheese, which must be made exclusively from raw or thermized whole sheep milk, according to the PDO specifications [27]. PDO Pecorino Romano cheese is the most popular ovine cheese produced in Italy and it has a very important role from the economic point of view. Indeed, Pecorino Romano is one of the most exported Italian cheeses in the world [28]. The United States, with an export quota of around 13,000 tons in 2019, is the leading export destination, where this cheese is mainly used as an ingredient in the food industry [29].
Typically Pecorino Romano cheese has about 32% of moisture, a water activity (aw) value of around 0.85, and a pH value between 5.07 and 5.31. This cheese usually has a high salt content that ranges from 4.5% to 8.3%. The minimum ripening period is 150 days for the table cheese, while grating cheese requires 240 days [28].
Although unpasteurized milk cheeses are commonly consumed in a large number of countries, some of them, the United States primarily, have doubts about the safety of these products. For instance, the Food and Drug Administration (FDA) is frequently evaluating whether to propose more restrictive requirements on the sale of unpasteurized milk cheeses, like Pecorino Romano cheese [30,31]. Despite the healthiness of this product, also indirectly attested from the lack of foodborne infection or intoxication episodes bound to the consumption of this cheese, experimental studies are important to investigate the fate of pathogenic bacteria during Pecorino Romano cheese manufacturing and ripening.
The main objective of this work was to investigate the survival of L. monocytogenes, Salmonella spp., E. coli O157:H7, and S. aureus, during the ripening of PDO Pecorino Romano cheese made from raw or thermized milk.
2. Materials and Methods
2.1. Experimental Design
Experimental productions of Pecorino Romano cheese were carried out according to PDO specifications, using cheese-making facilities of Agris Sardegna (Olmedo, Italy). Although PDO Pecorino Romano cheese is now exclusively produced from thermized milk, PDO specifications do not exclude the use of raw milk. Therefore, we performed two series of experimental cheese-makings, from raw milk (RM) and thermized milk (TM). Each series consisted of twelve cheese batches, three replicates for each selected pathogen. On the same day two experimental cheese-makings were performed starting from a single batch of raw whole sheep milk inoculated with a given pathogenic microorganism, the first one from raw milk and the second one after thermization of the same sheep milk.
2.2. Bacterial Strains and Inoculum Preparation
Seven strains of L. monocytogenes, two of Salmonella spp., three of E. coli O157:H7, and five of S. aureus were used (Table 1). We have chosen both reference and wild strains, the latter were isolated from milk and dairy products. Each inoculum was prepared from a culture containing different strains (reference and wild strains) of the same species.

Table 1.
Pathogen strains used in Pecorino Romano cheese-making trials.
Inoculum preparation included several steps. Briefly, all strains were conserved in Microbank beads (Biolife, Milan, Italy) at −18 °C. For each experiment, the strains were previously reactivated in brain heart infusion broth (Biolife, Milan, Italy). The pre-inoculation preparation included serial passages on trypticase soy broth (TSB, Oxoid, Thermo Fisher Scientific, Basingstoke, UK). Two milliliters of each strain culture were inoculated on 35 mL of TSB and incubated overnight at 37 °C under continuous stirring. Then, for each species (L. monocytogenes, Salmonella spp., E. coli O157:H7 and S. aureus), subcultures have been combined in equal quantity and centrifuged at 6000 rpm for 10 min. Subsequently, the supernatant was discarded and the pellet was resuspended in physiological saline solution (NaCl 0.85%, pH 7). The optical density at 600 nm (Perkin Elmer Lambda 25 UV/VIS Spectrophotometer, Perkin Elmer, Waltham, MA, USA) was determined and counts were confirmed by serial decimal dilution and inoculation in the following selective agar media: Agar Listeria according to Ottaviani and Agosti (ALOA, Biolife, Milan, Italy) plates for L. monocytogenes; Baird Parker plates with Rabbit Plasma Fibrinogen supplement (RPF, Biolife, Milan, Italy) plates for S. aureus; cefixime tellurite sorbitol MacConkey agar plates (CT-SMAC, Biolife, Milan, Italy) for E. coli O157:H7; xylose lysine deoxycholate agar plate (XLD, Microbiol, Uta, Italy) for Salmonella spp. The agar plates were incubated at 37 °C for 24 h.
2.3. Cheese Production and Sampling
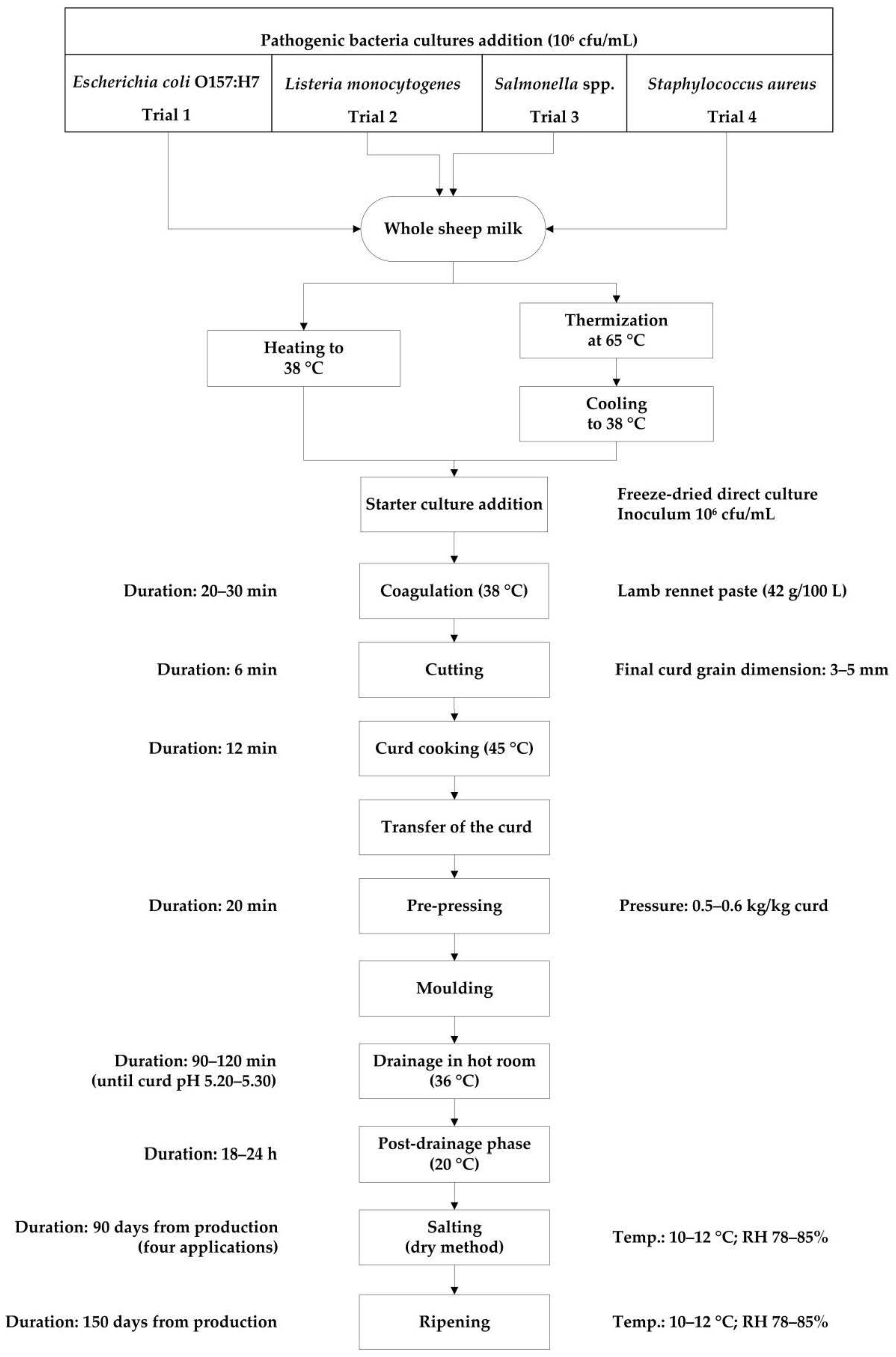
Each time, 700 L of raw whole bulk sheep milk collected from the “Bonassai” experimental farm of Agris Sardegna, were used. The milk was previously inoculated, under gentle stirring, with a multi-strain bacterial suspension of the same pathogenic microorganism to get a final concentration of approximately 106 CFU mL−1 and then was split into two 350 L aliquots, for RM and TM cheese-makings. In TM trials, milk was subsequently heated to 65 °C, without resting at the set temperature and quickly cooled down to coagulation temperature (38 °C). Then, the manufacturing process (RM and TM) followed the same procedure reported in Figure 1. Two vats were used alternately, one for RM trial and one for TM trial, in order to eliminate any vat variation. A thermophilic freeze-dried starter culture (FD-DVS CO-02, CHR Hansen, Hoersholm, Denmark) including Streptococcus thermophilus and Lactobacillus delbrueckii subsp. bulgaricus was added at a concentration of 106 CFU mL−1.
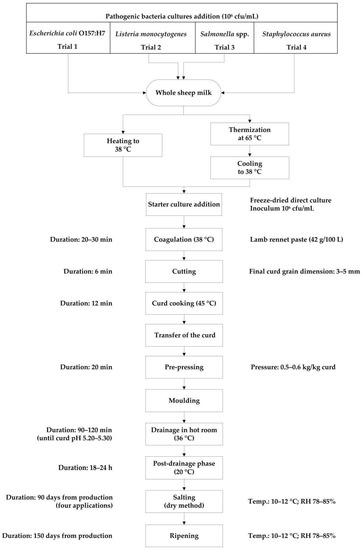
Figure 1.
Experimental flow diagram of protected designation of origin (PDO) Pecorino Romano cheese production. RH = relative humidity.
Three cheese wheels of approximately 25 kg at 1 day were obtained from each cheese-making, for a total of 36 RM and 36 TM cheeses wheels. After molding, cheeses were subjected to drainage in hot room at 36 °C until reaching pH 5.20–5.30 and then at 20 °C up to 18–24 h. Dry salting and ripening were conducted in controlled conditions (10–12 °C and 78–85% relative humidity). In particular, cheeses were dry salted after 48 h (first application) from the manufacture. Later, during the first 90 days of ripening, cheeses were salted three more times, respectively after 12, 26, and 56 days. After 90 days, at the end of the salting, cheeses were washed, dried, and aged for two more months, for a total of 5 months of ripening. The cheese was sampled after 1, 90, and 150 days for physico-chemical analysis while inoculated raw milk, thermized milk, and cheese were sampled to enumerate pathogenic bacteria.
2.4. Physico-Chemical Analysis
All physico-chemical analysis were performed in duplicate. Samples of curd and cheese were analyzed for pH (pH-meter Basic 20+, Crison Instruments S.A., Alella, Spain). The following parameters were determined for the cheese samples: moisture (ISO 5534:2004) [32]; sodium chloride, determined by potentiometric titration with AgNO3 (ISO 5943:2006) [33] (automatic titrator, model DL55, Mettler-Toledo GmbH, Schwerzenbach, Switzerland); water activity (aw), determined at 25 °C (Aw Sprint instrument, Axair Ltd., Novasina Division, Lachen, Switzerland).
2.5. Microbiological Analysis
Aliquots of 25 mL or g for qualitative detection and 10 mL or g for quantitative detection were taken from each sample and used to prepare a suspension with appropriate diluents. The following parameters have been then researched:
- (a)
- Listeria monocytogenes. Detection and enumeration were performed according to ISO 11290-1:2017 and ISO 11290-2:2017, respectively [34,35]. For detection, samples were mixed with Fraser broth base (Oxoid, Thermo Fisher Scientific, Basingstoke, UK), homogenized 90 s in a Stomacher Lab Blender 400 (International PBI S.p.A., Milan, Italy), and incubated for 24 h at 30 °C (primary enrichment). Subsequently, 100 μL of primary enrichment were transferred to 10 mL of Fraser broth supplemented by Fraser selective supplement (Oxoid, Thermo Fisher Scientific, Basingstoke, UK), which were incubated for 24 h at 37 °C (secondary enrichment). From primary and secondary enrichments, aliquots of 100 μL were streaked onto selective differential medium plates: Agar Listeria according to Ottaviani and Agosti (ALOA, Biolife, Milan, Italy) and Listeria selective agar (Oxford formulation, Oxoid, Thermo Fisher Scientific, Basingstoke, UK) and incubated for up to 24–48 h at 37 °C. All isolates with typical L. monocytogenes characteristics were subjected to morphological and biochemical proofs as confirmatory tests.
For the enumeration of L. monocytogenes ten-fold serial dilutions were made and aliquots of 100 μL were plated in duplicate on the surface of ALOA agar plates which were incubated for 48 h at 37 °C. Presumptive L. monocytogenes colonies were counted after confirmatory tests.
- (b)
- Staphylococcus aureus. Detection and enumeration were performed according to ISO 6888-2:2004 [36]. Samples were weighed and mixed with buffered peptone water (BPW, Microbiol, Uta, Italy), ten-fold serial dilutions were prepared and aliquots of 100 μL were plated in duplicate on Baird Parker plates with Rabbit Plasma Fibrinogen supplement (RPF, Biolife, Milan, Italy) and incubated for 24–48 h at 37 °C.
- (c)
- Escherichia coli O157:H7. The detection was performed according to ISO 16654:2001/A1:2017 and four successive stages were necessitated [37].
- (1)
- Enrichment of the test portion homogenized in modified tryptone soya broth containing novobiocin (mTSB + N, Biolife, Milan, Italy) with incubation at 41.5 °C for 6 h and subsequently for a further 12 h to 18 h.
- (2)
- Separation and concentration of microorganisms by means of immunomagnetic particles coated with antibodies to E. coli O157:H7.
- (3)
- Isolation by subculture of the immunomagnetic particles with adhering bacteria onto cefixime tellurite sorbitol MacConkey agar (CT-SMAC, Biolife, Milan, Italy) and sorbitol MacConkey agar (SMAC, Biolife, Milan, Italy) incubated at 37 °C for 24 h.
- (4)
- Confirmation of typical colonies.
E. coli O157:H7 count was determined by ten-fold serial dilutions and direct plating (100 μL in duplicate) on CT-SMAC agar plates incubated at 37 °C for 24 h. Typical E. coli O157:H7 colonies were subjected to confirmatory tests and were then enumerated.
- (d)
- Salmonella spp. The detection was performed according to ISO method 6579-1:2017 [38]. The method required the following successive stages. A pre-enrichment in buffered peptone water (BPW, Microbiol, Uta, Italy) at 37 °C for 24 h. A selective enrichment in Rappaport-Vassiliadis with soy broth (RVS, Oxoid, Basingstoke, UK) and Müller-Kauffmann tetrathionate-novobiocin broth (MKTTn, Microbiol, Uta, Italy) for 24 h at 41.5 and 37 °C, respectively. Aliquots of the selective broths were streaked onto two selective isolation agar media, xylose lysine deoxycholate agar (XLD, Microbiol, Uta, Italy) and Salmonella detection and identification agar (SMID, BioMérieux, Marcy L’Etoile, France). The agar plates were incubated at 37 °C for 24 h. Confirmation of suspect colonies was carried out by biochemical and serological testing.
For the enumeration of Salmonella spp. ten-fold serial dilutions were prepared and aliquots of 100 μL were double plated on XLD agar plates which were incubated at 37 °C for 24 h. Presumptive Salmonella spp. colonies were subjected to confirmatory tests and were then counted.
2.6. Statistical Analysis
Microbial loads were log transformed and expressed as means and standard deviation (SD). The means and SD of physico-chemical parameters were determined from twelve cheese-makings for each experimental series (RM and TM). Analysis of variance was carried out using Minitab statistical package release 16 (Minitab Inc., State College, PA, USA). The general linear model procedure was used to verify the effects of the two studied factors “milk heat treatment” (2 levels) and “ripening time” (3 levels) on the microbial loads and physico-chemical parameters, as far as their interaction is concerned. The comparison between means was performed using Tukey’s significant difference test (p < 0.05). Data were also analyzed by the Pearson correlation to measure the degree of the linear relationship.
3. Results and Discussion
3.1. Physico-Chemical Properties of Cheese
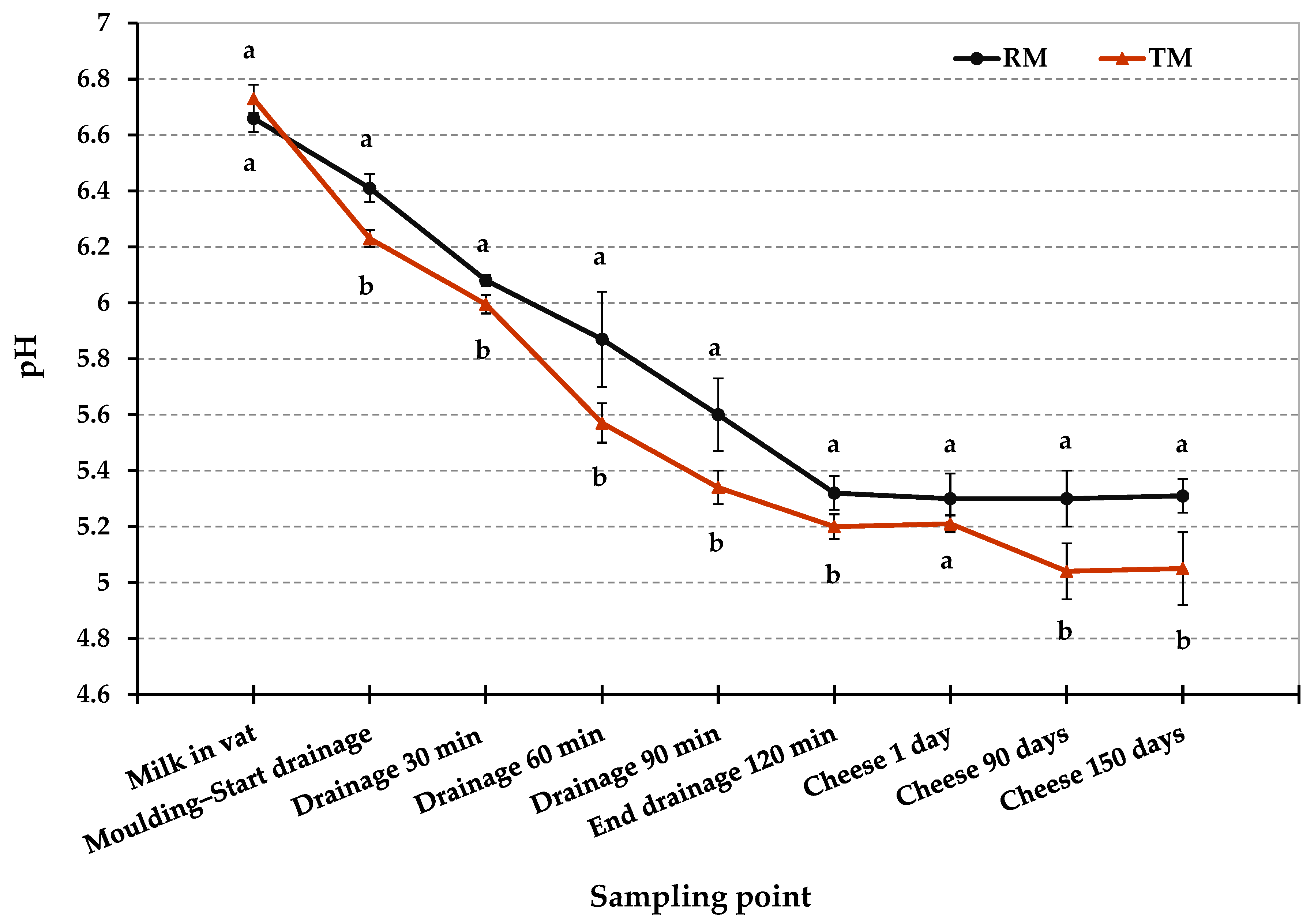
Figure 2 and Table 2 show the pH values in RM and TM cheese-making trials at different time. The pH values differed significantly between RM and TM (p < 0.05) in various step of cheese manufacturing process. As shown in Figure 2, after molding was completed, RM curd had a higher pH than TM curd (6.4 vs. 6.2, respectively; p < 0.05). This difference suggests a slight delay in the starter culture acidification activity in the early stage of RM cheese-making. This time lag could be due to the bacteriostatic activity of milk proteins such as lactoferrin, lactoperoxidase, and immunoglobulins since it normally decreases with milk heat treatments [39]. We cannot exclude that the competition of the starter culture with the native microflora of raw milk may also be involved in this delay of the acidification process. During drainage in hot room at 36 °C, a difference in pH values between RM and TM cheese-making was kept, however, this gap became less evident after two hours of drainage. Moreover, the lower SD in TM acidification curve shown in Figure 2 suggests a more regular and repeatable trend of the acidification process during TM cheese-making compared to RM one.
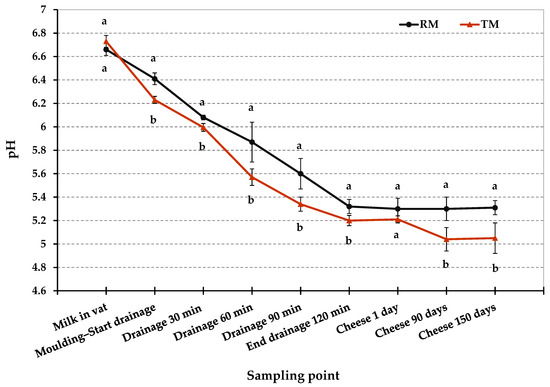
Figure 2.
Acidification profiles of Pecorino Romano cheese produced from raw (RM), and thermized milk (TM). Twelve replicates for each cheese-making technology. Error bars indicate standard deviations. Different letters at the same time point indicate significant differences (p < 0.05).

Table 2.
Physico-chemical parameters of Pecorino Romano cheese at different ripening times, obtained from raw (RM) and thermized milk (TM). Values are given as means ± standard deviation.
As shown in Table 2, the pH values in RM and TM cheeses were significantly affected by the milk thermization treatment (p < 0.01) and ripening time (p < 0.01), furthermore the interaction between these factors was significant (p < 0.01). Despite the slight delay in the acidification process, after 1 day, RM and TM cheeses were not statistically different for pH values (5.3 and 5.21, respectively). On the contrary, after 90 and 150 days of ripening, RM and TM cheeses differed significantly for pH values (p < 0.01). In particular, pH in RM cheeses remained substantially unchanged during ripening (around 5.3), while pH in TM cheeses showed a slight decrease, reaching an average pH value of 5.0 at 150 days. The evolution of pH during cheese ripening is due to several factors involved in the metabolism of the main components of the cheese: hydrolysis of the residual lactose, which involves a decrease in pH, and degradation of proteins that, instead, leads to an increase in pH value [40,41]. Pecorino Romano cheese is characterized by modest proteolysis due to the intense salting that limits the activity of the proteolytic enzymes. On the other hands, lipolysis is more accentuated mainly because of the use of exogenous lipases contained in the lamb paste rennet, used for milk coagulation. These enzymes catalyze the biochemical process of triglyceride hydrolysis resulting in the release of short-chain fatty acids that can contribute to a decrease in the pH during ripening [28,42]. Finally, it is important to point out that the pH values found both for RM and TM cheeses are in the range of variation found in commercial PDO Pecorino Romano cheese [28].
In Table 2 we report also the remaining physico-chemical properties of RM and TM cheese, at different time of ripening. The milk thermization treatment did not significantly affect the moisture content, which instead was significantly influenced by ripening time and its interaction with heat treatment (respectively, p < 0.001 and p < 0.05). No significant differences were found between RM and TM cheese at each observed ripening time. The major changes in moisture occur in the first 90 days of ripening in both TM and RM cheeses, while the decrease from 90 up to 150 days was statistically significant only in TM cheese (p < 0.05). Moisture content was approximately 42% in cheese after 1 day and 31–32% in cheese after 90 and 150 days of ripening, according to Addis et al. [28,42].
As reported for moisture content, also the aw values were significantly affected by the ripening time and its interaction with heat treatment (p < 0.001) (Table 2). The aw, significantly differed in RM and TM 1 day cheeses, and dropped significantly from approximately 0.98–0.97 (TM and RM, respectively) up to 0.89–0.88 at the end of the salting process (90 days), then slightly decreased tendentially (p > 0.05) at 150 days. The aw values at the end of ripening (150 days) were higher than those reported by Addis et al. [28]. However, these authors referred to longer aged Pecorino Romano cheese (7–8 months).
At the end of the salting process (90 days), the NaCl on dry matter (DM) content was significantly lower in RM cheeses (6.3%) compared to TM cheeses (7.2%). Salt content slightly increased, up to the end of ripening (6.4% and 7.3%, respectively in RM and TM cheeses). This difference in NaCl content between RM and TM cheeses could be due to the intrinsic variability of the dry salting technique. However, NaCl values are comparable to those of commercial Pecorino Romano cheese, which is characterized by high and variable salt content [28].
These results showed that the experimental cheeses met the typical requirements of PDO Pecorino Romano cheese. Moreover, the different cheese-making technology (RM and TM) seems to affect only in part the characteristics of the product at the end of the ripening period, which substantially has the same peculiarities of the Pecorino Romano cheese available on the market.
3.2. Pathogenic Bacteria Counts in RM Cheese-Making Trials
The pathogens counts in raw milk are given in Table 3. Pathogenic bacteria levels in raw milk pointed out the accuracy of the technique used for the inoculum preparation. Indeed, all the counts were around 6 log10 CFU mL−1, as established from the experimental protocol. Table 4 shows the pathogens counts in cheese at different ripening stages. A reduction in bacterial loads was observed for all pathogens in 1-day old cheese. L. monocytogenes and Salmonella spp. showed the major reduction, with a decrease of about 4 log10. The number of viable E. coli O157:H7 decreased by about 2.5 log10, while S. aureus counts showed a reduction by about 0.5 log10. The behavior of the pathogenic bacteria was likely in part attributed to the number of viable cells lost with the whey separation and partly to the combined effect of various hurdles occurring during the first phase of Pecorino Romano cheese manufacturing, such as competition with the starter culture, acidification, and curd cooking [17,43]. Conversely, other studies showed an increase in pathogens counts during the early stages of cheese-making in different unpasteurized milk cheeses. Bellio et al. [21] reported a growth of 2 log cycle in E. coli O157:H7 levels during the first 24 h of PDO Fontina cheese production, despite a curd cooking phase at 48 °C. Similar findings were also given by other authors in Cacioricotta, Cheddar, and Gouda cheeses [9,24]. Chatelard-Chauvin et al. [26] found that L. monocytogenes increased by about 3.5 log10 in Cantal cheese at 24 h. An increase in Salmonella spp. populations over 1 log10 was observed in Gouda and Cheddar cheeses in the first 24 h of manufacturing [25,44]. In our study, S. aureus fell slightly, however, some authors observed a growth in S. aureus counts in the early stages of cheese production [45,46]. These authors considered that the increase in pathogenic bacteria counts during the early stages of cheese-making could be due both to an actual bacterial growth and to the entrapment of pathogens cells within the curd, during curd contraction and whey separation. In our study, the reduction in pathogens population levels during the early stages of cheese manufacturing might be related not only to the aforementioned hurdles to bacteria growth and survival but also to a protracted curd breaking (about 6 min) into extremely fine grains (3–5 mm). This is a typical feature of Pecorino Romano cheese-making technology which could lead to a higher level of pathogens viable cells lost in the cheese whey.

Table 3.
Results of pathogens counts (log10 CFU mL−1) in sheep milk before and after thermization. Values are given as means ± standard deviation. Escherichia coli O157:H7: ATCC 43984, 47, 719. Salmonella spp.: Typhimurium ATCC 6994, Enteritidis 670. Listeria monocytogenes: ATCC 15313, ATCC 19114, ATCC 9525, ATCC 153/3, 2, 90, V7. Staphylococcus aureus: ATCC 14458, ATCC 25923, 401, 466, 64494.

Table 4.
Results of pathogens counts (log10 CFU g−1) in Pecorino Romano cheese at different ripening times, obtained from raw (RM), and thermized milk (TM). Values are given as means ± standard deviation. Escherichia coli O157:H7: ATCC 43984, 47, 719. Salmonella spp.: Typhimurium ATCC 6994, Enteritidis 670. Listeria monocytogenes: ATCC 15313, ATCC 19114, ATCC 9525, ATCC 153/3, 2, 90, V7. Staphylococcus aureus: ATCC 14458, ATCC 25923, 401, 466, 64494.
All tested pathogens, despite a reduction, were still present in 1-day old cheese in relevant bacterial loads. However, it must be pointed out that in the present study, high inoculum levels were used, which are difficult to find in practice, to simulate the worst-case scenario of contamination. Overall, our results are in agreement with other studies that indicate the ability of the pathogenic bacteria to overcome the obstacles of the first phase of cheese-making, first of all, acidification, probably owing to an acid tolerance response (ATR) widely reported in the literature [18,19,20].
Table 4 also shows the pathogens counts at the end of salting (90 days) and the end of the ripening period (150 days). At the experimental conditions, all inoculated pathogens except S. aureus, were not detectable after 90 days, even after selective enrichment. This result was confirmed in 150-days old cheese. S. aureus counts were below the detection limit for direct plating enumeration method (<1 log10 CFU g−1), both in cheese at 90 and 150 days of ripening. In the early phase of cheese-making (24 h), the pathogenic bacteria were subjected to initial stress because of the curd cooking and fast acidification. Afterwards, in the first 90 days of ripening, a significant correlation was found between microbial loads and aw, moisture and NaCl content. In particular, all the studied pathogens exhibited a strong positive correlation with moisture (E. coli O157:H7, R2 = 0.987, p < 0.001; L. monocytogenes, R2 = 0.982, p < 0.001; Salmonella spp., R2 = 0.987, p < 0.001; S. aureus, R2 = 0.993, p < 0.001) and aw (E. coli O157:H7, R2 = 0.971, p < 0.001; L. monocytogenes, R2 = 0.982, p < 0.001; Salmonella spp., R2 = 0.971, p < 0.001; S. aureus, R2 = 0.948, p < 0.001). Conversely, a strong negative correlation was found between microbial count and salt content (E. coli O157:H7, R2 = −0.998, p < 0.001; L. monocytogenes, R2 = −0.997, p < 0.001; Salmonella spp., R2 = −0.987, p < 0.001; S. aureus, R2 = −0.996, p < 0.001).
Several studies show the ability of pathogenic bacteria to survive in cheeses even beyond 90 days of ripening. Nevertheless, none of these had similar physico-chemical properties to those of PDO Pecorino Romano cheese (pH ≤ 5.4; moisture ~32%; aw < 0.90; NaCl 4–8%), together with a long ripening period. Ioanna et al. [9] reported that E. coli O157:H7, when inoculated in milk at 2 log10 CFU mL−1, survived in 90 days-old Cacioricotta cheese with a bacterial load greater than 4 log10 CFU g−1. Similar findings were observed in Fontina cheese at 80 days of ripening [21]. In Cheddar and Gouda cheeses, E. coli O157:H7 was detectable after an enrichment procedure for up to around 300 days, with an initial raw milk inoculation of about 20 CFU mL−1, which was far lower than that used in the present study [24]. E. coli O157:H7 appears to be one of the pathogens having the greatest ability to overcome environmental stresses and it is also infectious at very low doses (5–50 viable cells) [47]. Therefore, proving its complete inactivation is relevant. Viable cells of Salmonella spp. were detectable in Gouda cheese for more than 200 days [25]. However, in Feta and Tulum cheese, with a salt content close to that of Pecorino Romano cheese, Salmonella spp. was no more found after 20 and 90 days, respectively [48,49]. L. monocytogenes was detectable for more than 250 days in Cantal cheese [26]. In agreement with our study, Wusimanjiang et al. [50] observed that L. monocytogenes, when inoculated in milk at 3 log10 CFU mL−1, reached undetectable levels after 28–35 days of ripening in a cheese with a salt content similar to that of Pecorino Romano cheese (4–10% on DM basis).
According to Regulation (EC) No 2073/2005, Pecorino Romano cheese, already at 90 days, belongs to ready-to-eat foods that do not support the growth of L. monocytogenes, since its aw value is less than 0.92. For these products, the Regulation establishes a tolerance for L. monocytogenes up to 100 CFU g−1 [51]. The cheeses we obtained were Listeria-free at 90 days, despite the high initial inoculum in cheese milk.
S. aureus, unlike the other pathogens, was still present in high population levels in 1-day old cheese. Since S. aureus is halotolerant, it could find the proper environment to survive or even grow during or after the salting process, as some authors have already reported [52,53]. However, according to the growth limits of S. aureus stated by Valero et al. [54], the conditions of ripening and the characteristics of Pecorino Romano cheese: ripening temperature (10–12 °C), constant low pH (~5.3), and decreasing of aw from 0.97 to 0.89 in the first 90 days of ripening, were enough severe to prevent proliferation and also to reduce the survival of S. aureus as shown by the results (<1 log10 CFU g−1). These findings were in agreement with those observed by Pexara et al. [45] in Feta cheese with a salt content close to that of Pecorino Romano cheese.
Unlike the other bacteria tested in this study, the pathogenicity of S. aureus is due to the possible production of different enterotoxins in contaminated food. A level above 105 CFU mL−1 or g−1 of S. aureus can produce an infective enterotoxins concentration of about 1 μg [55]. Therefore, Regulation (EC) No 2073/2005 sets the limit in cheeses for coagulase-positive staphylococci (including S. aureus) at 105 CFU g−1 [51]. Above this limit, the presence of staphylococcal enterotoxins is expected, and there is a need to perform assays for enterotoxins detection, which must be absent in 25 g. However, staphylococcal enterotoxins production seems to be strongly limited in cheeses by the antagonistic effect of lactic acid bacteria. Microbial competition together with unfavorable environmental conditions, such as low pH, limits the growth of S. aureus and strongly downregulate virulence genes expression [52,53,56]. In particular, a rapid acidification in the first 6 h of cheese-making, as reported in our study (Figure 2), seems to be critical for S. aureus growth and enterotoxins production [46]. Furthermore, in our work, we simulated a worst-case contamination scenarios by employing unrealistically high starting S. aureus levels and despite this, the cheese-making conditions prevented the proliferation of S. aureus. Future studies will be carried out to examine the presence of staphylococcal enterotoxins in Pecorino Romano cheese produced from milk contaminated with S. aureus.
3.3. Pathogenic Bacteria Counts in TM Cheese-Making Trials
In Table 3 are reported the pathogens counts in milk (before and after thermization). Thermization is a sub-pasteurization heat treatment usually performed at 57–68 °C for 10–20 s to reduce the number of spoilage bacteria, with minimum collateral heat damage to milk components and thus provide a suitable environment for the growth of the added starter cultures [57]. Unlike the pasteurization, the thermization process is not able to ensure the complete inactivation of pathogenic microorganisms but, especially if they are present in high initial levels, it can only reduce their number, as confirmed by the pathogens counts in thermized milk shown in Table 3. In our study, we used a batch thermization at 65 °C, without resting at the set temperature. All the pathogens showed a reduction of around 3 log10 after thermization, starting from a bacterial count of about 6 log10 CFU mL−1 (Table 3). These rates of thermal inactivation are close to those reported by other authors, although a comparison with the time-temperature conditions reported in our work is not easy [58,59]. These studies highlight the heterogeneity in thermal resistance not only in different microbial groups but also between different strains of the same species. In particular, different strains of E. coli and S. aureus showed varying degrees of heat resistance, while L. monocytogenes and Salmonella spp. strains displayed a more uniform thermal susceptibility [59]. Hence, it is important to underline that a multi-strain inoculum should be used to represent the behavior of a given pathogen, as done in our work. The data reported in Table 3 show that counts levels in thermized milk did not differ significantly among the four pathogenic microorganisms, which indicate similar thermal tolerance under the conditions applied in the present study. However, we cannot exclude that some of the strains used in our study were more susceptible toward heat than others.
After 1 day from the production, L. monocytogenes, E. coli O157:H7, and Salmonella spp. dropped below the detection limit and were no longer detectable up to 150 days, even after an enrichment procedure, as shown in Table 4. S. aureus counts were also below the detection limit for the direct plating enumeration method (<1 log10 CFU g−1) from 1 day up to the end of the ripening period. As already reported for RM trials, it was not possible to assess exactly the amount of the pathogens decrease related to the bacteria loss through the whey separation compared to the pathogens counts reduction due to the several environmental hurdles during the first stage of cheese-making. The latter may have a greater impact on TM trials, especially as a consequence of milk thermization, a second less severe thermal stress (curd cooking), and the faster and more intense acidification. In fact, after molding, the curd had a lower average pH value in TM trials (6.24) compared to RM trials (6.41). This difference was kept during the drainage process (Figure 2). Besides, as reported in Table 4, the microbial counts in RM and TM cheeses were significantly affected by milk thermization treatment (p < 0.001), as well as ripening time (p < 0.001). Furthermore, the interaction between these factors was also significant for pathogens counts (p < 0.001).
The introduction of the thermization in Pecorino Romano cheese-making is a further obstacle to the possible growth and survival of pathogens. In addition to other hurdles (curd cooking and acidification), it makes the cheese microbiologically safe before the salting process. In the case of RM cheeses, it is instead necessary to wait until the end of the salting process (90 days) to consider safety of the product. In any case, this happens 60 days before the mandatory minimum period for marketing PDO Pecorino Romano cheese (150 days).
Hence, our study shows that the production process of Pecorino Romano cheese both from raw and thermized milk is enough restrictive for the survival of the pathogens we have examined, except for S. aureus which may be still present in such low levels that it does not represent a hazard to the consumers. Currently, thermization is practiced by all producers of PDO Pecorino Romano cheese since this treatment has the advantage in standardizing the microbiological properties of the cheese milk by reducing the total microbial count and containing the undesirable bacteria, to obtain a constant quality of the product. This is a key element for a product aimed at an international market, such as Pecorino Romano cheese.
4. Conclusions
Cheeses made from unpasteurized milk are known to pose a health risk to the consumers. According to PDO specifications, Pecorino Romano cheese can be produced from raw or thermized whole sheep milk. In this study, Pecorino Romano cheese was produced from unpasteurized (raw and thermized) milk inoculated with L. monocytogenes, Salmonella spp., E. coli O157:H7, or S. aureus, in high initial loads (106 CFU mL−1). The results demonstrated that the cheese-making process may ensure the microbiological safety of this cheese after the minimum ripening period (150 days) established by the PDO specifications. In particular, the obtained cheese was free from microbiological hazard after 90 days and 1 day, when manufactured from raw and thermized milk, respectively. These results could encourage cheese manufacturers to diversify Pecorino Romano cheese by using raw milk, which is currently almost not used, for its production, to provide the market with a product having a more intense flavor than that made from thermized milk. This is the first preliminary work to evaluate the survival of selected pathogenic bacteria during Pecorino Romano cheese ripening. Further studies are necessary to investigate the behavior of the pathogens in the early stages of manufacturing in order to understand more in depth on how their survival is hampered by different factors. The eventual production of staphylococcal enterotoxins will also be tested in subsequent investigations.
Author Contributions
Conceptualization, A.P. and A.F.; methodology, A.P., M.A. and A.F.; validation, G.L., R.M., M.P., M.A., A.F., and A.P.; formal analysis, G.L., R.M., and M.P.; investigation, G.L., R.M., and M.P.; resources, A.P., M.A., and A.F.; writing—original draft preparation, G.L., R.M., M.A., and M.P.; writing—review and editing, A.P. and A.F.; supervision A.P. and A.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The technical staff of both the Technology and Chemistry sectors of Agris Sardegna is gratefully acknowledged.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Colonna, A.; Durham, C.; Meunier-Goddik, L. Factors affecting consumers’ preferences for and purchasing decisions regarding pasteurized and raw milk specialty cheeses. J. Dairy Sci. 2011, 94, 5217–5226. [Google Scholar] [CrossRef] [PubMed]
- Montel, M.; Buchin, S.; Mallet, A.; Delbes-Paus, C.; Vuitton, D.A.; Desmasures, N.; Berthier, F. Traditional cheeses: Rich and diverse microbiota with associated benefits. Int. J. Food Microbiol. 2014, 177, 136–154. [Google Scholar] [CrossRef] [PubMed]
- Yoon, Y.; Lee, S.; Choi, K.-H. Microbial benefits and risks of raw milk cheese. Food Control 2016, 63, 201–215. [Google Scholar] [CrossRef]
- Kousta, M.; Mataragas, M.; Skandamis, P.; Drosinos, E.H. Prevalence and sources of cheese contamination with pathogens at farm and processing levels. Food Control 2010, 21, 805–815. [Google Scholar] [CrossRef]
- Verraes, C.; Vlaemynck, G.; Van Weyenberg, S.; De Zutter, L.; Daube, G.; Sindic, M.; Uyttendaelec, M.; Herman, L. A review of the microbiological hazards of dairy products made from raw milk. Int. Dairy J. 2015, 50, 32–44. [Google Scholar] [CrossRef]
- Langer, A.J.; Ayers, T.; Grass, J.; Lynch, M.; Angulo, F.J.; Mahon, B.E. Nonpasteurized Dairy Products, Disease Outbreaks, and State Laws—United States, 1993–2006. Emerg. Infect. Dis. 2012, 18, 385–391. [Google Scholar] [CrossRef]
- Costard, S.; Espejo, L.; Groenendaal, H.; Zagmutt, F.J. Outbreak-related disease burden associated with consumption of unpasteurized cow’s milk and cheese, United States, 2009–2014. Emerg. Infect. Dis. 2017, 23, 957–964. [Google Scholar] [CrossRef]
- Rocourt, J. Foodborne diseases: Foodborne diseases and vulnerable groups. In Encyclopedia of Food Safety, 1st ed.; Motarjemi, Y., Ed.; Academic Press: Waltham, MA, USA, 2014; Volume 1, pp. 323–331. ISBN 978-0-12-378613-5. [Google Scholar]
- Ioanna, F.; Quaglia, N.C.; Storelli, M.M.; Castiglia, D.; Goffredo, E.; Storelli, A.; De Rosa, M.; Normanno, G.; Jambrenghi, A.C.; Dambrosio, A. Survival of Escherichia coli O157:H7 during the manufacture and ripening of Cacioricotta goat cheese. Food Microbiol. 2018, 70, 200–205. [Google Scholar] [CrossRef]
- Thakur, M.; Asrani, R.K.; Patial, V. Listeria monocytogenes: A Food-Borne Pathogen. In Foodborne Diseases, 1st ed.; Holban, A.M., Grumezescu, A.M., Eds.; Academic Press: London, UK, 2018; Volume 15, pp. 157–192. ISBN 978-0-12-811444-5. [Google Scholar]
- Arqués, J.L.; Rodríguez, E.; Langa, S.; Landete, J.M.; Medina, M. Antimicrobial Activity of Lactic Acid Bacteria in Dairy Products and Gut: Effect on Pathogens. BioMed Res. Int. 2015, 2015, 584183. [Google Scholar] [CrossRef]
- Melero, B.; Stessl, B.; Manso, B.; Wagner, M.; Esteban-Carbonero, Ó.J.; Hernández, M.; Rovira, J.; Rodriguez-Lázaro, D. Listeria monocytogenes colonization in a newly established dairy processing facility. Int. J. Food Microbiol. 2019, 289, 64–71. [Google Scholar] [CrossRef]
- Castro, R.D.; Pedroso, S.H.S.P.; Sandes, S.H.C.; Silva, G.O.; Luiz, K.C.M.; Dias, R.S.; Filho, R.A.T.; Figueiredo, H.C.P.; Santos, S.G.; Nunes, A.C.; et al. Virulence factors and antimicrobial resistance of Staphylococcus aureus isolated from the production process of Minas artisanal cheese from the region of Campo das Vertentes, Brazil. J. Dairy Sci. 2020, 103, 2098–2110. [Google Scholar] [CrossRef] [PubMed]
- Lahou, E.; Uyttendaele, M. Growth potential of Listeria monocytogenes in soft, semi-soft and semi-hard artisanal cheeses after post-processing contamination in deli retail establishments. Food Control 2017, 76, 13–23. [Google Scholar] [CrossRef]
- Castañeda-Ruelas, G.M.; Soto-Beltrán, M.; Chaidez, C. Detecting Sources of Staphylococcus aureus in One Small-Scale Cheese Plant in Northwestern Mexico. J. Food Saf. 2016, 37, e12290. [Google Scholar] [CrossRef]
- Normanno, G.; Spinelli, E.; Caruso, M.; Fraccalvieri, R.; Capozzi, L.; Barlaam, A.; Parisi, A. Occurrence and characteristics of methicillin-resistant Staphylococcus aureus (MRSA) in buffalo bulk tank milk and the farm workers in Italy. Food Microbiol. 2020, 91, 103509. [Google Scholar]
- D’Amico, D.J.; Donelly, C.W. Growth and Survival of Microbial Pathogens in Cheese. In Cheese, 4th ed.; McSweeney, P.L.H., Fox, P.F., Cotter, P.D., Everett, D.W., Eds.; Academic Press: San Diego, CA, USA, 2017; Volume 1, pp. 573–594. ISBN 978-0-12-417012-4. [Google Scholar]
- Alvarez-Ordóñez, A.; Broussolle, V.; Colin, P.; Nguyen-The, C.; Prieto, M. The adaptive response of bacterial foodborne pathogens in the environment, host and food: Implications for food safety. Int. J. Food Microbiol. 2015, 213, 99–109. [Google Scholar] [CrossRef]
- Melo, J.; Andrew, P.W.; Faleiro, M.L. Listeria monocytogenes in cheese and the dairy environment remains a food safety challenge: The role of stress responses. Food Res. Int. 2015, 67, 75–90. [Google Scholar] [CrossRef]
- Ye, B.; He, S.; Zhou, X.; Cui, Y.; Zhou, M.; Shi, X. Response to Acid Adaptation in Salmonella enterica Serovar Enteritidis. J. Food Sci. 2019, 84, 599–605. [Google Scholar] [CrossRef]
- Bellio, A.; Bianchi, D.M.; Vitale, N.; Vernetti, L.; Gallina, S.; Decastelli, L. Behavior of Escherichia coli O157:H7 during the manufacture and ripening of Fontina Protected Designation of Origin cheese. J. Dairy Sci. 2018, 101, 4962–4970. [Google Scholar] [CrossRef]
- Dalzini, E.; Cosciani-Cunico, E.; Monastero, P.; Bernini, V.; Neviani, E.; Bellio, A.; Decastelli, L.; Losio, M.N.; Daminelli, P.; Varisco, G. Listeria monocytogenes in Gorgonzola cheese: Study of the behaviour throughout the process and growth prediction during shelf life. Int. J. Food Microbiol. 2017, 262, 71–79. [Google Scholar] [CrossRef]
- Peng, S.; Hoffmann, W.; Bockelmann, W.; Hummerjohann, J.; Stephan, R.; Hammer, P. Fate of Shiga toxin-producing and generic Escherichia coli during production and ripening of semihard raw milk cheese. J. Dairy Sci. 2013, 96, 815–823. [Google Scholar] [CrossRef]
- D’Amico, D.J.; Druart, M.J.; Donnelly, C.W. Behavior of Escherichia coli O157:H7 during the manufacture and aging of Gouda and stirred-curd cheddar cheeses manufactured from raw milk. J. Food Prot. 2010, 73, 2217–2224. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, D.J.; Druart, M.J.; Donnelly, C.W. Comparing the Behavior of Multidrug-Resistant and Pansusceptible Salmonella during the Production and Aging of a Gouda Cheese Manufactured from Raw Milk. J. Food Prot. 2014, 77, 903–913. [Google Scholar] [CrossRef] [PubMed]
- Chatelard-Chauvin, C.; Pelissier, F.; Hulin, S.; Montel, M.C. 2015. Behaviour of Listeria monocytogenes in raw milk Cantal type cheeses during cheese making, ripening and storage in different packaging conditions. Food Control 2015, 54, 53–65. [Google Scholar] [CrossRef]
- Consorzio per la Tutela del Formaggio Pecorino Romano. Specification and Regulations. Available online: https://www.pecorinoromano.com/en/pecorino-romano/specification-and-regulations (accessed on 27 May 2020).
- Addis, M.; Fiori, M.; Riu, G.; Pes, M.; Salvatore, E.; Pirisi, A. Physico-chemical and nutritional characteristics of PDO Pecorino Romano cheese: Seasonal variation. Small Rumin. Res. 2015, 126, 73–79. [Google Scholar] [CrossRef]
- CLAL. Italy: Export Pecorino e Fiore Sardo. 2020. Available online: https://www.clal.it/?section=impexpistat&cod=04069063&mov=E (accessed on 16 June 2020).
- Meunier-Goddik, L.; Waite-Cusic, J. Consumers Acceptance of Raw Milk and its Products. In Raw Milk, 1st ed.; Nero, L.A., De Carvalho, A.F., Eds.; Academic Press: San Diego, CA, USA, 2019; pp. 311–350. ISBN 978-0-12-810530-6. [Google Scholar]
- Brooks, J.C.; Martinez, B.; Stratton, J.; Bianchini, A.; Krokstrom, R.; Hutkins, R. Survey of raw milk cheeses for microbiological quality and prevalence of foodborne pathogens. Food Microbiol. 2012, 31, 154–158. [Google Scholar] [CrossRef]
- ISO 5534:2004. Cheese and Processed Cheese—Determination of the Total Solids content; International Organization for Standardization: Geneva, Switzerland, 2004.
- ISO 5943:2006. Cheese and Processed Cheese products—Determination of Chloride Content—Potentiometric Titration Method; International Organization for Standardization: Geneva, Switzerland, 2006.
- ISO 11290-1:2017. Microbiology of the Food Chain—Horizontal Method for the Detection and Enumeration of Listeria Monocytogenes and of Listeria spp.—Part 1: Detection Method; International Organization for Standardization: Geneva, Switzerland, 2017.
- ISO 11290-2:2017. Microbiology of the Food Chain—Horizontal Method for the Detection and Enumeration of Listeria Monocytogenes and of Listeria spp.—Part 2: Enumeration Method; International Organization for Standardization: Geneva, Switzerland, 2017.
- ISO 6888-2:2004. Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Coagulase-Positive Staphylococci (Staphylococcus aureus and Other Species)—Technique Using Rabbit Plasma Fibrinogen Agar Medium; International Organization for Standardization: Geneva, Switzerland, 2004.
- ISO 16654:2001/A1:2017. Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Detection of Escherichia Coli O157—Amendment 1: Annex B: Result of Interlaboratory Studies; International Organization for Standardization: Geneva, Switzerland, 2017.
- ISO 6579-1:2017. Microbiology of the Food Chain—Horizontal Method for the Detection, Enumeration and Serotyping of Salmonella—Part 1: Detection of Salmonella Spp.; International Organization for Standardization: Geneva, Switzerland, 2017.
- Xiong, L.; Li, C.; Boeren, S.; Vervoort, J.; Hettinga, K. Effect of heat treatment on bacteriostatic activity and protein profile of bovine whey proteins. Food Res. Int. 2020, 127, 108688. [Google Scholar] [CrossRef]
- McSweeney, P.L.H.; Fox, P.F.; Ciocia, F. Metabolism of Residual Lactose and of Lactate and Citrate. In Cheese: Chemistry, Physics and Microbiology, 4th ed.; Mc Sweeney, P., Fox, P., Cotter, P., Everett, D., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 411–421. ISBN 978-0-12-417012-4. [Google Scholar]
- McSweeney, P.L.H. Biochemistry of cheese ripening: Introduction and overview. In Cheese: Chemistry, Physics and Microbiology, 4th ed.; McSweeney, P., Fox, P., Cotter, P., Everett, D., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 379–387. ISBN 978-0-12-417012-4. [Google Scholar]
- Addis, M.; Piredda, G.; Pes, M.; Di Salvo, R.; Scintu, M.F.; Pirisi, A. Effect of the use of three different lamb paste rennets on lipolysis of the PDO Pecorino Romano Cheese. Int. Dairy J. 2005, 15, 563–569. [Google Scholar] [CrossRef]
- Peng, S.; Tasara, T.; Hummerjohann, J.; Stephan, R. An overview of molecular stress response mechanisms in Escherichia coli contributing to survival of Shiga toxin-producing Escherichia coli during raw milk cheese production. J. Food Prot. 2011, 74, 849–864. [Google Scholar] [CrossRef]
- Modi, R.; Hirvi, Y.; Hill, A.; Griffiths, M.W. Effect of phage on survival of Salmonella Enteritidis during manufacture and storage of cheddar cheese made from raw and pasteurized milk. J. Food Protect. 2001, 64, 927–933. [Google Scholar] [CrossRef]
- Pexara, A.; Solomakos, N.; Sergelidis, D.; Govaris, A. Fate of enterotoxigenic Staphylococcus aureus and staphylococcal enterotoxins in Feta and Galotyri cheeses. J. Dairy Res. 2012, 79, 405–413. [Google Scholar] [CrossRef]
- Delbes, C.; Alomar, J.; Chougui, N.; Martin, J.F.; Montel, M.C. Staphylococcus aureus growth and enterotoxin production during the manufacture of uncooked, semihard cheese from cows’ raw milk. J. Food Prot. 2006, 69, 2161–2167. [Google Scholar] [CrossRef] [PubMed]
- Farrokh, C.; Jordan, K.; Auvray, F.; Glass, K.; Oppegaard, H.; Raynaud, S.; Thevenot, D.; Condron, R.; De Reu, K.; Govaris, A.; et al. Review of Shiga-toxin-producing Escherichia coli (STEC) and their significance in dairy production. Int. J. Food Microbiol. 2013, 162, 190–212. [Google Scholar] [CrossRef]
- Calicioglu, M.; Dikici, A. Survival and Acid Adaptation Ability of Salmonella during Processing and Ripening of Savak Tulumi Cheese. J. Anim. Vet. Adv. 2009, 8, 1124–1130. [Google Scholar]
- Papadopoulou, C.; Maipa, V.; Dimitriou, D.; Pappas, C.; Voutsinas, L.; Malatou, H. Behavior of Salmonella enteritidis During the Manufacture, Ripening, and Storage of Feta Cheese Made from Unpasteurized Ewe’s Milk. J. Food Prot. 1993, 56, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Wusimanjiang, P.; Ozturk, M.; Ayhan, Z.; Mehmetoglu, A.Ç. Effect of salt concentration on acid- and salt-adapted Escherichia coli O157:H7 and Listeria monocytogenes in recombined nonfat cast cheese. J. Food Process Pres. 2019, 43, e14208. [Google Scholar] [CrossRef]
- Consolidated Text: Commission Regulation (EC) No 2073/2005 on Microbiological Criteria for Food Stuffs. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A02005R2073-20200308 (accessed on 30 October 2020).
- Al-Nabulsi, A.A.; Osaili, T.M.; AbuNaser, R.A.; Olaimat, A.N.; Ayyash, M.; Al-Holy, M.A.; Kadora, K.M.; Holley, R.A. Factors affecting the viability of Staphylococcus aureus and production of enterotoxin during processing and storage of white-brined cheese. J. Dairy Sci. 2020, 103, 6869–6881. [Google Scholar] [CrossRef]
- Mohammadi, K.; Hanifian, S. Growth and enterotoxin production of Staphylococcus aureus in Iranian ultra-filtered white cheese. Int. J. Dairy Technol. 2015, 68, 111–117. [Google Scholar] [CrossRef]
- Valero, A.; Pérez-Rodríguez, F.; Carrasco, E.; Fuentes-Alventosa, J.M.; García-Gimeno, R.; Zurera, G. Modelling the growth boundaries of Staphylococcus aureus: Effect of temperature, pH and water activity. Int. J. Food Microbiol. 2009, 133, 186–194. [Google Scholar] [CrossRef]
- Ahmed, A.A.-H.; Maharik, N.M.S.; Valero, A.; Kamal, S.M. Incidence of enterotoxigenic Staphylococcus aureus in milk and Egyptian artisanal dairy products. Food Control 2019, 104, 20–27. [Google Scholar] [CrossRef]
- Viçosa, G.N.; Botelho, C.V.; Botta, C.; Bertolino, M.; de Carvalho, A.F.; Nero, L.A.; Cocolin, L. Impact of co-cultivation with Enterococcus faecalis over growth, enterotoxin production and gene expression of Staphylococcus aureus in broth and fresh cheeses. Int. J. Food Microbiol. 2019, 308, 108291. [Google Scholar] [CrossRef]
- Rukke, E.O.; Sørhaug, T.; Stepaniak, L. Heat Treatment of Milk|Thermization of Milk. In Encyclopedia of Dairy Sciences, 2nd ed.; Fuquay, J.W., Ed.; Academic Press: San Diego, CA, USA, 2011; pp. 693–698. ISBN 978-0-12-374407-4. [Google Scholar]
- Gabriel, A.A.; Bayaga, C.L.T.; Magallanes, E.A.; Aba, R.P.M.; Tanguilig, K.M.N. Fates of pathogenic bacteria in time-temperature-abused and Holder-pasteurized human donor-, infant formula-, and full cream cow’s milk. Food Microbiol. 2020, 89, 103450. [Google Scholar] [CrossRef] [PubMed]
- Pearce, L.E.; Smythe, B.W.; Crawford, R.A.; Oakley, E.; Hathaway, S.C.; Shepherd, J.M. Pasteurization of milk: The heat inactivation kinetics of milk-borne dairy pathogens under commercial-type conditions of turbulent flow. J. Dairy Sci. 2012, 95, 20–35. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).