Synthesis and Properties of Cobalt/Nickel-Iron-Antimony(III, V)-Oxo Tartrate Cluster-Based Compounds
Abstract
:1. Introduction
2. Results and Discussion
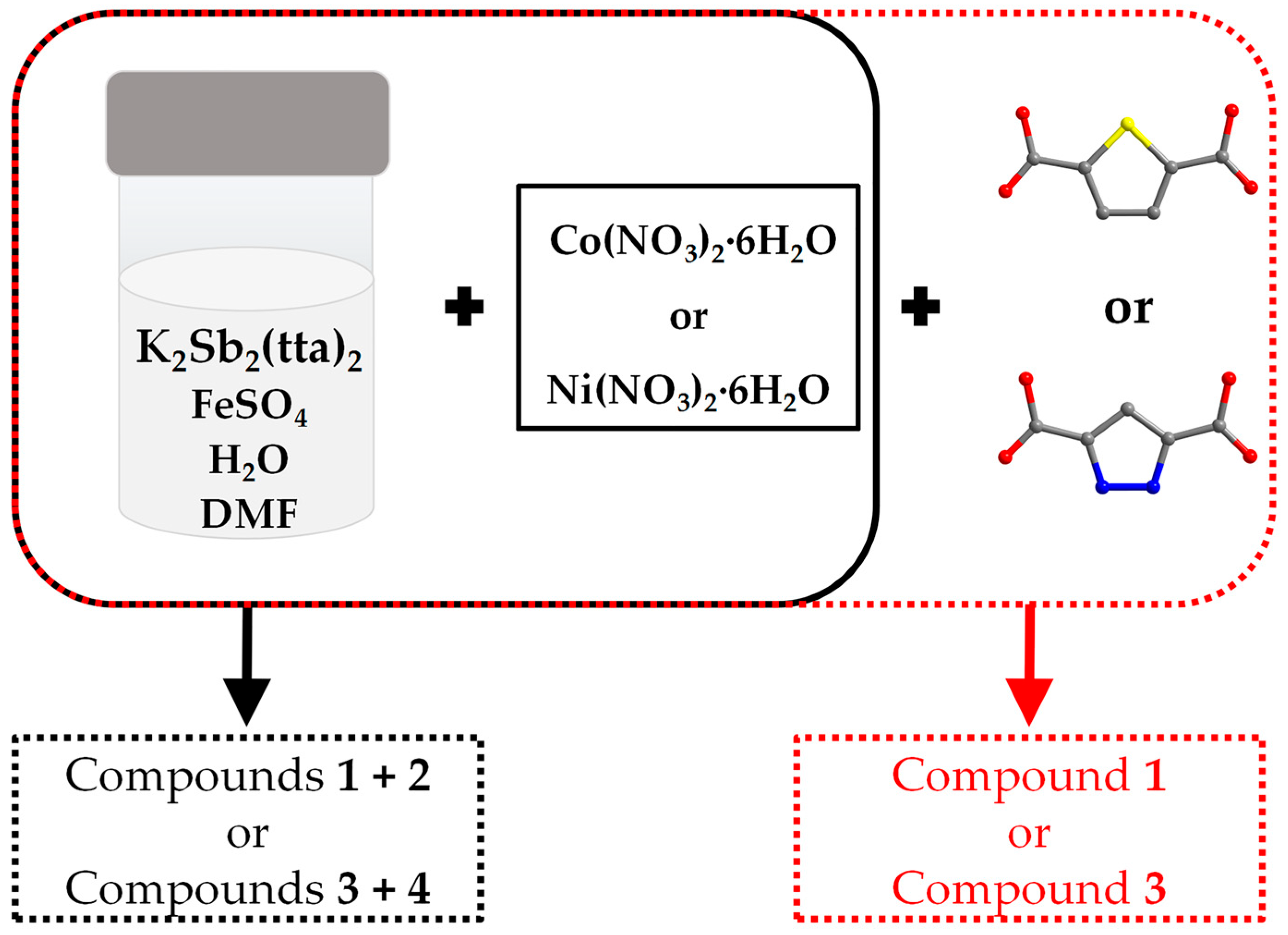
2.1. Discussion of Synthesis Methods
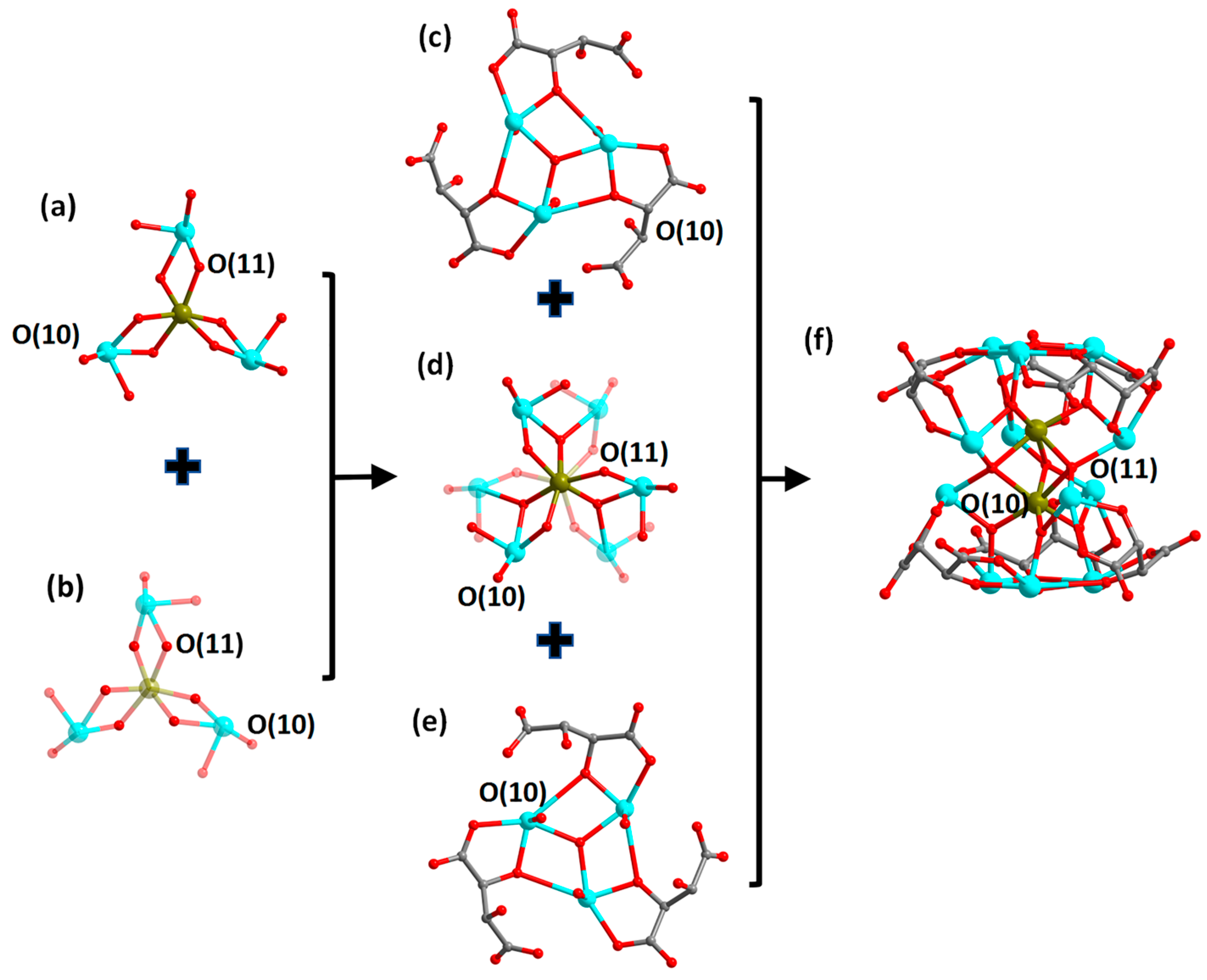
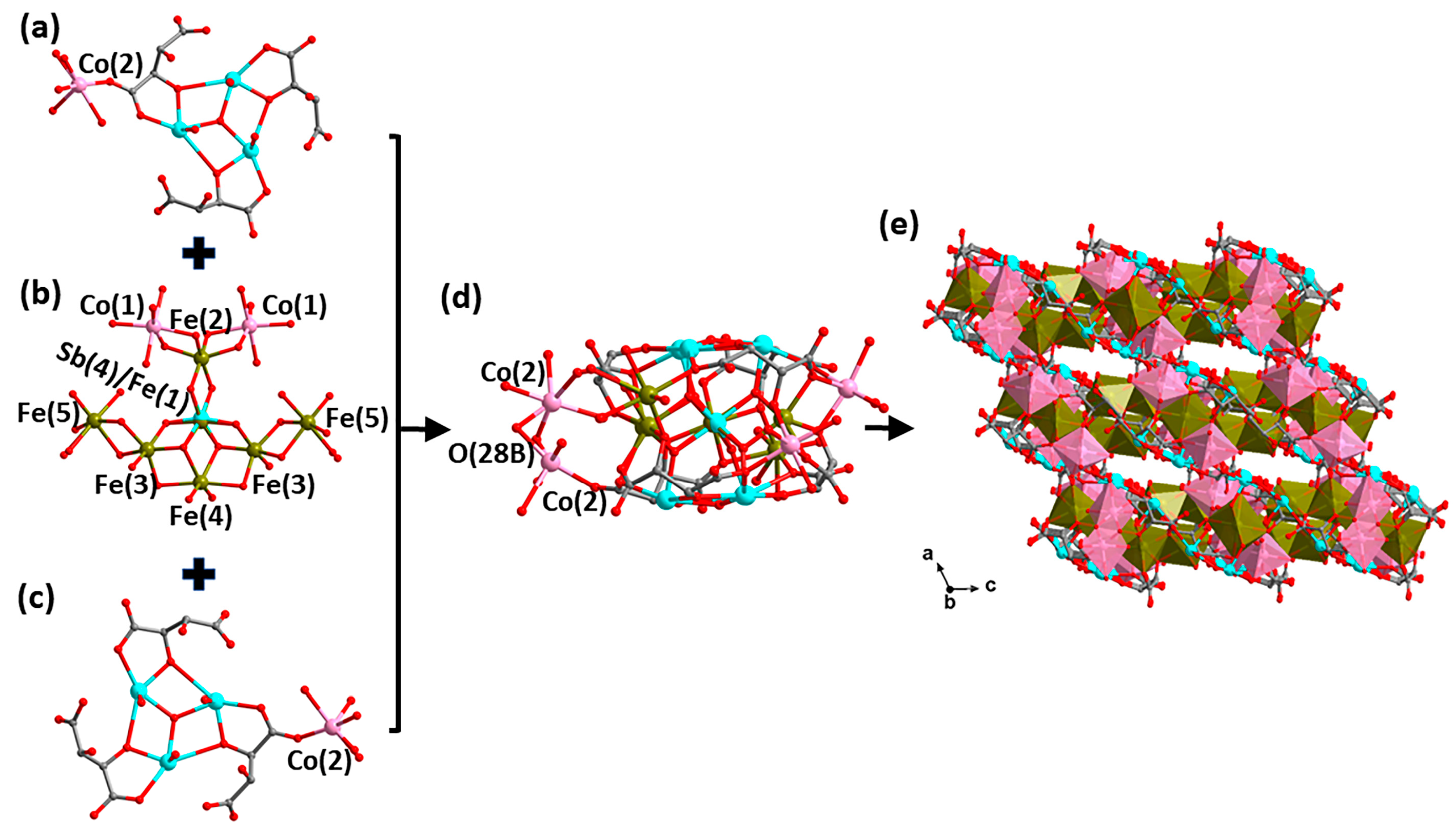
2.2. Description of the Structures
2.3. Thermal Stability and UV-Vis Analysis
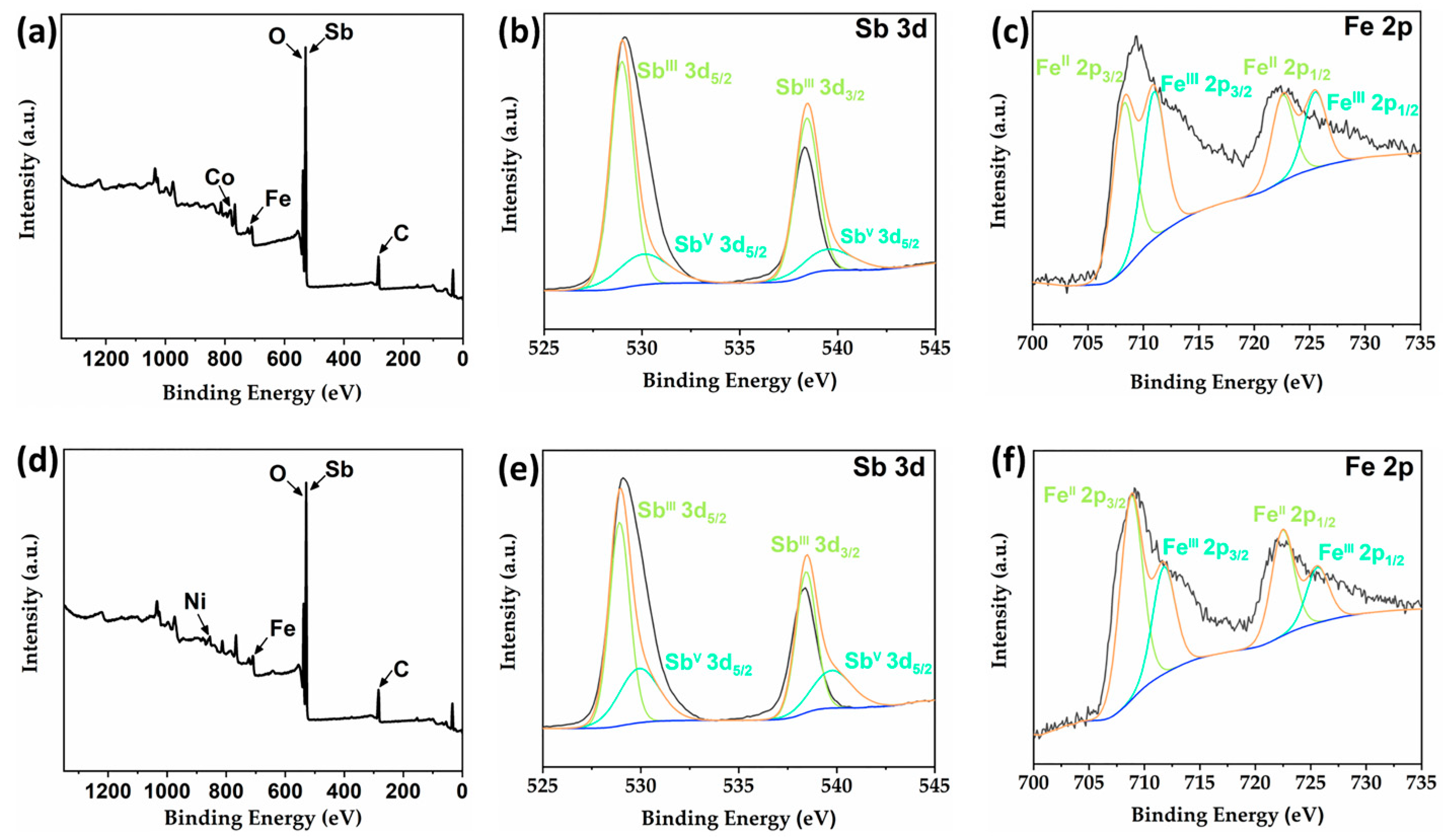
2.4. X-ray Photoelectron Spectroscopy (XPS) Analysis of 2 and 4
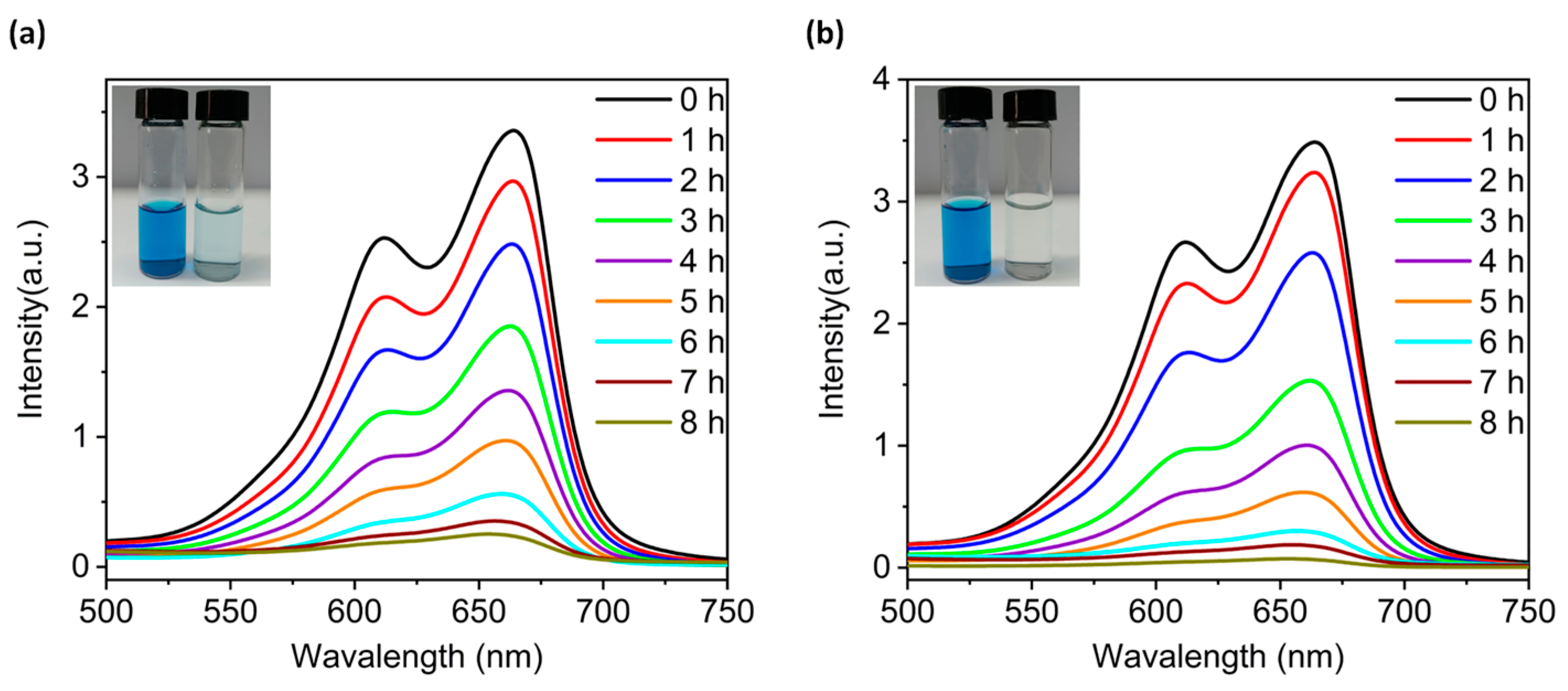
2.5. Photodegradation Performance of Compounds 2 and 4
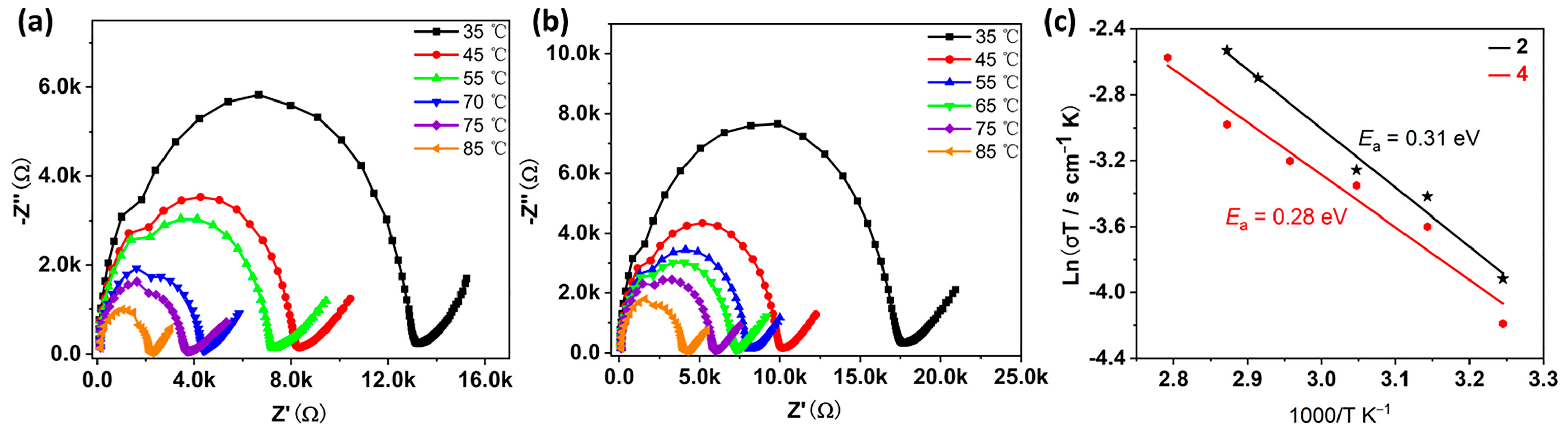
2.6. Proton Conduction of Compounds 2 and 4
2.7. Magnetic Measurements of Compounds 2 and 4
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wei, Q.; He, C.; Wang, K.; Duan, X.-F.; An, X.-T.; Li, J.-H.; Wang, G.-M. Sb6O7(SO4)2: A Promising Ultraviolet Nonlinear Optical Material with an Enhanced Second-Harmonic-Generation Response Activated by SbIII Lone-Pair Stereoactivity. Chem. Eur. J. 2021, 27, 5880–5884. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Wang, K.; He, C.; Wei, L.; Li, X.-F.; Zhang, S.; An, X.-T.; Li, J.-H.; Wang, G.-M. Linear and Nonlinear Optical Properties of Centrosymmetric Sb4O5SO4 and Noncentrosymmetric Sb4O4(SO4)(OH)2 Induced by Lone Pair Stereoactivity. Inorg. Chem. 2021, 60, 11648–11654. [Google Scholar] [CrossRef] [PubMed]
- Li, X.F.; Wang, K.; He, C.; Li, J.H.; An, X.T.; Pan, J.; Wei, Q.; Wang, G.M.; Yang, G.Y. Sb4O3(TeO3)2(HSO4)(OH): An Antimony Tellurite Sulfate Exhibiting Large Optical Anisotropy Activated by Lone Pair Stereoactivity. Inorg. Chem. 2023, 62, 7123–7129. [Google Scholar] [CrossRef] [PubMed]
- Yi, G.; Zou, G. Recent advances on the synthesis of Sb(III)-based inorganic ultraviolet nonlinear optical materials. Chin. J. Struct. Chem. 2023, 42, 100020. [Google Scholar] [CrossRef]
- Yin, J.; Fei, H. Cationic two-dimensional inorganic networks of antimony oxide hydroxide for Lewis acid catalysis. Dalton Trans. 2018, 47, 4054–4058. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Hyeon-Deuk, K.; Yamamoto, T.; Yabuuchi, M.; Karakulina, O.M.; Noda, Y.; Kurihara, T.; Chang, I.Y.; Higashi, M.; Tomita, O.; et al. Polyoxocationic antimony oxide cluster with acidic protons. Sci. Adv. 2022, 8, eabm5379. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Wen, W.-Y.; Sun, H.-Y.; Wang, Y.-Q.; Du, K.-Z.; Ma, W.; Zou, G.-D.; Wu, Z.-F.; Huang, X.-Y. Single-crystal superstructures via hierarchical assemblies of giant rubik’s cubes as tertiary building units. Angew. Chem. Int. Ed. 2023, 135, e202219025. [Google Scholar] [CrossRef]
- Wen, W.-Y.; Hu, B.; Pan, T.-Y.; Li, Z.-W.; Hu, Q.-Q.; Huang, X.-Y. Structural Evolution and Properties of Praseodymium Antimony Oxochlorides Based on a Chain-like Tertiary Building Unit. Molecules 2023, 28, 2725. [Google Scholar] [CrossRef]
- Wu, Z.-F.; Hu, B.; Fu, Z.-H.; Wang, H.; Xu, G.; Gong, L.-K.; Zou, G.-D.; Huang, X.-Y.; Li, J. Ba13Sb36Cl34O548−: High-nuclearity cluster for the assembly of nanocluster-based compounds. Chem. Commun. 2019, 55, 7442–7445. [Google Scholar] [CrossRef]
- Baskar, V.; Shanmugam, M.; Helliwell, M.; Teat, S.J.; Winpenny, R.E.P. Reverse-keggin ions: Polycondensation of antimonate ligands give inorganic cryptand. J. Am. Chem. Soc. 2007, 129, 3042–3043. [Google Scholar] [CrossRef]
- Nicholson, B.K.; Clark, C.J.; Telfer, S.G.; Groutso, T. Isopolyoxometalates derived from arylstibonic acids with “reverse-Keggin ion” structures based on [M(RSb)12O28] cores, M = Co(II) or Zn(II). Dalton Trans. 2012, 41, 9964–9970. [Google Scholar] [CrossRef] [PubMed]
- Sharutin, V.V.; Sharutina, O.K.; Rybakova, A.V.; Andreev, P.V. Synthesis and Structure of Oxygen-Containing Antimony Complexes (Ar2SbO)4(O2)2. Russ. J. Gen. Chem. 2019, 89, 1637–1641. [Google Scholar] [CrossRef]
- Gao, Q.; Wang, X.; Conato, M.T.; Makarenko, T.; Jacobson, A.J. Microporous, Homochiral Structures Containing Iron Oxo-Clusters Supported by Antimony(III) Tartrate Scaffolds. Cryst. Growth Des. 2011, 11, 4632–4638. [Google Scholar] [CrossRef]
- Meng, W.; Xu, F.; Xu, W. An Anionic Heptacopper(II) Oxo-Cluster {Cu7(II)} with an S=7/2 Ground State. Inorg. Chem. 2016, 55, 540–542. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Hu, B.; Li, J.-L.; Zhang, Z.-Z.; Zeng, X.; Jin, J.-C.; Li, Z.; Zheng, S.-T.; Feng, M.-L.; Huang, X.-Y. The Uptake of Hazardous Metal Ions into a High-Nuclearity Cluster-Based Compound with Structural Transformation and Proton Conduction. ACS Appl. Mater. Interfaces 2020, 12, 26222–26231. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Wang, X.; Tapp, J.; Moeller, A.; Jacobson, A.J. Antimony Tartrate Transition-Metal-Oxo Chiral Clusters. Inorg. Chem. 2013, 52, 6610–6616. [Google Scholar] [CrossRef]
- Gao, Q.; Wang, X.; Jacobson, A.J. A homochiral diamond framework constructed from Fe(III) and Mn(II) oxo-clusters supported by Sb(III) tartrate scaffolds. Chem. Commun. 2012, 48, 3990–3992. [Google Scholar] [CrossRef]
- Meng, W.; Qin, Y.; Hou, Q.; He, W.; Li, J.; Xu, F. Dinuclear cage-core Co2/Ni2 oxo-clusters supported by Sb(III) tartrate scaffolds: Synthesis, structure and magnetic properties. Polyhedron 2018, 153, 76–81. [Google Scholar] [CrossRef]
- Ma, W.; Hu, B.; Jing, K.-Q.; Li, Z.; Jin, J.-C.; Zheng, S.-T.; Huang, X.-Y. Proton-conducting layered structures based on transition metal oxo-clusters supported by Sb(III) tartrate scaffolds. Dalton Trans. 2020, 49, 3849–3855. [Google Scholar] [CrossRef]
- Wang, Y.-M.; Peng, Z.-W.; Liao, J.-M.; Li, A.; Liu, Y.-Y.; Zhang, J.-J.; Zhou, N.; Li, X.-D.; Li, S.; Meng, W. A New Heterometallic 3d-3d Transition Metal Oxo-cluster {CuII6MnIII}: Synthesis, Crystal Structure and Magnetic Property. Chin. J. Struct. Chem. 2021, 40, 1661–1667. [Google Scholar]
- Wen, W.-Y.; Ma, W.; Hu, B.; Xiao, H.-P.; Pan, T.-Y.; Liu, J.-T.; Lin, H.-W.; Li, X.-X.; Huang, X.-Y. Mixed-valence compounds based on heterometal-oxo-clusters containing Sb(III, V): Crystal structures and proton conduction. Dalton Trans. 2024, 53, 1156–1162. [Google Scholar] [CrossRef] [PubMed]
- Brown, I.D.; Altermatt, D. Bond-Valence Parameters Obtained From a Systematic Analysis of The Inorganic Crystal-Structure Database. Acta Crystallogr. Sect. B-Struct. Sci. 1985, 41, 244–247. [Google Scholar] [CrossRef]
- Birchall, T.; Connor, J.A.; Hillier, I.H. High-energy Photoelectron-Spectroscopy of Some Antimony Compounds. J. Chem. Soc. Dalton Trans. 1975, 2003–2006. [Google Scholar] [CrossRef]
- Mosallanejad, S.; Dlugogorski, B.Z.; Kennedy, E.M.; Stockenhuber, M. Adsorption of 2-Chlorophenol on the Surface of Silica- and Alumina-Supported Iron Oxide: An FTIR and XPS Study. ChemCatChem 2017, 9, 481–491. [Google Scholar] [CrossRef]
- El Messaoudi, N.; El Khomri, M.; El Mouden, A.; Bouich, A.; Jada, A.; Lacherai, A.; Iqbal, H.M.N.; Mulla, S.I.; Kumar, V.; Américo-Pinheiro, J.H.P. Regeneration and reusability of non-conventional low-cost adsorbents to remove dyes from wastewaters in multiple consecutive adsorption-desorption cycles: A review. Biomass Convers. Biorefin. 2022. [Google Scholar] [CrossRef]
- Hamad, H.N.; Idrus, S. Recent Developments in the Application of Bio-Waste-Derived Adsorbents for the Removal of Methylene Blue from Wastewater: A Review. Polymers 2022, 14, 783. [Google Scholar] [CrossRef] [PubMed]
- Ungureanu, E.L.; Mocanu, A.L.; Stroe, C.A.; Panciu, C.M.; Berca, L.; Sionel, R.M.; Mustatea, G. Agricultural Byproducts Used as Low-Cost Adsorbents for Removal of Potentially Toxic Elements from Wastewater: A Comprehensive Review. Sustainability 2023, 15, 5999. [Google Scholar] [CrossRef]
- Chintakrinda, K.; Narayanam, N.; Chen, G.-H.; Zhang, J.; Zhang, L. Ionothermal Synthesis and Photoactivity of Ti17 and Ti19-Oxo Clusters Functionalized by Sulfate and 1,10-Phenanthroline Ligands. Chin. J. Chem. 2023, 41, 3605–3610. [Google Scholar] [CrossRef]
- Wu, G.-Q.; Wu, K.; Wang, Y.; Luo, J.; Zhou, C.-Y.; Lu, W. Highly stable Ni8-pyrazolate metal-organic frameworks for adsorption of methylene blue from water. New J. Chem. 2023, 47, 16189–16196. [Google Scholar] [CrossRef]
- Ramaswamy, P.; Wong, N.E.; Shimizu, G.K.H. MOFs as proton conductors-challenges and opportunities. Chem. Soc. Rev. 2014, 43, 5913–5932. [Google Scholar] [CrossRef]
- Li, S.-R.; Wang, H.-Y.; Su, H.-F.; Chen, H.-J.; Du, M.-H.; Long, L.-S.; Kong, X.-J.; Zheng, L.-S. A Giant 3d-4f Polyoxometalate Super-Tetrahedron with High Proton Conductivity. Small Methods 2021, 5, 2000777. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.-P.; Zhang, R.-T.; Li, Z.; Xie, Y.-F.; Wang, M.; Ye, Y.-D.; Sun, C.; Sun, Y.-Q.; Li, X.-X.; Zheng, S.-T. Organoamine-Directed Assembly of 5p-4f Heterometallic Cluster Substituted Polyoxometalates: Luminescence and Proton Conduction Properties. Inorg. Chem. 2021, 60, 13718–13726. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with Shelxl. Acta Crystallogr. Sect. C-Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, W.; Wang, Y.; Pan, T.; Hu, Q.; Xiao, H.; Wang, N.; Li, X.; Li, X.; Hu, B.; Huang, X. Synthesis and Properties of Cobalt/Nickel-Iron-Antimony(III, V)-Oxo Tartrate Cluster-Based Compounds. Molecules 2024, 29, 591. https://doi.org/10.3390/molecules29030591
Wen W, Wang Y, Pan T, Hu Q, Xiao H, Wang N, Li X, Li X, Hu B, Huang X. Synthesis and Properties of Cobalt/Nickel-Iron-Antimony(III, V)-Oxo Tartrate Cluster-Based Compounds. Molecules. 2024; 29(3):591. https://doi.org/10.3390/molecules29030591
Chicago/Turabian StyleWen, Weiyang, Yanqi Wang, Tianyu Pan, Qianqian Hu, Huiping Xiao, Nannan Wang, Xiaoqi Li, Xinxiong Li, Bing Hu, and Xiaoying Huang. 2024. "Synthesis and Properties of Cobalt/Nickel-Iron-Antimony(III, V)-Oxo Tartrate Cluster-Based Compounds" Molecules 29, no. 3: 591. https://doi.org/10.3390/molecules29030591
APA StyleWen, W., Wang, Y., Pan, T., Hu, Q., Xiao, H., Wang, N., Li, X., Li, X., Hu, B., & Huang, X. (2024). Synthesis and Properties of Cobalt/Nickel-Iron-Antimony(III, V)-Oxo Tartrate Cluster-Based Compounds. Molecules, 29(3), 591. https://doi.org/10.3390/molecules29030591





