Abstract
Purpose: This in vitro study aimed to evaluate and compare the physical and antimicrobial properties of provisional resin modified with two different nanoparticles, namely, silicon dioxide (nano-SiO2) and titanium dioxide (nano-TiO2). Methods: A commonly used commercially available polymethyl methacrylate (PMMA) provisional resin (Unifast III; GC Corp) was modified with nano-SiO2 and nano-TiO2 at different concentrations (1% wt. and 2.5% wt. respectively), while one unmodified group was used as a control. Rectangular specimens (60 × 10 × 3.3 mm) for strength (MPa) and elastic modulus, and square specimens (10 × 10 × 3.4 mm) for surface roughness (Ra, µm), hardness (VHN), and Candida albicans adhesion (colony forming unit, CFU/mL) were prepared and grouped into five groups (n = 10) according to (nanoparticles) NPs type and concentration. After polymerization, the specimens were finished and polished and then subjected to thermal cycling (5000 cycles). Analysis of variance and post-hoc Tukey test were used for data analysis (α = 0.05). The scanning electron microscope (SEM) was used for fracture surface analysis and C. albicans count. Results: The addition of 1% nano-SiO2 significantly increased the flexural strength, and 1% nano-SiO2 contributed to the highest flexural strength value, while 2.5% nano-SiO2 and nano-TiO2 showed non-significant increases (p > 0.05). The elastic modulus increased significantly for both NPs. Among the NP-modified groups, the nano-SiO2 groups showed an increased elastic modulus compared to the nano-TiO2 groups. The hardness significantly increased with NPs addition with no significant differences between NPs-modified groups. Surface roughness increased with 2.5% nano-TiO2 addition, while 1% nano-TiO2 and nano-SiO2 showed non-significant differences. Nano-SiO2 and nano-TiO2 significantly decreased C. albicans adhesion, and nano-TiO2 groups were significantly superior in their antimicrobial effect compared with nano-SiO2. Conclusions: Low nano-SiO2 addition increased the flexural strength of provisional resin. The addition of NPs increased elastic modulus and hardness and decreased the C. albicans adhesion to provisional resin. Nano-SiO2 did not alter the surface roughness, while 2.5% of nano-TiO2 increased the surface roughness.
1. Introduction
Provisional crown and fixed partial denture (FPD) restorations play an important role during prosthodontic treatment. From its derived name, a provisional restoration should provide multiple purposes, including function [mastication, esthetics, and phonetics], protection [dental and periodontal], occlusal stability, and diagnostic assessment prior to fabrication of the final definitive restoration [1]. In addition, it prevents leakage into dentinal tubules by protecting the prepared dental tissue until permanent prostheses are placed [2].
Nevertheless, most provisional materials, regardless of chemistry, are prone to sorption, which results in changes in color when they are exposed to or immersed in staining media. Color stability of the provisional restoration can be affected by a variety of factors, such as material composition, polymerization method, degree of polymerization, surface properties, water sorption, and solubility, reactivity with surrounding media, chemical reactivity with different staining from dietary intake, and oral hygiene [3]. According to Barghi and Simmons’ qualitative assessment, auto-polymerizing acrylic resin provisional restorations frequently manifest deficiency in marginal adaptation [4]. In another study by Lepe et al., they found that polymerization shrinkage of the acrylic resin would have a significant influence on the fit of the provisional restorations; they reported volumetric polymerization shrinkage for PMMA is 6%, in contrast to 1% to 2% for composite materials [5].
Another important factor is susceptibility to microbial adhesion. Provisional restorations that are worn for extended periods of time allow bacterial colonization on their surfaces. This reveals that bacterial colonization on provisional prosthetic materials is greater than that on permanent prosthetic materials due to their high surface roughness and limited marginal adaptation [6]. On the other hand, the amount and quality of bacterial deposition on surfaces can be affected by a variety of surface properties. Surface roughness increases microbial adherence, which is difficult to remove from pits and grooves [7]. The species of bacteria is another important aspect of bacterial adhesion [8]. Streptococci are one of the most common “early colonizing bacteria” and are known as the primary pathogenesis of tooth caries. Candida albicans (C. albicans), is the most common opportunistic pathogen intra-orally [9].
Nanoparticles are tiny particles ranging in size from 1 to 100 nm. They have been introduced into different dental materials to enhance their mechanical properties and for reinforcement [10]. Efforts have been made to fulfill these objectives by adding structural components such as metals and ceramics, as well as filler particles containing zirconia, silica, alumina, glass fiber, tin, and copper [11]. It is important to realize that the type, size, shape, and concentration of inorganic nanoparticles determine how they alter the properties of the end product [11].
Silicon dioxide nanoparticles (nano-SiO2) have gained noticeable attention from researchers due to their promising performance. Silica nanoparticles are considered a valid additive for the reinforcement of dental resins [12]. From a biomedical perspective, silica nanoparticles have been implemented as reinforcement fillers for PMMA resins due to their high surface energy and total pore volume, which also promotes their antimicrobial behavior [13].
Titanium dioxide nanoparticles (nano-TiO2) have desirable properties. They are chemically inert, resistant to corrosion, biocompatible, and inexpensive [14]. They have a high refractive index, which aids in improving their optical properties in addition to their antibacterial properties, as reported in the literature [15]. Due to their favorable behavior, nano-TiO2 have been incorporated into PMMA in the dental field of research [16]. Nano-TiO2 primarily offer heat stability. As a result, the desired properties of NPs are not affected by the heat produced during the polymerization process, which exerts a positive impact on polymerization shrinkage [17]. Existing studies suggest that introducing surface-treated nano-TiO2 into a PMMA matrix leads to reduced shrinkage stress [18]. According to Acosta-Torres et al., a PMMA matrix containing TiO2 showed less surface porosity in comparison to unmodified PMMA [19]. On the other hand, other studies have presented adverse effects, especially on flexural strength, associated with nano-TiO2 and nano-SiO2 incorporated into dental resins [20].
Provisional restorations may require long-term presence in the oral cavity. For durability, provisional materials with high strength to withstand different types of stresses while also demonstrating appropriate surface characteristics with antimicrobial activities are required. The incorporation of nanoparticles was suggested, and it has the potential to overcome the drawbacks of unmodified resins. However, there is a lack of research that investigates the effect of different nanoparticles, such as nano-SiO2 and nano-TiO2, on the strength and antifungal activity of provisional resins. Therefore, this study was conducted to evaluate and compare the strength, surface properties, and C. albicans adherence to provisional resins modified with different nanoparticles, nano-SiO2, and nano-TiO2. The null hypothesis stated that the addition of nanoparticles had no significant effect on the strength, surface roughness, hardness, and C. albicans adhesion to nanocomposite provisional resins.
2. Materials and Methods
2.1. Sample Size Calculation
After sample size calculation with 80% power, 95% confidence interval, and 0.05 significance level, fifty bar shape specimens (60 × 10 × 3.3 mm) were prepared for flexural strength, and 100 square specimens (10 × 10 × 2 mm) were prepared for evaluating surface properties and C. albicans adhesion.
2.2. Specimens Fabrication and Distribution
2.2.1. Nanocomposite Mixture Preparations
The materials used and specifications are detailed in Table 1. Before nanocomposite resin mixture preparation, both nanopowders were treated with a silane coupling agent to create reactive groups that allowed good bonding between the resin matrix and nanoparticle surface. For the silanization process, nano-SiO2 and nano-TiO2 were treated with 3-(trimethoxysilyl) propyl methacrylate (Shanghai Richem International Co., Ltd., Shanghai, China). Concentrations of 1 and 2.5 wt% of silanized nanoparticles were measured using a digital scale and mixed with the auto-polymerizing acrylic resin powder (Unifast III; GC Corp., Yongin-si, Repubic of Korea). In order to achieve homogeneous mixtures with even particle distributions, the prepared mixtures were further mixed and stirred for 30 min using an electric mixer.

Table 1.
Nanomaterials used and their specifications.
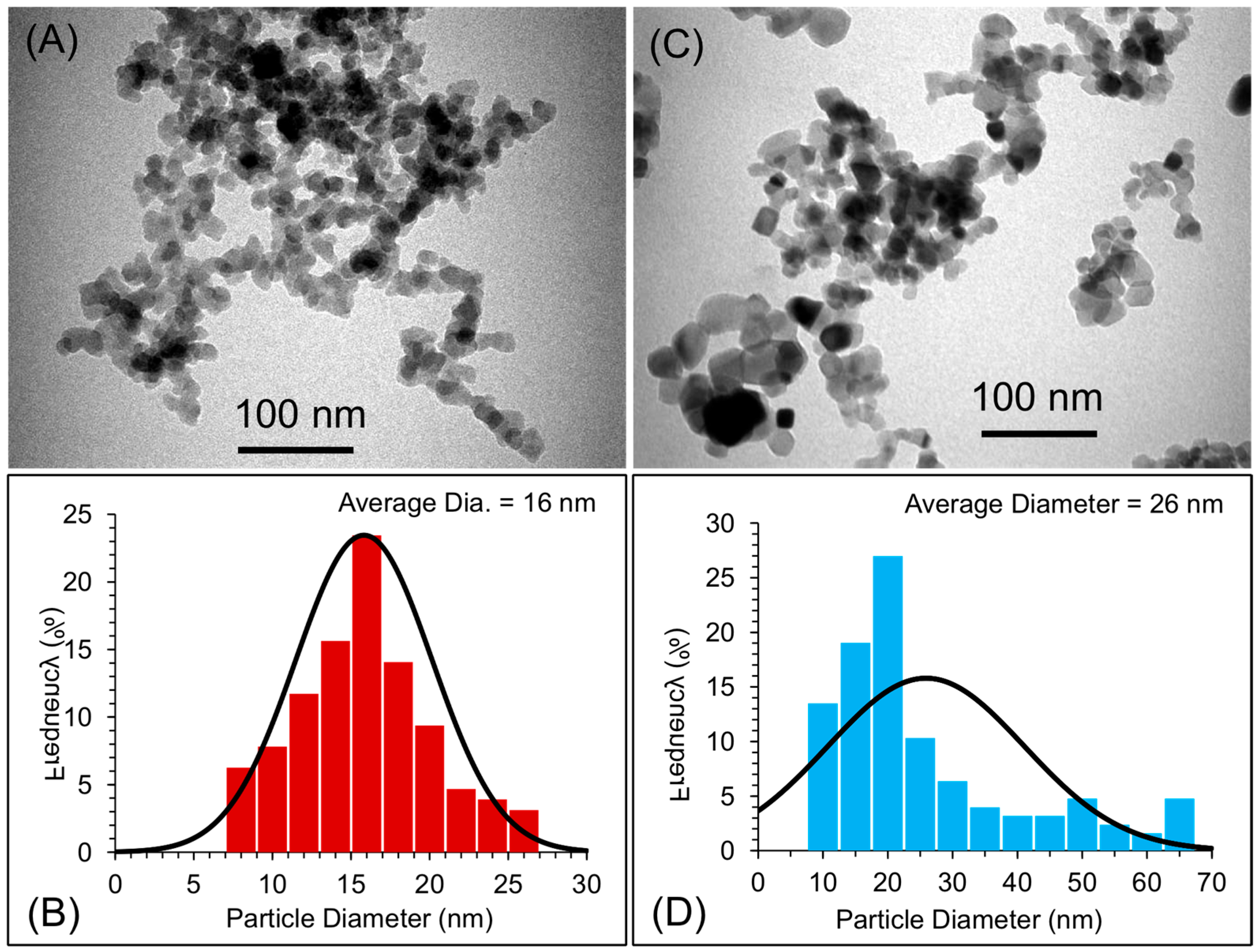
The particle size distributions of the two types of nanoparticles (nano-SiO2 and nano-TiO2) were evaluated using transmission electron microscopy (TEM; Morgagni 268, FEI, Czech Republic at 80 kV). For TEM, a small amount of the powder of the two types of nanoparticles was dispersed into ethanol using an ultrasound sonicator for about ten minutes. The dispersion of the nanoparticles was then deposited onto TEM grids with a carbon film. The grids were air-dried and mounted on a TEM machine for imaging. Several images were recorded to estimate the size of the nanoparticles [21,22,23]. The average size of the particles was measured to be about 16 and 26 nm for nano-SiO2 and nano-TiO2, respectively (Figure 1). The mixing of the nanoparticles into the PMMA matrix was confirmed and evaluated by scanning electron microscopy (SEM, TESCAN, VEGA3, Czech Republic at 20 kV). For SEM, powders of pure PMMA, PMMA coated with nano-SiO2, and PMMA coated with nano-TiO2 were dispersed onto a doubled-sided carbon film, and then samples were sputter-coated with a gold sputter coating machine (Q150R Plus—Rotary Pumped Coater) in order to make the samples suitable for SEM imaging. For comparison, SEM micrographs of the pure PMMA matrix are also shown in Figure 2. After mixing, the PMMA spheres were coated with either nano-SiO2 or nano-TiO2.
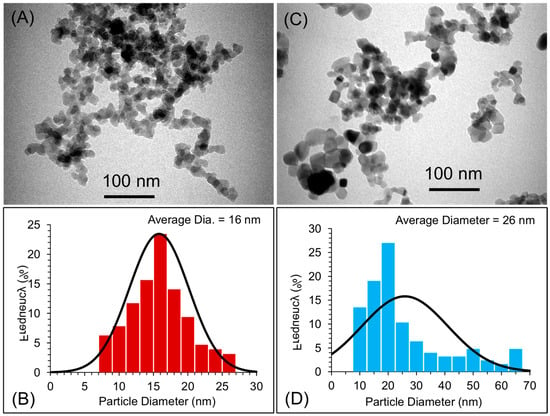
Figure 1.
(A,C) TEM images of nano-SiO2 and nano-TiO2 while (B,D) histogram image showing the particle distribution and average size of nano-SiO2 and nano-TiO2, respectively.

Figure 2.
SEM micrographs of (A) Pure PMMA matrix, (B) PMMA coated with nano-SiO2, and (C) PMMA coated with nano-TiO2. The pure PMMA spheres showed clear morphology without the appearance of any type of nanoparticles. The scale bars are 100 µm.
2.2.2. Mold Used for Disc Specimens Preparations
As per the recommendation of the International Organization for Standardization (ISO) 20795-1:2008.40, a split metal mold with dimensions of 60 × 10 × 3.3 mm was fabricated to produce 50 rectangular specimens for flexural strength tests [24,25]. For surface properties (surface roughness (Ra, µm), hardness (VHN)), and Candida albicans adhesion (CFU), square specimens (10 × 10 × 3.4 mm) were prepared and grouped into five groups (n = 10). The 50 specimens were divided into 5 groups (n = 10) based on nanoparticle concentration (1 and 2.5 wt%) of nano-SiO2 and nano-TiO2, and an unmodified control group was obtained.
2.2.3. Specimens’ Preparations
The polymer/monomer ratio was prepared according to the manufacturer’s recommendation. The mix was placed into molds with some overfill and then covered with a metal plate under pressure. The molds were covered with metal and placed in a pressure pot at 45 °C and 0.2 MPa for 15 min. After polymerization, any resin flashes were removed using a tungsten carbide bur and polished using a sandpaper disc. A digital caliper was used to evaluate the specimen dimensions, and specimens with proper dimensions and showing no voids and irregularities were stored in distilled water at 37 °C for 48 ± 2 h.
2.3. Thermal Cycling Process
Before testing, all specimens were subjected to thermal cycling (5000 cycles) in a thermal cycling machine (Thermocycler THE-1100, Mechatronik GmbH, Feldkirchen-Westerham, Bavaria, Germany) with 5–55 °C/30s of dwell time.
2.4. Specimens Testing
2.4.1. Flexural Strength (MPa) and Elastic Modulus (GPa) Measurement
To determine the flexural strength and elastic modulus of the material, a three-point bend test was performed (Electropuls E3000; Instron, High Wycombe, UK). A 3 point flexure apparatus with two 50 mm apart vertical rods was used to load the specimens horizontally at the midpoint with 5-kN at a crosshead speed of 5 mm/min until the specimen fractured, and then the fracture load was determined. Each specimen was measured for flexural strength using S = 3FL/2bh2, where S stands for flexural strength in MPa, F stands for fracture load in N, L stands for distance between the two vertical rods (50 mm), b stands for specimen width (10 mm), and h represents specimen thickness (3.3 mm). The elastic modulus was calculated from E = FL3/4bh3d, where E is the elastic modulus (MPa), F is the load (N) at a convenient point (P) along the straight line of the tension/deformation curve (that is, elastic deformation), and L, b, and h represent the same values in the previous equation of FS. Whereas d is the deflection at point P [10,24,25].
After fracture, the fractured surfaces were analyzed using scanning electron microscopy (SEM, TESCAN, VEGA3, Czech Republic at 20 kV) to determine the characteristics and topographical features of the fractured surfaces. The fractured samples were mounted onto a metallic stub using double-sided tape, keeping the fractured side upward, and then sputter-coated with gold (Q150R Plus—Rotary Pumped Coater). The gold coating was applied in order to minimize the charging effects and to improve the image quality during the scanning of the samples. The mode of fracture was analyzed according to the surface topography, which indicated ductile fracture, rougher and irregular, requiring higher energy before deformation and fracture (see Figure 3). The brittle fracture exhibited a smooth surface and consequently fractured relatively easily and immediately [25].
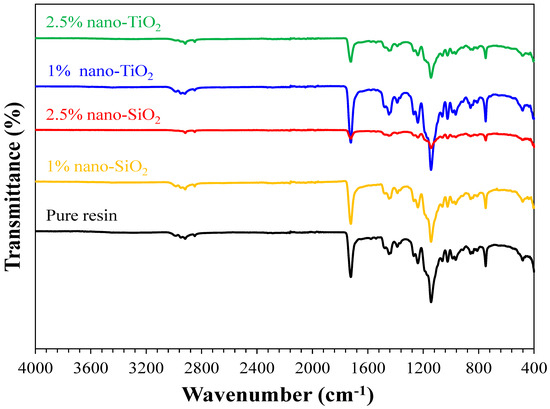
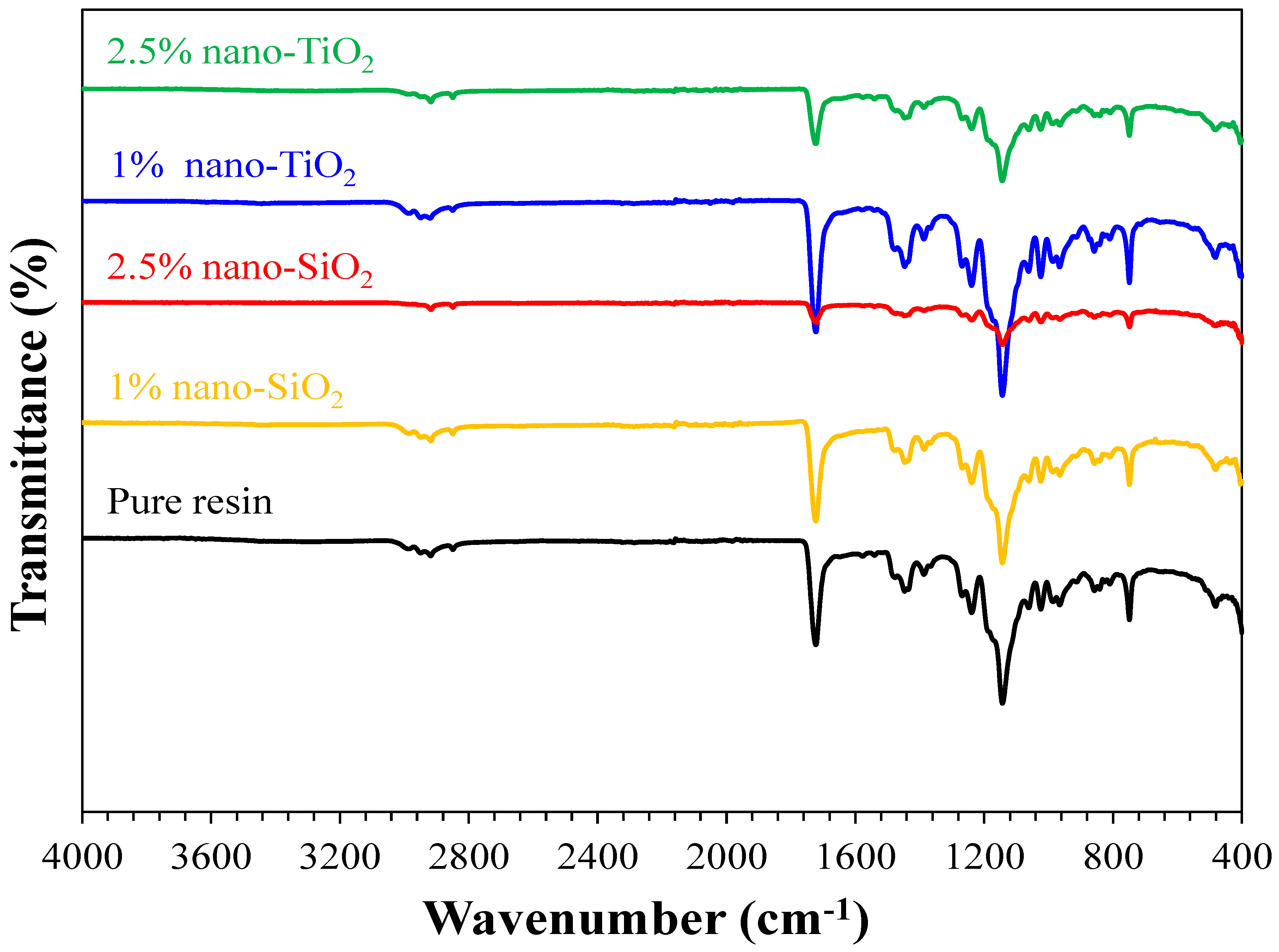
Figure 3.
FTIR spectra of the pure resin, 1% nano-SiO2, 2.5% nano-SiO2, 1% nano-TiO2, and 2.5% nano-TiO2 specimens showing the characteristics bands of PMMA matrix.
2.4.2. Hardness (VHN) Measurement
According to the ASTMC1327-15 standard, all specimens were tested for surface hardness using a Vickers hardness tester (MicroMet 6040, Buehler Ltd., Lake Bluff, IL, USA). Each specimen was subjected to a load of 50 g for 20 s on three different spots (at least 2 mm apart) by using a Vickers hardness indenter. The readings were digitally converted by a measuring machine, followed by an average calculation of 3 readings per specimen.
2.4.3. Surface Roughness (Ra, µM) Measurement
A non-contact optical profilometer (Contour Gt-K 3D optical profiler; Bruker Nano GmbH, Berlin, Germany) was used to obtain the initial surface roughness readings. Prior to evaluation, the specimen surface was finished and stored in distilled water for 72 h. Performing Ra of all samples was conducted by measuring three readings at equidistance in different areas. These areas were vertically scanned under 20× g magnification with speed set as 0.5 mm/s and cutoff at 0.8 mm. The traced image was analyzed with integrated software in accordance with International Organization for Standardization 4287 [26]. The average of these three readings was obtained for each sample. The arithmetic mean of the three measurements for each test sample was used to obtain the average surface roughness value of each sample [27].
2.4.4. Microbial Assay
Candida Conditions, Preparation, and Culture
The standard strain of C. albicans (ATCC 10231) was obtained from the American Type Culture Collection (ATCC) and stored in glycerol media (30%) at −86 °C in the College of Medicine, IAU. C. albicans were spread on Sabouraud dextrose agar (SDA) plates and cultured at 37 °C for 48 h. Inoculating a freshly isolated colony into 4 mL Sabouraud dextrose broth (SDB Acumedica Co., Manufacturers, Lansing, Michigan) was performed overnight, and it was shaken subsequently for 24 h followed by centrifuging to collect the cells. Collected cells were washed twice with phosphate-buffered saline (PBS) and were suspended again for concentration standardization by a spectrophotometer to 1.7 × 107 [21,22].
Biofilm Formation and Colony Forming Unit Assay
The prepared acrylic disks were cleaned ultrasonically, followed by sterilization by subjecting the specimens to ultraviolet (UV) light for 30 min [23]. The sterilized specimens were submerged in a 12-well plate containing 200 µL of SDB suspension with the reference strain C. albicans and incubated at 37 °C for 48 h. The specimens were then washed twice to remove non-adherent cells. Subsequently, the specimens were placed in sterile tubes containing 1 mL PBS and sonicated for 15 min. To detach adherent cells from the specimen surface, Candida colonies were counted. A vortex machine was used, followed by specimen centrifugation, as detailed in a previous study [28]. Ten milliliters of the centrifuged solution was serially diluted, spread on a petri dish containing SDA, and incubated for 24 h at 37 °C [28]. The colonies of C. albicans per quadrant were counted using a marker pen counter (colony counter “Scienceware- bel-art products”, Wayne, NJ, USA). Then the total number of colonies was multiplied by the dilution factor exhibited Candida colonies count (CFU/mL) [28].
2.5. Statistical Analysis
Data analysis was performed by using SPSS (Statistical package for social sciences). A test for normality checking was performed using the Shapiro–Wilk test. Results from the test were statistically insignificant; hence, the data were found to be normally distributed. Hence, parametric tests were performed for inferential analysis. One-way ANVOA was employed to study the effects of concentration levels of nanoparticles on the tested properties. In addition, Tukey’s post-hoc test was performed for pairwise comparison. Two-way ANOVA was used to study the interaction effect of groups and nanoparticles on the tested properties. All p-values considered statistically significant if they were less than 0.05.
3. Results
FTIR Finding
The formation of functional groups of pure, nano-SiO2, and nano-TiO2 incorporated resins was investigated by Fourier Transformed Infrared (FTIR) using transmission spectroscopy (Hartmann & Braun, MB series). The FTIR spectra were recorded between 4000 and 400 cm−1, as shown in Figure 3. The FTIR spectra of pure and nanoparticles incorporated resins (1% nano-SiO2, 2.5% nano-SiO2, 1% nano-TiO2, and 2.5% nano-TiO2 specimens) showed similar types of bands and characteristic features. FTIR results prove that the addition of NPs, either nano-SiO2 or nano-TiO2, did not change the chain structure of the nanocomposite but only varied the intensity of the bands.
Table 2 shows the two-way ANOVA results for all tested properties, and significant differences were found for all tested properties in terms of nanoparticle type and concentration (p < 0.001). Table 3 summarizes the mean, SD, and significance within the nano-modified groups and between all groups for all tested properties. The addition of 1%-nano-SiO2 significantly increased the flexural strength (p < 0.001) in comparison to the control group, while 2.5% showed no significant differences in flexural strength (p = 0.084). The addition of nano-TiO2 significantly increased the flexural strength (p < 0.001) at both concentrations, with no significant differences between 1% nano-TiO2 and 2.5% nano-TiO2 (p = 0.062). Among all NP-modified groups, the highest flexural strength value was recorded with 1% Nano-SiO2 significantly, while no significant differences were observed between other NP-modified groups (p > 0.05).

Table 2.
Two-way ANOVA results for all tested properties.

Table 3.
Mean, SD, and significance of tested properties for the pure and reinforced groups.
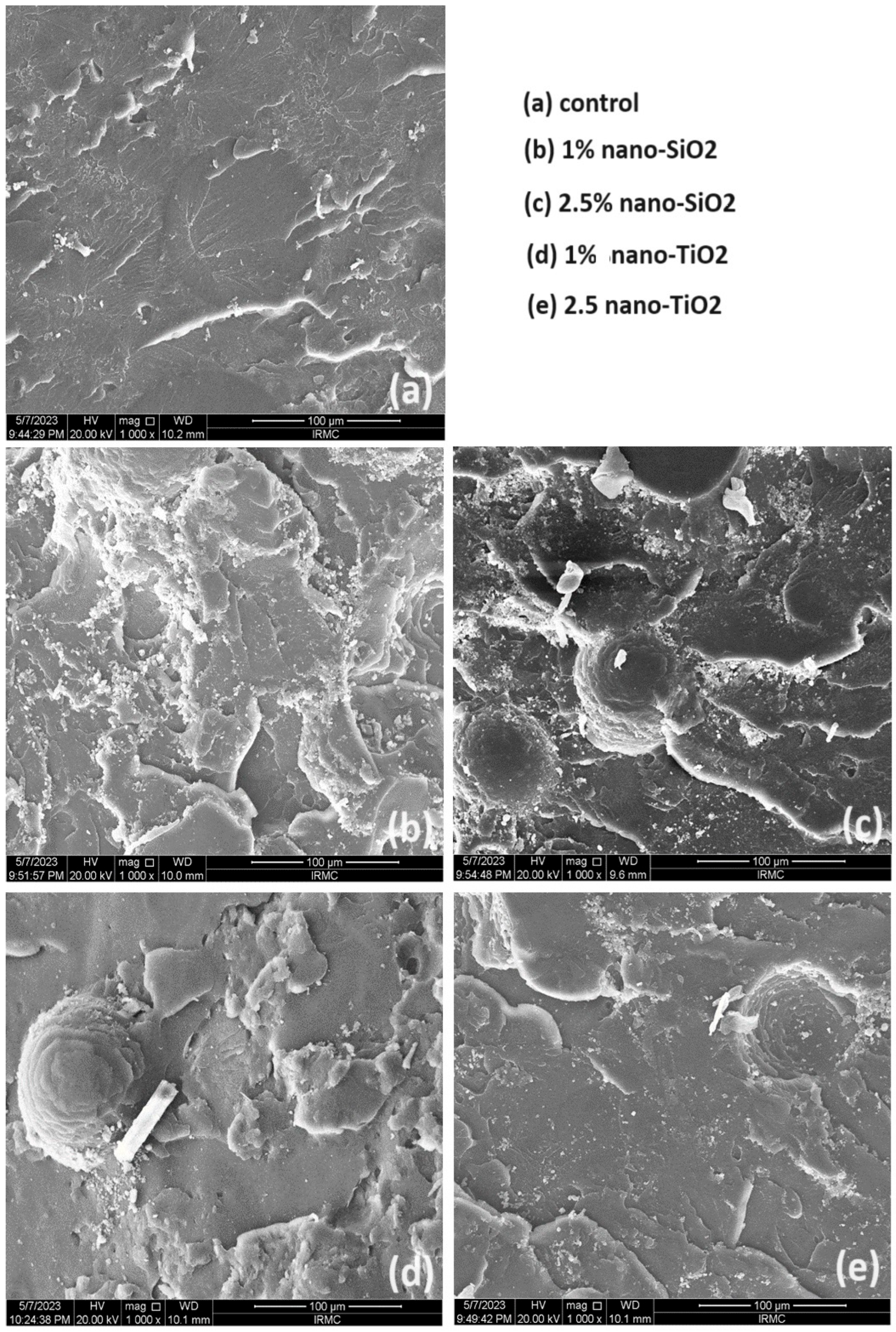
Figure 4 presents a comparative SEM analysis of the fractured surfaces of the unmodified and NP-modified groups. The unmodified group (no addition of NPs) exhibits a smooth background with faint lamellae as its main features. In contrast, the NP-modified groups reveal distinct surface features attributable to the addition of NPs. In the nano-SiO2 modified group, two different concentrations (1% and 2.5%) were investigated. At a nano-SiO2 concentration of 1 %, deeper and more uniform lamellae are clearly evident, signifying a pronounced effect of nano-SiO2 on the surface topography. However, with a higher concentration of 2.5% nano-SiO2, clusters become apparent, suggesting a shift in the surface morphology due to the increased NPs content. For the nano-TiO2 modified group, similar surface topography is observed with both concentrations. The fractured surfaces in this group are characterized by fewer lamellae, a lower number of NPs, and a limited formation of clusters compared to the nano-SiO2 group.
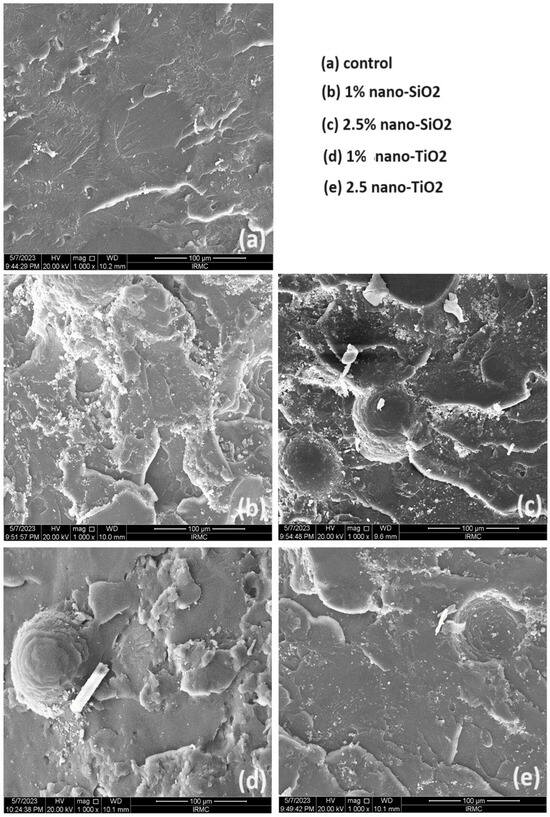
Figure 4.
Representative SEM images of tested groups at 1000× magnification. Scale bars represent 100 µm.
For elastic modulus, the addition of NS showed a significant increase in the elastic modules (p < 0.001) in comparison to the unmodified group with no significant change in 1% and 2.5% nano-SiO2 addition in elastic modules. The addition of nano-TiO2 significantly increased the elastic modulus (p < 0.001) at both concentrations, with no significant difference between 1% Nano-TiO2 vs. 2.5% Nano-TiO2. In between all NP-modified groups, the elastic modulus of nano-SiO2 showed an increase compared to nano-TiO2, while there were significant differences between other NP-modified groups (p > 0.05).
For surface roughness, the addition of nan-TiO2 did not show any significant change in surface roughness among all groups (p = 0.078). The addition of nano-TiO2 significantly decreased the surface roughness (p < 0.001) at both concentrations, with significant differences observed between 1% Nano-TiO2 and 2.5% Nano-TiO2. In between all NP-modified groups, the highest surface roughness value was recorded with the addition of Nano-TiO2 significantly, while no significant differences between other NP-modified groups.
The addition of 1%-Nano-SiO2 and 2.5% has significantly increased the hardness in comparison to the unmodified control group (p < 0.001), while 2.5% and 1% showed no significant differences between them. The addition of Nano-TiO2 significantly increased the hardness (p < 0.001) at both concentrations, with no significant differences between 1%Nano-TiO2 vs. 2.5%Nano-TiO2. Among all NP-modified groups, the highest hardness value was recorded with 2.5% Nano-TiO2 significantly, while no significant differences were observed between the other NP-modified groups (p > 0.05).
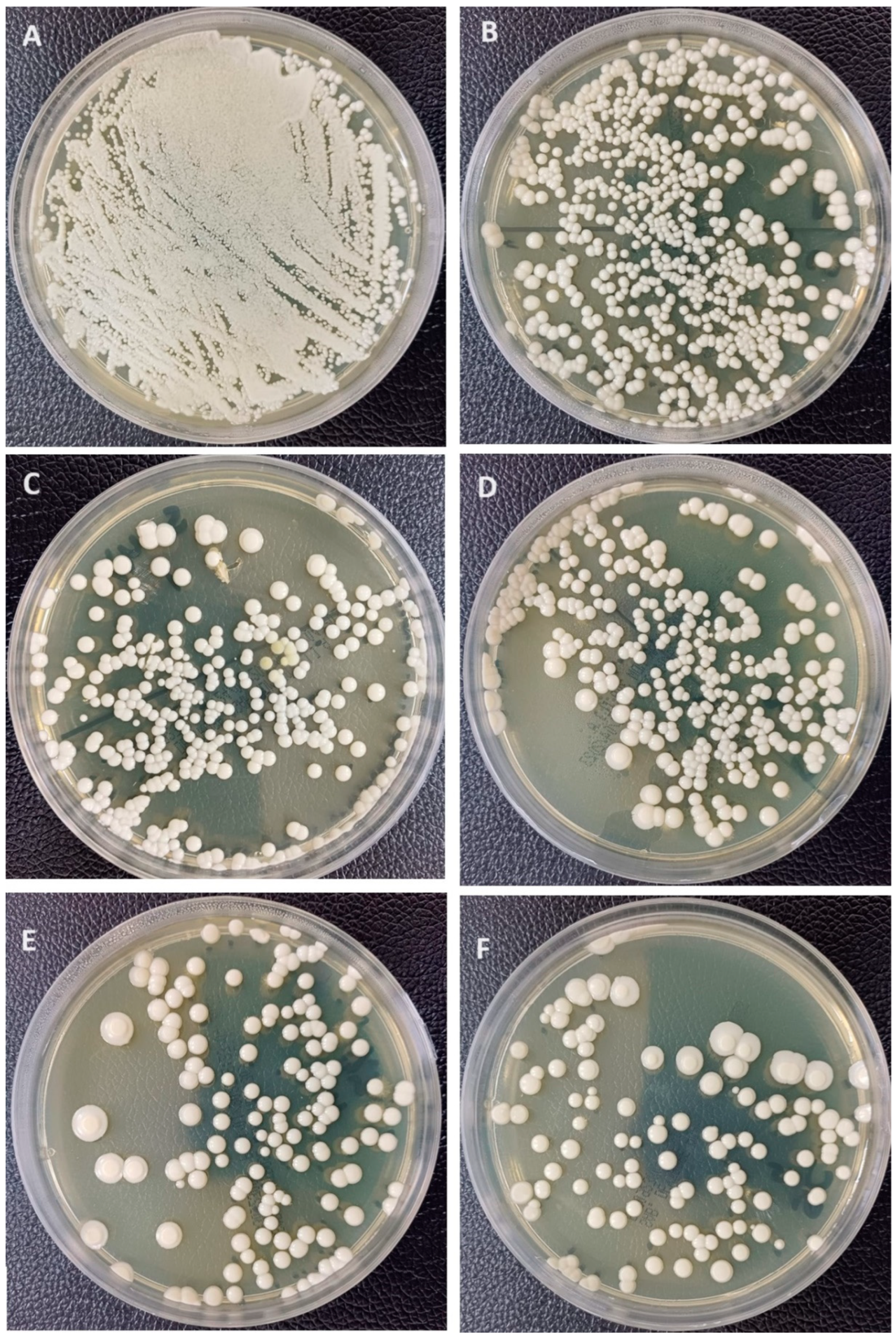
For C. albicans adhesion (Figure 5), the addition of 1%-Nano-SiO2 and 2.5% has significantly decreased Candida adhesion in comparison to the unmodified control group (p < 0.001), while 2.5% and 1% showed no significant differences between them. On the other hand, the addition of nano-TiO2 significantly decreased Candida adhesion (p < 0.001) at both concentrations, with no significant differences between 1% Nano-TiO2 and 2.5% Nano-TiO2. Among all NP-modified groups, the highest decrease was recorded with 1% Nano-TiO2 significantly, while there were significant differences between other NP-modified groups (p > 0.05).
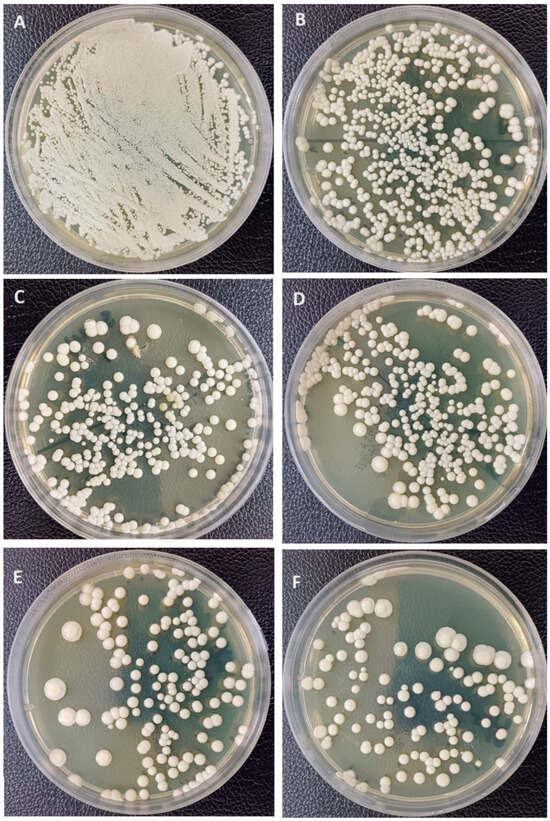
Figure 5.
Representative Candida albican adhesion images showing Candida colonies (CFU/mL). (A) Negative control; (B) unmodified group; (C) 1%Nano-SiO2; (D) 2.5%Nano-SiO2; (E) 1%Nano-TiO2; (F) 2.5%Nano-TiO2.
4. Discussion
PMMA is one of the most commonly used materials for the fabrication of provisional short- or long-span restorations in dental practice. However, several disadvantages of PMMA have led to more investigations on how to improve its mechanical and physical properties. One of these approaches is the addition of NPs to dental resins to improve their strength and biological behavior. Both nano-SiO2 and nano-TiO2 have demonstrated promising performance in enhancing the clinical behavior of modified dental resins in terms of mechanical properties and antimicrobial activities [20,29,30]. Thus, the present study utilized nano-SiO2 and nano-TiO2 as additives for provisional restoration to assess microbial adherence effects, strength, and surface properties.
Regarding the concentrations selected, different results have been reported in the literature concerning concentrations associated with higher strength values [20,30,31]. However, it was found that increasing the concentration of NPs to an extent above 5% would adversely affect the material properties [20,32]. This would reduce the homogeneity of the modified nanocomposites, which might worsen their surface structure [20,33]. Moreover, the higher the concentration, the greater the restriction of the polymer chain mobility, which makes the material more brittle. The flexural strength is also negatively affected by increased concentrations of NPs above 5%, as it was shown that high NPs concentrations formed clusters and the presence of these NPs at high concentrations could affect the degree of conversion and increase the residual monomer, which could act as plasticizers, eventually reducing the strength values [20,32]. As a result, NPs with concentrations of 1% and 2.5% were selected for this study to avoid any unfavorable effect of increased content of NPs.
All specimens were subjected to 5000 cycles of thermal cycling. Thermal aging is an experimental technique that exposes the tested specimens to a situation comparable to the actual situations in the oral cavity in terms of temperature values to assess how the material ‘properties would be affected under such conditions [14]. Hence, it is essential to evaluate the mechanical properties of the tested material that has been subjected to thermal aging to obtain valid results that are clinically relevant. Five thousands thermal cycles were set to simulate 6 month of clinical service, according to a study by Morresi et al. [34].
The null hypothesis was rejected, as the current results demonstrated that incorporating nano-SiO2 and nano-TiO2 into provisional restoration had an effect on physical, mechanical, and microbial adherence.
The addition of nano-SiO2 at low concentrations increased the flexural strength of the provisional resin, in agreement with Machado-Santos et al. [35]. This increase may be attributed to the NPs size, bonding between NPs and the resin matrix, and/or normal distribution of NPs within the resin matrix. Another possible reason for the increased strength after the addition of NPs is the crack deflection mechanism, in which, once impeded in the matrix, these particles tend to deviate from the propagated crack away and maintain the integrity of the polymer matrix. Moreover, they can prevent the disruption of polymer chains by filling in the cracks between the polymers [30,36]. In other words, the concentration of NPs has an impact on the strength of the provisional resins. As the concentration of nano-SiO2 increased above 1%, the strength decreased, which is in agreement with previous studies [14,30]. The increase in NPs concentration adversely affects the homogeneity of the matrix, which leads to increased susceptibility to crack formation [14,37]. Moreover, the higher the surface area of NPs, the higher their tendency toward agglomeration due to increased surface energy, which is one of the biggest issues in the utilization of NPs [38]. Therefore, low nano-SiO2 is recommended for the reinforcement and enhancement of the mechanical properties of provisional resins [14]. It is crucially important to follow the proper concentrations of NPs to achieve optimum properties; hence, adding nano-SiO2 at a low concentration was proven by a previous study to result in features of ductile nature, which positively improved the flexural strength of the resin [39]. The same study [39] reported the formation of loosely attached clusters, which increased stress formation when higher concentrations of NPs were used. This ultimately led to a decrease in flexural strength.
The effect of nano-TiO2 on the flexural strength of PMMA is multifactorial. The most important factors are the concentration of the particle, along with their interaction with PMMA [40]. Nano-TiO2 addition showed increased values compared with the unmodified group. However, this increase did not show any significant differences between the two concentrations. The slight increase may be attributed to the good bonding between the resin matrix and treated nano-TiO2 [41]. In addition, this effect on the stabilization of polymer chains has been reported in the literature in conjunction with nano-TiO2 [41]. It has been reported that the addition of TiO2 chemically reacts with PMMA through the ester functional group COOR (oxygen double-bound to carbon–carbonyl along with an OR group attached to the same carbon). The ester is a carboxylic acid derivative containing OH, which will be replaced by OR (oxygen reaction variable) during the interaction with PMMA, which creates a strong bond that retards polymer chain movements, providing a cross-linking action [41,42]. In contrast, a previous study reported a significant increase in the strength of self-cured PMMA when nano-TiO2 was added at a high concentration (5%). The usage of self-cured PMMA clarified the reason for the inconsistency between the results of that study and the current one. The finding of this study is in agreement with Chen et al. [43], who reported no adverse effect on flexural strength. Other studies [20,44] have reported a negative effect on the flexural strength of acrylic resins, even with different concentrations (0.5, 1%, and 3% of nano-TiO2). These variations in results may be attributed to the difference in resin type and nano-TiO2 concentrations.
Based on the results of this study, it is essential to restrict the concentration of the added NPs to a certain limit. As briefly mentioned, higher concentrations of NPs contribute to a higher tendency toward agglomeration. The agglomerates are weak areas within the matrix. Hence, once a load is applied, these weak spots are liable to collapse easily, which causes mechanical failure and degradation [37,38]. In addition, these areas result in defect formation by shrinkage at their contact area, which in turn prevents the proper transmission of forces to the entire matrix, negatively affecting the fracture toughness of the material [45].
The addition of NPs enhanced the elastic modulus of the provisional resin in comparison to the unmodified groups. There was a noticeable reduction in the elastic modulus with high concentrations of NPs; however, it was insignificant. The increase in the elastic modulus with the addition of NPs was in agreement with the results of Topouzi et al. [14], who found that the addition of NPs increased the elastic modulus in a concentration-dependent manner. A possible explanation could be related to the attachment of NPs to the modified organic matrix. Such strong attachment could restrain the macromolecular movement of the polymer chains, even at high temperatures [46]. However, this restriction acts only on the zone surrounding the NP, which is limited to a few nanometers. Hence, to achieve a higher elastic modulus throughout the entire matrix, the dispersion on NPs must be of proper homogeneity and concentration [47]. The increase in the elastic modulus in response to the increase in NPs concentration could be attributed to the agglomerates present in the matrix, which restrain the chain mobility, resulting in more contact area, causing a further increase in the elastic modulus [48]. However, this would occur at the expense of other mechanical properties, as discussed before, if no attention was given to the recommendations of proper concentrations to use.
Our results agree with those of Elkhouly et al. [49], who found that adding nano-TiO2 to PMMA increased the modulus of elasticity, and this increase was in relation to NPs concentrations. In addition, it has been reported that the modulus of elasticity increases with increasing NPs concentration, as expected by Chatterjee et al. [16], following the law of mixtures. In disagreement with the findings of the present study, Panyayong et al. [50] investigated the effect of nano-TiO2 at different concentrations (volume % of 0, 1.0, 2.0, and 3.0) mixed with nano-ZrO2 in provisional resins and evaluated their effects on the elastic modulus. They found that the mixture increased, and there was no significant difference between the control group and all percent by volume combinations in modulus of elasticity. The difference in results regarding the elastic modulus may be due to the fraction used as a mixture (nano-TiO2/nano-ZrO2).
Hardness is likewise impacted by the ratio of organic to inorganic substances. It is used to determine a material’s ability to withstand abrasion, resist indentations, and abrade the opposing structure, and it is used as an indirect way to determine the degree of polymerization [51]. This investigation revealed that the addition of nano-SiO2 and nano-TiO2 increased the hardness at a given level of concentration (1% or 2.5%). The controlled increase in the NPs content would reduce the spaces available in the matrix between the particles, creating a more solid structure resisting indentation and withstanding higher loads [52]. This is the most likely explanation for the increased hardness noted in the results with increasing concentrations of NPs. The above-mentioned hardness improvement is due to the strong interfacial adhesion of the NPs with the PMMA matrix.
In a previous study [49] that investigated the addition of different percentages of nano-TiO2 to auto-polymerized resins, it was reported that NPs with a weight of 1.2% increased the hardness of PMMA. Accordingly, Elkhouly et al. [49] reported that the hardness increased with increasing NPs concentration. This result is in good agreement with that of El-Tamimi et al. [53], who reported an 11% hardness improvement due to the incorporation of NPs. A study was conducted by Shirkavand and Moslehifrad to investigate the effect of nano-TiO2 on the mechanical properties of resins. Their findings suggested a positive impact of nano-TiO2 on the mechanical properties at a particular concentration (1 wt%), which then decreased with an additional higher amount of nano-TiO2 [18]. In disagreement with Abdelraouf et al. [41], it was found that no significant differences existed in microhardness with nano-TiO2 addition, and this variation may be due to the high concentration used (5%) in Abdelraouf et al. study.
The roughness of the provisional restoration is a major factor in achieving a good condition of the material surface, which includes no scratching on the surface and minimizing the collection of food or bacteria. Various mathematical models have been used to measure surface roughness based on geometric topography. However, researchers mainly use the average surface roughness (Ra) to analyze the surface roughness [54]. A study by Alwan and Alameer found an increase in surface roughness with the addition of 3 wt% of nano-TiO2 to PMMA denture base material and attributed this increase to the presence of nanoparticles on the surface of the specimens [55]. The characteristics of filler particles, such as their type, size, and shape, and their dispersion into the resin matrix are of great importance in determining the surface quality of the material [54]. Other important factors include the degree of conversion, type of resin matrix, and efficiency of bonding at the interface [54]. The results demonstrated a marginal increase in Ra of the modified PMMA in correlation with increased concentrations of nano-TiO2 and nano-SiO2. This could be due to the dispersion of the NPs. Most likely, the uncontrolled increase in NPs content would result in uneven distribution into the matrix, which produces an uneven surface [56].
In another study by Abdelraouf et al. [41], it was found that adding nano-TiO2 at high concentrations showed no significant differences in surface roughness. This finding was attributed to the reduced content of homogenously dispersed nano-TiO2 in the matrix. Although no significant differences were found between roughness among the tested groups, the reduced surface roughness may be clinically helpful in reducing staining and plaque accumulation. Abdelraouf et al. also analyzed the specimens’ (unmodified and modified) surfaces using SEM and reported irregularities and porosity associated with the surfaces of their control group. In contrast, the treated group demonstrated a homogenous structure with an evident reduction in surface porosity. These findings led to the assumption that the addition of NPs at the right concentrations produced a structure with improved properties in the presence of uniformly dispersed NPs [41].
Nanoparticles may interfere with gram-positive bacteria’s cell function and cause hyphae to deform, inhibiting their growth. In agreement with their results, adding nanoparticles was associated with an increased antibacterial effect in our study. The addition of NPs to the auto-polymerized resin resulted in a significant decrease in C. albicans adhesion, and the maximum reduction occurred with the addition of nano-TiO2. This improvement in resisting microbial adhesion may be attributed to the antimicrobial activity of nano-TiO2 or its potential aids in enhancing surface properties. It has also been shown that NPs have an antibacterial effect by inhibiting biofilm formation and cytoplasmic accumulation of bacteria [57]. Additionally, the effect of adding NPs on the surface characteristics of dental resins was demonstrated in a study by Yang et al. [58]. In their study, superior surface properties resulted in denture base materials after coating with nano-TiO2, which reduced bacterial and fungal adhesion.
In their study, Alzayyat et al. [21] found a significant decrease in C. albicans adhesion to PMMA when reinforced with nano-SiO2. This finding was attributed to the antifungal efficacy of nano-SiO2 and its effect on improving surface properties. According to a previous study [59], nano-SiO2 demonstrated antifungal behavior when they were introduced into soft liners at concentrations up to 0.5%. Similarly, Sodagar et al. [60] found a positive, direct correlation between the concentration of nano-SiO2 and their antimicrobial effect. The reported antifungal activity of nano-SiO2 might be due to the large surface area of the NPs in contact with C. albicans cell membranes, which is called the nanoparticle–Candida contact theory. This theory states that nano-SiO2 may break through C. albicans cell membranes, causing a disturbance in membrane permeability and abnormal diffusion of ions [61]. This could be highly destructive to the cell structure, leading to their deterioration [55]. Although further research is needed, the impact of nano-SiO2 on C. albicans adhesion could be similar to that of other antifungal nanoparticles, such as leached ions from the nanocomposite [55,60,62].
Surface roughness and hardness have a correlation with C. albicans adhesion [21,62]. As the roughness increased, the microbial adhesion increased. It is beneficial to decrease the surface roughness because it will lead to a decrease in the possibility of stains and plaque aggregation. Materials with high hardness are more resistant to abrasion, keeping this material with less surface deterioration and longevity of provisional prostheses. Based on our results, correlating surface roughness to decreased C. albicans adhesion supports the antifungal efficacy of provisional restoration using nanocomposite provisional resin investigated and revealed that it could be used to minimize bacterial adhesion and reduce the possibility of infection.
The introduced nanocomposites exhibited improved mechanical performance as all values were higher than the minimum values recommended for flexural strength (50 MPa) and modulus of elasticity [63]. In addition to the improved surface properties and reduced C. albicans adhesion, the experimental nanocomposite provisional materials tested in this study meet the requirements of the standard. Moreover, the impact resistance in the tested NPs groups exhibited superior performance when compared to that of the control group, indicating a positive impact of modifying the provisional resin with controlled concentrations of NPs to overcome the shortcomings related to its physicochemical behavior and limit the occurrence of failures.
Although incorporating two types of NPs into the PMMA matrix and subjecting them to thermal aging is of major strength to the current results, the utilization of one resin type is one limitation of this study. Despite the improvement in the properties of the introduced nanocomposites modified by the addition of NPs, the manual mixing of the two powders may exert some limitations in the conducted research as it cannot be completely controlled like mechanical mixing. Due to the absence of simulation of the oral environment, it is not known whether these differences in values will be observed clinically in the presence of saliva and other oral fluids and when prostheses are worn in their real configuration instead of bars as in the samples tested. The lack of these factors may be a limitation of this study, but it is an important research area for future in vivo studies. Further investigation is encouraged to employ a longer immersion time or use an accelerating protocol to compare the long-term mechanical performance of the materials. This is specifically beneficial when making decisions about whether temporary materials should be used for longer periods in more comprehensive cases.
5. Conclusions
Within the limitations of this study, the following conclusions could be drawn:
- The strength of the provisional resin increased with low nano-SiO2 addition, while high nano-SiO2 and both concentrations of nano-TiO2 did not affect the strength values.
- The addition of NPs enhanced the elastic modulus of the provisional resin.
- The addition of nano-SiO2 did not alter the surface roughness, while high concentrations of nano-TiO2 increased the surface roughness.
- The hardness of both nanocomposite provisional resins increased.
Author Contributions
Conceptualization, F.A.A. and M.M.G.; methodology, M.A. and D.A.; software, H.A.; validation, A.A.M., F.A.A. and M.M.G.; formal analysis, H.A., S.A. and S.Q.K.; investigation, M.A. and D.A.; resources, F.A. and F.A.A.; data curation, M.A. and D.A.; writing—original draft preparation, M.A., D.A., H.A., S.Q.K., S.A., A.-N.M.E. and F.A.; writing—review and editing, F.A.A., A.A.M., A.-N.M.E., F.A., H.A. and M.M.G.; visualization, M.M.G.; supervision, M.M.G.; project administration, F.A.A.; funding acquisition, A.-N.M.E., F.A. and F.A.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Doray, P.G.; Eldiwany, M.S.; Powers, J.M. Effect of resin surface sealers on improvement of stain resistance for a composite provisional material. J. Esthet. Restor. Dent. 2003, 15, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Ozel, G.S.; Guneser, M.B.; Inan, O.; Eldeniz, A.U. Evaluation of C. albicans and S. Mutans adherence on different provisional crown materials. J. Adv. Prosthodont. 2017, 9, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, C.A.; Hegde, D.; Sanjeevan, V.; Coutinho, I.F.; Priya, A. Comparative evaluation of color stability of three commercially available provisional restorative materials: An in vitro study. J. Ind. Prosthodont. Soc. 2021, 21, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Barghi, N.; Simmons, E.W., Jr. The marginal integrity of the temporary acrylic resin crown. J. Prosthet. Dent. 1976, 36, 274–277. [Google Scholar] [CrossRef] [PubMed]
- Lepe, X.; Bales, D.J.; Johnson, G.H. Retention of provisional crowns fabricated from two materials with the use of four temporary cements. J. Prosthet. Dent. 1999, 81, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Buergers, R.; Rosentritt, M.; Handel, G. Bacterial adhesion of Streptococcus mutans to provisional fixed prosthodontic material. J. Prosthet. Dent. 2007, 98, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Morgan, T.D.; Wilson, M. The effects of surface roughness and type of denture acrylic on biofilm formation by Streptococcus oralis in a constant depth film fermentor. J. Appl. Microbiol. 2001, 91, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, C.J.; Klier, C.M.; Kolenbrander, P.E. Mechanisms of adhesion by oral bacteria. Annu. Rev. Microbiol. 1996, 50, 513–552. [Google Scholar] [CrossRef]
- Odds, F.C. Candida and Candidosis, 2nd ed.; Bailliere Tindalll: London, UK, 1988; pp. 42–59. [Google Scholar]
- Raouf, L.; Faraj, S.; Azhdar, B. Evaluation of Flexural Strength of Heat Cure PMMA Denture Base Material Reinforced with Various Concentrations of Zirconium Oxide Nanoparticle: An In-vitro Study. Sulaimani Dent. J. 2019, 6, 22–30. [Google Scholar] [CrossRef]
- Hasratiningsih, Z.; Takarini, V.; Cahyanto, A.; Faza, Y.; Asri, L.; Purwasasmita, B. Hardness evaluation of PMMA reinforced with two different calcinations temperatures of ZrO2-Al2O3 filler system. Mater. Sci. Eng. 2017, 172, 012067. [Google Scholar] [CrossRef]
- Blackman, B.R.K.; Kinloch, A.J.; Sohn Lee, J.; Taylor, A.C.; Agarwal, R.; Schueneman, G.; Sprenger, S. The fracture and fatigue behaviour of nano-modified epoxy polymers. J. Mater. Sci. 2007, 42, 7049–7051. [Google Scholar] [CrossRef]
- Lee, J.H.; El-Fiqi, A.; Jo, J.K.; Kim, D.A.; Kim, S.C.; Jun, S.K.; Kim, H.W.; Lee, H.H. Development of long-term antimicrobial poly(methyl methacrylate) by incorporating mesoporous silica nanocarriers. Dent. Mater. 2016, 32, 1564–1574. [Google Scholar] [CrossRef]
- Topouzi, M.; Kontonasaki, E.; Bikiaris, D.; Papadopoulou, L.; Paraskevopoulos, K.M.; Koidis, P. Reinforcement of a PMMA resin for interim fixed prostheses with silica nanoparticles. J. Mech. Behav. Biomed. Mater. 2017, 69, 213–222. [Google Scholar] [CrossRef]
- Reijnders, L. The release of TiO2 and SiO2 nanoparticles from nanocomposites. Polym. Degrad. Stab. 2009, 94, 873–876. [Google Scholar] [CrossRef]
- Chatterjee, A. Effect of nanoTiO2 addition on poly(methylmethacrylate): An exciting nanocomposite. J. Appl. Polym. Sci. 2010, 116, 3396–3407. [Google Scholar] [CrossRef]
- Altaie, S.F. Tribological, microhardness and color stability properties of a heat-cured acrylic resin denture base after reinforcement with different types of nanofiller particles. Dent. Med. Probl. 2023, 60, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Shirkavand, S.; Moslehifard, E. Effect of TiO2 nanoparticles on tensile strength of dental acrylic resins. J. Dent. Res. Dent. Clin. Dent. Prospects 2014, 8, 197–203. [Google Scholar] [PubMed]
- Acosta-Torres, L.S.; López-Marín, L.M.; Núñez-Anita, R.E.; HernándezPadrón, G.; Castaño, V.M. Biocompatible metal-oxide nanoparticles: Nanotechnology improvement of conventional prosthetic acrylic resins. J. Nanomater. 2011, 2011, 941561. [Google Scholar] [CrossRef]
- Sodagar, A.; Bahador, A.; Khalil, S.; Shahroudi, A.S.; Kassaee, M.Z. The effect of TiO2 and SiO2 nanoparticles on flexural strength of poly (methyl methacrylate) acrylic resins. J. Prosthodont. Res. 2013, 57, 15–19. [Google Scholar] [CrossRef]
- Alzayyat, S.T.; Almutiri, G.A.; Aljandan, J.K.; Algarzai, R.M.; Khan, S.Q.; Akhtar, S. Antifungal efficacy and physical properties of poly(methylmethacrylate) denture base material reinforced with SiO2 nanoparticles. J. Prosthodont. 2021, 30, 500–508. [Google Scholar] [CrossRef]
- AlBin-Ameer, M.A.; Alsrheed, M.Y.; Aldukhi, I.A.; Matin, A.; Khan, S.Q.; Abualsaud, R.; Gad, M.M. Effect of protective coating on surface properties and Candida albicans adhesion to denture base materials. J. Prosthodont. 2020, 29, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Ren, L.; Pan, Y.; Gao, H.; Meng, X. Antifungal property of acrylic denture soft liner containing silver nanoparticles synthesized in situ. J. Dent. 2021, 106, 103589. [Google Scholar] [CrossRef] [PubMed]
- Alhavaz, A.; Rezaei Dastjerdi, M.; Ghasemi, A.; Ghasemi, A.; Alizadeh Sahraei, A. Effect of untreated zirconium oxide nanofiller on the flexural strength and surface hardness of autopolymerized interim fixed restoration resins. J. Esthet. Restor. Dent. 2017, 29, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Alshahrani, F.A.; Gad, M.M.; Al-Thobity, A.M.; Akhtar, S.; Kashkari, A.; Alzoubi, F.; Yilmaz, B. Effect of treated zirconium dioxide nanoparticles on the flexural properties of autopolymerized resin for interim fixed restorations: An in vitro study. J. Prosthet. Dent. 2023, 130, 257–264. [Google Scholar] [CrossRef]
- Geometrical Product Specifications (GPS)—Surface Texture: Profile Method—Terms, Definitions and Surface Texture Parameters. International Organization for Standardization: Geneva, Switzerland. 1997. Available online: https://www.iso.org/standard/10132.html (accessed on 10 August 2023).
- Sahin, Z.; Ozer, N.E.; Calı, A. Biofilm inhibition of denture cleaning tablets and carvacrol on denture bases produced with different techniques. Clin. Oral Investig. 2024, 28, 413. [Google Scholar] [CrossRef] [PubMed]
- Zupancic Cepic, L.; Dvorak, G.; Piehslinger, E.; Georgopoulos, A. In vitro adherence of Candida albicans to zirconia surfaces. Oral Dis. 2020, 26, 1072–1080. [Google Scholar] [CrossRef] [PubMed]
- Harini, P.; Mohamed, K.L.; Padmanabhan, T.V. Effect of Titanium dioxide nanoparticles on the flexural strength of polymethylmethacrylate: An in vitro study. Ind. J. Dent. Res. 2014, 25, 459–463. [Google Scholar] [CrossRef] [PubMed]
- Karci, M.; Demir, N.; Yazman, S. Evaluation of flexural strength of different denture base materials reinforced with different nanoparticles. J. Prosthodont. 2019, 28, 572–579. [Google Scholar] [CrossRef]
- Hafizah, N.N.; Mamat, M.H.; Abidin, M.H.; Said, C.M.S.; Rusop, M. Bonding and mechanical properties of PMMA/TiO2 nanocomposites. Adv. Mater. Res. 2013, 832, 700–705. [Google Scholar] [CrossRef]
- Bangera, M.K.; Kotian, R.; Ravishankar, N. Effect of titanium dioxide nanoparticle reinforcement on flexural strength of denture base resin: A systematic review and meta-analysis. Jpn. Dent. Sci. Rev. 2020, 56, 68–76. [Google Scholar] [CrossRef]
- Unnikrishnan, L.; Mohanty, S.; Nayak, S.K.; Ali, A. Preparation and characterization of poly(methyl methacrylate)–clay nanocomposites via melt intercalation: Effect of organoclay on thermal, mechanical and flammability properties. Mater. Sci. Eng. A 2015, 28, 3943–3951. [Google Scholar] [CrossRef]
- Morresi, A.L.; D’Amario, M.; Capogreco, M.; Gatto, R.; Marzo, G.; D’Arcangelo, C.; Monaco, A. Thermal cycling for restorative materials: Does a standardized protocol exist in laboratory testing? A literature review. J. Mech. Behav. Biomed. Mater. 2014, 29, 295–308. [Google Scholar] [CrossRef] [PubMed]
- Machado-Santos, L.; Baroudi, K.; Silikas, N.; Tribst, J.P.M.; Coelho Sinhoreti, M.A.; Brandt, W.C.; Liporoni, P.C.S. Physical analysis of an acrylic resin modified by metal and ceramic nanoparticles. Dent. Med. Probl. 2023, 60, 657–664. [Google Scholar] [CrossRef]
- Ferracane, J.L.; Berge, H.X.; Condon, J.R. In vitro aging of dental composites in water—Effect of degree of conversion, filler volume, and filler/matrix coupling. J. Biomed. Mater. Res. 1998, 42, 465–472. [Google Scholar] [CrossRef]
- Balos, S.; Pilic, B.; Markovic, D.; Pavlicevic, J.; Luzanin, O. Poly(methyl-methacrylate) nanocomposites with low silica addition. J. Prosthet. Dent. 2014, 111, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Kiat-amnuay, S.; Powers, J.M.; Zhao, Y. Effect of nano-oxide concentration on the mechanical properties of a maxillofacial silicone elastomer. J. Prosthet. Dent. 2008, 100, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Gad, M.M.; Abualsaud, R.; Al-Thobity, A.M.; Almaskin, D.F.; AlZaher, Z.A.; Abushowmi, T.H.; Qaw, M.S.; Akhtar, S.; Al-Harbi, F.A. Effect of SiO2 nanoparticles addition on the flexural strength of repaired acrylic denture base. Eur. J. Dent. 2020, 14, 19–23. [Google Scholar] [CrossRef]
- Gad, M.M.; Abualsaud, R. Behavior of PMMA denture base materials containing titanium dioxide nanoparticles: A literature review. Int. J. Biomater. 2019, 2019, 6190610. [Google Scholar] [CrossRef] [PubMed]
- Abdelraouf, R.M.; Bayoumi, R.E.; Hamdy, T.M. Influence of Incorporating 5% Weight Titanium Oxide Nanoparticles on Flexural Strength, Micro-Hardness, Surface Roughness and Water Sorption of Dental Self-Cured Acrylic Resin. Polymers 2022, 14, 3767. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, A. Properties improvement of PMMA using nano TiO2. J. Appl. Polym. Sci. 2010, 118, 2890–2897. [Google Scholar] [CrossRef]
- Chen, R.; Han, Z.; Huang, Z.; Karki, J.; Wang, C.; Zhu, B.; Zhang, X. Antibacterial activity, cytotoxicity and mechanical behavior of nano-enhanced denture base resin with different kinds of inorganic antibacterial agents. Dent. Mater. J. 2017, 36, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Nazirkar, G.; Bhanushali, S.; Singh, S.; Pattanaik, B.; Raj, N. Effect of anatase titanium dioxide nanoparticles on the flexural strength of heat cured poly methyl methacrylate resins: An in-vitro study. J. Indian. Prosthodont. Soc. 2014, 14, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.; Cai, A.; Zhou, W.; Shi, Z. Effect of flexibility of grafted polymer on the morphology and property of nano silica/PVC composites. Appl. Surf. Sci. 2008, 254, 3745–3752. [Google Scholar] [CrossRef]
- Madathingal, R.R.; Wunder, S.L. Effect of particle structure and surface chemistry on PMMA adsorption to silica nanoparticles. Langmuir 2010, 26, 5077–5087. [Google Scholar] [CrossRef] [PubMed]
- Bansal, A.; Yang, H.; Li, C.; Benicewicz, B.C.; Kumar, S.K.; Schadler, L.S. Controlling the thermo mechanical properties of polymer nano composites by tailoring the polymer-particle interface. J. Polym. Sci. Part B Polym. Phys. 2006, 44, 2944–2950. [Google Scholar] [CrossRef]
- Ramanathan, T.; Stankovich, S.; Dikin, D.A.; Liu, H.; Shen, H.; Nguyen, S.T.; Brinson, L.C. Graphitic nanofillers in PMMA nano composites-An investigation of particle size and dispersion and their influence on nano composite properties. J. Polym. Sci. Part B Polym. Phys. 2007, 45, 2097–2112. [Google Scholar] [CrossRef]
- Elkhouly, H.I.; Ali, E.M.; El-Sheikh, M.N.; Hassan, A.E.M. An investigated organic and inorganic reinforcement as an effective economical filler of poly (methyl methacrylate) nanocomposites. Sci. Rep. 2022, 12, 16416. [Google Scholar] [CrossRef] [PubMed]
- Panyayong, W.; Oshida, Y.; Andres, C.J.; Barco, T.M.; Brown, D.T.; Hovijitra, S. Reinforcement of acrylic resins for provisional fixed restorations. Part III: Effects of addition of titania and zirconia mixtures on some mechanical and physical properties. Biomed. Mater. Eng. 2002, 12, 353–366. [Google Scholar]
- Münchow, E.A.; Correa, M.B.; Ogliari, F.A.; Piva, E.; Zanchi, C.H. Correlation between Surface Roughness and Microhardness of Experimental Composites with Varying Filler Concentration. J. Contemp. Dent. Pract. 2012, 13, 299–304. [Google Scholar] [CrossRef]
- Tran, N.T.; Patterson, B.A.; Harris, D.E.; Napadensky, E.; Lenhart, J.L.; Knorr, D.B., Jr.; Bain, E.D. Influence of interfacial bonding on the mechanical and impact properties ring-opening metathesis polymer (ROMP) silica composites. ACS Appl. Mater. Interfaces 2020, 12, 53342–53355. [Google Scholar] [CrossRef]
- El-Tamimi, K.M.; Bayoumi, D.A.; Ahmed, M.M.; Albaijan, I.; El-Sayed, M.E. The effect of salinized nano ZrO2 particles on the microstructure, hardness, and wear behavior of acrylic denture tooth nanocomposite. Polymers 2022, 14, 302. [Google Scholar] [CrossRef] [PubMed]
- Lins, F.C.R.; Ferreira, R.C.; Silveira, R.R.; Pereira, C.N.B.; Moreira, A.N.; Magalhães, C.S. Surface roughness, microhardness, and microleakage of a silorane-based composite resin after immediate or delayed finishing/polishing. Int. J. Dent. 2016, 2016, 8. [Google Scholar] [CrossRef] [PubMed]
- Gad, M.M.; Fouda, S.M. Current perspectives and the future of Candida albicans associated denture stomatitis treatment. Dent. Med. Probl. 2020, 57, 95–102. [Google Scholar] [CrossRef]
- Alrahlah, A.; Khan, R.; Al-Odayni, A.B.; Saeed, W.S.; Bautista, L.S.; Vohra, F. Evaluation of synergic potential of rGO/SiO2 as hybrid filler for BisGMA/TEGDMA dental composites. Polymers 2020, 12, 3025. [Google Scholar] [CrossRef] [PubMed]
- Gad, M.M.; Abualsaud, R.; Rahoma, A.; Al-Thobity, A.M.; Akhtar, S.; Fouda, S.M. Double-layered acrylic resin denture base with nanoparticle additions: An in vitro study. J. Prosthet. Dent. 2020, 127, 174–183. [Google Scholar] [CrossRef]
- Yang, B.; Ginsburg, S.; Li, W.; Vilela, M.M.; Shahmohammadi, M.; Takoudis, C.G.; Wu, C.D. Effect of nano-ceramic coating on surface property and microbial adhesion to poly(methyl methacrylate). J. Biomed. Mater. Res. B Appl. Biomater. 2023, 111, 1480–1487. [Google Scholar] [CrossRef]
- Gad, M.M.; Bahgat, H.A.; Edrees, M.F.; Alhumaidan, A.; Khan, S.Q.; Ayad, N.M. Antifungal Activities and Some Surface Characteristics of Denture Soft Liners Containing Silicon Dioxide Nanoparticles. J. Int. Soc. Prev. Community Dent. 2022, 12, 109–116. [Google Scholar] [CrossRef]
- Sodagar, A.; Khalil, S.; Kassaee, M.Z.; Shahroudi, A.S.; Pourakbari, B.; Bahador, A. Antimicrobial properties of poly (methyl methacrylate) acrylic resins incorporated with silicon dioxide and titanium dioxide nanoparticles on cariogenic bacteria. J. Orthod. Sci. 2016, 5, 7–13. [Google Scholar]
- Ahmad, N.; Jafri, Z.; Khan, Z.H. Evaluation of nanomaterials to prevent oral candidiasis in PMMA based denture wearing patients: A systematic analysis. J. Oral Biol. Craniofacial Res. 2020, 10, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Vimbela, G.; Ngo, S.; Fraze, C.; Yang, L.; Stout, D.A. Antibacterial properties and toxicity from metallic nanomaterials. Int. J. Nanomed. 2017, 12, 3941–3965. [Google Scholar] [CrossRef]
- ISO 10477; Dentistry—Polymer-Based Crown and Veneering Materials. International Organization for Standardization: Geneva, Switzerland, 2020.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).