On the Effectiveness of Leukocytes Classification Methods in a Real Application Scenario
Abstract
:1. Introduction
2. Related Work
3. Materials and Methods
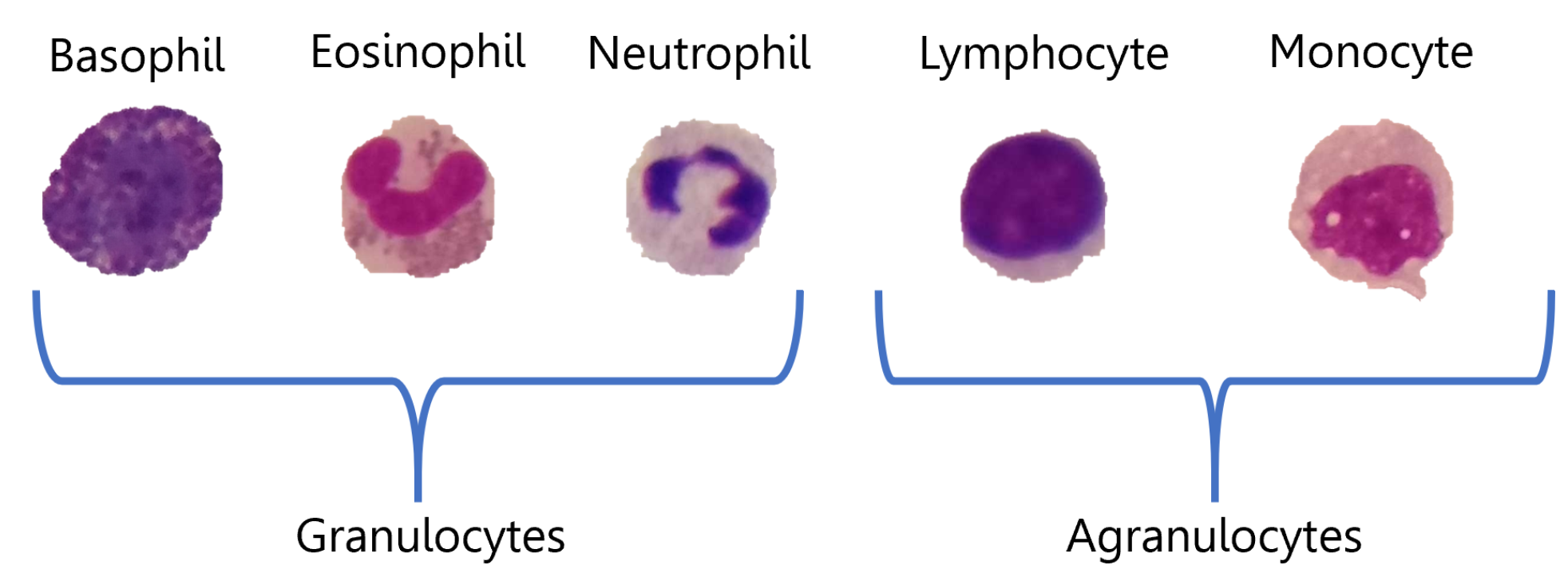
3.1. Data Sets
3.1.1. ALL-IDB2
3.1.2. R-WBC
3.2. Data Pre-Processing
3.3. Methods
3.3.1. Hand-Crafted Image Descriptors
3.3.2. Classic Machine Learning
3.3.3. Deep Learning
3.4. Experimental Setup
4. Experimental Results
4.1. Results
4.2. Discussion
- 1
- HC descriptors are more appropriate for both tasks when they are extracted from the tight version of the data sets (in particular invariant moments and texture), which makes them more robust to BBs variations;
- 2
- on the ALL-IDB2 task (ALL vs. Healthy cells detection), which is finer and more difficult, several HC descriptors (in particular Haar from large, and Gabor from tight) produced results in line with the descriptors extracted from CNNs;
- 3
- CNNs used as feature extractors produced better results than CNNs alone in practically all cases, although the large version is certainly more suitable than the tight one for feature extraction;
- 4
- however, CNNs alone, when trained on the tight versions, have proven to be very robust to every variation of BBs except the large case. Nevertheless, it is a rare case in real application scenarios.
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| PBS | Peripheral Blood Smear |
| RBC | Red Blood Cells |
| WBC | White Blood Cells |
| ALL | Acute Lymphoblastic Leukaemia |
| AML | Acute Myeloid Leukaemia |
| CLL | Chronic Lymphocytic Leukaemia |
| CML | Chronic Myeloid Leukaemia |
| CAD | Computer-Aided Diagnosis |
| ALL-IDB | Acute Lymphoblastic Leukaemia Image Database |
| R-WBC | Raabin-WBC |
| BB | Bounding Boxes |
| ML | Machine Learning |
| DL | Deep Learning |
| CNN | Convolutional Neural Network |
| LBP | Local Binary Pattern |
| GLCM | Gray Level Co-occurrence Matrix |
| FC | Fully Connected |
| TP | True Positive |
| TN | True Negative |
| FN | False Negative |
| FP | False Positive |
| A | Accuracy |
| P | Precision |
| R | Recall |
| S | Specificity |
| F1 | F1-score |
| WFS | Weighted F1-score |
References
- Ciesla, B. Hematology in Practice; FA Davis: Philadelphia, PA, USA, 2011. [Google Scholar]
- Biondi, A.; Cimino, G.; Pieters, R.; Pui, C.H. Biological and therapeutic aspects of infant leukemia. Blood 2000, 96, 24–33. [Google Scholar] [CrossRef]
- Labati, R.D.; Piuri, V.; Scotti, F. All-IDB: The acute lymphoblastic leukemia image database for image processing. In Proceedings of the IEEE ICIP International Conference on Image Processing, Brussels, Belgium, 29 December 2011; pp. 2045–2048. [Google Scholar]
- University Of Leeds The Histology Guide. 2021. Available online: https://www.histology.leeds.ac.uk/blood/blood_wbc.php (accessed on 10 June 2021).
- Bain, B.J. A Beginner’s Guide to Blood Cells; Wiley Online Library: New York, NY, USA, 2004. [Google Scholar]
- Cancer Treatment Centers of America, Types of Leukemia. 2021. Available online: https://www.cancercenter.com/cancer-types/leukemia/types (accessed on 11 June 2021).
- United States National Cancer Institute, Leukemia. 2021. Available online: https://www.cancer.gov/types/leukemia/hp (accessed on 11 June 2021).
- Madhloom, H.T.; Kareem, S.A.; Ariffin, H.; Zaidan, A.A.; Alanazi, H.O.; Zaidan, B.B. An automated white blood cell nucleus localization and segmentation using image arithmetic and automatic threshold. J. Appl. Sci. 2010, 10, 959–966. [Google Scholar] [CrossRef] [Green Version]
- Putzu, L.; Caocci, G.; Di Ruberto, C. Leucocyte classification for leukaemia detection using image processing techniques. AIM 2014, 62, 179–191. [Google Scholar] [CrossRef] [Green Version]
- Alomari, Y.M.; Sheikh Abdullah, S.N.H.; Zaharatul Azma, R.; Omar, K. Automatic detection and quantification of WBCs and RBCs using iterative structured circle detection algorithm. Comput. Math. Methods Med. 2014, 2014. [Google Scholar] [CrossRef] [Green Version]
- Mohapatra, S.; Patra, D.; Satpathy, S. An ensemble classifier system for early diagnosis of acute lymphoblastic leukemia in blood microscopic images. Neural Comput. Appl. 2014, 24, 1887–1904. [Google Scholar] [CrossRef]
- Ruberto, C.D.; Loddo, A.; Putzu, L. A leucocytes count system from blood smear images Segmentation and counting of white blood cells based on learning by sampling. Mach. Vis. Appl. 2016, 27, 1151–1160. [Google Scholar] [CrossRef]
- Vincent, I.; Kwon, K.; Lee, S.; Moon, K. Acute lymphoid leukemia classification using two-step neural network classifier. In Proceedings of the 2015 21st Korea-Japan Joint Workshop on Frontiers of Computer Vision, Mokpo, South Korea, 28–30 January 2015; pp. 1–4. [Google Scholar] [CrossRef]
- Singh, G.; Bathla, G.; Kaur, S. Design of new architecture to detect leukemia cancer from medical images. Int. J. Appl. Eng. Res. 2016, 11, 7087–7094. [Google Scholar]
- Di Ruberto, C.; Loddo, A.; Puglisi, G. Blob Detection and Deep Learning for Leukemic Blood Image Analysis. Appl. Sci. 2020, 10, 1176. [Google Scholar] [CrossRef]
- Krizhevsky, A.; Sutskever, I.; Hinton, G.E. ImageNet Classification with Deep Convolutional Neural Networks. In Proceedings of the 25th International Conference on Neural Information Processing Systems, Lake Tahoe, NV, USA, 3–6 December 2012; Volume 1, pp. 1097–1105. [Google Scholar]
- Qin, F.; Gao, N.; Peng, Y.; Wu, Z.; Shen, S.; Grudtsin, A. Fine-grained leukocyte classification with deep residual learning for microscopic images. Comput. Methods Programs Biomed. 2018, 162, 243–252. [Google Scholar] [CrossRef]
- Mahmood, N.H.; Lim, P.C.; Mazalan, S.M.; Razak, M.A.A. Blood cells extraction using color based segmentation technique. Int. J. Life Sci. Biotechnol. Pharma Res. 2013, 2. [Google Scholar] [CrossRef]
- Sipes, R.; Li, D. Using convolutional neural networks for automated fine grained image classification of acute lymphoblastic leukemia. In Proceedings of the 3rd International Conference on Computational Intelligence and Applications; Institute of Electrical and Electronics Engineers Inc.: New York, NY, USA, 2018; pp. 157–161. [Google Scholar]
- Ruberto, C.D.; Loddo, A.; Putzu, L. Detection of red and white blood cells from microscopic blood images using a region proposal approach. Comput. Biol. Med. 2020, 116, 103530. [Google Scholar] [CrossRef]
- Zhao, Z.; Zheng, P.; Xu, S.; Wu, X. Object Detection With Deep Learning: A Review. IEEE Trans. Neural Netw. Learn. Syst. 2019, 30, 3212–3232. [Google Scholar] [CrossRef] [Green Version]
- Ren, S.; He, K.; Girshick, R.B.; Sun, J. Faster R-CNN: Towards Real-Time Object Detection with Region Proposal Networks. IEEE Trans. Pattern Anal. Mach. Intell. 2017, 39, 1137–1149. [Google Scholar] [CrossRef] [Green Version]
- Çınar, A.; Tuncer, S.A. Classification of lymphocytes, monocytes, eosinophils, and neutrophils on white blood cells using hybrid Alexnet-GoogleNet-SVM. SN Appl. Sci. 2021, 3, 1–11. [Google Scholar] [CrossRef]
- Semerjian, S.; Khong, Y.F.; Mirzaei, S. White Blood Cells Classification Using Built-in Customizable Trained Convolutional Neural Network. In Proceedings of the 2021 International Conference on Emerging Smart Computing and Informatics (ESCI), Pune, India, 5–7 March 2021; pp. 357–362. [Google Scholar]
- Yao, X.; Sun, K.; Bu, X.; Zhao, C.; Jin, Y. Classification of white blood cells using weighted optimized deformable convolutional neural networks. Artif. Cells Nanomed. Biotechnol. 2021, 49, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Ridoy, M.A.R.; Islam, M.R. An automated approach to white blood cell classification using a lightweight convolutional neural network. In Proceedings of the 2020 2nd International Conference on Advanced Information and Communication Technology, Dhaka, Bangladesh, 28–29 November 2020; pp. 480–483. [Google Scholar]
- Pandey, P.; Kyatham, V.; Mishra, D.; Dastidar, T.R. Target-Independent Domain Adaptation for WBC Classification Using Generative Latent Search. IEEE Trans. Med. Imaging 2020, 39, 3979–3991. [Google Scholar] [CrossRef] [PubMed]
- Mooney, P. Blood Cell Images Data Set. 2014. Available online: https://github.com/Shenggan/BCCD_Dataset (accessed on 11 June 2021).
- Vogado, L.H.; Veras, R.M.; Araujo, F.H.; Silva, R.R.; Aires, K.R. Leukemia diagnosis in blood slides using transfer learning in CNNs and SVM for classification. Eng. Appl. Artif. Intell. 2018, 72, 415–422. [Google Scholar] [CrossRef]
- Sahlol, A.T.; Kollmannsberger, P.; Ewees, A.A. Efficient Classification of White Blood Cell Leukemia with Improved Swarm Optimization of Deep Features. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Toğaçar, M.; Ergen, B.; Cömert, Z. Classification of white blood cells using deep features obtained from Convolutional Neural Network models based on the combination of feature selection methods. Appl. Soft Comput. J. 2020, 97, 106810. [Google Scholar] [CrossRef]
- Ttp, T.; Pham, G.N.; Park, J.H.; Moon, K.S.; Lee, S.H.; Kwon, K.R. Acute leukemia classification using convolution neural network in clinical decision support system. CS IT Conf. Proc. 2017, 7, 49–53. [Google Scholar]
- Khandekar, R.; Shastry, P.; Jaishankar, S.; Faust, O.; Sampathila, N. Automated blast cell detection for Acute Lymphoblastic Leukemia diagnosis. Biomed. Signal Process. Control. 2021, 68, 102690. [Google Scholar] [CrossRef]
- Mondal, C.; Hasan, M.K.; Jawad, M.T.; Dutta, A.; Islam, M.R.; Awal, M.A.; Ahmad, M. Acute Lymphoblastic Leukemia Detection from Microscopic Images Using Weighted Ensemble of Convolutional Neural Networks. arXiv 2021, arXiv:2105.03995. [Google Scholar]
- Huang, Q.; Li, W.; Zhang, B.; Li, Q.; Tao, R.; Lovell, N.H. Blood Cell Classification Based on Hyperspectral Imaging with Modulated Gabor and CNN. IEEE J. Biomed. Health Inform. 2020, 24, 160–170. [Google Scholar] [CrossRef]
- Huang, P.; Wang, J.; Zhang, J.; Shen, Y.; Liu, C.; Song, W.; Wu, S.; Zuo, Y.; Lu, Z.; Li, D. Attention-Aware Residual Network Based Manifold Learning for White Blood Cells Classification. IEEE J. Biomed. Health Inform. 2020, 25, 1206–1214. [Google Scholar] [CrossRef] [PubMed]
- Duggal, R.; Gupta, A.; Gupta, R.; Mallick, P. SD-layer: Stain deconvolutional layer for CNNs in medical microscopic imaging. In International Conference on Medical Image Computing and Computer-Assisted Intervention; Springer: New York, NY, USA, 6–10 September 2017; pp. 435–443. [Google Scholar]
- Ahmed, N.; Yigit, A.; Isik, Z.; Alpkocak, A. Identification of Leukemia Subtypes from Microscopic Images Using Convolutional Neural Network. Diagnostics 2019, 9, 104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shafique, S.; Tehsin, S. Acute Lymphoblastic Leukemia Detection and Classification of Its Subtypes Using Pretrained Deep Convolutional Neural Networks. Technol. Cancer Res. Treat. 2018, 17, 1533033818802789. [Google Scholar] [CrossRef] [Green Version]
- Kouzehkanan, S.Z.M.; Saghari, S.; Tavakoli, E.; Rostami, P.; Abaszadeh, M.; Satlsar, E.S.; Mirzadeh, F.; Gheidishahran, M.; Gorgi, F.; Mohammadi, S.; et al. Raabin-WBC: A large free access dataset of white blood cells from normal peripheral blood. bioRxiv 2021. [Google Scholar] [CrossRef]
- Teague, M.R. Image analysis via the general theory of moments. J. Opt. Soc. Am. 1980, 70, 920–930. [Google Scholar] [CrossRef]
- Chong, C.W.; Raveendran, P.; Mukundan, R. Translation and scale invariants of Legendre moments. Pattern Recognit. 2004, 37, 119–129. [Google Scholar] [CrossRef]
- Ma, Z.; Kang, B.; Ma, J. Translation and scale invariant of Legendre moments for images retrieval. J. Inf. Comput. Sci. 2011, 8, 2221–2229. [Google Scholar]
- Oujaoura, M.; Minaoui, B.; Fakir, M. Image annotation by moments. Moments-Moment-Invariants Theory Appl. 2014, 1, 227–252. [Google Scholar]
- Di Ruberto, C.; Putzu, L.; Rodriguez, G. Fast and accurate computation of orthogonal moments for texture analysis. Pattern Recognit. 2018, 83, 498–510. [Google Scholar] [CrossRef] [Green Version]
- Putzu, L.; Di Ruberto, C. Rotation Invariant Co-occurrence Matrix Features. In 19th International Conference ICIAP on Image Analysis and Processing; Springer International Publishing: Cham, Switzerland, 2017; Volume 10484, pp. 391–401. [Google Scholar] [CrossRef]
- Ojala, T.; Pietikäinen, M.; Maenpaa, T. Multiresolution gray-scale and rotation invariant texture classification with local binary pattern. IEEE Trans. Pattern Anal. Mach. Intell. 2002, 24, 971–987. [Google Scholar] [CrossRef]
- Haralick, R.M.; Shanmugam, K.; Dinstein, I.H. Textural Features for Image Classification. IEEE Trans. Syst. Man Cybern. 1973, SMC-3, 610–621. [Google Scholar] [CrossRef] [Green Version]
- Mitro, J. Content-based image retrieval tutorial. arXiv 2016, arXiv:1608.03811. [Google Scholar]
- Samantaray, A.; Rahulkar, A. New design of adaptive Gabor wavelet filter bank for medical image retrieval. IET Image Process. 2020, 14, 679–687. [Google Scholar] [CrossRef]
- Singha, M.; Hemachandran, K.; Paul, A. Content-based image retrieval using the combination of the fast wavelet transformation and the colour histogram. IET Image Process. 2012, 6, 1221–1226. [Google Scholar] [CrossRef]
- Cover, T.M.; Hart, P.E. Nearest neighbor pattern classification. IEEE Trans. Inf. Theory 1967, 13, 21–27. [Google Scholar] [CrossRef]
- Lin, Y.; Lv, F.; Zhu, S.; Yang, M.; Cour, T.; Yu, K.; Cao, L.; Huang, T.S. Large-scale image classification: Fast feature extraction and SVM training. In Proceedings of the 24th IEEE Conference on Computer Vision and Pattern Recognition, Colorado Springs, CO, USA, 20–25 June 2011; pp. 1689–1696. [Google Scholar]
- Breiman, L. Random Forests. Mach. Learn. 2001, 4, 5–32. [Google Scholar] [CrossRef] [Green Version]
- Bagheri, M.A.; Montazer, G.A.; Escalera, S. Error correcting output codes for multiclass classification: Application to two image vision problems. In Proceedings of the 16th CSI International Symposium on Artificial Intelligence and Signal Processing, Shiraz, Iran, 2–3 May 2012; 3 May 2012; pp. 508–513. [Google Scholar]
- Deng, J.; Dong, W.; Socher, R.; Li, L.J.; Li, K.; Fei-Fei, L. Imagenet: A large-scale hierarchical image database. In Proceedings of the Computer Vision and Pattern Recognition (CVPR), Miami, FL, USA, 20–25 June 2009; pp. 248–255. [Google Scholar]
- Shin, H.; Roth, H.R.; Gao, M.; Lu, L.; Xu, Z.; Nogues, I.; Yao, J.; Mollura, D.; Summers, R.M. Deep Convolutional Neural Networks for Computer-Aided Detection: CNN Architectures, Dataset Characteristics and Transfer Learning. IEEE Trans. Med. Imaging 2016, 35, 1285–1298. [Google Scholar] [CrossRef] [Green Version]
- Simonyan, K.; Zisserman, A. Very Deep Convolutional Networks for Large-Scale Image Recognition. In Proceedings of the 3rd International Conference on Learning Representations, San Diego, CA, USA, 7–9 May 2015. [Google Scholar]
- He, K.; Zhang, X.; Ren, S.; Sun, J. Deep Residual Learning for Image Recognition. In Proceedings of the 2016 IEEE Conference on Computer Vision and Pattern Recognition, Las Vegas, NV, USA, 27–30 June 2016; pp. 770–778. [Google Scholar] [CrossRef] [Green Version]
- Szegedy, C.; Liu, W.; Jia, Y.; Sermanet, P.; Reed, S.; Anguelov, D.; Erhan, D.; Vanhoucke, V.; Rabinovich, A. Going deeper with convolutions. In Proceedings of the 2015 IEEE Conference on Computer Vision and Pattern Recognition, Boston, MA, USA, 7–12 June 2015; pp. 1–9. [Google Scholar] [CrossRef] [Green Version]
- Szegedy, C.; Vanhoucke, V.; Ioffe, S.; Shlens, J.; Wojna, Z. Rethinking the Inception Architecture for Computer Vision. In Proceedings of the 2016 IEEE Conference on Computer Vision and Pattern Recognition, Las Vegas, NV, USA, 26 June–1 July 2016; pp. 2818–2826. [Google Scholar] [CrossRef] [Green Version]



| Params | Value |
|---|---|
| Solver | Adam |
| Max Epochs | 50 |
| Mini Batch Size | 8 |
| Initial Learn Rate | 1e-4 |
| Learn Rate Drop Period | 10 |
| Learn Rate Drop Factor | 0.1 |
| L2 Regularisation | 0.1 |
| Classifier | Descriptor | Target Set | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Large | Tight | Eroded | Dilated | ||||||||||||||||||
| A | P | R | S | F1 | A | P | R | S | F1 | A | P | R | S | F1 | A | P | R | S | F1 | ||
| kNN | Legendre | 56.4 | 55.6 | 64.1 | 48.7 | 59.5 | 52.6 | 100.0 | 5.1 | 100.0 | 9.8 | 53.8 | 80.0 | 10.3 | 97.4 | 18.2 | 53.8 | 63.6 | 17.9 | 89.7 | 28.0 |
| Zernike | 35.9 | 33.3 | 28.2 | 43.6 | 30.6 | 10.3 | 10.3 | 10.3 | 10.3 | 10.3 | 19.2 | 16.7 | 15.4 | 23.1 | 16.0 | 21.8 | 25.0 | 28.2 | 15.4 | 26.5 | |
| HARri | 61.5 | 58.8 | 76.9 | 46.2 | 66.7 | 50.0 | 50.0 | 15.4 | 84.6 | 23.5 | 46.2 | 36.4 | 10.3 | 82.1 | 16.0 | 52.6 | 62.5 | 12.8 | 92.3 | 21.3 | |
| LBP18 | 53.8 | 52.5 | 82.1 | 25.6 | 64.0 | 47.4 | 48.7 | 94.9 | 0.0 | 64.3 | 50.0 | 50.0 | 100.0 | 0.0 | 66.7 | 47.4 | 48.7 | 94.9 | 0.0 | 64.3 | |
| Histogram | 64.1 | 60.8 | 79.5 | 48.7 | 68.9 | 46.2 | 33.3 | 7.7 | 84.6 | 12.5 | 47.4 | 37.5 | 7.7 | 87.2 | 12.8 | 43.6 | 27.3 | 7.7 | 79.5 | 12.0 | |
| Correlogram | 50.0 | 50.0 | 10.3 | 89.7 | 17.0 | 53.8 | 71.4 | 12.8 | 94.9 | 21.7 | 47.4 | 40.0 | 10.3 | 84.6 | 16.3 | 53.8 | 61.5 | 20.5 | 87.2 | 30.8 | |
| Haar wavelet | 75.6 | 69.2 | 92.3 | 59.0 | 79.1 | 70.5 | 73.5 | 64.1 | 76.9 | 68.5 | 53.8 | 53.7 | 56.4 | 51.3 | 55.0 | 79.5 | 87.1 | 69.2 | 89.7 | 77.1 | |
| Gabor wavelet | 43.6 | 45.1 | 59.0 | 28.2 | 51.1 | 60.3 | 63.3 | 48.7 | 71.8 | 55.1 | 53.8 | 54.3 | 48.7 | 59.0 | 51.4 | 52.6 | 52.2 | 61.5 | 43.6 | 56.5 | |
| AlexNet | 70.5 | 72.2 | 66.7 | 74.4 | 69.3 | 75.6 | 67.2 | 100.0 | 51.3 | 80.4 | 71.8 | 69.8 | 76.9 | 66.7 | 73.2 | 66.7 | 60.7 | 94.9 | 38.5 | 74.0 | |
| VGG-16 | 69.2 | 65.3 | 82.1 | 56.4 | 72.7 | 66.7 | 63.3 | 79.5 | 53.8 | 70.5 | 67.9 | 64.0 | 82.1 | 53.8 | 71.9 | 64.1 | 61.2 | 76.9 | 51.3 | 68.2 | |
| VGG-19 | 69.2 | 61.9 | 100.0 | 38.5 | 76.5 | 83.3 | 77.1 | 94.9 | 71.8 | 85.1 | 71.8 | 66.7 | 87.2 | 56.4 | 75.6 | 76.9 | 69.8 | 94.9 | 59.0 | 80.4 | |
| ResNet-18 | 75.6 | 67.9 | 97.4 | 53.8 | 80.0 | 66.7 | 69.7 | 59.0 | 74.4 | 63.9 | 60.3 | 60.0 | 61.5 | 59.0 | 60.8 | 73.1 | 73.7 | 71.8 | 74.4 | 72.7 | |
| ResNet-50 | 82.1 | 82.1 | 82.1 | 82.1 | 82.1 | 34.6 | 33.3 | 30.8 | 38.5 | 32.0 | 30.8 | 27.3 | 23.1 | 38.5 | 25.0 | 52.6 | 52.9 | 46.2 | 59.0 | 49.3 | |
| ResNet-101 | 76.9 | 80.0 | 71.8 | 82.1 | 75.7 | 46.2 | 38.5 | 12.8 | 79.5 | 19.2 | 39.7 | 10.0 | 2.6 | 76.9 | 4.1 | 50.0 | 50.0 | 20.5 | 79.5 | 29.1 | |
| GoogleNet | 82.1 | 77.8 | 89.7 | 74.4 | 83.3 | 51.3 | 100.0 | 2.6 | 100.0 | 5.0 | 50.0 | 0.0 | 0.0 | 100.0 | 0.0 | 57.7 | 100.0 | 15.4 | 100.0 | 26.7 | |
| Inceptionv3 | 76.9 | 70.6 | 92.3 | 61.5 | 80.0 | 33.3 | 31.4 | 28.2 | 38.5 | 29.7 | 39.7 | 30.0 | 15.4 | 64.1 | 20.3 | 26.9 | 30.4 | 35.9 | 17.9 | 32.9 | |
| AVG | 65.2 | 62.7 | 73.4 | 57.1 | 66.0 | 53.0 | 58.2 | 41.7 | 64.4 | 40.7 | 50.2 | 43.5 | 38.0 | 62.5 | 36.4 | 54.6 | 57.9 | 48.1 | 61.1 | 46.9 | |
| SVMRbf | Legendre | 60.3 | 68.2 | 38.5 | 82.1 | 49.2 | 48.7 | 40.0 | 5.1 | 92.3 | 9.1 | 47.4 | 40.0 | 10.3 | 84.6 | 16.3 | 51.3 | 100.0 | 2.6 | 100.0 | 5.0 |
| Zernike | 41.0 | 42.6 | 51.3 | 30.8 | 46.5 | 38.5 | 41.8 | 59.0 | 17.9 | 48.9 | 60.3 | 58.3 | 71.8 | 48.7 | 64.4 | 48.7 | 49.2 | 79.5 | 17.9 | 60.8 | |
| HARri | 59.0 | 55.7 | 87.2 | 30.8 | 68.0 | 52.6 | 52.9 | 46.2 | 59.0 | 49.3 | 52.6 | 53.3 | 41.0 | 64.1 | 46.4 | 56.4 | 57.1 | 51.3 | 61.5 | 54.1 | |
| LBP18 | 61.5 | 58.2 | 82.1 | 41.0 | 68.1 | 50.0 | 50.0 | 100.0 | 0.0 | 66.7 | 50.0 | 50.0 | 100.0 | 0.0 | 66.7 | 50.0 | 50.0 | 100.0 | 0.0 | 66.7 | |
| Histogram | 38.5 | 35.5 | 28.2 | 48.7 | 31.4 | 47.4 | 37.5 | 7.7 | 87.2 | 12.8 | 46.2 | 20.0 | 2.6 | 89.7 | 4.5 | 35.9 | 7.7 | 2.6 | 69.2 | 3.8 | |
| Correlogram | 55.1 | 53.6 | 76.9 | 33.3 | 63.2 | 51.3 | 51.4 | 48.7 | 53.8 | 50.0 | 47.4 | 47.2 | 43.6 | 51.3 | 45.3 | 52.6 | 52.8 | 48.7 | 56.4 | 50.7 | |
| Haar wavelet | 82.1 | 74.5 | 97.4 | 66.7 | 84.4 | 50.0 | 50.0 | 94.9 | 5.1 | 65.5 | 50.0 | 50.0 | 97.4 | 2.6 | 66.1 | 69.2 | 62.7 | 94.9 | 43.6 | 75.5 | |
| Gabor wavelet | 50.0 | 50.0 | 76.9 | 23.1 | 60.6 | 64.1 | 69.0 | 51.3 | 76.9 | 58.8 | 60.3 | 65.4 | 43.6 | 76.9 | 52.3 | 55.1 | 54.8 | 59.0 | 51.3 | 56.8 | |
| AlexNet | 44.9 | 46.6 | 69.2 | 20.5 | 55.7 | 67.9 | 93.8 | 38.5 | 97.4 | 54.5 | 61.5 | 100.0 | 23.1 | 100.0 | 37.5 | 69.2 | 75.9 | 56.4 | 82.1 | 64.7 | |
| VGG-16 | 71.8 | 64.9 | 94.9 | 48.7 | 77.1 | 69.2 | 64.7 | 84.6 | 53.8 | 73.3 | 59.0 | 56.1 | 82.1 | 35.9 | 66.7 | 67.9 | 63.5 | 84.6 | 51.3 | 72.5 | |
| VGG-19 | 62.8 | 57.4 | 100.0 | 25.6 | 72.9 | 74.4 | 66.1 | 100.0 | 48.7 | 79.6 | 70.5 | 62.9 | 100.0 | 41.0 | 77.2 | 70.5 | 62.9 | 100.0 | 41.0 | 77.2 | |
| ResNet-18 | 78.2 | 71.2 | 94.9 | 61.5 | 81.3 | 70.5 | 80.8 | 53.8 | 87.2 | 64.6 | 62.8 | 67.9 | 48.7 | 76.9 | 56.7 | 74.4 | 80.6 | 64.1 | 84.6 | 71.4 | |
| ResNet-50 | 83.3 | 81.0 | 87.2 | 79.5 | 84.0 | 35.9 | 33.3 | 28.2 | 43.6 | 30.6 | 28.2 | 27.0 | 25.6 | 30.8 | 26.3 | 44.9 | 44.1 | 38.5 | 51.3 | 41.1 | |
| ResNet-101 | 82.1 | 85.7 | 76.9 | 87.2 | 81.1 | 73.1 | 95.0 | 48.7 | 97.4 | 64.4 | 61.5 | 90.9 | 25.6 | 97.4 | 40.0 | 79.5 | 92.6 | 64.1 | 94.9 | 75.8 | |
| GoogleNet | 83.3 | 79.5 | 89.7 | 76.9 | 84.3 | 51.3 | 100.0 | 2.6 | 100.0 | 5.0 | 50.0 | 0.0 | 0.0 | 100.0 | 0.0 | 57.7 | 100.0 | 15.4 | 100.0 | 26.7 | |
| Inceptionv3 | 84.6 | 78.7 | 94.9 | 74.4 | 86.0 | 64.1 | 59.6 | 87.2 | 41.0 | 70.8 | 65.4 | 62.0 | 79.5 | 51.3 | 69.7 | 47.4 | 48.4 | 76.9 | 17.9 | 59.4 | |
| AVG | 64.9 | 62.7 | 77.9 | 51.9 | 68.4 | 56.8 | 61.6 | 53.5 | 60.1 | 50.2 | 54.6 | 53.2 | 49.7 | 59.5 | 46.0 | 58.2 | 62.6 | 58.7 | 57.7 | 53.9 | |
| RF | Legendre | 60.3 | 60.0 | 61.5 | 59.0 | 60.8 | 51.3 | 100.0 | 2.6 | 100.0 | 5.0 | 52.6 | 66.7 | 10.3 | 94.9 | 17.8 | 57.7 | 87.5 | 17.9 | 97.4 | 29.8 |
| Zernike | 25.6 | 25.6 | 25.6 | 25.6 | 25.6 | 35.9 | 36.6 | 38.5 | 33.3 | 37.5 | 26.9 | 17.9 | 12.8 | 41.0 | 14.9 | 43.6 | 40.7 | 28.2 | 59.0 | 33.3 | |
| HARri | 52.6 | 51.6 | 82.1 | 23.1 | 63.4 | 48.7 | 47.8 | 28.2 | 69.2 | 35.5 | 43.6 | 39.1 | 23.1 | 64.1 | 29.0 | 56.4 | 61.9 | 33.3 | 79.5 | 43.3 | |
| LBP18 | 47.4 | 48.6 | 92.3 | 2.6 | 63.7 | 50.0 | 0.0 | 0.0 | 100.0 | 0.0 | 50.0 | 0.0 | 0.0 | 100.0 | 0.0 | 51.3 | 100.0 | 2.6 | 100.0 | 5.0 | |
| Histogram | 65.4 | 61.5 | 82.1 | 48.7 | 70.3 | 60.3 | 72.2 | 33.3 | 87.2 | 45.6 | 50.0 | 50.0 | 28.2 | 71.8 | 36.1 | 52.6 | 57.1 | 20.5 | 84.6 | 30.2 | |
| Correlogram | 64.1 | 62.2 | 71.8 | 56.4 | 66.7 | 59.0 | 61.3 | 48.7 | 69.2 | 54.3 | 56.4 | 57.1 | 51.3 | 61.5 | 54.1 | 55.1 | 56.3 | 46.2 | 64.1 | 50.7 | |
| Haar wavelet | 46.2 | 44.8 | 33.3 | 59.0 | 38.2 | 51.3 | 53.8 | 17.9 | 84.6 | 26.9 | 51.3 | 53.8 | 17.9 | 84.6 | 26.9 | 53.8 | 60.0 | 23.1 | 84.6 | 33.3 | |
| Gabor wavelet | 42.3 | 45.2 | 71.8 | 12.8 | 55.4 | 35.9 | 40.7 | 61.5 | 10.3 | 49.0 | 46.2 | 46.5 | 51.3 | 41.0 | 48.8 | 39.7 | 43.1 | 64.1 | 15.4 | 51.5 | |
| AlexNet | 60.3 | 62.5 | 51.3 | 69.2 | 56.3 | 88.5 | 96.9 | 79.5 | 97.4 | 87.3 | 69.2 | 89.5 | 43.6 | 94.9 | 58.6 | 69.2 | 69.2 | 69.2 | 69.2 | 69.2 | |
| VGG-16 | 71.8 | 66.7 | 87.2 | 56.4 | 75.6 | 71.8 | 66.0 | 89.7 | 53.8 | 76.1 | 64.1 | 59.0 | 92.3 | 35.9 | 72.0 | 70.5 | 64.8 | 89.7 | 51.3 | 75.3 | |
| VGG-19 | 65.4 | 59.1 | 100.0 | 30.8 | 74.3 | 78.2 | 73.9 | 87.2 | 69.2 | 80.0 | 69.2 | 65.3 | 82.1 | 56.4 | 72.7 | 80.8 | 74.0 | 94.9 | 66.7 | 83.1 | |
| ResNet-18 | 76.9 | 70.6 | 92.3 | 61.5 | 80.0 | 67.9 | 79.2 | 48.7 | 87.2 | 60.3 | 57.7 | 59.4 | 48.7 | 66.7 | 53.5 | 76.9 | 88.9 | 61.5 | 92.3 | 72.7 | |
| ResNet-50 | 80.8 | 78.6 | 84.6 | 76.9 | 81.5 | 34.6 | 35.0 | 35.9 | 33.3 | 35.4 | 30.8 | 27.3 | 23.1 | 38.5 | 25.0 | 46.2 | 45.2 | 35.9 | 56.4 | 40.0 | |
| ResNet-101 | 79.5 | 81.1 | 76.9 | 82.1 | 78.9 | 59.0 | 66.7 | 35.9 | 82.1 | 46.7 | 52.6 | 55.6 | 25.6 | 79.5 | 35.1 | 62.8 | 70.8 | 43.6 | 82.1 | 54.0 | |
| GoogleNet | 83.3 | 79.5 | 89.7 | 76.9 | 84.3 | 51.3 | 100.0 | 2.6 | 100.0 | 5.0 | 50.0 | 0.0 | 0.0 | 100.0 | 0.0 | 56.4 | 100.0 | 12.8 | 100.0 | 22.7 | |
| InceptionV3 | 76.9 | 70.6 | 92.3 | 61.5 | 80.0 | 33.3 | 31.4 | 28.2 | 38.5 | 29.7 | 39.7 | 30.0 | 15.4 | 64.1 | 20.3 | 26.9 | 30.4 | 35.9 | 17.9 | 32.9 | |
| AVG | 62.4 | 60.5 | 74.7 | 50.2 | 65.9 | 54.8 | 60.1 | 39.9 | 69.7 | 42.1 | 50.6 | 44.8 | 32.9 | 68.4 | 35.3 | 56.3 | 65.6 | 42.5 | 70.0 | 45.5 | |
| CNN | AlexNet | 44.9 | 20.5 | 40.0 | 46.5 | 27.1 | 65.4 | 94.9 | 59.7 | 87.5 | 73.3 | 57.7 | 100.0 | 54.2 | 100.0 | 70.3 | 71.8 | 89.7 | 66.0 | 84.0 | 76.1 |
| VGG-16 | 61.5 | 23.1 | 100.0 | 56.5 | 37.5 | 73.1 | 56.4 | 84.6 | 67.3 | 67.7 | 67.9 | 43.6 | 85.0 | 62.1 | 57.6 | 70.5 | 51.3 | 83.3 | 64.8 | 63.5 | |
| VGG-19 | 57.7 | 15.4 | 100.0 | 54.2 | 26.7 | 71.8 | 59.0 | 79.3 | 67.3 | 67.6 | 66.7 | 51.3 | 74.1 | 62.7 | 60.6 | 70.5 | 48.7 | 86.4 | 64.3 | 62.3 | |
| ResNet-18 | 75.6 | 51.3 | 100.0 | 67.2 | 67.8 | 66.7 | 33.3 | 100.0 | 60.0 | 50.0 | 61.5 | 23.1 | 100.0 | 56.5 | 37.5 | 70.5 | 43.6 | 94.4 | 63.3 | 59.6 | |
| ResNet-50 | 75.6 | 64.1 | 83.3 | 70.8 | 72.5 | 35.9 | 35.9 | 35.9 | 35.9 | 35.9 | 25.6 | 20.5 | 22.9 | 27.9 | 21.6 | 41.0 | 41.0 | 41.0 | 41.0 | 41.0 | |
| ResNet-101 | 75.6 | 56.4 | 91.7 | 68.5 | 69.8 | 71.8 | 59.0 | 79.3 | 67.3 | 67.6 | 61.5 | 48.7 | 65.5 | 59.2 | 55.9 | 70.5 | 48.7 | 86.4 | 64.3 | 62.3 | |
| GoogleNet | 71.8 | 43.6 | 100.0 | 63.9 | 60.7 | 66.7 | 100.0 | 60.0 | 100.0 | 75.0 | 64.1 | 100.0 | 58.2 | 100.0 | 73.6 | 88.5 | 100.0 | 81.2 | 100.0 | 89.7 | |
| InceptionV3 | 78.2 | 64.1 | 89.3 | 72.0 | 74.6 | 34.6 | 41.0 | 36.4 | 32.3 | 38.5 | 39.7 | 64.1 | 43.1 | 30.0 | 51.5 | 29.5 | 23.1 | 26.5 | 31.8 | 24.6 | |
| AVG | 67.6 | 42.3 | 88.0 | 62.5 | 54.6 | 60.7 | 59.9 | 66.9 | 64.7 | 59.5 | 55.6 | 56.4 | 62.9 | 62.3 | 53.6 | 64.1 | 55.8 | 70.7 | 64.2 | 59.9 | |
| Classifier | Descriptor | Target Set | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Large | Tight | Eroded | Dilated | ||||||||||||||||||
| A | P | R | S | F1 | A | P | R | S | F1 | A | P | R | S | F1 | A | P | R | S | F1 | ||
| kNN | Legendre | 75.0 | 39.1 | 37.8 | 84.4 | 37.9 | 65.7 | 22.5 | 13.7 | 78.9 | 11.6 | 66.3 | 25.3 | 15.4 | 79.3 | 12.5 | 67.6 | 20.7 | 18.9 | 80.1 | 16.8 |
| Zernike | 71.3 | 28.9 | 28.5 | 82.3 | 28.3 | 65.8 | 13.1 | 15.7 | 78.4 | 12.6 | 69.0 | 17.1 | 22.7 | 80.8 | 16.4 | 68.3 | 20.3 | 20.6 | 80.3 | 16.7 | |
| HARri | 70.0 | 25.3 | 25.3 | 81.3 | 25.2 | 65.9 | 16.8 | 15.1 | 78.8 | 13.1 | 65.7 | 19.0 | 14.8 | 78.4 | 12.3 | 64.8 | 10.0 | 12.5 | 78.1 | 10.0 | |
| LBP18 | 78.3 | 47.2 | 46.2 | 86.6 | 46.4 | 68.1 | 3.6 | 18.9 | 81.1 | 6.0 | 68.1 | 3.6 | 18.9 | 81.1 | 6.0 | 68.1 | 3.6 | 18.9 | 81.1 | 6.0 | |
| Histogram | 70.2 | 25.6 | 26.2 | 81.4 | 25.7 | 69.0 | 23.3 | 23.5 | 80.4 | 21.8 | 70.1 | 24.0 | 26.5 | 80.9 | 23.2 | 67.2 | 18.9 | 18.6 | 79.2 | 17.9 | |
| Correlogram | 71.1 | 26.9 | 27.6 | 82.3 | 21.6 | 69.2 | 21.3 | 23.0 | 81.0 | 18.3 | 70.6 | 26.3 | 26.7 | 81.8 | 23.5 | 70.6 | 28.5 | 26.5 | 82.0 | 22.5 | |
| Haar wavelet | 79.6 | 49.9 | 49.7 | 87.3 | 49.3 | 63.8 | 14.7 | 9.3 | 77.4 | 10.9 | 64.4 | 12.8 | 11.6 | 77.6 | 11.0 | 63.0 | 13.2 | 7.0 | 77.1 | 8.8 | |
| Gabor wavelet | 74.4 | 36.9 | 36.0 | 84.1 | 35.1 | 71.5 | 28.8 | 28.8 | 82.0 | 23.5 | 69.6 | 24.1 | 23.8 | 81.2 | 19.2 | 72.0 | 31.3 | 29.7 | 82.5 | 25.5 | |
| AlexNet | 97.0 | 92.9 | 92.4 | 98.0 | 92.3 | 77.0 | 45.2 | 43.3 | 85.1 | 36.4 | 76.2 | 38.6 | 41.3 | 84.6 | 32.4 | 80.6 | 55.1 | 52.3 | 87.4 | 45.5 | |
| VGG-16 | 98.3 | 96.0 | 95.9 | 98.9 | 95.9 | 77.9 | 56.1 | 46.5 | 85.9 | 40.5 | 73.4 | 51.4 | 35.5 | 82.8 | 29.1 | 82.8 | 61.9 | 58.4 | 89.0 | 54.6 | |
| VGG-19 | 95.7 | 89.2 | 89.2 | 97.2 | 89.1 | 68.5 | 20.2 | 20.9 | 80.8 | 10.0 | 68.1 | 18.2 | 19.5 | 80.9 | 7.8 | 70.1 | 29.5 | 25.6 | 81.6 | 16.5 | |
| ResNet-18 | 96.3 | 90.6 | 90.7 | 97.7 | 90.6 | 72.2 | 33.6 | 30.2 | 83.0 | 20.2 | 69.7 | 42.7 | 23.3 | 81.9 | 13.6 | 74.2 | 40.4 | 35.5 | 84.2 | 26.5 | |
| ResNet-50 | 96.8 | 92.8 | 92.2 | 97.9 | 92.1 | 81.5 | 62.6 | 55.2 | 88.0 | 51.8 | 77.0 | 61.0 | 44.2 | 85.0 | 41.3 | 83.9 | 63.0 | 61.0 | 89.6 | 57.5 | |
| ResNet-101 | 98.6 | 96.5 | 96.5 | 99.1 | 96.5 | 73.5 | 37.2 | 35.8 | 82.8 | 28.5 | 69.7 | 35.4 | 26.2 | 80.2 | 16.2 | 78.1 | 54.5 | 47.1 | 86.0 | 39.5 | |
| GoogleNet | 97.7 | 94.4 | 94.2 | 98.5 | 94.1 | 77.5 | 46.6 | 44.5 | 85.7 | 34.6 | 75.9 | 43.2 | 40.7 | 84.7 | 28.7 | 82.5 | 55.7 | 57.3 | 89.0 | 51.3 | |
| Inceptionv3 | 98.4 | 96.4 | 96.2 | 99.0 | 96.2 | 70.1 | 59.5 | 27.3 | 80.5 | 19.0 | 69.3 | 62.8 | 25.3 | 79.9 | 15.8 | 72.7 | 59.4 | 33.7 | 82.3 | 28.7 | |
| AVG | 85.5 | 64.3 | 64.0 | 91.0 | 63.5 | 71.1 | 31.6 | 28.2 | 81.9 | 22.4 | 70.2 | 31.6 | 26.0 | 81.3 | 19.3 | 72.9 | 35.4 | 32.7 | 83.1 | 27.8 | |
| SVMRbf | Legendre | 77.1 | 43.1 | 43.0 | 85.6 | 43.0 | 64.6 | 18.6 | 10.8 | 78.5 | 6.9 | 64.5 | 43.5 | 11.0 | 78.1 | 10.2 | 69.0 | 34.3 | 21.8 | 81.1 | 13.8 |
| Zernike | 74.9 | 35.7 | 37.8 | 84.3 | 35.2 | 63.6 | 11.1 | 9.0 | 77.4 | 6.8 | 68.2 | 29.3 | 20.1 | 80.6 | 13.6 | 68.0 | 12.5 | 19.5 | 80.5 | 11.9 | |
| HARri | 74.7 | 37.4 | 36.6 | 84.6 | 35.0 | 71.6 | 23.1 | 29.4 | 81.8 | 24.0 | 73.1 | 35.1 | 33.1 | 82.8 | 28.9 | 70.4 | 22.8 | 26.5 | 81.3 | 23.2 | |
| LBP18 | 74.8 | 42.9 | 38.1 | 84.1 | 39.0 | 67.7 | 4.5 | 21.2 | 78.8 | 7.4 | 67.7 | 4.5 | 21.2 | 78.8 | 7.4 | 67.7 | 4.5 | 21.2 | 78.8 | 7.4 | |
| Histogram | 79.6 | 51.2 | 49.1 | 87.8 | 45.3 | 62.8 | 6.1 | 5.2 | 77.6 | 5.0 | 63.8 | 6.6 | 8.4 | 78.0 | 6.9 | 63.5 | 14.8 | 7.0 | 77.9 | 8.5 | |
| Correlogram | 78.4 | 48.4 | 46.8 | 86.5 | 47.3 | 72.2 | 44.4 | 31.7 | 82.1 | 30.5 | 71.8 | 43.3 | 30.8 | 81.7 | 28.7 | 73.8 | 46.5 | 35.5 | 83.1 | 35.4 | |
| Haar wavelet | 82.5 | 57.4 | 56.7 | 89.1 | 56.6 | 67.7 | 15.8 | 20.1 | 79.2 | 12.7 | 68.8 | 19.0 | 23.3 | 79.8 | 15.1 | 67.8 | 21.1 | 19.8 | 79.6 | 15.0 | |
| Gabor wavelet | 79.9 | 47.7 | 49.7 | 87.4 | 48.4 | 71.8 | 34.1 | 29.9 | 82.1 | 24.3 | 71.4 | 33.5 | 29.1 | 81.8 | 24.4 | 73.6 | 38.3 | 34.3 | 83.4 | 30.3 | |
| AlexNet | 98.0 | 95.2 | 95.1 | 98.7 | 95.0 | 76.3 | 50.3 | 41.6 | 84.4 | 33.4 | 75.2 | 46.3 | 39.0 | 83.7 | 29.8 | 80.4 | 57.6 | 51.7 | 87.1 | 44.6 | |
| VGG-16 | 98.7 | 96.8 | 96.8 | 99.2 | 96.8 | 79.2 | 55.2 | 49.4 | 86.9 | 42.2 | 75.1 | 45.5 | 39.5 | 84.1 | 32.2 | 83.2 | 59.5 | 59.3 | 89.5 | 53.8 | |
| VGG-19 | 96.1 | 90.0 | 90.1 | 97.5 | 90.1 | 68.0 | 4.6 | 19.2 | 80.7 | 7.1 | 68.0 | 3.7 | 18.9 | 80.9 | 6.2 | 68.3 | 26.4 | 20.3 | 80.8 | 9.6 | |
| ResNet-18 | 96.2 | 90.3 | 90.4 | 97.6 | 90.3 | 70.8 | 39.7 | 26.7 | 82.3 | 16.9 | 68.8 | 37.8 | 20.9 | 81.3 | 9.8 | 72.8 | 41.2 | 32.3 | 83.3 | 22.7 | |
| ResNet-50 | 97.9 | 95.0 | 94.8 | 98.6 | 94.7 | 79.6 | 59.8 | 50.6 | 86.8 | 47.7 | 73.8 | 58.2 | 36.3 | 82.9 | 32.2 | 84.3 | 62.8 | 61.9 | 89.8 | 58.0 | |
| ResNet-101 | 98.8 | 97.1 | 97.1 | 99.2 | 97.1 | 75.7 | 37.7 | 41.3 | 84.2 | 31.5 | 73.9 | 36.4 | 36.6 | 83.0 | 27.1 | 77.7 | 57.9 | 46.2 | 85.7 | 37.8 | |
| GoogleNet | 97.7 | 94.5 | 94.2 | 98.5 | 94.1 | 74.6 | 36.2 | 36.3 | 84.4 | 25.6 | 72.8 | 34.4 | 32.0 | 83.3 | 20.3 | 78.5 | 43.5 | 46.2 | 86.6 | 38.1 | |
| Inceptionv3 | 98.3 | 96.0 | 95.9 | 98.9 | 95.9 | 70.9 | 59.6 | 29.4 | 81.1 | 21.9 | 69.0 | 40.1 | 24.7 | 79.7 | 14.9 | 74.2 | 58.4 | 37.5 | 83.4 | 32.4 | |
| AVG | 87.7 | 69.9 | 69.5 | 92.3 | 69.0 | 71.1 | 31.3 | 28.2 | 81.8 | 21.5 | 70.4 | 32.3 | 26.6 | 81.3 | 19.2 | 73.3 | 37.6 | 33.8 | 83.2 | 27.7 | |
| RF | Legendre | 75.2 | 38.3 | 38.4 | 84.5 | 38.2 | 69.2 | 11.7 | 21.8 | 81.5 | 12.0 | 67.4 | 13.9 | 18.0 | 80.2 | 12.4 | 70.2 | 24.0 | 24.7 | 82.1 | 15.4 |
| Zernike | 73.2 | 31.3 | 33.1 | 83.5 | 31.5 | 65.4 | 7.7 | 12.8 | 79.0 | 6.3 | 68.7 | 16.3 | 20.9 | 81.2 | 12.1 | 68.3 | 16.9 | 19.8 | 80.9 | 11.2 | |
| HARri | 83.5 | 60.5 | 59.0 | 89.8 | 59.4 | 72.6 | 21.3 | 31.1 | 83.2 | 22.1 | 70.6 | 20.3 | 26.2 | 82.0 | 17.0 | 74.2 | 47.1 | 35.8 | 83.9 | 28.6 | |
| LBP18 | 79.9 | 53.5 | 49.4 | 87.8 | 49.9 | 68.1 | 9.8 | 22.1 | 79.2 | 11.0 | 67.8 | 13.9 | 21.5 | 78.9 | 9.4 | 68.8 | 15.3 | 23.8 | 79.7 | 14.3 | |
| Histogram | 81.0 | 53.9 | 52.9 | 88.2 | 52.6 | 66.4 | 46.9 | 15.7 | 79.4 | 13.6 | 66.2 | 31.8 | 14.8 | 79.3 | 13.2 | 67.1 | 29.4 | 17.4 | 79.6 | 17.3 | |
| Correlogram | 79.0 | 48.5 | 48.0 | 87.0 | 47.9 | 74.4 | 36.9 | 36.3 | 84.0 | 31.2 | 73.4 | 36.1 | 34.0 | 83.4 | 29.2 | 75.4 | 37.6 | 39.2 | 84.6 | 35.3 | |
| Haar wavelet | 88.3 | 71.0 | 70.6 | 92.7 | 70.6 | 63.7 | 17.6 | 8.4 | 77.7 | 8.4 | 63.7 | 17.0 | 8.1 | 77.7 | 7.5 | 65.7 | 30.5 | 13.7 | 78.9 | 14.3 | |
| Gabor wavelet | 81.3 | 51.2 | 53.2 | 88.3 | 51.8 | 72.3 | 36.3 | 30.8 | 82.5 | 26.4 | 70.6 | 30.6 | 26.7 | 81.4 | 22.5 | 72.9 | 32.8 | 32.3 | 83.0 | 27.9 | |
| AlexNet | 97.7 | 94.5 | 94.2 | 98.4 | 94.1 | 77.8 | 50.8 | 45.3 | 85.6 | 38.5 | 76.6 | 49.1 | 42.2 | 84.8 | 33.5 | 82.0 | 55.8 | 55.5 | 88.3 | 47.2 | |
| VGG-16 | 98.6 | 96.6 | 96.5 | 99.1 | 96.5 | 71.1 | 47.6 | 29.4 | 81.2 | 21.8 | 68.9 | 39.6 | 24.1 | 79.7 | 13.2 | 76.4 | 53.7 | 42.2 | 84.6 | 38.6 | |
| VGG-19 | 95.8 | 89.4 | 89.5 | 97.3 | 89.4 | 68.3 | 26.7 | 20.3 | 80.8 | 9.3 | 67.9 | 3.9 | 18.9 | 80.8 | 6.5 | 69.7 | 28.9 | 24.4 | 81.5 | 15.3 | |
| ResNet-18 | 96.3 | 90.5 | 90.7 | 97.7 | 90.6 | 71.7 | 39.3 | 29.4 | 82.6 | 18.6 | 69.2 | 40.4 | 22.4 | 81.4 | 12.0 | 72.8 | 38.9 | 32.6 | 83.2 | 22.3 | |
| ResNet-50 | 97.7 | 94.3 | 94.2 | 98.5 | 94.1 | 75.5 | 53.5 | 39.8 | 84.1 | 36.3 | 72.3 | 51.2 | 32.0 | 82.0 | 25.8 | 81.0 | 59.2 | 53.5 | 87.8 | 50.8 | |
| ResNet-101 | 98.7 | 96.8 | 96.8 | 99.2 | 96.8 | 70.3 | 26.8 | 25.9 | 81.3 | 15.7 | 69.8 | 27.0 | 24.4 | 81.1 | 12.5 | 74.7 | 50.9 | 37.2 | 84.1 | 29.1 | |
| GoogleNet | 97.3 | 93.7 | 93.3 | 98.2 | 93.3 | 76.9 | 46.0 | 43.3 | 85.3 | 33.5 | 75.6 | 39.5 | 40.1 | 84.5 | 28.4 | 81.0 | 56.0 | 53.5 | 87.9 | 48.3 | |
| Inceptionv3 | 98.3 | 96.0 | 95.9 | 99.0 | 95.9 | 69.4 | 50.7 | 25.0 | 80.2 | 14.3 | 68.6 | 28.9 | 23.3 | 79.6 | 11.8 | 72.0 | 51.8 | 31.1 | 82.1 | 23.0 | |
| AVG | 88.9 | 72.5 | 72.2 | 93.1 | 72.0 | 70.8 | 33.1 | 27.3 | 81.7 | 19.9 | 69.8 | 28.7 | 24.9 | 81.1 | 16.7 | 73.3 | 39.3 | 33.5 | 83.3 | 27.4 | |
| CNN | AlexNet | 98.1 | 95.5 | 95.3 | 98.9 | 95.4 | 64.7 | 82.6 | 43.9 | 96.1 | 50.2 | 63.3 | 80.2 | 38.7 | 95.4 | 46.2 | 72.9 | 86.6 | 51.4 | 96.9 | 58.8 |
| VGG-16 | 96.3 | 90.7 | 90.4 | 97.7 | 90.5 | 61.9 | 78.3 | 32.8 | 94.0 | 42.5 | 37.3 | 88.7 | 23.3 | 97.4 | 33.1 | 68.9 | 73.9 | 36.3 | 92.9 | 45.5 | |
| VGG-19 | 96.2 | 90.5 | 90.4 | 97.7 | 90.5 | 49.2 | 78.2 | 23.8 | 93.5 | 34.4 | 35.0 | 89.1 | 20.6 | 97.5 | 31.8 | 57.7 | 74.0 | 27.6 | 92.6 | 37.3 | |
| ResNet-18 | 98.9 | 97.4 | 97.4 | 99.3 | 97.4 | 57.5 | 89.9 | 41.0 | 98.0 | 49.9 | 44.2 | 87.9 | 30.5 | 97.6 | 39.1 | 66.6 | 90.8 | 47.7 | 98.1 | 56.6 | |
| ResNet-101 | 98.7 | 96.8 | 96.8 | 99.2 | 96.8 | 66.7 | 86.7 | 47.7 | 97.1 | 54.6 | 52.2 | 87.3 | 37.8 | 97.4 | 44.7 | 76.4 | 85.9 | 57.3 | 96.9 | 62.5 | |
| ResNet-50 | 98.3 | 96.0 | 95.9 | 99.0 | 95.9 | 70.0 | 86.2 | 52.6 | 97.1 | 57.0 | 56.6 | 85.4 | 41.0 | 97.0 | 46.2 | 78.4 | 86.8 | 60.8 | 97.1 | 65.4 | |
| GoogleNet | 98.4 | 96.0 | 95.9 | 99.0 | 95.9 | 73.0 | 85.3 | 42.4 | 96.4 | 54.1 | 70.4 | 86.7 | 38.7 | 95.9 | 52.5 | 77.5 | 82.9 | 50.0 | 96.1 | 58.3 | |
| InceptionV3 | 98.4 | 96.3 | 96.2 | 99.0 | 96.2 | 48.7 | 82.1 | 29.6 | 96.1 | 37.2 | 43.2 | 83.9 | 25.0 | 96.3 | 34.7 | 58.9 | 79.2 | 38.4 | 95.5 | 43.0 | |
| AVG | 97.9 | 94.9 | 94.8 | 98.7 | 94.8 | 61.5 | 83.7 | 39.2 | 96.0 | 47.5 | 50.3 | 86.1 | 31.9 | 96.8 | 41.0 | 69.7 | 82.5 | 46.2 | 95.8 | 53.4 | |
| Classifier | Descriptor | Target Set | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Large | Tight | Eroded | Dilated | ||||||||||||||||||
| A | P | R | S | F1 | A | P | R | S | F1 | A | P | R | S | F1 | A | P | R | S | F1 | ||
| kNN | Legendre | 47.4 | 48.7 | 94.9 | 0.0 | 64.3 | 69.2 | 68.3 | 71.8 | 66.7 | 70.0 | 73.1 | 87.5 | 53.8 | 92.3 | 66.7 | 60.3 | 56.3 | 92.3 | 28.2 | 69.9 |
| Zernike | 46.2 | 48.0 | 92.3 | 0.0 | 63.2 | 47.4 | 47.1 | 41.0 | 53.8 | 43.8 | 51.3 | 51.9 | 35.9 | 66.7 | 42.4 | 39.7 | 42.3 | 56.4 | 23.1 | 48.4 | |
| HARri | 47.4 | 46.9 | 38.5 | 56.4 | 42.3 | 62.8 | 61.9 | 66.7 | 59.0 | 64.2 | 59.0 | 61.3 | 48.7 | 69.2 | 54.3 | 52.6 | 51.8 | 74.4 | 30.8 | 61.1 | |
| LBP18 | 50.0 | 50.0 | 100.0 | 0.0 | 66.7 | 59.0 | 56.9 | 74.4 | 43.6 | 64.4 | 67.9 | 64.6 | 79.5 | 56.4 | 71.3 | 60.3 | 60.0 | 61.5 | 59.0 | 60.8 | |
| Histogram | 23.1 | 30.2 | 41.0 | 5.1 | 34.8 | 26.9 | 26.3 | 25.6 | 28.2 | 26.0 | 43.6 | 40.0 | 25.6 | 61.5 | 31.2 | 26.9 | 29.5 | 33.3 | 20.5 | 31.3 | |
| Correlogram | 62.8 | 59.3 | 82.1 | 43.6 | 68.8 | 56.4 | 55.3 | 66.7 | 46.2 | 60.5 | 53.8 | 53.1 | 66.7 | 41.0 | 59.1 | 51.3 | 50.9 | 69.2 | 33.3 | 58.7 | |
| Haar wavelet | 35.9 | 41.8 | 71.8 | 0.0 | 52.8 | 53.8 | 55.6 | 38.5 | 69.2 | 45.5 | 61.5 | 73.7 | 35.9 | 87.2 | 48.3 | 41.0 | 41.9 | 46.2 | 35.9 | 43.9 | |
| Gabor wavelet | 50.0 | 50.0 | 87.2 | 12.8 | 63.6 | 55.1 | 53.7 | 74.4 | 35.9 | 62.4 | 67.9 | 65.9 | 74.4 | 61.5 | 69.9 | 61.5 | 57.9 | 84.6 | 38.5 | 68.7 | |
| AlexNet | 50.0 | 50.0 | 97.4 | 2.6 | 66.1 | 74.4 | 66.7 | 97.4 | 51.3 | 79.2 | 50.0 | 50.0 | 100.0 | 0.0 | 66.7 | 53.8 | 52.0 | 100.0 | 7.7 | 68.4 | |
| VGG-16 | 32.1 | 25.0 | 17.9 | 46.2 | 20.9 | 65.4 | 66.7 | 61.5 | 69.2 | 64.0 | 64.1 | 69.0 | 51.3 | 76.9 | 58.8 | 64.1 | 65.7 | 59.0 | 69.2 | 62.2 | |
| VGG-19 | 60.3 | 55.7 | 100.0 | 20.5 | 71.6 | 66.7 | 60.3 | 97.4 | 35.9 | 74.5 | 57.7 | 54.3 | 97.4 | 17.9 | 69.7 | 55.1 | 52.8 | 97.4 | 12.8 | 68.5 | |
| ResNet-18 | 75.6 | 67.9 | 97.4 | 53.8 | 80.0 | 80.8 | 72.2 | 100.0 | 61.5 | 83.9 | 74.4 | 66.1 | 100.0 | 48.7 | 79.6 | 75.6 | 67.2 | 100.0 | 51.3 | 80.4 | |
| ResNet-50 | 59.0 | 64.0 | 41.0 | 76.9 | 50.0 | 75.6 | 72.7 | 82.1 | 69.2 | 77.1 | 47.4 | 37.5 | 7.7 | 87.2 | 12.8 | 47.4 | 41.7 | 12.8 | 82.1 | 19.6 | |
| ResNet-101 | 39.7 | 44.3 | 79.5 | 0.0 | 56.9 | 80.8 | 74.0 | 94.9 | 66.7 | 83.1 | 64.1 | 59.3 | 89.7 | 38.5 | 71.4 | 52.6 | 51.7 | 76.9 | 28.2 | 61.9 | |
| GoogleNet | 50.0 | 50.0 | 100.0 | 0.0 | 66.7 | 88.5 | 81.3 | 100.0 | 76.9 | 89.7 | 50.0 | 50.0 | 100.0 | 0.0 | 66.7 | 50.0 | 50.0 | 100.0 | 0.0 | 66.7 | |
| Inceptionv3 | 52.6 | 62.5 | 12.8 | 92.3 | 21.3 | 73.1 | 76.5 | 66.7 | 79.5 | 71.2 | 48.7 | 42.9 | 7.7 | 89.7 | 13.0 | 47.4 | 40.0 | 10.3 | 84.6 | 16.3 | |
| AVG | 48.9 | 49.6 | 72.1 | 25.6 | 55.6 | 64.7 | 62.2 | 72.4 | 57.1 | 66.2 | 58.4 | 57.9 | 60.9 | 55.9 | 55.1 | 52.5 | 50.7 | 67.1 | 37.8 | 55.4 | |
| SVMRbf | Legendre | 48.7 | 49.2 | 82.1 | 15.4 | 61.5 | 75.6 | 77.8 | 71.8 | 79.5 | 74.7 | 69.2 | 100.0 | 38.5 | 100.0 | 55.6 | 69.2 | 66.0 | 79.5 | 59.0 | 72.1 |
| Zernike | 50.0 | 50.0 | 100.0 | 0.0 | 66.7 | 62.8 | 60.9 | 71.8 | 53.8 | 65.9 | 74.4 | 67.3 | 94.9 | 53.8 | 78.7 | 59.0 | 54.9 | 100.0 | 17.9 | 70.9 | |
| HARri | 56.4 | 56.1 | 59.0 | 53.8 | 57.5 | 62.8 | 63.9 | 59.0 | 66.7 | 61.3 | 73.1 | 78.1 | 64.1 | 82.1 | 70.4 | 44.9 | 46.6 | 69.2 | 20.5 | 55.7 | |
| LBP18 | 50.0 | 50.0 | 100.0 | 0.0 | 66.7 | 62.8 | 58.9 | 84.6 | 41.0 | 69.5 | 67.9 | 63.0 | 87.2 | 48.7 | 73.1 | 61.5 | 58.8 | 76.9 | 46.2 | 66.7 | |
| Histogram | 10.3 | 17.0 | 20.5 | 0.0 | 18.6 | 43.6 | 41.9 | 33.3 | 53.8 | 37.1 | 55.1 | 61.1 | 28.2 | 82.1 | 38.6 | 33.3 | 34.9 | 38.5 | 28.2 | 36.6 | |
| Correlogram | 60.3 | 64.3 | 46.2 | 74.4 | 53.7 | 57.7 | 56.0 | 71.8 | 43.6 | 62.9 | 60.3 | 58.3 | 71.8 | 48.7 | 64.4 | 55.1 | 54.5 | 61.5 | 48.7 | 57.8 | |
| Haar wavelet | 50.0 | 50.0 | 100.0 | 0.0 | 66.7 | 79.5 | 71.7 | 97.4 | 61.5 | 82.6 | 78.2 | 70.4 | 97.4 | 59.0 | 81.7 | 61.5 | 56.7 | 97.4 | 25.6 | 71.7 | |
| Gabor wavelet | 46.2 | 47.9 | 89.7 | 2.6 | 62.5 | 62.8 | 60.4 | 74.4 | 51.3 | 66.7 | 56.4 | 55.1 | 69.2 | 43.6 | 61.4 | 61.5 | 57.9 | 84.6 | 38.5 | 68.7 | |
| AlexNet | 50.0 | 0.0 | 0.0 | 100.0 | 0.0 | 76.9 | 69.1 | 97.4 | 56.4 | 80.9 | 50.0 | 0.0 | 0.0 | 100.0 | 0.0 | 50.0 | 0.0 | 0.0 | 100.0 | 0.0 | |
| VGG-16 | 47.4 | 37.5 | 7.7 | 87.2 | 12.8 | 61.5 | 66.7 | 46.2 | 76.9 | 54.5 | 74.4 | 82.8 | 61.5 | 87.2 | 70.6 | 69.2 | 82.6 | 48.7 | 89.7 | 61.3 | |
| VGG-19 | 50.0 | 0.0 | 0.0 | 100.0 | 0.0 | 71.8 | 63.9 | 100.0 | 43.6 | 78.0 | 50.0 | 0.0 | 0.0 | 100.0 | 0.0 | 50.0 | 0.0 | 0.0 | 100.0 | 0.0 | |
| ResNet-18 | 62.8 | 100.0 | 25.6 | 100.0 | 40.8 | 82.1 | 73.6 | 100.0 | 64.1 | 84.8 | 50.0 | 0.0 | 0.0 | 100.0 | 0.0 | 50.0 | 0.0 | 0.0 | 100.0 | 0.0 | |
| ResNet-50 | 50.0 | 0.0 | 0.0 | 100.0 | 0.0 | 82.1 | 83.8 | 79.5 | 84.6 | 81.6 | 53.8 | 100.0 | 7.7 | 100.0 | 14.3 | 50.0 | 0.0 | 0.0 | 100.0 | 0.0 | |
| ResNet-101 | 48.7 | 49.4 | 97.4 | 0.0 | 65.5 | 66.7 | 60.0 | 100.0 | 33.3 | 75.0 | 61.5 | 57.9 | 84.6 | 38.5 | 68.7 | 60.3 | 56.1 | 94.9 | 25.6 | 70.5 | |
| GoogleNet | 50.0 | 50.0 | 100.0 | 0.0 | 66.7 | 88.5 | 81.3 | 100.0 | 76.9 | 89.7 | 50.0 | 50.0 | 100.0 | 0.0 | 66.7 | 50.0 | 50.0 | 100.0 | 0.0 | 66.7 | |
| Inceptionv3 | 48.7 | 45.5 | 12.8 | 84.6 | 20.0 | 76.9 | 81.8 | 69.2 | 84.6 | 75.0 | 59.0 | 66.7 | 35.9 | 82.1 | 46.7 | 51.3 | 52.4 | 28.2 | 74.4 | 36.7 | |
| AVG | 48.7 | 41.7 | 52.6 | 44.9 | 41.2 | 69.6 | 67.0 | 78.5 | 60.7 | 71.3 | 61.5 | 56.9 | 52.6 | 70.4 | 49.4 | 54.8 | 42.0 | 55.0 | 54.6 | 46.0 | |
| RF | Legendre | 50.0 | 50.0 | 100.0 | 0.0 | 66.7 | 80.8 | 83.3 | 76.9 | 84.6 | 80.0 | 76.9 | 88.9 | 61.5 | 92.3 | 72.7 | 56.4 | 53.5 | 97.4 | 15.4 | 69.1 |
| Zernike | 48.7 | 49.2 | 79.5 | 17.9 | 60.8 | 35.9 | 36.6 | 38.5 | 33.3 | 37.5 | 24.4 | 27.3 | 30.8 | 17.9 | 28.9 | 25.6 | 32.1 | 43.6 | 7.7 | 37.0 | |
| HARri | 47.4 | 48.7 | 94.9 | 0.0 | 64.3 | 79.5 | 72.5 | 94.9 | 64.1 | 82.2 | 80.8 | 74.0 | 94.9 | 66.7 | 83.1 | 52.6 | 51.4 | 97.4 | 7.7 | 67.3 | |
| LBP18 | 50.0 | 50.0 | 100.0 | 0.0 | 66.7 | 62.8 | 57.8 | 94.9 | 30.8 | 71.8 | 57.7 | 54.3 | 97.4 | 17.9 | 69.7 | 55.1 | 53.2 | 84.6 | 25.6 | 65.3 | |
| Histogram | 50.0 | 50.0 | 100.0 | 0.0 | 66.7 | 74.4 | 71.1 | 82.1 | 66.7 | 76.2 | 78.2 | 89.3 | 64.1 | 92.3 | 74.6 | 46.2 | 48.0 | 92.3 | 0.0 | 63.2 | |
| Correlogram | 52.6 | 51.7 | 76.9 | 28.2 | 61.9 | 60.3 | 58.3 | 71.8 | 48.7 | 64.4 | 61.5 | 59.6 | 71.8 | 51.3 | 65.1 | 61.5 | 58.8 | 76.9 | 46.2 | 66.7 | |
| Haar wavelet | 17.9 | 26.4 | 35.9 | 0.0 | 30.4 | 51.3 | 51.4 | 46.2 | 56.4 | 48.6 | 60.3 | 63.3 | 48.7 | 71.8 | 55.1 | 46.2 | 46.2 | 46.2 | 46.2 | 46.2 | |
| Gabor wavelet | 43.6 | 45.1 | 59.0 | 28.2 | 51.1 | 55.1 | 54.3 | 64.1 | 46.2 | 58.8 | 55.1 | 55.9 | 48.7 | 61.5 | 52.1 | 52.6 | 52.1 | 64.1 | 41.0 | 57.5 | |
| AlexNet | 61.5 | 56.7 | 97.4 | 25.6 | 71.7 | 76.9 | 69.1 | 97.4 | 56.4 | 80.9 | 70.5 | 62.9 | 100.0 | 41.0 | 77.2 | 69.2 | 61.9 | 100.0 | 38.5 | 76.5 | |
| VGG-16 | 37.2 | 36.8 | 35.9 | 38.5 | 36.4 | 62.8 | 63.9 | 59.0 | 66.7 | 61.3 | 50.0 | 50.0 | 30.8 | 69.2 | 38.1 | 59.0 | 62.1 | 46.2 | 71.8 | 52.9 | |
| VGG-19 | 50.0 | 0.0 | 0.0 | 100.0 | 0.0 | 66.7 | 60.7 | 94.9 | 38.5 | 74.0 | 50.0 | 0.0 | 0.0 | 100.0 | 0.0 | 50.0 | 0.0 | 0.0 | 100.0 | 0.0 | |
| ResNet-18 | 57.7 | 87.5 | 17.9 | 97.4 | 29.8 | 82.1 | 73.6 | 100.0 | 64.1 | 84.8 | 57.7 | 68.8 | 28.2 | 87.2 | 40.0 | 56.4 | 72.7 | 20.5 | 92.3 | 32.0 | |
| ResNet-50 | 57.7 | 80.0 | 20.5 | 94.9 | 32.7 | 78.2 | 76.2 | 82.1 | 74.4 | 79.0 | 46.2 | 20.0 | 2.6 | 89.7 | 4.5 | 48.7 | 33.3 | 2.6 | 94.9 | 4.8 | |
| ResNet-101 | 35.9 | 41.8 | 71.8 | 0.0 | 52.8 | 79.5 | 72.5 | 94.9 | 64.1 | 82.2 | 57.7 | 55.2 | 82.1 | 33.3 | 66.0 | 50.0 | 50.0 | 82.1 | 17.9 | 62.1 | |
| GoogleNet | 50.0 | 50.0 | 100.0 | 0.0 | 66.7 | 88.5 | 81.3 | 100.0 | 76.9 | 89.7 | 50.0 | 50.0 | 100.0 | 0.0 | 66.7 | 51.3 | 50.6 | 100.0 | 2.6 | 67.2 | |
| Inceptionv3 | 50.0 | 50.0 | 7.7 | 92.3 | 13.3 | 70.5 | 72.2 | 66.7 | 74.4 | 69.3 | 48.7 | 42.9 | 7.7 | 89.7 | 13.0 | 46.2 | 36.4 | 10.3 | 82.1 | 16.0 | |
| AVG | 47.5 | 48.4 | 62.3 | 32.7 | 48.2 | 69.1 | 65.9 | 79.0 | 59.1 | 71.3 | 57.9 | 53.9 | 54.3 | 61.4 | 50.4 | 51.7 | 47.6 | 60.3 | 43.1 | 49.0 | |
| CNN | AlexNet | 55.1 | 12.8 | 83.3 | 52.8 | 22.2 | 80.8 | 61.5 | 100.0 | 72.2 | 76.2 | 76.9 | 53.8 | 100.0 | 68.4 | 70.0 | 76.9 | 53.8 | 100.0 | 68.4 | 70.0 |
| VGG-16 | 51.3 | 7.7 | 60.0 | 50.7 | 13.6 | 78.2 | 56.4 | 100.0 | 69.6 | 72.1 | 76.9 | 53.8 | 100.0 | 68.4 | 70.0 | 82.0 | 64.1 | 100.0 | 73.6 | 78.1 | |
| VGG-19 | 57.7 | 15.4 | 100.0 | 54.2 | 26.7 | 74.4 | 48.7 | 100.0 | 66.1 | 65.5 | 67.9 | 38.5 | 93.7 | 61.3 | 54.5 | 66.7 | 33.3 | 100.0 | 60.0 | 50.0 | |
| ResNet-18 | 47.4 | 0.0 | 0.0 | 48.7 | 0.0 | 70.5 | 46.1 | 90.0 | 63.8 | 61.0 | 62.8 | 41.0 | 72.7 | 58.9 | 52.5 | 62.8 | 30.8 | 85.7 | 57.8 | 45.3 | |
| ResNet-50 | 53.8 | 7.7 | 100.0 | 52.0 | 14.3 | 70.5 | 41.0 | 100.0 | 62.9 | 58.2 | 56.4 | 20.5 | 72.7 | 53.7 | 32.0 | 71.8 | 43.6 | 100.0 | 63.9 | 60.7 | |
| ResNet-101 | 41.0 | 66.7 | 44.1 | 31.6 | 53.1 | 76.9 | 79.5 | 75.6 | 78.4 | 77.5 | 76.9 | 76.9 | 76.9 | 76.9 | 76.9 | 62.8 | 71.8 | 60.9 | 65.6 | 65.9 | |
| GoogleNet | 78.2 | 59.0 | 95.8 | 70.4 | 73.0 | 82.0 | 66.7 | 96.3 | 74.5 | 78.8 | 70.5 | 41.0 | 100.0 | 62.9 | 58.2 | 83.3 | 69.2 | 96.4 | 76.0 | 80.6 | |
| InceptionV3 | 43.6 | 2.6 | 14.3 | 46.5 | 4.3 | 57.7 | 28.2 | 68.7 | 54.8 | 40.0 | 53.8 | 25.6 | 58.8 | 52.5 | 35.7 | 57.7 | 30.8 | 66.7 | 55.0 | 42.1 | |
| AVG | 53.5 | 21.5 | 62.2 | 50.8 | 25.9 | 73.9 | 53.5 | 91.33 | 67.8 | 66.2 | 67.8 | 43.9 | 84.4 | 62.9 | 56.2 | 70.5 | 49.7 | 88.7 | 65.0 | 61.6 | |
| Classifier | Descriptor | Target Set | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Large | Tight | Eroded | Dilated | ||||||||||||||||||
| A | P | R | S | F1 | A | P | R | S | F1 | A | P | R | S | F1 | A | P | R | S | F1 | ||
| kNN | Legendre | 67.0 | 22.9 | 19.2 | 78.7 | 14.5 | 83.1 | 57.8 | 58.1 | 89.2 | 56.8 | 79.3 | 50.7 | 48.0 | 87.4 | 46.6 | 74.7 | 41.7 | 37.5 | 83.9 | 37.9 |
| Zernike | 68.1 | 8.1 | 20.9 | 79.8 | 11.3 | 84.0 | 60.7 | 60.5 | 89.9 | 60.3 | 78.7 | 47.0 | 47.4 | 86.5 | 46.6 | 73.3 | 43.7 | 34.3 | 82.9 | 32.7 | |
| HARri | 67.9 | 15.7 | 19.8 | 79.9 | 17.4 | 77.6 | 46.1 | 44.2 | 86.0 | 44.9 | 79.9 | 49.4 | 49.7 | 87.6 | 49.2 | 73.5 | 40.6 | 34.3 | 83.1 | 34.8 | |
| LBP18 | 67.7 | 4.5 | 21.2 | 78.8 | 7.4 | 87.5 | 70.5 | 68.6 | 92.4 | 69.2 | 84.6 | 62.8 | 61.6 | 90.2 | 60.8 | 84.8 | 64.6 | 62.2 | 90.5 | 61.7 | |
| Histogram | 68.7 | 20.4 | 22.4 | 80.1 | 16.6 | 76.5 | 42.6 | 41.6 | 85.2 | 41.6 | 78.5 | 46.3 | 46.5 | 86.6 | 46.2 | 76.3 | 45.9 | 41.0 | 85.0 | 40.6 | |
| Correlogram | 71.4 | 30.8 | 27.9 | 82.6 | 25.3 | 71.4 | 31.8 | 28.2 | 82.5 | 27.2 | 72.6 | 33.0 | 31.1 | 83.0 | 30.2 | 72.2 | 35.4 | 29.9 | 83.1 | 28.7 | |
| Haar wavelet | 68.0 | 21.1 | 20.9 | 79.6 | 16.4 | 89.6 | 75.1 | 74.1 | 93.5 | 74.1 | 88.8 | 71.6 | 72.1 | 93.0 | 71.6 | 83.9 | 65.6 | 59.9 | 89.9 | 59.3 | |
| Gabor wavelet | 70.9 | 28.1 | 27.3 | 81.7 | 26.9 | 74.8 | 38.2 | 37.5 | 84.0 | 35.6 | 71.7 | 28.5 | 29.7 | 82.1 | 28.5 | 74.1 | 35.3 | 35.5 | 83.6 | 34.5 | |
| AlexNet | 68.0 | 40.9 | 22.1 | 79.0 | 9.2 | 91.8 | 80.6 | 79.9 | 94.8 | 80.1 | 75.1 | 41.9 | 39.8 | 83.8 | 28.4 | 75.9 | 42.0 | 41.9 | 84.3 | 31.0 | |
| VGG-16 | 76.1 | 45.4 | 42.2 | 84.5 | 32.0 | 92.2 | 81.6 | 81.1 | 95.0 | 81.3 | 76.5 | 63.3 | 43.0 | 84.7 | 33.3 | 76.2 | 45.9 | 42.4 | 84.5 | 32.1 | |
| VGG-19 | 67.7 | 4.5 | 21.2 | 78.8 | 7.4 | 84.4 | 62.3 | 61.3 | 90.1 | 61.5 | 67.4 | 25.5 | 20.3 | 78.7 | 9.7 | 68.8 | 24.4 | 23.5 | 79.6 | 14.2 | |
| ResNet-18 | 67.7 | 4.5 | 21.2 | 78.8 | 7.4 | 87.0 | 67.9 | 67.7 | 91.8 | 67.0 | 67.7 | 4.9 | 20.9 | 78.9 | 7.9 | 67.4 | 4.8 | 20.3 | 78.7 | 7.7 | |
| ResNet-50 | 76.1 | 34.1 | 42.2 | 84.5 | 31.4 | 91.2 | 78.8 | 78.8 | 94.4 | 78.7 | 78.4 | 38.9 | 48.0 | 86.1 | 38.8 | 77.5 | 37.0 | 45.6 | 85.5 | 36.0 | |
| ResNet-101 | 71.2 | 33.1 | 29.9 | 81.1 | 20.9 | 90.6 | 77.9 | 77.0 | 93.9 | 77.1 | 81.3 | 64.2 | 55.5 | 88.0 | 48.3 | 79.7 | 63.2 | 51.5 | 87.0 | 44.7 | |
| GoogleNet | 67.8 | 5.7 | 21.5 | 78.9 | 8.5 | 92.5 | 82.0 | 81.7 | 95.2 | 81.4 | 68.8 | 40.4 | 23.5 | 79.7 | 13.3 | 68.1 | 33.0 | 22.1 | 79.2 | 10.5 | |
| Inceptionv3 | 74.1 | 45.7 | 37.2 | 83.1 | 26.6 | 89.8 | 74.3 | 74.7 | 93.6 | 74.4 | 83.5 | 59.6 | 60.2 | 89.6 | 53.4 | 83.5 | 60.4 | 60.2 | 89.6 | 54.0 | |
| AVG | 69.9 | 22.8 | 26.1 | 80.6 | 17.5 | 85.3 | 64.3 | 63.4 | 90.7 | 63.2 | 77.0 | 45.5 | 43.6 | 85.4 | 38.3 | 75.6 | 42.7 | 40.1 | 84.4 | 35.0 | |
| SVMRbf | Legendre | 66.8 | 8.1 | 19.2 | 78.3 | 11.2 | 85.8 | 65.1 | 64.5 | 91.0 | 64.6 | 84.5 | 65.2 | 61.3 | 90.4 | 60.4 | 76.6 | 47.0 | 41.6 | 85.1 | 39.9 |
| Zernike | 67.8 | 7.5 | 20.9 | 79.2 | 9.6 | 87.7 | 69.8 | 69.5 | 92.3 | 69.6 | 84.1 | 63.6 | 60.5 | 90.0 | 60.2 | 77.5 | 51.0 | 44.2 | 85.6 | 40.5 | |
| HARri | 67.5 | 14.6 | 19.2 | 79.6 | 15.4 | 83.2 | 58.9 | 58.4 | 89.4 | 58.6 | 83.5 | 58.2 | 58.7 | 89.7 | 57.6 | 78.4 | 51.2 | 46.5 | 86.0 | 45.7 | |
| LBP18 | 67.7 | 4.5 | 21.2 | 78.8 | 7.4 | 85.7 | 68.1 | 64.2 | 91.0 | 64.9 | 83.4 | 61.1 | 58.7 | 89.4 | 58.7 | 77.2 | 57.8 | 43.9 | 85.4 | 43.0 | |
| Histogram | 70.0 | 16.1 | 24.4 | 81.4 | 18.0 | 89.0 | 74.4 | 72.4 | 93.3 | 72.7 | 89.4 | 73.5 | 73.5 | 93.4 | 73.4 | 83.8 | 65.2 | 59.3 | 89.9 | 58.0 | |
| Correlogram | 77.1 | 42.6 | 43.3 | 85.7 | 41.1 | 81.6 | 55.5 | 54.1 | 88.5 | 54.5 | 79.7 | 50.3 | 49.7 | 87.3 | 49.5 | 78.7 | 49.8 | 46.5 | 87.0 | 46.8 | |
| Haar wavelet | 68.8 | 18.7 | 22.4 | 80.3 | 19.8 | 90.4 | 76.8 | 76.2 | 94.0 | 76.0 | 89.5 | 74.9 | 74.1 | 93.4 | 74.0 | 85.2 | 67.6 | 63.1 | 90.8 | 62.7 | |
| Gabor wavelet | 73.2 | 39.5 | 33.7 | 82.6 | 32.1 | 80.9 | 51.9 | 52.3 | 88.0 | 51.8 | 75.5 | 43.1 | 39.5 | 84.4 | 39.5 | 78.3 | 48.1 | 46.2 | 86.3 | 46.5 | |
| AlexNet | 67.7 | 4.5 | 21.2 | 78.8 | 7.4 | 96.4 | 91.6 | 91.3 | 97.7 | 91.3 | 67.7 | 4.5 | 21.2 | 78.8 | 7.4 | 67.7 | 4.5 | 21.2 | 78.8 | 7.4 | |
| VGG-16 | 76.5 | 46.8 | 42.7 | 84.9 | 33.8 | 92.5 | 82.3 | 81.7 | 95.2 | 81.7 | 72.7 | 62.7 | 33.7 | 82.1 | 25.3 | 74.5 | 45.6 | 38.1 | 83.4 | 27.3 | |
| VGG-19 | 61.0 | 1.3 | 3.5 | 75.4 | 1.8 | 86.2 | 66.9 | 65.7 | 91.2 | 65.5 | 66.6 | 4.5 | 16.3 | 79.6 | 6.9 | 65.9 | 4.2 | 14.8 | 79.1 | 6.5 | |
| ResNet-18 | 67.7 | 4.5 | 21.2 | 78.8 | 7.4 | 88.7 | 72.3 | 71.8 | 92.8 | 71.5 | 67.7 | 4.5 | 21.2 | 78.8 | 7.4 | 67.7 | 4.5 | 21.2 | 78.8 | 7.4 | |
| ResNet-50 | 75.6 | 36.2 | 41.0 | 84.1 | 29.8 | 93.1 | 83.7 | 83.4 | 95.6 | 83.5 | 78.4 | 63.3 | 48.3 | 86.1 | 39.9 | 77.7 | 41.6 | 46.5 | 85.6 | 37.6 | |
| ResNet-101 | 70.7 | 24.2 | 28.8 | 80.8 | 19.3 | 92.9 | 83.5 | 82.6 | 95.4 | 82.5 | 68.4 | 62.1 | 23.0 | 79.2 | 10.9 | 68.3 | 37.4 | 22.7 | 79.2 | 10.3 | |
| GoogleNet | 67.7 | 4.8 | 21.2 | 78.8 | 7.8 | 95.1 | 89.2 | 88.1 | 96.9 | 87.7 | 69.6 | 43.0 | 25.9 | 80.1 | 17.1 | 69.6 | 45.8 | 25.9 | 80.1 | 17.0 | |
| Inceptionv3 | 73.3 | 45.5 | 35.2 | 82.6 | 25.0 | 92.1 | 80.5 | 80.5 | 95.0 | 80.2 | 71.4 | 57.2 | 30.5 | 81.4 | 21.7 | 74.0 | 60.6 | 37.2 | 83.1 | 31.0 | |
| AVG | 69.9 | 20.0 | 26.2 | 80.6 | 17.9 | 88.8 | 73.2 | 72.3 | 93.0 | 72.3 | 77.0 | 49.5 | 43.5 | 85.3 | 38.1 | 75.1 | 42.6 | 38.7 | 84.0 | 33.0 | |
| RF | Legendre | 68.3 | 13.6 | 22.7 | 79.3 | 14.5 | 86.1 | 67.0 | 65.4 | 91.3 | 65.4 | 83.5 | 62.7 | 58.4 | 90.2 | 57.0 | 74.1 | 46.4 | 35.5 | 83.6 | 33.3 |
| Zernike | 67.6 | 6.5 | 20.9 | 78.8 | 7.9 | 87.6 | 69.6 | 69.2 | 92.1 | 69.3 | 85.1 | 65.8 | 63.4 | 90.5 | 63.1 | 76.2 | 48.7 | 41.3 | 84.6 | 38.5 | |
| HARri | 67.4 | 17.8 | 20.6 | 78.7 | 10.3 | 89.8 | 75.7 | 75.0 | 93.6 | 75.2 | 89.3 | 74.6 | 73.5 | 93.4 | 73.3 | 81.0 | 60.8 | 52.9 | 87.6 | 52.1 | |
| LBP18 | 75.3 | 24.2 | 40.1 | 83.9 | 27.6 | 89.7 | 74.0 | 74.1 | 93.6 | 74.0 | 83.9 | 63.3 | 60.2 | 89.8 | 58.9 | 85.8 | 69.2 | 64.5 | 91.2 | 63.5 | |
| Histogram | 69.2 | 17.6 | 23.0 | 80.9 | 16.0 | 88.5 | 72.0 | 71.2 | 92.9 | 71.4 | 89.1 | 72.6 | 73.0 | 93.3 | 72.4 | 82.4 | 60.7 | 55.8 | 89.0 | 54.3 | |
| Correlogram | 71.9 | 48.5 | 28.8 | 83.1 | 26.7 | 81.5 | 54.3 | 54.1 | 88.5 | 54.0 | 80.5 | 51.1 | 51.5 | 87.8 | 50.9 | 78.8 | 49.9 | 47.1 | 86.9 | 47.2 | |
| Haar wavelet | 73.1 | 17.9 | 32.8 | 83.4 | 21.3 | 89.8 | 75.7 | 74.4 | 93.7 | 74.3 | 89.9 | 74.3 | 74.7 | 93.7 | 74.4 | 85.5 | 69.6 | 63.7 | 91.0 | 63.6 | |
| Gabor wavelet | 71.8 | 36.7 | 29.9 | 81.9 | 28.3 | 82.3 | 55.1 | 55.8 | 89.0 | 54.7 | 75.7 | 39.9 | 39.8 | 84.6 | 39.3 | 79.3 | 48.2 | 48.3 | 87.1 | 48.0 | |
| AlexNet | 70.1 | 37.9 | 27.3 | 80.5 | 18.4 | 94.0 | 85.4 | 85.2 | 96.1 | 85.1 | 71.5 | 62.9 | 31.1 | 81.5 | 24.5 | 72.8 | 41.4 | 34.3 | 82.4 | 26.1 | |
| VGG-16 | 76.0 | 55.4 | 41.9 | 84.4 | 31.4 | 94.6 | 87.4 | 87.2 | 96.6 | 87.3 | 77.8 | 79.6 | 46.2 | 85.5 | 38.9 | 75.3 | 24.5 | 40.1 | 83.9 | 27.7 | |
| VGG-19 | 68.4 | 9.1 | 22.7 | 79.5 | 10.3 | 88.9 | 73.2 | 72.4 | 92.9 | 72.5 | 67.4 | 13.5 | 19.5 | 79.2 | 12.6 | 69.1 | 17.1 | 23.3 | 80.2 | 16.7 | |
| ResNet-18 | 67.9 | 4.9 | 20.9 | 79.2 | 7.9 | 89.8 | 75.1 | 74.4 | 93.6 | 74.4 | 67.4 | 9.5 | 18.6 | 79.4 | 9.6 | 67.7 | 26.9 | 19.5 | 79.5 | 10.4 | |
| ResNet-50 | 75.1 | 24.4 | 39.8 | 83.8 | 27.5 | 92.3 | 81.8 | 81.7 | 95.1 | 81.7 | 76.9 | 39.8 | 44.2 | 85.1 | 34.7 | 76.0 | 34.2 | 41.9 | 84.4 | 31.0 | |
| ResNet-101 | 71.6 | 41.8 | 31.1 | 81.4 | 22.0 | 92.6 | 82.7 | 82.0 | 95.2 | 82.0 | 77.0 | 63.4 | 44.5 | 85.0 | 35.6 | 76.1 | 60.5 | 42.2 | 84.4 | 31.5 | |
| GoogleNet | 69.0 | 10.9 | 24.4 | 79.6 | 12.4 | 94.6 | 87.7 | 86.9 | 96.5 | 86.6 | 69.7 | 22.1 | 25.6 | 80.2 | 16.0 | 69.9 | 21.9 | 26.2 | 80.3 | 16.7 | |
| Inceptionv3 | 74.5 | 48.4 | 38.1 | 83.4 | 27.4 | 92.6 | 81.6 | 81.7 | 95.3 | 81.5 | 80.3 | 61.2 | 52.6 | 87.3 | 46.9 | 80.4 | 59.9 | 52.6 | 87.3 | 47.3 | |
| AVG | 71.1 | 26.0 | 29.1 | 81.4 | 19.4 | 89.7 | 74.9 | 74.4 | 93.5 | 74.3 | 79.1 | 53.5 | 48.5 | 86.7 | 44.3 | 76.9 | 46.2 | 43.1 | 85.2 | 38.0 | |
| CNN | AlexNet | 70.6 | 75.1 | 39.8 | 93.2 | 50.3 | 97.7 | 94.7 | 94.5 | 98.7 | 94.5 | 96.7 | 92.7 | 91.9 | 98.2 | 92.0 | 96.4 | 91.6 | 91.3 | 97.9 | 91.2 |
| VGG-16 | 68.5 | 62.1 | 34.9 | 88.4 | 44.0 | 96.8 | 92.5 | 92.1 | 98.1 | 92.2 | 95.0 | 88.5 | 87.2 | 97.1 | 87.5 | 94.6 | 87.5 | 86.6 | 96.9 | 86.7 | |
| VGG-19 | 55.7 | 85.9 | 34.3 | 94.2 | 46.6 | 95.9 | 90.8 | 89.8 | 97.7 | 90.0 | 95.4 | 88.7 | 88.4 | 97.2 | 88.5 | 95.2 | 88.8 | 87.8 | 97.3 | 88.0 | |
| ResNet-18 | 72.0 | 71.9 | 46.5 | 91.4 | 52.6 | 98.4 | 96.2 | 96.2 | 99.0 | 96.2 | 98.0 | 95.5 | 95.3 | 98.8 | 95.3 | 97.9 | 95.1 | 95.1 | 98.7 | 95.1 | |
| ResNet-101 | 52.5 | 97.2 | 40.7 | 99.4 | 51.2 | 98.2 | 95.7 | 95.6 | 98.9 | 95.6 | 97.2 | 93.6 | 93.3 | 98.3 | 93.3 | 98.1 | 95.4 | 95.3 | 98.8 | 95.4 | |
| ResNet-50 | 71.1 | 87.8 | 50.0 | 97.4 | 58.3 | 98.3 | 95.9 | 95.9 | 98.9 | 95.9 | 97.7 | 94.6 | 94.5 | 98.6 | 94.5 | 97.9 | 95.0 | 95.1 | 98.7 | 95.0 | |
| GoogleNet | 59.6 | 83.3 | 35.2 | 95.3 | 45.5 | 97.7 | 94.5 | 94.5 | 98.6 | 94.5 | 97.0 | 93.7 | 93.0 | 98.4 | 93.0 | 95.5 | 90.2 | 89.2 | 97.6 | 89.2 | |
| InceptionV3 | 80.4 | 85.0 | 57.8 | 96.0 | 64.7 | 97.9 | 95.0 | 95.1 | 98.7 | 95.0 | 98.2 | 95.7 | 95.6 | 98.9 | 95.6 | 98.0 | 95.3 | 95.3 | 98.8 | 95.3 | |
| AVG | 66.3 | 81.0 | 42.4 | 94.4 | 51.7 | 97.6 | 94.4 | 94.2 | 98.6 | 94.2 | 96.9 | 92.9 | 92.4 | 98.2 | 92.5 | 96.7 | 92.4 | 92.0 | 98.1 | 92.0 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loddo, A.; Putzu, L. On the Effectiveness of Leukocytes Classification Methods in a Real Application Scenario. AI 2021, 2, 394-412. https://doi.org/10.3390/ai2030025
Loddo A, Putzu L. On the Effectiveness of Leukocytes Classification Methods in a Real Application Scenario. AI. 2021; 2(3):394-412. https://doi.org/10.3390/ai2030025
Chicago/Turabian StyleLoddo, Andrea, and Lorenzo Putzu. 2021. "On the Effectiveness of Leukocytes Classification Methods in a Real Application Scenario" AI 2, no. 3: 394-412. https://doi.org/10.3390/ai2030025






