Simultaneous Myopic Defocus for Myopia Control: Effect on Accommodation, Peripheral Refraction and Retinal Image Quality in Non-Presbyopic Patients
Abstract
:1. Introduction
2. Materials and Methods
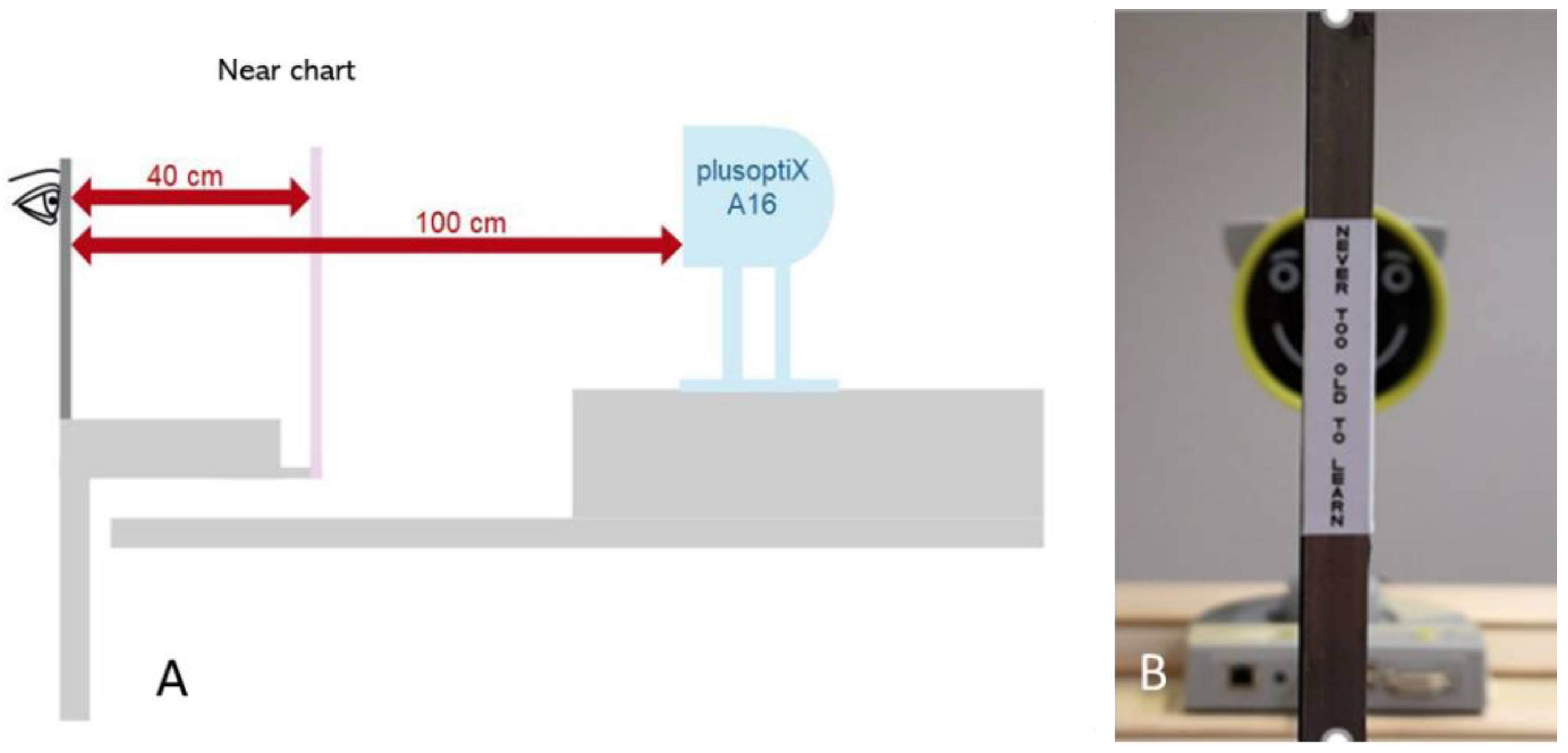
2.1. Experimental Setup
2.2. Participants
- Aged between 20 and 30 years;
- Refraction error between −0.50 and −4.00 D;
- Maximum astigmatism of −1.00 D;
- Corrected visual acuity of at least LogMAR 0.0 (20/20) or better.
- Amblyopia;
- Strabismus;
- Active eye disease that does not allow for wearing contact lenses;
- Pseudophakia.
2.3. Optical Simulation
2.4. Statistical Analysis
3. Results
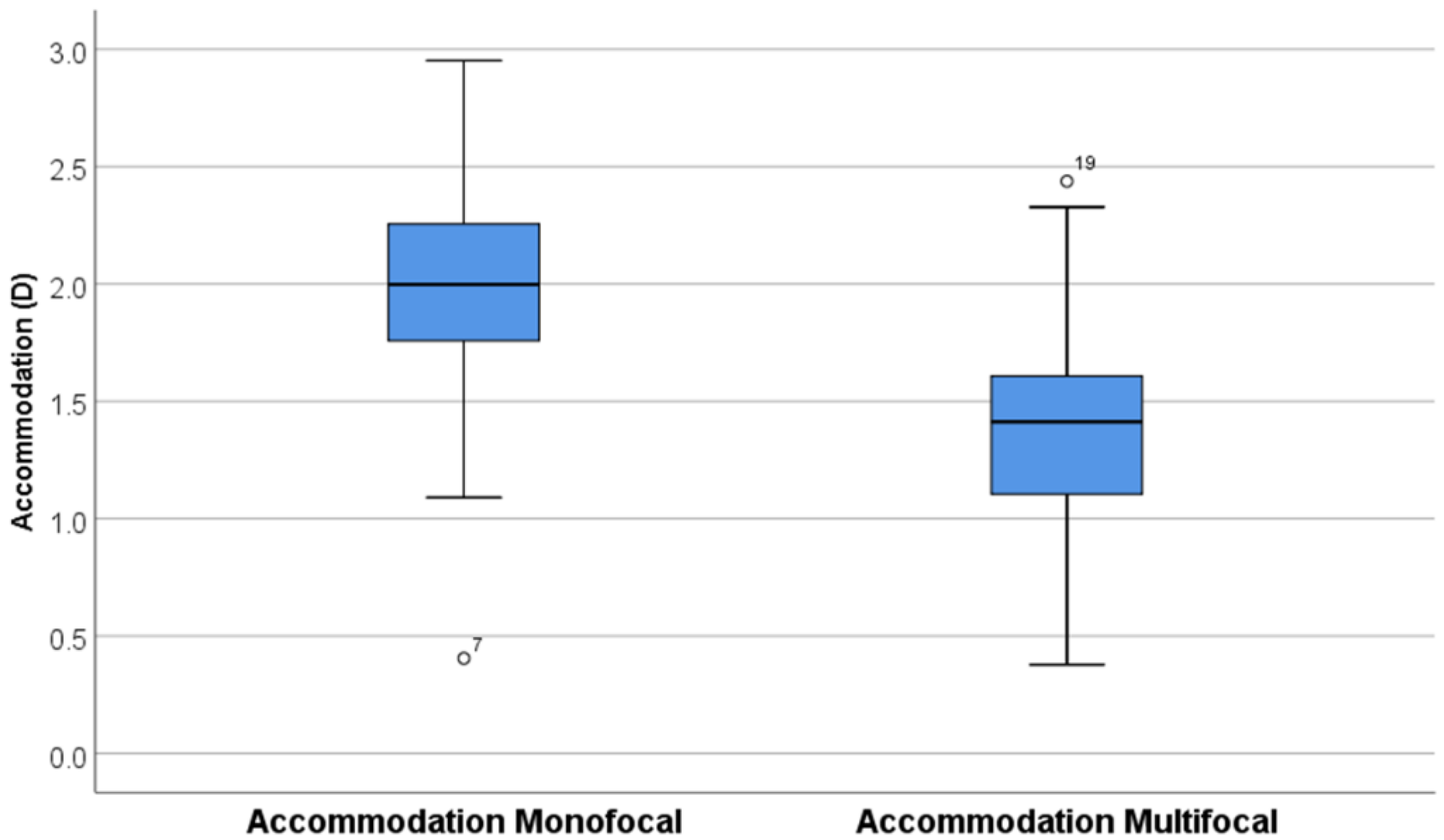
3.1. Accommodation
3.2. Pupil Size
3.3. Interpupillary Distance
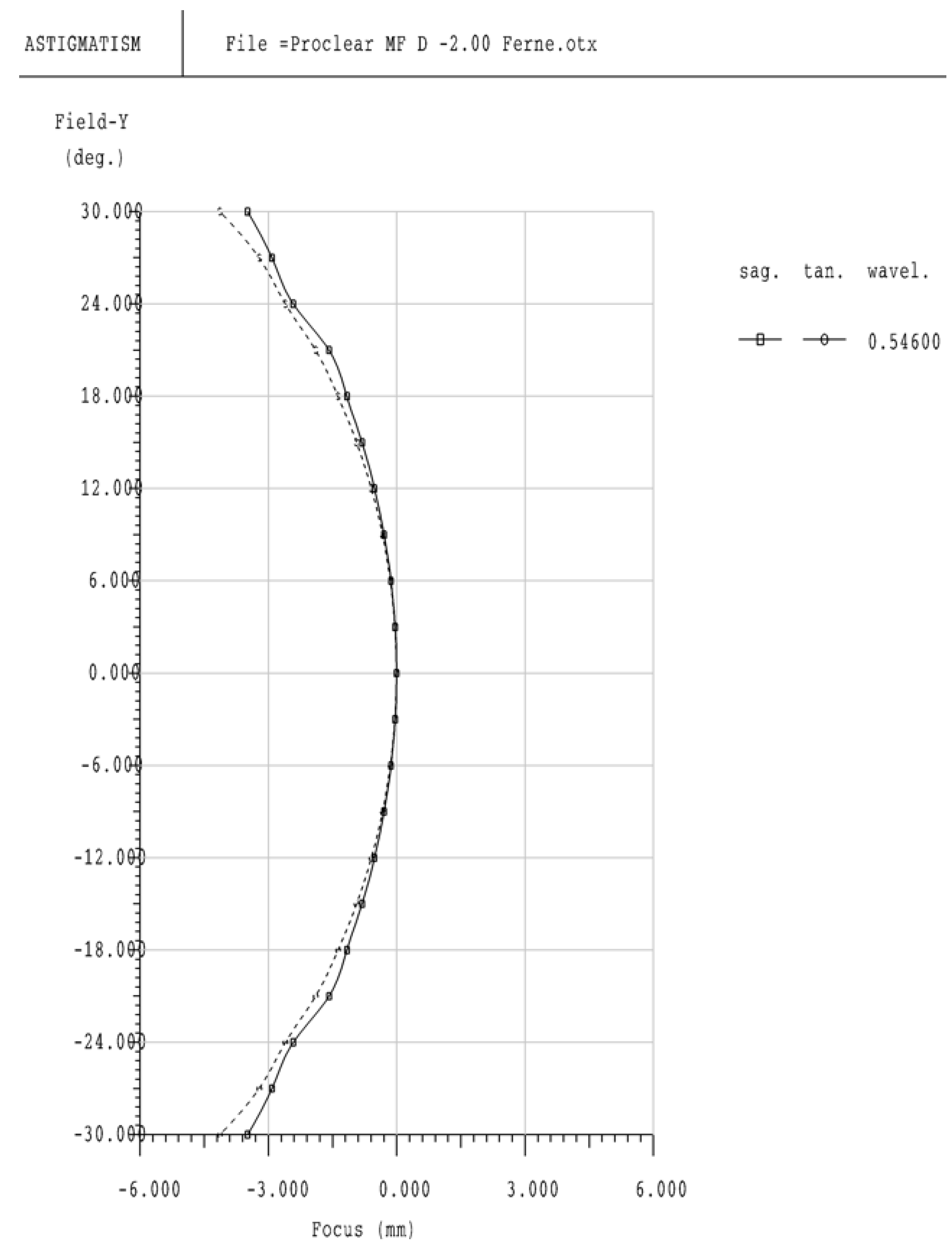
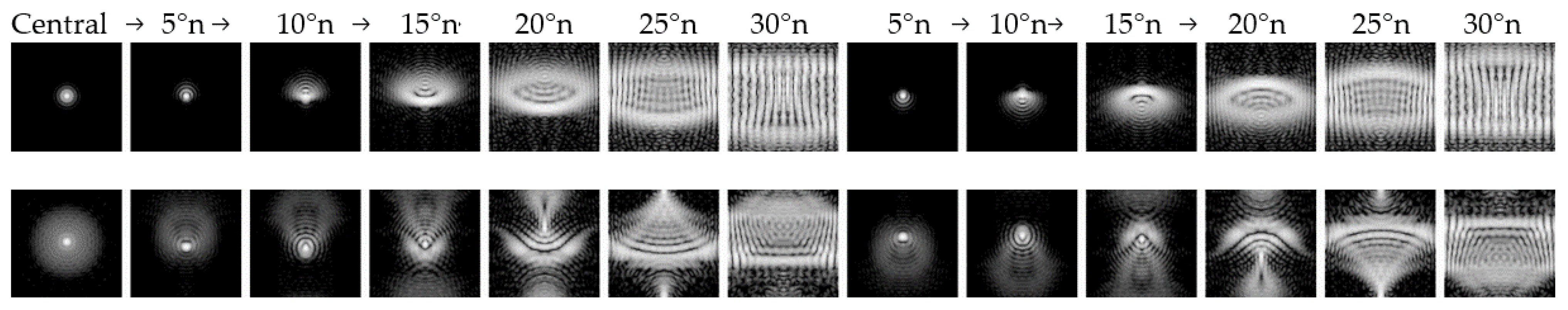
3.4. Optical Visualization
4. Discussion
4.1. Accommodation
4.2. Peripheral Refraction and Image Quality
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, J.; Li, Y.; Musch, D.C.; Wei, N.; Qi, X.; Ding, G.; Li, X.; Li, J.; Song, L.L.; Zhang, Y.; et al. Progression of Myopia in School-Aged Children After COVID-19 Home Confinement. JAMA Ophthalmol. 2021, 139, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.; Verhoeven, V.M.; Cumberland, P.; Bertelsen, G.; Wolfram, C.; Buitendijk, G.S.; Hofman, A.; van Duijn, C.M.; Vingerling, J.R.; Kuijpers, R.W.A.M.; et al. Prevalence of refractive error in Europe: The European Eye Epidemiology (E3) Consortium. Eur. J. Epidemiol. 2015, 30, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.-W.; Zheng, Y.-F.; Anuar, A.R.; Chew, M.; Gazzard, G.; Aung, T.; Cheng, C.-Y.; Wong, T.Y.; Saw, S.-M. Prevalence of Refractive Errors in a Multiethnic Asian Population: The Singapore Epidemiology of Eye Disease Study. Investig. Ophthalmol. Vis. Sci. 2013, 54, 2590–2598. [Google Scholar] [CrossRef] [Green Version]
- Yam, J.C.; Jiang, Y.; Tang, S.M.; Law, A.K.P.; Chan, J.J.; Wong, E.; Young, A.L.; Tham, C.C.; Chen, L.J.; Pang, C.P. Low-Concentration Atropine for Myopia Progression (LAMP) Study: A Randomized, Double-Blinded, Placebo-Controlled Trial of 0.05%, 0.025%, and 0.01% Atropine Eye Drops in Myopia Control. Ophthalmology 2019, 126, 113–124. [Google Scholar] [CrossRef]
- Fang, Y.T.; Chou, Y.J.; Pu, C.; Lin, P.J.; Liu, T.L.; Huang, N.; Chou, P. Prescription of atropine eye drops among children diagnosed with myopia in Taiwan from 2000 to 2007: A nationwide study. Eye 2013, 27, 418–424. [Google Scholar] [CrossRef] [Green Version]
- Chia, A.; Chua, W.H.; Wen, L.; Fong, A.; Goon, Y.Y.; Tan, D. Atropine for the Treatment of Childhood Myopia: Changes after Stopping Atropine 0.01%, 0.1% and 0.5%. Am. J. Ophthalmol. 2013, 3, 00642–00649. [Google Scholar] [CrossRef]
- Chia, A.; Chua, W.-H.; Cheung, Y.-B.; Wong, W.-L.; Lingham, A.; Fong, A.; Tan, D. Atropine for the Treatment of Childhood Myopia: Safety and Efficacy of 0.5%, 0.1%, and 0.01% Doses (Atropine for the Treatment of Myopia 2). Ophthalmology 2012, 119, 347–354. [Google Scholar] [CrossRef]
- Khanal, S.; Phillips, J.R. Which low-dose atropine for myopia control? Clin. Exp. Optom. 2020, 103, 230–232. [Google Scholar] [CrossRef] [Green Version]
- Cooper, J.; Eisenberg, N.; Schulman, E.; Wang, F.M. Maximum Atropine Dose Without Clinical Signs or Symptoms. Optom. Vis. Sci. 2013. Publish Ahead of Print. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.-X.; Hua, W.-J.; Jiang, X.; Wu, X.-Y.; Yang, J.-W.; Gao, G.-P.; Fang, Y.; Pei, C.L.; Wang, S.; Zhang, J.Z.; et al. Effect of outdoor activity on myopia onset and progression in school-aged children in northeast china: The sujiatun eye care study. BMC Ophthalmol. 2015, 15, 73. [Google Scholar] [CrossRef] [Green Version]
- Rose, K.A.; Morgan, I.G.; Ip, J.; Kifley, A.; Huynh, S.; Smith, W.; Mitchell, P. Outdoor activity reduces the prevalence of myopia in children. Ophthalmology 2008, 115, 1279–1285. [Google Scholar] [CrossRef]
- Torii, H.; Kurihara, T.; Seko, Y.; Negishi, K.; Ohnuma, K.; Inaba, T.; Kawashima, M.; Jiang, X.Y.; Kondo, S.; Miyauchi, M. Violet Light Exposure Can Be a Preventive Strategy Against Myopia Progression. EBioMedicine 2017, 15, 210–219. [Google Scholar] [CrossRef] [Green Version]
- Ticak, A.; Walline, J.J. Peripheral optics with bifocal soft and corneal reshaping contact lenses. Optom. Vis. Sci. 2013, 90, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, P.; Swarbrick, H. Peripheral refraction in myopic children wearing orthokeratology and gas-permeable lenses. Optom. Vis. Sci. 2011, 88, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Atchison, D.A.; Rosen, R. The Possible Role of Peripheral Refraction in Development of Myopia. Optom. Vis. Sci. 2016, 93, 1042–1044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chamberlain, P.; Peixoto-de-Matos, S.C.; Logan, N.S.; Ngo, C.; Jones, D.; Young, G. A 3-year Randomized Clinical Trial of MiSight Lenses for Myopia Control. Optom. Vis. Sci. 2019, 96, 556–567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.M.; Kang, M.T.; Wu, S.S.; Meng, B.; Sun, Y.Y.; Wei, S.F.; Liu, L.; Peng, X.X.; Chen, Z.; Zhang, F.J.; et al. Studies using concentric ring bifocal and peripheral add multifocal contact lenses to slow myopia progression in school-aged children: A meta-analysis. Ophthalmic Physiol. Opt. 2017, 37, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Anstice, N.S.; Phillips, J.R. Effect of dual-focus soft contact lens wear on axial myopia progression in children. Ophthalmology 2011, 118, 1152–1161. [Google Scholar] [CrossRef] [PubMed]
- Aller, T.A.; Wildsoet, C. Bifocal soft contact lenses as a possible myopia control treatment: A case report involving identical twins. Clin. Exp. Optom. 2008, 91, 394–399. [Google Scholar] [CrossRef] [PubMed]
- Loertscher, M.; Backhouse, S.; Phillips, J.R. Multifocal Orthokeratology versus Conventional Orthokeratology for Myopia Control: A Paired-Eye Study. J. Clin. Med. 2021, 10, 447. [Google Scholar] [CrossRef]
- Kim, E.; Bakaraju, R.C.; Ehrmann, K. Power Profiles of Commercial Multifocal Soft Contact Lenses. Optom. Vis. Sci. 2017, 94, 183–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phillips, J.R. Monovision slows juvenile myopia progression unilaterally. Br. J. Ophthalmol. 2005, 89, 1196–1200. [Google Scholar] [CrossRef] [PubMed]
- Llorente-Guillemot, A.; Garcia-Lazaro, S.; Ferrer-Blasco, T.; Perez-Cambrodi, R.J.; Cervino, A. Visual performance with simultaneous vision multifocal contact lenses. Clin. Exp. Optom. 2012, 95, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Walline, J.J.; Greiner, K.L.; McVey, M.E.; Jones-Jordan, L.A. Multifocal contact lens myopia control. Optom. Vis. Sci. 2013, 90, 1207–1214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seidemann, A.; Schaeffel, F. An evaluation of the lag of accommodation using photorefraction. Vis. Res. 2003, 43, 419–430. [Google Scholar] [CrossRef] [Green Version]
- Schaeffel, F.; Farkas, L.; Howland, H.C. Infrared photoretinoscope. Appl. Opt. 1987, 26, 1505–1509. [Google Scholar] [CrossRef] [PubMed]
- Tarrant, J.; Severson, H.; Wildsoet, C.F. Accommodation in emmetropic and myopic young adults wearing bifocal soft contact lenses. Ophthalmic Physiol. Opt. J. Br. Coll. Ophthalmic Opt. Optom. 2008, 28, 62–72. [Google Scholar] [CrossRef] [Green Version]
- Lam, C.S.; Tang, W.C.; Tse, D.Y.; Tang, Y.Y.; To, C.H. Defocus Incorporated Soft Contact (DISC) lens slows myopia progression in Hong Kong Chinese schoolchildren: A 2-year randomised clinical trial. Br. J. Ophthalmol. 2014, 98, 40–45. [Google Scholar] [CrossRef]
- Lopes-Ferreira, D.; Ribeiro, C.; Neves, H.; Faria-Ribeiro, M.; Queirós, A.; Villa-Collar, C.; Jorge, J.; González-Méijomea, J.M. Peripheral refraction with dominant design multifocal contact lenses in young myopes. J. Optom. 2013, 6, 85–94. [Google Scholar] [CrossRef] [Green Version]
- Duane, A. Normal Values of the accommodation at all ages. J. Am. Med Assoc. 1912, LIX, 1010–1013. [Google Scholar] [CrossRef]
- Ghoushchi, V.P.; Mompeán, J.; Prieto, P.M.; Artal, P. Binocular dynamics of accommodation, convergence, and pupil size in myopes. Biomed. Opt. Express 2021, 12, 3282–3295. [Google Scholar] [CrossRef]
- Gwiazda, J.; Thorn, F.; Bauer, J.; Held, R. Myopic children show insufficient accommodative response to blur. Investig. Ophthalmol. Vis. Sci. 1993, 34, 690–694. [Google Scholar]
- Kang, P.; Wildsoet, C.F. Acute and short-term changes in visual function with multifocal soft contact lens wear in young adults. Contact Lens Anterior Eye 2016, 39, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Gwiazda, J.E.; Hyman, L.; Norton, T.T.; Hussein, M.E.M.; Marsh-Tootle, W.; Manny, R.; Wang, Y.; Everett, D. Accommodation and Related Risk Factors Associated with Myopia Progression and Their Interaction with Treatment in COMET Children. Investig. Ophthalmol. Vis. Sci. 2004, 45, 2143–2151. [Google Scholar] [CrossRef] [PubMed]
- Berntsen, D.A.; Sinnott, L.T.; Mutti, D.O.; Zadnik, K. Accommodative lag and juvenile-onset myopia progression in children wearing refractive correction. Vision Res. 2011, 51, 1039–1046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, E.L., 3rd; Hung, L.F.; Arumugam, B. Visual regulation of refractive development: Insights from animal studies. Eye 2013, 13, 277. [Google Scholar] [CrossRef] [Green Version]
- Wagner, S.; Schaeffel, F.; Troilo, D. Changing accommodation behaviour during multifocal soft contact lens wear using auditory biofeedback training. Sci. Rep. 2020, 10, 5018. [Google Scholar] [CrossRef] [Green Version]
- Gong, C.R.; Troilo, D.; Richdale, K. Accommodation and Phoria in Children Wearing Multifocal Contact Lenses. Optom. Vis. Sci. 2017, 94, 353–360. [Google Scholar] [CrossRef] [Green Version]
- Altoaimi, B.H.; Almutairi, M.S.; Kollbaum, P.S.; Bradley, A. Accommodative Behavior of Young Eyes Wearing Multifocal Contact Lenses. Optom. Vis. Sci. 2018, 95, 416–427. [Google Scholar] [CrossRef]
- Ruiz-Pomeda, A.; Perez-Sanchez, B.; Canadas, P.; Prieto-Garrido, F.L.; Gutierrez-Ortega, R.; Villa-Collar, C. Binocular and accommodative function in the controlled randomized clinical trial MiSight(R) Assessment Study Spain (MASS). Graefes. Arch. Clin. Exp. Ophthalmol. 2018, 257, 207–215. [Google Scholar] [CrossRef]
- Guillon, M.; Dumbleton, K.; Theodoratos, P.; Gobbe, M.; Wooley, C.B.; Moody, K. The Effects of Age, Refractive Status, and Luminance on Pupil Size. Optom. Vis. Sci. 2016, 93, 1093–1100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van der Wel, P.; van Steenbergen, H. Pupil dilation as an index of effort in cognitive control tasks: A review. Psychon. Bull. Rev. 2018, 25, 2005–2015. [Google Scholar] [CrossRef] [PubMed]
- Benavente-Perez, A.; Nour, A.; Troilo, D. The effect of simultaneous negative and positive defocus on eye growth and development of refractive state in marmosets. Investig. Ophthalmol. Vis. Sci. 2012, 53, 6479–6487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, E.L., III; Ramamirtham, R.; Qiao-Grider, Y.; Hung, L.-F.; Huang, J.; Kee, C.-S.; Coats, D.; Paysse, E. Effects of Foveal Ablation on Emmetropization and Form-Deprivation Myopia. Investig. Ophthalmol. Vis. Sci. 2007, 48, 3914–3922. [Google Scholar] [CrossRef] [PubMed]
- Schippert, R.; Schaeffel, F. Peripheral defocus does not necessarily affect central refractive development. Vis. Res. 2006, 46, 3935–3940. [Google Scholar] [CrossRef] [Green Version]
- Tepelus, T.C.; Vazquez, D.; Seidemann, A.; Uttenweiler, D.; Schaeffel, F. Effects of lenses with different power profiles on eye shape in chickens. Vis. Res. 2012, 54, 12–19. [Google Scholar] [CrossRef]
- Smith Iii, E.L.; Arumugam, B.; Hung, L.-F.; She, Z.; Beach, K.; Sankaridurg, P. Eccentricity-dependent effects of simultaneous competing defocus on emmetropization in infant rhesus monkeys. Vis. Res. 2020, 177, 32–40. [Google Scholar] [CrossRef]
- Mutti, D.O.; Sinnott, L.T.; Mitchell, G.L.; Jones-Jordan, L.A.; Moeschberger, M.L.; Cotter, S.A.; Kleinstein, R.N.; Manny, R.E.; Twelker, J.D.; Zadnik, K.; et al. Relative peripheral refractive error and the risk of onset and progression of myopia in children. Investig. Ophthalmol. Vis. Sci. 2011, 52, 199–205. [Google Scholar] [CrossRef] [Green Version]
- Kwok, E.; Patel, B.; Backhouse, S.; Phillips, J.R. Peripheral Refraction in High Myopia with Spherical Soft Contact Lenses. Optom. Vis. Sci. 2012, 89, 263–270. [Google Scholar] [CrossRef]
- Backhouse, S.; Fox, S.; Ibrahim, B.; Phillips, J.R. Peripheral refraction in myopia corrected with spectacles versus contact lenses. Ophthalmic. Physiol. Opt. 2012, 32, 294–303. [Google Scholar] [CrossRef] [Green Version]
- Pauné, J.; Fonts, S.; Rodríguez, L.; Queirós, A. The Role of Back Optic Zone Diameter in Myopia Control with Orthokeratology Lenses. J. Clin. Med. 2021, 10, 336. [Google Scholar] [CrossRef] [PubMed]
- Santodomingo-Rubido, J.; Villa-Collar, C.; Gilmartin, B.; Gutierrez-Ortega, R. Myopia control with orthokeratology contact lenses in Spain: Refractive and biometric changes. Investig. Ophthalmol. Vis. Sci. 2012, 53, 5060–5065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, P.; Cheung, S.W. Retardation of Myopia in Orthokeratology (ROMIO) Study: A 2-year randomized clinical trial. Investig. Ophthalmol. Vis. Sci. 2012, 53, 7077–7085. [Google Scholar] [CrossRef] [PubMed]
- Kakita, T.; Hiraoka, T.; Oshika, T. Influence of overnight orthokeratology on axial length elongation in childhood myopia. Investig. Ophthalmol. Vis. Sci. 2011, 52, 2170–2174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charm, J.; Cho, P. High myopia—partial reduction orthokeratology (HM-PRO) study: Recruitment and one year result. Contact Lens Anterior Eye 2011, 34 (Suppl. 1), S3. [Google Scholar] [CrossRef]
- Walline, J.J.; Holden, B.A.; Bullimore, M.A.; Rah, M.J.; Asbell, P.A.; Barr, J.T.; Caroline, P.J.; Cavanagh, P.J.; Despotidis, N.; Desmond, F.; et al. The current state of corneal reshaping. Eye Contact Lens 2005, 31, 209–214. [Google Scholar] [CrossRef] [Green Version]
- Cho, P.; Cheung, S.W.; Edwards, M. The longitudinal orthokeratology research in children (LORIC) in Hong Kong: A pilot study on refractive changes and myopic control. Curr. Eye Res. 2005, 30, 71–80. [Google Scholar] [CrossRef]
- Rempt, F.; Hoogerheide, J.; Hoogenboom, W.P. Peripheral retinoscopy and the skiagram. Ophthalmologica 1971, 162, 1–10. [Google Scholar] [CrossRef]
- Bao, J.; Yang, A.; Huang, Y.; Li, X.; Pan, Y.; Ding, C.; Lim, E.W.; Zheng, J.; Spiegel, D.P.; Drobe, B.; et al. One-year myopia control efficacy of spectacle lenses with aspherical lenslets. Br. J. Ophthalmol. 2021. [Google Scholar] [CrossRef]
- Lam, C.S.Y.; Tang, W.C.; Tse, D.Y.; Lee, R.P.K.; Chun, R.K.M.; Hasegawa, K.; Qi, H.; Hatanaka, T.; To, C.H. Defocus Incorporated Multiple Segments (DIMS) spectacle lenses slow myopia progression: A 2-year randomised clinical trial. Br. J. Ophthalmol. 2020, 104, 363. [Google Scholar] [CrossRef] [Green Version]
- Radhakrishnan, H.; Allen, P.M.; Calver, R.I.; Theagarayan, B.; Price, H.; Rae, S.; Sailoganathan, A.; O’Leary, D.J. Peripheral refractive changes associated with myopia progression. Investig. Ophthalmol. Vis. Sci. 2013, 54, 1573–1581. [Google Scholar] [CrossRef] [Green Version]
- Sng, C.C.A.; Lin, X.Y.; Gazzard, G.; Chang, B.; Dirani, M.; Lim, L.; Selvaraj, P.; Ian, K.; Drobe, B.; Wong, T.-Y.; et al. Change in peripheral refraction over time in Singapore Chinese children. Investig. Ophthalmol. Vis. Sci. 2011, 52, 7880–7887. [Google Scholar] [CrossRef] [Green Version]
- Almeida, M.S.d.; Carvalho, L.A. Different schematic eyes and their accuracy to the in vivo eye: A quantitative comparison study. Braz. J. Phys. 2007, 37, 378–387. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Park, T.W.; Winawer, J.; Wallman, J. In a Matter of Minutes, the Eye Can Know Which Way to Grow. Investig. Ophthalmol. Vis. Sci. 2005, 46, 2238–2241. [Google Scholar] [CrossRef]
- Chiang, S.T.-H.; Chen, T.-L.; Phillips, J.R. Effect of Optical Defocus on Choroidal Thickness in Healthy Adults With Presbyopia. Investig. Ophthalmol. Vis. Sci. 2018, 59, 5188–5193. [Google Scholar] [CrossRef] [PubMed]
- Chiang, S.T.-H.; Phillips, J.R.; Backhouse, S. Effect of retinal image defocus on the thickness of the human choroid. Ophthalmic Physiol. Opt. 2015, 35, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Mathur, A.; Atchison, D.A. Peripheral refraction patterns out to large field angles. Optom. Vis. Sci. 2013, 90, 140–147. [Google Scholar] [CrossRef] [Green Version]






| Object | Radius (mm) |
|---|---|
| Anterior radius Proclear MF | 9.12 |
| Anterior peripheral radius MF | 8.52 |
| Anterior radius Proclear monofocal | 9.12 |
| Posterior radius MF and monofocal | 8.7 |
| Contact lens central thickness | 0.01 |
| Anterior cornea radius | 8.1 |
| Posterior corneal radius | 6.8 |
| Central corneal thickness | 0.5 |
| Anterior chamber depth | 3.6 |
| Pupil diameter without accommodation | 2.4 |
| Pupil diameter with accommodation | 2.2 |
| Anterior lens radius without accommodation | 10 |
| Posterior lens radius without accommodation | −6.0 |
| Anterior lens radius with accommodation | 9 |
| Posterior lens radius with accommodation | −5.4 |
| Retina radius | −11 |
| Axial length | 27.367 |
| Eccentricity | Proclear Monofocal Infinity | Proclear Multifocal Infinity | Proclear Monofocal 40 cm | Proclear Multifocal 40 cm |
|---|---|---|---|---|
| Central | 0.701 | 0.027 | 0.008 | 0.161 |
| 5° | 0.390 | 0.035 | 0.009 | 0.045 |
| 10° | 0.042 | 0.017 | 0.009 | 0.021 |
| 15° | 0.016 | 0.020 | 0.015 | 0.018 |
| 20° | 0.015 | 0.012 | 0.015 | 0.028 |
| 25° | 0.011 | 0.016 | 0.015 | 0.021 |
| 30° | 0.011 | 0.023 | 0.012 | 0.019 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fritschi, A.; Gerber, C.; Eggler, D.; Loertscher, M. Simultaneous Myopic Defocus for Myopia Control: Effect on Accommodation, Peripheral Refraction and Retinal Image Quality in Non-Presbyopic Patients. Optics 2021, 2, 200-215. https://doi.org/10.3390/opt2040019
Fritschi A, Gerber C, Eggler D, Loertscher M. Simultaneous Myopic Defocus for Myopia Control: Effect on Accommodation, Peripheral Refraction and Retinal Image Quality in Non-Presbyopic Patients. Optics. 2021; 2(4):200-215. https://doi.org/10.3390/opt2040019
Chicago/Turabian StyleFritschi, Alina, Chloe Gerber, Damian Eggler, and Martin Loertscher. 2021. "Simultaneous Myopic Defocus for Myopia Control: Effect on Accommodation, Peripheral Refraction and Retinal Image Quality in Non-Presbyopic Patients" Optics 2, no. 4: 200-215. https://doi.org/10.3390/opt2040019
APA StyleFritschi, A., Gerber, C., Eggler, D., & Loertscher, M. (2021). Simultaneous Myopic Defocus for Myopia Control: Effect on Accommodation, Peripheral Refraction and Retinal Image Quality in Non-Presbyopic Patients. Optics, 2(4), 200-215. https://doi.org/10.3390/opt2040019







