Active Carbon Modified by Rhenium Species as a Perspective Supercapacitor Electrode
Abstract
:1. Introduction
2. Experimental
2.1. Materials Preparation
2.2. Characterization
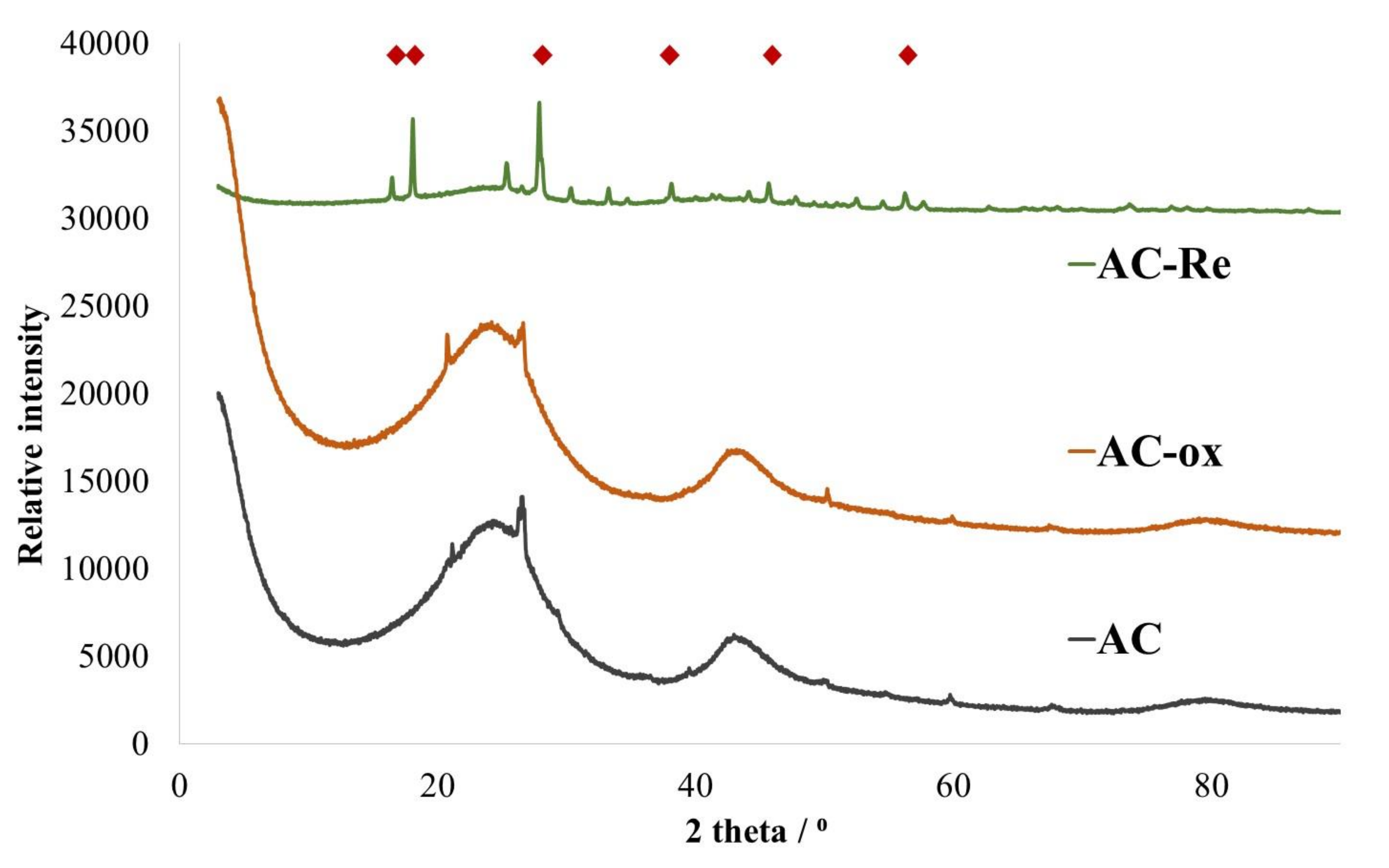
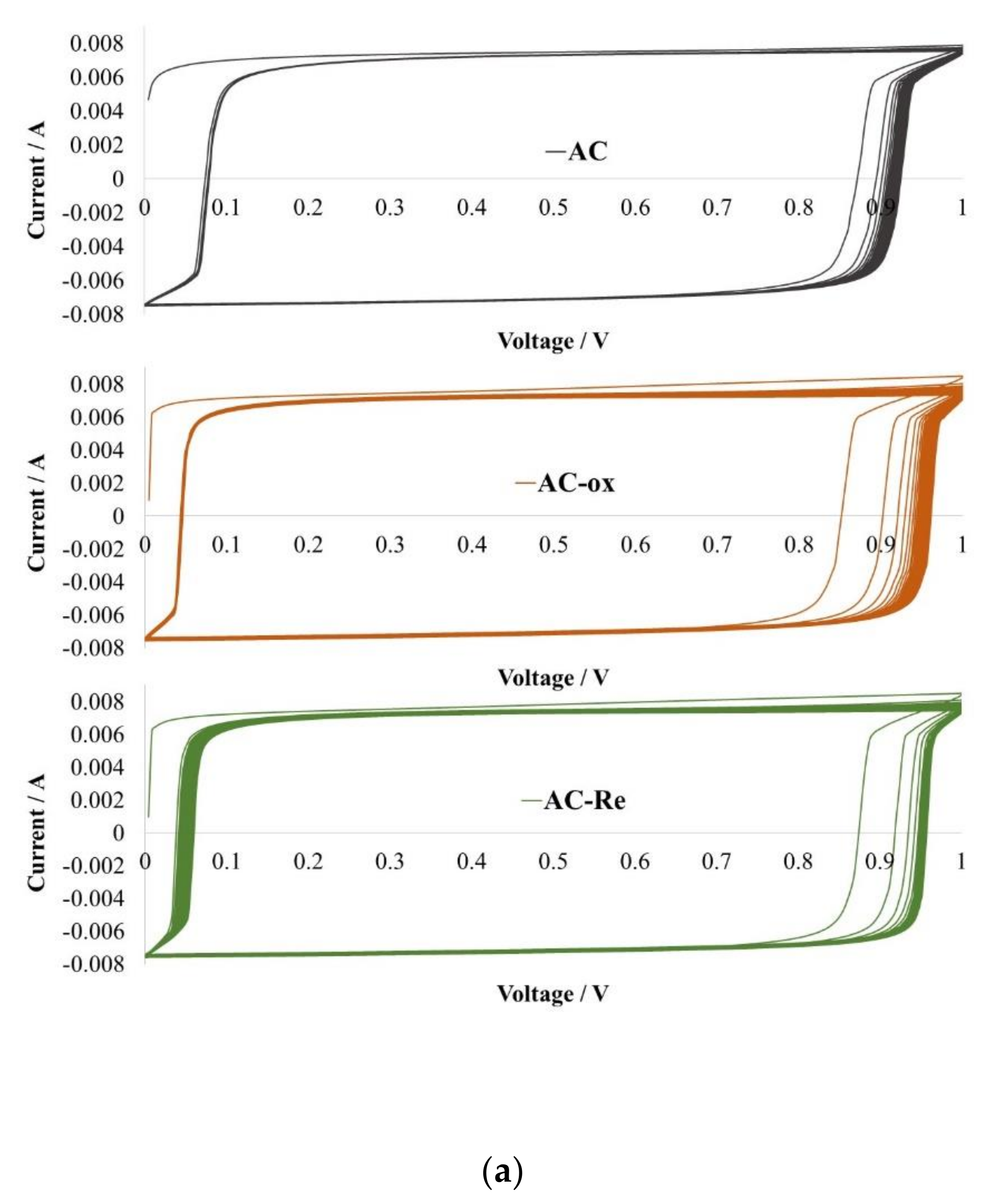
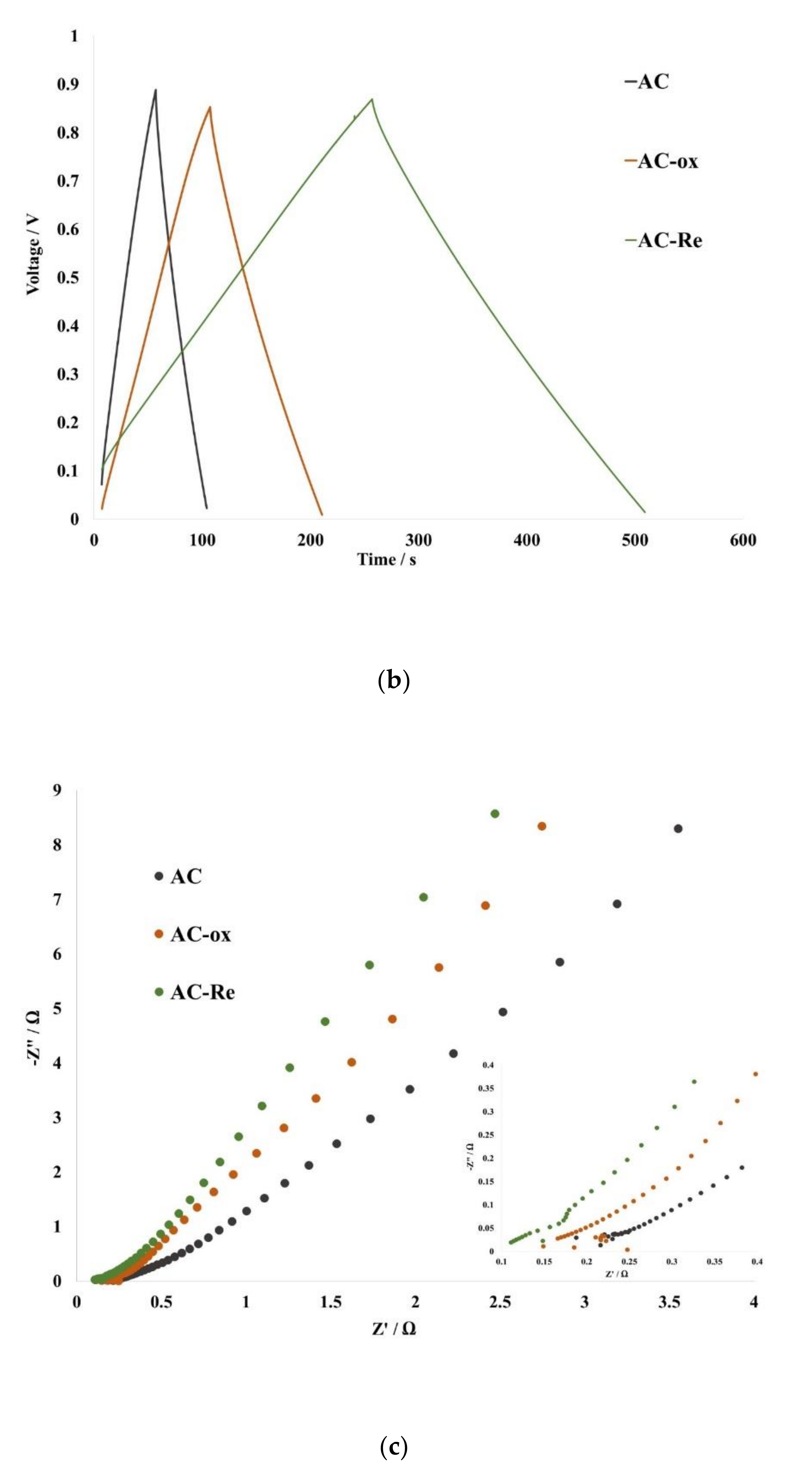
3. Results
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mazda ‘i-ELOOP’ Capacitor-Based Brake Energy Regeneration System. Available online: https://www2.mazda.com/en/publicity/release/2011/201111/111125a.html (accessed on 15 January 2020).
- Automotive. Available online: https://www.maxwell.com/solutions/transportation/auto (accessed on 15 January 2020).
- Samsung Galaxy Note 9 Review: This is Power User Perfection. Available online: https://www.sammobile.com/samsung/galaxy-note9/review/ (accessed on 15 June 2020).
- Boscaino, V.; Capponi, G.; Marino, F. Experimental test on a fuel cell—Supercapacitor hybrid power supply for a digital still camera. In Proceedings of the 2009 44th International Universities Power Engineering Conference (UPEC), Glasgow, UK, 1–4 September 2009. [Google Scholar]
- Meng, C.; Gall, O.Z.; Irazoqui, P.P. A flexible super-capacitive solid-state power supply for miniature implantable medical devices. Biomed. Microdevices 2013, 15, 973–983. [Google Scholar] [CrossRef] [PubMed]
- Najib, S.; Erdem, E. Current progress achieved in novel materials for supercapacitor electrodes: Mini review. Nanoscale Adv. 2019, 1, 2817–2827. [Google Scholar] [CrossRef] [Green Version]
- Zhao, B.; Chen, D.; Xiong, X.; Song, B.; Hu, R.; Zhang, Q.; Rainwater, B.H.; Waller, G.H.; Zhen, D.; Ding, Y.; et al. A high-energy, long cycle-life hybrid supercapacitor based on graphene composite electrodes. Energy Storage Mater. 2017, 7, 32–39. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.; de Fátima Montemor, M. Metal Oxide and Hydroxide–Based Aqueous Supercapacitors: From Charge Storage Mechanisms and Functional Electrode Engineering to Need-Tailored Devices. Adv. Sci. 2019, 6, 1801797. [Google Scholar] [CrossRef] [PubMed]
- Balandin, A.A.; Karpeiskaya, E.I.; Tolstopyatova, A.A. Catalytic properties of rhenium. Communication 1. Rhenium as dehydrogenation catalyst, Bulletin of the Academy of Sciences of the USSR. Bull. Acad. Sci. USSR Div. Chem. Sci. 1959, 8, 1318–1324. [Google Scholar] [CrossRef]
- Mol, J.C. Industrial applications of olefin metathesis. J. Mol. Catal. A Chem. 2004, 213, 39–45. [Google Scholar] [CrossRef]
- Kessler, V.G.; Seisenbaeva, G.A. Rhenium nanochemistry for catalyst preparation. Minerals 2012, 2, 244–257. [Google Scholar] [CrossRef] [Green Version]
- Tsoncheva, T.; Vankova, S.; Bozhkov, O.; Mehandjiev, D. Rhenium and manganese modified activated carbon as catalyst for methanol decomposition. Can. J. Chem. 2007, 85, 118–123. [Google Scholar] [CrossRef]
- Veerakumar, P.; Thanasekaran, P.; Lin, K.C.; Liu, S.B. Well-dispersed rhenium nanoparticles on three-dimensional carbon nanostructures: Efficient catalysts for the reduction of aromatic nitro compounds. J. Colloid Interface Sci. 2017, 506, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Di, X.; Shao, Z.; Li, C.; Li, W.; Liang, C. Hydrogenation of succinic acid over supported rhenium catalysts prepared by the microwaveassisted thermolytic method. Catal. Sci. Technol. 2015, 5, 2441–2448. [Google Scholar] [CrossRef]
- Kremlev, K.V.; Obiedkov, A.M.; Ketkov, S.Y.; Kaverin, B.S.; Semenov, N.M.; Domrachev, G.A.; Gusev, S.A.; Tatarskiy, D.A.; Yunin, P.A. New hybrid material based on multiwalled carbon nanotubes decorated with rhenium nanoparticles, Journal of Surface Investigation. X-ray. J. Surf. Investig. X-ray Synchrotron Neutron Tech. 2015, 9, 694–698. [Google Scholar] [CrossRef]
- Dobrzanska-Danikiewicz, A.D.; Wolany, W.; Benke, G.; Rdzawski, Z. The new MWCNTs–rhenium nanocomposite. Phys. Status Solidi B 2014, 251, 2485–2490. [Google Scholar] [CrossRef]
- Jeżowski, P.; Fic, K.; Crosnier, O.; Brousse, T.; Béguin, F. Lithium rhenium (VII) oxide as novel material for graphite pre-lithiation in high performance Lithium-ion capacitors. J. Mater. Chem. A 2016, 4, 12609–12615. [Google Scholar] [CrossRef]
- Qi, Y.; Meng, C.; Xu, X.; Deng, B.; Han, N.; Zhao, J.; Liu, M.; Fu, Q.; Hong, M.; Li, Y.; et al. Unique phase transformation from graphene to carbide on Re(0001) induced by strong carbon-metal interaction. J. Am. Chem. Soc. 2017, 139, 17574–17581. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Su, X.F.; Guan, W.; Yan, L.K.; Su, Z.M. Nanographene−rhenium complex as efficient catalyst for electrochemical reduction: A computational study. Mol. Catal. 2020, 484, 110736. [Google Scholar] [CrossRef]

| BET Surface Area m2/g | BJH Pore Volume (Adsorption) cm3/g | BJH Pore Volume (Desorption) cm3/g | BJH Pore Size (Adsorption) Å | BJH Pore Size (Desorption) Å | |
|---|---|---|---|---|---|
| AC | 1018.06 | 0.3068 | 0.3024 | 46.886 | 48.144 |
| AC-ox | 984.10 | 0.2700 | 0.2564 | 42.739 | 43.346 |
| AC-Re | 996.17 | 0.3416 | 0.3332 | 45.205 | 46.896 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciszewski, M.; Koszorek, A.; Hawełek, Ł.; Osadnik, M.; Szleper, K.; Drzazga, M. Active Carbon Modified by Rhenium Species as a Perspective Supercapacitor Electrode. Electrochem 2020, 1, 278-285. https://doi.org/10.3390/electrochem1030018
Ciszewski M, Koszorek A, Hawełek Ł, Osadnik M, Szleper K, Drzazga M. Active Carbon Modified by Rhenium Species as a Perspective Supercapacitor Electrode. Electrochem. 2020; 1(3):278-285. https://doi.org/10.3390/electrochem1030018
Chicago/Turabian StyleCiszewski, Mateusz, Andrzej Koszorek, Łukasz Hawełek, Małgorzata Osadnik, Katarzyna Szleper, and Michał Drzazga. 2020. "Active Carbon Modified by Rhenium Species as a Perspective Supercapacitor Electrode" Electrochem 1, no. 3: 278-285. https://doi.org/10.3390/electrochem1030018
APA StyleCiszewski, M., Koszorek, A., Hawełek, Ł., Osadnik, M., Szleper, K., & Drzazga, M. (2020). Active Carbon Modified by Rhenium Species as a Perspective Supercapacitor Electrode. Electrochem, 1(3), 278-285. https://doi.org/10.3390/electrochem1030018






