A Comprehensive Review on the Use of Metal–Organic Frameworks (MOFs) Coupled with Enzymes as Biosensors
Abstract
:1. Introduction
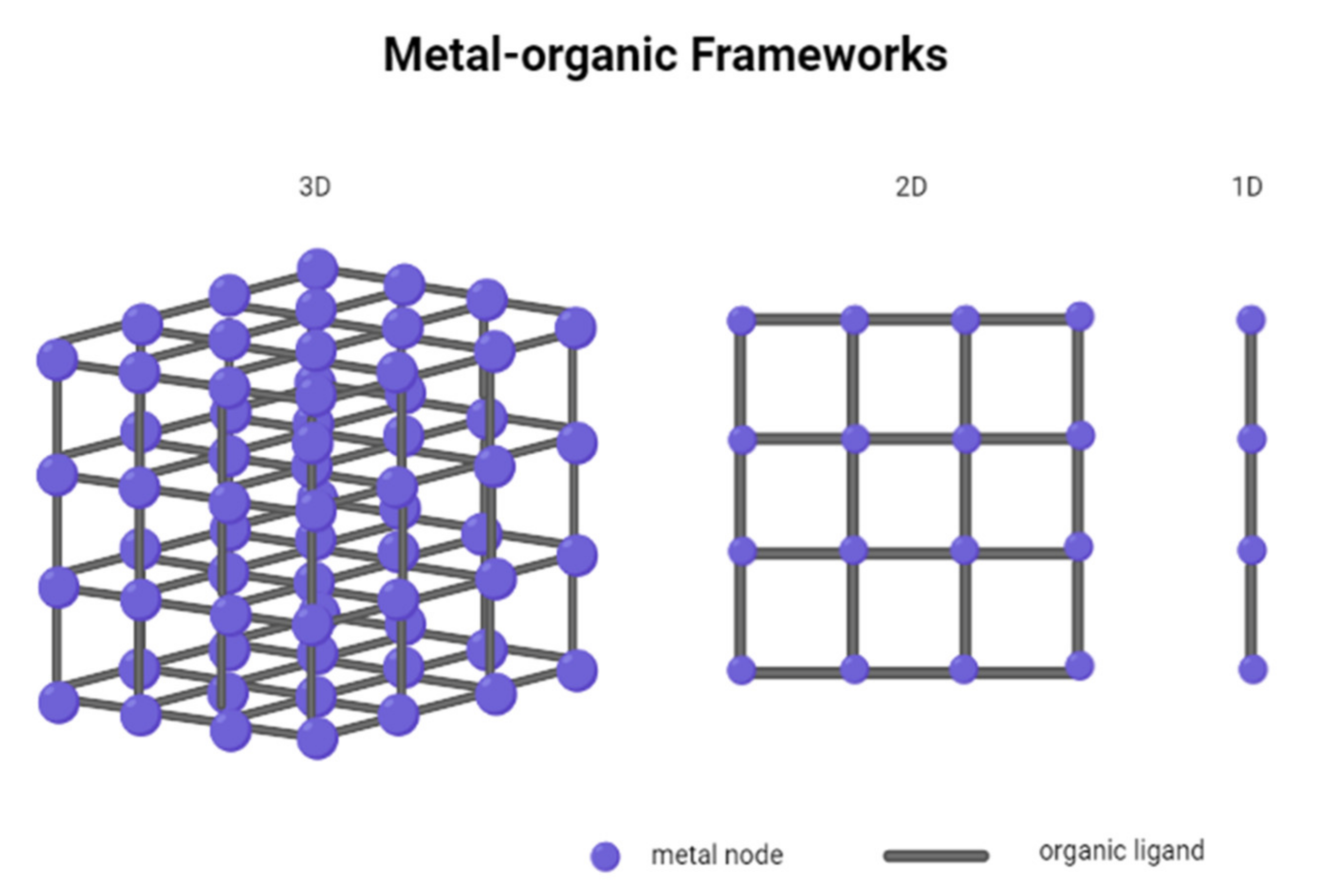
2. Metal–Organic Frameworks
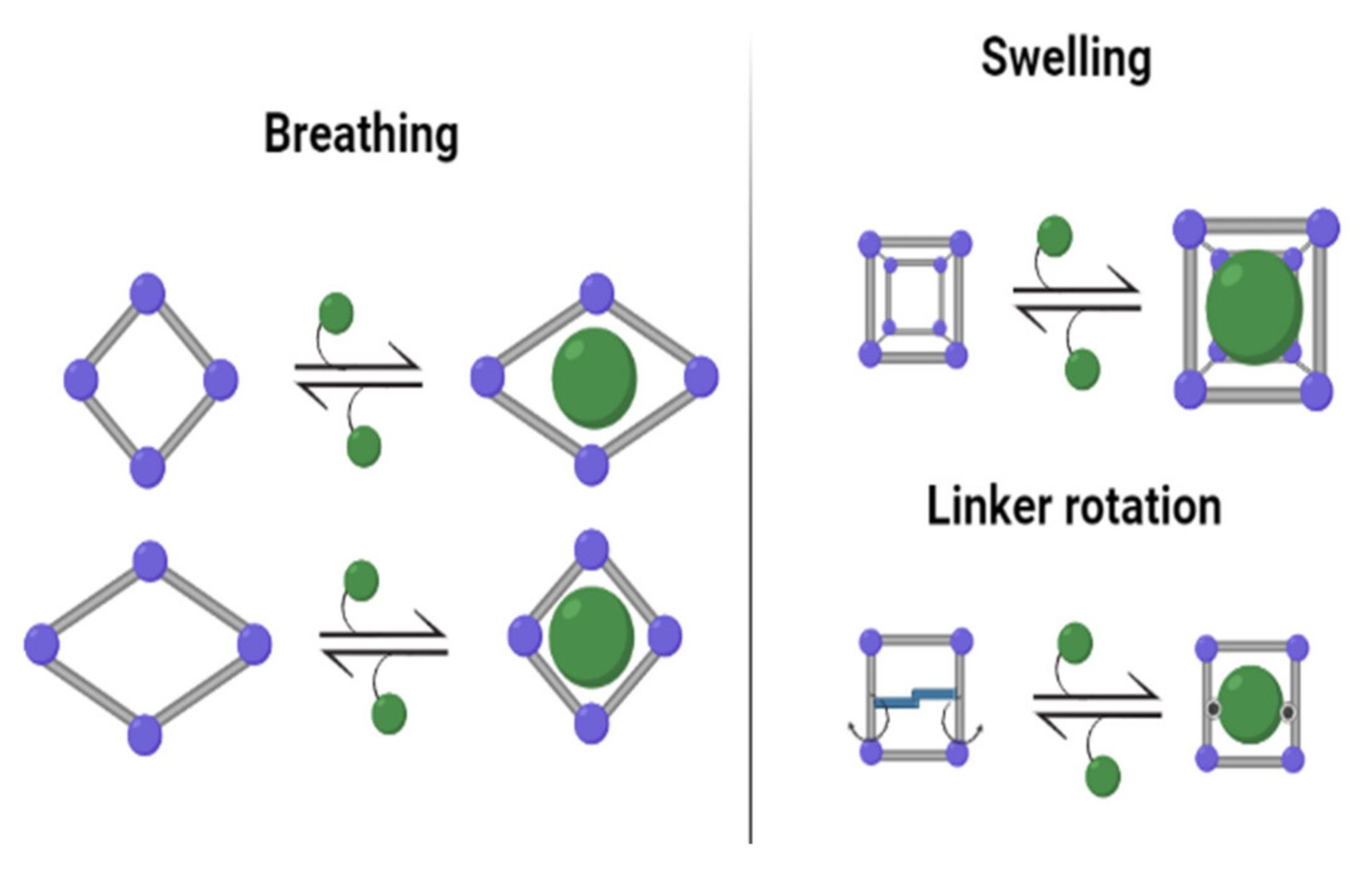
Properties of Metal–Organic Frameworks
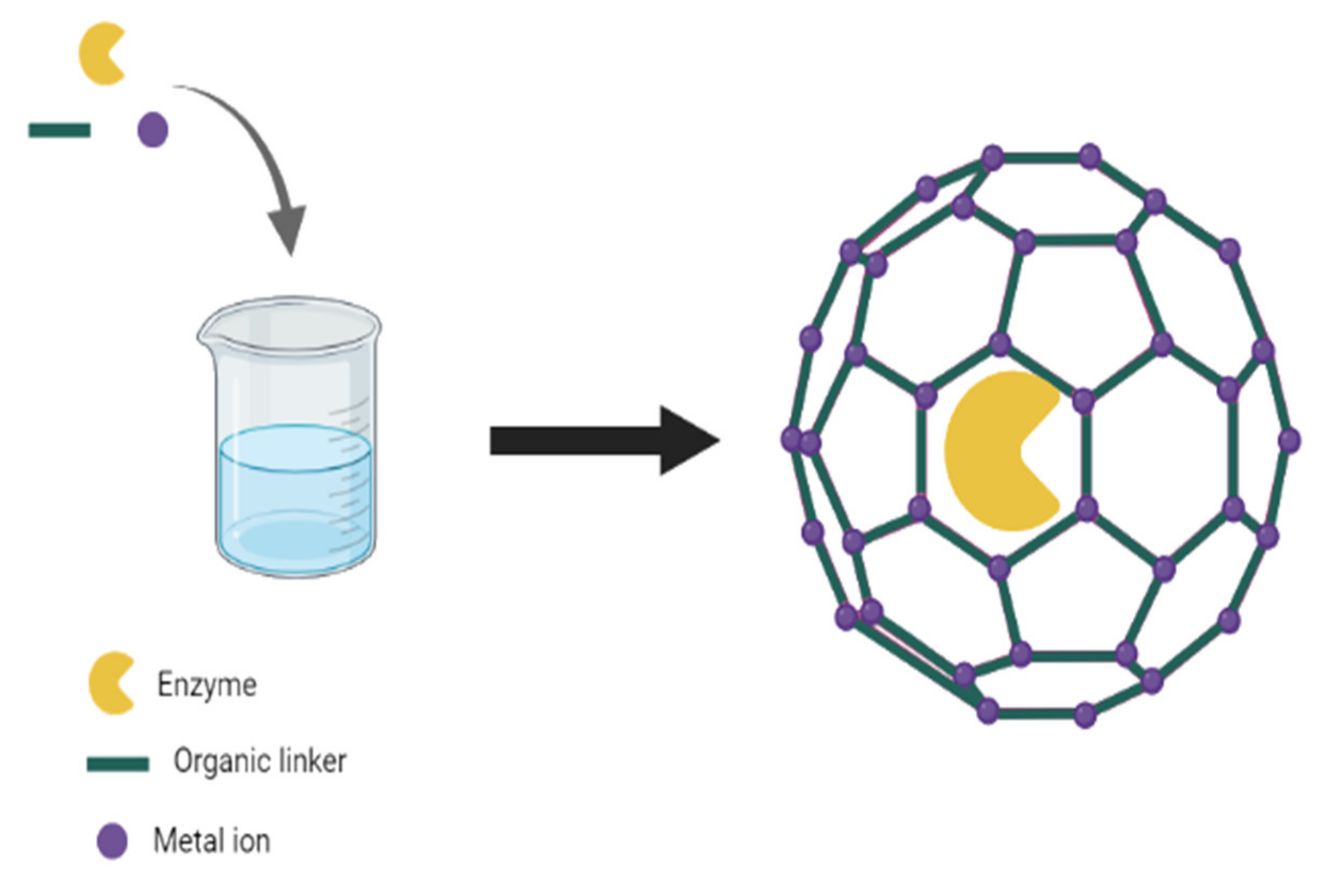
3. Preparation/Characterization of Enzyme–MOF Biosensors
3.1. Co-Precipitation
3.2. Covalent Linkage
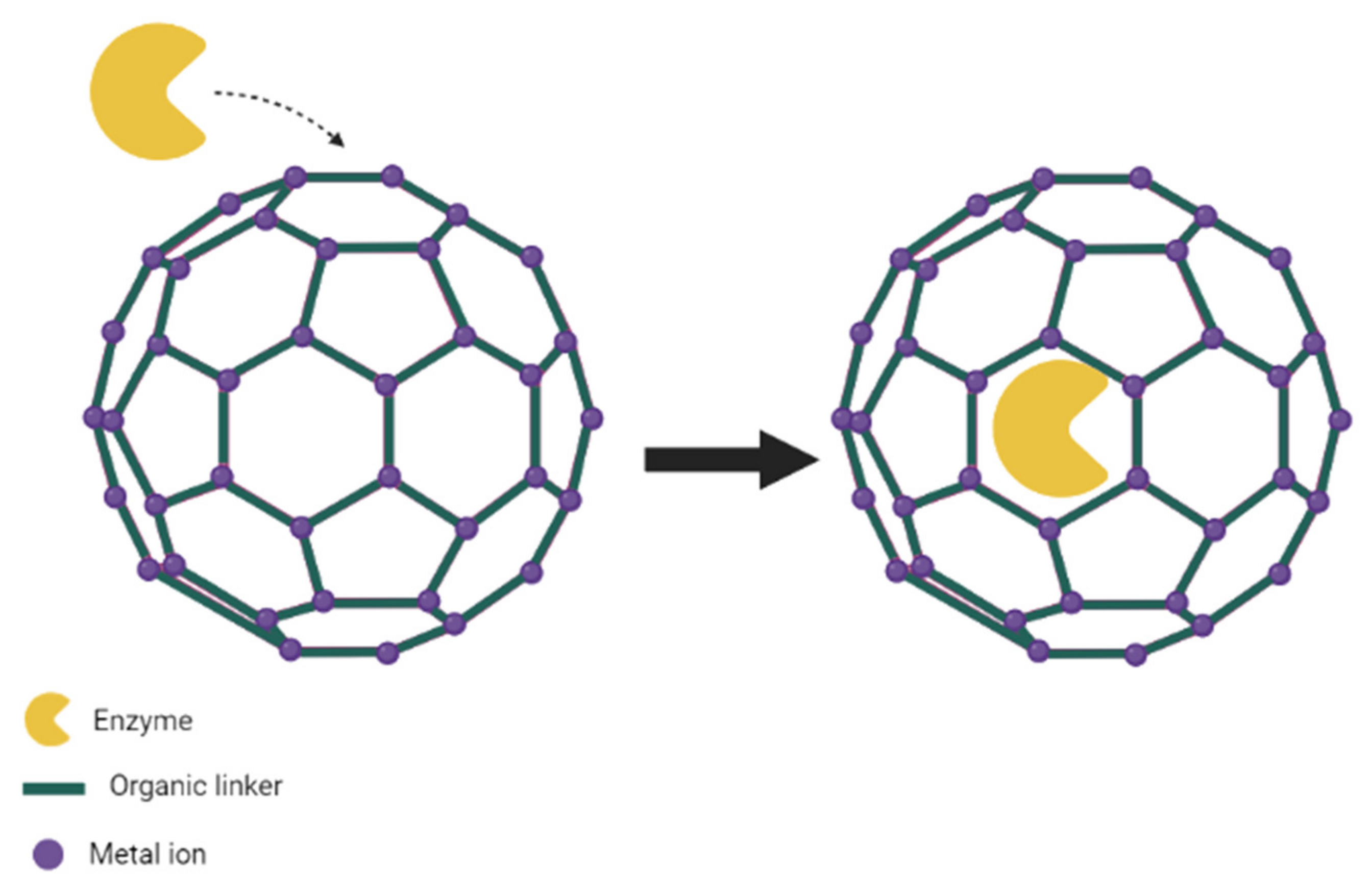
3.3. Entrapment
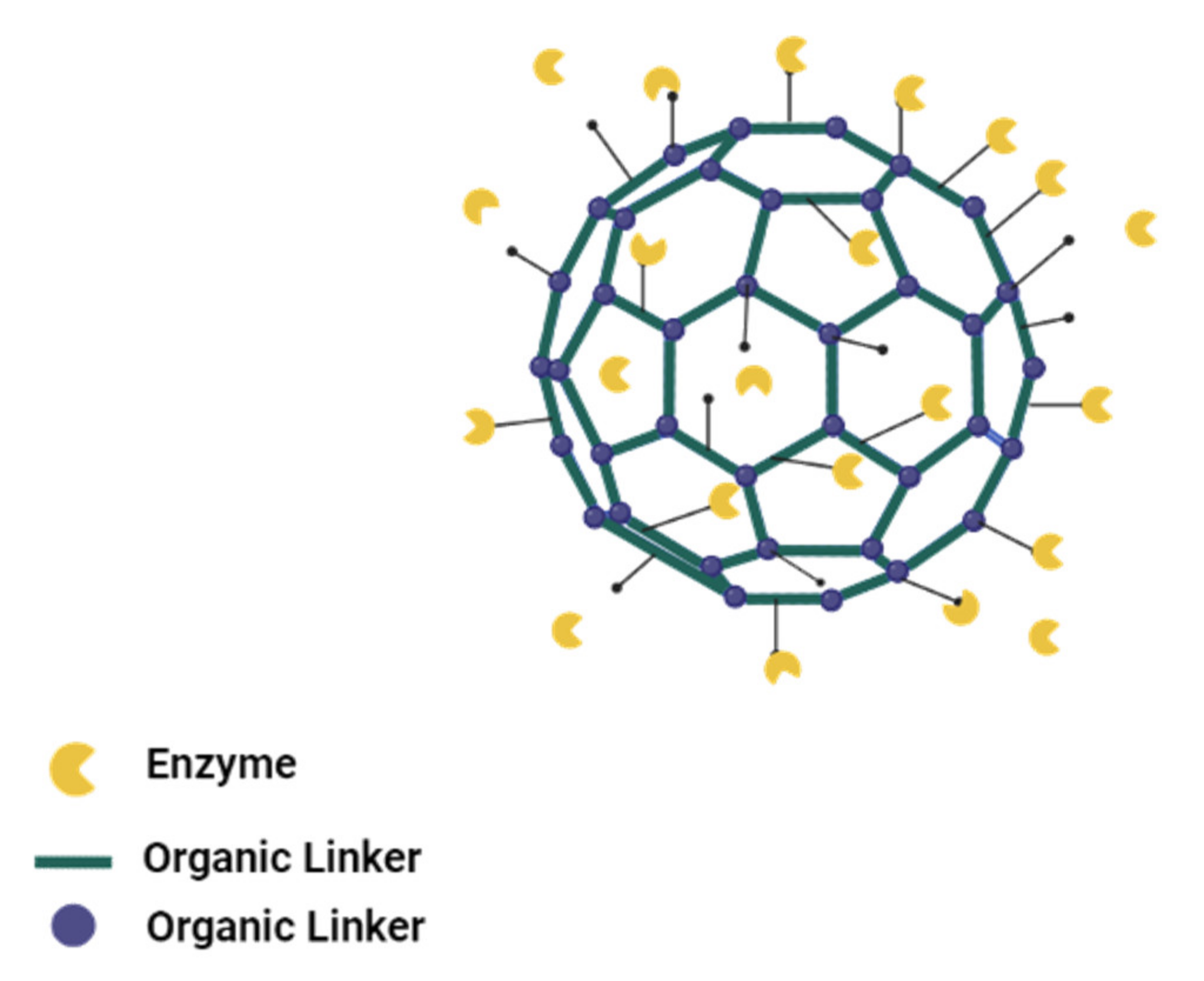
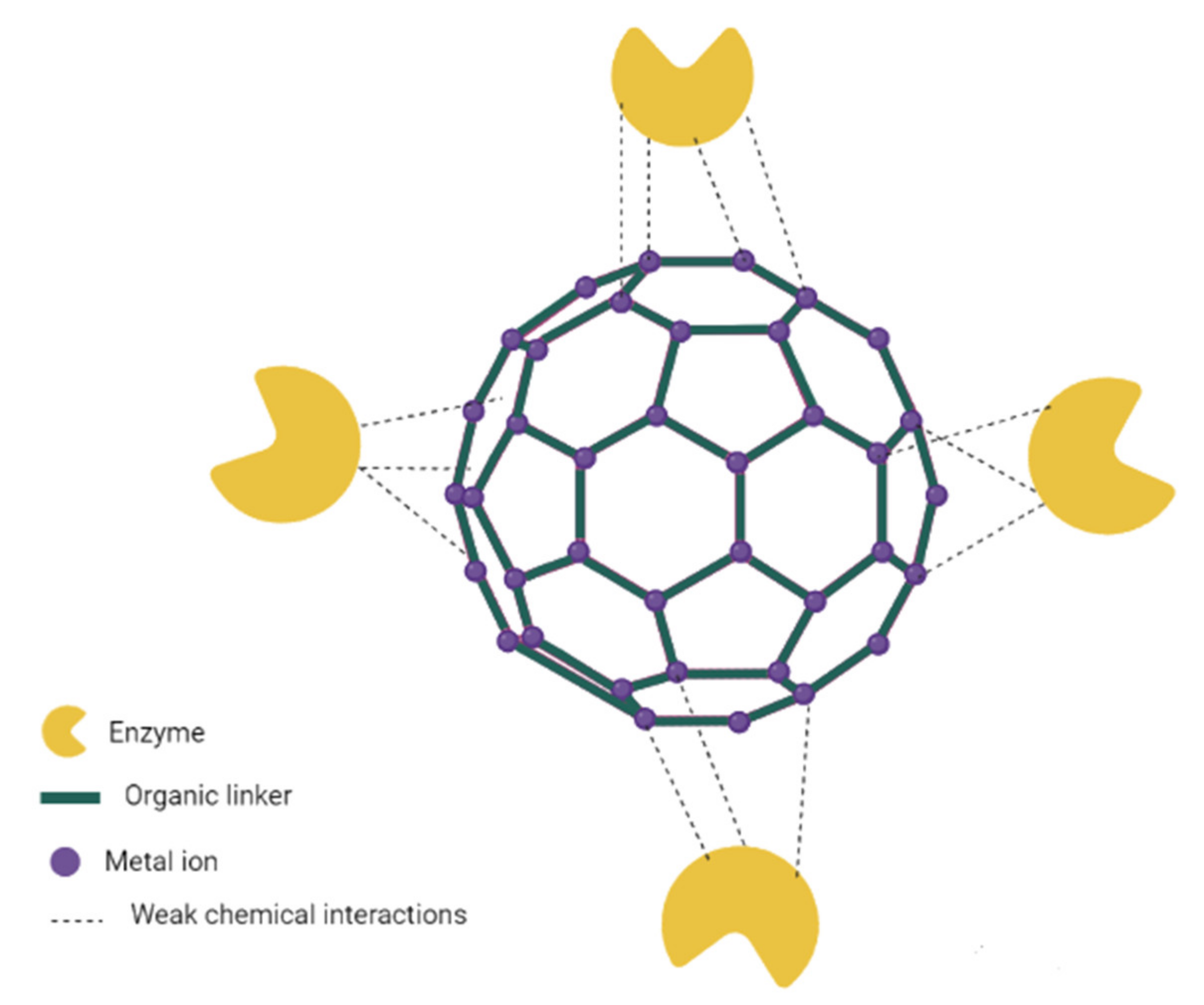
3.4. Surface Attachment
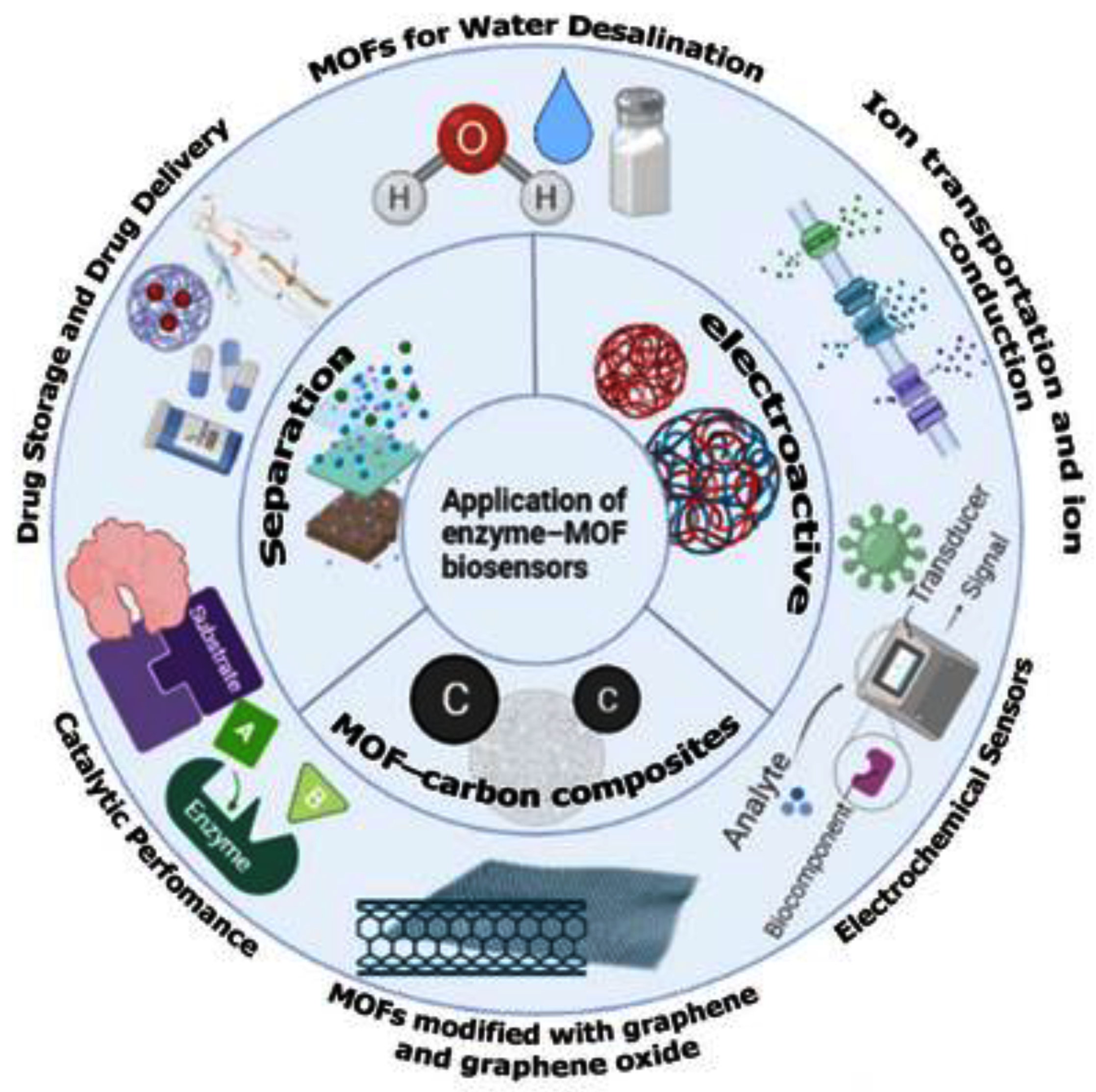
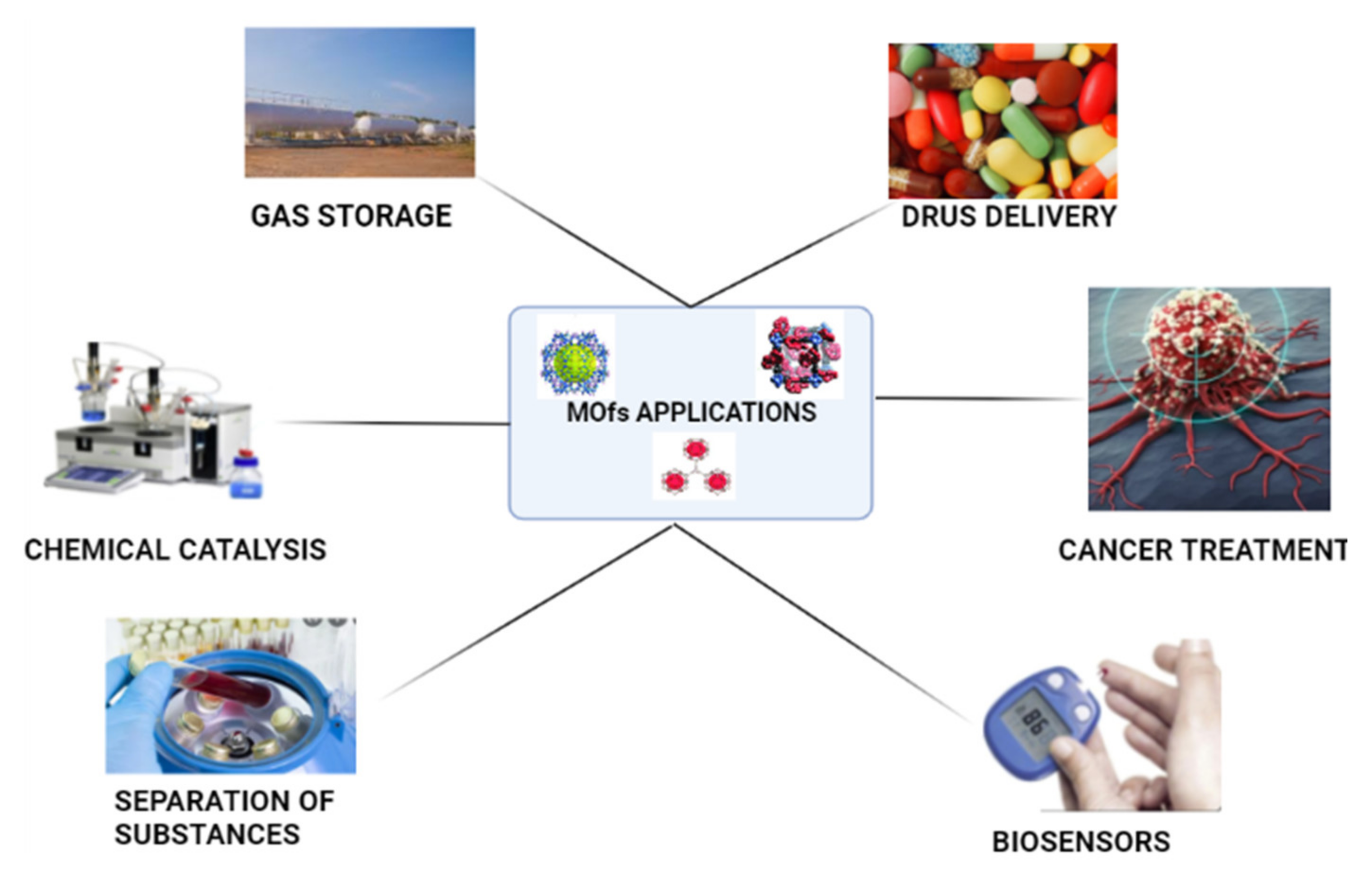
4. Application of Enzyme–MOF Biosensors
4.1. Biomedical Applications
4.1.1. Glucose Oxidase-Integrated MOFs as Biosensors
4.1.2. Detection of Hydrogen Peroxide Using MOF-Based Enzymes
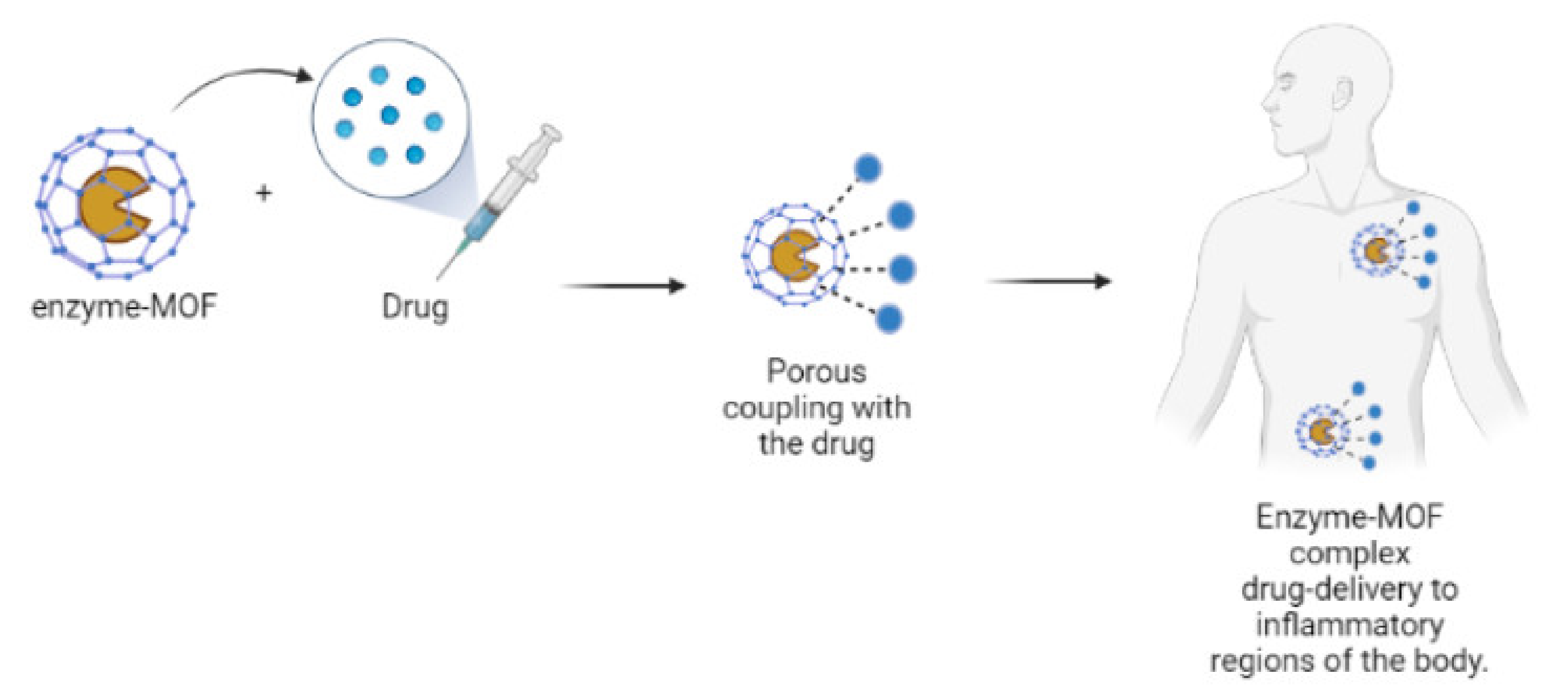
4.1.3. Enzymes Immobilized with MOFs for Drug Delivery as Immunosensors
4.1.4. MOF-Based Electrochemical Biosensors for Detecting Cancer Biomarkers
4.1.5. Detection of Other Analytes of Biomedical Interest
4.2. Environmental Applications
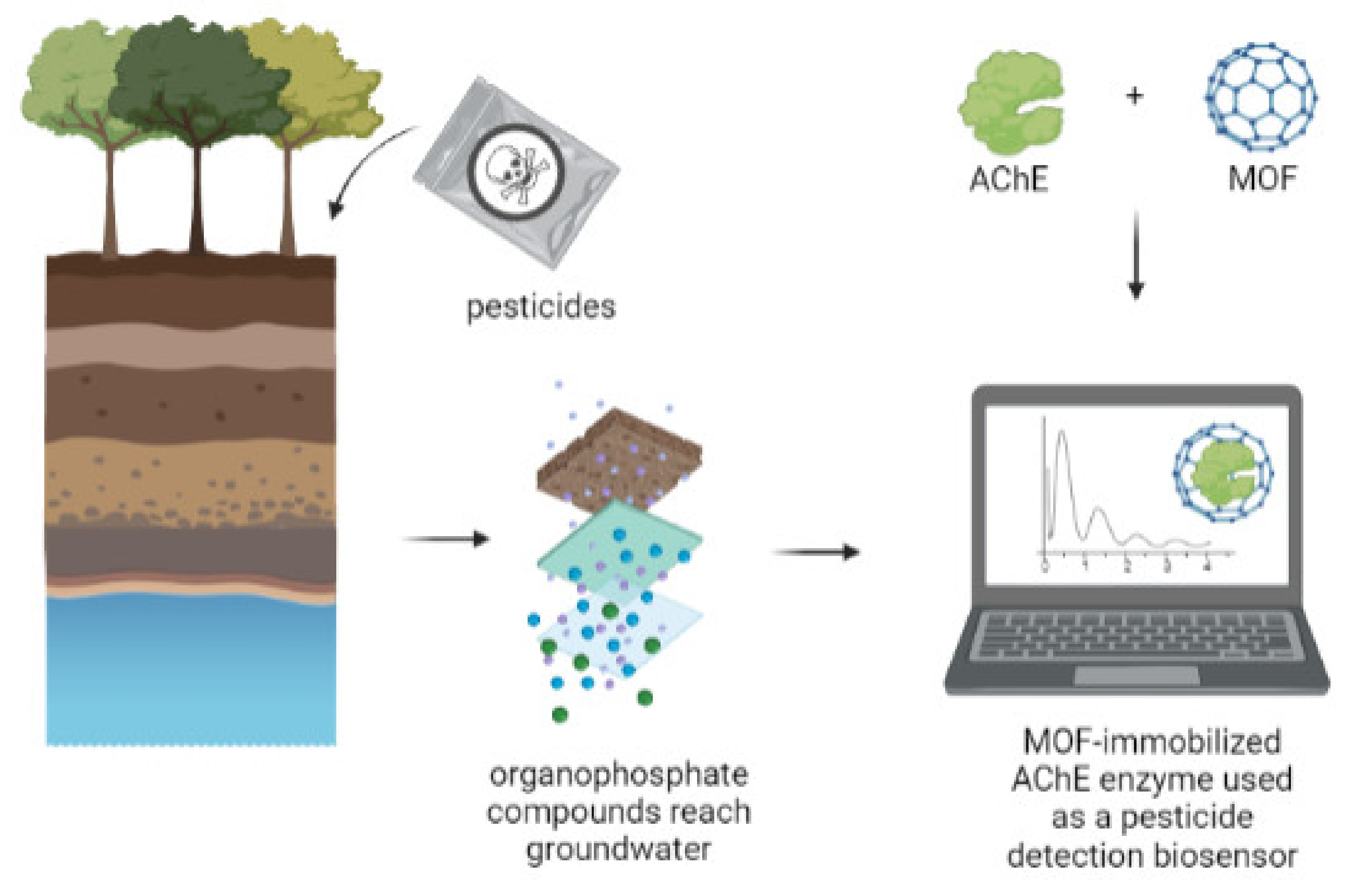
4.2.1. MOF-Based Biosensors for Detecting Environmental Pollutants
4.2.2. Enzyme–MOF as Biosensors with Improved Electrochemical Performance for Pesticides
4.2.3. Detoxification and Effluent Treatment Using MOF-Based Enzymes
4.3. Food Applications
4.4. General Applications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Mohammad, M.; Razmjou, A.; Liang, K.; Asadnia, M.; Chen, V. Metal–Organic-Framework-Based Enzymatic Microfluidic Biosensor via Surface Patterning and Biomineralization. ACS Appl. Mater. Interfaces 2019, 11, 1807–1820. [Google Scholar] [CrossRef] [PubMed]
- Mota, G.F.; de Sousa, I.G.; de Oliveira, A.L.; Cavalcante, A.L.; da Silva Moreira, K.; Cavalcante, F.T.; da Silva Souza, J.E.; de Aguiar Falcão, Í.R.; Rocha, T.G.; Valério, R.B.; et al. Biodiesel Production from Microalgae Using Lipase-Based Catalysts: Current Challenges and Prospects. Algal Res. 2022, 62, 102616. [Google Scholar] [CrossRef]
- Manoel, E.A.; Pinto, M.; dos Santos, J.C.S.; Tacias-Pascacio, V.G.; Freire, D.M.G.; Pinto, J.C.; Fernandez-Lafuente, R. Design of a Core–Shell Support to Improve Lipase Features by Immobilization. RSC Adv. 2016, 6, 62814–62824. [Google Scholar] [CrossRef]
- Cavalcante, F.T.T.; de A. Falcão, I.R.; da S. Souza, J.E.; Rocha, T.G.; de Sousa, I.G.; Cavalcante, A.L.G.; de Oliveira, A.L.B.; de Sousa, M.C.M.; dos Santos, J.C.S. Designing of Nanomaterials-Based Enzymatic Biosensors: Synthesis, Properties, and Applications. Electrochem 2021, 2, 149–184. [Google Scholar] [CrossRef]
- Lian, X.; Fang, Y.; Joseph, E.; Wang, Q.; Li, J.; Banerjee, S.; Lollar, C.; Wang, X.; Zhou, H.-C. Enzyme–MOF (Metal–Organic Framework) Composites. Chem. Soc. Rev. 2017, 46, 3386–3401. [Google Scholar] [CrossRef] [PubMed]
- Nadar, S.S.; Rathod, V.K. Magnetic-Metal Organic Framework (Magnetic-MOF): A Novel Platform for Enzyme Immobilization and Nanozyme Applications. Int. J. Biol. Macromol. 2018, 120, 2293–2302. [Google Scholar] [CrossRef]
- De Oliveira, A.L.B.; Cavalcante, F.T.T.; Moreira, K.S.; Monteiro, R.R.C.; Rocha, T.G.; Souza, J.E.S.; da Fonseca, A.M.; Lopes, A.A.S.; Guimarães, A.P.; de Lima, R.K.C.; et al. Lipases Immobilized onto Nanomaterials as Biocatalysts in Biodiesel Production: Scientific Context, Challenges, and Opportunities. Rev. Virtual Quím. 2021, 13, 875–891. [Google Scholar] [CrossRef]
- Carneiro, E.; Bastos, A.; de Oliveira, U.; de Matos, L.; Adriano, W.; Monteiro, R.; dos Santos, J.; Gonçalves, L. Improving the Catalytic Features of the Lipase from Rhizomucor miehei Immobilized on Chitosan-Based Hybrid Matrices by Altering the Chemical Activation Conditions. Quím. Nova 2020, 43, 1234–1239. [Google Scholar] [CrossRef]
- Melo, A.; Silva, F.; dos Santos, J.; Fernández-Lafuente, R.; Lemos, T.; Dias Filho, F. Synthesis of Benzyl Acetate Catalyzed by Lipase Immobilized in Nontoxic Chitosan-Polyphosphate Beads. Molecules 2017, 22, 2165. [Google Scholar] [CrossRef] [Green Version]
- Da Fonseca, A.M.; de Freitas, I.B.; Soares, N.B.; de Araújo, F.A.M.; Gaieta, E.M.; dos Santos, J.C.S.; Sobrinho, A.C.N.; Marinho, E.S.; Colares, R.P. Synthesis, Biological Activity, and In Silico Study of Bioesters Derived from Bixin by the CALB Enzyme. Biointerface Res. Appl. Chem. 2021, 12, 5901–5917. [Google Scholar] [CrossRef]
- Liao, P.-Q.; Shen, J.-Q.; Zhang, J.-P. Metal–Organic Frameworks for Electrocatalysis. Coord. Chem. Rev. 2018, 373, 22–48. [Google Scholar] [CrossRef]
- Li, Y.; Xu, H.; Ouyang, S.; Ye, J. Metal–Organic Frameworks for Photocatalysis. Phys. Chem. Chem. Phys. 2016, 18, 7563–7572. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhu, Q.-L.; Zou, R.; Xu, Q. Metal-Organic Frameworks for Energy Applications. Chem 2017, 2, 52–80. [Google Scholar] [CrossRef] [Green Version]
- Thorarinsdottir, A.E.; Harris, T.D. Metal–Organic Framework Magnets. Chem. Rev. 2020, 120, 8716–8789. [Google Scholar] [CrossRef]
- Liu, L.; Zhou, Y.; Liu, S.; Xu, M. The Applications of Metal−Organic Frameworks in Electrochemical Sensors. ChemElectroChem 2018, 5, 6–19. [Google Scholar] [CrossRef] [Green Version]
- Abednatanzi, S.; Gohari Derakhshandeh, P.; Depauw, H.; Coudert, F.-X.; Vrielinck, H.; van der Voort, P.; Leus, K. Mixed-Metal Metal–Organic Frameworks. Chem. Soc. Rev. 2019, 48, 2535–2565. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; McGuirk, C.M.; d’Aquino, A.; Mason, J.A.; Mirkin, C.A. Metal–Organic Framework Nanoparticles. Adv. Mater. 2018, 30, 1800202. [Google Scholar] [CrossRef]
- Dai, H.; Lü, W.; Zuo, X.; Zhu, Q.; Pan, C.; Niu, X.; Liu, J.; Chen, H.; Chen, X. A Novel Biosensor Based on Boronic Acid Functionalized Metal-Organic Frameworks for the Determination of Hydrogen Peroxide Released from Living Cells. Biosens. Bioelectron. 2017, 95, 131–137. [Google Scholar] [CrossRef]
- Ali, N.; Bilal, M.; Khan, A.; Ali, F.; Khan, H.; Khan, H.A.; Rasool, K.; Iqbal, H.M.N. Understanding the Hierarchical Assemblies and Oil/Water Separation Applications of Metal-Organic Frameworks. J. Mol. Liq. 2020, 318, 114273. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, B.; Alsalme, A.; Xiang, S.; Zhang, Z.; Chen, B. Design and Applications of Water-Stable Metal-Organic Frameworks: Status and Challenges. Coord. Chem. Rev. 2020, 423, 213507. [Google Scholar] [CrossRef]
- Saeed, T.; Naeem, A.; Din, I.U.; Alotaibi, M.A.; Alharthi, A.I.; Khan, I.W.; Khan, N.H.; Malik, T. Structure, Nomenclature and Viable Synthesis of Micro/Nanoscale Metal Organic Frameworks and Their Remarkable Applications in Adsorption of Organic Pollutants. Microchem. J. 2020, 159, 105579. [Google Scholar] [CrossRef]
- Wang, F.; Chen, L.; Liu, D.; Ma, W.; Dramou, P.; He, H. Nanozymes Based on Metal-Organic Frameworks: Construction and Prospects. TrAC Trends Anal. Chem. 2020, 133, 116080. [Google Scholar] [CrossRef]
- Alhumaimess, M.S. Metal–Organic Frameworks and Their Catalytic Applications. J. Saudi Chem. Soc. 2020, 24, 461–473. [Google Scholar] [CrossRef]
- Zhao, P.; Tsang, S.C.E.; Fairen-Jimenez, D. Structural Heterogeneity and Dynamics in Flexible Metal-Organic Frameworks. Cell Rep. Phys. Sci. 2021, 2, 100544. [Google Scholar] [CrossRef]
- Zulys, A.; Yulia, F.; Buhori, A.; Muhadzib, N.; Ghiyats, M.; Saha, B.B. Synthesis and Characterization of a Novel Microporous Lanthanide Based Metal-Organic Framework (MOF) Using Napthalenedicarboxylate Acid. J. Mater. Res. Technol. 2020, 9, 7409–7417. [Google Scholar] [CrossRef]
- Razavi, S.A.A.; Morsali, A. Linker Functionalized Metal-Organic Frameworks. Coord. Chem. Rev. 2019, 399, 213023. [Google Scholar] [CrossRef]
- Esrafili, L.; Tehrani, A.A.; Morsali, A.; Carlucci, L.; Proserpio, D.M. Ultrasound and Solvothermal Synthesis of a New Urea-Based Metal-Organic Framework as a Precursor for Fabrication of Cadmium(II) Oxide Nanostructures. Inorg. Chim. Acta 2019, 484, 386–393. [Google Scholar] [CrossRef]
- Jia, Z.; Hao, S.; Wen, J.; Li, S.; Peng, W.; Huang, R.; Xu, X. Electrochemical Fabrication of Metal–Organic Frameworks Membranes and Films: A Review. Microporous Mesoporous Mater. 2020, 305, 110322. [Google Scholar] [CrossRef]
- Peña-Rodríguez, R.; Márquez-López, E.; Guerrero, A.; Chiñas, L.E.; Hernández-González, D.F.; Rivera, J.M. Hydrothermal Synthesis of Cobalt (II) 3D Metal-Organic Framework Acid Catalyst Applied in the Transesterification Process of Vegetable Oil. Mater. Lett. 2018, 217, 117–119. [Google Scholar] [CrossRef]
- Thi Dang, Y.; Hoang, H.T.; Dong, H.C.; Bui, K.-B.T.; Nguyen, L.H.T.; Phan, T.B.; Kawazoe, Y.; Doan, T.L.H. Microwave-Assisted Synthesis of Nano Hf- and Zr-Based Metal-Organic Frameworks for Enhancement of Curcumin Adsorption. Microporous Mesoporous Mater. 2020, 298, 110064. [Google Scholar] [CrossRef]
- Ding, S.; He, L.; Bian, X.; Tian, G. Metal-Organic Frameworks-Based Nanozymes for Combined Cancer Therapy. Nano Today 2020, 35, 100920. [Google Scholar] [CrossRef]
- Latifi, L.; Sohrabnezhad, S. Drug Delivery by Micro and Meso Metal-Organic Frameworks. Polyhedron 2020, 180, 114321. [Google Scholar] [CrossRef]
- Li, B.; Wen, H.-M.; Yu, Y.; Cui, Y.; Zhou, W.; Chen, B.; Qian, G. Nanospace within Metal–Organic Frameworks for Gas Storage and Separation. Mater. Today Nano 2018, 2, 21–49. [Google Scholar] [CrossRef]
- Li, D.; Xu, H.-Q.; Jiao, L.; Jiang, H.-L. Metal-Organic Frameworks for Catalysis: State of the Art, Challenges, and Opportunities. EnergyChem 2019, 1, 100005. [Google Scholar] [CrossRef]
- Mehtab, T.; Yasin, G.; Arif, M.; Shakeel, M.; Korai, R.M.; Nadeem, M.; Muhammad, N.; Lu, X. Metal-Organic Frameworks for Energy Storage Devices: Batteries and Supercapacitors. J. Energy Storage 2019, 21, 632–646. [Google Scholar] [CrossRef]
- Wang, S.-J.; Joharian, M.; Naghiloo, M.; Yan, X.-W.; Rasuli, R.; Bigdeli, F.; Morsali, A. Synthesis of the Highly Porous Semiconductors with Different Electrical Features Using Isostructural Metal-Organic Frameworks as Precursor. Synth. Met. 2020, 270, 116600. [Google Scholar] [CrossRef]
- Cai, H.; Huang, Y.-L.; Li, D. Biological Metal–Organic Frameworks: Structures, Host–Guest Chemistry and Bio-Applications. Coord. Chem. Rev. 2019, 378, 207–221. [Google Scholar] [CrossRef]
- Tang, X.; Zhao, L.; Sun, W.; Wang, Y. Two-Dimensional Metal-Organic Framework Materials for Energy Conversion and Storage. J. Power Sources 2020, 477, 228919. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, Y.; He, Q.; Yan, J.; Xiong, H.; Wen, N.; Cai, S.; Peng, D.; Liu, Y.; Liu, Z. Metal-Organic Frameworks for Virus Detection. Biosens. Bioelectron. 2020, 169, 112604. [Google Scholar] [CrossRef]
- Meteku, B.E.; Huang, J.; Zeng, J.; Subhan, F.; Feng, F.; Zhang, Y.; Qiu, Z.; Aslam, S.; Li, G.; Yan, Z. Magnetic Metal–Organic Framework Composites for Environmental Monitoring and Remediation. Coord. Chem. Rev. 2020, 413, 213261. [Google Scholar] [CrossRef]
- Zhao, L.; Jing, X.; Li, X.; Guo, X.; Zeng, L.; He, C.; Duan, C. Catalytic Properties of Chemical Transformation within the Confined Pockets of Werner-Type Capsules. Coord. Chem. Rev. 2019, 378, 151–187. [Google Scholar] [CrossRef]
- Moeinian, M.; Akhbari, K. How the Guest Molecules in Nanoporous Zn(II) Metal-Organic Framework Can Prevent Agglomeration of ZnO Nanoparticles. J. Solid State Chem. 2015, 225, 459–463. [Google Scholar] [CrossRef]
- So, P.B.; Chen, H.-T.; Lin, C.-H. De Novo Synthesis and Particle Size Control of Iron Metal Organic Framework for Diclofenac Drug Delivery. Microporous Mesoporous Mater. 2020, 309, 110495. [Google Scholar] [CrossRef]
- Zhao, S.-N.; Zhang, Y.; Song, S.-Y.; Zhang, H.-J. Design Strategies and Applications of Charged Metal Organic Frameworks. Coord. Chem. Rev. 2019, 398, 113007. [Google Scholar] [CrossRef]
- Adesina Adegoke, K.; Samuel Agboola, O.; Ogunmodede, J.; Oluyomi Araoye, A.; Solomon Bello, O. Metal-Organic Frameworks as Adsorbents for Sequestering Organic Pollutants from Wastewater. Mater. Chem. Phys. 2020, 253, 123246. [Google Scholar] [CrossRef]
- Duo, H.; Lu, X.; Wang, S.; Liang, X.; Guo, Y. Preparation and Applications of Metal-Organic Framework Derived Porous Carbons as Novel Adsorbents in Sample Preparation. TrAC Trends Anal. Chem. 2020, 133, 116093. [Google Scholar] [CrossRef]
- Wang, Q.; Zhangsun, H.; Zhao, Y.; Zhuang, Y.; Xu, Z.; Bu, T.; Li, R.; Wang, L. Macro-Meso-Microporous Carbon Composite Derived from Hydrophilic Metal-Organic Framework as High-Performance Electrochemical Sensor for Neonicotinoid Determination. J. Hazard. Mater. 2021, 411, 125122. [Google Scholar] [CrossRef]
- Hao, Y.; Kang, Y.; Mi, Y.; Wang, W.; Lei, Z. Highly Ordered Micro-Meso-Macroporous Co-N-Doped Carbon Polyhedrons from Bimetal-Organic Frameworks for Rechargeable Zn-Air Batteries. J. Colloid Interface Sci. 2021, 598, 83–92. [Google Scholar] [CrossRef]
- Vaidya, L.B.; Nadar, S.S.; Rathod, V.K. Biological Metal Organic Framework (Bio-MOF) of Glucoamylase with Enhanced Stability. Colloids Surf. B Biointerfaces 2020, 193, 111052. [Google Scholar] [CrossRef]
- Jia, X.; Li, S.; Sun, T.; Wang, Y.; Fan, Y.; Zhang, C.; Xu, Y.; Liang, Z.; Lei, H.; Zhang, W.; et al. Single Crystal Metal-Organic Framework Constructed by Vertically Self-Pillared Nanosheets and Its Derivative for Oriented Lithium Plating. Chin. J. Catal. 2021, 42, 1553–1560. [Google Scholar] [CrossRef]
- Sai, T.; Ran, S.; Guo, Z.; Fang, Z. A Zr-Based Metal Organic Frameworks towards Improving Fire Safety and Thermal Stability of Polycarbonate. Compos. Part B Eng. 2019, 176, 107198. [Google Scholar] [CrossRef]
- Park, J.M.; Jhung, S.H. CO2 Adsorption at Low Pressure over Polymers-Loaded Mesoporous Metal Organic Framework PCN-777: Effect of Basic Site and Porosity on Adsorption. J. CO2 Util. 2020, 42, 101332. [Google Scholar] [CrossRef]
- Guo, F.; Su, C.; Fan, Y.; Shi, W.; Zhang, X. Construction of a Dual-Response Luminescent Metal-Organic Framework with Excellent Stability for Detecting Fe3+ and Antibiotic with High Selectivity and Sensitivity. J. Solid State Chem. 2020, 284, 121183. [Google Scholar] [CrossRef]
- Ahmadijokani, F.; Mohammadkhani, R.; Ahmadipouya, S.; Shokrgozar, A.; Rezakazemi, M.; Molavi, H.; Aminabhavi, T.M.; Arjmand, M. Superior Chemical Stability of UiO-66 Metal-Organic Frameworks (MOFs) for Selective Dye Adsorption. Chem. Eng. J. 2020, 399, 125346. [Google Scholar] [CrossRef]
- Ohsaki, S.; Nakazawa, R.; Teranishi, A.; Nakamura, H.; Watano, S. Control of Gate Adsorption Characteristics of Flexible Metal-Organic Frameworks by Crystal Defect. Microporous Mesoporous Mater. 2020, 302, 110215. [Google Scholar] [CrossRef]
- Formalik, F.; Neimark, A.v.; Rogacka, J.; Firlej, L.; Kuchta, B. Pore Opening and Breathing Transitions in Metal-Organic Frameworks: Coupling Adsorption and Deformation. J. Colloid Interface Sci. 2020, 578, 77–88. [Google Scholar] [CrossRef]
- Abdolalian, P.; Morsali, A. Flexible and Breathing Metal–Organic Framework with High and Selective Carbon Dioxide Storage versus Nitrogen. Polyhedron 2019, 161, 56–62. [Google Scholar] [CrossRef]
- Wei, Q.; Wang, C.; Zhou, X.; Wu, T.; Wang, Y.; Li, C.; Yang, N. Ionic Liquid and Spatially Confined Gold Nanoparticles Enhanced Photoelectrochemical Response of Zinc-Metal Organic Frameworks and Immunosensing Squamous Cell Carcinoma Antigen. Biosens. Bioelectron. 2019, 142, 111540. [Google Scholar] [CrossRef]
- Wang, K.; Li, S.; Jiang, Y.; Hu, M.; Zhai, Q.-G. A Pillar-Layered Metal-Organic Framework as Luminescent Sensor for Selective and Reversible Response of Chloroform. J. Solid State Chem. 2017, 247, 39–42. [Google Scholar] [CrossRef]
- Tang, P.; Wang, Y.; He, F. Electrochemical Sensor Based on Super-Magnetic Metal–Organic Framework@molecularly Imprinted Polymer for Sarcosine Detection in Urine. J. Saudi Chem. Soc. 2020, 24, 620–630. [Google Scholar] [CrossRef]
- Qiao, Y.; Guo, J.; Li, D.; Li, H.; Xue, X.; Jiang, W.; Che, G.; Guan, W. Fluorescent Sensing Response of Metal-Organic Frameworks for the Highly Sensitive Detection of Hg2+ and Nitrobenzene in Aqueous Media. J. Solid State Chem. 2020, 290, 121610. [Google Scholar] [CrossRef]
- Wang, H.; Jiang, L.; Chen, J.; Fu, M.; Diao, Z.; Liu, H.; Guo, H. Enhanced Bioelectrochemical Performance Caused by Porous Metal-Organic Framework MIL-53(Fe) as the Catalyst in Microbial Fuel Cells. Process Biochem. 2020, 99, 147–153. [Google Scholar] [CrossRef]
- Bazzazzadeh, A.; Dizaji, B.F.; Kianinejad, N.; Nouri, A.; Irani, M. Fabrication of Poly(Acrylic Acid) Grafted-Chitosan/Polyurethane/Magnetic MIL-53 Metal Organic Framework Composite Core-Shell Nanofibers for Co-Delivery of Temozolomide and Paclitaxel against Glioblastoma Cancer Cells. Int. J. Pharm. 2020, 587, 119674. [Google Scholar] [CrossRef] [PubMed]
- Al Sharabati, M.; Sabouni, R. Selective Removal of Dual Dyes from Aqueous Solutions Using a Metal Organic Framework (MIL-53(Al)). Polyhedron 2020, 190, 114762. [Google Scholar] [CrossRef]
- Elsaidi, S.K.; Mohamed, M.H.; Banerjee, D.; Thallapally, P.K. Flexibility in Metal–Organic Frameworks: A Fundamental Understanding. Coord. Chem. Rev. 2018, 358, 125–152. [Google Scholar] [CrossRef]
- Lee, J.H.; Jeoung, S.; Chung, Y.G.; Moon, H.R. Elucidation of Flexible Metal-Organic Frameworks: Research Progresses and Recent Developments. Coord. Chem. Rev. 2019, 389, 161–188. [Google Scholar] [CrossRef]
- Jeoung, S.; Kim, S.; Kim, M.; Moon, H.R. Pore Engineering of Metal-Organic Frameworks with Coordinating Functionalities. Coord. Chem. Rev. 2020, 420, 213377. [Google Scholar] [CrossRef]
- Mendes, R.F.; Almeida Paz, F.A. Dynamic Breathing Effect in Metal-Organic Frameworks: Reversible 2D-3D-2D-3D Single-Crystal to Single-Crystal Transformation. Inorg. Chim. Acta 2017, 460, 99–107. [Google Scholar] [CrossRef]
- Moreira, K.S.; Moura Júnior, L.S.; Monteiro, R.R.C.; de Oliveira, A.L.B.; Valle, C.P.; Freire, T.M.; Fechine, P.B.A.; de Souza, M.C.M.; Fernandez-Lorente, G.; Guisan, J.M.; et al. Optimization of the Production of Enzymatic Biodiesel from Residual Babassu Oil (Orbignya Sp.) via RSM. Catalysts 2020, 10, 414. [Google Scholar] [CrossRef] [Green Version]
- Da Fonseca, A.M.; dos Santos, J.C.S.; de Souza, M.C.M.; de Oliveira, M.M.; Colares, R.P.; de Lemos, T.L.G.; Braz-Filho, R. The Use of New Hydrogel Microcapsules in Coconut Juice as Biocatalyst System for the Reaction of Quinine. Ind. Crops Prod. 2020, 145, 111890. [Google Scholar] [CrossRef]
- Bilal, M.; Adeel, M.; Rasheed, T.; Iqbal, H.M.N. Multifunctional Metal–Organic Frameworks-Based Biocatalytic Platforms: Recent Developments and Future Prospects. J. Mater. Res. Technol. 2019, 8, 2359–2371. [Google Scholar] [CrossRef]
- Cui, J.; Ren, S.; Sun, B.; Jia, S. Optimization Protocols and Improved Strategies for Metal-Organic Frameworks for Immobilizing Enzymes: Current Development and Future Challenges. Coord. Chem. Rev. 2018, 370, 22–41. [Google Scholar] [CrossRef]
- Gkaniatsou, E.; Sicard, C.; Ricoux, R.; Mahy, J.-P.; Steunou, N.; Serre, C. Metal–Organic Frameworks: A Novel Host Platform for Enzymatic Catalysis and Detection. Mater. Horiz. 2017, 4, 55–63. [Google Scholar] [CrossRef]
- Liang, K.; Ricco, R.; Doherty, C.M.; Styles, M.J.; Bell, S.; Kirby, N.; Mudie, S.; Haylock, D.; Hill, A.J.; Doonan, C.J.; et al. Biomimetic Mineralization of Metal-Organic Frameworks as Protective Coatings for Biomacromolecules. Nat. Commun. 2015, 6, 7240. [Google Scholar] [CrossRef] [Green Version]
- Wei, W.; Dong, S.; Huang, G.; Xie, Q.; Huang, T. MOF-Derived Fe2O3 Nanoparticle Embedded in Porous Carbon as Electrode Materials for Two Enzyme-Based Biosensors. Sens. Actuators B Chem. 2018, 260, 189–197. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, X.; Huang, L.; Zhang, Z.; Dong, S. GOx@ZIF-8(NiPd) Nanoflower: An Artificial Enzyme System for Tandem Catalysis. Angew. Chem. Int. Ed. 2017, 56, 16082–16085. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, H.; Liu, J.; Hou, J.; Zhang, Y. Enzyme-Embedded Metal–Organic Framework Membranes on Polymeric Substrates for Efficient CO2 Capture. J. Mater. Chem. A 2017, 5, 19954–19962. [Google Scholar] [CrossRef]
- Qin, F.-X.; Jia, S.-Y.; Wang, F.-F.; Wu, S.-H.; Song, J.; Liu, Y. Hemin@metal–Organic Framework with Peroxidase-like Activity and Its Application to Glucose Detection. Catal. Sci. Technol. 2013, 3, 2761. [Google Scholar] [CrossRef]
- Cao, S.-L.; Yue, D.-M.; Li, X.-H.; Smith, T.J.; Li, N.; Zong, M.-H.; Wu, H.; Ma, Y.-Z.; Lou, W.-Y. Novel Nano-/Micro-Biocatalyst: Soybean Epoxide Hydrolase Immobilized on UiO-66-NH2 MOF for Efficient Biosynthesis of Enantiopure (R)-1,2-Octanediol in Deep Eutectic Solvents. ACS Sustain. Chem. Eng. 2016, 4, 3586–3595. [Google Scholar] [CrossRef]
- Fernandez-Lopez, L.; Bartolome-Cabrero, R.; Rodriguez, M.D.; dos Santos, C.S.; Rueda, N.; Fernandez-Lafuente, R. Stabilizing Effects of Cations on Lipases Depend on the Immobilization Protocol. RSC Adv. 2015, 5, 83868–83875. [Google Scholar] [CrossRef]
- Cao, Y.; Wu, Z.; Wang, T.; Xiao, Y.; Huo, Q.; Liu, Y. Immobilization of Bacillus Subtilis Lipase on a Cu-BTC Based Hierarchically Porous Metal–Organic Framework Material: A Biocatalyst for Esterification. Dalton Trans. 2016, 45, 6998–7003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, G.; Kou, X.; Huang, S.; Tong, L.; Shen, Y.; Zhu, W.; Zhu, F.; Ouyang, G. Modulating the Biofunctionality of Metal–Organic-Framework-Encapsulated Enzymes through Controllable Embedding Patterns. Angew. Chem. Int. Ed. 2020, 59, 2867–2874. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liao, K. Recent Advances in Emerging Metal– and Covalent–Organic Frameworks for Enzyme Encapsulation. ACS Appl. Mater. Interfaces 2021, 13, 56752–56776. [Google Scholar] [CrossRef] [PubMed]
- Lykourinou, V.; Chen, Y.; Wang, X.-S.; Meng, L.; Hoang, T.; Ming, L.-J.; Musselman, R.L.; Ma, S. Immobilization of MP-11 into a Mesoporous Metal–Organic Framework, MP-11@mesoMOF: A New Platform for Enzymatic Catalysis. J. Am. Chem. Soc. 2011, 133, 10382–10385. [Google Scholar] [CrossRef]
- Li, P.; Moon, S.-Y.; Guelta, M.A.; Harvey, S.P.; Hupp, J.T.; Farha, O.K. Encapsulation of a Nerve Agent Detoxifying Enzyme by a Mesoporous Zirconium Metal–Organic Framework Engenders Thermal and Long-Term Stability. J. Am. Chem. Soc. 2016, 138, 8052–8055. [Google Scholar] [CrossRef]
- Monteiro, R.R.C.; Lima, P.J.M.; Pinheiro, B.B.; Freire, T.M.; Dutra, L.M.U.; Fechine, P.B.A.; Gonçalves, L.R.B.; de Souza, M.C.M.; dos Santos, J.C.S.; Fernandez-Lafuente, R. Immobilization of Lipase A from Candida Antarctica onto Chitosan-Coated Magnetic Nanoparticles. Int. J. Mol. Sci. 2019, 20, 4018. [Google Scholar] [CrossRef] [Green Version]
- De Oliveira, U.M.F.; de Matos, L.J.B.L.; de Souza, M.C.M.; Pinheiro, B.B.; dos Santos, J.C.S.; Gonçalves, L.R.B. Efficient Biotechnological Synthesis of Flavor Esters Using a Low-Cost Biocatalyst with Immobilized Rhizomucor miehei Lipase. Mol. Biol. Rep. 2019, 46, 597–608. [Google Scholar] [CrossRef]
- Rios, N.S.; Neto, D.M.A.; dos Santos, J.C.S.; Fechine, P.B.A.; Fernández-Lafuente, R.; Gonçalves, L.R.B. Comparison of the Immobilization of Lipase from Pseudomonas Fluorescens on Divinylsulfone or P-Benzoquinone Activated Support. Int. J. Biol. Macromol. 2019, 134, 936–945. [Google Scholar] [CrossRef]
- Bezerra, R.M.; Monteiro, R.R.C.; Neto, D.M.A.; da Silva, F.F.M.; de Paula, R.C.M.; de Lemos, T.L.G.; Fechine, P.B.A.; Correa, M.A.; Bohn, F.; Gonçalves, L.R.B.; et al. A New Heterofunctional Support for Enzyme Immobilization: PEI Functionalized Fe3O4 MNPs Activated with Divinyl Sulfone. Application in the Immobilization of Lipase from Thermomyces lanuginosus. Enzym. Microb. Technol. 2020, 138, 109560. [Google Scholar] [CrossRef]
- Bonazza, H.L.; Manzo, R.M.; dos Santos, J.C.S.; Mammarella, E.J. Operational and Thermal Stability Analysis of Thermomyces lanuginosus Lipase Covalently Immobilized onto Modified Chitosan Supports. Appl. Biochem. Biotechnol. 2018, 184, 182–196. [Google Scholar] [CrossRef]
- Rueda, N.; dos Santos, J.C.S.; Torres, R.; Barbosa, O.; Ortiz, C.; Fernandez-Lafuente, R. Reactivation of Lipases by the Unfolding and Refolding of Covalently Immobilized Biocatalysts. RSC Adv. 2015, 5, 55588–55594. [Google Scholar] [CrossRef] [Green Version]
- Rios, N.S.; Morais, E.G.; dos Santos Galvão, W.; Andrade Neto, D.M.; dos Santos, J.C.S.; Bohn, F.; Correa, M.A.; Fechine, P.B.A.; Fernandez-Lafuente, R.; Gonçalves, L.R.B. Further Stabilization of Lipase from Pseudomonas fluorescens Immobilized on Octyl Coated Nanoparticles via Chemical Modification with Bifunctional Agents. Int. J. Biol. Macromol. 2019, 141, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, R.R.C.; Neto, D.M.A.; Fechine, P.B.A.; Lopes, A.A.S.; Gonçalves, L.R.B.; dos Santos, J.C.S.; de Souza, M.C.M.; Fernandez-Lafuente, R. Ethyl Butyrate Synthesis Catalyzed by Lipases A and B from Candida antarctica Immobilized onto Magnetic Nanoparticles. Improvement of Biocatalysts’ Performance under Ultrasonic Irradiation. Int. J. Mol. Sci. 2019, 20, 5807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nunes, Y.L.; de Menezes, F.L.; de Sousa, I.G.; Cavalcante, A.L.G.; Cavalcante, F.T.T.; da Silva Moreira, K.; de Oliveira, A.L.B.; Mota, G.F.; da Silva Souza, J.E.; de Aguiar Falcão, I.R.; et al. Chemical and Physical Chitosan Modification for Designing Enzymatic Industrial Biocatalysts: How to Choose the Best Strategy? Int. J. Biol. Macromol. 2021, 181, 1124–1170. [Google Scholar] [CrossRef]
- De Souza, T.C.; de Sousa Fonseca, T.; de Sousa Silva, J.; Lima, P.J.M.; Neto, C.A.C.G.; Monteiro, R.R.C.; Rocha, M.V.P.; de Mattos, M.C.; dos Santos, J.C.S.; Gonçalves, L.R.B. Modulation of Lipase B from Candida Antarctica Properties via Covalent Immobilization on Eco-Friendly Support for Enzymatic Kinetic Resolution of Rac-Indanyl Acetate. Bioprocess Biosyst. Eng. 2020, 43, 2253–2268. [Google Scholar] [CrossRef]
- Pinheiro, M.P.; Monteiro, R.R.C.; Silva, F.F.M.; Lemos, T.L.G.; Fernandez-Lafuente, R.; Gonçalves, L.R.B.; dos Santos, J.C.S. Modulation of Lecitase Properties via Immobilization on Differently Activated Immobead-350: Stabilization and Inversion of Enantiospecificity. Process Biochem. 2019, 87, 128–137. [Google Scholar] [CrossRef]
- Liu, W.-L.; Lo, S.-H.; Singco, B.; Yang, C.-C.; Huang, H.-Y.; Lin, C.-H. Novel Trypsin–FITC@MOF Bioreactor Efficiently Catalyzes Protein Digestion. J. Mater. Chem. B 2013, 1, 928. [Google Scholar] [CrossRef]
- Patra, S.; Hidalgo Crespo, T.; Permyakova, A.; Sicard, C.; Serre, C.; Chaussé, A.; Steunou, N.; Legrand, L. Design of Metal Organic Framework–Enzyme Based Bioelectrodes as a Novel and Highly Sensitive Biosensing Platform. J. Mater. Chem. B 2015, 3, 8983–8992. [Google Scholar] [CrossRef]
- Liu, W.-L.; Yang, N.-S.; Chen, Y.-T.; Lirio, S.; Wu, C.-Y.; Lin, C.-H.; Huang, H.-Y. Lipase-Supported Metal-Organic Framework Bioreactor Catalyzes Warfarin Synthesis. Chem. Eur. J. 2015, 21, 115–119. [Google Scholar] [CrossRef]
- Megías-Sayago, C.; Ivanova, S.; López-Cartes, C.; Centeno, M.A.; Odriozola, J.A. Gold Catalysts Screening in Base-Free Aerobic Oxidation of Glucose to Gluconic Acid. Catal. Today 2017, 279, 148–154. [Google Scholar] [CrossRef]
- Du, L.; Chen, W.; Zhu, P.; Tian, Y.; Chen, Y.; Wu, C. Applications of Functional Metal-Organic Frameworks in Biosensors. Biotechnol. J. 2021, 16, 1900424. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yang, Y. Metal–Organic Frameworks for Biomedical Applications. Small 2020, 16, 1906846. [Google Scholar] [CrossRef] [PubMed]
- Ferri, S.; Kojima, K.; Sode, K. Review of Glucose Oxidases and Glucose Dehydrogenases: A Bird’s Eye View of Glucose Sensing Enzymes. J. Diabetes Sci. Technol. 2011, 5, 1068–1076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Wang, Z.; Zhang, Y.; Cao, F.; Dong, K.; Ren, J.; Qu, X. Erythrocyte Membrane Cloaked Metal–Organic Framework Nanoparticle as Biomimetic Nanoreactor for Starvation-Activated Colon Cancer Therapy. ACS Nano 2018, 12, 10201–10211. [Google Scholar] [CrossRef] [PubMed]
- Ispas, C.R.; Crivat, G.; Andreescu, S. Review: Recent Developments in Enzyme-Based Biosensors for Biomedical Analysis. Anal. Lett. 2012, 45, 168–186. [Google Scholar] [CrossRef]
- Fritea, L.; Tertis, M.; Sandulescu, R.; Cristea, C. Enzyme–Graphene Platforms for Electrochemical Biosensor Design with Biomedical Applications. Methods Enzymol. 2018, 609, 293–333. [Google Scholar] [CrossRef] [PubMed]
- Nandini, S.; Nalini, S.; Manjunatha, R.; Shanmugam, S.; Melo, J.S.; Suresh, G.S. Electrochemical Biosensor for the Selective Determination of Hydrogen Peroxide Based on the Co-Deposition of Palladium, Horseradish Peroxidase on Functionalized-Graphene Modified Graphite Electrode as Composite. J. Electroanal. Chem. 2013, 689, 233–242. [Google Scholar] [CrossRef]
- Horcajada, P.; Gref, R.; Baati, T.; Allan, P.K.; Maurin, G.; Couvreur, P.; Férey, G.; Morris, R.E.; Serre, C. Metal–Organic Frameworks in Biomedicine. Chem. Rev. 2012, 112, 1232–1268. [Google Scholar] [CrossRef]
- Datta, S.; Christena, L.R.; Rajaram, Y.R.S. Enzyme Immobilization: An Overview on Techniques and Support Materials. 3 Biotech 2013, 3, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Falcaro, P.; Ricco, R.; Doherty, C.M.; Liang, K.; Hill, A.J.; Styles, M.J. MOF Positioning Technology and Device Fabrication. Chem. Soc. Rev. 2014, 43, 5513–5560. [Google Scholar] [CrossRef] [Green Version]
- Zhai, Q.-G.; Bu, X.; Zhao, X.; Li, D.-S.; Feng, P. Pore Space Partition in Metal–Organic Frameworks. Acc. Chem. Res. 2017, 50, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Rong, F.; Guo, C.; Duan, F.; He, L.; Wang, M.; Zhang, Z.; Kang, M.; Du, M. Metal–Organic Frameworks (MOFs) Based Electrochemical Biosensors for Early Cancer Diagnosis In Vitro. Coord. Chem. Rev. 2021, 439, 213948. [Google Scholar] [CrossRef]
- Filik, H.; Avan, A.A. Nanostructures for Nonlabeled and Labeled Electrochemical Immunosensors: Simultaneous Electrochemical Detection of Cancer Markers: A Review. Talanta 2019, 205, 120153. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ding, S.-N. General Strategy to Fabricate Electrochemiluminescence Sandwich-Type Nanoimmunosensors Using CdTe@ZnS Quantum Dots as Luminescent Labels and Fe3O4@SiO2 Nanoparticles as Magnetic Separable Scaffolds. ACS Sens. 2016, 1, 358–365. [Google Scholar] [CrossRef]
- Cui, L.; Hu, J.; Li, C.; Wang, C.; Zhang, C. An Electrochemical Biosensor Based on the Enhanced Quasi-Reversible Redox Signal of Prussian Blue Generated by Self-Sacrificial Label of Iron Metal-Organic Framework. Biosens. Bioelectron. 2018, 122, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Navarro, J.A.; Hernandez-Garcia, F.; Alvarez Romero, G.A. Novel Applications of Metal-Organic Frameworks (MOFs) as Redox-Active Materials for Elaboration of Carbon-Based Electrodes with Electroanalytical Uses. Coord. Chem. Rev. 2020, 412, 213263. [Google Scholar] [CrossRef]
- Chen, J.; Jiang, S.; Wang, M.; Xie, X.; Su, X. Self-Assembled Dual-Emissive Nanoprobe with Metal−organic Frameworks as Scaffolds for Enhanced Ascorbic Acid and Ascorbate Oxidase Sensing. Sens. Actuators B Chem. 2021, 339, 129910. [Google Scholar] [CrossRef]
- Xue, J.; Liu, J.; Yong, J.; Liang, K. Biomedical Applications of Metal–Organic Frameworks at the Subcellular Level. Adv. NanoBiomed Res. 2021, 1, 2100034. [Google Scholar] [CrossRef]
- Huang, Y.; Tan, J.; Cui, L.; Zhou, Z.; Zhou, S.; Zhang, Z.; Zheng, R.; Xue, Y.; Zhang, M.; Li, S.; et al. Graphene and Au NPs Co-Mediated Enzymatic Silver Deposition for the Ultrasensitive Electrochemical Detection of Cholesterol. Biosens. Bioelectron. 2018, 102, 560–567. [Google Scholar] [CrossRef]
- Yu, J.; Wei, Z.; Li, Q.; Wan, F.; Chao, Z.; Zhang, X.; Lin, L.; Meng, H.; Tian, L. Advanced Cancer Starvation Therapy by Simultaneous Deprivation of Lactate and Glucose Using a MOF Nanoplatform. Adv. Sci. 2021, 8, 2101467. [Google Scholar] [CrossRef]
- Tu, X.; Gao, F.; Ma, X.; Zou, J.; Yu, Y.; Li, M.; Qu, F.; Huang, X.; Lu, L. Mxene/Carbon Nanohorn/β-Cyclodextrin-Metal-Organic Frameworks as High-Performance Electrochemical Sensing Platform for Sensitive Detection of Carbendazim Pesticide. J. Hazard. Mater. 2020, 396, 122776. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, K.; Li, C.; Gu, J. In Situ Coupling of Catalytic Centers into Artificial Substrate Mesochannels as Super-Active Metalloenzyme Mimics. Small 2021, 17, 2101455. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, H.N.; Sharmoukh, W. Intrinsic Catalase-Mimicking MOFzyme for Sensitive Detection of Hydrogen Peroxide and Ferric Ions. Microchem. J. 2021, 163, 105873. [Google Scholar] [CrossRef]
- Xu, D.; Qi, S.; Chen, Y.; Yin, M.; Zhang, L.; Ge, K.; Wei, X.; Tian, X.; Wang, P.; Li, M.; et al. Hierarchical Mesoporous Hollow Ce-MOF Nanosphere as Oxidase Mimic for Highly Sensitive Colorimetric Detection of Ascorbic Acid. Chem. Phys. Lett. 2021, 777, 138749. [Google Scholar] [CrossRef]
- Zhang, C.; Hong, S.; Liu, M.-D.; Yu, W.-Y.; Zhang, M.-K.; Zhang, L.; Zeng, X.; Zhang, X.-Z. PH-Sensitive MOF Integrated with Glucose Oxidase for Glucose-Responsive Insulin Delivery. J. Control. Release 2020, 320, 159–167. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, B. A Point-of-Care Diagnostics Logic Detector Based on Glucose Oxidase Immobilized Lanthanide Functionalized Metal–Organic Frameworks. Nanoscale 2019, 11, 22946–22953. [Google Scholar] [CrossRef]
- Li, M.; Chen, J.; Wu, W.; Fang, Y.; Dong, S. Oxidase-like MOF-818 Nanozyme with High Specificity for Catalysis of Catechol Oxidation. J. Am. Chem. Soc. 2020, 142, 15569–15574. [Google Scholar] [CrossRef]
- Phipps, J.; Chen, H.; Donovan, C.; Dominguez, D.; Morgan, S.; Weidman, B.; Fan, C.; Beyzavi, H. Catalytic Activity, Stability, and Loading Trends of Alcohol Dehydrogenase Enzyme Encapsulated in a Metal–Organic Framework. ACS Appl. Mater. Interfaces 2020, 12, 26084–26094. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, X.; Zhou, H.-C. Direct Synthesis of Functionalized PCN-333 via Linker Design for Fe3+ Detection in Aqueous Media. Dalton Trans. 2018, 47, 11806–11811. [Google Scholar] [CrossRef]
- Gkaniatsou, E.; Ricoux, R.; Kariyawasam, K.; Stenger, I.; Fan, B.; Ayoub, N.; Salas, S.; Patriarche, G.; Serre, C.; Mahy, J.-P.; et al. Encapsulation of Microperoxidase-8 in MIL-101(Cr)-X Nanoparticles: Influence of Metal–Organic Framework Functionalization on Enzymatic Immobilization and Catalytic Activity. ACS Appl. Nano Mater. 2020, 3, 3233–3243. [Google Scholar] [CrossRef]
- Haghighi, E.; Zeinali, S. Nanoporous MIL-101(Cr) as a Sensing Layer Coated on a Quartz Crystal Microbalance (QCM) Nanosensor to Detect Volatile Organic Compounds (VOCs). RSC Adv. 2019, 9, 24460–24470. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Zhang, H.; Zha, Q.; Zhai, C.; Li, W.; Zeng, L.; Zhu, M. Photo-Electrochemical Detection of Dopamine in Human Urine and Calf Serum Based on MIL-101 (Cr)/Carbon Black. Microchim. Acta 2020, 187, 526. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Cheng, L.; Qi, R.; Zhang, M.; Zhao, J.; Zhu, L.; Dong, M. A Metal-Organic Zeolitic Framework with Immobilized Urease for Use in a Tapered Optical Fiber Urea Biosensor. Microchim. Acta 2020, 187, 72. [Google Scholar] [CrossRef] [PubMed]
- Hassanzadeh, J.; Khataee, A.; Eskandari, H. Encapsulated Cholesterol Oxidase in Metal-Organic Framework and Biomimetic Ag Nanocluster Decorated MoS2 Nanosheets for Sensitive Detection of Cholesterol. Sens. Actuators B Chem. 2018, 259, 402–410. [Google Scholar] [CrossRef]
- Fang, B.; Guo, P.; Yang, M.; Ma, Y.; Yan, X.; Jia, Z.; Gao, W.; Ahmad, S.; Xu, C.; Liu, C.; et al. A Novel Fluorescent Enhancing Platform Based on DNA-Scaffolded Silver Nanoclusters for Potential Inflammatory Bowel Disease-Associated MicroRNA Detection. Talanta 2020, 218, 121122. [Google Scholar] [CrossRef] [PubMed]
- Nadar, S.S.; Rathod, V.K. Encapsulation of Lipase within Metal-Organic Framework (MOF) with Enhanced Activity Intensified under Ultrasound. Enzym. Microb. Technol. 2018, 108, 11–20. [Google Scholar] [CrossRef]
- Moghaddam, Z.S.; Kaykhaii, M.; Khajeh, M.; Oveisi, A.R. Synthesis of UiO-66-OH Zirconium Metal-Organic Framework and Its Application for Selective Extraction and Trace Determination of Thorium in Water Samples by Spectrophotometry. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 194, 76–82. [Google Scholar] [CrossRef]
- Zhou, J.; Long, Z.; Tian, Y.; Ding, X.; Wu, L.; Hou, X. A Chemiluminescence Metalloimmunoassay for Sensitive Detection of Alpha-Fetoprotein in Human Serum Using Fe-MIL-88B-NH2 as a Label. Appl. Spectrosc. Rev. 2016, 51, 517–526. [Google Scholar] [CrossRef]
- Aguado, S.; Quirós, J.; Canivet, J.; Farrusseng, D.; Boltes, K.; Rosal, R. Antimicrobial Activity of Cobalt Imidazolate Metal–Organic Frameworks. Chemosphere 2014, 113, 188–192. [Google Scholar] [CrossRef]
- Meng, W.; Wen, Y.; Dai, L.; He, Z.; Wang, L. A Novel Electrochemical Sensor for Glucose Detection Based on Ag@ZIF-67 Nanocomposite. Sens. Actuators B Chem. 2018, 260, 852–860. [Google Scholar] [CrossRef]
- Xu, W.; Qin, Z.; Hao, Y.; He, Q.; Chen, S.; Zhang, Z.; Peng, D.; Wen, H.; Chen, J.; Qiu, J.; et al. A Signal-Decreased Electrochemical Immunosensor for the Sensitive Detection of LAG-3 Protein Based on a Hollow Nanobox-MOFs/AuPt Alloy. Biosens. Bioelectron. 2018, 113, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Li, C.; Tang, B.; Zhang, C. Advances in the Integration of Quantum Dots with Various Nanomaterials for Biomedical and Environmental Applications. Analyst 2018, 143, 2469–2478. [Google Scholar] [CrossRef] [PubMed]
- Salouti, M.; Khadivi Derakhshan, F. Biosensors and Nanobiosensors in Environmental Applications. In Biogenic Nano-Particles and their Use in Agro-Ecosystems; Springer: Singapore, 2020; pp. 515–591. [Google Scholar]
- Van Dorst, B.; Mehta, J.; Bekaert, K.; Rouah-Martin, E.; de Coen, W.; Dubruel, P.; Blust, R.; Robbens, J. Recent Advances in Recognition Elements of Food and Environmental Biosensors: A Review. Biosens. Bioelectron. 2010, 26, 1178–1194. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, F.; Zhang, S.; Cai, Y.; Cao, S.; Li, S.; Zhao, W.; Yuan, S.; Feng, X.; Cao, A.; et al. Facile Fabrication of Multifunctional Metal–Organic Framework Hollow Tubes To Trap Pollutants. J. Am. Chem. Soc. 2017, 139, 16482–16485. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wen, L.; Svec, F.; Tan, T.; Lv, Y. Magnetic Metal–Organic Frameworks as Scaffolds for Spatial Co-Location and Positional Assembly of Multi-Enzyme Systems Enabling Enhanced Cascade Biocatalysis. RSC Adv. 2017, 7, 21205–21213. [Google Scholar] [CrossRef] [Green Version]
- Ma, L.; He, Y.; Wang, Y.; Wang, Y.; Li, R.; Huang, Z.; Jiang, Y.; Gao, J. Nanocomposites of Pt Nanoparticles Anchored on UiO66-NH2 as Carriers to Construct Acetylcholinesterase Biosensors for Organophosphorus Pesticide Detection. Electrochim. Acta 2019, 318, 525–533. [Google Scholar] [CrossRef]
- Pan, S.; Chen, X.; Li, X.; Jin, M. Nonderivatization Method for Determination of Glyphosate, Glufosinate, Bialaphos, and Their Main Metabolites in Environmental Waters Based on Magnetic Metal-Organic Framework Pretreatment. J. Sep. Sci. 2019, 42, 1045–1050. [Google Scholar] [CrossRef]
- Ma, X.; Chai, Y.; Li, P.; Wang, B. Metal–Organic Framework Films and Their Potential Applications in Environmental Pollution Control. Acc. Chem. Res. 2019, 52, 1461–1470. [Google Scholar] [CrossRef]
- Bagheri, N.; Khataee, A.; Hassanzadeh, J.; Habibi, B. Sensitive Biosensing of Organophosphate Pesticides Using Enzyme Mimics of Magnetic ZIF-8. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 209, 118–125. [Google Scholar] [CrossRef]
- Yang, J.-M.; Ying, R.-J.; Han, C.-X.; Hu, Q.-T.; Xu, H.-M.; Li, J.-H.; Wang, Q.; Zhang, W. Adsorptive Removal of Organic Dyes from Aqueous Solution by a Zr-Based Metal–Organic Framework: Effects of Ce(III) Doping. Dalton Trans. 2018, 47, 3913–3920. [Google Scholar] [CrossRef]
- Mehta, J.; Dhaka, S.; Paul, A.K.; Dayananda, S.; Deep, A. Organophosphate Hydrolase Conjugated UiO-66-NH2 MOF Based Highly Sensitive Optical Detection of Methyl Parathion. Environ. Res. 2019, 174, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Sule, R.; Mishra, A.K. MOFs-Carbon Hybrid Nanocomposites in Environmental Protection Applications. Environ. Sci. Pollut. Res. 2020, 27, 16004–16018. [Google Scholar] [CrossRef] [PubMed]
- Majewski, M.B.; Howarth, A.J.; Li, P.; Wasielewski, M.R.; Hupp, J.T.; Farha, O.K. Enzyme Encapsulation in Metal–Organic Frameworks for Applications in Catalysis. CrystEngComm 2017, 19, 4082–4091. [Google Scholar] [CrossRef]
- Cortés-Súarez, J.; Celis-Arias, V.; Beltrán, H.I.; Tejeda-Cruz, A.; Ibarra, I.A.; Romero-Ibarra, J.E.; Sánchez-González, E.; Loera-Serna, S. Synthesis and Characterization of an SWCNT@HKUST-1 Composite: Enhancing the CO2 Adsorption Properties of HKUST-1. ACS Omega 2019, 4, 5275–5282. [Google Scholar] [CrossRef] [Green Version]
- Zou, R.; Gong, Q.; Shi, Z.; Zheng, J.; Xing, J.; Liu, C.; Jiang, Z.; Wu, A. A ZIF-90 Nanoplatform Loaded with an Enzyme-Responsive Organic Small-Molecule Probe for Imaging the Hypoxia Status of Tumor Cells. Nanoscale 2020, 12, 14870–14881. [Google Scholar] [CrossRef]
- Yang, G.; Jiang, X.; Xu, H.; Zhao, B. Applications of MOFs as Luminescent Sensors for Environmental Pollutants. Small 2021, 17, 2005327. [Google Scholar] [CrossRef]
- Safaei, M.; Foroughi, M.M.; Ebrahimpoor, N.; Jahani, S.; Omidi, A.; Khatami, M. A Review on Metal-Organic Frameworks: Synthesis and Applications. TrAC Trends Anal. Chem. 2019, 118, 401–425. [Google Scholar] [CrossRef]
- Wu, T.; Liu, X.; Liu, Y.; Cheng, M.; Liu, Z.; Zeng, G.; Shao, B.; Liang, Q.; Zhang, W.; He, Q.; et al. Application of QD-MOF Composites for Photocatalysis: Energy Production and Environmental Remediation. Coord. Chem. Rev. 2020, 403, 213097. [Google Scholar] [CrossRef]
- Aguilera-Sigalat, J.; Bradshaw, D. Synthesis and Applications of Metal-Organic Framework–Quantum Dot (QD@MOF) Composites. Coord. Chem. Rev. 2016, 307, 267–291. [Google Scholar] [CrossRef] [Green Version]
- Marty, J.-L.; Garcia, D.; Rouillon, R. Biosensors: Potential in Pesticide Detection. TrAC Trends Anal. Chem. 1995, 14, 329–333. [Google Scholar] [CrossRef]
- Marco, M.-P.; Barceló, D. Environmental Applications of Analytical Biosensors. Meas. Sci. Technol. 1996, 7, 1547–1562. [Google Scholar] [CrossRef]
- Xia, H.; Li, N.; Zhong, X.; Jiang, Y. Metal-Organic Frameworks: A Potential Platform for Enzyme Immobilization and Related Applications. Front. Bioeng. Biotechnol. 2020, 8, 695. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Zhao, D.; Lu, Y.; Sun, W.-Y. Photoluminescent Metal–Organic Frameworks and Their Application for Sensing Biomolecules. J. Mater. Chem. A 2019, 7, 22744–22767. [Google Scholar] [CrossRef]
- Cammarota, M.C.; Freire, D.M.G. A Review on Hydrolytic Enzymes in the Treatment of Wastewater with High Oil and Grease Content. Bioresour. Technol. 2006, 97, 2195–2210. [Google Scholar] [CrossRef]
- Lyu, F.; Zhang, Y.; Zare, R.N.; Ge, J.; Liu, Z. One-Pot Synthesis of Protein-Embedded Metal–Organic Frameworks with Enhanced Biological Activities. Nano Lett. 2014, 14, 5761–5765. [Google Scholar] [CrossRef]
- Feng, D.; Liu, T.-F.; Su, J.; Bosch, M.; Wei, Z.; Wan, W.; Yuan, D.; Chen, Y.-P.; Wang, X.; Wang, K.; et al. Stable Metal-Organic Frameworks Containing Single-Molecule Traps for Enzyme Encapsulation. Nat. Commun. 2015, 6, 5979. [Google Scholar] [CrossRef] [Green Version]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The Chemistry and Applications of Metal-Organic Frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef] [Green Version]
- Huo, J.; Aguilera-Sigalat, J.; El-Hankari, S.; Bradshaw, D. Magnetic MOF Microreactors for Recyclable Size-Selective Biocatalysis. Chem. Sci. 2015, 6, 1938–1943. [Google Scholar] [CrossRef] [Green Version]
- Ahuja, S.K.; Ferreira, G.M.; Moreira, A.R. Utilization of Enzymes for Environmental Applications. Crit. Rev. Biotechnol. 2004, 24, 125–154. [Google Scholar] [CrossRef]
- Vaisocherová-Lísalová, H.; Víšová, I.; Ermini, M.L.; Špringer, T.; Song, X.C.; Mrázek, J.; Lamačová, J.; Scott Lynn, N.; Šedivák, P.; Homola, J. Low-Fouling Surface Plasmon Resonance Biosensor for Multi-Step Detection of Foodborne Bacterial Pathogens in Complex Food Samples. Biosens. Bioelectron. 2016, 80, 84–90. [Google Scholar] [CrossRef]
- Griffiths, E.; Dooley, D.; Graham, M.; van Domselaar, G.; Brinkman, F.S.L.; Hsiao, W.W.L. Context Is Everything: Harmonization of Critical Food Microbiology Descriptors and Metadata for Improved Food Safety and Surveillance. Front. Microbiol. 2017, 8, 1068. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-S.; Sun, C.-X.; Tian, J.-Y.; Wang, Z.-W.; Ji, H.-F.; Song, Y.-P.; Zhang, S.; Zhang, Z.-H.; He, L.-H.; Du, M. Highly Stable Aluminum-Based Metal-Organic Frameworks as Biosensing Platforms for Assessment of Food Safety. Biosens. Bioelectron. 2017, 91, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Sharanyakanth, P.S.; Radhakrishnan, M. Synthesis of Metal-Organic Frameworks (MOFs) and Its Application in Food Packaging: A Critical Review. Trends Food Sci. Technol. 2020, 104, 102–116. [Google Scholar] [CrossRef]
- Luo, X.; Han, Y.; Chen, X.; Tang, W.; Yue, T.; Li, Z. Carbon Dots Derived Fluorescent Nanosensors as Versatile Tools for Food Quality and Safety Assessment: A Review. Trends Food Sci. Technol. 2020, 95, 149–161. [Google Scholar] [CrossRef]
- Huang, X.; Liu, Y.; Yung, B.; Xiong, Y.; Chen, X. Nanotechnology-Enhanced No-Wash Biosensors for in Vitro Diagnostics of Cancer. ACS Nano 2017, 11, 5238–5292. [Google Scholar] [CrossRef]
- Yuan, S.; Qin, J.; Su, J.; Li, B.; Li, J.; Chen, W.; Drake, H.F.; Zhang, P.; Yuan, D.; Zuo, J.; et al. Sequential Transformation of Zirconium(IV)-MOFs into Heterobimetallic MOFs Bearing Magnetic Anisotropic Cobalt(II) Centers. Angew. Chem. Int. Ed. 2018, 57, 12578–12583. [Google Scholar] [CrossRef]
- Wang, M.; Hu, M.; Li, Z.; He, L.; Song, Y.; Jia, Q.; Zhang, Z.; Du, M. Construction of Tb-MOF-on-Fe-MOF Conjugate as a Novel Platform for Ultrasensitive Detection of Carbohydrate Antigen 125 and Living Cancer Cells. Biosens. Bioelectron. 2019, 142, 111536. [Google Scholar] [CrossRef]
- Kumar, P.; Deep, A.; Kim, K.-H. Metal Organic Frameworks for Sensing Applications. TrAC Trends Anal. Chem. 2015, 73, 39–53. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, Y.; Binyam, A.; Liu, M.; Wu, Y.; Li, F. Discovering the Enzyme Mimetic Activity of Metal-Organic Framework (MOF) for Label-Free and Colorimetric Sensing of Biomolecules. Biosens. Bioelectron. 2016, 86, 432–438. [Google Scholar] [CrossRef]
- Bhardwaj, R.; Sharma, T.; Nguyen, D.D.; Cheng, C.K.; Lam, S.S.; Xia, C.; Nadda, A.K. Integrated Catalytic Insights into Methanol Production: Sustainable Framework for CO2 Conversion. J. Environ. Manag. 2021, 289, 112468. [Google Scholar] [CrossRef]
- Shukla, P. Synthetic Biology Perspectives of Microbial Enzymes and Their Innovative Applications. Indian J. Microbiol. 2019, 59, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Khanmohammadi, A.; Aghaie, A.; Vahedi, E.; Qazvini, A.; Ghanei, M.; Afkhami, A.; Hajian, A.; Bagheri, H. Electrochemical Biosensors for the Detection of Lung Cancer Biomarkers: A Review. Talanta 2020, 206, 120251. [Google Scholar] [CrossRef] [PubMed]
- Sri Abirami Saraswathi, M.S.; Rana, D.; Divya, K.; Gowrishankar, S.; Sakthivel, A.; Alwarappan, S.; Nagendran, A. Highly Permeable, Antifouling and Antibacterial Poly(ether imide) Membranes Tailored with Poly(hexamethylenebiguanide) Coated Copper Oxide Nanoparticles. Mater. Chem. Phys. 2020, 240, 122224. [Google Scholar] [CrossRef]
- Yang, L.; Xu, C.; Ye, W.; Liu, W. An Electrochemical Sensor for H2O2 Based on a New Co-Metal-Organic Framework Modified Electrode. Sens. Actuators B Chem. 2015, 215, 489–496. [Google Scholar] [CrossRef]
- Nguyen, H.H.; Lee, S.H.; Lee, U.J.; Fermin, C.D.; Kim, M. Immobilized Enzymes in Biosensor Applications. Materials 2019, 12, 121. [Google Scholar] [CrossRef] [Green Version]
- Bhalla, N.; Jolly, P.; Formisano, N.; Estrela, P. Introduction to Biosensors. Essays Biochem. 2016, 60, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Hou, C.; Zhang, Y.; He, F.; Liu, M.; Li, X. Preparation of Graphene Nano-Sheet Bonded PDA/MOF Microcapsules with Immobilized Glucose Oxidase as a Mimetic Multi-Enzyme System for Electrochemical Sensing of Glucose. J. Mater. Chem. B 2016, 4, 3695–3702. [Google Scholar] [CrossRef]
- Montañez, J.L.; Ramos, E.G.; Alegret, S.; Delgado, R.J. Biosensor de Glucosa Basado En Un Biocompósito Disperso de Grafito-Epoxi-Platino-Glucosa Oxidasa. Inf. Tecnol. 2011, 22, 29–40. [Google Scholar] [CrossRef] [Green Version]
- Zacco, E.; Pividori, M.I.; Alegret, S.; Galve, R.; Marco, M.-P. Electrochemical Magnetoimmunosensing Strategy for the Detection of Pesticides Residues. Anal. Chem. 2006, 78, 1780–1788. [Google Scholar] [CrossRef]
- Komathi, S.; Muthuchamy, N.; Lee, K.-P.; Gopalan, A.-I. Fabrication of a Novel Dual Mode Cholesterol Biosensor Using Titanium Dioxide Nanowire Bridged 3D Graphene Nanostacks. Biosens. Bioelectron. 2016, 84, 64–71. [Google Scholar] [CrossRef]
- Chen, Y.; Meng, X.-Z.; Gu, H.-W.; Yi, H.-C.; Sun, W.-Y. A Dual-Response Biosensor for Electrochemical and Glucometer Detection of DNA Methyltransferase Activity Based on Functionalized Metal-Organic Framework Amplification. Biosens. Bioelectron. 2019, 134, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Gkaniatsou, E.; Sicard, C.; Ricoux, R.; Benahmed, L.; Bourdreux, F.; Zhang, Q.; Serre, C.; Mahy, J.; Steunou, N. Enzyme Encapsulation in Mesoporous Metal–Organic Frameworks for Selective Biodegradation of Harmful Dye Molecules. Angew. Chem. Int. Ed. 2018, 57, 16141–16146. [Google Scholar] [CrossRef] [PubMed]
- Kempahanumakkagari, S.; Vellingiri, K.; Deep, A.; Kwon, E.E.; Bolan, N.; Kim, K.-H. Metal–Organic Framework Composites as Electrocatalysts for Electrochemical Sensing Applications. Coord. Chem. Rev. 2018, 357, 105–129. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, J.; He, H.; Qian, G. Photonic Functional Metal–Organic Frameworks. Chem. Soc. Rev. 2018, 47, 5740–5785. [Google Scholar] [CrossRef]
- Reyes-De-Corcuera, J.I.; Olstad, H.E.; García-Torres, R. Stability and Stabilization of Enzyme Biosensors: The Key to Successful Application and Commercialization. Annu. Rev. Food Sci. Technol. 2018, 9, 293–322. [Google Scholar] [CrossRef]
- Hu, Y.; Dai, L.; Liu, D.; Du, W.; Wang, Y. Progress & Prospect of Metal-Organic Frameworks (MOFs) for Enzyme Immobilization (Enzyme/MOFs). Renew. Sustain. Energy Rev. 2018, 91, 793–801. [Google Scholar] [CrossRef]
- Luo, F.; Yan, C.; Dang, L.; Krishna, R.; Zhou, W.; Wu, H.; Dong, X.; Han, Y.; Hu, T.-L.; O’Keeffe, M.; et al. UTSA-74: A MOF-74 Isomer with Two Accessible Binding Sites per Metal Center for Highly Selective Gas Separation. J. Am. Chem. Soc. 2016, 138, 5678–5684. [Google Scholar] [CrossRef]
- Li, P.; Moon, S.-Y.; Guelta, M.A.; Lin, L.; Gómez-Gualdrón, D.A.; Snurr, R.Q.; Harvey, S.P.; Hupp, J.T.; Farha, O.K. Nanosizing a Metal–Organic Framework Enzyme Carrier for Accelerating Nerve Agent Hydrolysis. ACS Nano 2016, 10, 9174–9182. [Google Scholar] [CrossRef]
- Chen, Y.; Lykourinou, V.; Vetromile, C.; Hoang, T.; Ming, L.-J.; Larsen, R.W.; Ma, S. How Can Proteins Enter the Interior of a MOF? Investigation of Cytochrome c Translocation into a MOF Consisting of Mesoporous Cages with Microporous Windows. J. Am. Chem. Soc. 2012, 134, 13188–13191. [Google Scholar] [CrossRef]
- Liao, F.-S.; Lo, W.-S.; Hsu, Y.-S.; Wu, C.-C.; Wang, S.-C.; Shieh, F.-K.; Morabito, J.V.; Chou, L.-Y.; Wu, K.C.-W.; Tsung, C.-K. Shielding against Unfolding by Embedding Enzymes in Metal–Organic Frameworks via a de novo Approach. J. Am. Chem. Soc. 2017, 139, 6530–6533. [Google Scholar] [CrossRef]
- Hu, S.; Yan, J.; Huang, X.; Guo, L.; Lin, Z.; Luo, F.; Qiu, B.; Wong, K.-Y.; Chen, G. A Sensing Platform for Hypoxanthine Detection Based on Amino-Functionalized Metal Organic Framework Nanosheet with Peroxidase Mimic and Fluorescence Properties. Sens. Actuators B Chem. 2018, 267, 312–319. [Google Scholar] [CrossRef]
- Shen, K.; Zhang, L.; Chen, X.; Liu, L.; Zhang, D.; Han, Y.; Chen, J.; Long, J.; Luque, R.; Li, Y.; et al. Ordered Macro-Microporous Metal-Organic Framework Single Crystals. Science 2018, 359, 206–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.; Wang, X.; Hou, M.; Li, X.; Wu, X.; Ge, J. Immobilization on Metal–Organic Framework Engenders High Sensitivity for Enzymatic Electrochemical Detection. ACS Appl. Mater. Interfaces 2017, 9, 13831–13836. [Google Scholar] [CrossRef] [PubMed]
- Anand, R.; Borghi, F.; Manoli, F.; Manet, I.; Agostoni, V.; Reschiglian, P.; Gref, R.; Monti, S. Host–Guest Interactions in Fe(III)-Trimesate MOF Nanoparticles Loaded with Doxorubicin. J. Phys. Chem. B 2014, 118, 8532–8539. [Google Scholar] [CrossRef]
- Zhou, J.; Tian, G.; Zeng, L.; Song, X.; Bian, X. Nanoscaled Metal-Organic Frameworks for Biosensing, Imaging, and Cancer Therapy. Adv. Healthc. Mater. 2018, 7, 1800022. [Google Scholar] [CrossRef]
- Ma, Y.; Qu, X.; Liu, C.; Xu, Q.; Tu, K. Metal-Organic Frameworks and Their Composites Towards Biomedical Applications. Front. Mol. Biosci. 2021, 8, 1223. [Google Scholar] [CrossRef]
- Cavalcante, F.T.T.; Cavalcante, A.L.G.; de Sousa, I.G.; Neto, F.S.; dos Santos, J.C.S. Current Status and Future Perspectives of Supports and Protocols for Enzyme Immobilization. Catalysts 2021, 11, 1222. [Google Scholar] [CrossRef]
- Ge, X.; Wong, R.; Anisa, A.; Ma, S. Recent Development of Metal-Organic Framework Nanocomposites for Biomedical Applications. Biomaterials 2022, 281, 121322. [Google Scholar] [CrossRef]



| Synthesis of Metal–Organic Frameworks (MOFs) for Enzyme Immobilization | ||
|---|---|---|
| Immobilization Strategies | Main Advantage | Main Disadvantage |
| Co-precipitation | More enzymes can be added to the MOF structure [71,72,73]. | As synthesis and immobilization occur concomitantly, enzyme clusters may form, reducing the immobilization yield [74,75,76,77]. |
| Covalent linkage | High binding strength usually involves several enzyme residues, providing outstanding structural rigidity [78,79]. | Partial inactivation or reduction of catalytic activity may occur due to conformational changes in the enzyme structure [80,81]. |
| Entrapment | Reduces enzyme exposure to unnatural environments [82,83]. | Difficulty in controlling pore size facilitates enzyme desorption; this also causes problems of mass transfer limitations and diffusion of substrates in the pores [84,85]. |
| Surface attachment | Reduces changes in the enzyme’s active site [79]. | Ease of desorption due to weak interactions between enzyme and support [81]. |
| No. | MOFs | Enzyme | Detection Ranges (mM) | LOD (μM) | Biomedical Applications | References |
|---|---|---|---|---|---|---|
| 1 | ZIF-8 | Lactate/glucose oxidase | 0.01–0.3 | 9.2 | Tumor cell mapping and energy reduction in tumor cycle | [120] |
| 2 | QDs/CDs @ MOFs | Ascorbate oxidase | 0.003–0.01 | 1.0 | Improved ascorbic acid detection | [117,121] |
| 3 | OMUiO-66 (Ce) | Glutamate oxidase | 0.125–8 | 1.2 | Potential for screening for specific chiral amino acids in complex biological samples | [102,122] |
| 4 | ZIF-90/Ce-MOF | Catalase | 0.008–0.056 | 0.03 | Sensitive detection and degradation of hydrogen peroxide | [123,124] |
| 5 | L-MOFs | Glucose oxidase | 0.01–10 | 0.2 | Insulin delivery | [125,126] |
| 6 | MOF-818 @ RGO/MWCNTs/GCE | Polyphenol oxidase | 0.002–0.6 | 6.1 | Mapping of oxidoreductase activity on phenols | [127] |
| 7 | PCN-333(Fe) | Alcohol Dehydrogenase | 0.01–0.2 | 6.2 | Catalysis of the conversion of toxic alcohols to aldehydes in cells | [128,129] |
| 8 | MIL-101(Cr) | Microperoxidase 8 | 0.001–2.22 | 3.0 | Dual catalytic activity in the selective oxidation of organic molecules | [130,131,132] |
| 9 | ZIF-8 | Urease | 0–0.8 | 5.0 | Sensitive urea detection | [133] |
| 10 | AgNC/Mo(II)-NS | Cholesterol oxidase | 0.05–0.6 | 0.018 | Detections and concentration measurements in blood vessels or body tissues | [134,135] |
| 11 | UiO-66 | Lipase | 0.001–0.2 | 0.35 | Drug synthesis against venous thromboembolism | [136,137] |
| 12 | ZIF-8 | Glucose oxidase | 0.008–5 | 8.0 | Electrochemical glucose detection | [76] |
| 13 | MIL-88B-NH2(Cr) | Trypsin | 0.05–1 | 3.0 | Protein degradation by enzymatic hydrolysis | [5,138] |
| 14 | CYCU-4 | Trypsin | 0.001–0.2 | 0.5 | Protein digestion | [84] |
| 15 | Tb-mesoMOF | Mb | 0.01–5 | 5.0 | Oxidation of ABTS and THB | [139] |
| 16 | ZIF-67 | Glucose oxidase | 0.002–1 | 0.66 | Antimicrobial action | [140] |
| No. | MOFs | Enzyme | Detection Ranges (mM) | LOD (μM) | Environmental Applications | References |
|---|---|---|---|---|---|---|
| 1 | MOF-199 | Laccase | 0.015–0.1 | 9.8 | Removal of heavy metals from fluids and aquatic environments | [145,146] |
| 2 | UIO66-NH2 | Acetylcholinesterase | 0–50 | 3.0 | Organophosphorus pesticide detection | [147,148] |
| 3 | ZIF-8 | Choline oxidase | 0.01–0.8 | 7.8 | Detection and removal of water pollutants | [149,150] |
| 4 | Ce (III)/UiO-66 | Hydrolases | 0.005–1 | 7.4 | Adsorptive removal of organic dyes from aqueous solution | [151,152] |
| 5 | ZIF-90 | Catalase | 0–0.3 | 5.8 | Effluent treatment for wastewaters | [153,154] |
| 6 | HKUST-1 | Peroxidase | 0.03–0.9 | 7.5 | CO2 adsorption | [5,155,156] |
| 7 | L-MOFs | Lipase | 0.01–10 | 2.0 | Luminescent sensors for environmental pollutants | [157,158] |
| 8 | QD-MOF | Oxidase | 0.005–1 | 0.05 | Degradation of organic dyes in industrial waters | [159,160] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Souza, J.E.d.S.; Oliveira, G.P.d.; Alexandre, J.Y.N.H.; Neto, J.G.L.; Sales, M.B.; Junior, P.G.d.S.; Oliveira, A.L.B.d.; Souza, M.C.M.d.; Santos, J.C.S.d. A Comprehensive Review on the Use of Metal–Organic Frameworks (MOFs) Coupled with Enzymes as Biosensors. Electrochem 2022, 3, 89-113. https://doi.org/10.3390/electrochem3010006
Souza JEdS, Oliveira GPd, Alexandre JYNH, Neto JGL, Sales MB, Junior PGdS, Oliveira ALBd, Souza MCMd, Santos JCSd. A Comprehensive Review on the Use of Metal–Organic Frameworks (MOFs) Coupled with Enzymes as Biosensors. Electrochem. 2022; 3(1):89-113. https://doi.org/10.3390/electrochem3010006
Chicago/Turabian StyleSouza, José E. da S., Gabriel P. de Oliveira, Jeferson Y. N. H. Alexandre, José G. L. Neto, Misael B. Sales, Paulo G. de S. Junior, André L. B. de Oliveira, Maria C. M. de Souza, and José C. S. dos Santos. 2022. "A Comprehensive Review on the Use of Metal–Organic Frameworks (MOFs) Coupled with Enzymes as Biosensors" Electrochem 3, no. 1: 89-113. https://doi.org/10.3390/electrochem3010006
APA StyleSouza, J. E. d. S., Oliveira, G. P. d., Alexandre, J. Y. N. H., Neto, J. G. L., Sales, M. B., Junior, P. G. d. S., Oliveira, A. L. B. d., Souza, M. C. M. d., & Santos, J. C. S. d. (2022). A Comprehensive Review on the Use of Metal–Organic Frameworks (MOFs) Coupled with Enzymes as Biosensors. Electrochem, 3(1), 89-113. https://doi.org/10.3390/electrochem3010006









