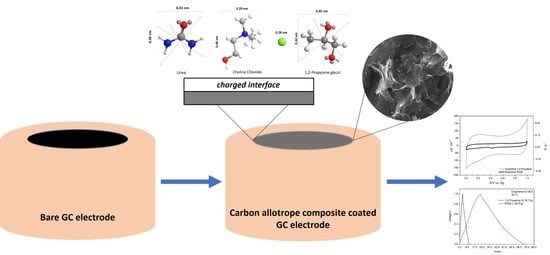
Hydrogen Bond Donors Influence on the Electrochemical Performance of Composite Graphene Electrodes/Deep Eutectic Solvents Interface
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Solvents
2.2. Physicochemical Characterization
2.2.1. Viscosity
2.2.2. Water Content
2.2.3. Ionic Conductivity
2.3. Electrochemical Measurements
2.4. Carbon Material Characterization
3. Results and Discussion
3.1. Deep Eutectic Solvent Characterization
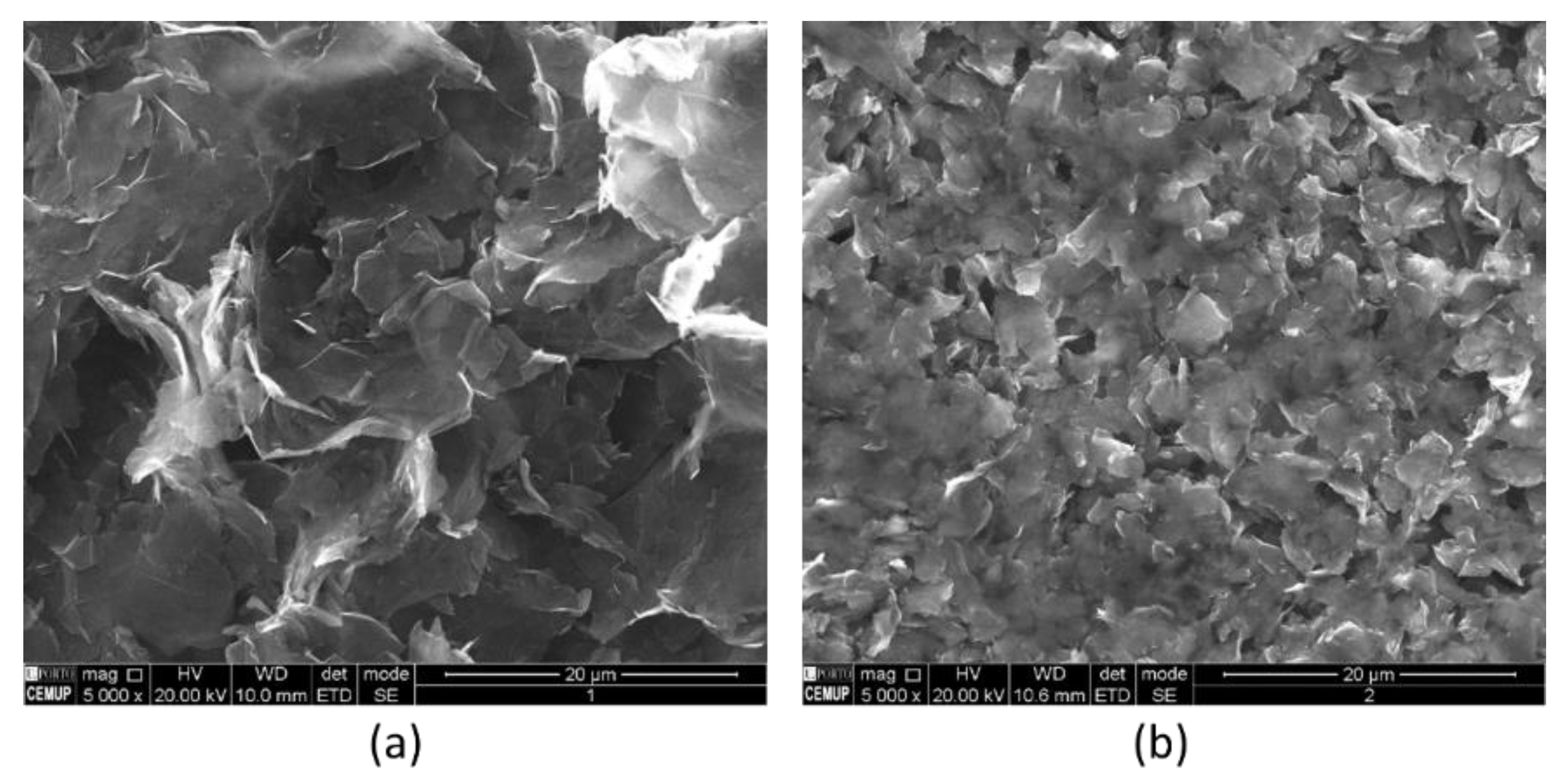
3.2. Carbon Materials Characterization
3.3. Electrochemical Characterization
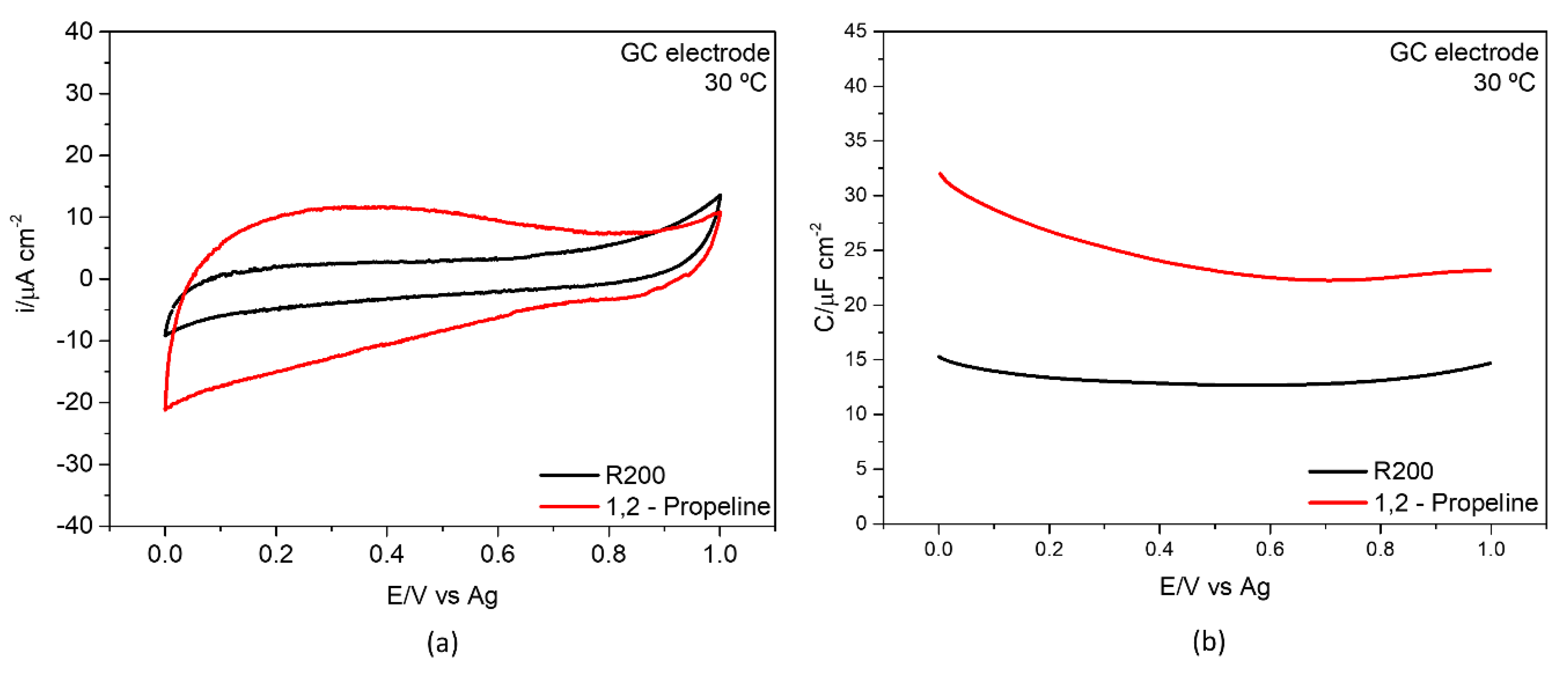
3.3.1. Glassy Carbon/DES Interface
3.3.2. Graphene and Graphite/ DES Interface
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ratajczak, P.; Suss, M.E.; Kaasik, F.; Béguin, F. Carbon electrodes for capacitive technologies. Energy Storage Mater. 2019, 16, 126–145. [Google Scholar] [CrossRef]
- Pareek, A.; Shanthi Sravan, J.; Venkata Mohan, S. Exploring chemically reduced graphene oxide electrode for power generation in microbial fuel cell. Mater. Sci. Energy Technol. 2019, 2, 600–606. [Google Scholar] [CrossRef]
- Shin, D.; Lee, K.; Chang, N. Fuel economy analysis of fuel cell and supercapacitor hybrid systems. Int. J. Hydrog. Energy 2016, 41, 1381–1390. [Google Scholar] [CrossRef]
- Iqbal, M.Z.; Siddique, S.; Khan, A.; Haider, S.S.; Khalid, M. Recent developments in graphene based novel structures for efficient and durable fuel cells. Mater. Res. Bull. 2019, 122, 110674. [Google Scholar] [CrossRef]
- Iro, Z.S.; Subramani, C.; Dash, S.S. A brief review on electrode materials for supercapacitor. Int. J. Electrochem. Sci. 2016, 11, 10628–10643. [Google Scholar] [CrossRef]
- Eftekhari, A. On the mechanism of microporous carbon supercapacitors. Mater. Today Chem. 2018, 7, 1–4. [Google Scholar] [CrossRef]
- Yang, Z.; Tian, J.; Yin, Z.; Cui, C.; Qian, W.; Wei, F. Carbon nanotube- and graphene-based nanomaterials and applications in high-voltage supercapacitor: A review. Carbon N. Y. 2019, 141, 467–480. [Google Scholar] [CrossRef]
- Ge, J.; Zhang, Y.; Heo, Y.-J.; Park, S.-J. Advanced Design and Synthesis of Composite Photocatalysts for the Remediation of Wastewater: A Review. Catalysts 2019, 9, 122. [Google Scholar] [CrossRef] [Green Version]
- Laskar, M.A.; Siddiqui, S. Chapter 12-Nanomaterials—Based on graphene oxide and its derivatives—For separation and preconcentration of metal ions. In Micro and Nano Technologies, Graphene-Based Nanotechnologies for Energy and Environmental Applications; Jawaid, M., Ahmad, A., Lokhat, D., Eds.; Elsevier: Durban, South Africa, 2019; pp. 205–219. ISBN 978-0-12-815811-1. [Google Scholar]
- Centeno, T.A.; Hahn, M.; Fernández, J.A.; Kötz, R.; Stoeckli, F. Correlation between capacitances of porous carbons in acidic and aprotic EDLC electrolytes. Electrochem. Commun. 2007, 9, 1242–1246. [Google Scholar] [CrossRef] [Green Version]
- Paek, E.; Pak, A.J.; Hwang, G.S. A Computational Study of the Interfacial Structure and Capacitance of Graphene in [BMIM][PF6] Ionic Liquid. J. Electrochem. Soc. 2012, 160, A1–A10. [Google Scholar] [CrossRef] [Green Version]
- Uysal, A.; Zhou, H.; Feng, G.; Lee, S.S.; Li, S.; Fenter, P.; Cummings, P.T.; Fulvio, P.F.; Dai, S.; McDonough, J.K.; et al. Structural Origins of Potential Dependent Hysteresis at the Electrified Graphene/Ionic Liquid Interface. J. Phys. Chem. C 2014, 118, 569–574. [Google Scholar] [CrossRef]
- Oll, O.; Romann, T.; Lust, E. An infrared study of the few-layer graphene | ionic liquid interface: Reintroduction of in situ electroreflectance spectroscopy. Electrochem. Commun. 2014, 46, 22–25. [Google Scholar] [CrossRef]
- Méndez-Morales, T.; Carrete, J.; Pérez-Rodríguez, M.; Cabeza, Ó.; Gallego, L.J.; Lynden-Bell, R.M.; Varela, L.M. Molecular dynamics simulations of the structure of the graphene–ionic liquid/alkali salt mixtures interface. Phys. Chem. Chem. Phys. 2014, 16, 13271–13278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ivaništšev, V.; Fedorov, M.V.; Lynden-Bell, R.M. Screening of Ion–Graphene Electrode Interactions by Ionic Liquids: The Effects of Liquid Structure. J. Phys. Chem. C 2014, 118, 5841–5847. [Google Scholar] [CrossRef]
- Paek, E.; Pak, A.J.; Hwang, G.S. On the influence of polarization effects in predicting the interfacial structure and capacitance of graphene-like electrodes in ionic liquids. J. Chem. Phys. 2015, 142, 24701. [Google Scholar] [CrossRef]
- Begić, S.; Jónsson, E.; Chen, F.; Forsyth, M. Molecular dynamics simulations of pyrrolidinium and imidazolium ionic liquids at graphene interfaces. Phys. Chem. Chem. Phys. 2017, 19, 30010–30020. [Google Scholar] [CrossRef]
- Williams, C.D.; Dix, J.; Troisi, A.; Carbone, P. Effective Polarization in Pairwise Potentials at the Graphene–Electrolyte Interface. J. Phys. Chem. Lett. 2017, 8, 703–708. [Google Scholar] [CrossRef]
- Shen, Y.; He, X.; Hung, F.R. Structural and Dynamical Properties of a Deep Eutectic Solvent Confined Inside a Slit Pore. J. Phys. Chem. C 2015, 119, 24489–24500. [Google Scholar] [CrossRef]
- Kaur, S.; Sharma, S.; Kashyap, H.K. Bulk and interfacial structures of reline deep eutectic solvent: A molecular dynamics study. J. Chem. Phys. 2017, 147, 194507. [Google Scholar] [CrossRef]
- Atilhan, M.; Costa, L.T.; Aparicio, S. Elucidating the Properties of Graphene–Deep Eutectic Solvents Interface. Langmuir 2017, 33, 5154–5165. [Google Scholar] [CrossRef]
- Chen, Z.; McLean, B.; Ludwig, M.; Stefanovic, R.; Warr, G.G.; Webber, G.B.; Page, A.J.; Atkin, R. Nanostructure of Deep Eutectic Solvents at Graphite Electrode Interfaces as a Function of Potential. J. Phys. Chem. C 2016, 120, 2225–2233. [Google Scholar] [CrossRef]
- Fuchs, D.; Bayer, B.C.; Gupta, T.; Szabo, G.L.; Wilhelm, R.A.; Eder, D.; Meyer, J.C.; Steiner, S.; Gollas, B. Electrochemical Behavior of Graphene in a Deep Eutectic Solvent. ACS Appl. Mater. Interfaces 2020, 12, 40937–40948. [Google Scholar] [CrossRef] [PubMed]
- Brandão, A.T.S.C.; Costa, R.; Silva, A.F.; Pereira, C.M. Sustainable Preparation of Nanoporous Carbons via Dry Ball Milling: Electrochemical Studies Using Nanocarbon Composite Electrodes and a Deep Eutectic Solvent as Electrolyte. Nanomaterials 2021, 11, 3258. [Google Scholar] [CrossRef] [PubMed]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; John Wiley & Sons, Inc.: New York, NY, USA, 2001; ISBN 978-0-471-04372-0. [Google Scholar]
- Costa, R.; Pereira, C.M.; Silva, A.F. Charge storage on ionic liquid electric double layer: The role of the electrode material. Electrochim. Acta 2015, 167, 421–428. [Google Scholar] [CrossRef]
- Figueiredo, M.; Gomes, C.; Costa, R.; Martins, A.; Pereira, C.M.; Silva, F. Differential capacity of a deep eutectic solvent based on choline chloride and glycerol on solid electrodes. Electrochim. Acta 2009, 54, 2630–2634. [Google Scholar] [CrossRef]
- Dean, W.; Klein, J.; Gurkan, B. Do Deep Eutectic Solvents Behave Like Ionic Liquid Electrolytes? A Perspective from the Electrode-Electrolyte Interface. J. Electrochem. Soc. 2021, 168, 026503. [Google Scholar] [CrossRef]
- Wu, J.; Liu, S.; Tan, Z.; Guo, Y.; Zhou, J.-Z.; Mao, B.; Yan, J. Effect of Hydrogen Bond Donor Molecules Ethylene Glycerol and Lactic Acid on Electrochemical Interfaces in Choline Chloride Based-Deep Eutectic Solvents. J. Chem. Phys. 2021, 155, 244702. [Google Scholar] [CrossRef]
- Costa, R.; Pereira, C.M.; Silva, A.F. Insight on the effect of surface modification by carbon materials on the Ionic Liquid Electric Double Layer Charge Storage properties. Electrochim. Acta 2015, 176, 880–886. [Google Scholar] [CrossRef]
- Yan, J.; Wang, Q.; Wei, T.; Fan, Z. Recent Advances in Design and Fabrication of Electrochemical Supercapacitors with High Energy Densities. Adv. Energy Mater. 2014, 4, 1300816. [Google Scholar] [CrossRef]
- Pandolfo, A.G.; Hollenkamp, A.F. Carbon properties and their role in supercapacitors. J. Power Sources 2006, 157, 11–27. [Google Scholar] [CrossRef]
- Qu, D.; Shi, H. Studies of activated carbons used in double-layer capacitors. J. Power Sources 1998, 74, 99–107. [Google Scholar] [CrossRef]
- Shi, H. Activated carbons and double layer capacitance. Electrochim. Acta 1996, 41, 1633–1639. [Google Scholar] [CrossRef]
- Raymundo-Piñero, E.; Kierzek, K.; Machnikowski, J.; Béguin, F. Relationship between the nanoporous texture of activated carbons and their capacitance properties in different electrolytes. Carbon N. Y. 2006, 44, 2498–2507. [Google Scholar] [CrossRef]
- Pohlmann, S.; Lobato, B.; Centeno, T.A.; Balducci, A. The influence of pore size and surface area of activated carbons on the performance of ionic liquid based supercapacitors. Phys. Chem. Chem. Phys. 2013, 15, 17287–17294. [Google Scholar] [CrossRef] [Green Version]
- Salomé, S.; Pereira, N.M.; Ferreira, E.S.; Pereira, C.M.; Silva, A.F. Tin electrodeposition from choline chloride based solvent: Influence of the hydrogen bond donors. J. Electroanal. Chem. 2013, 703, 80–87. [Google Scholar] [CrossRef]
- Brandão, A.T.S.C.; Rosoiu, S.; Costa, R.; Lazar, O.A.; Silva, A.F.; Anicai, L.; Pereira, C.M.; Enachescu, M. Characterization and electrochemical studies of MWCNTs decorated with Ag nanoparticles through pulse reversed current electrodeposition using a deep eutectic solvent for energy storage applications. J. Mater. Res. Technol. 2021, 15, 342–359. [Google Scholar] [CrossRef]
- Silva, F.; Gomes, C.; Figueiredo, M.; Costa, R.; Martins, A.; Pereira, C.M. The electrical double layer at the [BMIM][PF6] ionic liquid/electrode interface—Effect of temperature on the differential capacitance. J. Electroanal. Chem. 2008, 622, 153–160. [Google Scholar] [CrossRef]
- Stoller, M.D.; Ruoff, R.S. Best practice methods for determining an electrode material’s performance for ultracapacitors. Energy Environ. Sci. 2010, 3, 1294–1301. [Google Scholar] [CrossRef]
- Protsenko, V.S.; Kityk, A.A.; Shaiderov, D.A.; Danilov, F.I. Effect of water content on physicochemical properties and electrochemical behavior of ionic liquids containing choline chloride, ethylene glycol and hydrated nickel chloride. J. Mol. Liq. 2015, 212, 716–722. [Google Scholar] [CrossRef]
- Protsenko, V.S.; Bobrova, L.S.; Danilov, F.I. Physicochemical properties of ionic liquid mixtures containing choline chloride, chromium (III) chloride and water: Effects of temperature and water content. Ionics 2017, 23, 637–643. [Google Scholar] [CrossRef]
- Al-Murshedi, A.Y.M.; Alesary, H.F.; Al-Hadrawi, R. Thermophysical properties in deep eutectic solvents with/without water. J. Phys. Conf. Ser. 2019, 1294, 052041. [Google Scholar] [CrossRef]
- Gajardo-Parra, N.F.; Cotroneo-Figueroa, V.P.; Aravena, P.; Vesovic, V.; Canales, R.I. Viscosity of Choline Chloride-Based Deep Eutectic Solvents: Experiments and Modeling. J. Chem. Eng. Data 2020, 65, 5581–5592. [Google Scholar] [CrossRef]
- Shah, D.; Mjalli, F.S. Effect of water on the thermo-physical properties of Reline: An experimental and molecular simulation based approach. Phys. Chem. Chem. Phys. 2014, 16, 23900–23907. [Google Scholar] [CrossRef] [PubMed]
- Brandão, A.T.S.C.; Rosoiu, S.; Costa, R.; Silva, A.F.; Anicai, L.; Enachescu, M.; Pereira, C.M. Characterization of Carbon Nanomaterials Dispersions: Can Metal Decoration of MWCNTs Improve Their Physicochemical Properties? Nanomaterials 2022, 12, 99. [Google Scholar] [CrossRef] [PubMed]
- Shahbaz, K.; Mjalli, F.S.; Hashim, M.A.; AlNashef, I.M. Prediction of the surface tension of deep eutectic solvents. Fluid Phase Equilib. 2012, 319, 48–54. [Google Scholar] [CrossRef]
- Cotroneo-Figueroa, V.P.; Gajardo-Parra, N.F.; López-Porfiri, P.; Leiva, Á.; Gonzalez-Miquel, M.; Garrido, J.M.; Canales, R.I. Hydrogen bond donor and alcohol chain length effect on the physicochemical properties of choline chloride based deep eutectic solvents mixed with alcohols. J. Mol. Liq. 2022, 345, 116986. [Google Scholar] [CrossRef]
- García, G.; Aparicio, S.; Ullah, R.; Atilhan, M. Deep eutectic solvents: Physicochemical properties and gas separation applications. Energy Fuels 2015, 29, 2616–2644. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Meyer, J.C.; Geim, A.K.; Katsnelson, M.I.; Novoselov, K.S.; Booth, T.J.; Roth, S. The structure of suspended graphene sheets. Nature 2007, 446, 60–63. [Google Scholar] [CrossRef]
- Bourlinos, A.B.; Steriotis, T.A.; Karakassides, M.; Sanakis, Y.; Tzitzios, V.; Trapalis, C.; Kouvelos, E.; Stubos, A. Synthesis, characterization and gas sorption properties of a molecularly-derived graphite oxide-like foam. Carbon N. Y. 2007, 45, 852–857. [Google Scholar] [CrossRef]
- Gao, Y.; Ma, D.; Wang, C.; Guan, J.; Bao, X. Reduced graphene oxide as a catalyst for hydrogenation of nitrobenzene at room temperature. Chem. Commun. 2011, 47, 2432–2434. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.; Pereira, C.M.; Silva, A.F. Dicationic ionic liquid: Insight in the electrical double layer structure at mercury, glassy carbon and gold surfaces. Electrochim. Acta 2014, 116, 306–313. [Google Scholar] [CrossRef]
- Voroshylova, I.V.; Teixeira, F.; Costa, R.; Pereira, C.M.; Cordeiro, M.N.D.S. Interactions in the ionic liquid [EMIM][FAP]: A coupled experimental and computational analysis. Phys. Chem. Chem. Phys. 2015, 2617–2628. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.; Figueiredo, M.; Pereira, C.M.; Silva, F. Electrochemical double layer at the interfaces of Hg/choline chloride based solvents. Electrochim. Acta 2010, 55, 8916–8920. [Google Scholar] [CrossRef]
- AlOmar, M.K.; Hayyan, M.; Alsaadi, M.A.; Akib, S.; Hayyan, A.; Hashim, M.A. Glycerol-based deep eutectic solvents: Physical properties. J. Mol. Liq. 2016, 215, 98–103. [Google Scholar] [CrossRef]
- Lewandowski, A.; Olejniczak, A.; Galinski, M.; Stepniak, I. Performance of carbon–carbon supercapacitors based on organic, aqueous and ionic liquid electrolytes. J. Power Sources 2010, 195, 5814–5819. [Google Scholar] [CrossRef]
- Kwon, H.-N.; Jang, S.-J.; Kang, Y.C.; Roh, K.C. The effect of ILs as co-salts in electrolytes for high voltage supercapacitors. Sci. Rep. 2019, 9, 1180. [Google Scholar] [CrossRef] [Green Version]
- Abdallah, T.; Lemordant, D.; Claude-Montigny, B. Are room temperature ionic liquids able to improve the safety of supercapacitors organic electrolytes without degrading the performances? J. Power Sources 2012, 201, 353–359. [Google Scholar] [CrossRef]
- Zarrougui, R.; Hachicha, R.; Rjab, R.; Messaoudi, S.; Ghodbane, O. Physicochemical characterizations of novel dicyanamide-based ionic liquids applied as electrolytes for supercapacitors. RSC Adv. 2018, 8, 31213–31223. [Google Scholar] [CrossRef] [Green Version]
- Galek, P.; Slesinski, A.; Fic, K.; Menzel, J. Peculiar role of the electrolyte viscosity in the electrochemical capacitor performance. J. Mater. Chem. A 2021, 9, 8644–8654. [Google Scholar] [CrossRef]
- Mohd Zaid, N.A.; Idris, N.H. Enhanced Capacitance of Hybrid Layered Graphene/Nickel Nanocomposite for Supercapacitors. Sci. Rep. 2016, 6, 32082. [Google Scholar] [CrossRef] [PubMed]
- illars, F.B.; Fletcher, S.I.; Mirzaeian, M.; Hall, P.J. Variation of electrochemical capacitor performance with room temperature ionic liquid electrolyte viscosity and ion size. Phys. Chem. Chem. Phys. 2012, 14, 6094–6100. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, K.; Yamashita, R.; Sakuragawa, S.; Hasumi, K.; Mukai, Y.; Kobayakawa, H.; Wakabayashi, S.; Saito, Y. Pore structure and the properties of electric double layer capacitor electrode of bamboo-derived activated carbon prepared by superheated steam. J. Wood Sci. 2018, 64, 642–649. [Google Scholar] [CrossRef] [Green Version]
- Luo, Z.; Zhang, B.; Qian, H.; Lu, Z.; Cui, S. Effect of the size of solvent molecules on the single-chain mechanics of poly (ethylene glycol): Implications on a novel design of a molecular motor. Nanoscale 2016, 8, 17820–17827. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Wu, F.; Chen, R.; Cao, G.; Chen, S.; Wang, G.; Yang, Y. Room temperature molten salt as electrolyte for carbon nanotube-based electric double layer capacitors. J. Power Sources 2006, 158, 773–778. [Google Scholar] [CrossRef]
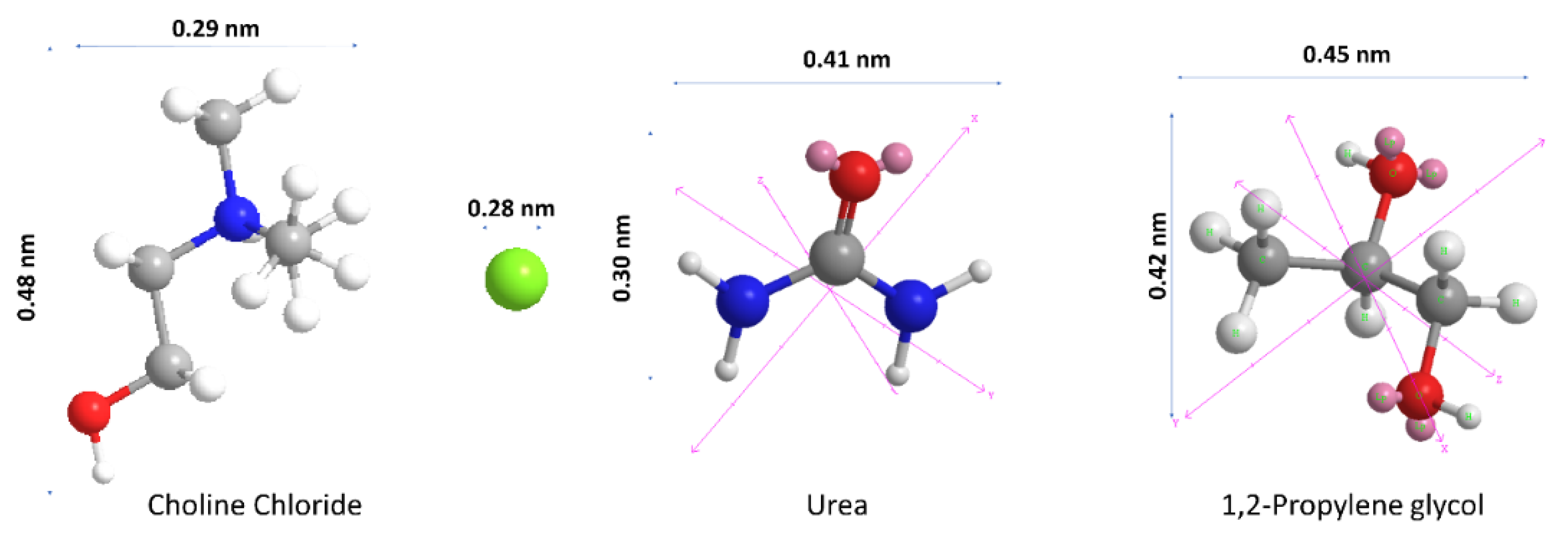
| DES | Composition | Molar Ratio | Choline Chloride | HBD |
|---|---|---|---|---|
| 1,2-Propeline (12P) | Choline chloride (ChCl) + 1,2-propylene glycol (1,2-PG) | 1 (ChCl): 2 (1,2-PG) | 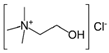 | 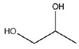 |
| reline 200 * (R200) | Choline chloride (ChCl) + Urea (U) | 1 (ChCl):2 U |  | 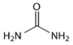 |
| DES | Water Content **/wt.% | T/°C | Viscosity ***/mPa s | Ionic Conductivity/mS cm−1 |
|---|---|---|---|---|
| 1,2–Propeline * | 6.9 ± 1.1 | 30 °C | 71.1 ± 0.9 56.8 ± 1.1 40.8 ± 0.8 31.5 ± 1.0 | 3.11 ± 0.09 4.09 ± 0.05 4.78 ± 0.09 5.4 ± 0.1 |
| 40 °C | ||||
| 50 °C | ||||
| 60 °C | ||||
| reline 200 * (R200) | 5.5 ± 0.4 | 30 °C | 350.3 ± 4.3 322.2 ± 1.9 257.1 ± 6.0 203.7 ± 9.6 | 0.34 ± 0.01 0.76 ± 0.03 1.21 ± 0.09 1.96 ± 0.07 |
| 40 °C | ||||
| 50 °C | ||||
| 60 °C |
| SBET/m2 g−1 | Pore Volume/cm3 g−1 | Average Pore Width/nm | |
|---|---|---|---|
| Commercial Graphene | 45.14 ± 0.43 | 0.00461 ± 0.00012 | 0.9049 |
| Commercial Graphite | 10.74 ± 0.12 | 0.00028 ± 0.00009 | 0.9251 |
| DES | Specific Capacitance/ F g−1 | Capacitance Retention after 1000 Cycles/% | IR/V | |
|---|---|---|---|---|
| Commercial Graphene | 1,2-Propeline | 6.19 | 59.78 | 0.0064 |
| R200 | 1.06 | 79.71 | 0.0278 | |
| Commercial Graphite | 1,2-Propeline | 1.70 | 65.47 | 0.0104 |
| R200 | 1.34 | 79.55 | 0.0289 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brandão, A.T.S.C.; Costa, R.; Silva, A.F.; Pereira, C.M. Hydrogen Bond Donors Influence on the Electrochemical Performance of Composite Graphene Electrodes/Deep Eutectic Solvents Interface. Electrochem 2022, 3, 129-142. https://doi.org/10.3390/electrochem3010009
Brandão ATSC, Costa R, Silva AF, Pereira CM. Hydrogen Bond Donors Influence on the Electrochemical Performance of Composite Graphene Electrodes/Deep Eutectic Solvents Interface. Electrochem. 2022; 3(1):129-142. https://doi.org/10.3390/electrochem3010009
Chicago/Turabian StyleBrandão, Ana T. S. C., Renata Costa, A. Fernando Silva, and Carlos M. Pereira. 2022. "Hydrogen Bond Donors Influence on the Electrochemical Performance of Composite Graphene Electrodes/Deep Eutectic Solvents Interface" Electrochem 3, no. 1: 129-142. https://doi.org/10.3390/electrochem3010009
APA StyleBrandão, A. T. S. C., Costa, R., Silva, A. F., & Pereira, C. M. (2022). Hydrogen Bond Donors Influence on the Electrochemical Performance of Composite Graphene Electrodes/Deep Eutectic Solvents Interface. Electrochem, 3(1), 129-142. https://doi.org/10.3390/electrochem3010009