Electrochemical Properties of Sputtered Ruthenium Oxide Neural Stimulation and Recording Electrodes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Planar Microelectrode Fabrication
2.2. Electrolyte Preparation
2.3. Cyclic Voltammetry
2.4. Electrochemical Impedance Spectroscopy
2.5. Voltage Transient Measurements
2.6. Statistical Analysis
3. Results
3.1. Cyclic Voltammetry
3.2. Electrochemical Impedance Characteristics
3.3. Impedance Modelling Study
3.4. Contribution of Circuit Components in Charge Injection
3.5. Charge-Injection Reversibility
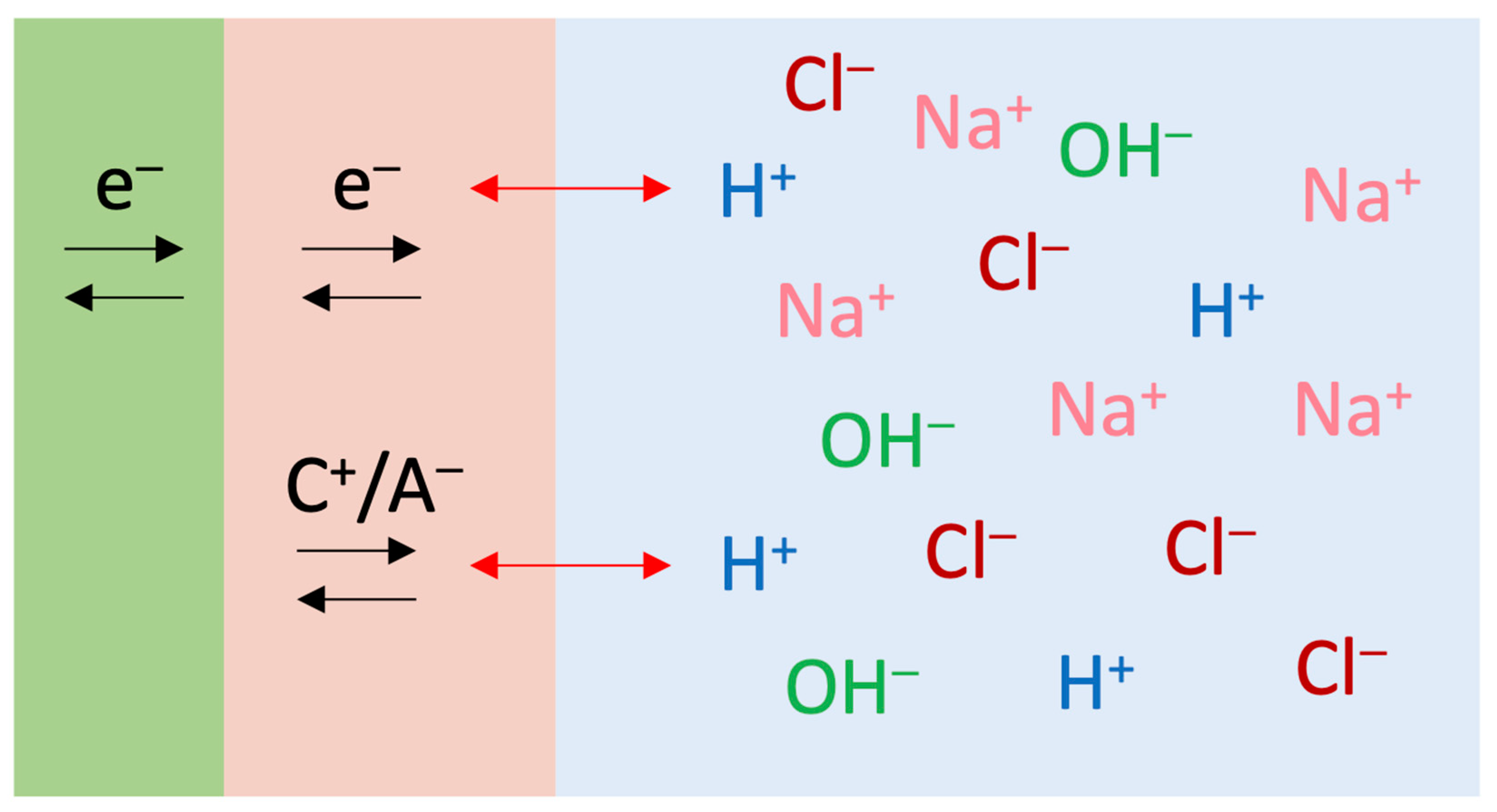
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Flesher, S.N.; Collinger, J.L.; Foldes, S.T.; Weiss, J.M.; Downey, J.E.; Tyler-Kabara, E.C.; Bensmaia, S.J.; Schwartz, A.B.; Boninger, M.L.; Gaunt, R.A. Intracortical microstimulation of human somatosensory cortex. Sci. Transl. Med. 2016, 8, 361ra141. [Google Scholar] [CrossRef]
- Dhillon, G.S.; Horch, K.W. Direct neural sensory feedback and control of a prosthetic arm. IEEE Trans. Neural Syst. Rehabil. Eng. 2005, 13, 468–472. [Google Scholar] [CrossRef]
- Tomlinson, T.; Miller, L.E. Toward a proprioceptive neural interface that mimics natural cortical activity. Prog. Mot. Control Theor. Transl. 2016, 957, 367–388. [Google Scholar] [CrossRef] [Green Version]
- Humayun, M.S.; Weiland, J.D.; Fujii, G.Y.; Greenberg, R.; Williamson, R.; Little, J.; Mech, B.; Cimmarusti, V.; Van Boemel, G.; Dagnelie, G.; et al. Visual perception in a blind subject with a chronic microelectronic retinal prosthesis. Vis. Res. 2003, 43, 2573–2581. [Google Scholar] [CrossRef] [Green Version]
- Bradley, D.C.; Troyk, P.R.; Berg, J.A.; Bak, M.; Cogan, S.; Erickson, R.; Kufta, C.; Mascaro, M.; McCreery, D.; Schmidt, E.M.; et al. Visuotopic mapping through a multichannel stimulating implant in primate V1. J. Neurophysiol. 2005, 93, 1659–1670. [Google Scholar] [CrossRef] [Green Version]
- Cogan, S.F. Neural stimulation and recording electrodes. Annu. Rev. Biomed. Eng. 2008, 10, 275–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merrill, D.R.; Bikson, M.; Jefferys, J.G. Electrical stimulation of excitable tissue: Design of efficacious and safe protocols. J. Neurosci. Methods 2005, 141, 171–198. [Google Scholar] [CrossRef] [PubMed]
- Barrese, J.C.; Rao, N.; Paroo, K.; Triebwasser, C.; Vargas-Irwin, C.; Franquemont, L.; Donoghue, J.P. Failure mode analysis of silicon-based intracortical microelectrode arrays in non-human primates. J. Neural Eng. 2013, 10, 066014. [Google Scholar] [CrossRef]
- Negi, S.; Bhandari, R.; Rieth, L.; Solzbacher, F. In vitro comparison of sputtered iridium oxide and platinum-coated neural implantable microelectrode arrays. Biomed. Mater. 2010, 5, 015007. [Google Scholar] [CrossRef]
- Rose, T.L.; Robblee, L.S. Electrical stimulation with Pt electrodes. VIII. Electrochemically safe charge injection limits with 0.2 ms pulses (neuronal application). IEEE Trans. Biomed. Eng. 1990, 37, 1118–1120. [Google Scholar] [CrossRef]
- Weiland, J.D.; Anderson, D.J.; Humayun, M.S. In vitro electrical properties for iridium oxide versus titanium nitride stimulating electrodes. IEEE Trans. Biomed. Eng. 2002, 49, 1574–1579. [Google Scholar] [CrossRef] [PubMed]
- Beebe, X.; Rose, T.L. Charge injection limits of activated iridium oxide electrodes with 0.2 ms pulses in bicarbonate buffered saline (neurological stimulation application). IEEE Trans. Biomed. Eng. 1988, 35, 494–495. [Google Scholar] [CrossRef]
- Du, Z.J.; Luo, X.; Weaver, C.L.; Cui, X.T. Poly (3, 4-ethylenedioxythiophene)-ionic liquid coating improves neural recording and stimulation functionality of MEAs. J. Mater. Chem. C 2015, 3, 6515–6524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chalupczok, S.; Kurzweil, P.; Hartmann, H.; Schell, C. The redox chemistry of ruthenium dioxide: A cyclic voltammetry study—Review and revision. Int. J. Electrochem. 2018, 2018, 1273768. [Google Scholar] [CrossRef] [Green Version]
- Hu, C.C.; Chen, W.C.; Chang, K.H. How to achieve maximum utilization of hydrous ruthenium oxide for supercapacitors. J. Electrochem. Soc. 2004, 151, A281. [Google Scholar] [CrossRef]
- Chakraborty, B.; Joshi-Imre, A.; Maeng, J.; Cogan, S.F. Sputtered ruthenium oxide coatings for neural stimulation and recording electrodes. J. Biomed. Mater. Res. Part B Appl. Biomater. 2021, 109, 643–653. [Google Scholar] [CrossRef]
- Chakraborty, B.; Joshi-Imre, A.; Cogan, S.F. Charge injection characteristics of sputtered ruthenium oxide electrodes for neural stimulation and recording. J. Biomed. Mater. Res. Part B Appl. Biomater. 2022, 110, 229–238. [Google Scholar] [CrossRef]
- Chakraborty, B.; Joshi-Imre, A.; Cogan, S.F. Sputtered Ruthenium Oxide Neural Stimulation Electrodes. In Proceedings of the 43rd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Virtual, 1–5 November 2021; pp. 6655–6658. [Google Scholar]
- Randles, J.E.B. Kinetics of rapid electrode reactions. Discuss. Faraday Soc. 1947, 1, 11–19. [Google Scholar] [CrossRef]
- Maeng, J.; Chakraborty, B.; Geramifard, N.; Kang, T.; Rihani, R.T.; Joshi-Imre, A.; Cogan, S.F. High-charge-capacity sputtered iridium oxide neural stimulation electrodes deposited using water vapor as a reactive plasma constituent. J. Biomed. Mater. Res. Part B: Appl. Biomater. 2020, 108, 880–891. [Google Scholar] [CrossRef]
- Cogan, S.F.; Troyk, P.R.; Ehrlich, J.; Gasbarro, C.M.; Plante, T.D. The influence of electrolyte composition on the in vitro charge-injection limits of activated iridium oxide (AIROF) stimulation electrodes. J. Neural Eng. 2007, 4, 79. [Google Scholar] [CrossRef]
- Deku, F.; Joshi-Imre, A.; Mertiri, A.; Gardner, T.J.; Cogan, S.F. Electrodeposited iridium oxide on carbon fiber ultramicroelectrodes for neural recording and stimulation. J. Electrochem. Soc. 2018, 165, D375. [Google Scholar] [CrossRef] [Green Version]
- Fontes, M.B.A. Electrodes for bio-application: Recording and stimulation. J. Phys. Conf. Ser. 2013, 421, 012019. [Google Scholar] [CrossRef]
- Frederick, R.A.; Meliane, I.Y.; Joshi-Imre, A.; Troyk, P.R.; Cogan, S.F. Activated iridium oxide film (AIROF) electrodes for neural tissue stimulation. J. Neural Eng. 2020, 17, 056001. [Google Scholar] [CrossRef]
- Pauporté, T.; Durand, R. Impedance spectroscopy study of electrochromism in sputtered iridium oxide films. J. Appl. Electrochem. 2000, 30, 35–41. [Google Scholar] [CrossRef]
- Caldwell, R.; Sharma, R.; Takmakov, P.; Street, M.G.; Solzbacher, F.; Tathireddy, P.; Rieth, L. Neural electrode resilience against dielectric damage may be improved by use of highly doped silicon as a conductive material. J. Neurosci. Methods 2018, 293, 210–225. [Google Scholar] [CrossRef] [PubMed]
- Franks, W.; Schenker, I.; Schmutz, P.; Hierlemann, A. Impedance characterization and modeling of electrodes for biomedical applications. IEEE Trans. Biomed. Eng. 2005, 52, 1295–1302. [Google Scholar] [CrossRef]
- Brug, G.J.; van den Eeden, A.L.; Sluyters-Rehbach, M.; Sluyters, J.H. The analysis of electrode impedances complicated by the presence of a constant phase element. J. Electroanal. Chem. Interfacial Electrochem. 1984, 176, 275–295. [Google Scholar] [CrossRef]
- Dellis, J.L.; Carpentier, J.L. Nelder and Mead algorithm in impedance spectra fitting. Solid State Ion. 1993, 62, 119–123. [Google Scholar] [CrossRef]
- Sunde, S.; Lervik, I.A.; Tsypkin, M.; Owe, L.E. Impedance analysis of nanostructured iridium oxide electrocatalysts. Electrochim. Acta 2010, 55, 7751–7760. [Google Scholar] [CrossRef]
- Weiland, J.D.; Anderson, D.J. Chronic neural stimulation with thin-film, iridium oxide electrodes. IEEE Trans. Biomed. Eng. 2000, 47, 911–918. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, J.; Gharbi, O.; Vivier, V.; Gao, M.; Orazem, M.E. Electrochemical impedance spectroscopy. Nat. Rev. Methods Primers 2021, 1, 41. [Google Scholar] [CrossRef]
- Zheng, X.S.; Tan, C.; Castagnola, E.; Cui, X.T. Electrode materials for chronic electrical microstimulation. Adv. Healthc. Mater. 2021, 10, 2100119. [Google Scholar] [CrossRef] [PubMed]
- Normann, R.A.; Campbell, P.K.; Jones, K.E.; University of Utah. Three-Dimensional Electrode Device. U.S. Patent US5215088A, 1 June 1993. [Google Scholar]
- Musk, E. An integrated brain-machine interface platform with thousands of channels. J. Med. Internet Res. 2019, 21, e16194. [Google Scholar] [CrossRef] [PubMed]
- McKeown, D.A.; Hagans, P.L.; Carette, L.P.; Russell, A.E.; Swider, K.E.; Rolison, D.R. Structure of hydrous ruthenium oxides: Implications for charge storage. J. Phys. Chem. B 1999, 103, 4825–4832. [Google Scholar] [CrossRef]
- Atmaramani, R.; Chakraborty, B.; Rihani, R.T.; Usoro, J.; Hammack, A.; Abbott, J.; Nnoromele, P.; Black, B.J.; Pancrazio, J.J.; Cogan, S.F. Ruthenium oxide based microelectrode arrays for in vitro and in vivo neural recording and stimulation. Acta Biomater. 2020, 101, 565–574. [Google Scholar] [CrossRef]
- Available online: https://pmm.umicore.com/en/prices/ (accessed on 10 June 2023).



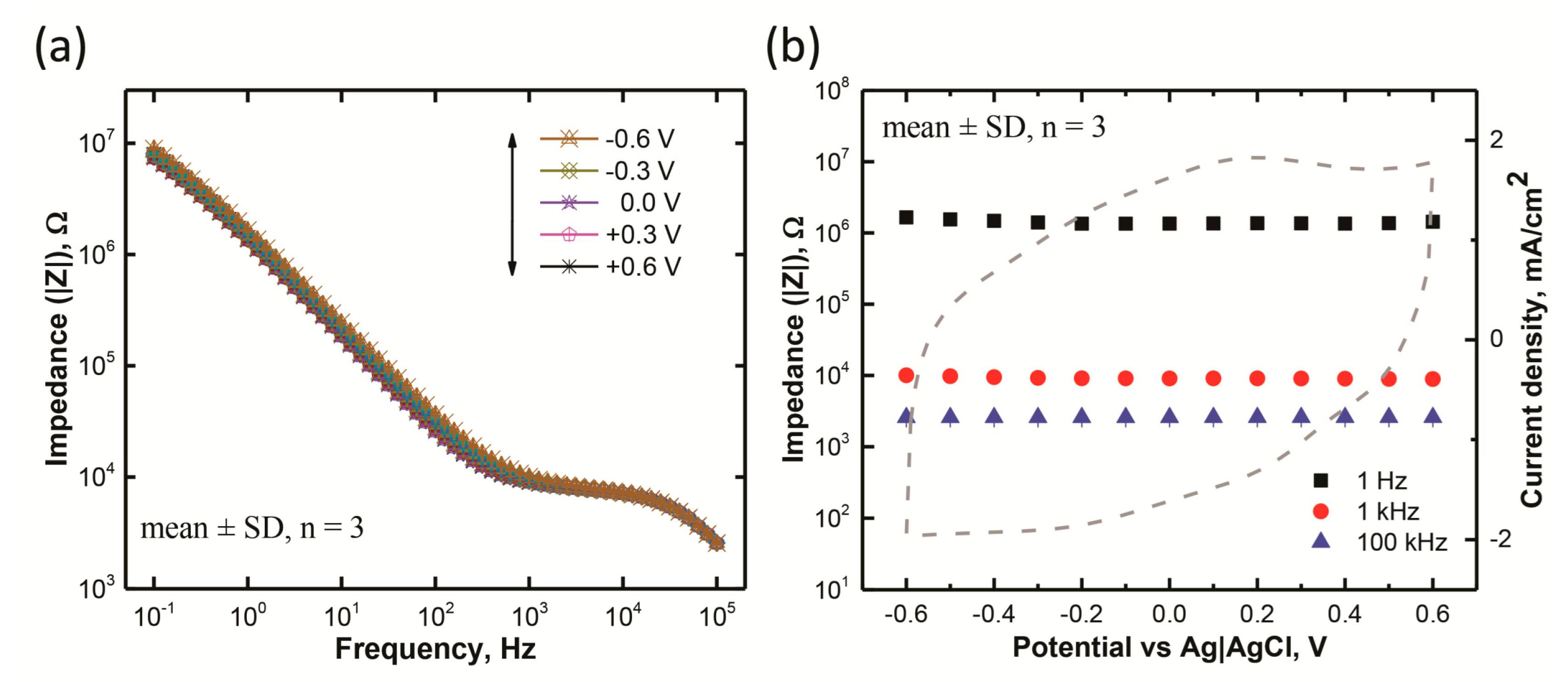




| Electrolyte | Rs (kΩ) Mean ± SD | Rf (kΩ) Mean ± SD | Cdl (nF) Mean ± SD | Yo (nS s−a) Mean ± SD | a Mean ± SD |
|---|---|---|---|---|---|
| PBS | 2.32 ± 0.12 | 1.08 ± 0.14 | 6.54 ± 1.05 | 601.70 ± 30.90 | 0.85 ± 0.00 |
| model-ISF | 4.95 ± 0.62 | 2.96 ± 0.31 | 8.86 ± 3.75 | 328.90 ± 33.40 | 0.87 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chakraborty, B. Electrochemical Properties of Sputtered Ruthenium Oxide Neural Stimulation and Recording Electrodes. Electrochem 2023, 4, 350-364. https://doi.org/10.3390/electrochem4030023
Chakraborty B. Electrochemical Properties of Sputtered Ruthenium Oxide Neural Stimulation and Recording Electrodes. Electrochem. 2023; 4(3):350-364. https://doi.org/10.3390/electrochem4030023
Chicago/Turabian StyleChakraborty, Bitan. 2023. "Electrochemical Properties of Sputtered Ruthenium Oxide Neural Stimulation and Recording Electrodes" Electrochem 4, no. 3: 350-364. https://doi.org/10.3390/electrochem4030023
APA StyleChakraborty, B. (2023). Electrochemical Properties of Sputtered Ruthenium Oxide Neural Stimulation and Recording Electrodes. Electrochem, 4(3), 350-364. https://doi.org/10.3390/electrochem4030023







