High C-Rate Performant Electrospun LiFePO4/Carbon Nanofiber Self-Standing Cathodes for Lithium-Ion Batteries
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
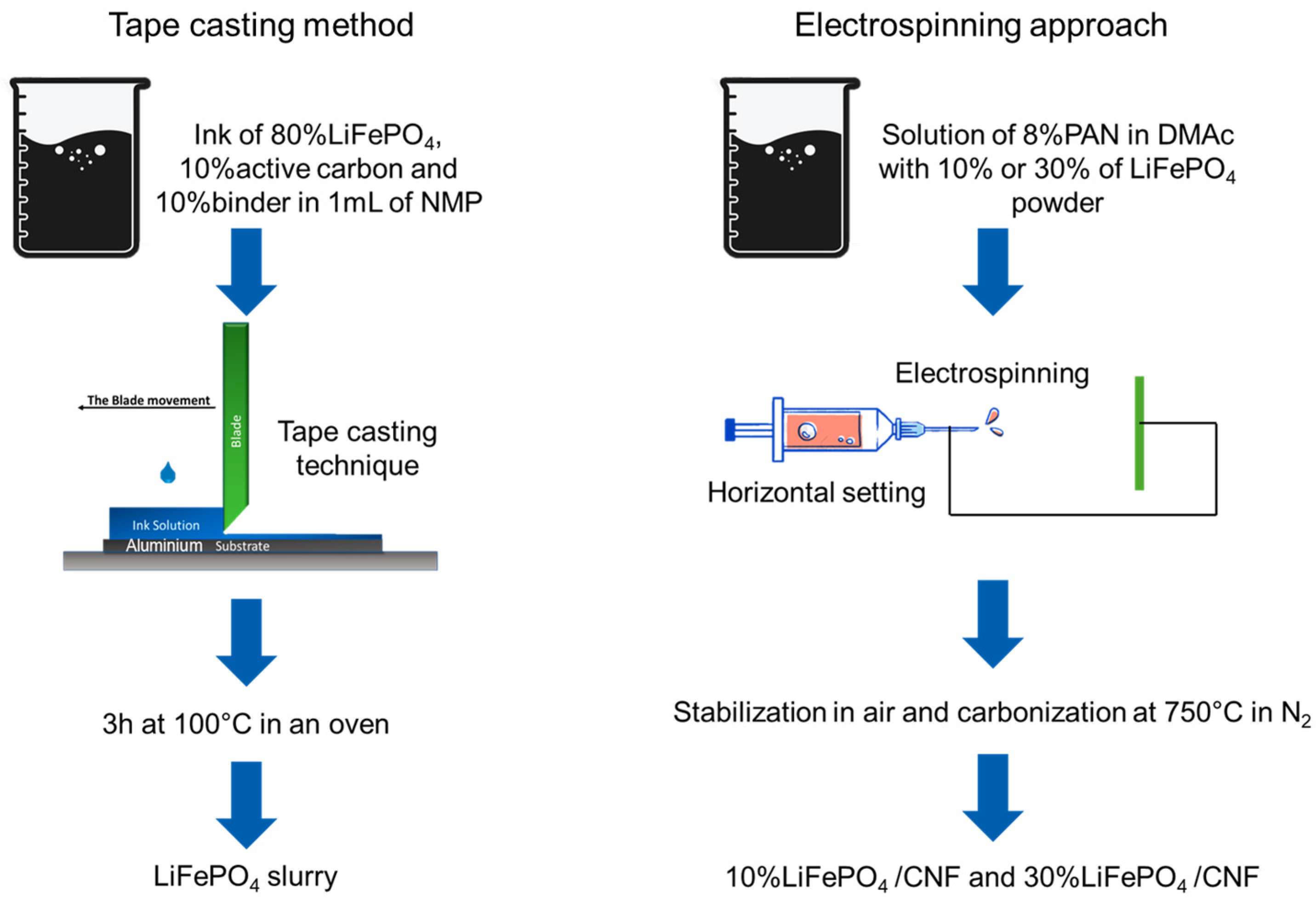
2.2. Synthesis
2.2.1. LiFePO4 Self-Standing Cathodes
2.2.2. Tape-Casting Cathode
2.3. Cell Assembly
2.4. Characterization Techniques
3. Results and Discussion
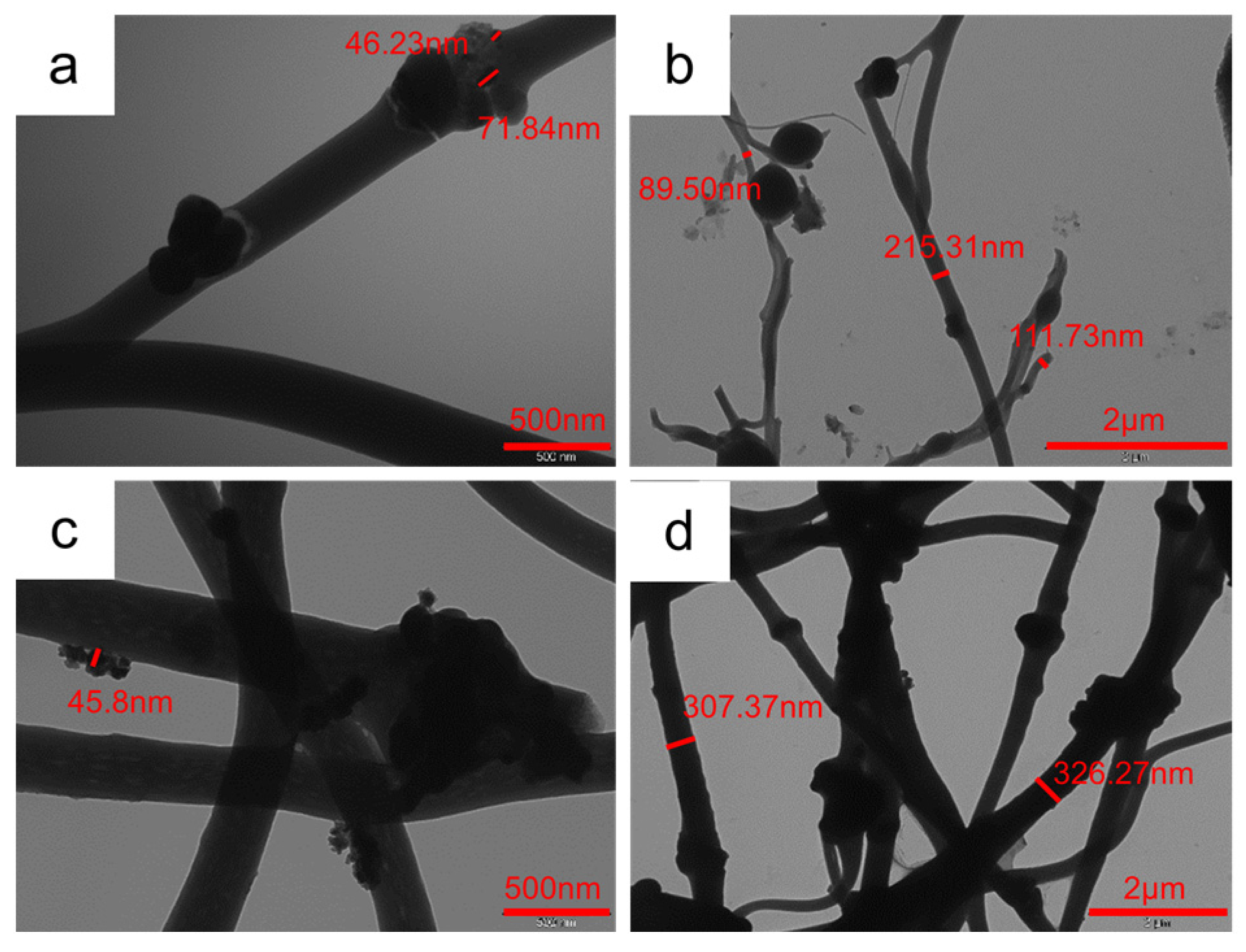
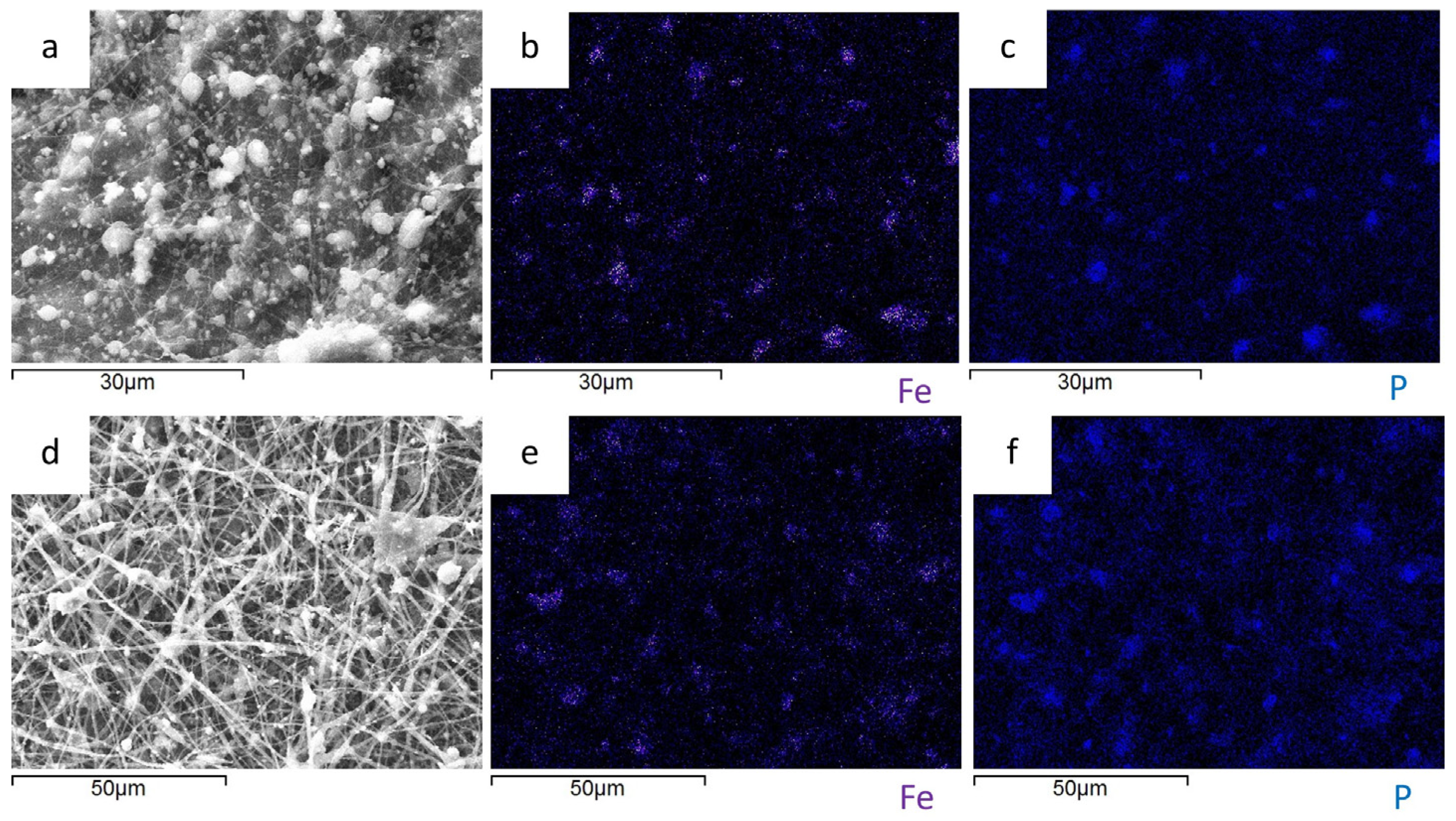
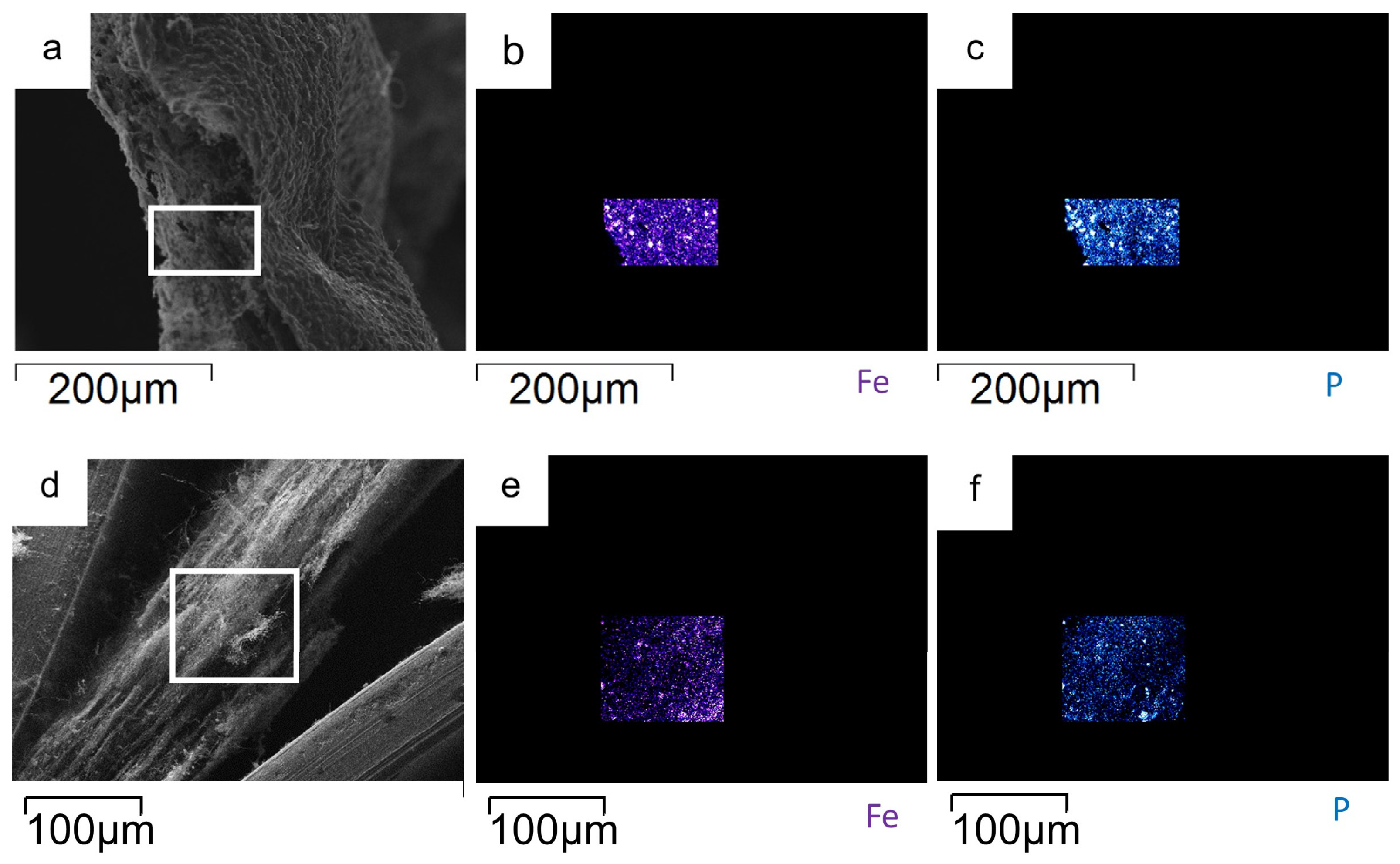
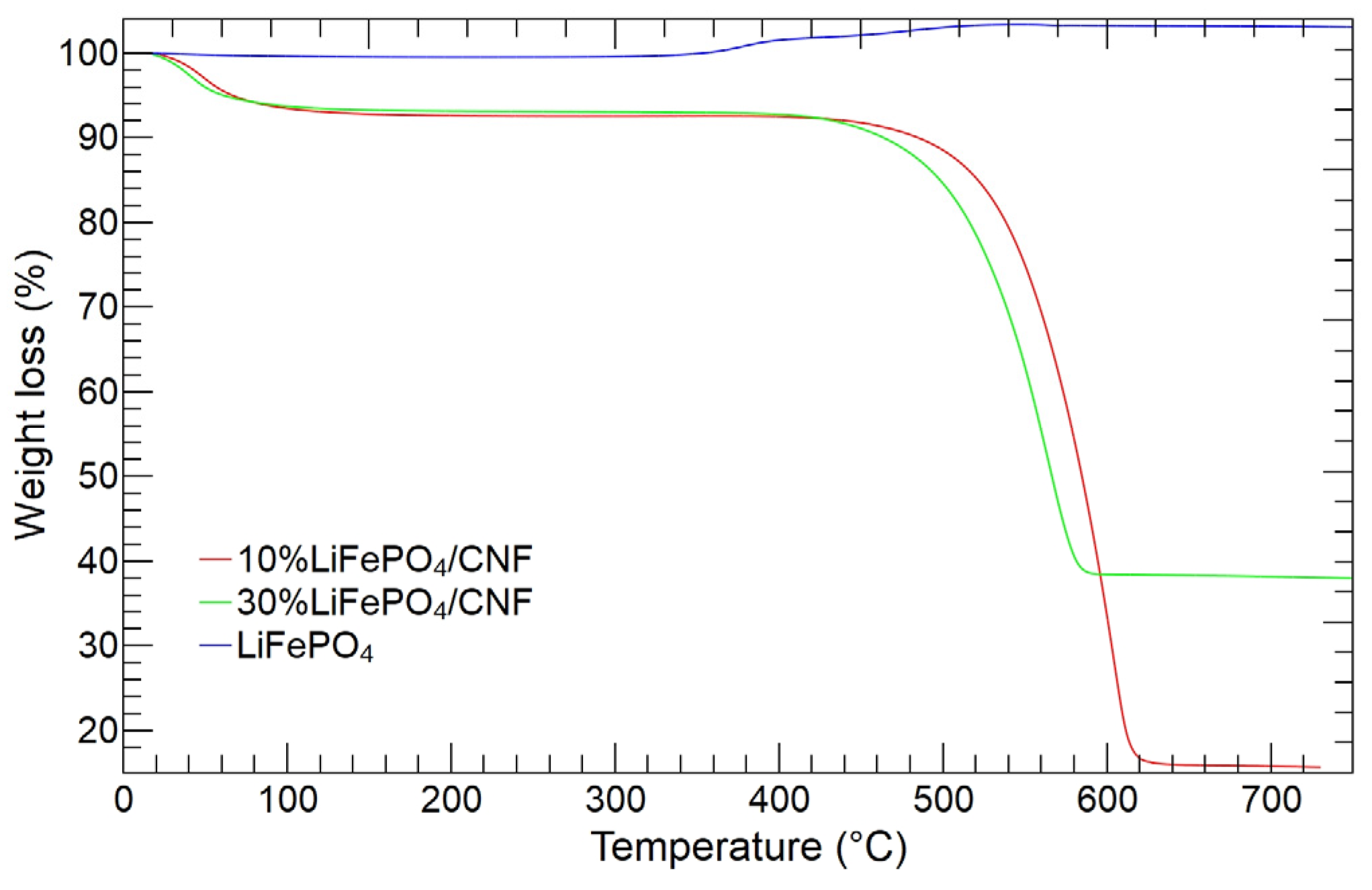
3.1. LiFePO4 and LiFePO4/CNF Characterization
3.2. LiFePO4 and LiFePO4/CNF Electrochemical Characterization
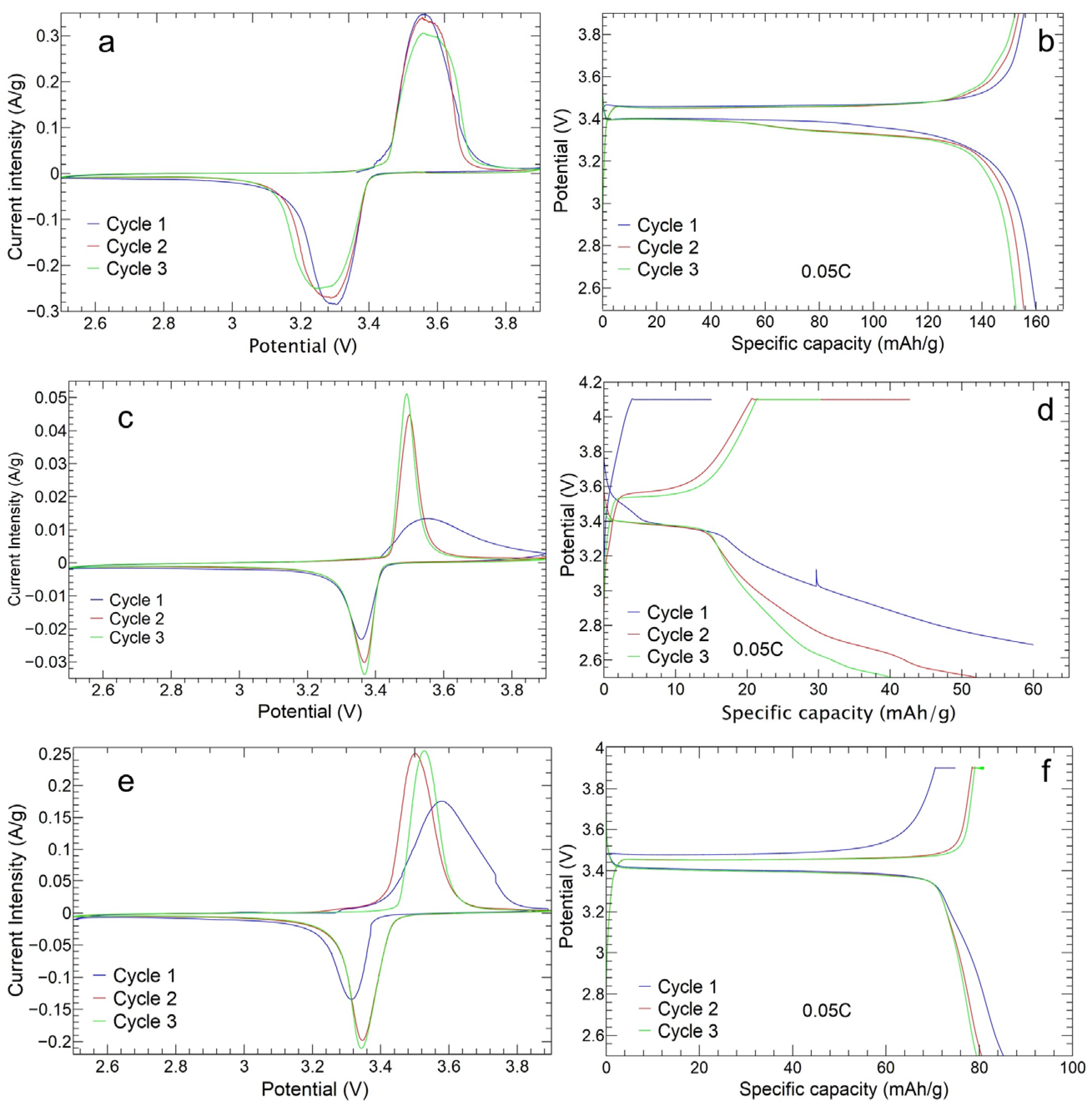
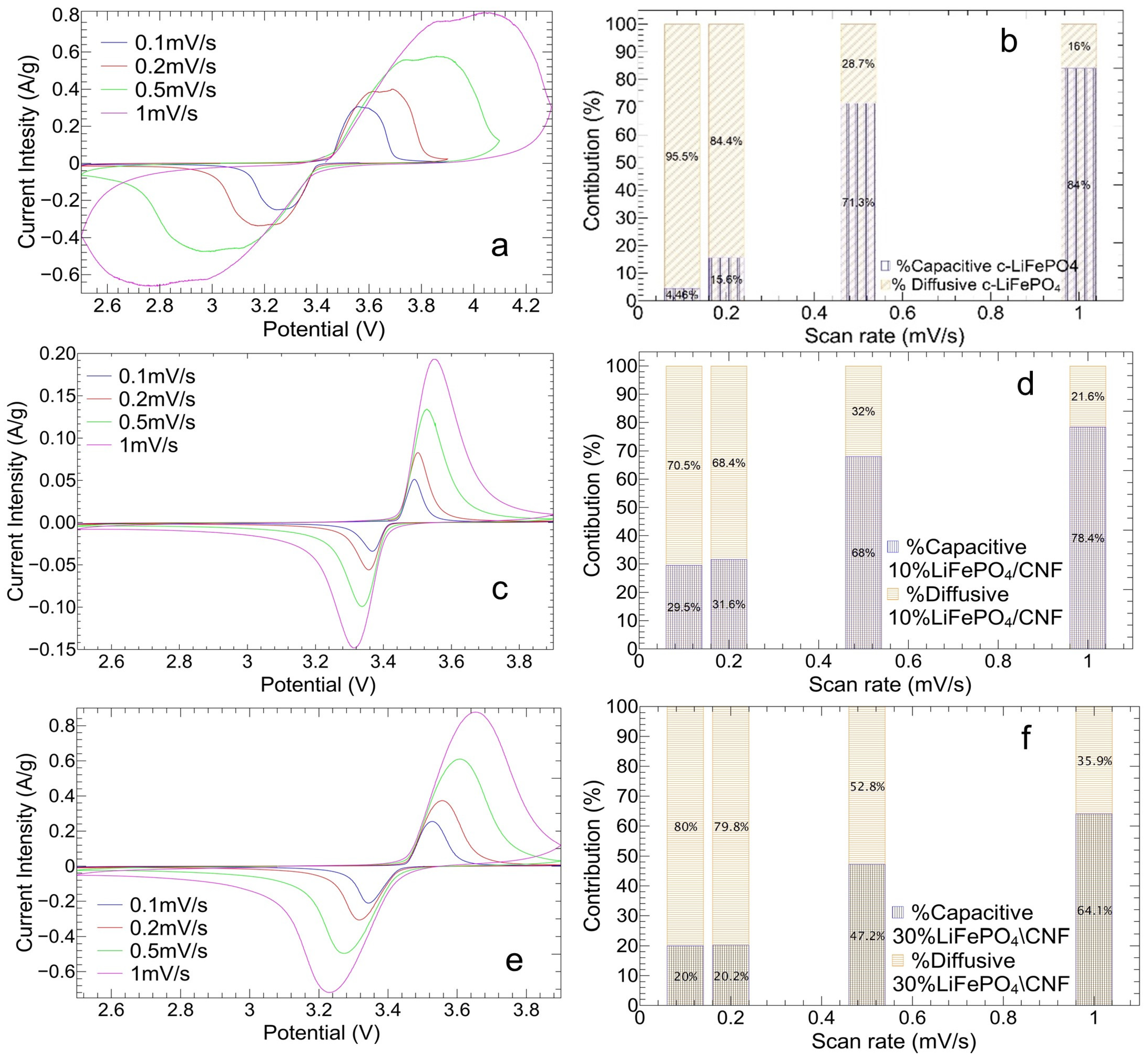
3.2.1. Cyclic Voltammetry Analysis
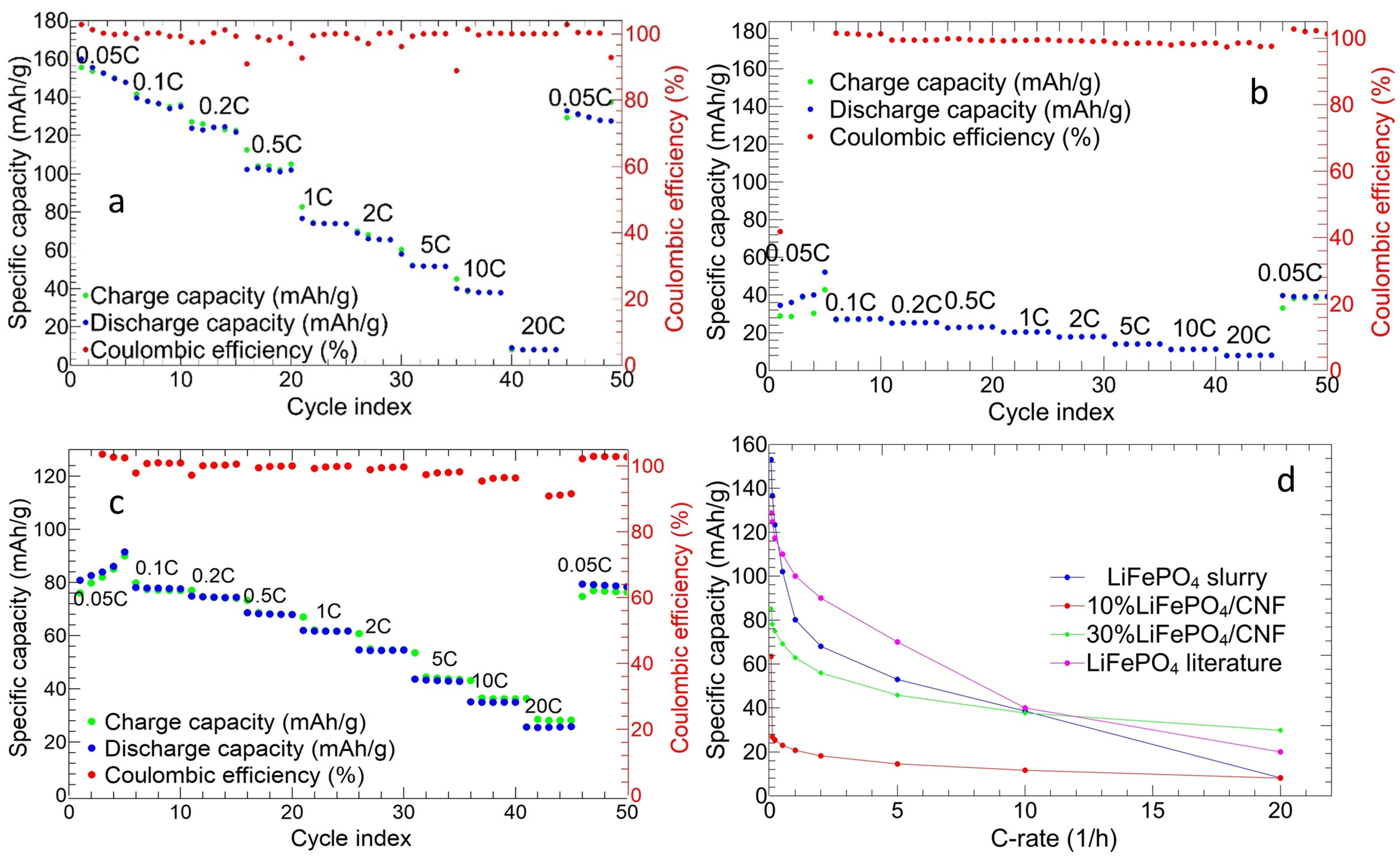
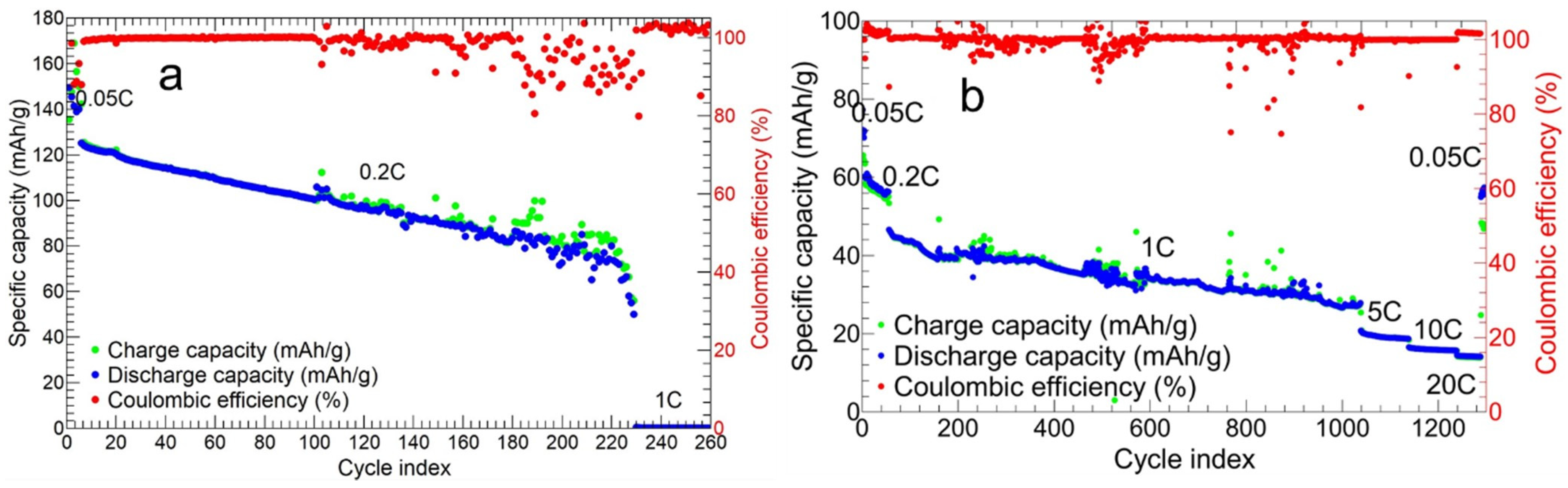
3.2.2. Charge and Discharge Cycles
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oskouei, M.Z.; Şeker, A.A.; Tunçel, S.; Demirbaş, E.; Gözel, T.; Hocaoğlu, M.H.; Abapour, M.; Mohammadi-Ivatloo, B. A Critical Review on the Impacts of Energy Storage Systems and Demand-Side Management Strategies in the Economic Operation of Renewable-Based Distribution Network. Sustainability 2022, 14, 2110. [Google Scholar] [CrossRef]
- Dunn, B.; Kamath, H.; Tarascon, J.-M. Electrical Energy Storage for the Grid: A Battery of Choices. Science 2011, 334, 928–935. [Google Scholar] [CrossRef]
- Wang, B.; Li, X.; Zhang, X.; Luo, B.; Zhang, Y.; Zhi, L. Contact-Engineered and Void-Involved Silicon/Carbon Nanohybrids as Lithium-Ion-Battery Anodes. Adv. Mater. 2013, 25, 3560–3565. [Google Scholar] [CrossRef]
- Chen, L.; Yan, B.; Xu, J.; Wang, C.; Chao, Y.; Jiang, X.; Yang, G. Bicontinuous Structure of Li3V2(PO4)3 Clustered via Carbon Nanofiber as High-Performance Cathode Material of Li-Ion Batteries. ACS Appl. Mater. Interfaces 2015, 7, 13934–13943. [Google Scholar] [CrossRef]
- Gholami, T.; Salavati-Niasari, M.; Varshoy, S. Electrochemical Hydrogen Storage Capacity and Optical Properties of NiAl2O4/NiO Nanocomposite Synthesized by Green Method. Int. J. Hydrogen Energy 2017, 42, 5235–5245. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, K.; Hu, Z.; Tao, Z.; Mai, L.; Kang, Y.-M.; Chou, S.-L.; Chen, J. Recent Developments on and Prospects for Electrode Materials with Hierarchical Structures for Lithium-Ion Batteries. Adv. Energy Mater. 2018, 8, 1701415. [Google Scholar] [CrossRef]
- Eftekhari, A. Lithium Batteries for Electric Vehicles: From Economy to Research Strategy. ACS Sustain. Chem. Eng. 2019, 7, 5602–5613. [Google Scholar] [CrossRef]
- Duan, J.; Tang, X.; Dai, H.; Yang, Y.; Wu, W.; Wei, X.; Huang, Y. Building Safe Lithium-Ion Batteries for Electric Vehicles: A Review. Electrochem. Energy Rev. 2020, 3, 1–42. [Google Scholar] [CrossRef]
- Wang, J.; Chen, L.; Zeng, L.; Wei, Q.; Wei, M. In Situ Synthesis of WSe2/CMK-5 Nanocomposite for Rechargeable Lithium-Ion Batteries with a Long-Term Cycling Stability. ACS Sustain. Chem. Eng. 2018, 6, 4688–4694. [Google Scholar] [CrossRef]
- Sun, J.; Lian, G.; Jing, L.; Wu, D.; Cui, D.; Wang, Q.; Yu, H.; Zhang, H.; Wong, C.-P. Assembly of Flower-like VS2/N-Doped Porous Carbon with Expanded (001) Plane on rGO for Superior Na-Ion and K-Ion Storage. Nano Res. 2022, 15, 4108–4116. [Google Scholar] [CrossRef]
- Hou, Z.; Zhang, X.; Chen, J.; Qian, Y.; Chen, L.-F.; Lee, P.S. Towards High-Performance Aqueous Sodium Ion Batteries: Constructing Hollow NaTi2(PO4)3@C Nanocube Anode with Zn Metal-Induced Pre-Sodiation and Deep Eutectic Electrolyte. Adv. Energy Mater. 2022, 12, 2104053. [Google Scholar] [CrossRef]
- Liu, Y.-R.; Lei, Z.-W.; Liu, R.-P.; Li, X.-Y.; Xiong, P.-X.; Luo, Y.-J.; Chen, Q.-H.; Wei, M.-D.; Zeng, L.-X.; Qian, Q.-R. Sn-Doped Induced Stable 1T-WSe2 Nanosheets Entrenched on N-Doped Carbon with Extraordinary Half/Full Sodium/Potassium Storage Performance. Rare Met. 2023, 42, 1557–1569. [Google Scholar] [CrossRef]
- Kong, F.; Liang, C.; Longo, R.C.; Zheng, Y.; Cho, K. Atomic-Scale Understanding of Non-Stoichiometry Effects on the Electrochemical Performance of Ni-Rich Cathode Materials. J. Power Sources 2018, 378, 750–758. [Google Scholar] [CrossRef]
- Yasuhara, S.; Yasui, S.; Teranishi, T.; Chajima, K.; Yoshikawa, Y.; Majima, Y.; Taniyama, T.; Itoh, M. Enhancement of Ultrahigh Rate Chargeability by Interfacial Nanodot BaTiO3 Treatment on LiCoO2 Cathode Thin Film Batteries. Nano Lett. 2019, 19, 1688–1694. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Yao, J.; Huang, H.; Du, Z.; Gu, H.; Wang, Z. Enhanced Cathode Performance of LiFePO4/C Composite by Novel Reaction of Ethylene Glycol with Different Carboxylic Acids. Mater. Chem. Phys. 2019, 224, 293–300. [Google Scholar] [CrossRef]
- Pang, P.; Wang, Z.; Deng, Y.; Nan, J.; Xing, Z.; Li, H. Delayed Phase Transition and Improved Cycling/Thermal Stability by Spinel LiNi0.5Mn1.5O4 Modification for LiCoO2 Cathode at High Voltages. ACS Appl. Mater. Interfaces 2020, 12, 27339–27349. [Google Scholar] [CrossRef]
- Abou-Rjeily, J.; Bezza, I.; Laziz, N.A.; Autret-Lambert, C.; Sougrati, M.T.; Ghamouss, F. High-Rate Cyclability and Stability of LiMn2O4 Cathode Materials for Lithium-Ion Batteries from Low-Cost Natural β−MnO2. Energy Storage Mater. 2020, 26, 423–432. [Google Scholar] [CrossRef]
- Tabassam, R.; Alvi, F.; Aslam, N.; Raza, R.; Saifur-Rehman; Sherin, L.; Ajaml, M.; Ali, A. Electrochemical Investigation of LiMn2O4/Asphalt and LiMn2O4/Bituminous Coal Based Cathode Composites for Efficient Lithium-Ion Battery. Mater. Lett. 2021, 302, 130275. [Google Scholar] [CrossRef]
- Ha, Y.; Harvey, S.P.; Teeter, G.; Colclasure, A.M.; Trask, S.E.; Jansen, A.N.; Burrell, A.; Park, K. Long-Term Cyclability of Li4Ti5O12/LiMn2O4 Cells Using Carbonate-Based Electrolytes for behind-the-Meter Storage Applications. Energy Storage Mater. 2021, 38, 581–589. [Google Scholar] [CrossRef]
- Lin, J.; Sun, Y.-H.; Lin, X. Metal-Organic Framework-Derived LiFePO4 Cathode Encapsulated in O,F-Codoped Carbon Matrix towards Superior Lithium Storage. Nano Energy 2022, 91, 106655. [Google Scholar] [CrossRef]
- Armand, M.; Tarascon, J.-M. Building Better Batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef]
- Bruce, P.G.; Freunberger, S.A.; Hardwick, L.J.; Tarascon, J.-M. Li–O2 and Li–S Batteries with High Energy Storage. Nat. Mater. 2012, 11, 19–29. [Google Scholar] [CrossRef]
- Jo, C.-H.; Cho, D.-H.; Noh, H.-J.; Yashiro, H.; Sun, Y.-K.; Myung, S.T. An Effective Method to Reduce Residual Lithium Compounds on Ni-Rich Li[Ni0.6Co0.2Mn0.2]O2 Active Material Using a Phosphoric Acid Derived Li3PO4 Nanolayer. Nano Res. 2015, 8, 1464–1479. [Google Scholar] [CrossRef]
- Lu, J.; Tian, X.; Zhou, Y.; Zhu, Y.; Tang, Z.; Ma, B.; Wu, G.; Jiang, T.; Tu, X.; Chen, G.Z. A Novel “Holey-LFP/Graphene/Holey-LFP” Sandwich Nanostructure with Significantly Improved Rate Capability for Lithium Storage. Electrochim. Acta 2019, 320, 134566. [Google Scholar] [CrossRef]
- Cao, Z.; Sang, M.; Chen, S.; Jia, J.; Yang, M.; Zhang, H.; Li, X.; Yang, S. In Situ Constructed (010)-Oriented LiFePO4 Nanocrystals/Carbon Nanofiber Hybrid Network: Facile Synthesis of Free-Standing Cathodes for Lithium-Ion Batteries. Electrochim. Acta 2020, 333, 135538. [Google Scholar] [CrossRef]
- Adepoju, A.A.; Williams, Q.L. High C-Rate Performance of LiFePO4/Carbon Nanofibers Composite Cathode for Li-Ion Batteries. Curr. Appl. Phys. 2020, 20, 1–4. [Google Scholar] [CrossRef]
- Wang, B.; Wang, D.; Wang, Q.; Liu, T.; Guo, C.; Zhao, X. Improvement of the Electrochemical Performance of Carbon-Coated LiFePO4 Modified with Reduced Graphene Oxide. J. Mater. Chem. A 2012, 1, 135–144. [Google Scholar] [CrossRef]
- Yang, S.; Hu, M.; Xi, L.; Ma, R.; Dong, Y.; Chung, C.Y. Solvothermal Synthesis of Monodisperse LiFePO4 Micro Hollow Spheres as High Performance Cathode Material for Lithium Ion Batteries. ACS Appl. Mater. Interfaces 2013, 5, 8961–8967. [Google Scholar] [CrossRef]
- Ramasubramanian, B.; Sundarrajan, S.; Chellappan, V.; Reddy, M.V.; Ramakrishna, S.; Zaghib, K. Recent Development in Carbon-LiFePO4 Cathodes for Lithium-Ion Batteries: A Mini Review. Batteries 2022, 8, 133. [Google Scholar] [CrossRef]
- Johnson, I.D.; Lübke, M.; Wu, O.Y.; Makwana, N.M.; Smales, G.J.; Islam, H.U.; Dedigama, R.Y.; Gruar, R.I.; Tighe, C.J.; Scanlon, D.O.; et al. Pilot-Scale Continuous Synthesis of a Vanadium-Doped LiFePO4/C Nanocomposite High-Rate Cathodes for Lithium-Ion Batteries. J. Power Sources 2016, 302, 410–418. [Google Scholar] [CrossRef]
- Habedank, J.B.; Günter, F.J.; Billot, N.; Gilles, R.; Neuwirth, T.; Reinhart, G.; Zaeh, M.F. Rapid Electrolyte Wetting of Lithium-Ion Batteries Containing Laser Structured Electrodes: In Situ Visualization by Neutron Radiography. Int. J. Adv. Manuf. Technol. 2019, 102, 2769–2778. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhou, J.; Zeng, G.; Ren, S. Effect of Lanthanum and Yttrium Doped LiFePO4 Cathodes on Electrochemical Performance of Lithium-Ion Battery. J. Mater. Sci. 2023, 58, 8463–8477. [Google Scholar] [CrossRef]
- Yang, S.; Zhou, X.; Zhang, J.; Liu, Z. Morphology-Controlled Solvothermal Synthesis of LiFePO4 as a Cathode Material for Lithium-Ion Batteries. J. Mater. Chem. 2010, 20, 8086–8091. [Google Scholar] [CrossRef]
- Shi, Y.; Chou, S.-L.; Wang, J.-Z.; Wexler, D.; Li, H.-J.; Liu, H.-K.; Wu, Y. Graphene Wrapped LiFePO4/C Composites as Cathode Materials for Li-Ion Batteries with Enhanced Rate Capability. J. Mater. Chem. 2012, 22, 16465–16470. [Google Scholar] [CrossRef]
- Li, W.; Li, M.; Adair, K.R.; Sun, X.; Yu, Y. Carbon Nanofiber-Based Nanostructures for Lithium-Ion and Sodium-Ion Batteries. J. Mater. Chem. A 2017, 5, 13882–13906. [Google Scholar] [CrossRef]
- Weng, W.; Kurihara, R.; Wang, J.; Shiratori, S. Electrospun Carbon Nanofiber-Based Composites for Lithium-Ion Batteries: Structure Optimization towards High Performance. Compos. Commun. 2019, 15, 135–148. [Google Scholar] [CrossRef]
- Ma, D.; Zhang, P.; Li, Y.; Ren, X. Li1.2Mn0.54Ni0.13Co0.13O2-Encapsulated Carbon Nanofiber Network Cathodes with Improved Stability and Rate Capability for Li-Ion Batteries. Sci. Rep. 2015, 5, 11257. [Google Scholar] [CrossRef]
- Yu, X.; Deng, J.; Yang, X.; Li, J.; Huang, Z.-H.; Li, B.; Kang, F. A Dual-Carbon-Anchoring Strategy to Fabricate Flexible LiMn2O4 Cathode for Advanced Lithium-Ion Batteries with High Areal Capacity. Nano Energy 2020, 67, 104256. [Google Scholar] [CrossRef]
- Dimesso, L.; Spanheimer, C.; Jaegermann, W.; Zhang, Y.; Yarin, A.L. LiFePO4—3D Carbon Nanofiber Composites as Cathode Materials for Li-Ions Batteries. J. Appl. Phys. 2012, 111, 064307. [Google Scholar] [CrossRef]
- von Hagen, R.; Lepcha, A.; Song, X.; Tyrra, W.; Mathur, S. Influence of Electrode Design on the Electrochemical Performance of Li3V2(PO4)3/C Nanocomposite Cathode in Lithium Ion Batteries. Nano Energy 2013, 2, 304–313. [Google Scholar] [CrossRef]
- Shin, J.; Yang, J.; Sergey, C.; Song, M.-S.; Kang, Y.-M. Carbon Nanofibers Heavy Laden with Li3V2(PO4)3 Particles Featuring Superb Kinetics for High-Power Lithium Ion Battery. Adv. Sci. 2017, 4, 1700128. [Google Scholar] [CrossRef]
- Choi, M.; Kim, H.-S.; Lee, Y.M.; Choi, W.-K.; Jin, B.-S. The High Electrochemical Performance of Li3V2(PO4)3 Supported by Graphene and Carbon-Nanofibers for Advanced Li-Ion Batteries. Mater. Res. Bull. 2016, 73, 211–218. [Google Scholar] [CrossRef]
- Zhang, S.; Lin, Z.; Ji, L.; Li, Y.; Xu, G.; Xue, L.; Li, S.; Lu, Y.; Toprakci, O.; Zhang, X. Cr-Doped Li2MnSiO4/Carbon Composite Nanofibers as High-Energy Cathodes for Li-Ion Batteries. J. Mater. Chem. 2012, 22, 14661–14666. [Google Scholar] [CrossRef]
- Park, H.; Song, T.; Tripathi, R.; Nazar, L.F.; Paik, U. Li2MnSiO4/Carbon Nanofiber Cathodes for Li-Ion Batteries. Ionics 2014, 20, 1351–1359. [Google Scholar] [CrossRef]
- Song, H.J.; Kim, J.-C.; Choi, M.; Choi, C.; Dar, M.A.; Lee, C.W.; Park, S.; Kim, D.-W. Li2MnSiO4 Nanorods-Embedded Carbon Nanofibers for Lithium-Ion Battery Electrodes. Electrochim. Acta 2015, 180, 756–762. [Google Scholar] [CrossRef]
- Zhu, C.; Mu, X.; van Aken, P.A.; Yu, Y.; Maier, J. Single-Layered Ultrasmall Nanoplates of MoS2 Embedded in Carbon Nanofibers with Excellent Electrochemical Performance for Lithium and Sodium Storage. Angew. Chem. Int. Ed. 2014, 53, 2152–2156. [Google Scholar] [CrossRef]
- Bachtin, K.; Kaus, M.; Pfaffmann, L.; Indris, S.; Knapp, M.; Roth, C.; Ehrenberg, H. Comparison of Electrospun and Conventional LiFePO4/C Composite Cathodes for Li-Ion Batteries. Mater. Sci. Eng. B 2016, 213, 98–104. [Google Scholar] [CrossRef]
- Bachtin, K.; Kramer, D.; Chakravadhanula, V.S.K.; Mu, X.; Trouillet, V.; Kaus, M.; Indris, S.; Ehrenberg, H.; Roth, C. Activation and Degradation of Electrospun LiFePO4 Battery Cathodes. J. Power Sources 2018, 396, 386–394. [Google Scholar] [CrossRef]
- Meligrana, G.; Ferrari, S.; Lucherini, L.; Celè, J.; Colò, F.; Brugger, J.; Ricciardi, C.; Ruffo, R.; Gerbaldi, C. Na3V2(PO4)3-Supported Electrospun Carbon Nanofiber Nonwoven Fabric as Self-Standing Na-Ion Cell Cathode. ChemElectroChem 2020, 7, 1652–1659. [Google Scholar] [CrossRef]
- Conti, D.M.; Fusaro, C.; Bruni, G.; Galinetto, P.; Albini, B.; Milanese, C.; Berbenni, V.; Capsoni, D. ZnS–rGO/CNF Free-Standing Anodes for SIBs: Improved Electrochemical Performance at High C-Rate. Nanomaterials 2023, 13, 1160. [Google Scholar] [CrossRef]
- Nishide, H.; Oyaizu, K. Toward Flexible Batteries. Science 2008, 319, 737–738. [Google Scholar] [CrossRef]
- Zeng, W.; Shu, L.; Li, Q.; Chen, S.; Wang, F.; Tao, X.-M. Fiber-Based Wearable Electronics: A Review of Materials, Fabrication, Devices, and Applications. Adv. Mater. 2014, 26, 5310–5336. [Google Scholar] [CrossRef]
- Liu, W.; Song, M.-S.; Kong, B.; Cui, Y. Flexible and Stretchable Energy Storage: Recent Advances and Future Perspectives. Adv. Mater. 2017, 29, 1603436. [Google Scholar] [CrossRef]
- Chang, J.; Huang, Q.; Gao, Y.; Zheng, Z. Pathways of Developing High-Energy-Density Flexible Lithium Batteries. Adv. Mater. 2021, 33, 2004419. [Google Scholar] [CrossRef]
- Zhang, F.; Qi, L. Recent Progress in Self-Supported Metal Oxide Nanoarray Electrodes for Advanced Lithium-Ion Batteries. Adv. Sci. 2016, 3, 1600049. [Google Scholar] [CrossRef]
- Lee, S.-H.; Jung, M.-J.; Im, J.S.; Sheem, K.-Y.; Lee, Y.-S. Preparation and Characterization of Electrospun LiFePO4/Carbon Complex Improving Rate Performance at High C-Rate. Res. Chem. Intermed. 2010, 36, 591–602. [Google Scholar] [CrossRef]
- Hu, Y.; Guo, P.; Thompson, J.; Wang, Z. Rational Design of Mesoporous LiFePO4@C Nanofibers as Cathode Materials for Energy Storage. Ceram. Int. 2017, 43, 10201–10206. [Google Scholar] [CrossRef]
- Zhang, C.; Yao, L.; Qiu, Y. Synthesis and Characterization of LiFePO4–Carbon Nanofiber–Carbon Nanotube Composites Prepared by Electrospinning and Thermal Treatment as a Cathode Material for Lithium-Ion Batteries. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Liu, J.; Hu, X.; Ran, F.; Wang, K.; Dai, J.; Zhu, X. Electrospinning-Assisted Construction of 3D LiFePO4@rGO/Carbon Nanofibers as Flexible Cathode to Boost the Rate Capabilities of Lithium-Ion Batteries. Ceram. Int. 2023, 49, 1401–1408. [Google Scholar] [CrossRef]
- Zhang, W.; He, X.; Pu, W.; Li, J.; Wan, C. Effect of Slurry Preparation and Dispersion on Electrochemical Performances of LiFePO4 Composite Electrode. Ionics 2011, 17, 473–477. [Google Scholar] [CrossRef]
- Bruker AXS TOPAS V3.0: General Profile and Structural Analysis Software for Powder Diffraction Data. In User Manual Bruker AXS; Bruker AXS: Karlsruhe, Germany, 2005.
- Bini, M.; Ferrari, S.; Capsoni, D.; Mustarelli, P.; Spina, G.; Giallo, F.D.; Lantieri, M.; Leonelli, C.; Rizzuti, A.; Massarotti, V. Pair Distribution Function Analysis and Mössbauer Study of Defects in Microwave-Hydrothermal LiFePO4. RSC Adv. 2011, 2, 250–258. [Google Scholar] [CrossRef]
- Zaghib, K.; Mauger, A.; Goodenough, J.B.; Gendron, F.; Julien, C.M. Design and Properties of LiFePO4 Positive Electrode Materials for Li-Ion Batteries. In Advanced Materials and Methodes for Lithium-Ion Batteries; Zhang, S.S., Ed.; Transworld Research Network: Trivandrum, India, 2007; ISBN 978-81-7895-279-6. [Google Scholar]
- Kumar, A.; Thomas, R.; Karan, N.K.; Saavedra-Arias, J.J.; Singh, M.K.; Majumder, S.B.; Tomar, M.S.; Katiyar, R.S. Structural and Electrochemical Characterization of Pure LiFePO4 and C-LiFePO4 Nanocomposite C- Cathodes for Lithium Ion Rechargeable Batteries. J. Nanotechnol. 2009, 2009, 176517. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman Spectra of Disordered and Amorphous Carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef]
- Belharouak, I.; Johnson, C.; Amine, K. Synthesis and Electrochemical Analysis of Vapor-Deposited Carbon-Coated LiFePO4. Electrochem. Commun. 2005, 7, 983–988. [Google Scholar] [CrossRef]
- Hamelet, S.; Gibot, P.; Casas-Cabanas, M.; Bonnin, D.; Grey, C.P.; Cabana, J.; Leriche, J.-B.; Rodriguez-Carvajal, J.; Courty, M.; Levasseur, S.; et al. The Effects of Moderate Thermal Treatments under Air on LiFePO4-Based Nano Powders. J. Mater. Chem. 2009, 19, 3979–3991. [Google Scholar] [CrossRef]
- Delaporte, N.; Trudeau, M.L.; Bélanger, D.; Zaghib, K. Protection of LiFePO4 against Moisture. Materials 2020, 13, 942. [Google Scholar] [CrossRef]
- Padhi, A.K.; Nanjundaswamy, K.S.; Goodenough, J.B. Phospho-olivines as Positive-Electrode Materials for Rechargeable Lithium Batteries. J. Electrochem. Soc. 1997, 144, 1188–1194. [Google Scholar] [CrossRef]
- Malik, R.; Abdellahi, A.; Ceder, G. A Critical Review of the Li Insertion Mechanisms in LiFePO4 Electrodes. J. Electrochem. Soc. 2013, 160, A3179. [Google Scholar] [CrossRef]
- Yu, D.Y.W.; Donoue, K.; Inoue, T.; Fujimoto, M.; Fujitani, S. Effect of Electrode Parameters on LiFePO4 Cathodes. J. Electrochem. Soc. 2006, 153, A835. [Google Scholar] [CrossRef]
- Hu, Y.; Gu, D.; Jiang, H.; Wang, L.; Sun, H.; Wang, J.; Shen, L. Electrochemical Performance of LiFePO4/C via Coaxial and Uniaxial Electrospinning Method. Adv. Chem. Eng. Sci. 2016, 6, 149–157. [Google Scholar] [CrossRef]
- Chen, Q.; Qiao, X.; Peng, C.; Zhang, T.; Wang, Y.; Wang, X. Electrochemical Performance of Electrospun LiFePO4/C Submicrofibers Composite Cathode Material for Lithium Ion Batteries. Electrochim. Acta 2012, 78, 40–48. [Google Scholar] [CrossRef]
- Vu, A.; Qian, Y.; Stein, A. Porous Electrode Materials for Lithium-Ion Batteries—How to Prepare Them and What Makes Them Special. Adv. Energy Mater. 2012, 2, 1056–1085. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, J.; Xue, J.; Zhang, K.; Wen, L.; Liang, G. Effect of Particle Dispersion on the Properties of LiFePO4 Slurry and the Electrochemical Properties of the Battery. Ionics 2022, 28, 1547–1558. [Google Scholar] [CrossRef]
- Dominko, R.; Bele, M.; Gaberscek, M.; Remskar, M.; Hanzel, D.; Pejovnik, S.; Jamnik, J. Impact of the Carbon Coating Thickness on the Electrochemical Performance of LiFePO4/C Composites. J. Electrochem. Soc. 2005, 152, A607. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conti, D.M.; Urru, C.; Bruni, G.; Galinetto, P.; Albini, B.; Berbenni, V.; Capsoni, D. High C-Rate Performant Electrospun LiFePO4/Carbon Nanofiber Self-Standing Cathodes for Lithium-Ion Batteries. Electrochem 2024, 5, 223-242. https://doi.org/10.3390/electrochem5020014
Conti DM, Urru C, Bruni G, Galinetto P, Albini B, Berbenni V, Capsoni D. High C-Rate Performant Electrospun LiFePO4/Carbon Nanofiber Self-Standing Cathodes for Lithium-Ion Batteries. Electrochem. 2024; 5(2):223-242. https://doi.org/10.3390/electrochem5020014
Chicago/Turabian StyleConti, Debora Maria, Claudia Urru, Giovanna Bruni, Pietro Galinetto, Benedetta Albini, Vittorio Berbenni, and Doretta Capsoni. 2024. "High C-Rate Performant Electrospun LiFePO4/Carbon Nanofiber Self-Standing Cathodes for Lithium-Ion Batteries" Electrochem 5, no. 2: 223-242. https://doi.org/10.3390/electrochem5020014
APA StyleConti, D. M., Urru, C., Bruni, G., Galinetto, P., Albini, B., Berbenni, V., & Capsoni, D. (2024). High C-Rate Performant Electrospun LiFePO4/Carbon Nanofiber Self-Standing Cathodes for Lithium-Ion Batteries. Electrochem, 5(2), 223-242. https://doi.org/10.3390/electrochem5020014







