Glucagon-like Peptide-1 Receptor Agonists: Are They as Good as They Seem? A Systematic Review of Severe Adverse Effects
Abstract
1. Background
2. Materials and Methods
2.1. Study Design
2.2. Search Strategy
2.3. Eligibility Criteria
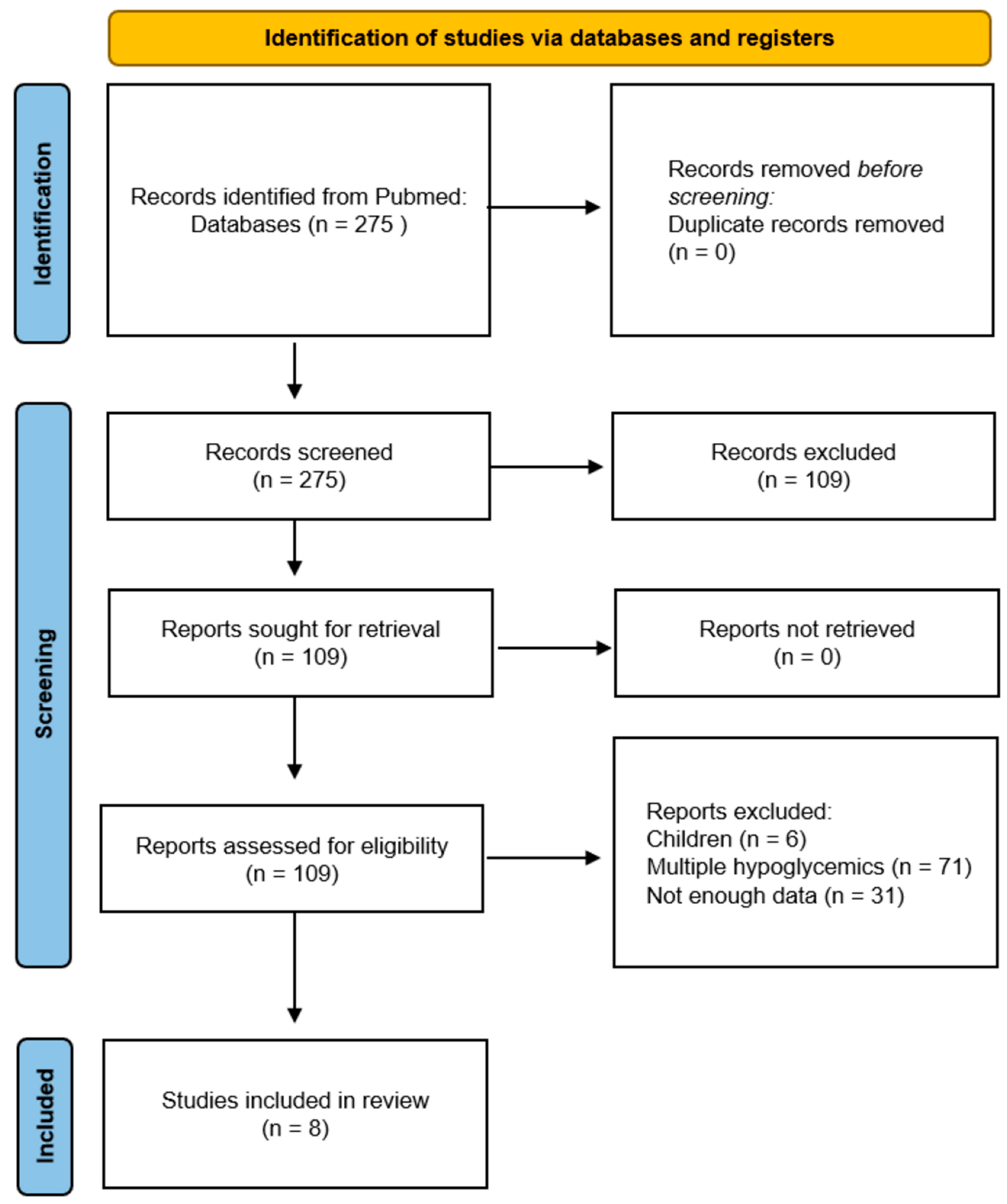
2.4. Study Selection
2.5. Data Extraction
2.6. Statistical Analysis
3. Results
3.1. Outcomes
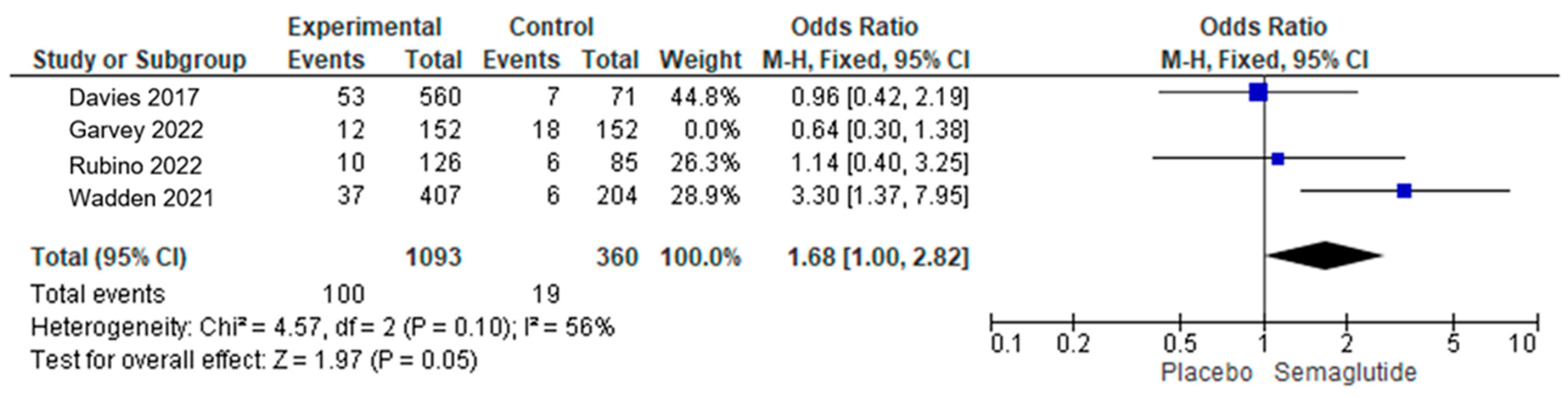
3.2. Analysis of SAEs of Semaglutide
3.3. Analysis of SAEs of Liraglutide
3.4. Specific Severe Adverse Effects
| Study, Year | Drug | Duration of Study (Days) | Age GLP-1 RA | Age Placebo | BMI GLP-1 RA | BMI Placebo |
|---|---|---|---|---|---|---|
| Wadden et al., 2021 [16] | Semaglutide | 78 | 46 | 46 | 38.1 | 37.8 |
| Rubino et al., 2022 [13] | Semaglutide | 476 | 48 | 51 | 37 | 38.8 |
| Garvey et al., 2022 [11] | Semaglutide | 728 | 47.3 | 37.4 | 38.6 | 38.5 |
| Davies et al., 2017 [14] | Semaglutide | 217 | 40 | 32.6 | ||
| Larsen et al., 2017 [17] | Liraglutide | 112 | 42.1 | 43 | 33.7 | 33.9 |
| Rubino et al., 2022 [13] | Liraglutide | 476 | 49 | N/A | 37.2 | N/A |
| Astrup et al., 2012 [15] | Liraglutide | 365 (of 730) | 45.9 | 34.9 | ||
| Pi-Sunyer et al., 2015 [12] | Liraglutide | 392 | 45.2 | 45 | 38.3 | 38.3 |
| Study, Year | Drug | SAEs GLP | SAEs Placebo | No Withdrawn: GLP | No Withdrawn: Placebo |
|---|---|---|---|---|---|
| Wadden et al., 2021 [16] | Semaglutide | 23 | 0 | 24 | 6 |
| Rubino et al., 2022 [13] | Semaglutide | 10 | 6 | 4 | 3 |
| Garvey et al., 2022 [11] | Semaglutide | 12 | 18 | 10 | 7 |
| Davies et al., 2017 [14] | Semaglutide | 53 | 7 | 91 | 0 |
| Larsen et al., 2017 [17] | Liraglutide | 6 | 13 | 3 | 0 |
| Rubino et al., 2022 [13] | Liraglutide | 14 | 6 | 6 | 0 |
| Astrup et al., 2012 [15] | Liraglutide | 20 | 3 | 5 | 3 |
| Pi-Sunyer et al., 2015 [12] | Liraglutide | 154 | 62 | 246 | 47 |
| Study, Year | Drug | Incidence of Cancer: GLP1-RA N (%) | Types of Cancer | Incidence of Cancer: Placebo N (%) | Types of Cancer |
|---|---|---|---|---|---|
| Wadden et al., 2021 [16] | Semaglutide | 3 (0.7) | Basal cell carcinoma, breast cancer, and papillary thyroid cancer | 1 (0.5) | Invasive lobular breast carcinoma |
| Rubino et al., 2022 [13] | Semaglutide | 3 (2.4) | Basal cell carcinoma, clear cell renal cell carcinoma, and invasive ductal breast carcinoma | 1 (1.2) | Invasive ductal breast carcinoma |
| Garvey et al., 2022 [11] | Semaglutide | 2 (1.3) | Basal cell carcinoma and Bowen’s disease | 4 (2.6) | 2 X Invasive ductal breast carcinoma, lung adenocarcinoma, and small cell lung cancer |
| Davies et al., 2022 [14] | Semaglutide | 3 | Basal cell carcinoma, GI tract adenoma [benign], and keratoacanthoma [benign]) | 0 | N/A |
| Larsen et al., 2017 [17] | Liraglutide | N/A | N/A | N/A | N/A |
| Rubino et al., 2022 [13] | Liraglutide | 3 (2.4) | Basal cell carcinoma, invasive ductal breast carcinoma, and invasive lobular breast carcinoma | 1 (1.2) | Invasive lobular breast carcinoma |
| Astrup et al., 2012 [15] | Liraglutide | 4 (all noted in the second year of study) | Breast cancer, intestinal adenocarcinoma, uterine leiomyoma, and prostate cancer | 0 | N/A |
| Pi-Sunyer et al., 2015 [12] | Liraglutide | 9 (1 patient had malignant neoplasm detected X 2) | Breast cancer: 3 pre-malignant and 6 malignant | 3 | Breast cancer: 1 pre-malignant and 2 malignant |
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AE | Adverse effect; |
| AKI | Acute kidney injury; |
| ASVD | Atherosclerotic cardiovascular disease; |
| BMI | Basic metabolic index; |
| DM | Diabetes mellitus; |
| GI | Gastrointestinal; |
| GLP-1 RA | Glucagon-like peptide-1 receptor agonist; |
| MEN | Multiple endocrine neoplasia; |
| N | No. of subjects or incidents; |
| N/A | Not applicable; |
| SAE | Serious adverse effect or event. |
References
- Davies, M.J.; Aroda, V.R.; Collins, B.S.; Gabbay, R.A.; Green, J.; Maruthur, N.M.; Rosas, S.E.; Del Prato, S.; Mathieu, C.; Mingrone, G.; et al. Management of hyperglycaemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2022, 65, 1925–1966. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Smits, M.M.; Van Raalte, D.H. Safety of Semaglutide. Front. Endocrinol. 2021, 12, 645563. [Google Scholar] [CrossRef] [PubMed]
- Nauck, M.A.; Petrie, J.R.; Sesti, G.; Mannucci, E.; Courrèges, J.-P.; Lindegaard, M.L.; Jensen, C.B.; Atkin, S.L.; Study 1821 Investigators. A Phase 2, Randomized, Dose-Finding Study of the Novel Once-Weekly Human GLP-1 Analog, Semaglutide, Compared with Placebo and Open-Label Liraglutide in Patients with Type 2 Diabetes. Diabetes Care 2016, 39, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Mehta Ambalal, S.; Monga, V.; Kashyap, R. Exploring the Dorian Gray Trait: Unveiling the Complexities of Perceived Aging and Self-Image. Cureus 2023, 15, e47326. [Google Scholar] [CrossRef] [PubMed]
- Perkovic, V.; Tuttle, K.R.; Rossing, P.; Mahaffey, K.W.; Mann, J.F.E.; Bakris, G.; Baeres, F.M.M.; Idorn, T.; Bosch-Traberg, H.; Lausvig, N.L.; et al. Effects of Semaglutide on Chronic Kidney Disease in Patients with Type 2 Diabetes. N. Engl. J. Med. 2024, 391, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Patti, A.M.; Nikolic, D.; Magan-Fernandez, A.; Giglio, R.V.; Castellino, G.; Chianetta, R.; Citarrella, R.; Corrado, E.; Provenzano, F.; Provenzano, V.; et al. Exenatide once-weekly improves metabolic parameters, endothelial dysfunction and carotid intima-media thickness in patients with type-2 diabetes: An 8-month prospective study. Diabetes Res. Clin. Pract. 2019, 149, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Patti, A.M.; Rizvi, A.A.; Giglio, R.V.; Stoian, A.P.; Ligi, D.; Mannello, F. Impact of Glucose-Lowering Medications on Cardiovascular and Metabolic Risk in Type 2 Diabetes. J. Clin. Med. 2020, 9, 912. [Google Scholar] [CrossRef]
- Giglio, R.V.; Nikolic, D.; Volti, G.L.; Stoian, A.P.; Banerjee, Y.; Magan-Fernandez, A.; Castellino, G.; Patti, A.M.; Chianetta, R.; Castracani, C.C.; et al. Liraglutide Increases Serum Levels of MicroRNA-27b, -130a and -210 in Patients with Type 2 Diabetes Mellitus: A Novel Epigenetic Effect. Metabolites 2020, 10, 391. [Google Scholar] [CrossRef]
- Available online: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=488579 (accessed on 31 March 2024).
- Pranjal, S.; Venkata, B.; Gagandeep, D.; Kishun, V.R.; Ramprakash, D.; Ripudaman, M.; Harpreet, G.; Rahul, K. Glucagon Like Peptide-1 Receptor Agonists: Is It As Good as It Seems? A Systematic Review of Severe Adverse Effects. Available online: https://ssrn.com/abstract=4780856 (accessed on 2 April 2024). [CrossRef]
- Garvey, W.T.; Batterham, R.L.; Bhatta, M.; Buscemi, S.; Christensen, L.N.; Frias, J.P.; Jódar, E.; Kandler, K.; Rigas, G.; Wadden, T.A.; et al. STEP 5 Study Group. Two-year effects of semaglutide in adults with overweight or obesity: The STEP 5 trial. Nat. Med. 2022, 28, 2083–2091. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pi-Sunyer, X.; Astrup, A.; Fujioka, K.; Greenway, F.; Halpern, A.; Krempf, M.; Lau, D.C.; le Roux, C.W.; Violante Ortiz, R.; Jensen, C.B.; et al. A Randomized, Controlled Trial of 3.0 mg of Liraglutide in Weight Management. N. Engl. J. Med. 2015, 373, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Rubino, D.M.; Greenway, F.L.; Khalid, U.; O′Neil, P.M.; Rosenstock, J.; Sørrig, R.; Wadden, T.A.; Wizert, A.; Garvey, W.T.; STEP 8 Investigators. Effect of Weekly Subcutaneous Semaglutide vs Daily Liraglutide on Body Weight in Adults with Overweight or Obesity without Diabetes: The STEP 8 Randomized Clinical Trial. JAMA 2022, 327, 138–150. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Davies, M.; Pieber, T.R.; Hartoft-Nielsen, M.L.; Hansen, O.K.H.; Jabbour, S.; Rosenstock, J. Effect of Oral Semaglutide Compared with Placebo and Subcutaneous Semaglutide on Glycemic Control in Patients with Type 2 Diabetes: A Randomized Clinical Trial. JAMA 2017, 318, 1460–1470. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Astrup, A.; Carraro, R.; Finer, N.; Harper, A.; Kunesova, M.; Lean, M.E.; Niskanen, L.; Rasmussen, M.F.; Rissanen, A.; Rössner, S.; et al. Safety, tolerability and sustained weight loss over 2 years with the once-daily human GLP-1 analog, liraglutide. Int. J. Obes. 2012, 36, 843–854. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wadden, T.A.; Bailey, T.S.; Billings, L.K.; Davies, M.; Frias, J.P.; Koroleva, A.; Lingvay, I.; O’Neil, P.M.; Rubino, D.M.; Skovgaard, D.; et al. STEP 3 Investigators. Effect of Subcutaneous Semaglutide vs Placebo as an Adjunct to Intensive Behavioral Therapy on Body Weight in Adults With Overweight or Obesity: The STEP 3 Randomized Clinical Trial. JAMA 2021, 325, 1403–1413. [Google Scholar] [CrossRef] [PubMed]
- Larsen, J.R.; Vedtofte, L.; Jakobsen, M.S.L.; Jespersen, H.R.; Jakobsen, M.I.; Svensson, C.K.; Koyuncu, K.; Schjerning, O.; Oturai, P.S.; Kjaer, A.; et al. Effect of Liraglutide Treatment on Prediabetes and Overweight or Obesity in Clozapine- or Olanzapine-Treated Patients With Schizophrenia Spectrum Disorder: A Randomized Clinical Trial. JAMA Psychiatry 2017, 74, 719–728. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lisco, G.; De Tullio, A.; Disoteo, O.; Piazzolla, G.; Guastamacchia, E.; Sabbà, C.; De Geronimo, V.; Papini, E.; Triggiani, V. Glucagon-like peptide 1 receptor agonists and thyroid cancer: Is it the time to be concerned? Endocr. Connect. 2023, 12, e230257. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lean, M.E.; Carraro, R.; Finer, N.; Hartvig, H.; Lindegaard, M.L.; Rössner, S.; Van Gaal, L.; Astrup, A.; NN8022-1807 Investigators. Tolerability of nausea and vomiting and associations with weight loss in a randomized trial of liraglutide in obese, non-diabetic adults. Int. J. Obes. 2014, 38, 689–697. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nikolaus, M.; Husain, M.; Lehrke, M.; Verma, S.; Sattar, N. GLP-1 receptor agonists for the reduction of atherosclerotic cardiovascular risk in patients with type 2 diabetes. Circulation 2022, 146, 1882–1894. [Google Scholar]
- Yumuk, V.; Tsigos, C.; Fried, M.; Schindler, K.; Busetto, L.; Micic, D.; Toplak, H. European Guidelines for Obesity Management in Adults. Obes. Facts 2015, 8, 402–424. [Google Scholar] [CrossRef]
- Wharton, S.; Calanna, S.; Davies, M.; Dicker, D.; Goldman, B.; Lingvay, I.; Mosenzon, O.; Rubino, D.M.; Thomsen, M.; Wadden, T.A.; et al. Gastrointestinal tolerability of once-weekly semaglutide 2.4 mg in adults with overweight or obesity, and the relationship between gastrointestinal adverse events and weight loss. Diabetes Obes. Metab. 2022, 24, 94–105. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- He, L.; Wang, J.; Ping, F.; Yang, N.; Huang, J.; Li, Y.; Xu, L.; Li, W.; Zhang, H. Association of glucagon-like peptide-1 receptor agonist use with risk of gallbladder and biliary diseases: A systematic review and meta-analysis of randomized clinical trials. JAMA Intern. Med. 2022, 182, e220338. [Google Scholar] [CrossRef] [PubMed]
- Elashoff, M.; Matveyenko, A.V.; Gier, B.; Elashoff, R.; Butler, P.C. Pancreatitis, pancreatic, and thyroid cancer with glucagon-like peptide-1-based therapies. Gastroenterology 2011, 141, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Wilding, J.P.H.; Batterham, R.L.; Calanna, S.; Davies, M.; Van Gaal, L.F.; Lingvay, I.; McGowan, B.M.; Rosenstock, J.; Tran, M.T.D.; Wadden, T.A.; et al. Once-Weekly Semaglutide in Adults with Overweight or Obesity. N. Engl. J. Med. 2021, 384, 989–1002. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.; Færch, L.; Jeppesen, O.K.; Pakseresht, A.; Pedersen, S.D.; Perreault, L.; Rosenstock, J.; Shimomura, I.; Viljoen, A.; Wadden, T.A.; et al. STEP 2 Study Group. Semaglutide 2·4 mg once a week in adults with overweight or obesity, and type 2 diabetes (STEP 2): A randomised, double-blind, double-dummy, placebo-controlled, phase 3 trial. Lancet 2021, 397, 971–984. [Google Scholar] [CrossRef] [PubMed]
- Filippatos, T.D.; Panagiotopoulou, T.V.; Elisaf, M.S. Adverse Effects of GLP-1 Receptor Agonists. Rev. Diabet. Stud. 2014, 11, 202–230. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tobaiqy, M.; Elkout, H. Psychiatric adverse events associated with semaglutide, liraglutide and tirzepatide: A pharmacovigilance analysis of individual case safety reports submitted to the EudraVigilance database. Int. J. Clin. Pharm. 2024, 46, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Kohli, A.; Trivedi, S.; Kanagala, S.G.; Anamika, F.N.U.; Garg, N.; Patel, M.A.; Munjal, R.S.; Jain, R. The contentious relationship between artificial sweeteners and cardiovascular health. Egypt J. Intern. Med. 2023, 35, 43. [Google Scholar] [CrossRef]
- Jangid, G.; Popoola-Samuel, H.A.O.; Goda, K.; Anamika, F.N.U.; Gupta, V.; Kanagala, S.G.; Munjal, R.S. Influence of Plant-Based Diet on the Cardiovascular System: A Narrative Review. Cardiol. Rev. 2023. [Google Scholar] [CrossRef] [PubMed]
- Darapaneni, H.; Lakhanpal, S.; Chhayani, H.; Parikh, K.; Patel, M.; Gupta, V.; Anamika, F.; Munjal, R.; Jain, R. Shedding light on weight loss: A narrative review of medications for treating obesity. Rom. J. Intern. Med. 2023, 62, 3–11. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, P.; Buddhavarapu, V.; Dhillon, G.; Verma, R.K.; Devadoss, R.; Raynor, J.; Munjal, R.; Grewal, H.; Kashyap, R. Glucagon-like Peptide-1 Receptor Agonists: Are They as Good as They Seem? A Systematic Review of Severe Adverse Effects. Endocrines 2024, 5, 323-333. https://doi.org/10.3390/endocrines5030023
Sharma P, Buddhavarapu V, Dhillon G, Verma RK, Devadoss R, Raynor J, Munjal R, Grewal H, Kashyap R. Glucagon-like Peptide-1 Receptor Agonists: Are They as Good as They Seem? A Systematic Review of Severe Adverse Effects. Endocrines. 2024; 5(3):323-333. https://doi.org/10.3390/endocrines5030023
Chicago/Turabian StyleSharma, Pranjal, Venkata Buddhavarapu, Gagandeep Dhillon, Ram Kishun Verma, Ramprakash Devadoss, James Raynor, Ripudaman Munjal, Harpreet Grewal, and Rahul Kashyap. 2024. "Glucagon-like Peptide-1 Receptor Agonists: Are They as Good as They Seem? A Systematic Review of Severe Adverse Effects" Endocrines 5, no. 3: 323-333. https://doi.org/10.3390/endocrines5030023
APA StyleSharma, P., Buddhavarapu, V., Dhillon, G., Verma, R. K., Devadoss, R., Raynor, J., Munjal, R., Grewal, H., & Kashyap, R. (2024). Glucagon-like Peptide-1 Receptor Agonists: Are They as Good as They Seem? A Systematic Review of Severe Adverse Effects. Endocrines, 5(3), 323-333. https://doi.org/10.3390/endocrines5030023












