A Review of Catalyst Integration in Hydrothermal Gasification
Abstract
1. Introduction
2. Role of Temperature and Catalysts in Hydrothermal Conversion Systems
2.1. Supercritical High Temperature—Regime I
2.2. Supercritical Intermediate Temperature—Regime II
2.3. Subcritical below 374 °C of Water—Regime III
3. Catalysts in Hydrothermal Gasification Systems
3.1. Homogeneous Catalysts
3.2. Heterogeneous Catalysts
3.3. Transitional Metal Catalysis
4. Effect of Catalysts on Reactor and the Gasification Reactions
5. Types of Reactors and Reactor Configurations Used for Catalytic Gasification
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| HTG | Hydrothermal gasification |
| HTL | Hydrothermal liquefaction |
| SCWG | Supercritical water gasification |
| Syngas | Synthesis gas |
| H2 | Hydrogen |
| CH4 | Methane |
| CO | Carbon monoxide |
| CO2 | Carbon dioxide |
| Pt | Platinum |
| AC | Activated carbon |
| Ni | Nickel |
| Ru | Ruthenium |
| ZrO2 | Zirconium oxide |
| Co | Cobalt |
| Ti | Titanium |
| Ir | Iridium |
| Zi | Zirconium |
| NaOH | Sodium hydroxide |
| Na2CO3 | Sodium carbonate |
| Ca(OH)2 | Calcium hydroxide |
| KOH | Potassium hydroxide |
| K2CO3 | Potassium carbonate |
| H2O2 | Hydrogen peroxide |
| γ-Al2O3 | Gamma alumina |
| OD | Outer diameter |
| ID | Inner diameter |
| CSTR | Continuous stirred tank reactor |
| HPLC | High-performance liquid chromatography |
| CMC | Sodium carboxymethyl cellulose |
| S/V | Surface area-to-volume ratios |
| LCA | Life cycle assessment |
| TEA | Technoeconomic analysis |
References
- Kusenberg, M.; Eschenbacher, A.; Djokic, M.R.; Zayoud, A.; Ragaert, K.; De Meester, S.; Van Geem, K.M. Opportunities and challenges for the application of post-consumer plastic waste pyrolysis oils as steam cracker feedstocks: To decontaminate or not to decontaminate? Waste Manag. 2022, 138, 83–115. [Google Scholar] [CrossRef]
- Alper, K.; Tekin, K.; Karagöz, S.; Ragauskas, A.J. Sustainable energy and fuels from biomass: A review focusing on hydrothermal biomass processing. Sustain. Energy Fuels 2020, 4, 4390–4414. [Google Scholar] [CrossRef]
- Habib, M.A.; Haque, M.A.; Harale, A.; Paglieri, S.; Alrashed, F.S.; Al-Sayoud, A.; Ben-Mansour, R. Palladium-alloy membrane reactors for fuel reforming and hydrogen production: Hydrogen Production Modeling. Case Stud. Therm. Eng. 2023, 49, 103359. [Google Scholar] [CrossRef]
- Li, C.; Chen, C.; Wu, X.; Tsang, C.W.; Mou, J.; Yan, J.; Liu, Y.; Lin, C.S.K. Recent advancement in lignin biorefinery: With special focuson enzymatic degradation and valorization. Bioresour. Technol. 2019, 291, 121898. [Google Scholar] [CrossRef] [PubMed]
- Aziz, M.; Darmawan, A.; Juangsa, F.B. Hydrogen production from biomasses and wastes: A technological review. Int. J. Hydrogen Energy 2021, 46, 33756–33781. [Google Scholar] [CrossRef]
- Pal, D.B.; Singh, A.; Bhatnagar, A. A review on biomass based hydrogen production technologies. Int. J. Hydrogen Energy 2022, 47, 1461–1480. [Google Scholar] [CrossRef]
- Okolie, J.A.; Nanda, S.; Dalai, A.K.; Berruti, F.; Kozinski, J.A. A review on subcritical and supercritical water gasification of biogenic, polymeric and petroleum wastes to hydrogen-rich synthesis gas. Renew. Sustain. Energy Rev. 2020, 119, 109546. [Google Scholar] [CrossRef]
- Ibrahim, A.B.A.; Akilli, H. Supercritical water gasification of wastewater sludge for hydrogen production. Int. J. Hydrogen Energy 2019, 44, 10328–10349. [Google Scholar] [CrossRef]
- Bröll, D.; Kaul, C.; Krämer, A.; Krammer, P.; Richter, T.; Jung, M.; Vogel, H.; Zehner, P. Chemistry in supercritical water. Angew. Chem. Int. Ed 1999, 38, 2998–3014. [Google Scholar]
- Chen, J.; Wang, Q.; Xu, Z.; Leng, J.E.; Zhang, E.F.; Liao, G. Process in supercritical water gasification of coal: A review of fundamentals, mechanisms, catalysts and element transformation. Energy Convers. Manag. 2021, 237, 10–1016. [Google Scholar] [CrossRef]
- Sun, J.; Xu, L.; Dong, G.H.; Nanda, S.; Li, H.; Fang, Z.; Kozinski, J.A.; Dalai, A.K. Subcritical water gasification of lignocellulosic wastes for hydrogen production with Co modified Ni/Al2O3 catalysts. J. Supercrit. Fluids 2020, 162, 104863. [Google Scholar] [CrossRef]
- Su, H.; Hantoko, D.; Yan, M.; Cai, Y.; Kanchanatip, E.; Liu, J.; Zhou, X.; Zhang, S. Evaluation of catalytic subcritical water gasification of food waste for hydrogen production: Effect of process conditions and different types of catalyst loading. Int. J. Hydrogen Energy 2019, 44, 21451–21463. [Google Scholar] [CrossRef]
- Song, Y.Q.; Nanda, S.; Cong, W.J.; Sun, J.; Dong, G.H.; Magdziarz, A.; Fang, Z.; Dalai, A.K.; Kozinski, J.A. Hydrogen production from cotton stalk over Ni-La catalysts supported on spent bleaching clay via hydrothermal gasification. Ind. Crop. Prod. 2022, 186, 115228. [Google Scholar] [CrossRef]
- Xu, X.; Matsumura, Y.; Stenberg, J.; Antal, M.J. Carbon-catalyzed gasification of organic feedstocks in supercritical water. Ind. Eng. Chem. Res 1996, 35, 2522–2530. [Google Scholar] [CrossRef]
- Antal, M.J., Jr.; Allen, S.G.; Schulman, D.; Xu, X.; Divilio, R.J. Biomass gasification in supercritical water. Ind. Eng. Chem. Res. 2000, 39, 4040–4053. [Google Scholar] [CrossRef]
- Kruse, A.; Meier, D.; Rimbrecht, P.; Schacht, M. Gasification of pyrocatechol in supercritical water in the presence of potassium hydroxide. Ind. Eng. Chem. Res. 2000, 39, 4842–4848. [Google Scholar] [CrossRef]
- Schmieder, H.; Abeln, J.; Boukis, N.; Dinjus, E.; Kruse, A.; Kluth, M.; Petrich, G.; Sadri, E.; Schacht, M. Hydrothermal gasification of biomass and organic wastes. J. Supercrit. Fluids 2000, 17, 145–153. [Google Scholar] [CrossRef]
- Sztancs, G.; Juhasz, L.; Nagy, B.J.; Nemeth, A.; Selim, A.; Andre, A.; Toth, A.J.; Mizsey, P.; Fozer, D. Co-Hydrothermal gasification of Chlorella vulgaris and hydrochar: The effects of waste-to-solid biofuel production and blending concentration on biogas generation. Bioresour. Technol. 2020, 302, 122793. [Google Scholar] [CrossRef]
- Nanda, S.; Reddy, S.N.; Hunter, H.N.; Vo, D.V.N.; Kozinski, J.A.; Gökalp, I. Catalytic subcritical and supercritical water gasification as a resource recovery approach from waste tires for hydrogen-rich syngas production. J. Supercrit. Fluids 2019, 154, 104627. [Google Scholar] [CrossRef]
- Elliott, D.C. Catalytic hydrothermal gasification of biomass. Biorefining 2008, 2, 254–265. [Google Scholar] [CrossRef]
- Jin, H.; Zhao, X.; Su, X.; Zhu, C.; Cao, C.; Guo, L. Supercritical water synthesis of bimetallic catalyst and its application in hydrogen production by furfural gasification in supercritical water. Int. J. Hydrogen Energy 2017, 42, 4943–4950. [Google Scholar] [CrossRef]
- Lu, Y.; Li, S.; Guo, L.; Zhang, X. Hydrogen production by biomass gasification in supercritical water over Ni/γAl2O3 and Ni/CeO2-γAl2O3 catalysts. Int. J. Hydrogen Energy 2010, 35, 7161–7168. [Google Scholar] [CrossRef]
- Onwudili, J.A.; Lea-Langton, A.R.; Ross, A.B.; Williams, P.T. Catalytic hydrothermal gasification of algae for hydrogen production: Composition of reaction products and potential for nutrient recycling. Bioresour. Technol. 2013, 127, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Dreher, M.; De Boni, E.; Nachtegaal, M.; Wambach, J.; Vogel, F. Design of a continuous-flow reactor for in situ x-ray absorption spectroscopy of solids in supercritical fluids. Rev. Sci. Instruments 2012, 83, 054101. [Google Scholar] [CrossRef] [PubMed]
- Adar, E.; Ince, M.; Bilgili, M.S. Supercritical water gasification of sewage sludge by continuous flow tubular reactor: A pilot scale study. J. Chem. Eng. 2020, 391, 123499. [Google Scholar] [CrossRef]
- Chen, Y.; Yi, L.; Yin, J.; Jin, H.; Guo, L. Sewage sludge gasification in supercritical water with fluidized bed reactor: Reaction and product characteristics. Energy 2022, 239, 122115. [Google Scholar] [CrossRef]
- Osada, M.; Sato, T.; Watanabe, M.; Adschiri, T.; Arai, K. Low-temperature catalytic gasification of lignin and cellulose with a ruthenium catalyst in supercritical water. Energy Fuels 2004, 18, 327–333. [Google Scholar] [CrossRef]
- Sasaki, M.; Adschiri, T.; Arai, K. Kinetics of cellulose conversion at 25 MPa in sub-and supercritical water. AIChE J. 2004, 50, 192–202. [Google Scholar] [CrossRef]
- Elliott, D.C.; Hart, T.R.; Neuenschwander, G.G.; Rotness, L.J.; Roesijadi, G.; Zacher, A.H.; Magnuson, J.K. Hydrothermal processing of macroalgal feedstocks in continuous-flow reactors. ACS Sustain. Chem. Eng. 2014, 2, 207–215. [Google Scholar] [CrossRef]
- Elliott, D.C.; Sealock, L.J., Jr.; Baker, E.G. Chemical processing in high-pressure aqueous environments. 2. Development of catalysts for gasification. Ind. Eng. Chem. Res. 1993, 32, 1542–1548. [Google Scholar] [CrossRef]
- Elliott, D.C.; Phelps, M.R.; Sealock, L.J., Jr.; Baker, E.G. Chemical processing in high-pressure aqueous environments. 4. Continuous-flow reactor process development experiments for organics destruction. Ind. Eng. Chem. Res. 1994, 33, 566–574. [Google Scholar] [CrossRef]
- Elliott, D.C.; Neuenschwander, G.G.; Hart, T.R.; Butner, R.S.; Zacher, A.H.; Engelhard, M.H.; McCready, D.E. Chemical processing in high-pressure aqueous environments. 7. Process development for catalytic gasification of wet biomass feedstocks. Ind. Eng. Chem. Res. 2004, 43, 1999–2004. [Google Scholar] [CrossRef]
- Elliott, D.C.; Biller, P.; Ross, A.B.; Schmidt, A.J.; Jones, S.B. Hydrothermal liquefaction of biomass: Developments from batch to continuous process. Bioresour. Technol. 2015, 178, 147–156. [Google Scholar] [CrossRef]
- Nanda, S.; Gong, M.; Hunter, H.N.; Dalai, A.K.; Gökalp, I.; Kozinski, J.A. An assessment of pinecone gasification in subcritical, near-critical and supercritical water. Fuel Process. Technol. 2017, 168, 84–96. [Google Scholar] [CrossRef]
- Seif, S.; Tavakoli, O.; Fatemi, S.; Bahmanyar, H. Subcritical water gasification of beet-based distillery wastewater for hydrogen production. J. Supercrit. Fluids 2015, 104, 212–220. [Google Scholar] [CrossRef]
- Minowa, T.; Zhen, F.; Ogi, T. Cellulose decomposition in hot-compressed water with alkali or nickel catalyst. J. Supercrit. Fluids 1998, 13, 253–259. [Google Scholar] [CrossRef]
- Minowa, T.; Ogi, T. Hydrogen production from cellulose using a reduced nickel catalyst. Catal. Today 1998, 45, 411–416. [Google Scholar] [CrossRef]
- Azadi, P.; Syed, K.M.; Farnood, R. Catalytic gasification of biomass model compound in near-critical water. Appl. Catal. A-Gen. 2009, 358, 65–72. [Google Scholar] [CrossRef]
- Azadi, P.; Farnood, R. Review of heterogeneous catalysts for sub-and supercritical water gasification of biomass and wastes. Int. J. Hydrogen Energy 2011, 36, 9529–9541. [Google Scholar] [CrossRef]
- Azadi, P.; Afif, E.; Foroughi, H.; Dai, T.; Azadi, F.; Farnood, R. Catalytic reforming of activated sludge model compounds in supercritical water using nickel and ruthenium catalysts. Appl. Catal. B-Environ. 2013, 134, 265–273. [Google Scholar] [CrossRef]
- Azadi, P.; Afif, E.; Azadi, F.; Farnood, R. Screening of nickel catalysts for selective hydrogen production using supercritical water gasification of glucose. Green Chem. 2012, 14, 1766–1777. [Google Scholar] [CrossRef]
- Muhlke, R.; Castaing, J.B.; Morel, M.; Donat, V. Hydrothermal Gasification-White Paper. 2023. Available online: https://inis.iaea.org/search/search.aspx?orig_q=RN:54034649 (accessed on 7 April 2024).
- Ghavami, N.; Özdenkçi, K.; Salierno, G.; Björklund-Sänkiaho, M.; De Blasio, C. Analysis of operational issues in hydrothermal liquefaction and supercritical water gasification processes: A review. Biomass Convers. Biorefinery 2021, 13, 12367–12394. [Google Scholar] [CrossRef]
- Kruse, A.; Bernolle, P.; Dahmen, N.; Dinjus, E.; Maniam, P. Hydrothermal gasification of biomass: Consecutive reactions to long-living intermediates. Energy Environ. Sci. 2010, 3, 136–143. [Google Scholar] [CrossRef]
- García-Jarana, M.B.; Portela, J.R.; Sánchez-Oneto, J.; Martinez de la Ossa, E.J.; Al-Duri, B. Analysis of the supercritical water gasification of cellulose in a continuous system using short residence times. Appl. Sci. 2020, 10, 5185. [Google Scholar] [CrossRef]
- Rönnlund, I.; Myréen, L.; Lundqvist, K.; Ahlbeck, J.; Westerlund, T. Waste to energy by industrially integrated supercritical water gasification–Effects of alkali salts in residual by-products from the pulp and paper industry. Energy 2011, 36, 2151–2163. [Google Scholar] [CrossRef]
- Feng, H.; Zhou, Z.; Hantoko, D.; Zhong, L.; Rahim, D.A.; Fang, W.; Yan, M. Effect of alkali additives on desulfurization of syngas during supercritical water gasification of sewage sludge. Waste Manag. 2021, 131, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Nanda, S.; Dalai, A.K.; Kozinski, J.A. Supercritical water gasification of timothy grass as an energy crop in the presence of alkali carbonate and hydroxide catalysts. Biomass Bioenergy 2016, 95, 378–387. [Google Scholar] [CrossRef]
- Sınaǧ, A.; Kruse, A.; Rathert, J. Influence of the heating rate and the type of catalyst on the formation of key intermediates and on the generation of gases during hydropyrolysis of glucose in supercritical water in a batch reactor. Ind. Eng. Chem. Res. 2004, 43, 502–508. [Google Scholar]
- Madenoğlu, T.G.; Sağlam, M.; Yüksel, M.; Ballice, L. Hydrothermal gasification of biomass model compounds (cellulose and lignin alkali) and model mixtures. J. Supercrit. Fluids 2016, 115, 79–85. [Google Scholar] [CrossRef]
- Kang, K.; Azargohar, R.; Dalai, A.K.; Wang, H. Hydrogen production from lignin, cellulose and waste biomass via supercritical water gasification: Catalyst activity and process optimization study. Energy Convers. Manag. 2016, 117, 528–537. [Google Scholar] [CrossRef]
- Su, W.; Zhao, M.; Xing, Y.; Ma, H.; Liu, P.; Li, X.; Zhang, H.; Wu, Y.; Xia, C. Supercritical water gasification of hyperaccumulators for hydrogen production and heavy metal immobilization with alkali metal catalysts. Environ. Res. 2022, 214, 114093. [Google Scholar] [CrossRef]
- Ge, Z.; Jin, H.; Guo, L. Hydrogen production by catalytic gasification of coal in supercritical water with alkaline catalysts: Explore the way to complete gasification of coal. Int. J. Hydrogen Energy 2014, 39, 19583–19592. [Google Scholar] [CrossRef]
- Yanik, J.; Ebale, S.; Kruse, A.; Saglam, M.; Yüksel, M. Biomass gasification in supercritical water: II. Effect of catalyst. Int. J. Hydrogen Energy 2008, 33, 4520–4526. [Google Scholar] [CrossRef]
- Ferreira-Pinto, L.; Feirhrmann, A.C.; Corazza, M.L.; Fernandes-Machado, N.R.C.; dos Reis Coimbra, J.S.; Saldaña, M.D.; Cardozo-Filho, L. Hydrogen production and TOC reduction from gasification of lactose by supercritical water. Int. J. Hydrogen Energy 2015, 40, 12162–12168. [Google Scholar] [CrossRef]
- Zhong, J.; Zhu, W.; Wang, C.; Mu, B.; Lin, N.; Chen, S.; Li, Z. Transformation mechanism of polycyclic aromatic hydrocarbons and hydrogen production during the gasification of coking sludge in supercritical water. Chemosphere 2022, 300, 134467. [Google Scholar] [CrossRef]
- Zaera, F. Designing sites in heterogeneous catalysis: Are we reaching selectivities competitive with those of homogeneous catalysts? Chem. Rev. 2022, 122, 8594–8757. [Google Scholar] [CrossRef] [PubMed]
- Fadhel, A.Z.; Pollet, P.; Liotta, C.L.; Eckert, C.A. Combining the benefits of homogeneous and heterogeneous catalysis with tunable solvents and nearcritical water. Molecules 2010, 15, 8400–8424. [Google Scholar] [CrossRef]
- Onwudili, J.A.; Williams, P.T. Role of sodium hydroxide in the production of hydrogen gas from the hydrothermal gasification of biomass. Int. J. Hydrogen Energy 2009, 34, 5645–5656. [Google Scholar] [CrossRef]
- Karakuş, Y.; Aynacı, F.; Kıpçak, E.; Akgün, M. Hydrogen production from 2-propanol over Pt/Al2O3 and Ru/Al2O3 catalysts in supercritical water. Int. J. Hydrogen Energy 2013, 38, 7298–7306. [Google Scholar] [CrossRef]
- Lu, Y.; Jin, H.; Zhang, R. Evaluation of stability and catalytic activity of Ni catalysts for hydrogen production by biomass gasification in supercritical water. Carbon Resour. Convers. 2019, 2, 95–101. [Google Scholar] [CrossRef]
- Hossain, M.Z.; Chowdhury, M.B.; Jhawar, A.K.; Charpentier, P.A. Supercritical water gasification of glucose using bimetallic aerogel Ru-Ni-Al2O3 catalyst for H2 production. Biomass Bioenergy 2017, 107, 39–51. [Google Scholar] [CrossRef]
- Chowdhury, M.B.; Hossain, M.Z.; Mazumder, J.; Jhawar, A.K.; Charpentier, P.A. La-based catalysts to enhance hydrogen production during supercritical water gasification of glucose. Fuel 2018, 217, 166–174. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, C.C.; Champagne, P. Activity and stability of a novel Ru modified Ni catalyst for hydrogen generation by supercritical water gasification of glucose. Fuel 2012, 96, 541–545. [Google Scholar] [CrossRef]
- Sato, T.; Osada, M.; Watanabe, M.; Shirai, M.; Arai, K. Gasification of alkylphenols with supported noble metal catalysts in supercritical water. Ind. Eng. Chem. Res. 2003, 42, 4277–4282. [Google Scholar] [CrossRef]
- Shen, L.; Xu, J.; Zhu, M.; Han, Y.F. Essential role of the support for nickel-based CO2 methanation catalysts. ACS Catal. 2020, 10, 14581–14591. [Google Scholar] [CrossRef]
- Furusawa, T.; Sato, T.; Saito, M.; Ishiyama, Y.; Sato, M.; Itoh, N.; Suzuki, N. The evaluation of the stability of Ni/MgO catalysts for the gasification of lignin in supercritical water. Appl. Catal. A-Gen. 2007, 327, 300–310. [Google Scholar] [CrossRef]
- Zhang, L.; Champagne, P.; Xu, C.C. Screening of supported transition metal catalysts for hydrogen production from glucose via catalytic supercritical water gasification. Int. J. Hydrogen Energy 2011, 36, 9591–9601. [Google Scholar] [CrossRef]
- Zhang, B.; Tang, X.; Li, Y.; Xu, Y.; Shen, W. Hydrogen production from steam reforming of ethanol and glycerol over ceria-supported metal catalysts. Int. J. Hydrogen Energy 2007, 32, 2367–2373. [Google Scholar] [CrossRef]
- Chakinala, A.G.; Brilman, D.W.; van Swaaij, W.P.; Kersten, S.R. Catalytic and non-catalytic supercritical water gasification of microalgae and glycerol. Ind. Eng. Chem. Res. 2010, 49, 1113–1122. [Google Scholar] [CrossRef]
- Byrd, A.J.; Kumar, S.; Kong, L.; Ramsurn, H.; Gupta, R.B. Hydrogen production from catalytic gasification of switchgrass biocrude in supercritical water. Int. J. Hydrogen Energy 2011, 36, 3426–3433. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, S.Z.; Xu, D.H.; Gong, Y.M.; Ma, H.H.; Tang, X.Y. Review of catalytic supercritical water gasification for hydrogen production from biomass. Renew. Sustain. Energy Rev. 2010, 14, 334–343. [Google Scholar] [CrossRef]
- De Vlieger, D.J.M.; Chakinala, A.G.; Lefferts, L.; Kersten, S.R.A.; Seshan, K.; Brilman, D.W.F. Hydrogen from ethylene glycol by supercritical water reforming using noble and base metal catalysts. Appl. Catal. B-Environ. 2010, 111, 536–544. [Google Scholar] [CrossRef]
- May, A.; Salvadó, J.; Torras, C.; Montané, D. Catalytic gasification of glycerol in supercritical water. Chem. Eng. 2010, 160, 751–759. [Google Scholar] [CrossRef]
- Tushar, M.S.; Dutta, A.; Xu, C.C. Catalytic supercritical gasification of biocrude from hydrothermal liquefaction of cattle manure. Appl. Catal. B-Environ. 2016, 189, 119–132. [Google Scholar] [CrossRef]
- Li, Y.; Guo, L.; Zhang, X.; Jin, H.; Lu, Y. Hydrogen production from coal gasification in supercritical water with a continuous flowing system. Int. J. Hydrogen Energy 2010, 35, 3036–3045. [Google Scholar] [CrossRef]
- Elliott, D.C.; Hart, T.R.; Schmidt, A.J.; Neuenschwander, G.G.; Rotness, L.J.; Olarte, M.V.; Zacher, A.H.; Albrecht, K.O.; Hallen, R.T.; Holladay, J.E. Process development for hydrothermal liquefaction of algae feedstocks in a continuous-flow reactor. Algal Res. 2013, 2, 445–454. [Google Scholar] [CrossRef]
- Lee, C.H.; Kim, S.; Yoon, H.J.; Yoon, C.W.; Lee, K.B. Water gas shift and sorption-enhanced water gas shift reactions using hydrothermally synthesized novel Cu–Mg–Al hydrotalcite-based catalysts for hydrogen production. Renew. Sustain. Energy Rev. 2021, 145, 111064. [Google Scholar] [CrossRef]
- Gradisher, L.; Dutcher, B.; Fan, M. Catalytic hydrogen production from fossil fuels via the water gas shift reaction. Appl. Energy 2015, 139, 335–349. [Google Scholar] [CrossRef]
- Sert, M.; Gökkaya, D.S.; Cengiz, N.; Ballice, L.; Yüksel, M.; Sağlam, M. Hydrogen production from olive-pomace by catalytic hydrothermal gasification. J. Taiwan Inst. Chem. Eng. 2018, 83, 90–98. [Google Scholar] [CrossRef]
- Hussin, F.; Hazani, N.N.; Khalil, M.; Aroua, M.K. Environmental life cycle assessment of biomass conversion using hydrothermal technology: A review. Fuel Process. Technol. 2023, 246, 107747. [Google Scholar] [CrossRef]
- Samanmulya, T.; Matsumura, Y. Effect of activated carbon catalytic on supercritical water gasification of glycine as a model compound of protein. J. Jpn. Inst. Energy 2013, 92, 894–899. [Google Scholar] [CrossRef]
- Lee, I.G.; Ihm, S.K. Catalytic gasification of glucose over Ni/activated charcoal in supercritical water. Ind. Eng. Chem. Res. 2009, 48, 1435–1442. [Google Scholar] [CrossRef]
- Yamamura, T.; Mori, T.; Park, K.C.; Fujii, Y.; Tomiyasu, H. Ruthenium (IV) dioxide-catalyzed reductive gasification of intractable biomass including cellulose, heterocyclic compounds, and sludge in supercritical water. J. Supercrit. Fluids 2009, 51, 43–49. [Google Scholar] [CrossRef]
- Osada, M.; Yamaguchi, A.; Hiyoshi, N.; Sato, O.; Shirai, M. Gasification of sugarcane bagasse over supported ruthenium catalysts in supercritical water. Energy Fuel 2012, 26, 3179–3186. [Google Scholar] [CrossRef]
- Akizuki, M.; Tomita, K.; Oshima, Y. Kinetics of solid acid catalyzed 1-octene reactions with TiO2 in sub-and supercritical water. J. Supercrit. Fluids 2011, 56, 14–20. [Google Scholar] [CrossRef]
- Matsumura, Y.; Yamamura, Y.S.; Nakamura, Y.T.; Kiyonaga, H.; Minowa, T.; Noda, Y. Biomass Yamamura Method and Biomass Gasification System. Japan Patent No. JP2009149773 A, JP5463524 B2, 2007. [Google Scholar]
- Ryu, S.H.C.H.; Noh, M.K.; Lee, J.H.; Ji, J.W. Apparatus for Manufacturing Biofuel Using Supercritical Water Gasification. Korea Patent No. KR101416755 B1, 2014. [Google Scholar]
- Dooher, J.P.; Castaldi, M.J.; Modroukas, D.P. Advanced concepts in modular coal and biomass gasifiers. J. Energy Resour. 2019, 141, 012001. [Google Scholar] [CrossRef]
- Casademont, P.; García-Jarana, M.B.; Sánchez-Oneto, J.; Portela, J.R.; Martinez de la Ossa, E.J. Supercritical water gasification: A patents review. Rev. Chem. Eng. 2017, 33, 237–261. [Google Scholar] [CrossRef]
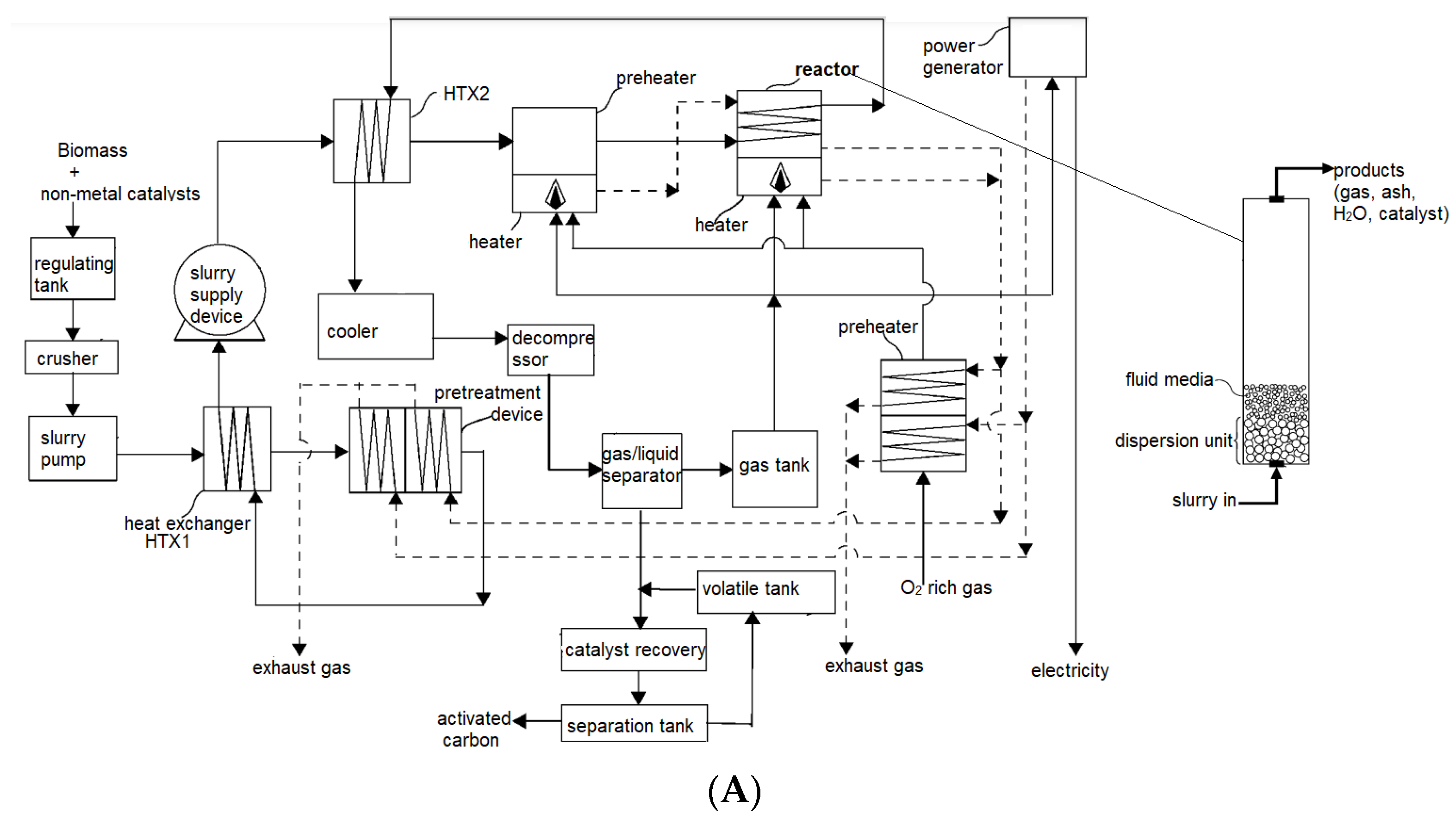

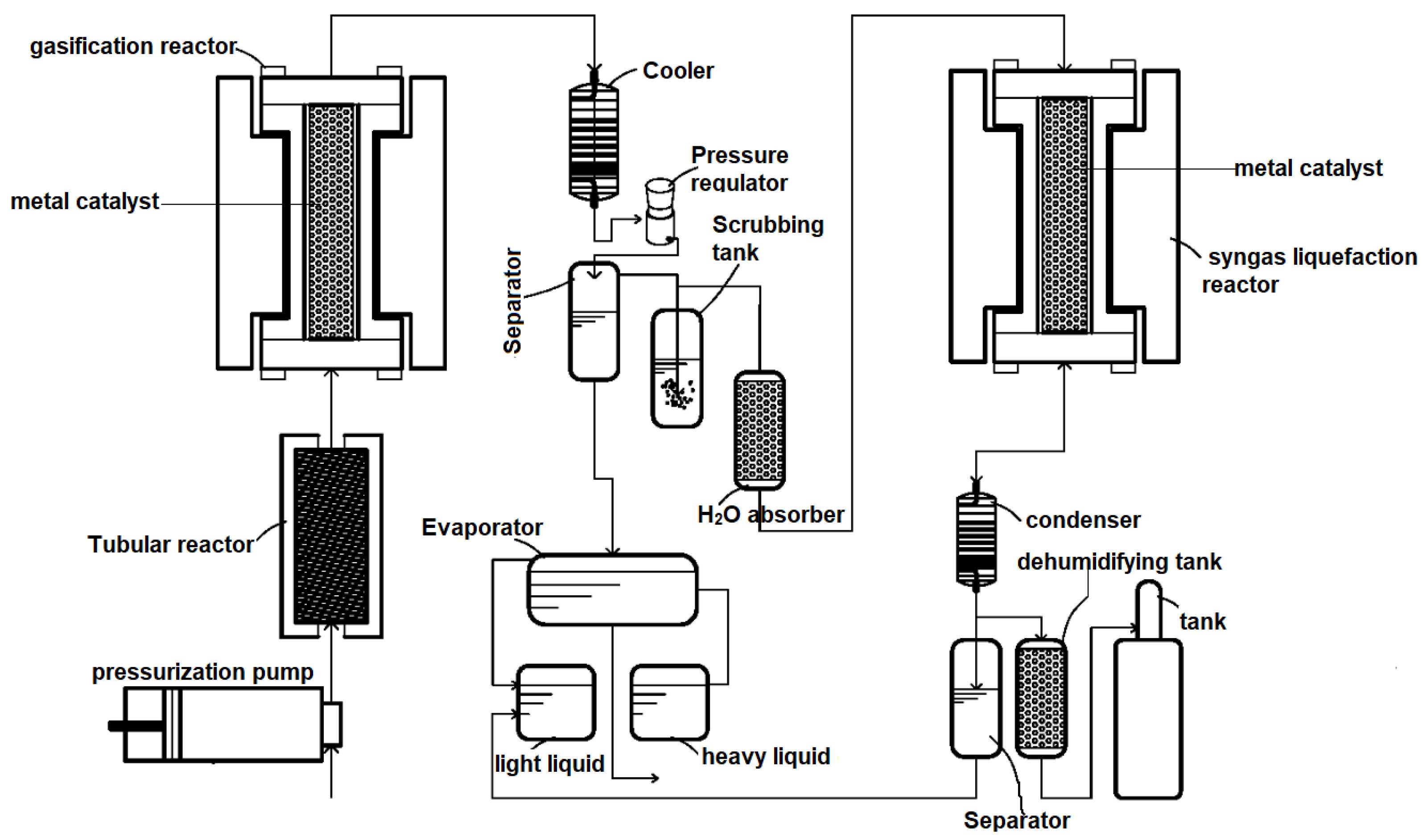

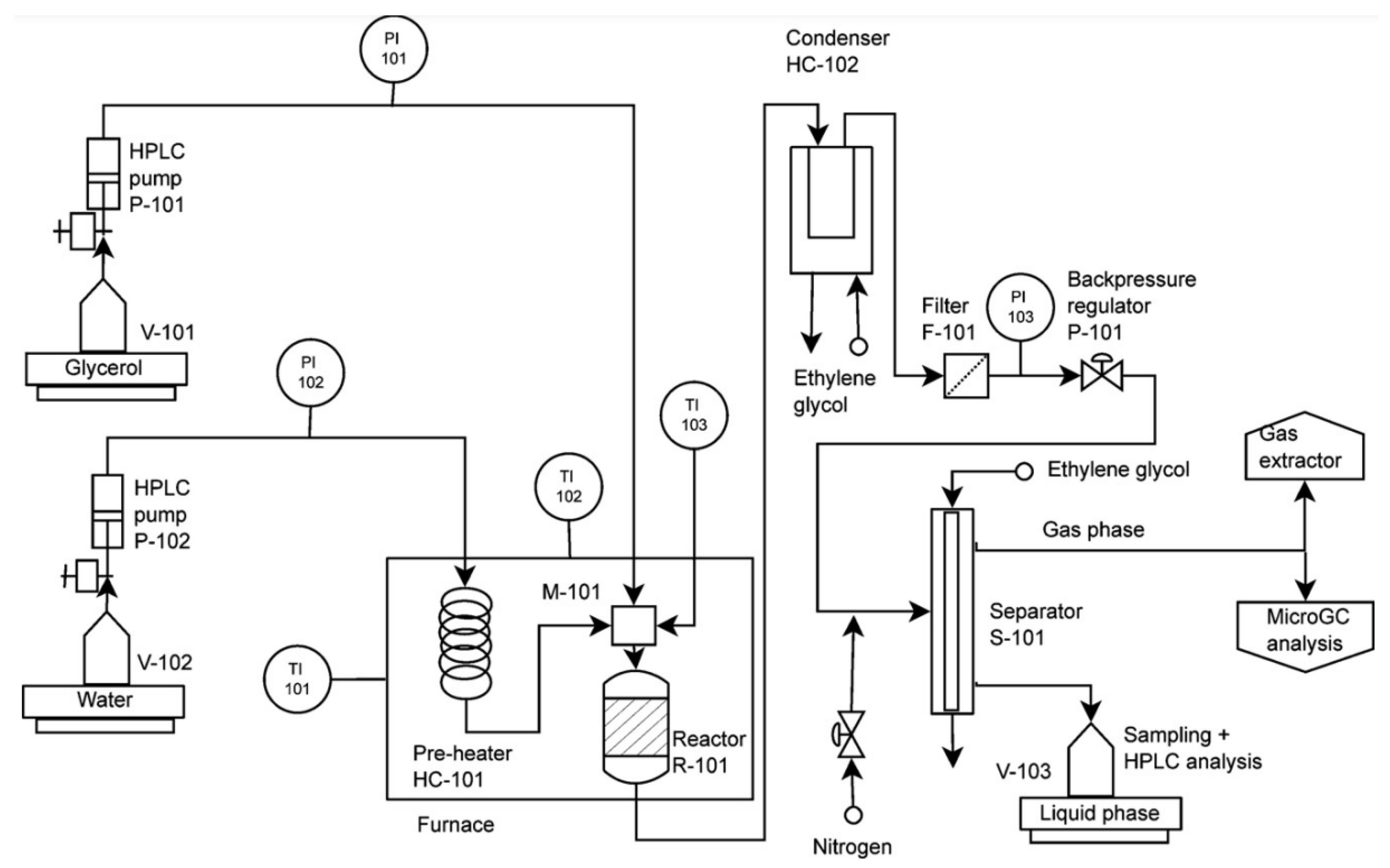



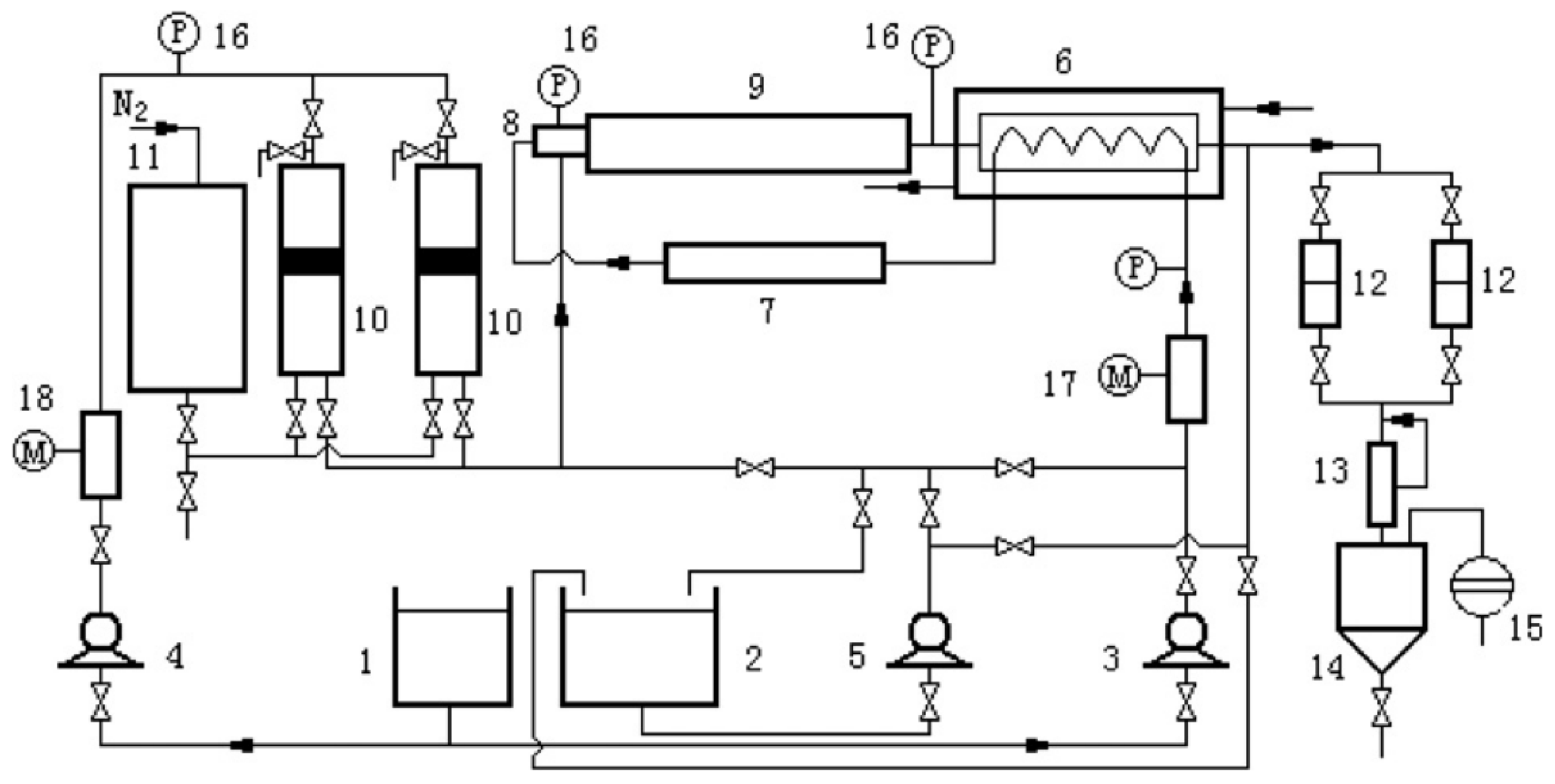
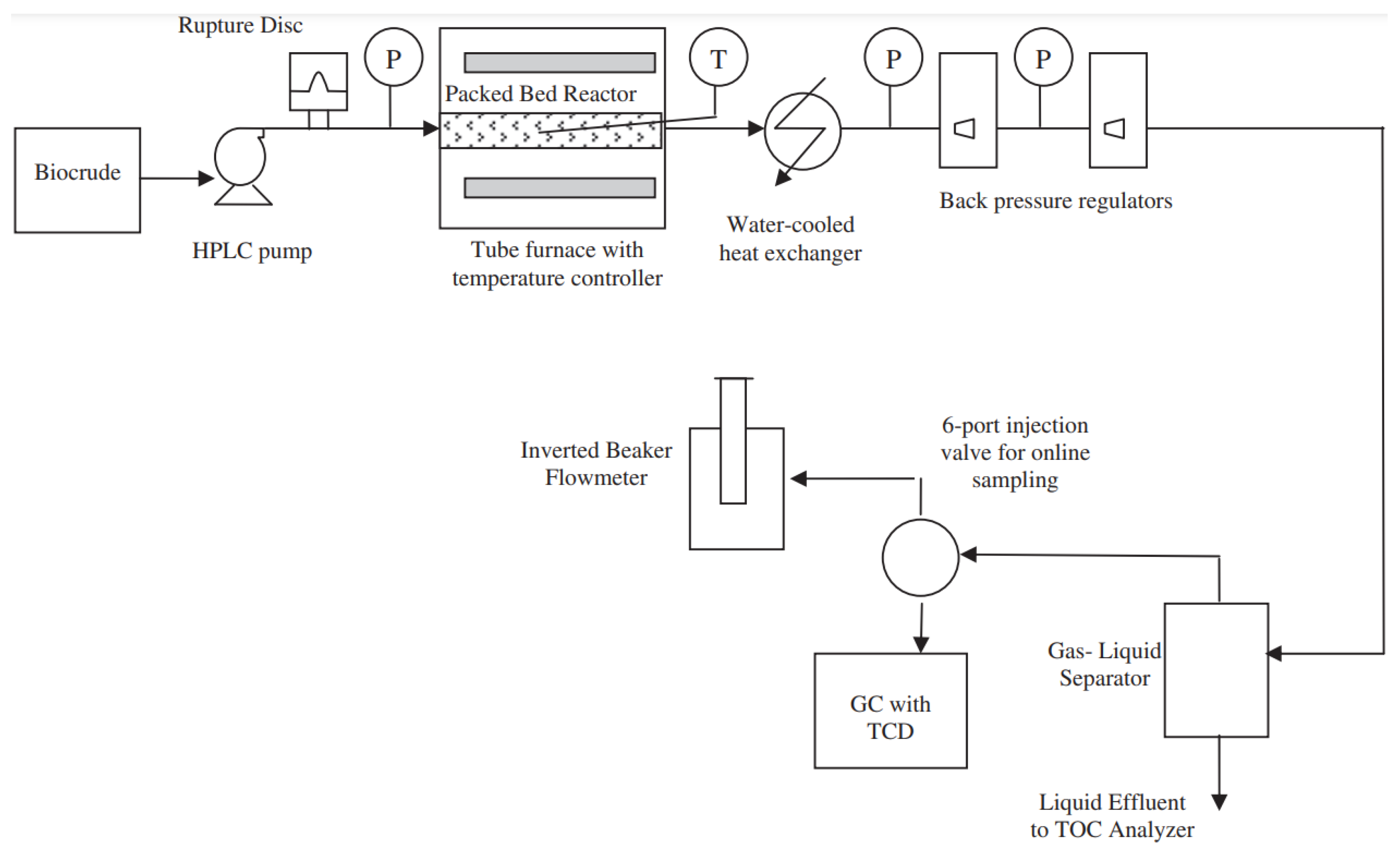
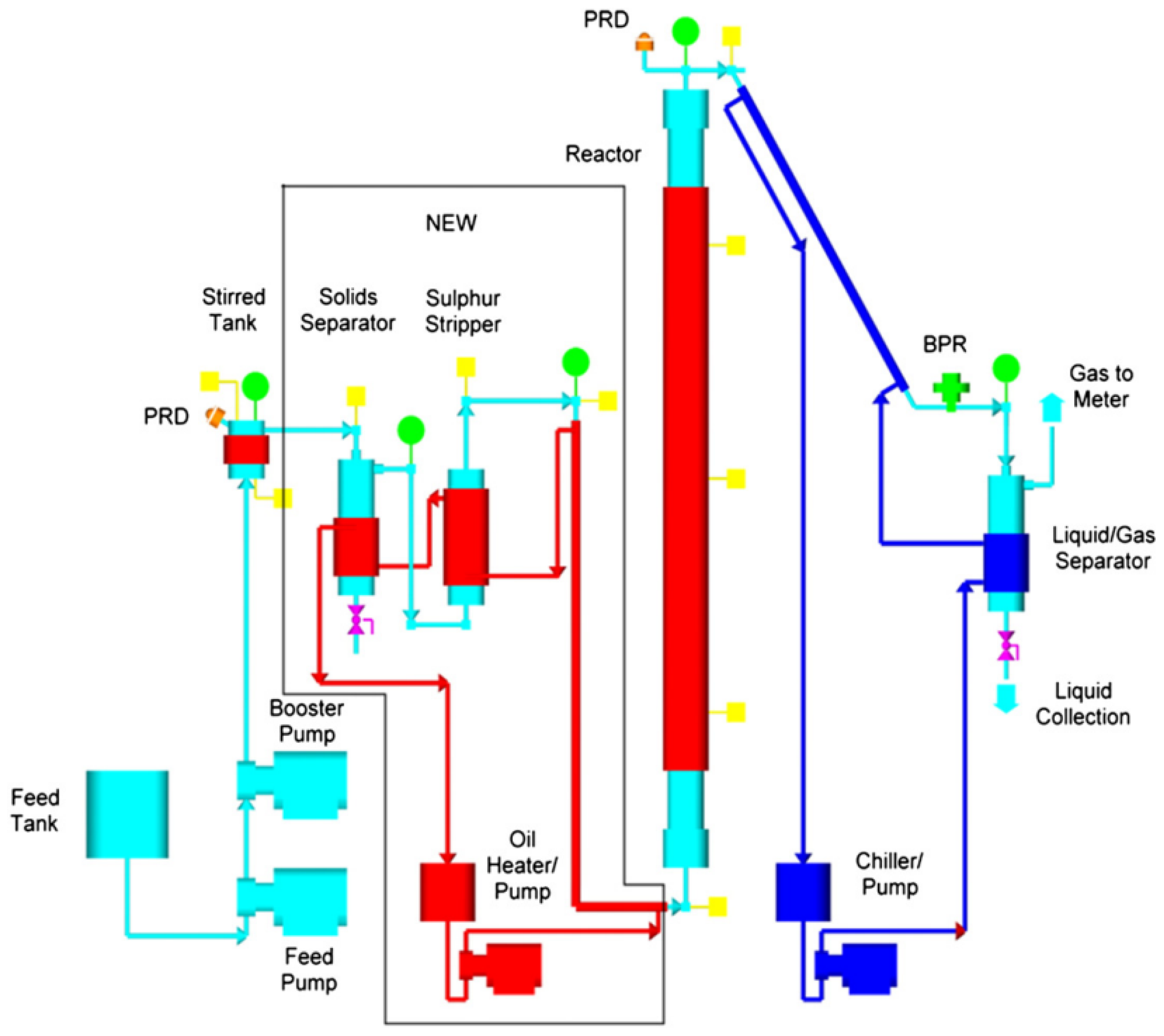
| Catalyst | Conditions | Feedstock | Comments | Reference |
|---|---|---|---|---|
| NaOH, Na2CO3 Ca (OH)2 |
| Sedum plumbizincicola |
| [52] |
| KOH, K2CO3, Na2CO3, Ca(OH)2, NaOH |
| Coal (100–150 μm) |
| [53] |
| Red-mud, Ranney Ni, trona, K2CO3 |
| Cotton stalk, corncob, tannery waste (<200 μm) |
| [54] |
| KOH, NaOH, Na2CO3 |
| Lactose monohydrate |
| [55] |
| KOH, K2CO3, H2O2 |
| Coking sludge |
| [56] |
| Catalyst | Conditions | Feedstock | Comments | Reference |
|---|---|---|---|---|
|
| Ethylene glycol |
| [73] |
|
| Glycerol (<0.2 μm) |
| [74] |
|
| Cattle manure oil |
| [75] |
|
| Coal |
| [76] |
|
| Switch grass biocrude |
| [71] |
|
|
|
| [77] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galiwango, E.; Butler, J.; Lotfi, S. A Review of Catalyst Integration in Hydrothermal Gasification. Fuels 2024, 5, 375-393. https://doi.org/10.3390/fuels5030022
Galiwango E, Butler J, Lotfi S. A Review of Catalyst Integration in Hydrothermal Gasification. Fuels. 2024; 5(3):375-393. https://doi.org/10.3390/fuels5030022
Chicago/Turabian StyleGaliwango, Emmanuel, James Butler, and Samira Lotfi. 2024. "A Review of Catalyst Integration in Hydrothermal Gasification" Fuels 5, no. 3: 375-393. https://doi.org/10.3390/fuels5030022
APA StyleGaliwango, E., Butler, J., & Lotfi, S. (2024). A Review of Catalyst Integration in Hydrothermal Gasification. Fuels, 5(3), 375-393. https://doi.org/10.3390/fuels5030022








