High-Temperature Fermentation and Its Downstream Processes for Compact-Scale Bioethanol Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strain and Media
2.2. Determination of Specific Growth Rates at Different Temperatures
2.3. Ethanol Production by SSF and SHF with Rice as Biomass Followed by RPD
2.4. Simulation of Heat Generation and Heat Dissipation During Fermentation
2.5. Membrane Separation
2.6. Measurement of Glucose and Ethanol Concentrations in the Culture Liquid
2.7. Determination of Cell Numbers of Contaminating Microorganisms in a Non-Sterile Medium
3. Results
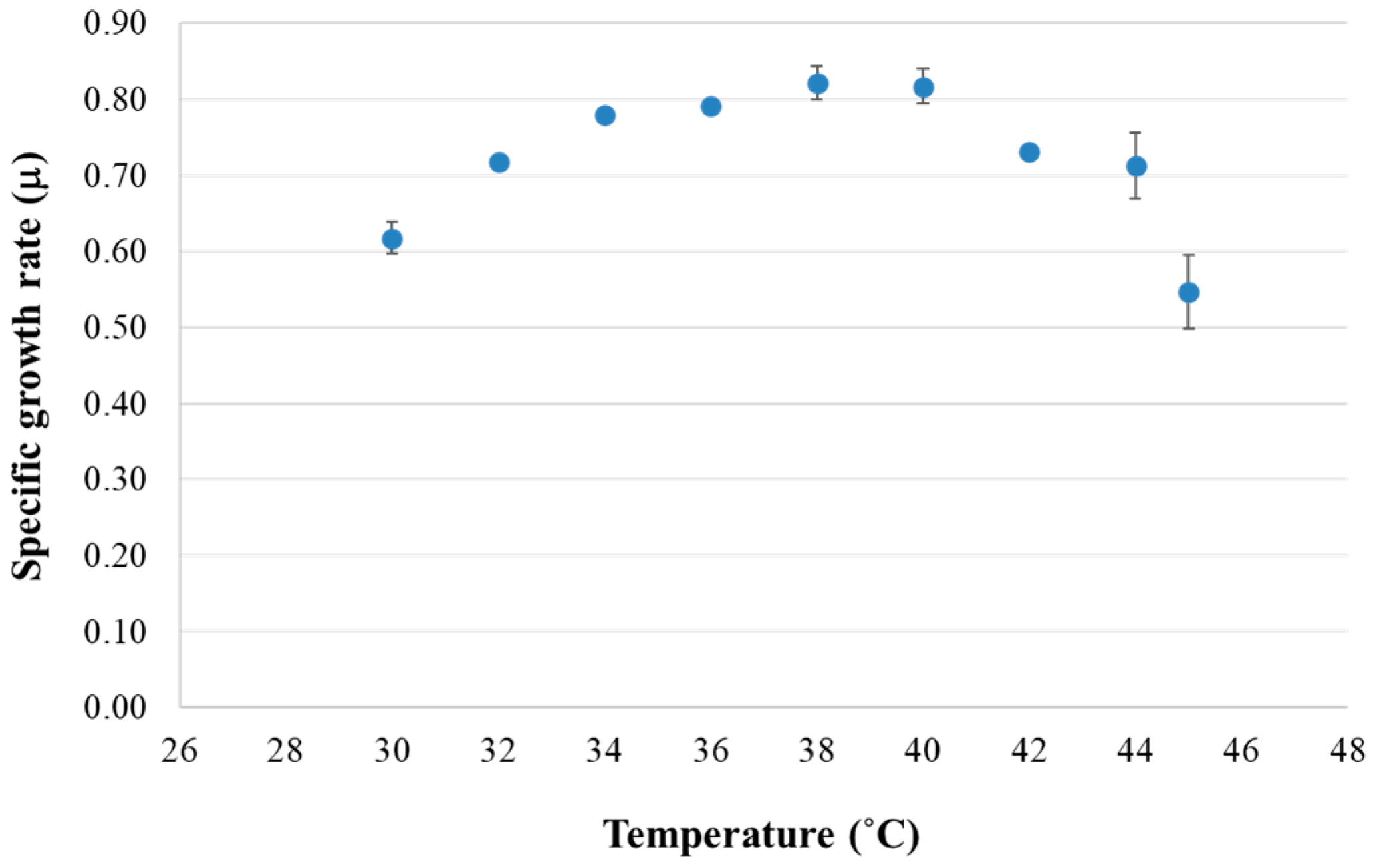
3.1. Optimal Temperature for Specific Growth Rate of K. marxianus DMKU 3-1042
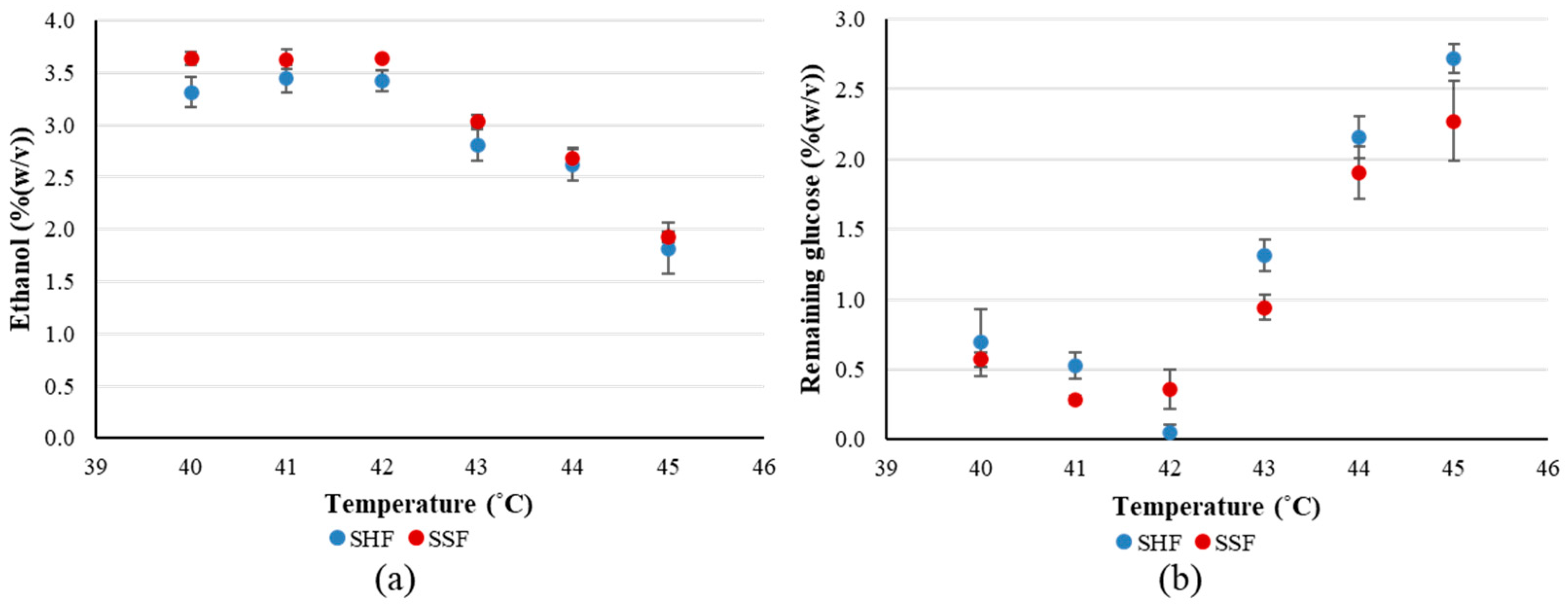
3.2. Ethanol Production at High Temperatures Using Rice as a Raw Material
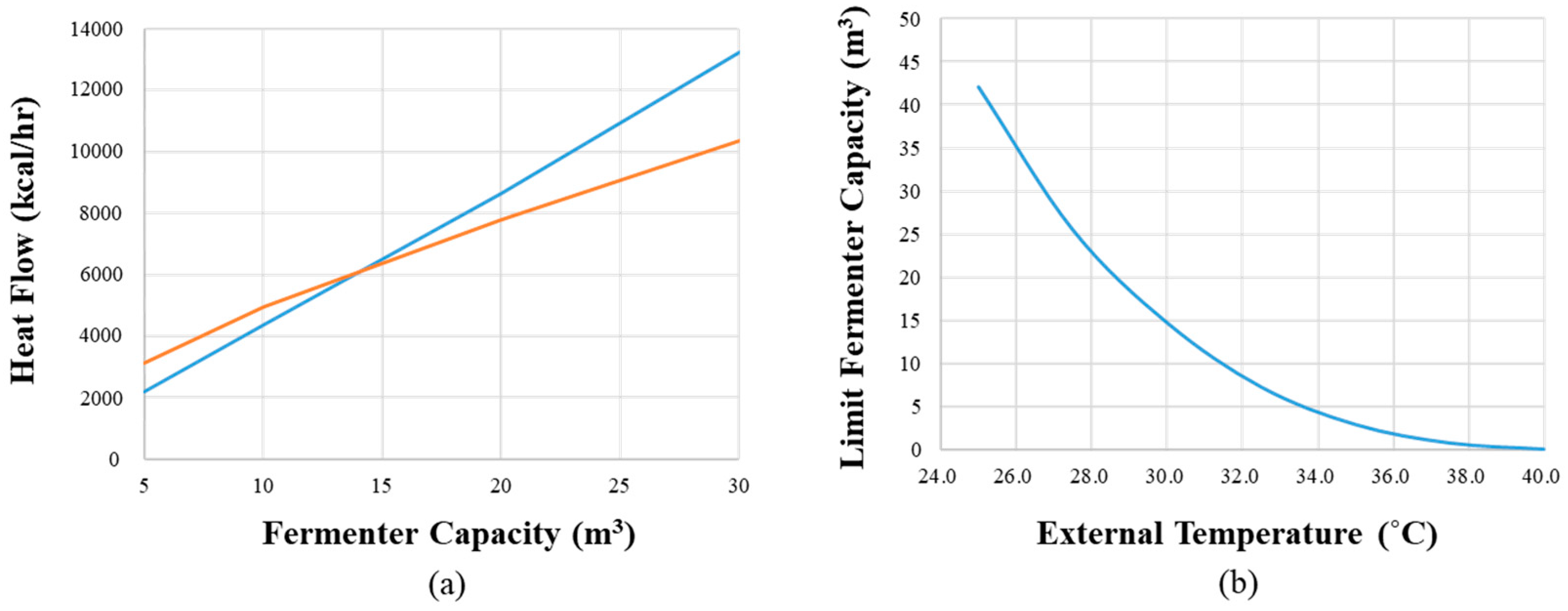
3.3. Estimating the Size of a Fermenter That Does Not Require Cooling in HTF by Simulating Heat Generation and Heat Dissipation During Fermentation
3.4. Ethanol Recovery from Fermentation Liquid by RPD
3.5. Ethanol Concentration Using a Zeolite Membrane
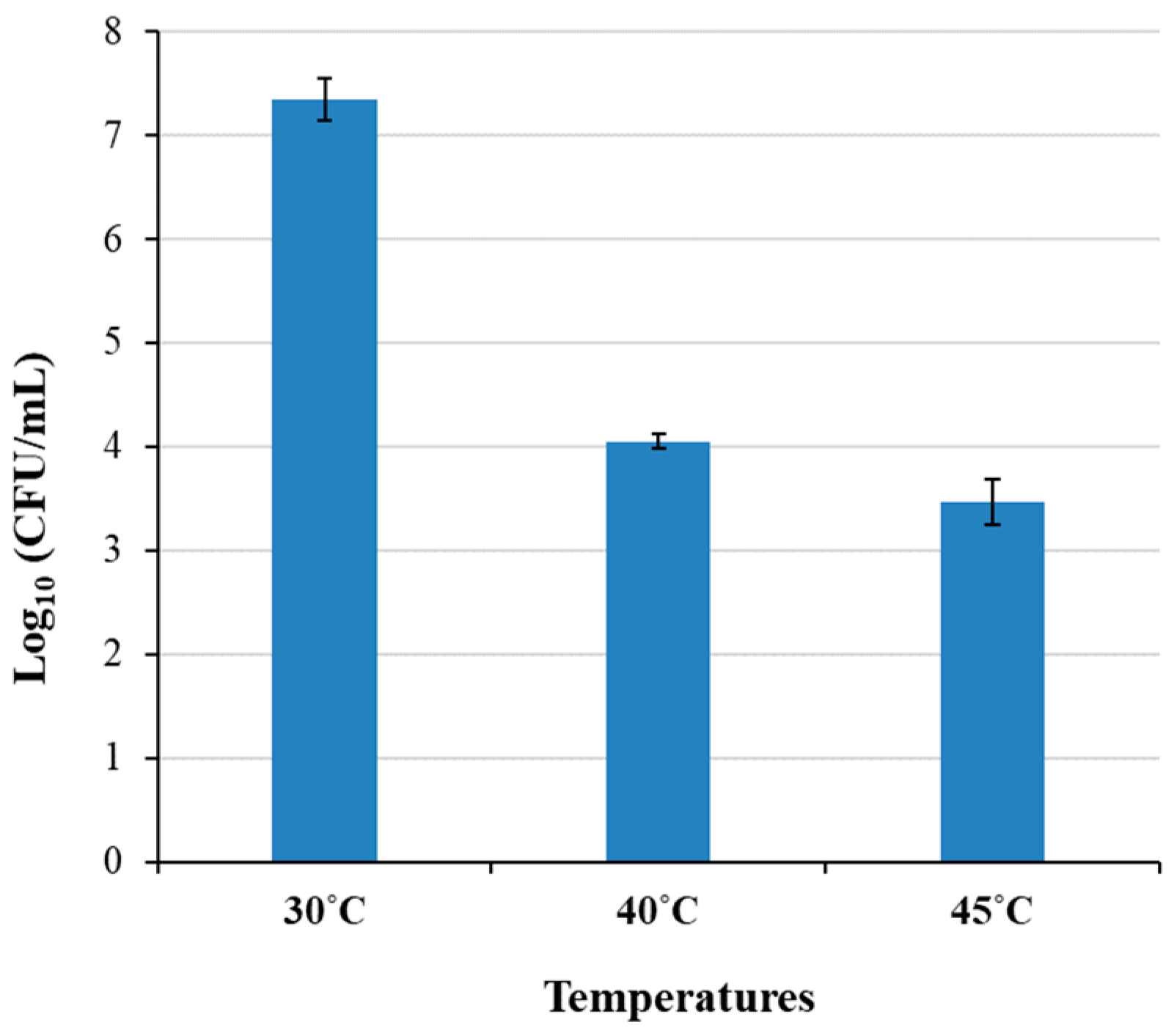
3.6. Suppression of the Growth of Contaminating Microorganisms by High Temperatures
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sebos, I. Fossil fraction of CO2 emissions of biofuels. Carbon Manag. 2022, 13, 154–163. [Google Scholar] [CrossRef]
- Jeswani, H.K.; Chilvers, A.; Azapagic, A. Environmental sustainability of biofuels: A review. Proc. R. Soc. A 2020, 476, 20200351. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, S.; Nam, H.; Chakraborty, J.P. Conversion of Solid Wastes to Fuels and Chemicals Through Pyrolysis. In Waste Biorefinery; Bhaskar, T., Pandey, A., Mohan, S.V., Lee, D.-J., Khanal, S.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 239–263. [Google Scholar]
- Bakari, R.; Asha, R.; Hossein, M.; Huang, X.; Islan, N.F.; Liew, R.K.; Narayan, M.; Lam, S.S.; Sarma, H. Converting food waste to biofuel: A sustainable energy solution for Sub-Saharan Africa. Sustain. Chem. Environ. 2024, 7, 100126. [Google Scholar] [CrossRef]
- Li, J.; Zhao, R.; Xu, Y.; Wu, X.; Bean, S.R.; Wang, D. Fuel ethanol production from starchy grain and other crops: An overview on feedstocks, affecting factors, and technical advances. Renew. Energy 2022, 188, 223–239. [Google Scholar] [CrossRef]
- Bai, F.W.; Anderson, W.A.; Moo-Young, M. Ethanol fermentation technologies from sugar and starch feedstocks. Biotechnol. Adv. 2008, 26, 89–105. [Google Scholar] [CrossRef]
- Huang, H.-J.; Ramaswamy, S.; Tschirner, U.W.; Ramarao, B.V. A review of separation technologies in current and future biorefineries. Sep. Purif. Technol. 2008, 62, 1–21. [Google Scholar] [CrossRef]
- Bušić, A.; Marđetko, N.; Kundas, S.; Morzak, G.; Belskaya, H.; Šantek, M.I.; Komes, D.; Novak, S.; Šantek, B. Bioethanol Production from Renewable Raw Materials and Its Separation and Purification: A Review. Food Technol. Biotechnol. 2018, 56, 289–311. [Google Scholar] [CrossRef]
- Zhu, M.; Li, P.; Gong, X.; Wang, J. A comparison of the production of ethanol between simultaneous saccharification and fermentation and separate hydrolysis and fermentation using unpretreated cassava pulp and enzyme cocktail. Biosci. Biotechnol. Biochem. 2014, 76, 671–678. [Google Scholar] [CrossRef]
- Lertwattanasakul, N.; Rodrussamee, N.; Kumakiri, I.; Pattanakittivorakul, S.; Yamada, M. Potential of thermo-tolerant microorganisms for production of cellulosic bioethanol. In The Handbook of Biorefinery Research and Technology; Bisaria, V., Ed.; Springer: Dordrecht, The Netherlands, 2023; pp. 33–62. [Google Scholar]
- Murata, M.; Nitiyon, S.; Lertwattanasakul, N.; Sootsuwan, K.; Kosaka, T.; Thanonkeo, P.; Limtong, S.; Yamada, M. High-temperaturefer-mentation technology for low-cost bioethanol. J. Jpn. Inst. Energy 2015, 94, 1154–1162. [Google Scholar] [CrossRef]
- Abdel-Banat, B.A.; Hoshida, H.; Ano, A.; Nongklang, S.; Akada, R. High-temperature fermentation: How can processes for ethanol production at high temperatures become superior to traditional process using mesophilic yeast? Appl. Microbiol. Biotechnol. 2010, 85, 861–867. [Google Scholar] [CrossRef]
- Kumakiri, I.; Yokota, M.; Tanaka, R.; Shimada, Y.; Kiatkittipong, W.; Lim, J.W.; Murata, M.; Yamada, M. Process intensification in bio-ethanol production–recent developments in membrane separation. Processes 2021, 9, 1028. [Google Scholar] [CrossRef]
- Kwon, Y.J.; Ma, A.Z.; Li, Q.; Wang, F.; Zhuang, G.Q.; Liu, C.Z. Effect of lignocellulosic inhibitory compounds on growth and ethanol fermentation of newly-isolated thermotolerant Issatchenkia orientalis. Bioresour. Technol. 2011, 102, 8099–8104. [Google Scholar] [CrossRef] [PubMed]
- Ryabova, O.B.; Chmil, O.M.; Sibirny, A.A. Xylose and cellobiose fermentation to ethanol by the thermotolerant methylotorophic yeast Hansenula polymorpha. FEMS Yeast Res. 2003, 4, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, I.; Nakamura, T.; Shima, J. Characterization of a spontaneous flocculation mutant derived from Candida glabrata: A useful strain for bioethanol production. J. Biosci. Bioeng. 2009, 107, 379–382. [Google Scholar] [CrossRef]
- Prasetyo, J.; Naruse, K.; Kato, T.; Boonchird, C.; Harashima, S.; Park, E.Y. Bioconversion of paper sludge to biofuel by simultaneous saccharification and fermentation using a cellulase of paper sludge origin and thermotolerant Saccharomyces cerevisiae TJ14. Biotechnol. Biofuels 2007, 4, 35. [Google Scholar] [CrossRef]
- Limtong, S.; Sringiew, C.; Yongmanitchai, W. Production of fuel ethanol at high temperature from sugar cane juice by a newly isolated Kluyveromyces marxianus. Bioresour. Technol. 2007, 98, 3367–3374. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, G.G.; Heinzle, E.; Wittmann, C.; Gombert, A.K. The yeast Kluyveromyces marxianus and its biotechnological potential. Appl. Microbiol. Biotechnol. 2008, 79, 339–354. [Google Scholar] [CrossRef]
- Lane, M.M.; Morrissey, J.P. Kluyveromyces marxianus: A yeast emerging from its sister’s shadow. Fungal Biol. Rev. 2010, 24, 17–26. [Google Scholar] [CrossRef]
- Lertwattanasakul, N.; Nurcholis, M.; Rodrussamee, N.; Kosaka, T.; Murata, M.; Yamada, M. Kluyveromyces marxianus as a platform in synthetic biology for producing useful materials. In Syn-Thetic Biology of Yeasts; Harzevili, F.D., Ed.; Springer: Cham, Switzerland, 2022; pp. 293–335. [Google Scholar]
- Lertwattanasakul, N.; Rodrussamee, N.; Suprayogi; Limtong, S.; Thanonkeo, P.; Kosaka, T.; Yamada, M. Utilization capability of sucrose, raffinose and inulin and its less-sensitiveness to glucose repression in thermotolerant yeast Kluyveromyces marxianus DMKU 3-1042. AMB Express 2011, 1, 20. [Google Scholar] [CrossRef]
- Saini, P.; Beniwal, A.; Kokkiligadda, A.; Vij, S. Evolutionary adaptation of Kluyveromyces marxianus strain for efficient conversion of whey lactose to bioethanol. Process Biochem. 2017, 62, 69–79. [Google Scholar] [CrossRef]
- Sharma, N.K.; Behera, S.; Arora, R.; Kumar, S. Evolutionary adaptation of Kluyveromyces marxianus NIRE-K3 for enhanced xylose utilization. Front. Energy Res. 2017, 5, 32. [Google Scholar] [CrossRef]
- Pattanakittivorakul, S.; Tsuzuno, T.; Kosaka, T.; Murata, M.; Kanesaki, Y.; Yoshikawa, H.; Limtong, S.; Yamada, M. Evolu-tionary adaptation by repetitive long-term cultivation with gradual increase in temperature for acquiring multi-stress tolerance and high ethanol productivity in Kluyveromyces marxianus DMKU 3-1042. Microorganisms 2022, 10, 798. [Google Scholar] [CrossRef]
- Zhu, M.H.; Xia, S.L.; Hua, X.M.; Feng, Z.J.; Hu, N.; Zhang, F.; Kumakiri, I.; Lu, Z.H.; Chen, X.S.; Kita, H. Rapid preparation of acid-stable and high dehydration performance mordenite membranes. Ind. Eng. Chem. Res. 2014, 53, 19168–19174. [Google Scholar] [CrossRef]
- Sakai, K.; Murata, Y.; Yamazumi, H.; Yau, Y.; Mori, M.; Moriguchi, M.; Shirai, Y. Selective proliferation of lactic acid bacteria and accumulation of lactic acid during open fermentation of kitchen refuse with intermittent pH adjustment. Food Sci. Technol. Res. 2000, 6, 140–145. [Google Scholar] [CrossRef]
- Nonklang, S.; Abdel-Banat, B.M.; Cha-aim, K.; Moonjai, N.; Hoshida, H.; Limtong, S.; Yamada, M.; Akada, R. High-temperature ethanol fermentation and transformation with linear DNA in the thermotolerant yeast Kluyveromyces marxianus DMKU 3-1042. Appl. Environ. Microbiol. 2008, 74, 7514–7521. [Google Scholar] [CrossRef] [PubMed]
- Nedwell, D.B. Effect of low temperature on microbial growth: Lowered affinity for substrates limits growth at low temperature. FEMS Microbiol. Ecol. 1999, 30, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Morigami, Y.; Kondo, M.; Abe, J.; Kita, H. The first large-scale pervaporation plant using tubular-type module with zeolite NaA membrane. Sep. Purif. Technol. 2001, 25, 251–260. [Google Scholar] [CrossRef]
- Krajang, M.; Malairuang, K.; Sukna, J.; Rattanapradit, K.; Chamsart, S. Single-step ethanol production from raw cassava starch using a combination of raw starch hydrolysis and fermentation, scale-up from 5-L laboratory and 200-L pilot plant to 3000-L industrial fermenters. Biotechnol. Biofuels 2021, 14, 68. [Google Scholar] [CrossRef]
- Zhang, P.; Chen, C.; Shen, Y.; Ding, T.; Ma, D.; Hua, Z.; Sun, D. Starch saccharification and fermentation of uncooked sweet potato roots for fuel ethanol production. Bioresour. Technol. 2013, 128, 835–838. [Google Scholar] [CrossRef]
- Kumar, D.; Singh, V. Dry-grind processing using amylase corn and superior yeast to reduce the exogenous enzyme requirements in bioethanol production. Biotechnol. Biofuels 2016, 9, 228. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pattanakittivorakul, S.; Kumakiri, I.; Nutaratat, P.; Hara, M.; Yokota, M.; Murata, M.; Kosaka, T.; Thanonkeo, P.; Limtong, S.; Yamada, M. High-Temperature Fermentation and Its Downstream Processes for Compact-Scale Bioethanol Production. Fuels 2024, 5, 857-867. https://doi.org/10.3390/fuels5040048
Pattanakittivorakul S, Kumakiri I, Nutaratat P, Hara M, Yokota M, Murata M, Kosaka T, Thanonkeo P, Limtong S, Yamada M. High-Temperature Fermentation and Its Downstream Processes for Compact-Scale Bioethanol Production. Fuels. 2024; 5(4):857-867. https://doi.org/10.3390/fuels5040048
Chicago/Turabian StylePattanakittivorakul, Sornsiri, Izumi Kumakiri, Pumin Nutaratat, Marino Hara, Morihisa Yokota, Masayuki Murata, Tomoyuki Kosaka, Pornthap Thanonkeo, Savitree Limtong, and Mamoru Yamada. 2024. "High-Temperature Fermentation and Its Downstream Processes for Compact-Scale Bioethanol Production" Fuels 5, no. 4: 857-867. https://doi.org/10.3390/fuels5040048
APA StylePattanakittivorakul, S., Kumakiri, I., Nutaratat, P., Hara, M., Yokota, M., Murata, M., Kosaka, T., Thanonkeo, P., Limtong, S., & Yamada, M. (2024). High-Temperature Fermentation and Its Downstream Processes for Compact-Scale Bioethanol Production. Fuels, 5(4), 857-867. https://doi.org/10.3390/fuels5040048







