Production of Biodiesel Employing Chlorella vulgaris Biomass Cultivated in Poultry Effluents
Abstract
1. Introduction
2. Materials and Methods
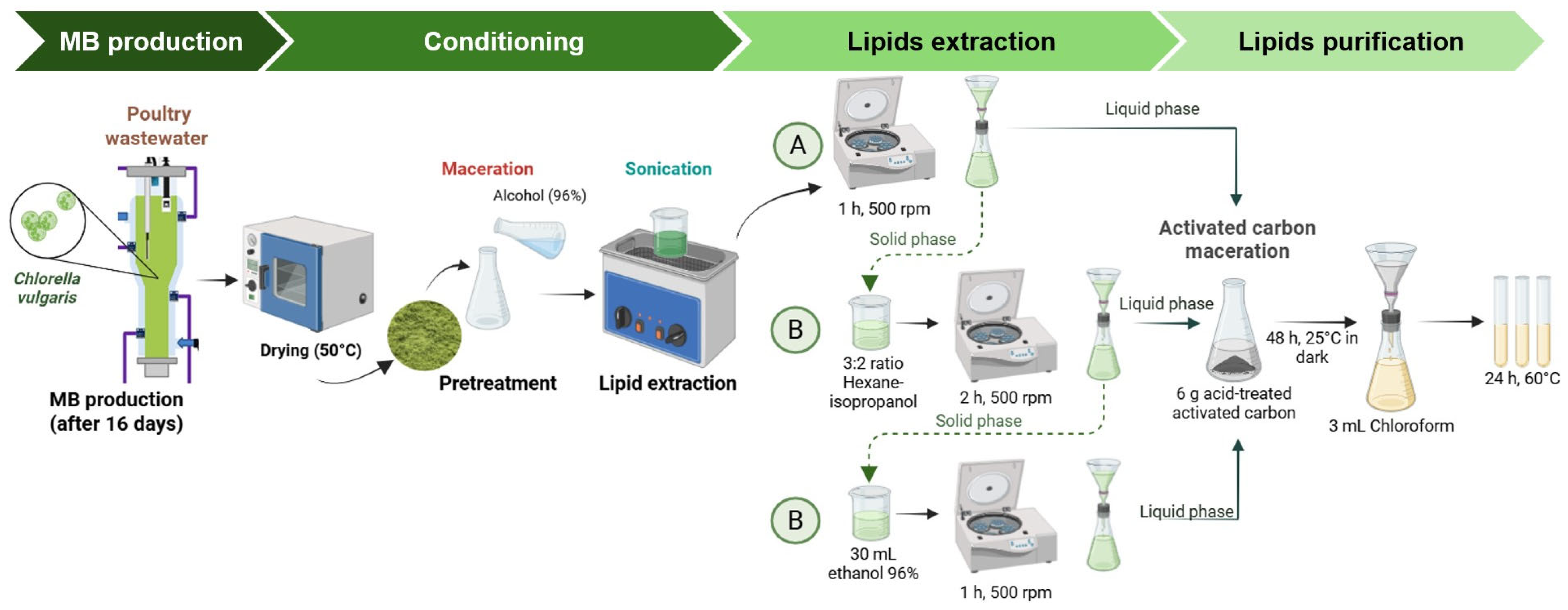
2.1. Sample Conditioning and Biochemical Characterization of MB
2.2. Lipid Extraction and Purification
Lipid Characterization
2.3. Biodiesel Production by Alkaline Transesterification
2.4. Physicochemical and Rheological Characterization of Biodiesel
3. Results
3.1. Lipid Characteristics
3.2. Microalgal Metabolism and Biofuel Production Pathways

3.3. Biodiesel Yields and Characterization
3.4. FAME Characterization of Biodiesel
3.5. Statistical Analysis of Quality Biodiesel Production
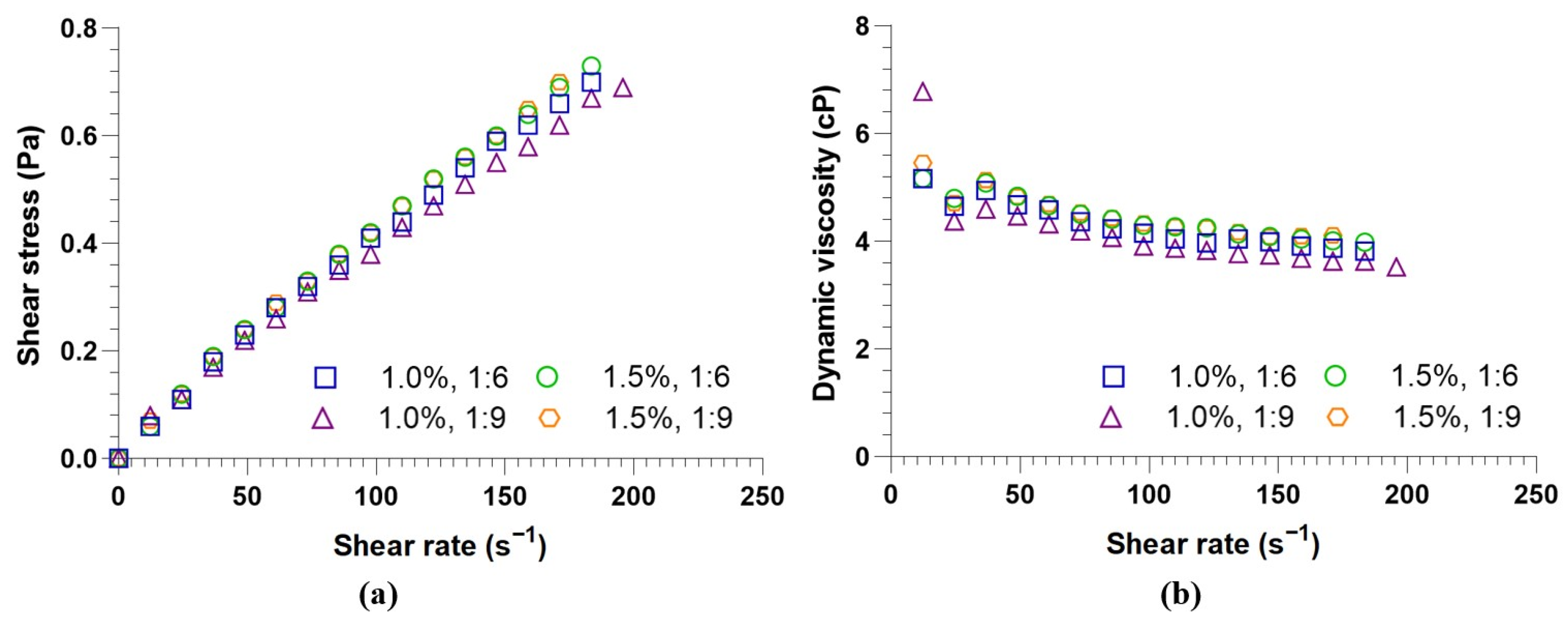
3.6. Rheological Characterization of Biodiesel
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alotaibi, M.M.; Alturki, A.A. Optimizing Renewable Energy Integration for Sustainable Fuel Production: A Techno-Economic Assessment of Dimethyl Ether Synthesis via a Hybrid Microgrid-Hydrogen System. Fuels 2024, 5, 176–209. [Google Scholar] [CrossRef]
- Ghasemian, S.; Faridzad, A.; Abbaszadeh, P.; Taklif, A.; Ghasemi, A.; Hafezi, R. An overview of global energy scenarios by 2040: Identifying the driving forces using cross-impact analysis method. Int. J. Environ. Sci. Technol. 2024, 21, 7749–7772. [Google Scholar] [CrossRef]
- Kalair, A.; Abas, N.; Saleem, M.S.; Kalair, A.R.; Khan, N. Role of energy storage systems in energy transition from fossil fuels to renewables. Energy Storage 2020, 3, e135. [Google Scholar] [CrossRef]
- Mahapatra, S.; Kumar, D.; Singh, B.; Sacham, P.K. Biofuels and their sources of production: A review on cleaner sustainable alternative against conventional fuel, in the framework of the food and energy nexus. Energy Nexus 2021, 4, 100036. [Google Scholar] [CrossRef]
- Awogbemi, O.; Kallon, D.V.V.; Onuh, E.I.; Aigbodion, V.S. An overview of the classification, production and utilization of biofuels for internal combustion engine applications. Energies 2021, 14, 5687. [Google Scholar] [CrossRef]
- Mat Aron, N.S.; Khoo, K.S.; Chew, K.W.; Show, P.L.; Chen, W.H.; Nguyen, T.H.P. Sustainability of the four generations of biofuels–a review. Int. J. Energy Res. 2020, 44, 9266–9282. [Google Scholar] [CrossRef]
- Sarwer, A.; Hussain, M.; Al-Muhtaseb, A.A.H.; Inayat, A.; Rafiq, S.; Khurram, M.S.; Ul-Haq, N.; Shah, N.S.; Din, A.A.; Ahmad, I.; et al. Suitability of biofuels production on commercial scale from various feedstocks: A critical review. ChemBioEng Rev. 2022, 9, 423–441. [Google Scholar] [CrossRef]
- Singh, A.; Prajapati, P.; Vyas, S.; Gaur, V.K.; Sindhu, R.; Binod, P.; Kumar, V.; Singhania, R.R.; Awasthi, M.K.; Zhang, Z.; et al. A Comprehensive review of feedstocks as sustainable substrates for next-generation biofuels. Bioenergy Res. 2023, 16, 105–122. [Google Scholar] [CrossRef]
- Leong, W.-H.; Lim, J.-W.; Lam, M.-K.; Uemura, Y.; Ho, Y.-C. Third generation biofuels: A nutritional perspective in enhancing microbial lipid production. Renew. Sustain. Energy Rev. 2018, 91, 950–961. [Google Scholar] [CrossRef]
- Razzak, S.A.; Lucky, R.A.; Hossain, M.M.; deLasa, H. Valorization of microalgae biomass to biofuel production: A review. Energy Nexus 2022, 7, 100139. [Google Scholar] [CrossRef]
- Antar, M.; Lyu, D.; Nazari, M.; Shah, A.; Zhou, X.; Smith, D.L. Biomass for a sustainable bioeconomy: An overview of world biomass production utilization. Renew. Sustain. Energy Rev. 2021, 139, 110691. [Google Scholar] [CrossRef]
- Mehariya, S.; Goswami, R.K.; Karthikeysan, O.P.; Verma, P. Microalgae for high-value products: A way towards green nutraceutical and pharmaceutical compounds. Chemosphere 2021, 280, 130553. [Google Scholar] [CrossRef] [PubMed]
- Basheer, S.; Huo, S.; Zhu, F.; Qian, J.; Xu, L.; Cui, F.; Zou, B. Microalgae in human health and medicine. In Microalgae Biotechnology for Food, Health and High Value Products, 1st ed.; Alam, M., Xu, J.L., Wang, Z., Eds.; Springer: Singapore, 2020; pp. 149–174. [Google Scholar] [CrossRef]
- Barreiro-Vescovo, S.; Barbera, E.; Bertucco, A.; Sforza, E. Integration of Microalgae Cultivation in a Biogas Production Process from Organic Municipal Solid Waste: From Laboratory to Pilot Scale. ChemEngineering 2020, 4, 25. [Google Scholar] [CrossRef]
- Velmozhina, K.; Shinkevich, P.; Zhazhkov, V.; Politaeva, N.; Korablev, V.; Vladimirov, I.; Morales, T.C. Production of Biohydrogen from Microalgae Biomass after Wastewater Treatment and Air Purification from CO2. Processes 2023, 11, 2978. [Google Scholar] [CrossRef]
- Branco-Vieira, M.; Mata, T.M.; Martins, A.A.; Freitas, M.A.V.; Caetano, N.S. Economic analysis of microalgae biodiesel production in a small-scale facility. Energy Rep. 2020, 6, 325–332. [Google Scholar] [CrossRef]
- Safi, C.; Zebib, B.; Merah, O.; Pontalier, P.Y.; Vaca-Garcia, C. Morphology, composition, production, processing and applications of Chlorella vulgaris: A review. Renew. Sustain. Energy Rev. 2014, 35, 265–278. [Google Scholar] [CrossRef]
- Adochite, C.; Andronic, L. Aquatic Toxicity of Photocatalyst Nanoparticles to Green Microalgae Chlorella vulgaris. Water 2021, 13, 77. [Google Scholar] [CrossRef]
- Gutiérrez-Casiano, N.; Hernández-Aguilar, E.; Alvarado-Lassman, A.; Méndez-Contreras, J.M. Removal of carbon and nitrogen in wastewater from a poultry processing plant in a photobioreactor cultivated with the microalga Chlorella vulgaris. J. Environ. Sci. Health Part A 2022, 57, 620–633. [Google Scholar] [CrossRef]
- Mata, T.M.; Martins, A.A.; Caetano, N.S. Microalgae for biodiesel production and other applications: A review. Renew. Sustain. Energy Rev. 2010, 14, 217–232. [Google Scholar] [CrossRef]
- Gaur, A.; Dwivedi, G.; Baredar, P.; Jain, S. Influence of blending additives in biodiesel on physicochemical properties, engine performance, and emission characteristics. Fuel 2022, 321, 124072. [Google Scholar] [CrossRef]
- Palani, Y.; Devarajan, C.; Manickam, D.; Thanikodi, S. Performance and emission characteristics of biodiesel-blend in diesel engine: A review. Environ. Eng. Res. 2022, 27, 200338. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Bhatia, R.K.; Jeon, J.-M.; Pugazhendhi, A.; Awasthie, M.K.; Kumar, D.; Kumar, G.; Yoon, J.-J.; Yang, Y.-H. An overview on advancements in biobased transesterification methods for biodiesel production: Oil resources, extraction, biocatalysts, and process intensification technologies. Fuel 2021, 285, 119117. [Google Scholar] [CrossRef]
- Gutiérrez-Casiano, N.; Cobos-Murcia, J.A.; Ortiz-Sánchez, C.A.; Pérez-Guzmán, S.M.; Hernández-Aguilar, E. Valorization of Poultry Waste Oils Recovered from Water Treatment Through the Degumming–Transesterification Process to Produce Biodiesel. Fuels 2025, 6, 7. [Google Scholar] [CrossRef]
- Singh, D.; Sharma, D.; Soni, S.L.; Sharma, S.; Sharma, P.K.; Jhalani, A. A review on feedstocks, production processes, and yield for different generations of biodiesel. Fuel 2020, 262, 116553. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Gurav, R.; Choi, T.-R.; Joong, K.H.; Yang, S.-Y.; Song, H.-S.; Park, J.Y.; Park, Y.-L.; Han, Y.-H.; Choi, Y.-K.; et al. Conversion of waste cooking oil into biodiesel using heterogenous catalyst derived from cork biochar. Bioresour. Technol. 2020, 302, 122872. [Google Scholar] [CrossRef]
- Wang, B.; Wang, B.; Shukla, S.K.; Wang, R. Enabling Catalysts for Biodiesel Production via Transesterification. Catalysts 2023, 13, 740. [Google Scholar] [CrossRef]
- Jain, S.; Kumar, N.; Singh, V.P.; Mishra, S.; Sharma, N.K.; Bajaj, M.; Khan, T.M.Y. Transesterification of Algae Oil and Little Amount of Waste Cooking Oil Blend at Low Temperature in the Presence of NaOH. Energies 2023, 16, 1293. [Google Scholar] [CrossRef]
- Varol, P.M.; Çakan, A.; Kiren, B.; Ayas, N. Microwave-assisted catalytic transesterification of soybean oil using KOH/γ-Al2O3. Biomass Conv. Bioref. 2023, 13, 633–645. [Google Scholar] [CrossRef]
- Pranyoto, N.; Dewi Susanti, Y.; Joseph Ondang, I.; Angkawijaya, A.E.; Edi Soetaredjo, F.; Santoso, S.P.; Yuliana, M.; Ismadji, S.; Budi Hartono, S. Facile Synthesis of Silane-Modified Mixed Metal Oxide as Catalyst in Transesterification Processes. Nanomaterials 2022, 12, 245. [Google Scholar] [CrossRef]
- Saleem, M.; Jamil, F.; Qamar, O.A.; Akhter, P.; Hussain, M.; Khurram, M.S.; Al-Muhtaseb, A.H.; Inayat, A.; Shah, N.S. Enhancing the Catalytic Activity of Eggshell-Derived CaO Catalyst and Its Application in Biodiesel Production from Waste Chicken Fat. Catalysts 2022, 12, 1627. [Google Scholar] [CrossRef]
- Agarwal, M.; Chauhan, G.; Chaurasia, S.P.; Singh, K. Study of catalytic behavior of KOH as homogeneous and heterogeneous catalyst for biodiesel production. J. Taiwan Inst. Chem. Eng. 2012, 43, 89–94. [Google Scholar] [CrossRef]
- Koh, M.Y.; Ghazi, T.I.M. A review of biodiesel production form Jatropha curcas L. oil. Renew. Sustain. Energy Rev. 2011, 15, 2240–2251. [Google Scholar] [CrossRef]
- Eze, V.C.; Phan, A.N.; Harvey, A.P. A more robust model of the biodiesel reaction, allowing identification of process conditions for significantly enhanced rate and water tolerance. Bioresour. Technol. 2014, 156, 222–231. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Salam, K.A.; Velasquez-Orta, S.B.; Harvey, A.P. Kinetics of fast alkali reactive extraction/in situ transesterification of Chlorella vulgaris that identifies process conditions for a significant enhanced rate and water tolerance. Fuel Process Technol. 2016, 144, 212–219. [Google Scholar] [CrossRef]
- Alavijeh, R.S.; Karimi, K.; Wijffels, R.H.; van Den Berg, C.; Eppink, M. Combined bead milling and enzymatic hydrolysis for efficient fractionation of lipids, proteins, and carbohydrates of Chlorella vulgaris microalgae. Bioresour. Technol. 2020, 309, 123321. [Google Scholar] [CrossRef]
- Madhubalaji, C.K.; Mudaliar, S.N.; Chauhan, V.S.; Sarada, R. Evaluation of drying methods on nutritional constituents and antioxidant activities of Chlorella vulgaris cultivated in an outdoor open raceway pond. J. Appl. Phycol. 2021, 33, 1419–1434. [Google Scholar] [CrossRef]
- Asadi, P.; Rad, H.A.; Qaderi, F. Lipid and biodiesel production by cultivation isolated strain Chlorella sorokiniana pa. 91 and Chlorella vulgaris in dairy wastewater treatment plant effluents. J. Environ. Health Sci. Eng. 2020, 18, 573–585. [Google Scholar] [CrossRef]
- Xiao, G.; Wang, Y.; Guo, W.; Song, Y.; Dong, C.; Zhu, X. Pyrolysis Characteristics and Kinetics of Laminated Glass Interlayer. Fuel Process. Technol. 2012, 104, 276–282. [Google Scholar] [CrossRef]
- Park, J.-Y.; Lee, K.; Choi, S.-A.; Jeong, M.-J.; Kim, B.; Lee, J.-S.; Oh, Y.-K. Sonication-assisted homogenization system for improved lipid extraction from Chlorella vulgaris. Renew. Energy 2015, 79, 3–8. [Google Scholar] [CrossRef]
- Brennan, L.; Owende, P. Biofuels from microalgae—A review of technologies for production, processing, and extractions of biofuels and co-products. Renew. Sustain. Energy Rev. 2010, 14, 557–577. [Google Scholar] [CrossRef]
- Widjaja, A.; Chien, C.-C.; Ju, Y.-H. Study of increasing lipid production from fresh water microalgae Chlorella vulgaris. J. Taiwan Inst. Chem. Eng. 2009, 40, 13–20. [Google Scholar] [CrossRef]
- Harris, J.P.; Glick, B.N. Purification and deodorization by absorptive carbons: A discussion of modern methods of bleaching as applied to oils and fats. Oil Fat Ind. 1928, 5, 46–47. [Google Scholar] [CrossRef]
- Nazario, L.H.; Cabrales, M.M.Q.; Quevedo, H.J.M. Obtención de glicerol a partir de la microalga Dunaliella salina. Rev. Cuba. Farm. 2000, 34, 134–137. [Google Scholar]
- Gutiérrez-Casiano, N.; Hernández-Aguilar, E.; Méndez-Contreras, J.M. Biodiesel production as an alternative for energetic valorization of biomass from poultry wastewater through Chlorella vulgaris and Novozyme 435. Can. J. Chem. Eng. 2025, 1–13. [Google Scholar] [CrossRef]
- Mayorga Betancourt, M.A.; López Santamarina, C.A.; López Gómez, M.; Gonzalez Caranton, A.R. Experimental analysis of biodiesel synthesis from palm kernel oil: Empirical model and surface response variables. Reac. Kinet. Mech. Cat. 2020, 131, 297–317. [Google Scholar] [CrossRef]
- ASTM D1298-17; Standard Test Method for Density, Relative Density, or API Gravity of Crude Petroleum and Liquid Petroleum Products by Hydrometer Method. ASTM International: West Conshohocken, PA, USA, 2017.
- ASTM D2500-17; Standard Test Method for Cloud Point of Petroleum Products. ASTM International: West Conshohocken, PA, USA, 2017.
- ASTM D1218-12; Standard Test Method for Refractive Index and Refractive Dispersion of Hydrocarbon Liquids. ASTM International: West Conshohocken, PA, USA, 2012.
- ASTM D130-19; Standard Test Method for Corrosiveness to Copper from Petroleum Products by Copper Strip Test. ASTM International: West Conshohocken, PA, USA, 2019.
- ASTM D93-20; Standard Test Methods for Flash Point by Pensky-Martens Closed Cup Tester. ASTM International: West Conshohocken, PA, USA, 2020.
- ASTM D4530-15; Standard Test Method for Determination of Carbon Residue (Micro Method). ASTM International: West Conshohocken, PA, USA, 2015.
- ASTM D664-11; Standard Test Method for Acid Number of Petroleum Products by Potentiometric Titration. ASTM International: West Conshohocken, PA, USA, 2011.
- EN 14104:2003; Fat and Oil Derivatives—Fatty Acid Methyl Esters (FAME)—Determination of Acid Number. European Committee for Standardization (CEN): Brussels, Belgium, 2003.
- ASTM D445-19; Standard Test Method for Kinematic Viscosity of Transparent and Opaque Liquids (and Calculation of Dynamic Viscosity). ASTM International: West Conshohocken, PA, USA, 2019.
- Estrada-García, J.; Hernández-Aguilar, E.; Romero-Mota, D.I.; Méndez-Contreras, J.M. Influence of anaerobic biotransformation process of agro-industrial waste with Lactobacillus acidophilus on the rheological parameters: Case of study of pig manure. Arch. Microbiol. 2023, 205, 99. [Google Scholar] [CrossRef]
- Malagón-Micán, M.L.; Suárez-Chaparro, M.Y. Influence of the initial concentration of Chlorella vulgaris and CO2 in the production of lipids. Rev. Lasallista Investig. 2021, 17, 59–69. [Google Scholar] [CrossRef]
- Vitova, M.; Bisova, K.; Kawano, S.; Zachleder, V. Accumulation of energy reserves in algae: Form cell cycles to biotechnological applications. Biotechnol. Adv. 2015, 33, 1204–1218. [Google Scholar] [CrossRef]
- Beardall, J.; Raven, J.A. Acquisition of inorganic carbon by microalgae and cyanobacteria. In Microbial Photosynthesis, 1st ed.; Wang, Q., Ed.; Springer: Singapore, 2020; pp. 151–168. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, Z. Advances in the biological fixation of carbon dioxide by microalgae. J. Chem. Technol. Biotechnol. 2021, 96, 1475–1495. [Google Scholar] [CrossRef]
- Naseef, H.H.; Tulaimat, R.H. Transesterification and esterification for biodiesel production: A comprehensive review of catalysts and palm oil feedstocks. Energy Convers. Manag. 2025, 26, 100931. [Google Scholar] [CrossRef]
- Derner, R.B.; Ohse, S.; Villela, M.; Carvalho, S.M.D.; Fett, R. Microalgas, produtos e aplicações. Ciência Rural 2006, 36, 1959–1967. [Google Scholar] [CrossRef]
- Alamu, O.J.; Waheed, M.A.; Jekayinfa, S.O. Determination of optimum temperature for the laboratory preparation of biodiesel from Nigerian palm kernel oil. Energy Sources Part A 2009, 31, 1105–1114. [Google Scholar] [CrossRef]
- Komers, K.; Skopal, F.; Stloulkal, R.; Machek, J. Kinetics and mechanism of the KOH-catalyzed methanolysis of rapeseed oil for biodiesel production. Eur. J. Lipid Sci. Technol. 2002, 104, 728–737. [Google Scholar] [CrossRef]
- Mandal, S.; Kundu, K. Synthesis of biodiesel by KOH-catalyzed methanolysis of flaxseed and determination of fuel properties. Biofuels 2019, 12, 999–1005. [Google Scholar] [CrossRef]
- López, L.; Bocanegra, J.; Malagón-Romero, D. Production of biodiesel from waste cooking oil by transesterification. Ing. Univ. 2015, 19, 155–172. [Google Scholar] [CrossRef]
- Pandey, S.; Narayanan, I.; Selvaraj, R.; Varadavenkatesan, T.; Vinayagam, R. Biodiesel production from microalgae: A comprehensive review on influential factors, transesterification processes, and challenges. Fuel 2024, 367, 131547. [Google Scholar] [CrossRef]
- Bajwa, W.; Ikram, A.; Malik, M.A.I.; Razzaq, L.; Khan, A.R.; Lafit, A.; Hussain, F.; Qazi, A. Optimization of biodiesel yield from waste cooking oil and sesame oil using RSM and ANN techniques. Heliyon 2024, 10, e34804. [Google Scholar] [CrossRef]
- Ashouri, R.; Jafari, D.; Esfandyari, M.; Vatankhah, G.; Mahdavi, M. Valorization of slaughterhouse wastes through transesterification for sustainable biodiesel production using potassium hydroxide as a heterogeneous catalyst. J. Clean. Prod. 2024, 447, 141596. [Google Scholar] [CrossRef]
- Ulukardesler, A.H. Sustainable Biodiesel Production from Turkish Coffee Waste Oil: A Comparative Study with Homogeneous and Heterogeneous Catalysts. Processes 2025, 13, 1002. [Google Scholar] [CrossRef]
- Rajanren, J.R.; Ismail, H.M. Investigation of Chlorella vulgaris as a source for renewable fuel. Biofuels 2016, 8, 37–47. [Google Scholar] [CrossRef]
- Gouveia, L.; Oliveira, A.C. Microalgae as a raw material for biofuels production. J. Ind. Microbiol. Biotechnol. 2009, 36, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Rocha, J.G., Jr.; dos Santos, M.D.R.; Madeira, F.B.; Rocha, S.F.L.S.; Bauerfeldt, G.F.; da Silva, W.L.G.; Salomão, A.A.; Tubino, M. Influence of fatty acid methyl ester composition, acid value, and water content on metallic copper corrosion caused by biodiesel. J. Braz. Chem. Soc. 2019, 30, 1751–1761. [Google Scholar] [CrossRef]
- Sanjurjo, C.; Rivera, N.; Rodríguez, E.; Fernández-González, A.; Battez, A.H. Biodiesel derived from the microalgae Nannochloropsis gaditana and Haematococcus pluvialis: Physicochemical and tribological properties. J. Mol. Liq. 2024, 408, 125391. [Google Scholar] [CrossRef]
- Farrokheh, A.; Tahvildari, K.; Nozari, M. comparison of biodiesel production using the oil of Chlorella vulgaris microalgae by electrolysis and reflux methods using CaO/KOH-Fe3O4 and KF/KOH-Fe3O4 as magnetic nano catalysts. Waste Biomass Valor. 2021, 12, 3315–3329. [Google Scholar] [CrossRef]
- Adbullah, M.; Ali, Z.; Yasin, M.T.; Amarat, K.; Sarwar, F.; Khan, J.; Ahmad, K. Advancements in sustainable production of biofuel by microalgae: Recent insights and future directions. Environ. Res. 2024, 262, 119902. [Google Scholar] [CrossRef]
- Wang, H.; Hu, X.; Elshobary, M.; Sobhi, M.; Zhu, F.; Cui, Y.; Xu, X.; Ni, J.; El-Sheekh, M.; Huo, S. Integrated partial nitrification and Tribonema minus cultivation for cost-effective ammonia recovery and lipid production from slaughterhouse wastewater. Chem. Eng. J. 2024, 492, 152199. [Google Scholar] [CrossRef]
- Ghannam, M.T.; Selim, M.Y.E. Rheological properties of the jojoba biofuel. Sustainability 2021, 13, 6047. [Google Scholar] [CrossRef]
- Kass, M.; Kaul, B.; Armstrong, B.; Szybist, J.; Lobodin, V. Stability, rheological and combustion properties of biodiesel blends with a very-low sulfur fuel oil (VLSFO). Fuel 2022, 316, 123365. [Google Scholar] [CrossRef]
- Köse, S.; Aylanşık, G.; Babagiray, M.; Kocakulak, T. Biodiesel production from waste sunflower oil and engine performance tests. Int. J. Automot. Technol. Manag. 2020, 4, 206–212. [Google Scholar] [CrossRef]
- Zapevalov, M.V.; Sergev, N.S.; Redreev, G.V. Rapeseed oil is the base for biodiesel fuel. IOP Conf. Ser. Earth Environ. Sci. 2021, 688, 012013. [Google Scholar] [CrossRef]
- Zakaria, F.; Lujaji, F.; Kivevele, T. Rheological and Physicochemical Analysis of Nonedible Oils Used for Biodiesel Production. ASC Omega 2022, 7, 37133–37141. [Google Scholar] [CrossRef] [PubMed]


| Parameter | Methods |
|---|---|
| Saponification index | NMX-F-174-SCFI-2014 |
| Acidity index | NMX-F-101-SCFI-2012 |
| Density | NMX-F-075-SCFI-2012 |
| Moisture and volatile material | NMX-F-211-1987 |
| Refractive index | NMX-F-074-S-1981 |
| Random Sequence | Run Order | Concentrations of Catalyst (%) | Molar Ratio |
|---|---|---|---|
| 7 | 1 | 1.0 | 1:9 |
| 5 | 2 | 1.0 | 1:6 |
| 11 | 3 | 1.0 | 1:9 |
| 10 | 4 | 1.5 | 1:6 |
| 1 | 5 | 1.0 | 1:6 |
| 3 | 6 | 1.0 | 1:9 |
| 2 | 7 | 1.5 | 1:6 |
| 12 | 8 | 1.5 | 1:9 |
| 8 | 9 | 1.5 | 1:9 |
| 4 | 10 | 1.5 | 1:9 |
| 6 | 11 | 1.5 | 1:6 |
| 9 | 12 | 1.0 | 1:6 |
| Parameter | Methods | References |
|---|---|---|
| Density | ASTM D1298 | [47] |
| Cloud point | ASTM D2500-17 | [48] |
| Refractive index | ASTM D1218-12 | [49] |
| Copper foil corrosion | ASTM D130-19 | [50] |
| Flash point | ASTM D93 | [51] |
| Carbon residues | ASTM D4530-15 | [52] |
| Acid number | ASTM D664 and EN 14104 | [53,54] |
| Kinematic viscosity | ASTM D445 | [55] |
| FAME | Culture Medium | ||
|---|---|---|---|
| FAME Obtained (%) | Bold Basal (%) [71] | INETI 58 (%) [72] | |
| 14:0 | 1.74 | 2.5 | 3.07 |
| 16:0 | 25.08 | 25.20 | 25.07 |
| 16:1 | 1.63 | 1.80 | 5.25 |
| 16:3 | 0.31 | 0 | 1.27 |
| 16:4 | 1.02 | 0 | 4.06 |
| 18:0 | 1.75 | 8.75 | 0.63 |
| 18:1 | 27.75 | 22.5 | 12.64 |
| 18:2 | 11.98 | 17.25 | 7.19 |
| 18:3 | 20.08 | 18.5 | 19.05 |
| 20:0 | 0 | 0 | 0.09 |
| 20:1 | 0.23 | 0 | 0.93 |
| 20:3 | 0.21 | 0 | 0.83 |
| 20:4 | 0 | 0 | 0.23 |
| 20:5 | 0.12 | 0 | 0.46 |
| Saturated | 28.57 | 36.45 | 28.86 |
| Unsaturated | 63.33 | 60.05 | 51.91 |
| Test (% KOH, Molar Ratio Methanol to Oil) | Density (g/mL) | Refractive Index (-) | FP (°C) | Carbon Residue (-) | Acid Number (mg KOH/g) |
|---|---|---|---|---|---|
| 1.0%, 1:6 | 0.863 ± 0.005 a | 1.476 ± 0.003 a | 161.333 ± 5.033 ab | 0.035 ± 0.005 b | 0.263 ± 0.041 b |
| 1.0%, 1:9 | 0.829 ± 0.016 b | 1.447 ± 0.011 b | 152.666 ± 3.214 b | 0.024 ± 0.004 a | 0.393 ± 0.031 a |
| 1.5%, 1:6 | 0.879 ± 0.010 a | 1.435 ± 0.002 b | 179.666 ± 5.033 a | 0.046 ± 0.006 b | 0.216 ± 0.058 b |
| 1.5%, 1:9 | 0.879 ± 0.011 a | 1.445 ± 0.009 b | 160.666 ± 12.503 ab | 0.043 ± 0.002 a | 0.473 ± 0.011 a |
| Test (% KOH, Oil–Methanol Ratio) | Herschel–Bulkley | Ostwald–de Waele | Newton | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 1.0, 1:6 | 0.0042 a | 0.0081 a | 0.8554 a | 0.9991 | 0.0077 a | 0.8657 a | 0.9991 | 3.99 × 10−3 a | 0.9900 |
| 1.5, 1:6 | 0.0043 a | 0.0085 a | 0.8541 a | 0.9995 | 0.0080 a | 0.8644 a | 0.9995 | 4.15 × 10−3 a | 0.9902 |
| 1.0, 1:9 | 0.0020 b | 0.0080 a | 0.8450 a | 0.9992 | 0.0082 a | 0.8400 a | 0.9992 | 3.74 × 10−3 a | 0.9857 |
| 1.5, 1:9 | 0.0001 c | 0.0080 a | 0.8660 a | 0.9995 | 0.0080 a | 0.8657 a | 0.9995 | 4.21 × 10−3 a | 0.9905 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutiérrez-Casiano, N.; Estrada-García, J.; Díaz-Castellanos, K.; Vicente-Martínez, J.; Ortiz-Sánchez, C.A.; Hernández-Aguilar, E. Production of Biodiesel Employing Chlorella vulgaris Biomass Cultivated in Poultry Effluents. Fuels 2025, 6, 53. https://doi.org/10.3390/fuels6030053
Gutiérrez-Casiano N, Estrada-García J, Díaz-Castellanos K, Vicente-Martínez J, Ortiz-Sánchez CA, Hernández-Aguilar E. Production of Biodiesel Employing Chlorella vulgaris Biomass Cultivated in Poultry Effluents. Fuels. 2025; 6(3):53. https://doi.org/10.3390/fuels6030053
Chicago/Turabian StyleGutiérrez-Casiano, Nayeli, Joaquín Estrada-García, Karla Díaz-Castellanos, José Vicente-Martínez, César Antonio Ortiz-Sánchez, and Eduardo Hernández-Aguilar. 2025. "Production of Biodiesel Employing Chlorella vulgaris Biomass Cultivated in Poultry Effluents" Fuels 6, no. 3: 53. https://doi.org/10.3390/fuels6030053
APA StyleGutiérrez-Casiano, N., Estrada-García, J., Díaz-Castellanos, K., Vicente-Martínez, J., Ortiz-Sánchez, C. A., & Hernández-Aguilar, E. (2025). Production of Biodiesel Employing Chlorella vulgaris Biomass Cultivated in Poultry Effluents. Fuels, 6(3), 53. https://doi.org/10.3390/fuels6030053







