Regioselective Transfer Hydrogenative Defluorination of Polyfluoroarenes Catalyzed by Bifunctional Azairidacycle
Abstract
:1. Introduction
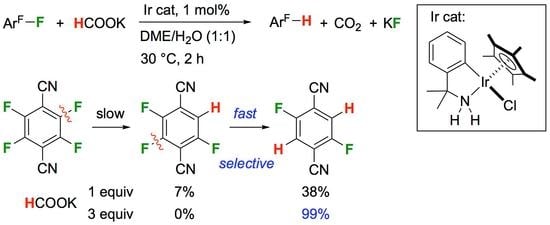
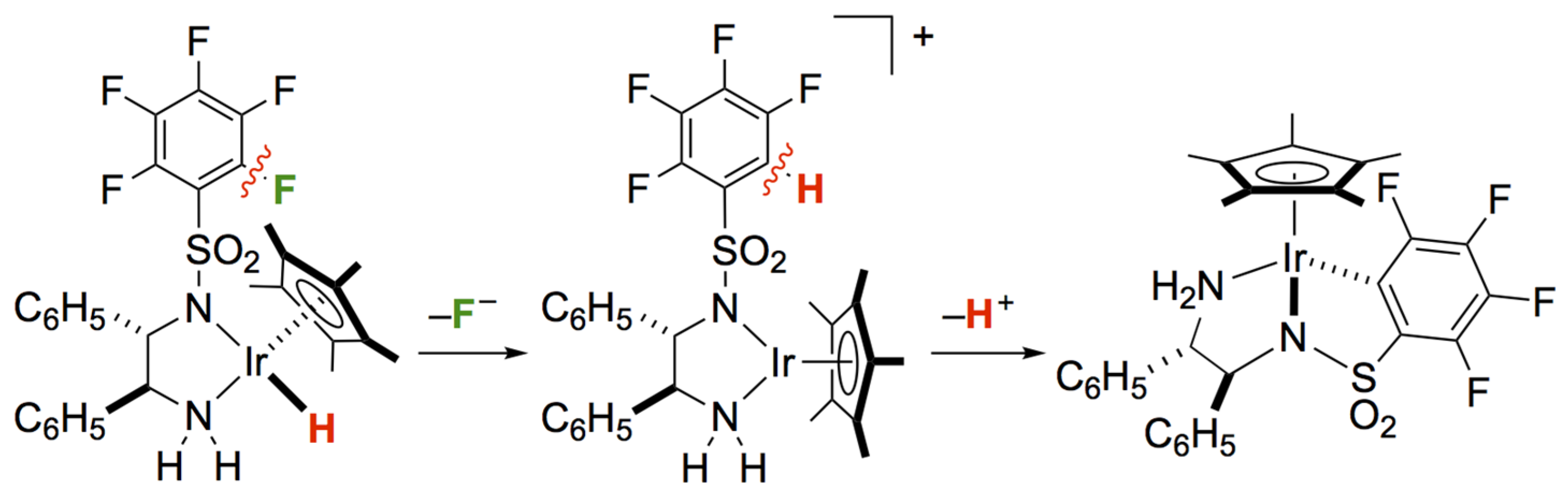
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. Representative Procedures of HDF of Fluoroarenes Using Potassium Formate under the Conditions of S/C = 100 at 30 °C
3.3. Catalytic Hydration of 9b
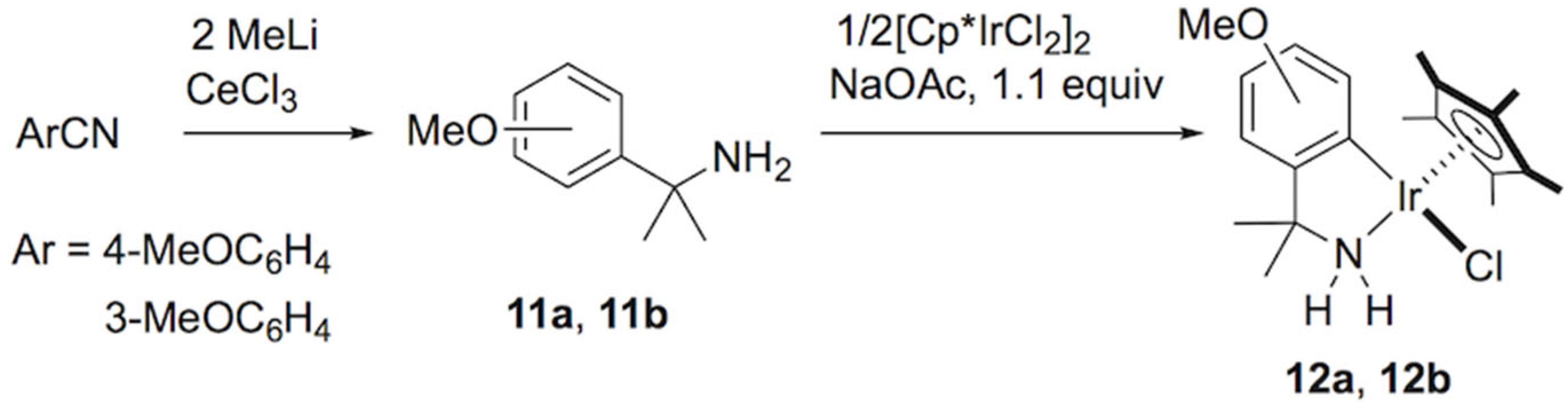
3.4. Synthesis of 11a and 11b
3.5. Synthesis of 12a
3.6. Synthesis of 12b
3.7. Scale-Up Experiment in the Synthesis of 7b
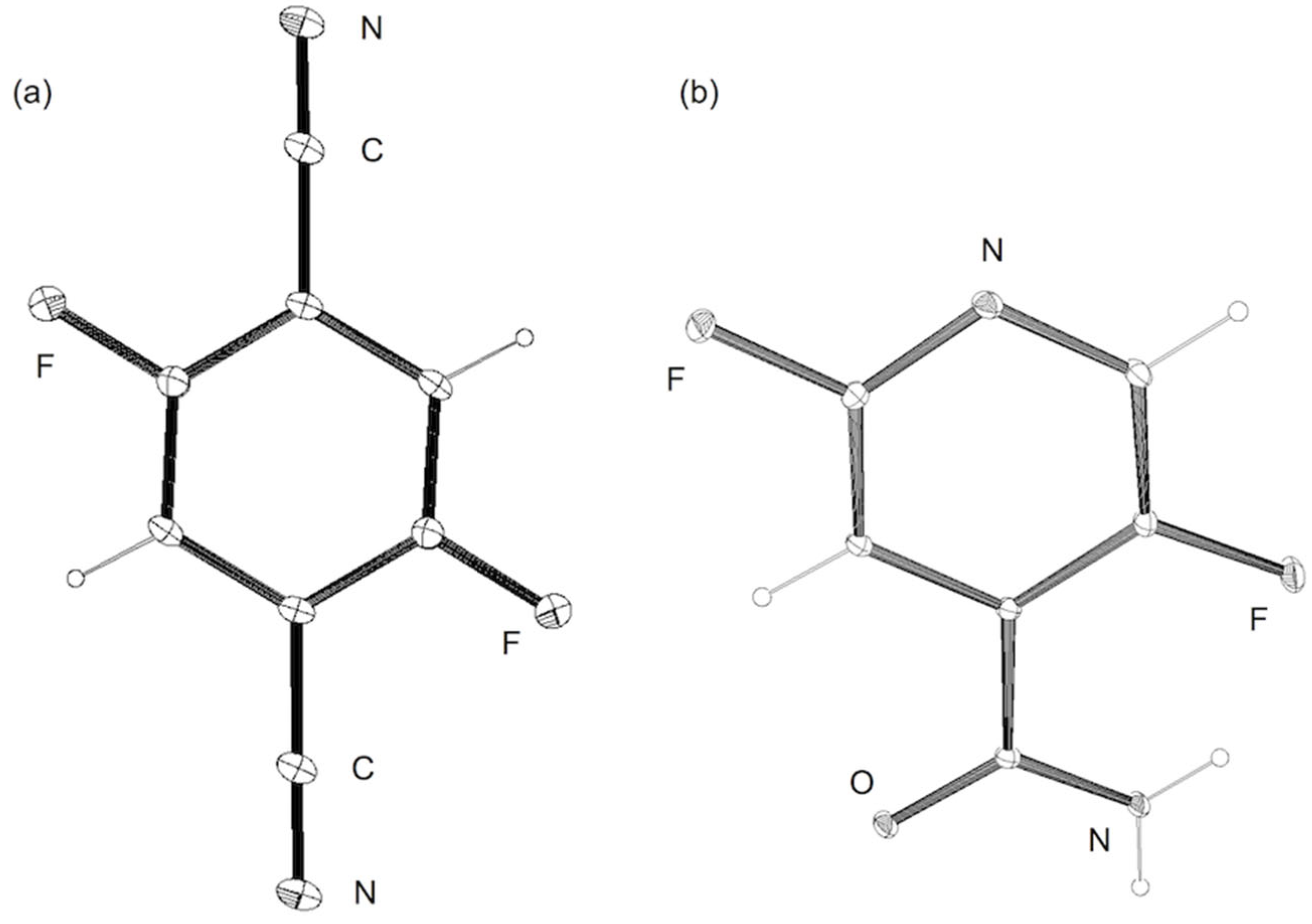
3.8. X-ray Crystal Structure Determination
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Senaweera, S.M.; Singh, A.; Weaver, J.D. Photocatalytic Hydrodefluorination: Facile Access to Partially, Fluorinated Aromatics. J. Am. Chem. Soc. 2014, 136, 3002–3005. [Google Scholar] [CrossRef] [PubMed]
- Weaver, J.; Senaweera, S. C−F activation and functionalization of perfluoro- and polyfluoroarenes. Tetrahedron 2014, 70, 7413–7428. [Google Scholar] [CrossRef]
- Amii, H.; Uneyama, K. C−F Bond Activation in Organic Synthesis. Chem. Rev. 2009, 109, 2119–2183. [Google Scholar] [CrossRef]
- Liang, T.; Neumann, C.N.; Ritter, T. Introduction of Fluorine and Fluorine-Containing Functional Groups. Angew. Chem. Int. Ed. 2013, 52, 8214–8264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campbell, M.G.; Ritter, T. Modern Carbon-Fluorine Bond Forming Reactions for Aryl Fluoride Synthesis. Chem. Rev. 2015, 115, 612–633. [Google Scholar] [CrossRef] [PubMed]
- Champagne, P.A.; Desroches, J.; Hamel, J.-D.; Vandamme, M.; Paquin, J.-F. Monofluorination of Organic Compounds: 10 Years of Innovation. Chem. Rev. 2015, 115, 9073–9174. [Google Scholar] [CrossRef]
- Sather, A.C.; Buchwald, S.L. The Evolution of Pd0/PdII-Catalyzed Aromatic Fluorination. Acc. Chem. Res. 2016, 49, 2146–2157. [Google Scholar] [CrossRef]
- Szpera, R.; Moseley, D.F.J.; Smith, L.B.; Sterling, A.J.; Gouverneur, V. The Fluorinayion of C–H Bonds: Developments and Perspectives. Angew. Chem. Int. Ed. 2019, 58, 14824–14848. [Google Scholar] [CrossRef]
- See, Y.Y.; Morales-Colón, M.T.; Bland, D.C.; Sanford, M.S. Development of SNAr Nucleophilic Fluorination: A Fruitful Academia-Industry Collaboration. Acc. Chem. Res. 2020, 53, 2372–2383. [Google Scholar] [CrossRef]
- Rozatian, N.; Hodgson, D.R.W. Reactivities of electrophilic N–F fluorinating reagents. Chem. Commun. 2021, 57, 683–712. [Google Scholar] [CrossRef]
- Kuehnel, M.F.; Lentz, D.; Braun, T. Synthesis of Fluorinated Building Blocks by Transition-Metal-Mediated Hydrodefluorination Reactions. Angew. Chem. Int. Ed. 2013, 52, 3328–3348. [Google Scholar] [CrossRef] [PubMed]
- Whittlesey, M.K.; Peris, E. Catalytic Hydrodefluorination with Late Transition Metal Complexes. ACS Catal. 2014, 4, 3152–3159. [Google Scholar] [CrossRef]
- Hu, J.-Y.; Zhang, J.-L. Hydrodefluorination Reactions Catalyzed by Transition-Metal Complexes. In Organometallic Fluorine Chemistry; Braun, T., Hughes, R.P., Eds.; Topics in Organometallic Chemistry; Springer: Cham, Switzerland, 2015; Volume 52, pp. 143–196. [Google Scholar]
- Aizenberg, M.; Milstein, D. Catalytic Activation of Carbon-Fluorine Bonds by a Soluble Transition Metal Complex. Science 1994, 265, 359–361. [Google Scholar] [CrossRef] [PubMed]
- Vela, J.; Smith, J.M.; Yu, Y.; Ketterer, N.A.; Flaschenriem, C.J.; Lachicotte, R.J.; Holland, P.L. Synthesis and Reactivity of Low-Coordinate Iron(II) Fluoride Complexes and Their Use in the Catalytic Hydroefluorination of Fluorocarbons. J. Am. Chem. Soc. 2005, 127, 7857–7870. [Google Scholar] [CrossRef]
- Reade, S.P.; Mahon, M.F.; Whittlesey, M.K. Catalytic Hydrodefluorination of Aromatic Fluorocarbons by Ruthenium N-Heterocyclic Carbene Complexes. J. Am. Chem. Soc. 2009, 131, 1847–1861. [Google Scholar] [CrossRef] [PubMed]
- Beltrán, T.F.; Feliz, M.; Llusar, R.; Mata, J.A.; Safont, V.S. Mechanism of the Catalytic Hydrodefluorination of Pentafluoropyridine by Group Six Triangular Cluster Hydrides Containing Phosphines: A Combined Experimental and Theoretical Study. Organometallics 2011, 30, 290–297. [Google Scholar] [CrossRef]
- Fischer, P.; Götz, K.; Eichhorn, A.; Radius, U. Decisive Steps of the Hydrodefluorination of Fluoroaromatics Using [Ni(NHC)2]. Organometallics 2012, 31, 1374–1383. [Google Scholar] [CrossRef]
- Lv, H.; Zhan, J.-H.; Cai, Y.-B.; Yu, Y.; Wang, B.; Zhang, J.-L. π–π Interaction Assisted Hydrodefluorination of Perfluoroarenes by Gold Hydride: A Case of Synergistic Effect on C–F Bond Activation. J. Am. Chem. Soc. 2012, 134, 16216–16227. [Google Scholar] [CrossRef]
- Chen, Z.; He, C.-Y.; Yin, Z.; Chen, L.; He, Y.; Zhang, X. Palladium-Catalyzed Ortho-Selective C–F Activation of Polyfluoroarenes with Triethylsilane: A Facile Access to Partially Fluorinated Aromatics. Angew. Chem. Int. Ed. 2013, 52, 5813–5817. [Google Scholar] [CrossRef]
- Podolan, G.; Lentz, D.; Reissig, H.-U. Selective Catalytic Hydrdefluorination as a Key Step for the Synthesis of Hitherto Inaccessible Aminopyridine Derivatives. Angew. Chem. Int. Ed. 2013, 52, 9491–9494. [Google Scholar] [CrossRef]
- Arévalo, A.; Tlahuext-Aca, A.; Flores-Alamo, M.; García, J.J. On the Catalytic Hydrodefluorination of Fluoroaromatics Using Nickel Complexes: The True Role of the Phosphine. J. Am. Chem. Soc. 2014, 136, 4634–4639. [Google Scholar] [CrossRef] [PubMed]
- Hein, N.M.; Pick, F.S.; Fryzuk, M.D. Synthesis and Reactivity of a Low-Coordinate Iron(II) Hydride Complex: Applications in Catalytic Hydrodefluorination. Inorg. Chem. 2017, 56, 14513–14523. [Google Scholar] [CrossRef] [PubMed]
- Yow, S.; Gates, S.J.; White, A.J.P.; Crimmin, M.R. Zirconocene Dichloride Catalyzed Hydrodefluorination of Csp3−F bonds. Angew. Chem. Int. Ed. 2012, 51, 12559–12563. [Google Scholar] [CrossRef] [PubMed]
- Ekkert, O.; Strudley, S.D.A.; Rozenfeld, A.; White, A.J.P.; Crimmin, M.R. Rhodium Catalyzed, Carbon−Hydrogen Bond Directed Hydrodefluorination of Fluoroarenes. Organometallics 2014, 33, 7027–7030. [Google Scholar] [CrossRef]
- Aizenberg, M.; Milstein, D. Homogeneous Rhodium Complex-Catalyzed Hydrogenolysis of C–F Bonds. J. Am. Chem. Soc. 1995, 117, 8674–8675. [Google Scholar] [CrossRef]
- Edelbach, B.L.; Jones, W.D. Mechanism of Carbon–Fluorine Bond Activation by (C5Me5)Rh(PMe3)H2. J. Am. Chem. Soc. 1997, 119, 7734–7742. [Google Scholar] [CrossRef]
- Young, R.J., Jr.; Grushin, V.V. Catalytic C–F Bond Activation of Nonactivated Monofluoroarenes. Organometallics 1999, 18, 294–296. [Google Scholar] [CrossRef]
- Braun, T.; Noveski, D.; Ahijado, M.; Wehmeier, F. Hydrodefluorination of pentafluoropyridine at rhodium using dihydrogen: Detection of unusual rhodium hydrido complexes. Dalton Trans. 2007, 34, 3820–3825. [Google Scholar] [CrossRef]
- Konnick, M.M.; Bischof, S.M.; Periana, R.A.; Hashiguchi, B.G. The Hydroxide-Promoted Catalytic Hydrodefluoriation of Fluorocarbons by Ruthenium in Aqueous Media. Adv. Synth. Catal. 2013, 355, 632–636. [Google Scholar] [CrossRef]
- Nakai, H.; Jeong, K.; Matsumoto, T.; Ogo, S. Catalytic C–F Bond Hydrogenolysis of Fluoroaromatics by [(η2-C5Me5)RhI(2,2’-bipyridine)]. Organometallics 2014, 33, 4349–4352. [Google Scholar] [CrossRef]
- Moore, J.T.; Lu, C.C. Catalytic Hydrogenolysis of Aryl C–F Bonds Using a Bimetallic Rhodium–Indium Complex. J. Am. Chem. Soc. 2020, 142, 11641–11646. [Google Scholar] [CrossRef] [PubMed]
- Matsunami, A.; Kuwata, S.; Kayaki, Y. Hydrodefluorination of Fluoroarenes Using Hydrogen Transfer Catalysts with a Bifunctional Iridium/NH Moiety. ACS Catal. 2016, 6, 5181–5185. [Google Scholar] [CrossRef]
- Matsunami, A.; Kuwata, S.; Kayaki, Y. Hydrogen Evolution from Formic Acid and Hydrodefluorination of Fluoroarenes by Bifunctional Iridium Catalysts—Beyond the Transfer Hydrogenation. J. Synth. Org. Chem. Jpn. 2018, 76, 315–324. [Google Scholar] [CrossRef]
- Matsunami, A.; Kayaki, Y.; Kuwata, S.; Ikariya, T. Nucleophilic Aromatic Substitution in Hydrodefluorination Exemplified by Hydridoiridium(III) Complexes with Fluorinated Phenylsulfonyl-1,2-diphenylethylenediamine Ligands. Organometallics 2018, 37, 1958–1969. [Google Scholar] [CrossRef]
- Dub, P.A.; Wang, H.; Matsunami, A.; Gridnev, I.D.; Kuwata, S.; Ikariya, T. C−F Bond Breaking through Aromatic Nucleophilic Substitution with a Hydroxo Ligand Mediated via Water Bifunctional Activation. Bull. Chem. Soc. Jpn. 2013, 86, 557–568. [Google Scholar] [CrossRef] [Green Version]
- Dub, P.A.; Matsunami, A.; Kuwata, S.; Kayaki, Y. Cleavage of N−H Bond of Ammonia via Metal–Ligand Cooperation Enables Rational Design of a Conceptually New Noyori-Ikariya Catalyst. J. Am. Chem. Soc. 2019, 141, 2661–2677. [Google Scholar] [CrossRef]
- McKay, D.; Riddlestone, I.M.; Macgregor, S.A.; Mahon, M.F.; Whittlesey, M.K. Mechanistic Study of Ru-NHC-Catalyzed Hydrodefluorination of Fluoropyridines: The Influence of the NHC on the Regioselectivity of C–F Activation and Chemoselectivity of C–F versus C–H Bond Cleavage. ACS Catal. 2015, 5, 776–787. [Google Scholar] [CrossRef] [Green Version]
- Cybulski, M.K.; McKay, D.; Macgregor, S.A.; Mahon, M.F.; Whittlesey, M.K. Room Temperature Regioselective Catalytic Hydrodefluorination of Fluoroarenes with trans-[Ru(NHC)4H2] through a Concerted Nucleophilic Ru–H Attack Pathway. Angew. Chem. Int. Ed. 2017, 56, 1515–1519. [Google Scholar] [CrossRef] [Green Version]
- Kvíčala, J.; Beneš, M.; Paleta, O.; Král, V. Regiospecific nucleophilic substitution in 2,3,4,5,6-pentafluorobiphenyl as model compound for supramolecular systems. Theoretical study of transition states and energy profiles, evidence for tetrahedral SN2 mechanism. J. Fluorine Chem. 2010, 131, 1327–1337. [Google Scholar] [CrossRef]
- Kikushima, K.; Koyama, H.; Kodama, K.; Dohi, T. Nucleophilic Aromatic Substitution of Polyfluoroarene to Access Highly Functionalized 10-Phenylphenothiazine Derivatives. Molecules 2021, 26, 1365. [Google Scholar] [CrossRef]
- Zimmerman, H.E. Orientation in Metal Ammonia Reduction. Tetrahedron 1961, 16, 169–176. [Google Scholar] [CrossRef]
- Chambers, R.D.; Seabury, M.J.; Lyn, D.; Williams, H.; Hughes, N. Mechanisms for Reactions of Halogenated Compounds. Part 5. Orientating Effects of Fluorine Substituents on Nucleophilic Substitution in Naphthalene and Other Polycyclic Systems. J. Chem. Soc. PerkinTrans. 1988, 1, 251–254. [Google Scholar] [CrossRef]
- Baker, J.; Muir, M. The Meisenheimer model for predicting the principal site for nucleophilic substitution in aromatic perfluorocarbons—Generalization to include ring-nitrogen atoms and non-fluorine ring substituents. Can. J. Chem. 2010, 88, 588–597. [Google Scholar] [CrossRef]
- Liljenberg, M.; Brinck, T.; Herschend, B.; Rein, T.; Tomasi, S.; Svensson, M. Predicting Regioselectivity in Nucleophilic Aromatic Substitutiton. J. Org. Chem. 2012, 77, 3262–3269. [Google Scholar] [CrossRef] [PubMed]
- Liljenberg, M.; Brinck, T.; Rein, T.; Svensson, M. Utilizing the σ-complex stability for quantifying reactivity in nucleophilic substitution of aromatic fluorides. Beilstein J. Org. Chem. 2013, 9, 791–799. [Google Scholar] [CrossRef]
- Sadowsky, D.; McNeill, K.; Cramer, C.J. Thermochemical Factors Affecting Dehalogenation of Aromatics. Environ. Sci. Technol. 2013, 47, 14194–14203. [Google Scholar] [CrossRef]
- Sadowsky, D.; McNeill, K.; Cramer, C.J. Dehalogenation of Aromatics by Nucleophilic Aromatic Substitution. Environ. Sci. Technol. 2014, 48, 10904–10911. [Google Scholar] [CrossRef]
- Crucius, G.; Lyubimtsev, A.; Kramer, M.; Hanack, M.; Ziegler, T. 1,4,8,11,15,18,22,25-Octafluorophthalocyaninato Zinc (F8PcZn). Synlett 2012, 23, 2501–2503. [Google Scholar]
- Banks, R.E.; Burgess, J.E.; Cheng, W.M.; Haszeldine, R.N. Heterocyclic Polyfluoro-Compounds. Part IV. Nucleophilic Substitution in Pentafluoropyridine: The Preparation and Properties of Some 4-Substituted 2,3,5,6-Tetrafluoropyridines. J. Chem. Soc. 1965, 67, 575–581. [Google Scholar] [CrossRef]
- Schoch, T.D.; Mondal, M.; Weaver, J.D. Catalyst-Free Hydrodefluorination of Perfluoroarenes with NaBH4. Org. Lett. 2021, 23, 1588–1593. [Google Scholar] [CrossRef]
- Beregovaya, I.V.; Shchegoleva, L.N. Potential Energy Surface and dissociative cleavage of chlorobenzene radial anion. Chem. Phys. Lett. 2001, 348, 501–506. [Google Scholar] [CrossRef]
- Marcé, P.; Lynch, J.; Blacker, A.J.; Williams, J.M.J. A mild hydration of nitriles catalyzed by copper(II) acetate. Chem. Commun. 2016, 52, 1436–1438. [Google Scholar] [CrossRef] [PubMed]
- Ciganek, E.J. Tertiary Carbinamines by Addition of Organocerium Reagents to Nitriles and Ketimines. Org. Chem. 1992, 57, 4521–4527. [Google Scholar] [CrossRef]
- Arita, S.; Koike, T.; Kayaki, Y.; Ikariya, T. Synthesis and Reactivities of Cp*Ir Amide and Hydride Complexes Bearing C−N Chelate Ligands. Organometallics 2008, 27, 2795–2802. [Google Scholar] [CrossRef]
- Sato, Y.; Kawata, Y.; Yasui, S.; Kayaki, Y.; Ikariya, T. New Bifunctional Bis(azairidacycle) with Axial Chirality via Double Cyclometalation of 2,2’-Bis(aminomethyl)-1,1’-binaphthyl. Molecules 2021, 26, 1145. [Google Scholar] [CrossRef] [PubMed]
- Gallardo-Macias, R.; Kumar, P.; Jaskowski, M.; Richmann, T.; Shrestha, R.; Russo, R.; Singleton, E.; Zimmerman, M.D.; Ho, H.P.; Dartois, V.; et al. Optimization of N-benzyl-5-nitrofuran-2-carboxamide as an antitubercular agent. Bioorg. Med. Chem. Lett. 2019, 29, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Jaudzems, K.; Tars, K.; Maurops, G.; Ivdra, N.; Otikovs, M.; Leitans, J.; Kanepe-Lapsa, I.; Domraceva, I.; Mutule, I.; Trapencieris, P.; et al. Plasmepsin Inhibitory Activity and Structure-Guided Optimization of a Potent Hydroxyethylamine-Based Antimalarial Hit. ACS Med. Chem. Lett. 2014, 5, 373–377. [Google Scholar] [CrossRef] [Green Version]
- Rigaku Coorporation. CrystalStructure 4.1: Crystal Structure Analysis Package; Rigaku Coorporation: Tokyo, Japan, 2015. [Google Scholar]
- Altomare, A.; Cascarano, G.; Giacovazzo, C.; Guagliardi, A.; Burla, M.; Polidori, G.; Camalli, M. SIR92—A program for automatic solution of crystal structures by direct methods. J. Appl. Cryst. 1994, 27, 435. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef]



| Entry | Substrate | HCOOK, eq | Time, h | % Yield 2 | |
|---|---|---|---|---|---|
| 1 |  2a | 2 | 1 | 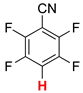 3a, 100 | |
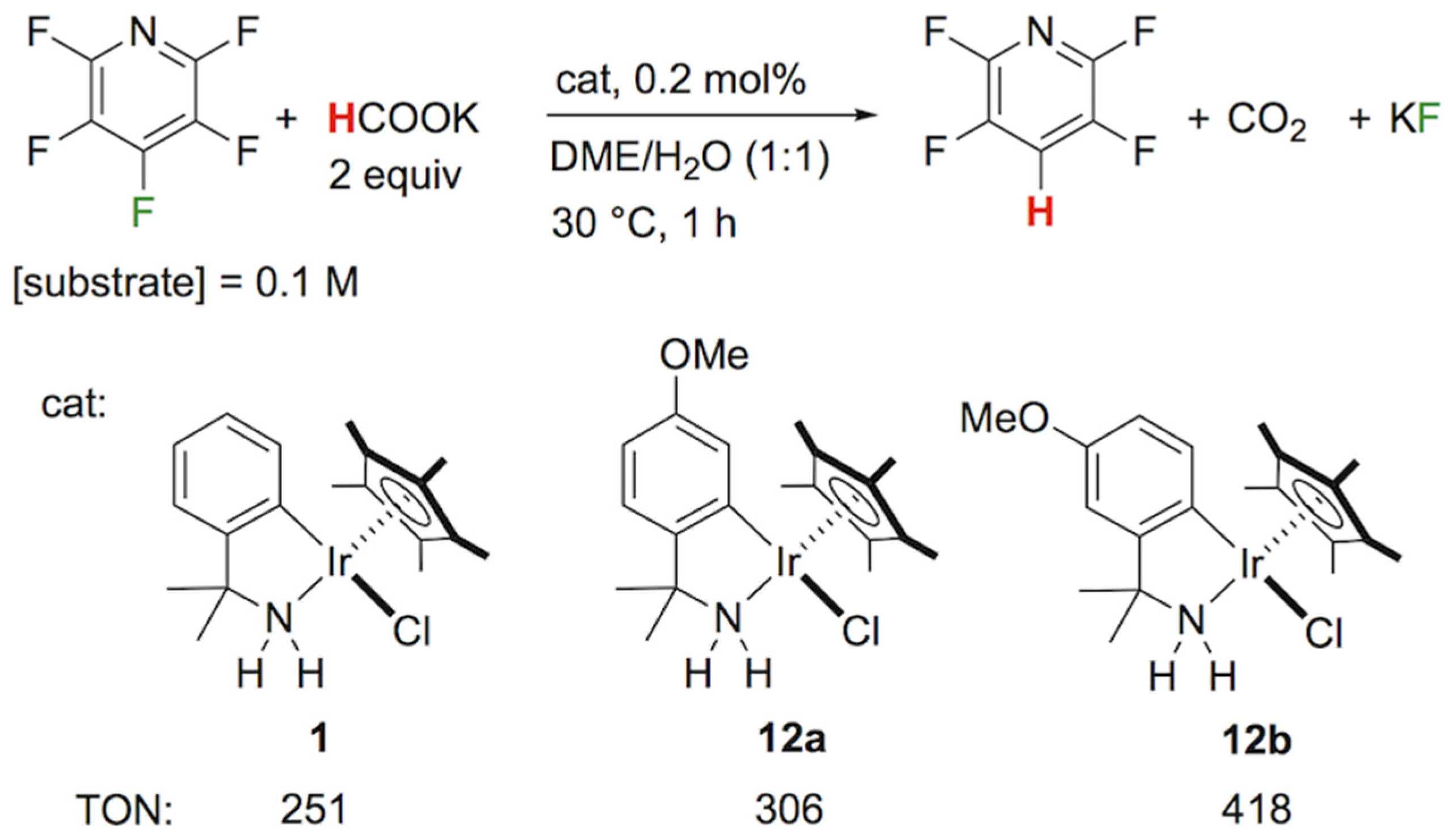
| 2 | 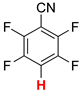 3a | 2 | 12 |  4, 20 | |
| 3 3 |  3b | 2 | 2 | 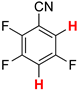 4, 100 | |
| 4 4 | C6F6 2b | 2 | 12 | N.R. | |
| 5 |  5a | 1 | 2 |  6a, 7 |  7a, 38 |
| 6 | 5a | 3 | 2 | 6a, 0 | 7a, 99 |
| 7 | 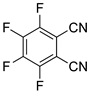 5b | 1 | 1 | 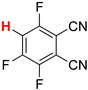 6b, 90(88) 5 | 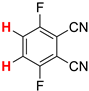 7b, 2 |
| 8 | 5b | 3 | 1 | 6b, 0 | 7b, 100(86) 5 |
| 9 |  8a | 2 | 1 | 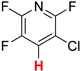 9a, 100 | |
| 10 | 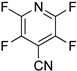 8b | 2 | 1 | 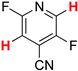 9b, 98 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsunami, A.; Kuwata, S.; Kayaki, Y. Regioselective Transfer Hydrogenative Defluorination of Polyfluoroarenes Catalyzed by Bifunctional Azairidacycle. Organics 2022, 3, 150-160. https://doi.org/10.3390/org3030012
Matsunami A, Kuwata S, Kayaki Y. Regioselective Transfer Hydrogenative Defluorination of Polyfluoroarenes Catalyzed by Bifunctional Azairidacycle. Organics. 2022; 3(3):150-160. https://doi.org/10.3390/org3030012
Chicago/Turabian StyleMatsunami, Asuka, Shigeki Kuwata, and Yoshihito Kayaki. 2022. "Regioselective Transfer Hydrogenative Defluorination of Polyfluoroarenes Catalyzed by Bifunctional Azairidacycle" Organics 3, no. 3: 150-160. https://doi.org/10.3390/org3030012