Abstract
Organic molecules are gaining special attention over the last years in the corrosion area thanks to their general low achievable cytotoxicity, structural versatility, and environmentally friendly obtainment methods. Under those approaches, synthetic organic motifs have attracted the interest of researchers due to their variated methods of obtention through molecular manipulation via diverse chemical reactions, allowing the production of adequately planned structures or repurposing their original application in the case of drugs. This review summarizes general aspects that are desired in organic molecules as corrosion inhibitors, presenting selected works published in the 2022–2023 period and emphasizing the importance of finding novel and different organic corrosion inhibitors. Patents were not considered in this review. Scifinder, Google Scholar, and Web of Science were employed as databases. Mathematical and analytical methods involved in the search for corrosion inhibitors are out of this review’s scope.
1. Introduction
1.1. Corrosion Process Fundamentals: Chemistry, Society, Economy, and Environment
The corrosion process can be generically described as a situation where an electron is lost from a substance or material, leading to its oxidation. It has been chemically described so far as M(s) → M+ + e−, wherein M is the oxidized metallic material, i.e., iron. Due to its exergonic nature, the corrosion process is a thermodynamically favorable phenomenon that occurs in metallic structures, given the metal’s lower energy in its oxidized state. A key point that must be noticed is that oxidation occurs simultaneously with reduction, characterizing a redox process. Therefore, there must be an oxidizing agent to receive those lost electrons. Many conditions accelerate the process of oxidation, such as the presence of salt water, acidic medium (pH < 4 to remove protective oxide films), temperature, metal reactivity, metal impurities, carbon dioxide, and high levels of oxygen [1,2]. Being a process contrary to a sustainable purpose, corrosion demands the constant replacement of disposable pieces in corrosion-favorable environments, which boosts waste production and pollution. This is particularly disadvantageous in high-productivity industries with processes relying on long pipelines, i.e., oil/gas distribution [3]. Additionally, in cases where the parts cannot be replaced, such as in monuments and large bleachers, the repair costs, and hazardous conditions for humans are the primary drawbacks of the corrosion process. Globally, corrosion is estimated to cost up to USD four trillion annually. [4].
1.2. Organic Molecules in the Corrosion Inhibition Process
Organic corrosion inhibitors form a uniform passive film on the metal surface, specifically in the case of green inhibitors protecting the structure/material from the aggressive oxidizing agents [5]. That film is adsorbed in the metal’s surface physically, chemically, or in a mixed type of both. Additionally, the ideal molecule should be in an optimal range of interaction force with the metal’s surface according to Sabatier’s principle, independently of the interaction type. If it is too strong, it might weaken neighboring atomic interactions on the metal structure and cause metal dissolution. Otherwise, if it is too weak, the molecule will not protect the surface adequately, as it will not persist on the metal surface [6]. So, in the end, the type of adsorption does not determine whether the organic molecule will achieve its goal or fail. Moreover, to reduce and/or avoid the corrosion process, corrosion inhibitors should be employed with the lowest concentration possible. If a given inhibitor needs to be in a high concentration to be effective, a concerning collateral effect is that it might act against the structure preservation due to the over-formation of inhibitor–metal interactions, which will exert a similar effect as strong interactions [7].
Many mathematic methods for the adsorption mechanisms, namely adsorption isotherms (i.e., Langmuir, Freundlich, Langmuir–Freundlich), and experimental methods to evaluate the corrosion inhibition potential (i.e., weight loss measurement, electrochemical impedance spectroscopy, and scanning electron microscopy) are extensively described in the literature [8,9,10,11]. However, it is out of this article’s scope to conduct mathematical and experimental approaches rather than focus on the chemical aspects regarding the corrosion inhibition potential of organic molecules.
Although there have been some good correlations, relying solely on the difference between HOMO and LUMO energies to predict inhibition efficiency is not enough, and other patterns or factors should be taken into consideration to improve the accuracy of predictions, as many factors play key roles in the corrosion process, such as the surface model, force fields, electrified solid/liquid interfaces, and formed and broke bonds [12,13,14]. As Kokalj and Costa [15] elegantly said, “correlation does not imply causation”. While it poses a challenge, predicting viable organic molecules as corrosion inhibitors is still a worthwhile and feasible task. To achieve better results, the corrosion inhibitor molecules must comply with some requisites, such as being water soluble, having proper functional groups, being synthesized through greener synthetic routes, and having recurrent molecular frameworks [16,17]. In this article, we emphasize the following factors that have been established as key molecular features to achieve promising corrosion inhibition, which encompasses functional groups and molecular frameworks, such as the presence of atoms with an unpaired electron pair (P, N, O, and S), unsaturated bonds (sp2 and sp carbons) and aromatic portions π-electrons, heterocyclic nucleus, and electron donor substituents (i.e., -OCH3, -OH, -SH, -CH3, and -NH2) [2,7]. It is noteworthy that organic frameworks can easily achieve those characteristics if they are not intrinsic through many known types of reactions (i.e., reduction, oxidation, cyclization, and nucleophilic substitution) [18]. With the pertinent structural components, the inhibitor’s unpaired and π-electrons can adequately interact with the metal atoms’ d-orbitals and initiate the formation of a protective film through donor–acceptor interaction, characterizing chemical adsorption. However, when the molecule and the metal surface are electrically charged, electrostatic attraction forces can rule their interaction and cause physical adsorption (Figure 1) [5,19].
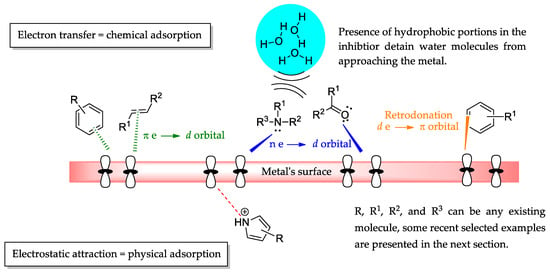
Figure 1.
Generic organic molecules’ structural characteristics and their role in corrosion inhibition.
To overcome the environmental, economic, and social challenges regarding the corrosion process, organic corrosion inhibitors emerged as an elegant solution in the early 1980s [20]. We can highlight, as their main benefits, the lower or lack of significative toxicity, which directs them towards a greener solution when compared, for example, with the inorganic ones based on chromates [21] and molybdates [22]. As a direct consequence of the achievement of greener inhibitors, drugs have been explored as corrosion inhibitors [23,24,25]. As examples, the antitubercular drug streptomycin [26] and the antibacterial drug penicillin G [27] demonstrated very good results, inhibiting as high as 90% of the corrosion process (Figure 2). It is worth noting that drugs usually detain the major structural aspects desired to achieve high corrosion inhibition potentials, such as heteroatoms and balance between hydrophobic and hydrophilic groups. Additionally, amino acids, natural extracts, carbohydrates, polymers, and gums are possible green chemistry solutions as organic compounds against corrosion (Figure 2) [8,14,28,29,30,31,32,33,34,35]. Moreover, macrocyclic compounds are a particularly interesting class of organic molecular motifs. The organized heteroatoms, specifically nitrogen and oxygen, present in certain molecules facilitate closer sp2 hybridization and improve electronic interactions with the metal surface. As a result, these molecules can form more efficient hydrophobic films, leading to better metal–inhibitor interactions and improved corrosion inhibition [8]. Those aspects can be observed in the promising corrosion inhibition potential of MOAT and BMOAT, where the incorporation of hydrophobic benzene units in the macrocycle motif (BMOAT) greatly increased the corrosion inhibition potency (Figure 2) [36].
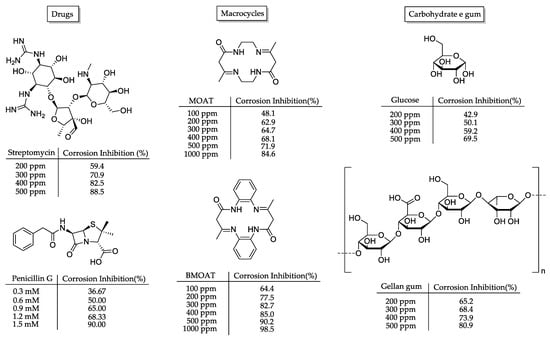
Figure 2.
Examples of organic molecules (i.e., drugs [26,27], macrocycles [36], carbohydrates, and gum [29]) and their related corrosion inhibition potential relative to weight loss assays in acid medium.
2. Synthetic Organic Corrosion Inhibitors
Using the search input “synthetic and organic corrosion inhibitor -review -extract” Google Scholar found a great number of almost 1500 works published in the 2022–2023 period. Web of Science was consulted using the search input “(synthetic AND organic corrosion inhibitors) NOT review NOT extract” and found only one pertinent reference. Lastly, SciFinder found 121 references employing the same search input of Web of Science. Therefore, we selected examples to exemplify each of the desired molecular characteristics of the synthetic organic corrosion inhibitors presented in the last section, summarized the diversity of molecular functionalities comprising the organic chemistry world, and wrote a reader-friendly revision work. The main points taken into consideration to select the works were green synthetic approaches and the different molecular motifs/organic functions. Ionic liquids, metal–organic frameworks, covalent organic frameworks, nanoparticles, and complexes were not considered for this article’s scope. Many organic molecules present two or more functions/motifs (i.e., a triazole nucleus and a Schiff base in the same molecule), and they will be designated by their generic names assigned by the authors.
2.1. Mannich Bases
Since the early 1960s, Mannich bases attracted the attention of synthetic chemists [37,38], mainly due to their abundance of organic functions (ketone, amine, and alcohol), leading to a versatile molecular motif with application purposes that vary from medicinal chemistry [39] to polymer chemistry [40]. After more than 60 years, Zhang and colleagues [41] synthesized in a single-step protocol three Bis-Mannich bases differing only by the size of the central aliphatic chain (Figure 3) as corrosion inhibitors. M4M, M6M, and M8M contain central chains with four, six, and eight carbon atoms, respectively. Briefly, the synthesis protocol consisted of the dissolution of the adequate diamine compound in the alcoholic medium at acidic pH followed by the addition of formaldehyde and acetophenone. This simple reactional workup is in line with a green chemistry initiative using benign solvents and reagents and presents excellent atomic efficiency (over 70% in this example) [42,43]. In the corrosion inhibition assays based on the weight loss method, all the compounds presented excellent results (over 85% corrosion inhibition) under the lowest concentrations tested (0.05% wt) and at high temperatures. This is indicative of their excellent stability and surface adhesion. In high-temperature assays, M8M demonstrated exceptional potential as a corrosion inhibitor compared to commercially available inhibitors HB-3 and HT-196. All three compounds achieved over 99% inhibition at 110 °C and 120 °C. Lastly, contact angle assays pointed higher angles for longer aliphatic chains, and this implies a better displacement of water molecules by the M8M derivative, which is more hydrophobic.
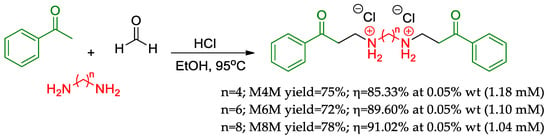
Figure 3.
Mannich bases (M4M, M6M, and M8M) synthesized by Zhang and colleagues [41] through a single-step reaction with high atomic efficiency and presented outstanding corrosion inhibition potential (η).
2.2. Schiff Bases
By reacting different primary amines with vanillin, a natural nontoxic compound, Fernandes and coworkers [44] synthesized three imines as corrosion inhibitors, namely VTRIS, VCNG, and VTOS (Figure 4). The synthetic approach involved a single-step protocol employing low-toxicity solvent and reagents with excellent atomic efficiency and allowed the recovery of almost 87% of the solvent used in every reaction. Weight loss measurements pointed out that at the highest concentration tested for each compound, only VCNG achieved over 90% corrosion inhibition. The analysis of the VTRIS and VTOS structures shows that spatial disposition also plays a key role in the formation of the covering film. VTRIS achieved the same inhibition potential as VTOS but with twice its concentration, implying that the sp3 carbon configuration was not as efficient as the planar structure of an aromatic ring.
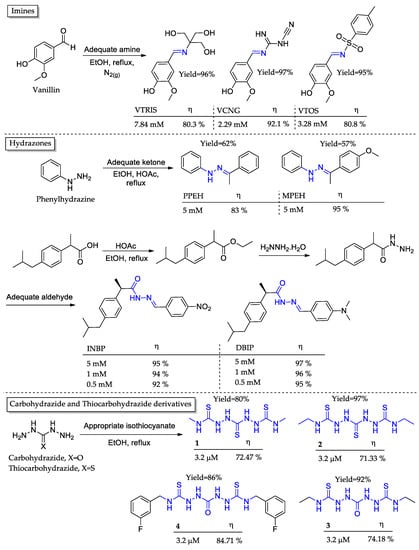
Figure 4.
Synthesized Schiff bases and Schiff base derivative (1–4), detached in blue as corrosion inhibitors and its corrosion inhibition potential (η) in acidic media and at 303 K.
Under a different approach, Zinad and coworkers [45] synthesized two hydrazone derivatives as corrosion inhibitors, namely PPEH and MPEH (Figure 4). The single-step synthetic protocol involved the reflux of both reactants (phenylhydrazine and an adequate ketone) under alcoholic media with catalytic acetic acid. The weight loss assay pointed out that the presence of an electron donor substituent exerted major improvement in the anti-corrosive potential, as MPEH presented an 8% increase compared to PPEH at the same concentration (5 mM).
Moreover, in the Schiff bases approach, Haitout and coworkers [46] synthesized two hydrazones as corrosion inhibitors (Figure 4). The only difference between the compounds was the substituent at one of the aromatic rings. INBP and DBIP present a nitrous group and a diethylamino group as substituents, respectively. Weight loss assays showed that the electron donor substituent demonstrated similar corrosion inhibition potential. Specifically, at a concentration of 5 mM, DBIP and INBP exhibited corrosion inhibition rates of 97% and 95%, respectively. The explanation relied on the acidic media potential to reduce the nitro group to an electron-rich amino group, which leads to similar corrosion inhibition potentials. Moreover, the saturation of inhibitors at higher concentrations led to metal dissolution without a significant increase in corrosion inhibition.
Another work comprising Schiff bases derivatives reports the synthesis of two thiocarbohydrazide (1 and 2) and two carbohydrazide (3 and 4) derivatives (Figure 4). [47] The synthetic protocol comprised a single-step procedure where the reactants were mixed under reflux in the ethanolic medium. The excellent yields (80–97%) corroborated the atomic efficiency premise of green chemistry. The weight loss assay evidenced that there was no significant difference between the sulfonated and oxygenated derivatives, as well as methylated or ethylated terminations. However, the exception is the aromatic derivative 4, which has approximately 10% more inhibition activity than the others). Despite being an electron-poor system due to fluoride electronegativity, its planarity and plenty of unsaturated bonds from the aromatic system seemed to be paramount for hydrophobic interactions and to form a more effective protection film on the metal’s surface.
In line with the examples of Schiff base derivatives discussed herein, Bimoussa and colleagues synthesized a semicarbazone (SMC) and a thiosemicarbazone (TSC) from the enone (Figure 5) [48]. Potentiodynamic polarization was employed to measure the inhibition potential of the three compounds. It was observed, via theoretical and experimental approaches, that C = S and C = O groups had a minor influence on the anticorrosive activity, and nitrogen atoms played a key role in the increase in enone activity.
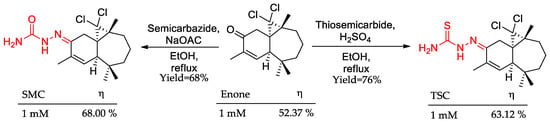
Figure 5.
Carbazone and thiosemicarbazone, detached in red, as examples of Schiff base planned as corrosion inhibition and its inhibition potential (η).
2.3. Six-Membered Heterocyclic Nucleus
Mehta and coworkers [49] designed two pyrimidine derivatives (5–6) as corrosion inhibitors (Figure 6). The synthetic protocol to obtain those six-membered heterocyclic derivatives bearing two nitrogen atoms consisted of a single-step procedure employing low toxicity or nontoxic cheap reagents/solvent. The weight loss assays pointed to better inhibition for derivative 6 at every tested concentration (up to 97% against 93.9% from 5 at 250 ppm). This difference was explained by the superior electron donating potential for the empty metal’s d-orbitals of the cyano group over chloride atom and sulfur over oxygen. Interestingly, a further increase in the inhibitor’s concentration did not result in better inhibition potential since, at this point, the metal surface is completely covered.
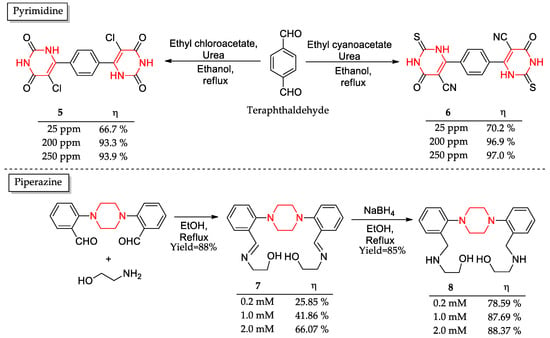
Figure 6.
Synthesized molecules containing a six-membered heterocyclic nucleus detached in red as corrosion inhibitors and its corrosion inhibition potential (η).
Another class of six-membered heterocyclic compounds synthesized as corrosion inhibitors involves piperazine base ligands (Figure 6) [50]. Piperazine derivative 7 was achieved in a single-step reaction with excellent yields as well as its reduced derivative 8. To evaluate the corrosion inhibition potential of the compounds, electrochemical impedance spectroscopy was used. The results showed that the reduced derivative 8 was more effective, exhibiting over 20% higher inhibition than compound 7. Further analysis showed that the enhanced inhibition efficiency of compound 8 could be attributed to its superior stability in acidic media compared to compound 7. Lastly, a theoretical assay comprising HOMO and LUMO energy difference, namely GAP, pointed out that compound 7 is the best inhibitor, contrary to the experimental assays. The authors attributed this divergence to the complexity involving this kind of data, given that many variables play key roles in corrosion assays, as explained in Section 1.2.
2.4. Five-Membered Heterocyclic Nucleus
Five-membered heterocyclic compounds belong to a particularly special class of organic molecular motifs. The interest in this kind of molecular motif relies on many field areas. Firstly, promiscuous medicinal chemistry applications as antitumoral, antimicrobial, antibacterial, and antifungal agents [51,52,53,54]. Secondly, material chemistry with liquid crystals research [55]. Lastly, this class of molecular motifs hinges on agricultural chemistry (insecticides and herbicides) [56]. Regarding their molecular aspects, they differ in position and type of heteroatom, as demonstrated in the following selected examples.
Imidazole nucleus is particularly interesting among the many five-membered heterocyclic compounds due to its aromaticity and two nitrogen atoms capable of donating their unpaired par of π-electrons [57]. Under this concept, three imidazole derivatives (PDI, PAI, and PMI) were prepared as corrosion inhibitors (Figure 7) [58]. The synthetical procedure formed all the derivatives in good yields and comprised four steps: bromination of ketone 9; reduction of cyanopyridine 10a-c; obtainment of intermediate amidines 11a-c; cyclization between 11a-c and 12. Potential dynamic polarization was responsible for the corrosion inhibition potential for the three tested molecules at the concentration of 4g/L.
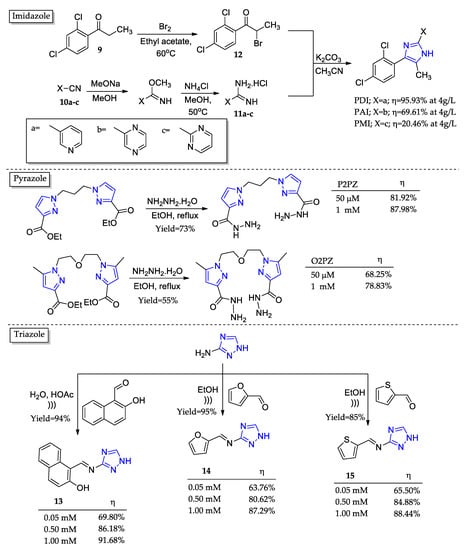
Figure 7.
Five-membered nitrogenated heterocyclic compounds as corrosion inhibitors and their corrosion inhibition potential (η).
The values for PDI, PAI, and PMI were ranked in the following order: 95.93%, 69.61%, and 20.46%. The results were explained by the difference in the position and number of nitrogen atoms, which systematically decreased the aromatic nucleus electron density.
Two bis-pyrazole derivatives were prepared by Cherrak and coworkers (Figure 7) [59]. The only difference between them was the linker of the two pyrazoles; while P2PZ presents an aliphatic chain, O2PZ has an ether linker. The preparation involved a single-step procedure consisting of reflux under an ethanolic medium in the presence of hydrazine monohydrate. Both inhibitors presented good activity at the highest concentration tested (1 mM). However, the difference in geometrical disposition and the influence of protonated form given the acidic media turned a simpler aliphatic chain more advantageous to adequately interact with the metal’s surface. The result was an almost 10% better activity for P2PZ (87.98%) compared to OP2Z (78.83%).
The triazole nucleus is so versatile that it represents a research area on its own [51,60,61]. As a relevant example for this review focus and size, we present the triazoles (13–15) synthesized by Abdelsalam and coworkers using ultrasound-assisted methods in an ethanolic or aqueous medium (Figure 7) [62]. Considering the achieved yields of 85–95%, this is a great example of an environmentally friendly synthetic protocol given the non-toxic nature of solvents, excellent atomic efficiency, and clean low energy necessary for the reactional system work. Electrochemical frequency modulation was employed to assess the corrosion inhibition potential of the triazole compounds. It was noted that they presented a similar behavior under the different concentrations tested and responded well to discretionary increases in their concentration, with better corrosion inhibition (up to 87.29–91.68% at 1.00 mM). Overall, the triazole nucleus has proven to be a very reliable choice in terms of the obtainment of corrosion inhibition.
Two thiophene derivatives (OXM and TET) were synthesized as corrosion inhibitors in single-step procedures and with high yields (Figure 8) [63]. Electrochemical impedance measurements pointed to very similar behaviors between the tetrazole-substituted TET and oxime-substituted OXM, as both compounds presented good responses in terms of activity as the tested concentration increased. At the highest concentration tested (1 mM), both compounds presented over 90% anticorrosive inhibition.
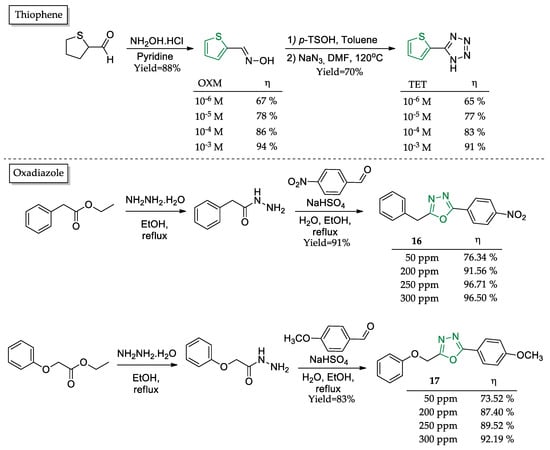
Figure 8.
Five-membered sulfonated and oxygenated heterocyclic compounds as corrosion inhibitors and their corrosion inhibition potential (η).
One last example of a five-membered heterocyclic compound comprises two oxadiazole derivatives (16–17) (Figure 8) [64]. The synthetic procedure required two steps and furnished the products with excellent yields (>80%). Weight loss assays pointed to a slight advantage (about 5%) in every tested concentration for 16 over 17. A plausible reason may lie in the reduction of the nitro group to amino given the acid media in the presence of iron as a catalyst. At the highest concentrations tested of 250 and 300 ppm, 16 achieved the optimal concentration, as its anticorrosive activities started to decrease, while 17 started to achieve its maximum potential, as further increments did not show an appreciable upgrade in corrosion inhibition.
2.5. Polycyclic-Based Compounds
The indole’s impressive track record in medicinal chemistry makes it unnecessary to further highlight its widespread and effective use. [65,66]. However, a gossypol indole (GI) was proposed as a novel green corrosion inhibitor in an aggressive alkaline–saline medium composed of NaOH 1 M and NaCl 1 M (Figure 9) [67,68]. The synthetic procedure involved a one-pot methodology furnishing the desired GI in high yields (69.7%). Electrochemical impedance spectroscopy revealed the promising potential use of GI as a corrosion inhibitor in the aggressive alkaline–saline medium since high inhibition values (88.54%) were found under the lowest concentration tested (2.8 μM).
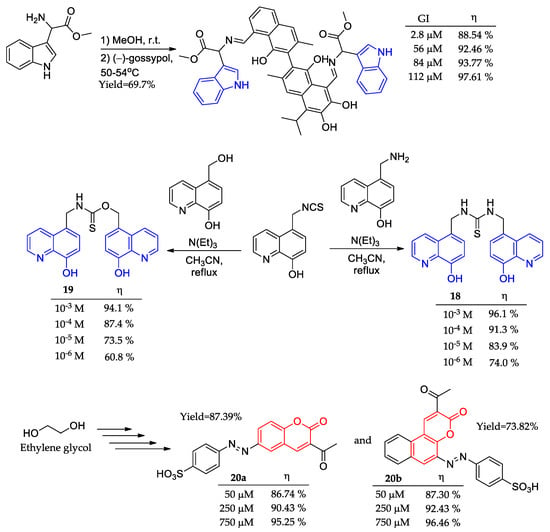
Figure 9.
Polycyclic-based compounds as corrosion inhibitors and their corrosion inhibition potential (η).
Similar to the indole nucleus, quinoline has a significant and well-respected history in the field of medicinal chemistry [69,70]. Therefore, under a different approach, quinoline analogs 18 and 19 were prepared to explore their anticorrosive potential (Figure 9) [71]. Weight loss assays pointed to almost the same potential for corrosion inhibition at the highest concentration tested (10−3 M) for both compounds. However, it was observed that the extra amino group in the BQMT structure led to the acquisition of higher anticorrosive potential, 18 (74.0%) > 19 (60.8%), in the lowest concentration tested (10−6 M) along with a better response to concentration increments.
The coumarin nucleus was also explored in corrosion inhibitor development [72]. Two coumarin derivatives (20a-b) were prepared in the quite extensive synthetic procedure, in which we highlight the starting material ethylene glycol (Figure 9). Electrochemical impedance spectroscopy pointed to almost the same behavior against corrosion inhibition for both derivatives, with activities superior to 95% in the highest tested concentration of 750 μM. Moreover, with melting points bearing 300 °C, both coumarin dyes were presented as potential thermodynamically resistant corrosion inhibitors.
Under a similar approach with polycyclic compounds, Caihong and coworkers [73] prepared a hybrid compound 21 consisting of three different fused heterocycles: pyrazole, pyran, and pyrimidine (Figure 10). The green synthetical procedure took only a single-step multicomponent one-pot methodology, furnishing 21 in high yields (92%) [74]. Weight loss measurement allowed the observation of high corrosion inhibition at 200 mg/L (94.46%), proving the potential application of multiple heterocyclic nuclei fused in one molecule as a viable approach to obtain novel promising anticorrosive compounds. Moreover, the weight loss assays conducted at higher temperatures demonstrated the high activity of the compound, even at 330 K (over 80%). Furthermore, the compound’s high melting point (266–268 °C) underscores its potential as a thermodynamically resistant inhibitor.
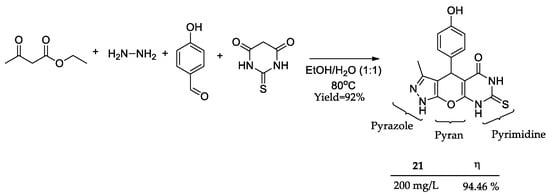
Figure 10.
Three heterocycles hybrid compound 21 prepared as a corrosion inhibitor and its anticorrosive activity (η).
2.6. Carbazones and Carboxamides
Carboxamides (22–24) have been prepared by different synthetic protocols with excellent yields to serve as anticorrosive agents (Figure 11) [75]. The weight loss assays were carried out in various concentrations. We will focus on the lowest, where the compounds showed the most significant difference, and the highest ones, where the compounds achieved their maximum anticorrosive capacity due to the complete covering of the metal’s surface. This work is quite interesting as the reason for the anticorrosive activity results is justified by structural details. Derivative 23 has the advantage of possessing more aromatic nuclei. However, carboxamide sp3 nitrogen, which detains the stronger electronic density, is involved in resonance with its adjacent aromatic nucleus. As a result, this derivative is unable to chemically bind to the metal surface, which explains why its activity is more than 10% lower than the other two. As for the other two derivatives, this is explained via the difference between the number of nitrogen atoms.
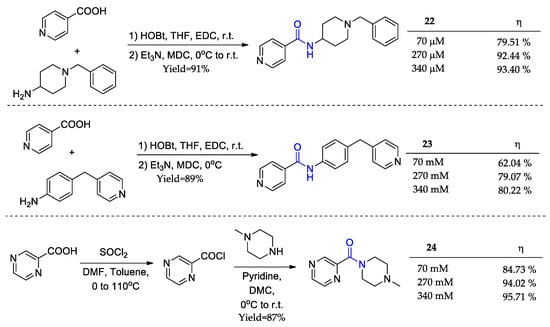
Figure 11.
Carboxamide derivatives synthesized by Ramachandran and colleagues [62] as corrosion inhibitors and their respective anticorrosive potential (η) at 303 K.
2.7. Miscellaneous
Despite the great myriad of functions and molecular motifs covered in the previous topics, there are some additional interesting suitable uses of organic molecules to be employed as corrosion inhibitors due to intrinsic characteristics already listed, such as the abundance of heteroatoms, low cytotoxicity, presence of unsaturations, and abundant occurrence in nature. For example, mono-isoxazoline MIC [76] and bis-isoxazoline BMIC [77] fused (R)-carvone derivatives were prepared to employ the same procedure consecutively (Figure 12). The weight loss assay showed that incorporating a second isoxazole nucleus significantly increased the compound’s anticorrosive activity. This increased activity was observed even at lower concentrations, indicating a higher efficiency compared to the compound without the second isoxazole nucleus. Unfortunately, the conjugated alkene from the α,β-unsaturated ketone is in resonance with the carbonyl group, which means that its π-electrons are not as available as they should be to boost higher yields in a 3 + 2 cycloaddition reaction.
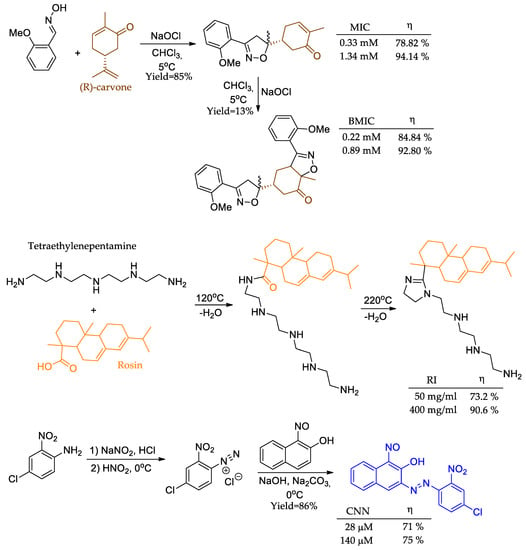
Figure 12.
Carvone derivatives, natural resin-based inhibitors, and dye as corrosion inhibitors.
Rosin, a resin obtainable from pine trees, had its structure modified via two synthetic procedures involving heating and dehydration, furnishing RI (Figure 12) [78]. Weight loss assay showed its promising anticorrosive activity at both the lowest and highest tested concentrations. Another example encompasses a synthetic dye (CNN) obtained by a diazonium salt procedure in high yields (Figure 12) [79]. The weight loss assay demonstrated a linear relationship between the tested concentrations, with the exception of the lowest (28 μM) and highest (140 μM) concentrations, where only a 4% increase was observed. The dye approach was considered an optimal resource for the petroleum industry, mainly because it maintained up to 55% inhibition at 140 mM and 338 K. Moreover, CNN presented a high melting point of 230–232 °C, indicating promising thermal stability.
Organoselenium compounds (NMSeCN and NMSe2) were prepared to be used as corrosion inhibitors (Figure 13) [80]. The synthetical procedure encompassed two steps for NMSeCN and three steps for NMSe2, and both furnished the desired compounds in yields above 95%. Weight loss measurements showed that both derivatives met the expectation as potential corrosion inhibitors with over 90% activity at the highest concentration tested (6.4 mM). This is in line with the fact that a selenium atom is as good as a sulfur atom in terms of nucleophilicity/polarizability, allowing great interaction with a metal’s valence orbitals [81]. Nonetheless, the symmetrical compound bearing the double number of functions, and heteroatoms presented better potential in all tested concentrations. Moreover, NMSe2’s high melting point (149–150 °C) indicated a good thermal resistance.
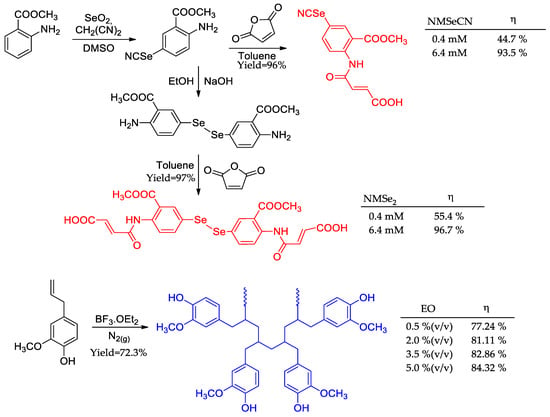
Figure 13.
Selenium compounds and eugenol-based corrosion inhibitors.
A eugenol oligomer (EO) was prepared in a single step, using BF3·OEt2 as an initiator, with a high yield (72.3%) as a green corrosion inhibitor (Figure 13) [82]. One of its main features was thermal stability since the first structural cracking only occurred above 629 K due to polymeric chain rupture. At all tested concentrations used in the weight loss assays (at 333 K), EO showed minimal increase in corrosion inhibition potential, meaning that it already has high potential at low concentrations.
Two sweetener-based compounds were explored as novel corrosion inhibitions (Figure 14). The sucrose derivative (SDCI) [83] was identified as a green and thermally stable compound, retaining its molecular motif even at temperatures as high as 420 K. In addition, potentiodynamic polarization results demonstrated the potential application of SDCI as a high-temperature corrosion inhibitor. At a concentration range of 2.2–9.0 mM and a temperature of 363 K, SDCI showed a corrosion inhibition range of 80.6–94.3%. The synthetical procedure employed a one-pot methodology. As for saccharin derivative (NPS), [84] a green inhibition was achieved through a one-step procedure, employing a relatively low-cost, abundant, renewable, and non-toxic feedstock with an inhibition potential of up to 92.31% at the highest tested concentration.
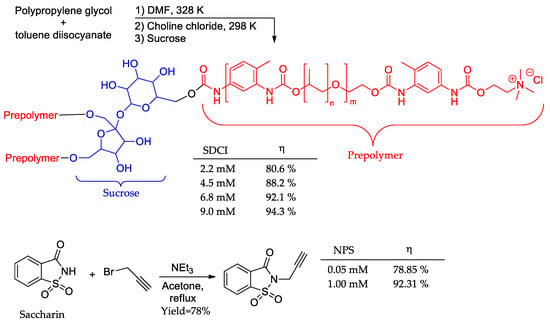
Figure 14.
Sweeteners as corrosion inhibitors.
Phosphonic acids derived from natural products such as undecylenic acid and oleic acid were synthesized by Ruf and coworkers (Figure 15) [85]. The synthetic procedures furnished the corresponding derivatives PA1 and PA2 in high yields (>80%) employing one-pot methodologies. In this work, the compounds’ anticorrosive activity was evaluated in different concentrations of saline solution. Two commercially available corrosion inhibitors, octylphosphonic acid (OPA) and 1,3,5-triazine-2,4,6-triaminocaproic acid (TC), were employed as control compounds. It was found that PA1 was so potent that it achieved outstanding corrosion inhibition (99.74%) in the most challenging media (3.0% NaCl), where control inhibitors did not show significant results.
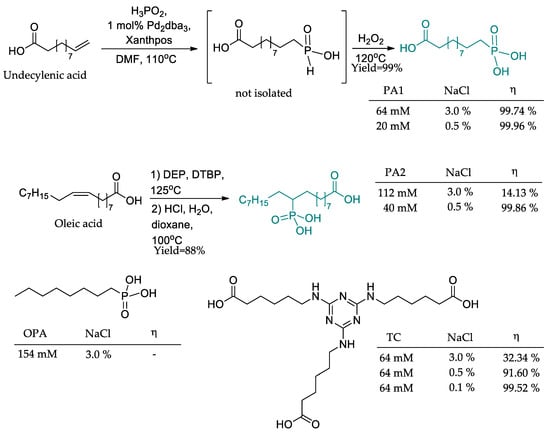
Figure 15.
Natural product-based phosphonic acids as corrosion inhibitors.
3. Conclusions
The versatility in the synthesis of organic molecules that can be used as corrosion inhibitors can be justified by the abundant reactions which allow a plethora of functional and structural manipulations in different molecular frameworks (i.e., the addition of heteroatoms in several carbon motifs and exploration of diverse substituents, especially aromatic ones), which contributes to elevate their corrosion inhibition performance. Moreover, environmentally friendly synthetic strategies (one-step protocols, multi-component approaches, and cheap reagents), and the absence of toxic components, i.e., metals, are important points that contribute to the use of organic molecules in the corrosion inhibition market. Furthermore, the structural features of many organic compounds designed or used as drugs in medicinal chemistry make them compatible with corrosion inhibition applications, and they have been shown to be effective at extremely low concentrations (ppm range). That point enlarges the meaning of drug repurposing beyond the “same drug, different diseases” to “same organic compound, different applications”. In a general aspect, there are no preferred organic molecular motifs, as all of the different examples successfully showed high corrosion inhibition potential (quinolines, triazoles, hydrazones, etc.). So, the real challenge comprising organic molecules in corrosion inhibition lies in achieving low or no toxicity levels through friendly and cost-effective obtainment methods.
Although computational and theoretical methods in chemistry have evolved significantly, relying solely on these approaches to search for novel corrosion inhibitors may not be entirely reliable. Instead, they provide valuable predictions and explain more deeply the inhibitor’s behavior after the correct experimental procedures to further clarify the phenomena and bring robustness to this research environment.
When it comes to logistics, corrosion inhibitors can simultaneously save time and money by delaying complex repair services and dismissing oxidized material substitution, especially in hard-to-reach places, such as extensive oil/gas pipelines. Within the previous points, it becomes clear why these kinds of molecules have attracted the special interest of researchers as an elegant solution for the corrosion process consequences.
Funding
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Renato C. S. Lessa acknowledges Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES) for the scholarship received, Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) and CNPq.
Conflicts of Interest
The author declares no conflict of interest.
References
- Aslam, R.; Serdaroglu, G.; Zehra, S.; Kumar Verma, D.; Aslam, J.; Guo, L.; Verma, C.; Ebenso, E.E.; Quraishi, M.A. Corrosion Inhibition of Steel Using Different Families of Organic Compounds: Past and Present Progress. J. Mol. Liq. 2022, 348, 118373. [Google Scholar] [CrossRef]
- Popoola, L.T. Organic Green Corrosion Inhibitors (OGCIs): A Critical Review. Corros. Rev. 2019, 37, 71–102. [Google Scholar] [CrossRef]
- Quraishi, M.A.; Chauhan, D.S.; Ansari, F.A. Development of Environmentally Benign Corrosion Inhibitors for Organic Acid Environments for Oil-Gas Industry. J. Mol. Liq. 2021, 329, 115514. [Google Scholar] [CrossRef]
- Lou, Y.; Chang, W.; Cui, T.; Wang, J.; Qian, H.; Ma, L.; Hao, X.; Zhang, D. Microbiologically Influenced Corrosion Inhibition Mechanisms in Corrosion Protection: A Review. Bioelectrochemistry 2021, 141, 107883. [Google Scholar] [CrossRef]
- Wei, H.; Heidarshenas, B.; Zhou, L.; Hussain, G.; Li, Q.; Ostrikov, K.K. Green Inhibitors for Steel Corrosion in Acidic Environment: State of Art. Mater. Today Sustain. 2020, 10, 100044. [Google Scholar] [CrossRef]
- Kokalj, A. Corrosion Inhibitors: Physisorbed or Chemisorbed? Corros. Sci. 2022, 196, 109939. [Google Scholar] [CrossRef]
- Verma, C.; Quraishi, M.A.; Rhee, K.Y. Electronic Effect vs. Molecular Size Effect: Experimental and Computational Based Designing of Potential Corrosion Inhibitors. Chem. Eng. J. 2022, 430, 132645. [Google Scholar] [CrossRef]
- Chauhan, D.S.; Verma, C.; Quraishi, M.A. Molecular Structural Aspects of Organic Corrosion Inhibitors: Experimental and Computational Insights. J. Mol. Struct. 2021, 1227, 129374. [Google Scholar] [CrossRef]
- Fateh, A.; Aliofkhazraei, M.; Rezvanian, A.R. Review of Corrosive Environments for Copper and Its Corrosion Inhibitors. Arab. J. Chem. 2020, 13, 481–544. [Google Scholar] [CrossRef]
- da Silva, A.D.; do Nascimento, G.X.; Quattrociocchi, D.G.S.; Martinazzo, A.P. Quantum chemical properties using the DFT method: A theoretical tool applied in the study of corrosion inhibitors. Res. Soc. Dev. 2020, 12, e2291210499. [Google Scholar]
- Quattrociocchi, D.G.S.; Santoro, A.S.; da Fonseca, T.N.M.; da Conceição Júnior, V.; Paes, L.W.C.; Campos, V.R. Técnicas Experimentais e Teóricas Aplicadas Ao Estudo de Inibidores Orgânicos de Corrosão Em Meio Ácido. Res. Soc. Dev. 2022, 11, e57811932321. [Google Scholar] [CrossRef]
- Kokalj, A. On the Alleged Importance of the Molecular Electron-Donating Ability and the HOMO–LUMO Gap in Corrosion Inhibition Studies. Corros. Sci. 2021, 180, 109016. [Google Scholar] [CrossRef]
- Kokalj, A. Molecular Modeling of Organic Corrosion Inhibitors: Calculations, Pitfalls, and Conceptualization of Molecule–Surface Bonding. Corros Sci 2021, 193, 109650. [Google Scholar] [CrossRef]
- Verma, D.K.; Aslam, R.; Aslam, J.; Quraishi, M.A.; Ebenso, E.E.; Verma, C. Computational Modeling: Theoretical Predictive Tools for Designing of Potential Organic Corrosion Inhibitors. J. Mol. Struct. 2021, 1236, 130294. [Google Scholar] [CrossRef]
- Kokalj, A.; Costa, D. Molecular Modeling of Corrosion Inhibitors. In Encyclopedia of Interfacial Chemistry: Surface Science and Electrochemistry; Elsevier: Amsterdam, The Netherlands, 2018; pp. 332–345. ISBN 9780128098943. [Google Scholar]
- Verma, C.; Ebenso, E.E.; Quraishi, M.A. Ultrasound and microwave heating for the synthesis of green corrosion inhibitors: A literature study. In Environmentally Sustainable Corrosion Inhibitors: Fundamentals and Industrial Applications; Elsevier: Amsterdam, The Netherlands, 2021; pp. 303–319. ISBN 978-0-323-85405-4. [Google Scholar]
- Dewangan, A.K.; Dewangan, Y.; Verma, D.K.; Verma, C. Synthetic environment-friendly corrosion inhibitors. In Environmentally Sustainable Corrosion Inhibitors: Fundamentals and Industrial Applications; Elsevier: Amsterdam, The Netherlands, 2022; pp. 71–95. ISBN 978-0-323-85405-4. [Google Scholar]
- Fernandes, C.M.; Alvarez, L.X.; dos Santos, N.E.; Maldonado Barrios, A.C.; Ponzio, E.A. Green Synthesis of 1-Benzyl-4-Phenyl-1H-1,2,3-Triazole, Its Application as Corrosion Inhibitor for Mild Steel in Acidic Medium and New Approach of Classical Electrochemical Analyses. Corros. Sci. 2019, 149, 185–194. [Google Scholar] [CrossRef]
- Ebenso, E.E.; Verma, C.; Olasunkanmi, L.O.; Akpan, E.D.; Verma, D.K.; Lgaz, H.; Guo, L.; Kayah, S.; Quraishi, M.A. Molecular modelling of compounds used for corrosion inhibition studies: A review. Phys. Chem. Chem. Phys. 2021, 23, 19987–20027. [Google Scholar] [CrossRef]
- Sanyal, B. Organic compounds as corrosion inhibitors in different environments—A Review. Process Org. Coat. 1981, 9, 165–236. [Google Scholar] [CrossRef]
- Samiee, R.; Ramezanzadeh, B.; Mahdavian, M.; Alibakhshi, E. Assessment of the Smart Self-Healing Corrosion Protection Properties of a Water-Base Hybrid Organo-Silane Film Combined with Non-Toxic Organic/Inorganic Environmentally Friendly Corrosion Inhibitors on Mild Steel. J. Clean. Prod. 2019, 220, 340–356. [Google Scholar] [CrossRef]
- Mu, G.; Li, X.; Qu, Q.; Zhou, J. Molybdate and Tungstate as Corrosion Inhibitors for Cold Rolling Steel in Hydrochloric Acid Solution. Corros. Sci. 2006, 48, 445–459. [Google Scholar] [CrossRef]
- Raja, P.B.; Ismail, M.; Ghoreishiamiri, S.; Mirza, J.; Ismail, M.C.; Kakooei, S.; Rahim, A.A. Reviews on Corrosion Inhibitors: A Short View. Chem. Eng. Commun. 2016, 203, 1145–1156. [Google Scholar] [CrossRef]
- Gece, G. Drugs: A Review of Promising Novel Corrosion Inhibitors. Corros. Sci. 2011, 53, 3873–3898. [Google Scholar] [CrossRef]
- Iroha, N.B.; Madueke, N.A.; Mkpenie, V.; Ogunyemi, B.T.; Nnanna, L.A.; Singh, S.; Akpan, E.D.; Ebenso, E.E. Experimental, Adsorption, Quantum Chemical and Molecular Dynamics Simulation Studies on the Corrosion Inhibition Performance of Vincamine on J55 Steel in Acidic Medium. J. Mol. Struct. 2021, 1227, 129533. [Google Scholar] [CrossRef]
- Shukla, S.K.; Singh, A.K.; Ahamad, I.; Quraishi, M.A. Streptomycin: A Commercially Available Drug as Corrosion Inhibitor for Mild Steel in Hydrochloric Acid Solution. Mater. Lett. 2009, 63, 819–822. [Google Scholar] [CrossRef]
- Eddy, N.O.; Odoemelam, S.A.; Ekwumemgbo, P. Inhibition of the Corrosion of Mild Steel in H2SO4 by Penicillin G. Sci. Res. Essay 2009, 4, 33–038. [Google Scholar]
- Ituen, E.; James, A.; Akaranta, O.; Sun, S. Eco-friendly corrosion inhibitor from Pennisetum purpureum biomass and synergistic intensifiers for mild steel. Chin. J. Chem. Eng. 2016, 24, 1442–1447. [Google Scholar] [CrossRef]
- Rajeswari, V.; Kesavan, D.; Gopiraman, M.; Viswanathamurthi, P. Physicochemical Studies of Glucose, Gellan Gum, and Hydroxypropyl Cellulose—Inhibition of Cast Iron Corrosion. Carbohydr. Polym. 2013, 95, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ma, X.; Tabish, M.; Wang, J. Sunflower-head extract as a sustainable and eco-friendly corrosion inhibitor for carbon steel in hydrochloric acid and sulfuric acid solutions. J. Mol. Liq. 2022, 367, 120429. [Google Scholar] [CrossRef]
- Wang, Q.; Zheng, H.; Liu, L.; Zhang, Q.; Wu, X.; Yan, Z.; Sun, Y.; Li, X. Insight into the anti–corrosion behavior of Reineckia Carnea leaves extract as an eco–friendly and high–efficiency corrosion inhibitor. Ind. Crops Prod. 2022, 188, 115640. [Google Scholar] [CrossRef]
- Nazari, A.; Ramezanzadeh, B.; Guo, L.; Dehghani, A. Application of green active bio-molecules from the aquatic extract of Mint leaves for steel corrosion control in hydrochloric acid (1M) solution: Surface, electrochemical, and theoretical explorations. Colloids Surf. A 2023, 656, 130540. [Google Scholar] [CrossRef]
- Haque, J.; Srivastava, V.; Chauhan, D.S.; Quraishi, M.A.; Madhan Kumar, A.; Lgaz, H. Electrochemical and Surface Studies on Chemically Modified Glucose Derivatives as Environmentally Benign Corrosion Inhibitors. Sustain. Chem. Pharm. 2020, 16, 100260. [Google Scholar] [CrossRef]
- Chaubey, N.; Savita; Qurashi, A.; Chauhan, D.S.; Quraishi, M.A. Frontiers and Advances in Green and Sustainable Inhibitors for Corrosion Applications: A Critical Review. J. Mol. Liq. 2021, 321, 114385. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, J.; Nishtha. Natural gums as corrosion inhibitor: A review. Mater. Today Proc. 2022, 64, 141–146. [Google Scholar] [CrossRef]
- Quaraishi, M.A.; Rawat, J. A review on macrocyclics as corrosion inhibitors. Corros. Rev. 2001, 19, 273–299. [Google Scholar] [CrossRef]
- Wight, J.B. Some Reactions of Mannich Bases Derived from a-Phenoxyacetophenone and a-Phenoxypropiophenone. J. Org. Chem. 1960, 25, 1867–1972. [Google Scholar] [CrossRef]
- Tramontini, M. Advances in the Chemistry of Mannich Bases. Synthesis 1973, 1973, 703–775. [Google Scholar] [CrossRef]
- Roman, G. Mannich bases in medicinal chemistry and drug design. Eur. J. Med. Chem. 2015, 89, 743–816. [Google Scholar] [CrossRef] [PubMed]
- Tramontini, M.; Angiolini, L.; Ghedini, N. Mannich bases in polymer chemistry. Polymer 1988, 29, 771–788. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, M.; Zhang, Z.; Li, Q.; Lv, R.; Wu, W. Bis-Mannich Bases as Effective Corrosion Inhibitors for N80 Steel in 15% HCl Medium. J. Mol. Liq. 2022, 347, 117957. [Google Scholar] [CrossRef]
- Schaub, T. Efficient Industrial Organic Synthesis and the Principles of Green Chemistry. Chem. A Eur. J. 2021, 27, 1865–1869. [Google Scholar] [CrossRef]
- Anastas, P.; Eghbali, N. Green Chemistry: Principles and Practice. Chem. Soc. Rev. 2010, 39, 301–312. [Google Scholar] [CrossRef]
- Fernandes, C.M.; Pina, V.G.S.S.; Alfaro, C.G.; de Sampaio, M.T.G.; Massante, F.F.; Alvarez, L.X.; Barrios, A.M.; Silva, J.C.M.; Alves, O.C.; Briganti, M.; et al. Innovative Characterization of Original Green Vanillin-Derived Schiff Bases as Corrosion Inhibitors by a Synergic Approach Based on Electrochemistry, Microstructure, and Computational Analyses. Colloids Surf. A Physicochem. Eng. Asp. 2022, 641, 128540. [Google Scholar] [CrossRef]
- Zinad, D.S.; Salim, R.D.; Betti, N.; Shaker, L.M.; Al-Amiery, A.A. Comparative Investigations of the Corrosion Inhibition Efficiency of a 1-Phenyl-2-(1-Phenylethylidene)Hydrazine and Its Analog Against Mild Steel Corrosion in Hydrochloric Acid Solution. Prog. Color Color. Coat. 2021, 15, 53–63. [Google Scholar] [CrossRef]
- El-Haitout, B.; Selatnia, I.; Lgaz, H.; Al-Hadeethi, M.R.; Lee, H.S.; Chaouiki, A.; Ko, Y.G.; Ali, I.H.; Salghi, R. Exploring the Feasibility of New Eco-Friendly Heterocyclic Compounds for Establishing Efficient Corrosion Protection for N80 Steel in a Simulated Oil Well Acidizing Environment: From Molecular-Level Prediction to Experimental Validation. Colloids Surf. A Physicochem. Eng. Asp. 2023, 656, 130372. [Google Scholar] [CrossRef]
- Çavuş, M.S.; Yakan, H.; Özorak, C.; Muğlu, H.; Bakır, T.K. New N,N′-Bis(Thioamido)Thiocarbohydrazones and Carbohydrazones: Synthesis, Structure Characterization, Antioxidant Activity, Corrosion Inhibitors and DFT Studies. Res. Chem. Intermed. 2022, 48, 1593–1613. [Google Scholar] [CrossRef]
- Bimoussa, A.; Koumya, Y.; Oubella, A.; Kaddouri, Y.; Fawzi, M.; Laamari, Y.; Abouelfida, A.; Ait Itto, M.Y.; Touzani, R.; Benyaich, A.; et al. Synthesis, Experimental and Theoretical Studies of Sesquiterpenic Thiosemicarbazone and Semicarbazone as Organic Corrosion Inhibitors for Stainless Steel 321 in H2SO4 1M. J. Mol. Struct. 2022, 1253, 132276. [Google Scholar] [CrossRef]
- Mehta, R.K.; Gupta, S.K.; Yadav, M. Studies on Pyrimidine Derivative as Green Corrosion Inhibitor in Acidic Environment: Electrochemical and Computational Approach. J. Environ. Chem. Eng. 2022, 10, 108499. [Google Scholar] [CrossRef]
- Rezaeivala, M.; Karimi, S.; Sayin, K.; Tüzün, B. Experimental and Theoretical Investigation of Corrosion Inhibition Effect of Two Piperazine-Based Ligands on Carbon Steel in Acidic Media. Colloids Surf. A Physicochem. Eng. Asp. 2022, 641, 128538. [Google Scholar] [CrossRef]
- Lessa, R.C.S. 1,2,3-Triazole Nucleus as a Versatile Tool for the Obtainment of Novel Biologically Active Compounds: An Overview. Rev. Virtual Quim. 2021, 13, 74–89. [Google Scholar] [CrossRef]
- Sahiba, N.; Sethiya, A.; Soni, J.; Agarwal, D.K.; Agarwal, S. Saturated Five-Membered Thiazolidines and Their Derivatives: From Synthesis to Biological Applications. Top. Curr. Chem. 2020, 378, 34. [Google Scholar] [CrossRef]
- Grunberg, E.; Titsworth, E.H. Properties of Heterocyclic Compounds: Monocyclic compounds with five-membered rings. Annu. Rev. Microbiol. 1973, 27, 317–346. [Google Scholar] [CrossRef]
- Ambhaikar, N.B.; Uppaluri, S. Five-membered ring systems: With N and S atom. Prog. Heterocycl. Chem. 2023, 34, 305–340. [Google Scholar] [CrossRef]
- Kotian, S.Y.; Mohan, C.D.; Merlo, A.A.; Rangappa, S.; Nayak, S.C.; Rai, K.M.L.; Rangappa, K.S. Small Molecule Based Five-Membered Heterocycles: A View of Liquid Crystalline Properties beyond the Biological Applications. J. Mol. Liq. 2020, 297, 111686. [Google Scholar] [CrossRef]
- Filho, J.R.D.F.; Freitas, J.J.R.D.; Freitas, J.C.R.D.; Ramos, C.D.S.; Souza, F.A.M.D.; Ramos, J.F.; Tavares, R.; Oliveira, D.E.T.B.d. Synthesis, Biological Activity and Applications of 1,2,5-Oxadiazol: A Brief Review. Int. Res. J. Pure Appl. Chem. 2023, 24, 1–26. [Google Scholar] [CrossRef]
- Ouakki, M.; Galai, M.; Cherkaoui, M. Imidazole Derivatives as Efficient and Potential Class of Corrosion Inhibitors for Metals and Alloys in Aqueous Electrolytes: A Review. J. Mol. Liq. 2022, 345, 117815. [Google Scholar] [CrossRef]
- Hou, Y.; Zhu, L.; He, K.; Yang, Z.; Ma, S.; Lei, J. Synthesis of Three Imidazole Derivatives and Corrosion Inhibition Performance for Copper. J. Mol. Liq. 2022, 348, 118432. [Google Scholar] [CrossRef]
- Cherrak, K.; Khamaysa, O.M.A.; Bidi, H.; Massaoudi, M.e.; Ali, I.A.; Radi, S.; el Ouadi, Y.; El-Hajjaji, F.; Zarrouk, A.; Dafali, A. Performance Evaluation of Newly Synthetized Bi-Pyrazole Derivatives as Corrosion Inhibitors for Mild Steel in Acid Environment. J. Mol. Struct. 2022, 1261, 132925. [Google Scholar] [CrossRef]
- Li, W.; Tan, B.; Zhang, S.; Guo, L.; Ji, J.; Yan, M.; Wang, R. Insights into Triazole Derivatives as Potential Corrosion Inhibitors in CMP Process: Experimental Evaluation and Theoretical Analysis. Appl. Surf. Sci. 2022, 602, 154165. [Google Scholar] [CrossRef]
- Hrimla, M.; Bahsis, L.; Laamari, M.R.; Julve, M.; Stiriba, S.E. An Overview on the Performance of 1,2,3-Triazole Derivatives as Corrosion Inhibitors for Metal Surfaces. Int. J. Mol. Sci. 2022, 23, 16. [Google Scholar] [CrossRef]
- Abdelsalam, M.M.; Bedair, M.A.; Hassan, A.M.; Heakal, B.H.; Younis, A.; Elbialy, Z.I.; Badawy, M.A.; Fawzy, H.E.D.; Fareed, S.A. Green Synthesis, Electrochemical, and DFT Studies on the Corrosion Inhibition of Steel by Some Novel Triazole Schiff Base Derivatives in Hydrochloric Acid Solution. Arab. J. Chem. 2022, 15, 103491. [Google Scholar] [CrossRef]
- Arrousse, N.; Fernine, Y.; Al-Zaqri, N.; Boshaala, A.; Ech-Chihbi, E.; Salim, R.; el Hajjaji, F.; Alami, A.; Touhami, M.E.; Taleb, M. Thiophene Derivatives as Corrosion Inhibitors for 2024-T3 Aluminum Alloy in Hydrochloric Acid Medium. RSC Adv. 2022, 12, 10321–10335. [Google Scholar] [CrossRef]
- Kumar, S.; Kalia, V.; Goyal, M.; Jhaa, G.; Kumar, S.; Vashisht, H.; Dahiya, H.; Quraishi, M.A.; Verma, C. Newly Synthesized Oxadiazole Derivatives as Corrosion Inhibitors for Mild Steel in Acidic Medium: Experimental and Theoretical Approaches. J. Mol. Liq. 2022, 357, 119077. [Google Scholar] [CrossRef]
- Mathada, B.S.; Yernale, N.G.; Basha, J.N. The Multi-Pharmacological Targeted Role of Indole and its Derivatives: A review. ChemistrySelect 2023, 8, e202204181. [Google Scholar] [CrossRef]
- Li, T.; Xu, H. Recent Progress of Bioactivities, Mechanisms of Action, Total Synthesis, Structural Modifications and Structure-activity Relationships of Indole Derivatives: A Review. Mini Rev. Med. Chem. 2022, 22, 2702–2725. [Google Scholar] [CrossRef]
- Yan, F.; Cao, X.X.; Jiang, H.X.; Zhao, X.L.; Wang, J.Y.; Lin, Y.H.; Liu, Q.L.; Zhang, C.; Jiang, B.; Guo, F. A Novel Water-Soluble Gossypol Derivative Increases Chemotherapeutic Sensitivity and Promotes Growth Inhibition in Colon Cancer. J. Med. Chem. 2010, 53, 5502–5510. [Google Scholar] [CrossRef] [PubMed]
- Berdimurodov, E.; Kholikov, A.; Akbarov, K.; Guo, L.; Kaya, S.; Katin, K.P.; Verma, D.K.; Rbaa, M.; Dagdag, O.; Haldhar, R. Novel Gossypol–Indole Modification as a Green Corrosion Inhibitor for Low–Carbon Steel in Aggressive Alkaline–Saline Solution. Colloids Surf. A Physicochem. Eng. Asp. 2022, 637, 128207. [Google Scholar] [CrossRef]
- Ajani, O.O.; Iyaye, K.T.; Ademosum, O.T. Recent advances in chemistry and therapeutic potential of functionalized quinoline motifs—A review. RSC Adv. 2022, 12, 18594–18614. [Google Scholar] [CrossRef]
- Rajendran, S.; Sivalingam, K.; Jayarampillai, R.P.K.; Wang, W.L.; Salas, C.O. Friedländer’s synthesis of quinolines as a pivotal step in the development of bioactive heterocyclic derivatives in the current era of medicinal chemistry. Chem. Biol. Drug Des. 2022, 100, 1042–1085. [Google Scholar] [CrossRef]
- El Faydy, M.; Benhiba, F.; Warad, I.; Saoiabi, S.; Alharbi, A.; Alluhaybi, A.A.; Lakhrissi, B.; Abdallah, M.; Zarrouk, A. Bisquinoline Analogs as Corrosion Inhibitors for Carbon Steel in Acidic Electrolyte: Experimental, DFT, and Molecular Dynamics Simulation Approaches. J. Mol. Struct. 2022, 1265, 133389. [Google Scholar] [CrossRef]
- Elaryian, H.M.; Bedair, M.A.; Bedair, A.H.; Aboushahba, R.M.; Fouda, A.E.A.S. Synthesis, Characterization of Novel Coumarin Dyes as Corrosion Inhibitors for Mild Steel in Acidic Environment: Experimental, Theoretical, and Biological Studies. J. Mol. Liq. 2022, 346, 118310. [Google Scholar] [CrossRef]
- Caihong, Y.; Singh, A.; Ansari, K.R.; Ali, I.H.; Kumar, R. Novel Nitrogen Based Heterocyclic Compound as Q235 Steel Corrosion Inhibitor in 15% HCl under Dynamic Condition: A Detailed Experimental and Surface Analysis. J. Mol. Liq. 2022, 362, 119720. [Google Scholar] [CrossRef]
- Ramesh, R.; Tamilselvi, V.; Vadivel, P.; Lalitha, A. Innovative Green Synthesis of 4-Aryl-Pyrazolo[5,6]Pyrano [2,3-d]Pyrimidines under Catalyst-Free Conditions. Polycycl. Aromat. Compd. 2020, 40, 811–823. [Google Scholar] [CrossRef]
- Ramachandran, A.; Anitha, P.; Gnanavel, S. Structural and Electronic Impacts on Corrosion Inhibition Activity of Novel Heterocyclic Carboxamides Derivatives on Mild Steel in 1 M HCl Environment: Experimental and Theoretical Approaches. J. Mol. Liq. 2022, 359, 119218. [Google Scholar] [CrossRef]
- Elqars, E.; Oubella, A.; Eddine Hachim, M.; Byadi, S.; Auhmani, A.; Guennoun, M.; Essadki, A.; Riahi, A.; Robert, A.; Itto, M.Y.A.; et al. New 3-(2-Methoxyphenyl)-Isoxazole-Carvone: Synthesis, Spectroscopic Characterization, and Prevention of Carbon Steel Corrosion in Hydrochloric Acid. J. Mol. Liq. 2022, 347, 118311. [Google Scholar] [CrossRef]
- Elqars, E.; Oubella, A.; Byadi, S.; Hachim, M.E.; Auhmani, A.; Guennoun, M.; Essadki, A.; Riahi, A.; Robert, A.; Itto, M.Y.A.; et al. Synthesis, Spectroscopic Characterization, and Prevention of Carbon Steel Corrosion in Hydrochloric Acid of a New Bis-Isoxazoline-Carvone. J. Mol. Struct. 2022, 1256, 132526. [Google Scholar] [CrossRef]
- Geng, S.; Hu, J.; Yu, J.; Zhang, C.; Wang, H.; Zhong, X. Rosin Imidazoline as an Eco-Friendly Corrosion Inhibitor for the Carbon Steel in CO2-Containing Solution and Its Synergistic Effect with Thiourea. J. Mol. Struct. 2022, 1250, 131778. [Google Scholar] [CrossRef]
- Akinyele, O.F.; Adekunle, A.S.; Olayanju, D.S.; Oyeneyin, O.E.; Durodola, S.S.; Ojo, N.D.; Akinmuyisitan, A.A.; Ajayeoba, T.A.; Olasunkanmi, L.O. Synthesis and Corrosion Inhibition Studies of (E)-3-(2-(4-chloro-2-Nitrophenyl)Diazenyl)-1-Nitrosonaphthalen-2-Ol on Mild Steel Dissolution in 0.5 M HCl Solution- Experimental, DFT and Monte Carlo Simulations. J. Mol. Struct. 2022, 1268, 133738. [Google Scholar] [CrossRef]
- El-Lateef, H.M.A.; Shaaban, S.; Shalabi, K.; Khalaf, M.M. Novel Organoselenium-Based N-Mealanilic Acids as Efficacious Corrosion Inhibitors for 6061 Aluminum Alloy in Molar HCl: In-Silico Modeling, Electrochemical, and Surface Morphology Studies. J. Taiwan Inst. Chem. Eng. 2022, 133, 104258. [Google Scholar] [CrossRef]
- El-Lateef, H.M.A.; Shaaban, S.; Khalaf, M.M.; Toghan, A.; Shalabi, K. Synthesis, experimental, and computational studies of water soluble anthranilic organoselenium compounds as safe corrosion inhibitors for J55 pipeline steel in acidic oilfield formation water. Colloids Surf. A 2021, 625, 126894. [Google Scholar] [CrossRef]
- Furtado, L.B.; Nascimento, R.C.; Guimarães, M.J.O.C.; Brasil, S.L.D.C.; Barra, S.H.R. Green Eugenol Oligomers as Corrosion Inhibitors for Carbon Steel in 1M HCl. Mater. Res. 2022, 25, e20220012. [Google Scholar] [CrossRef]
- Rahimi, A.; Farhadian, A.; Berisha, A.; Shaabani, A.; Varfolomeev, M.A.; Mehmeti, V.; Zhong, X.; Yousefzadeh, S.; Djimasbe, R. Novel Sucrose Derivative as a Thermally Stable Inhibitor for Mild Steel Corrosion in 15% HCl Medium: An Experimental and Computational Study. Chem. Eng. J. 2022, 446, 136938. [Google Scholar] [CrossRef]
- Ould Abdelwedoud, B.; Damej, M.; Tassaoui, K.; Berisha, A.; Tachallait, H.; Bougrin, K.; Mehmeti, V.; Benmessaoud, M. Inhibition Effect of N-Propargyl Saccharin as Corrosion Inhibitor of C38 Steel in 1 M HCl, Experimental and Theoretical Study. J. Mol. Liq. 2022, 354, 118784. [Google Scholar] [CrossRef]
- Ruf, E.; Naundorf, T.; Seddig, T.; Kipphardt, H.; Maison, W. Natural Product-Derived Phosphonic Acids as Corrosion Inhibitors for Iron and Steel. Molecules 2022, 27, 1778. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).