An Overview of Quinolones as Potential Drugs: Synthesis, Reactivity and Biological Activities
Abstract
1. Introduction
2. Methods of Synthesis of Quinolone Derivatives
2.1. Synthesis from Derivatives of Aniline and an Arylamine
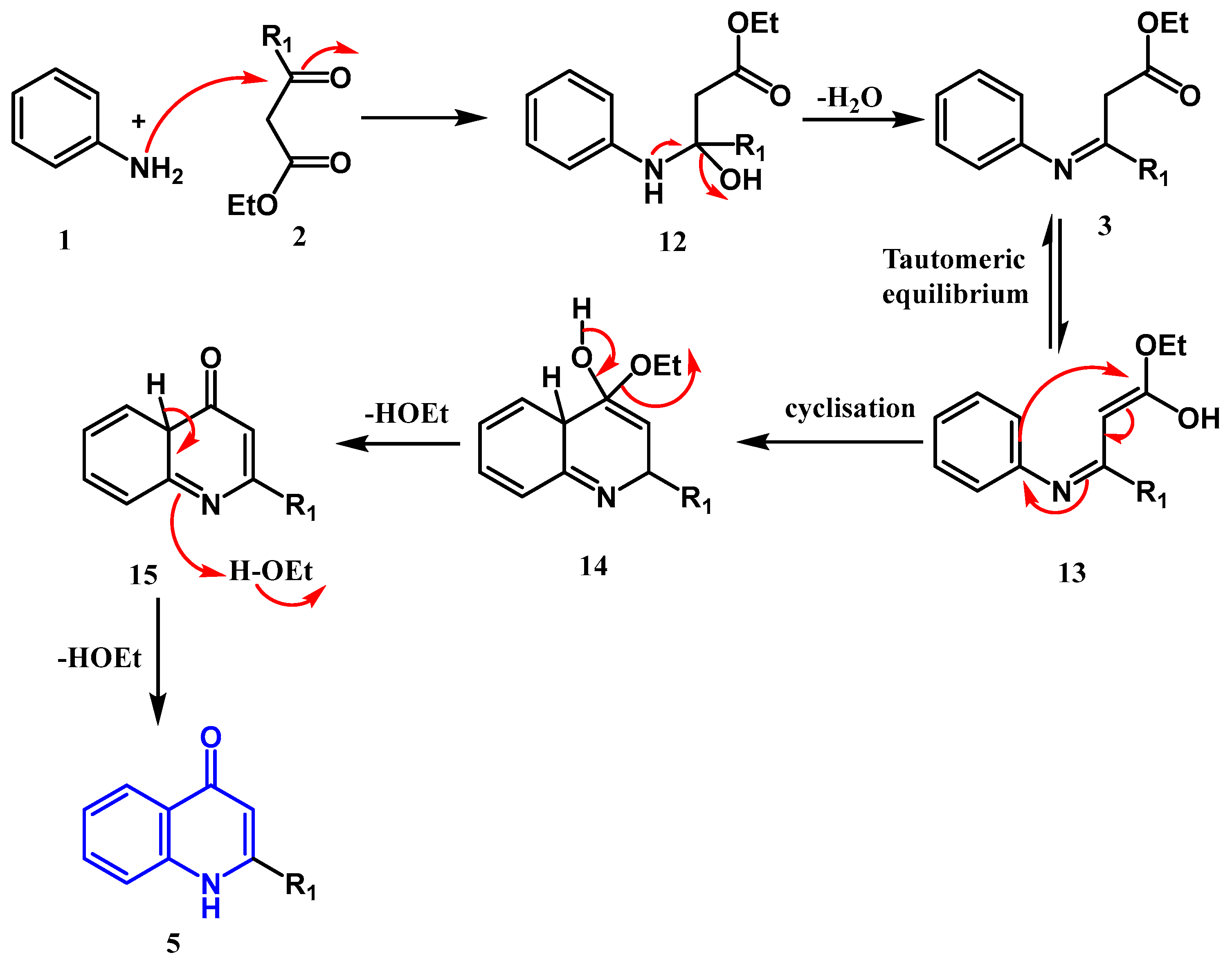
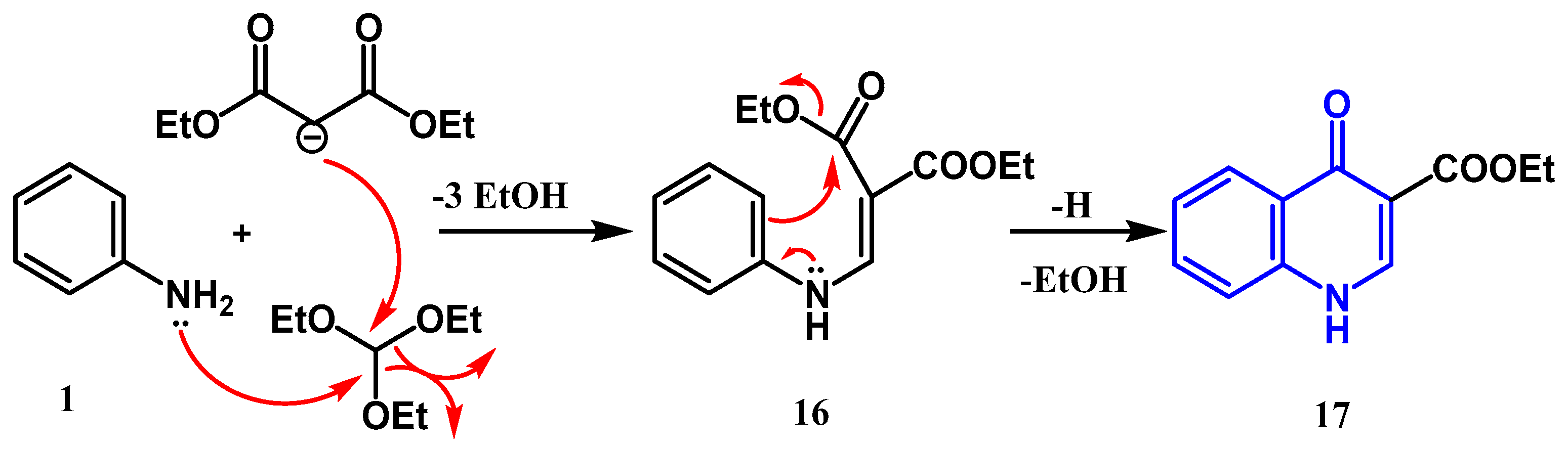
2.1.1. Conrad–Limpach–Knorr Reaction
2.1.2. Doebner Multicomponent Reaction
2.1.3. Gould–Jacobs Reactions
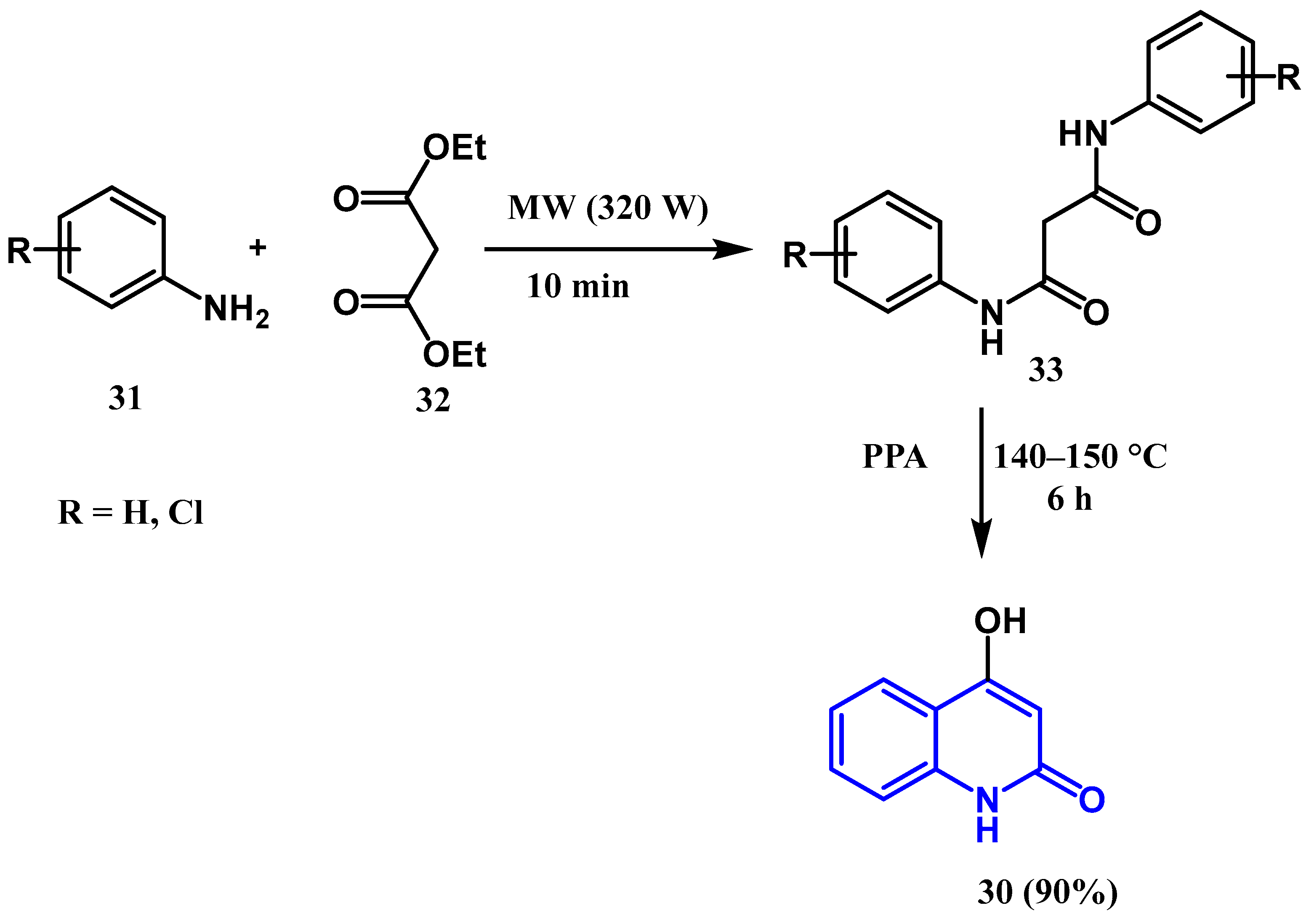
2.1.4. Jampilek Reaction
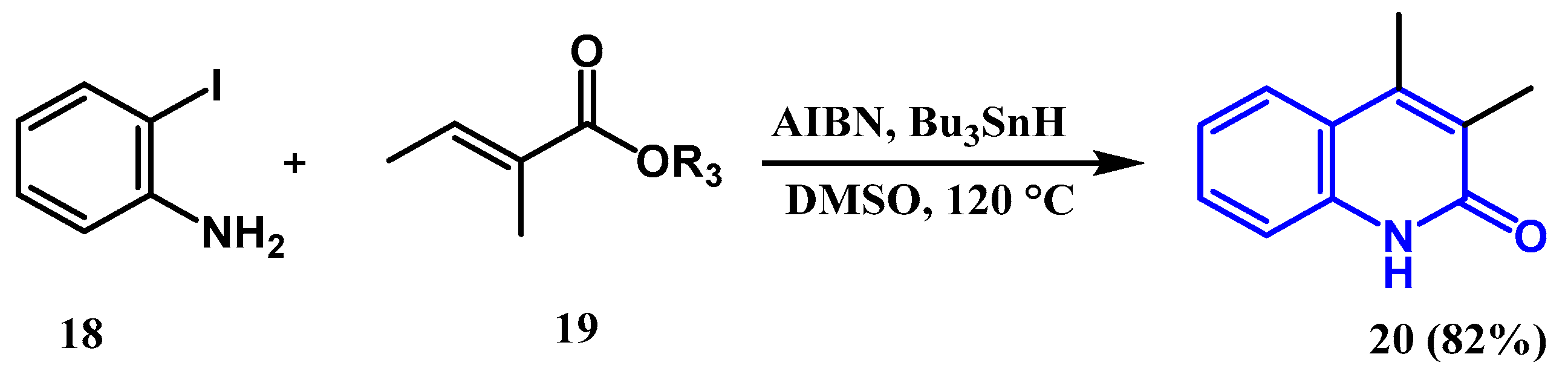
2.1.5. Cheng Reaction
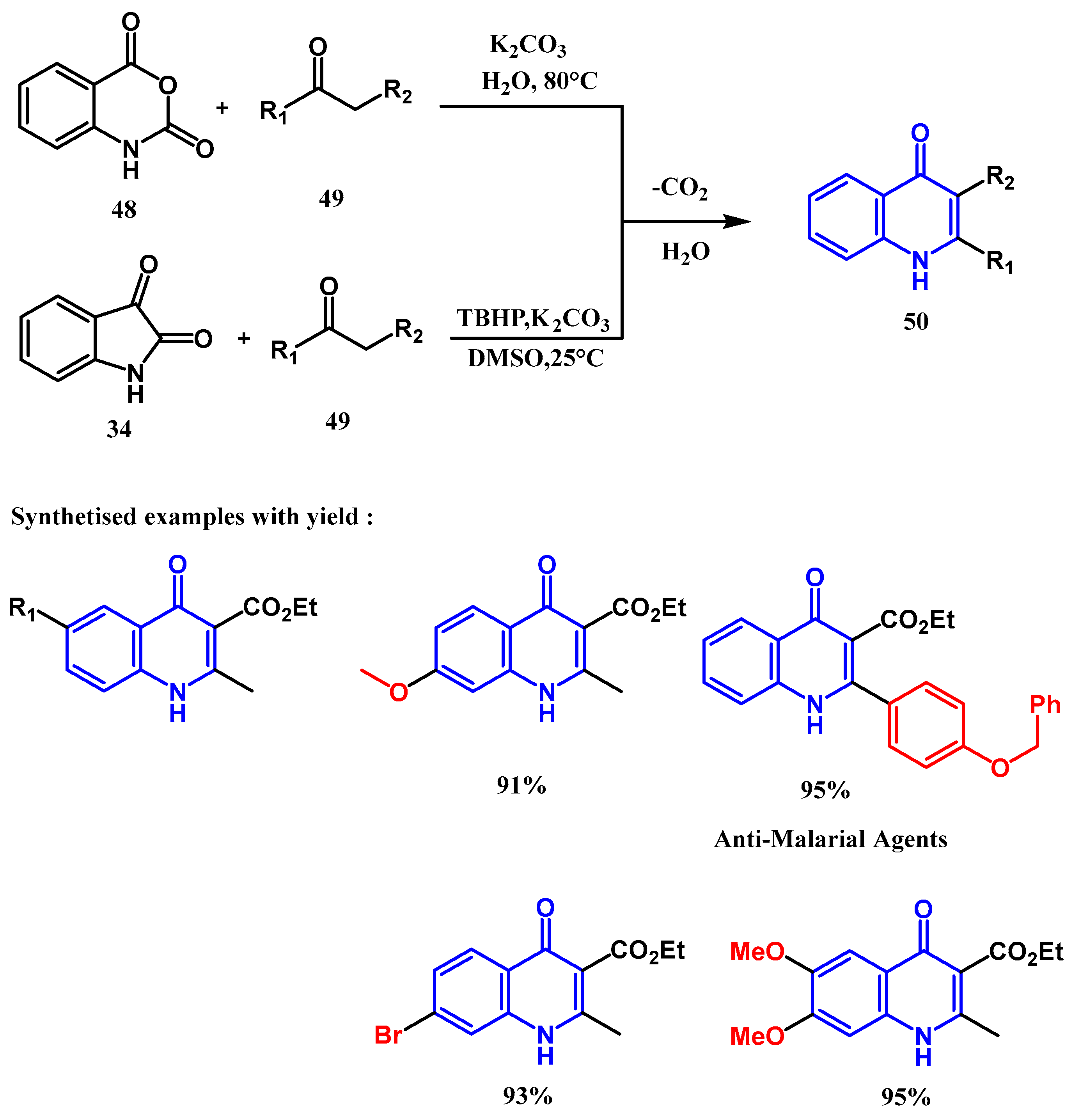
2.2. Syntheses from the Indole-2,3-dione Ring
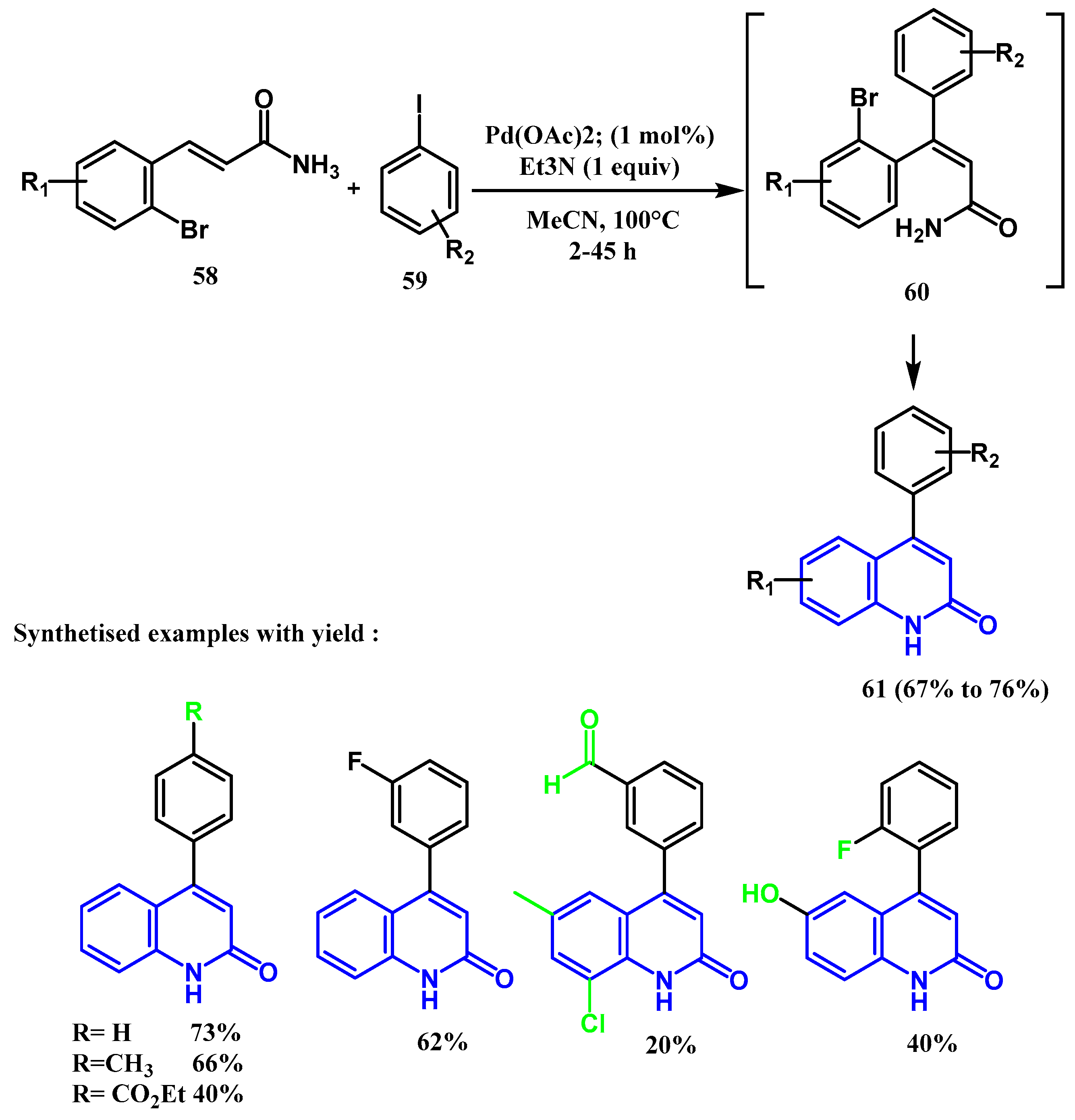
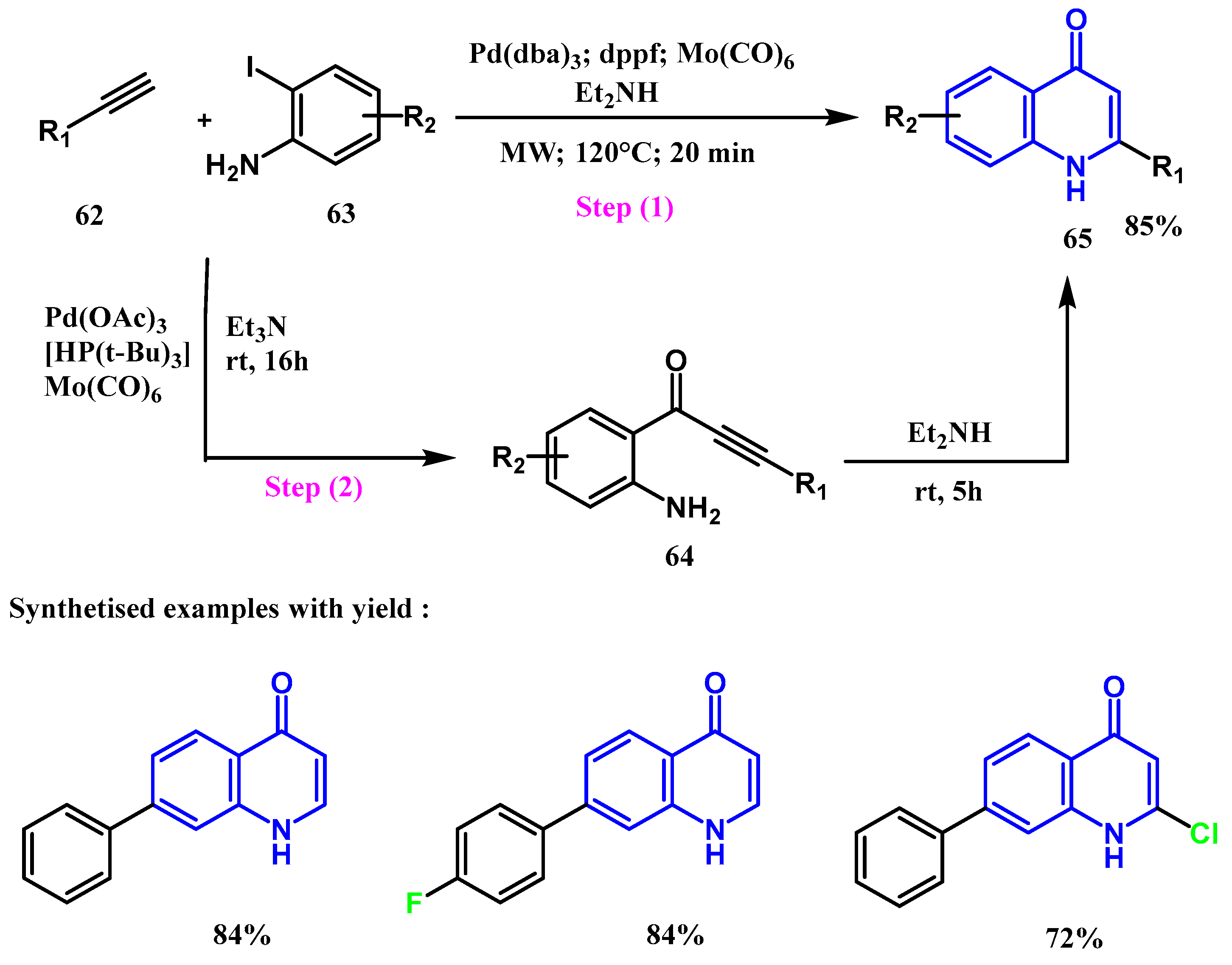
2.3. Reactions Catalyzed by Pd
2.4. Catalytic Reduction
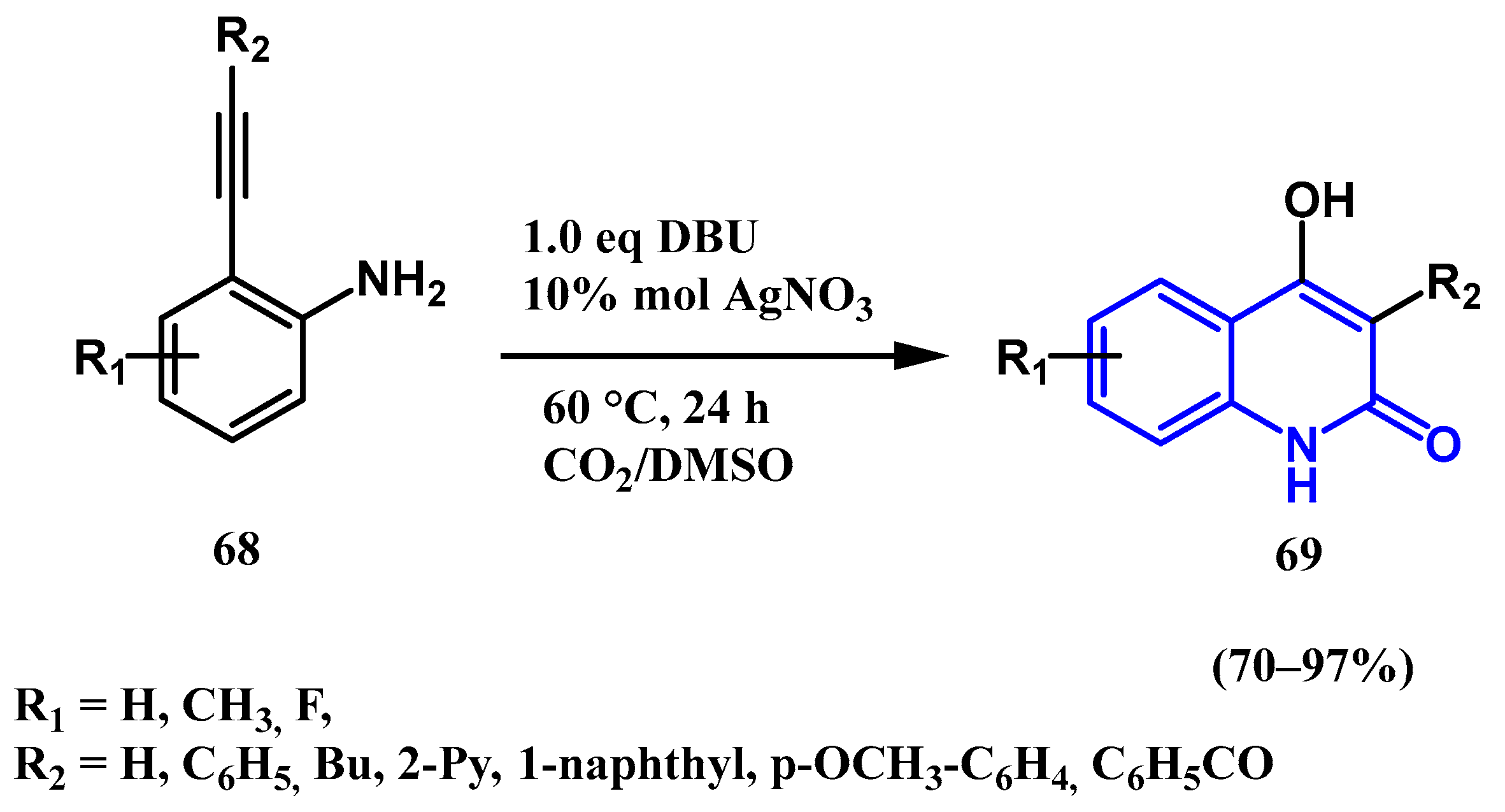
2.5. Silver-Catalyzed Carboxylation
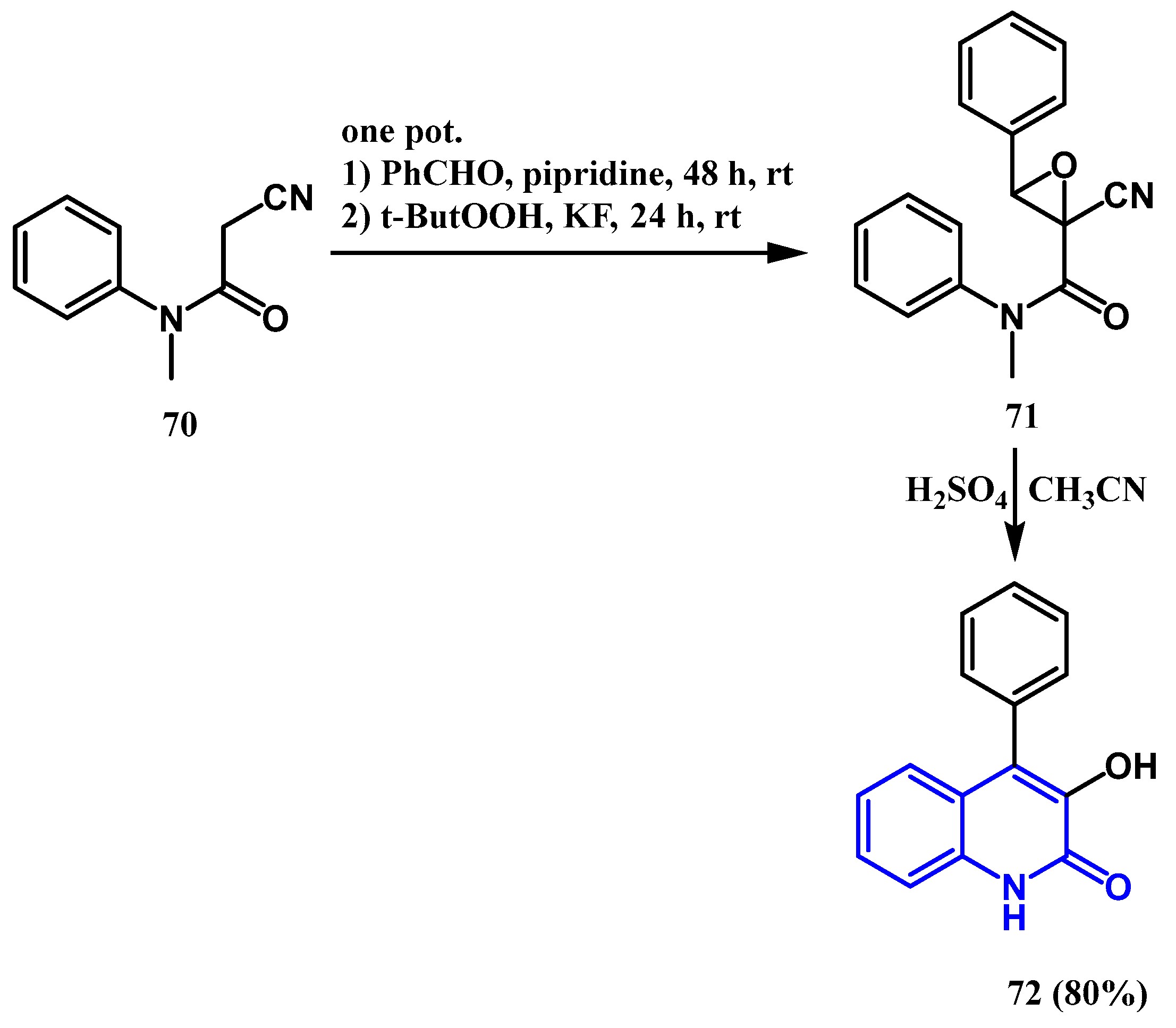
2.6. One-Pot Reaction
2.7. PIFA Catalyzed Oxidative Reaction
3. Reactivity of Quinolones’ Derivatives
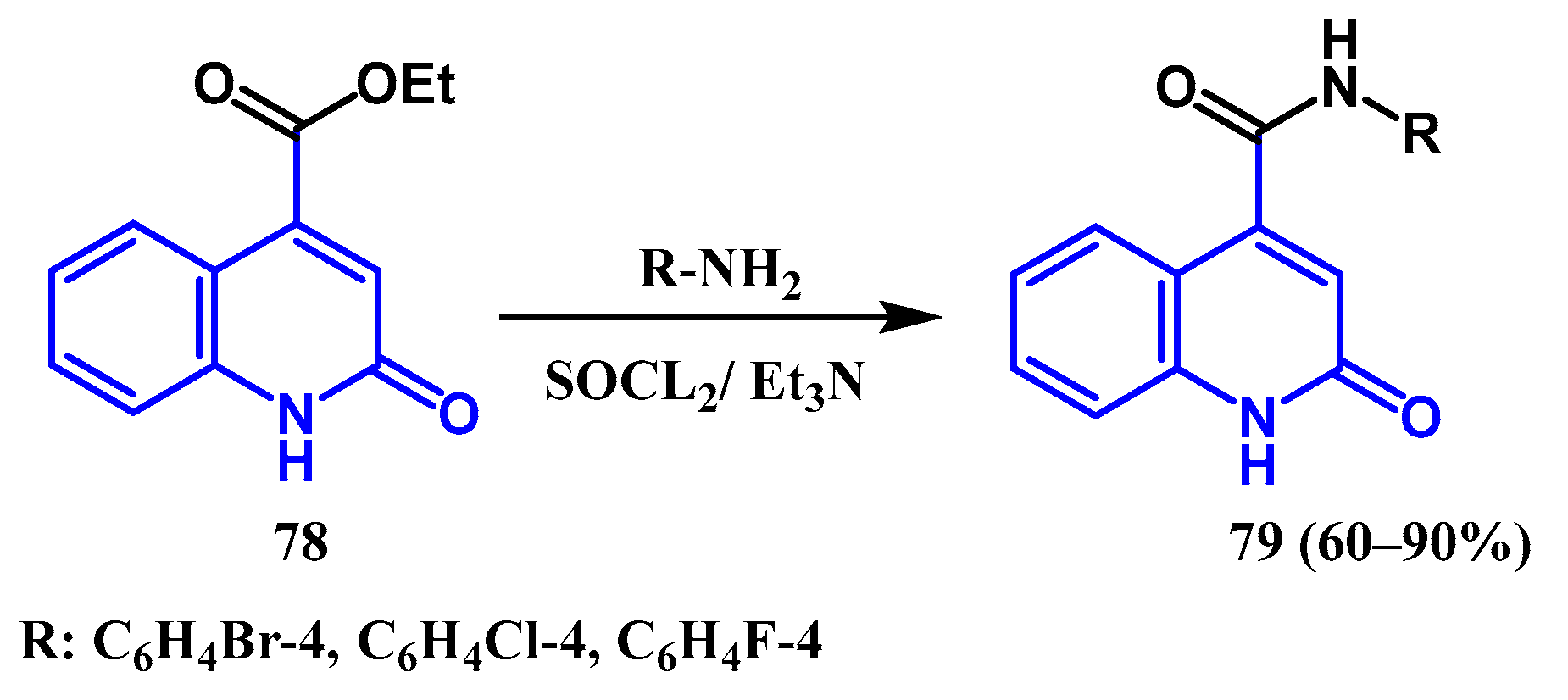
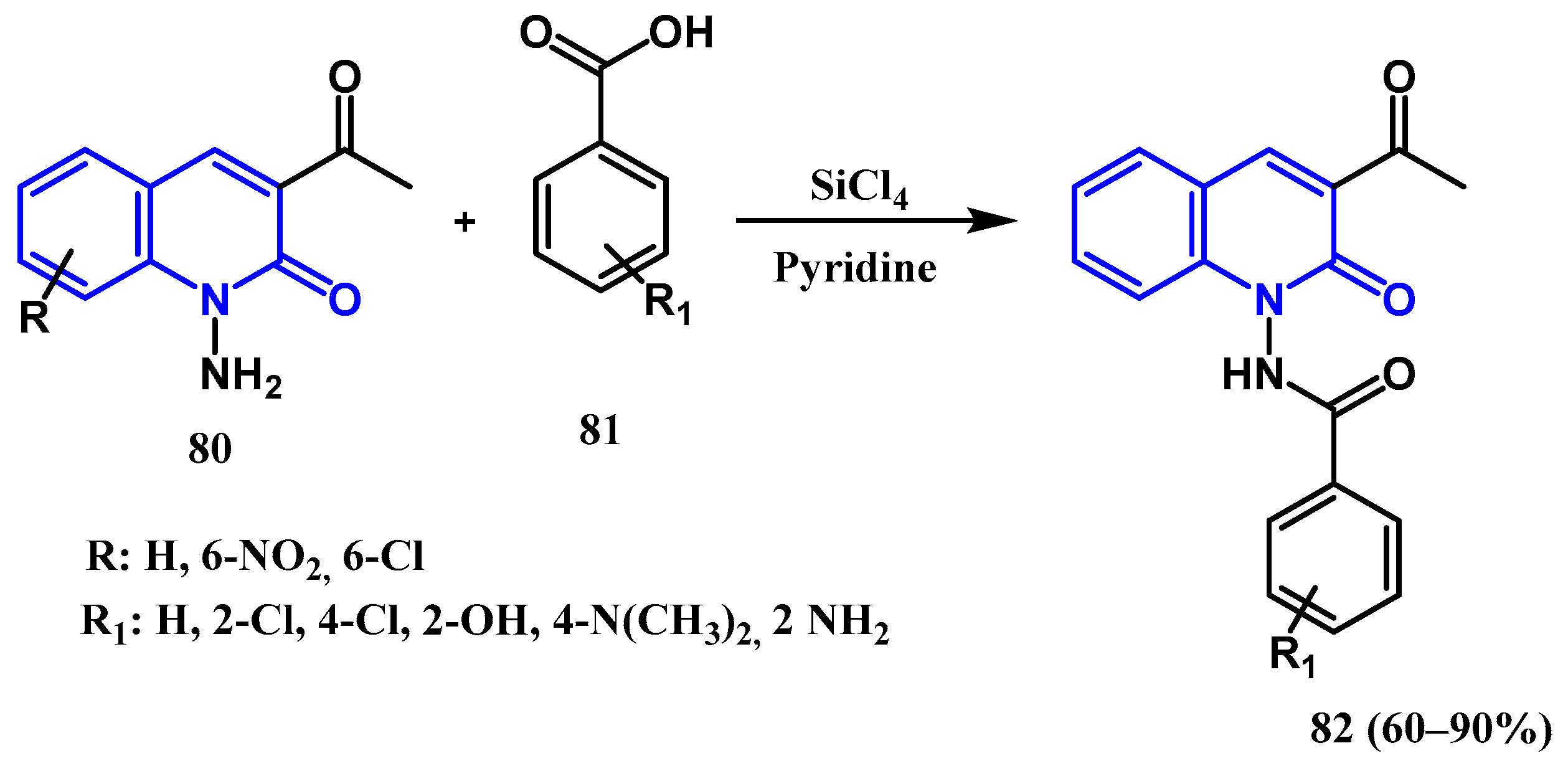
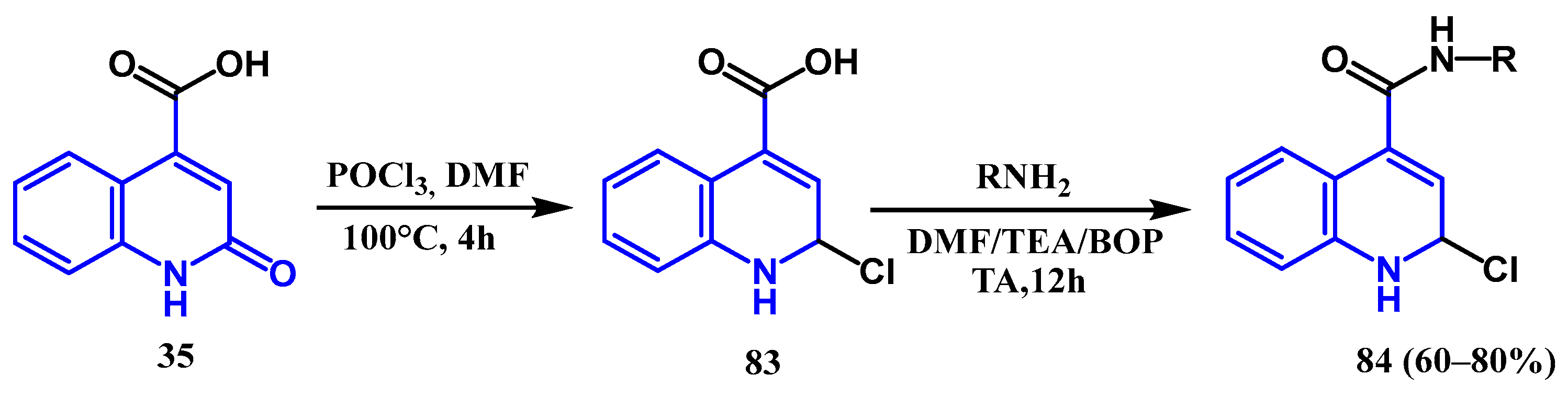
3.1. Amidation and Amination
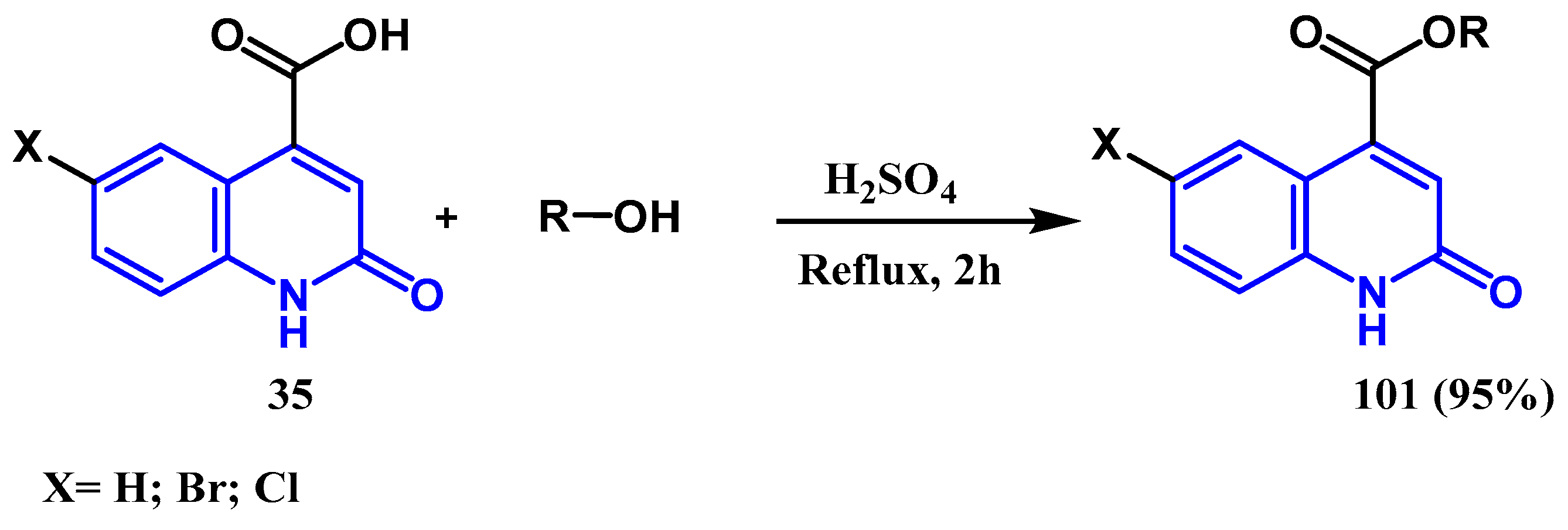
3.2. Esterification
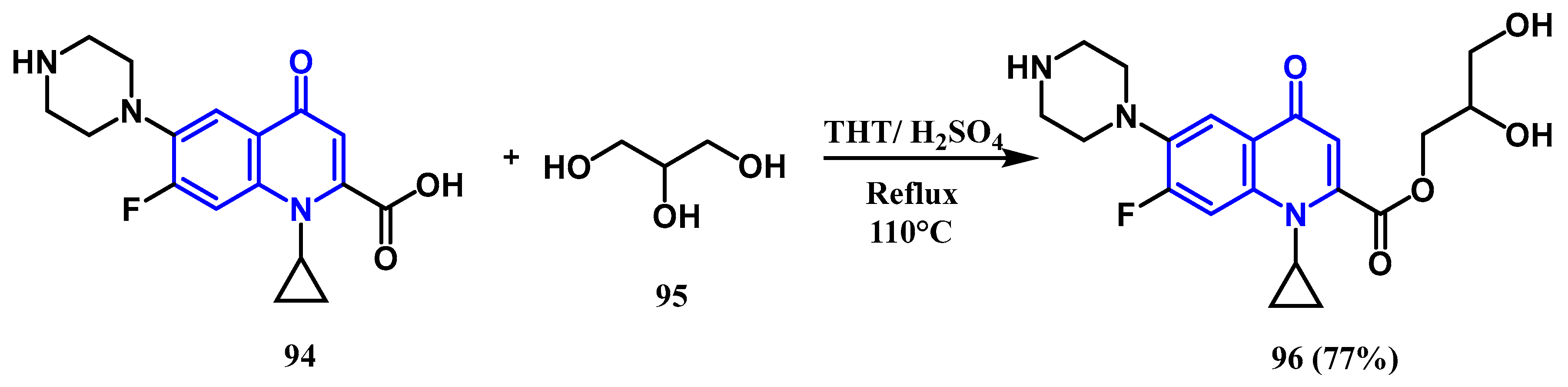
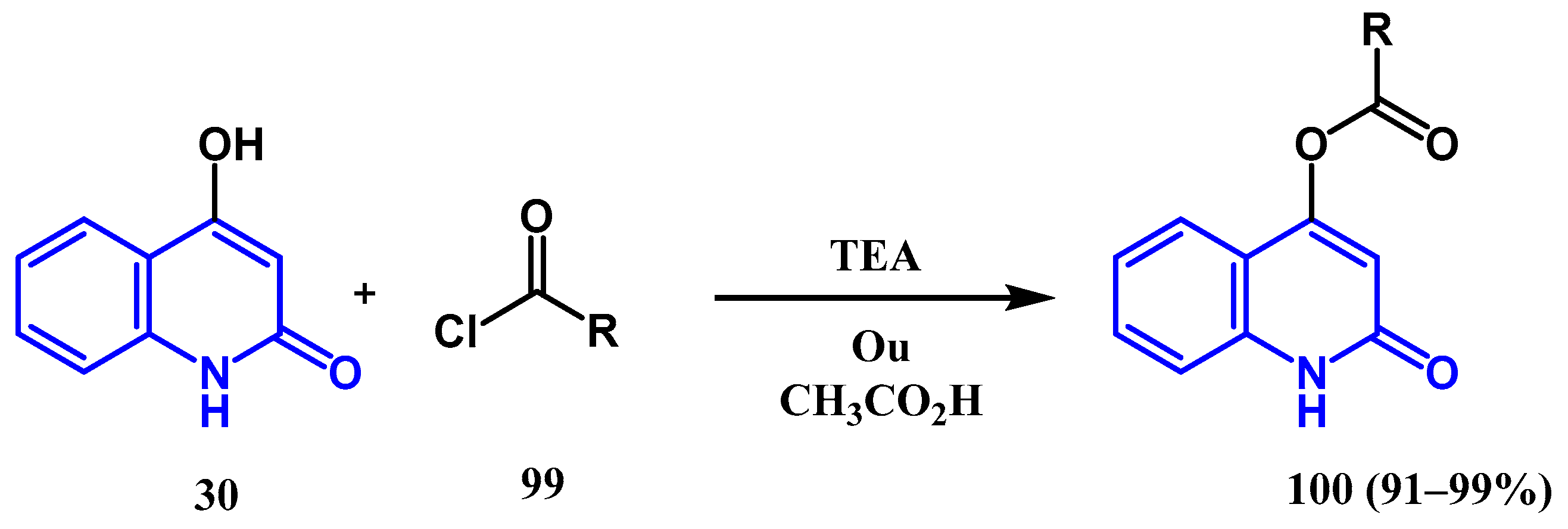
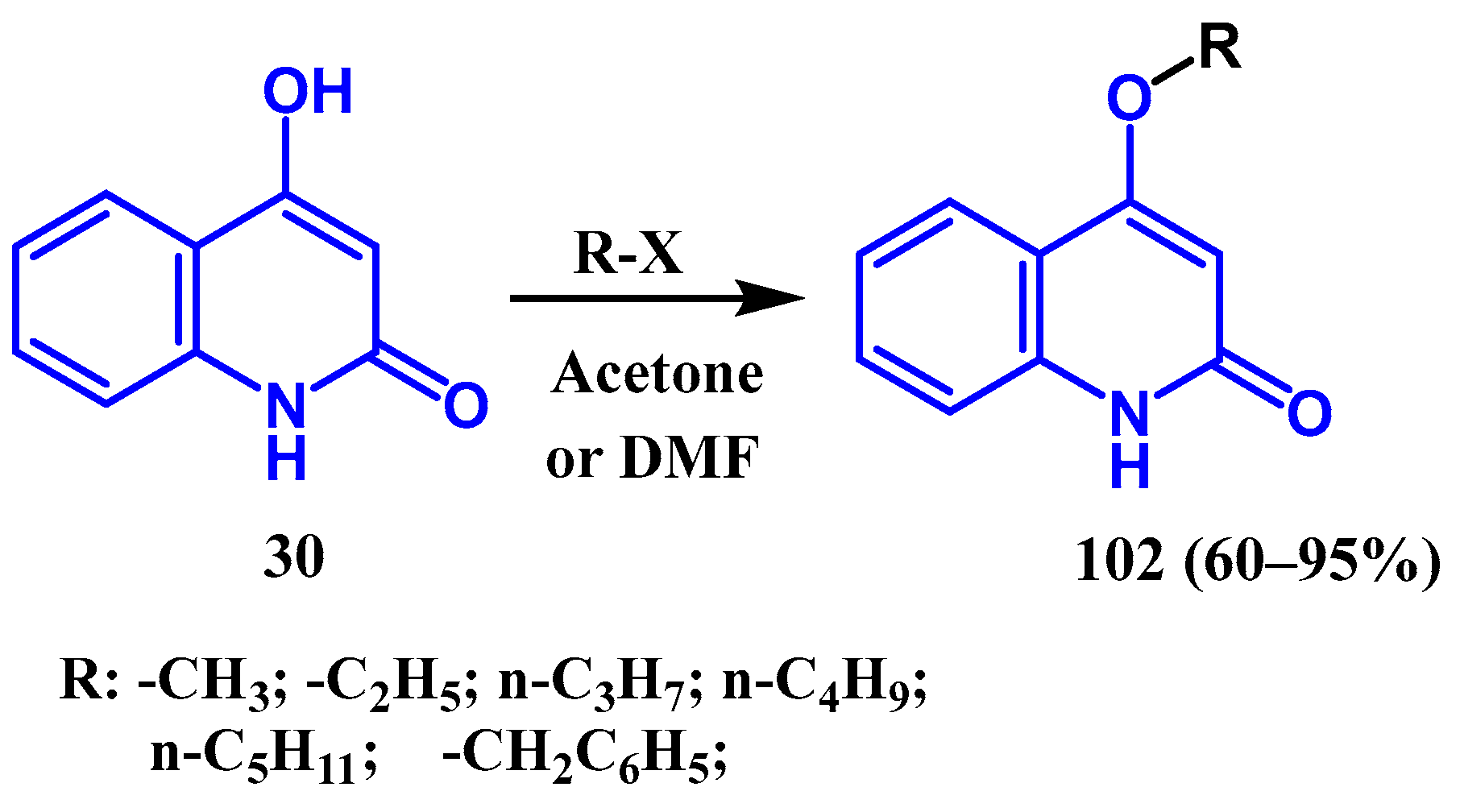
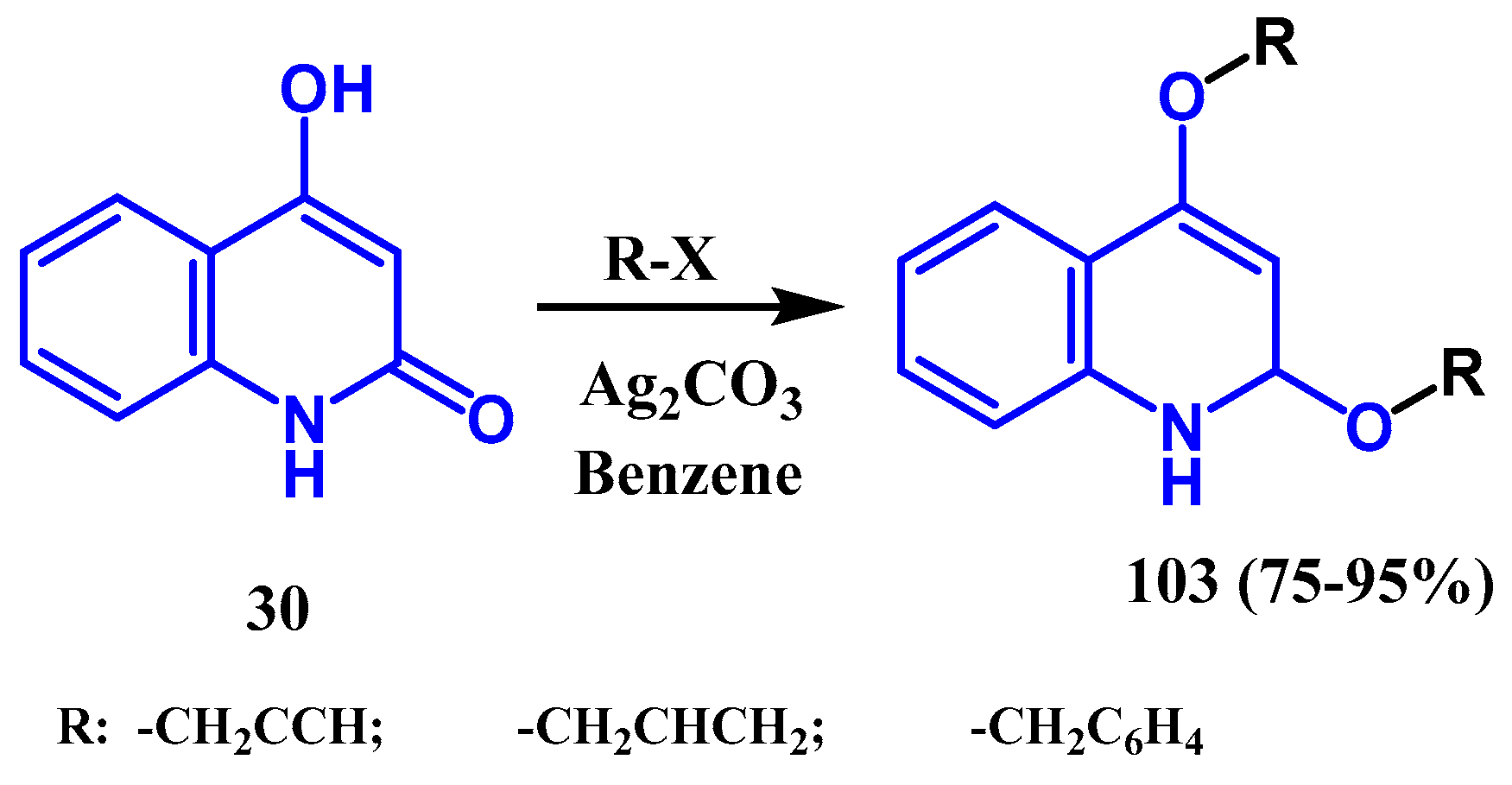
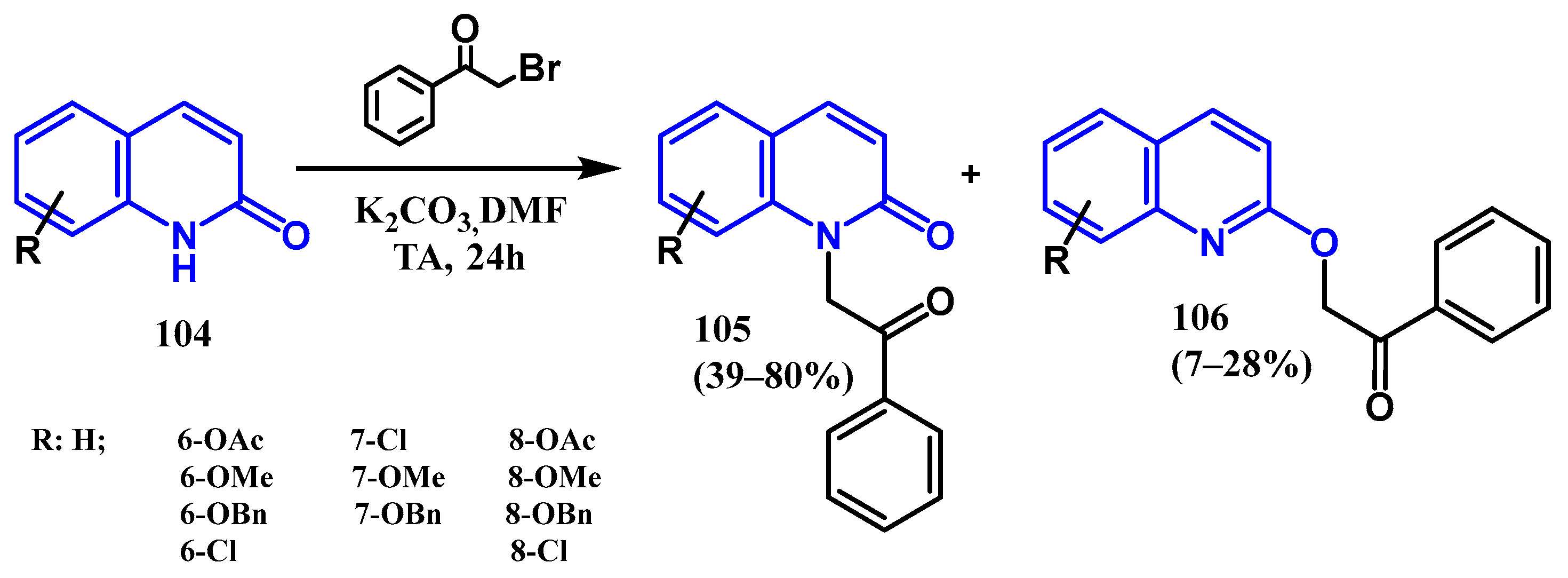
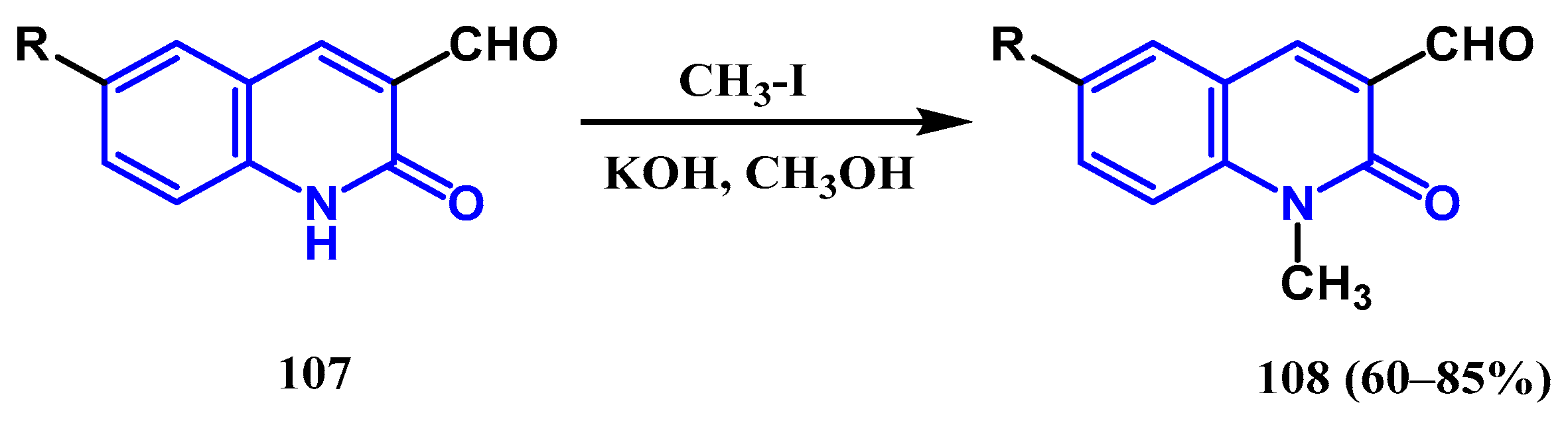
3.3. N-, O- and C-Alkylation
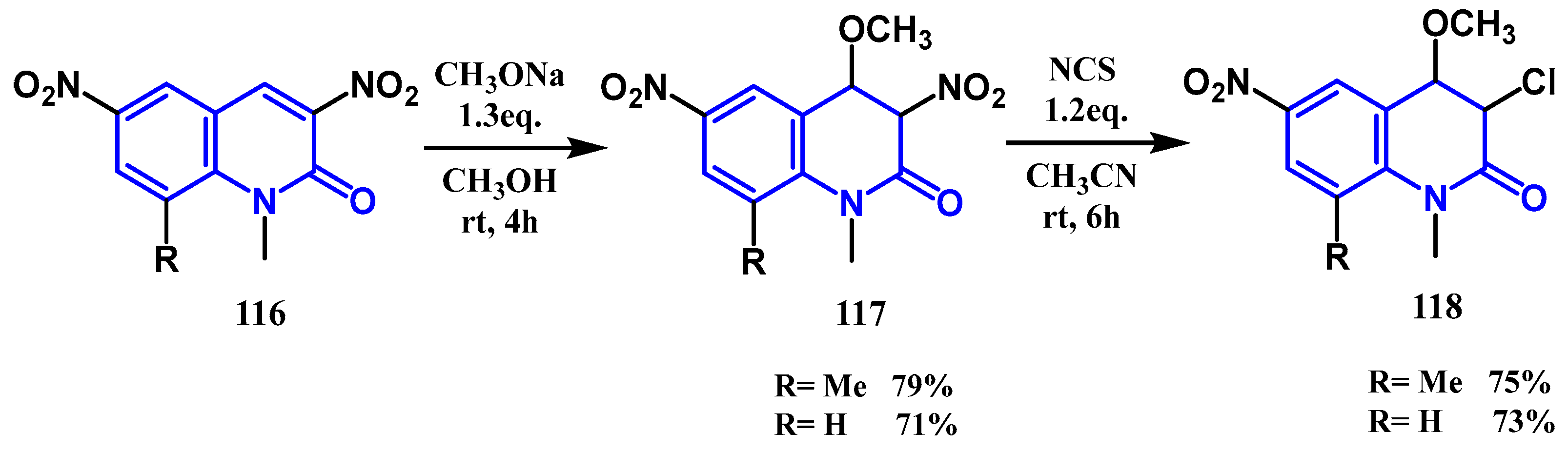
3.4. Halogenation
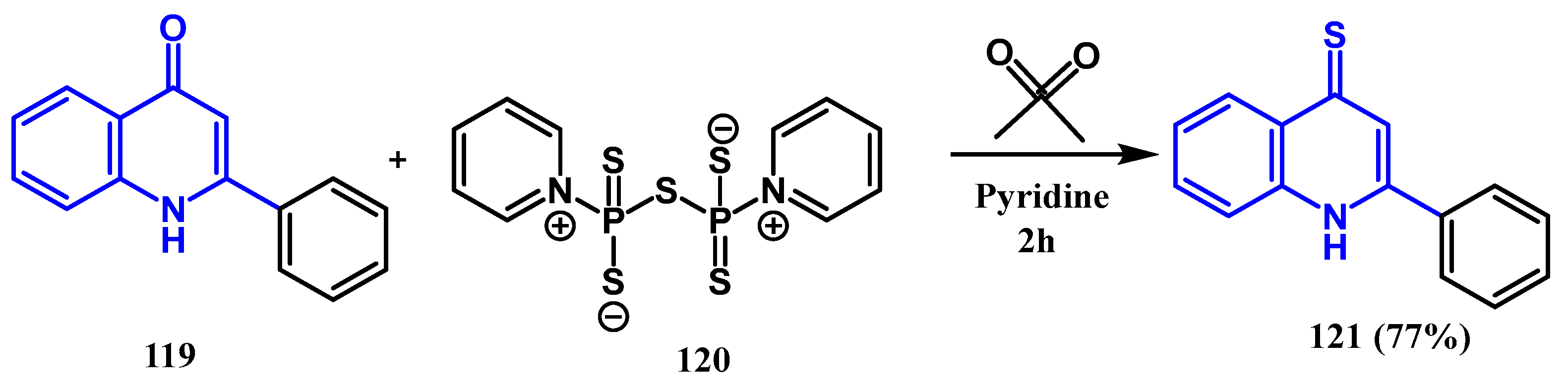
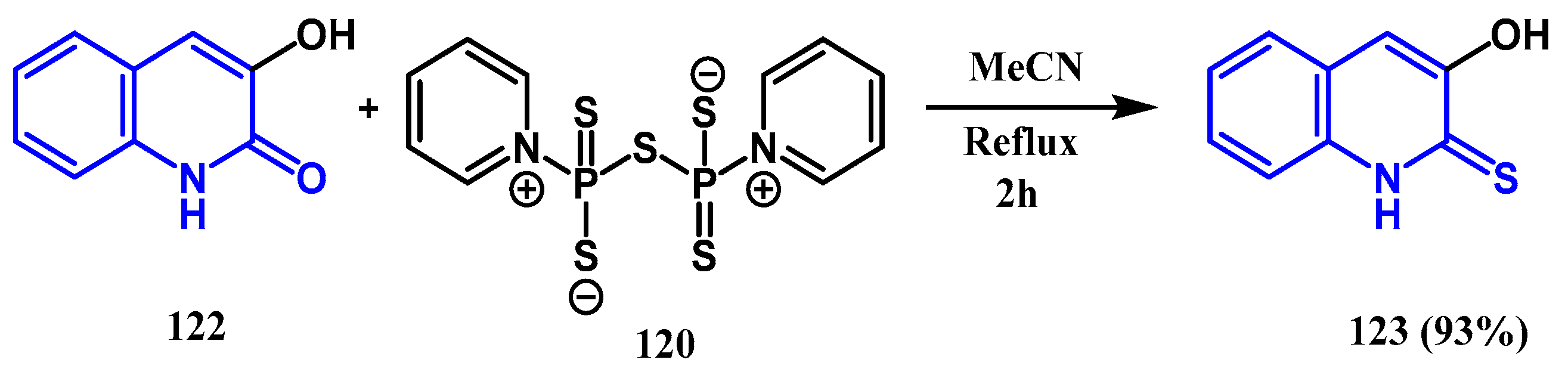
3.5. Thionation
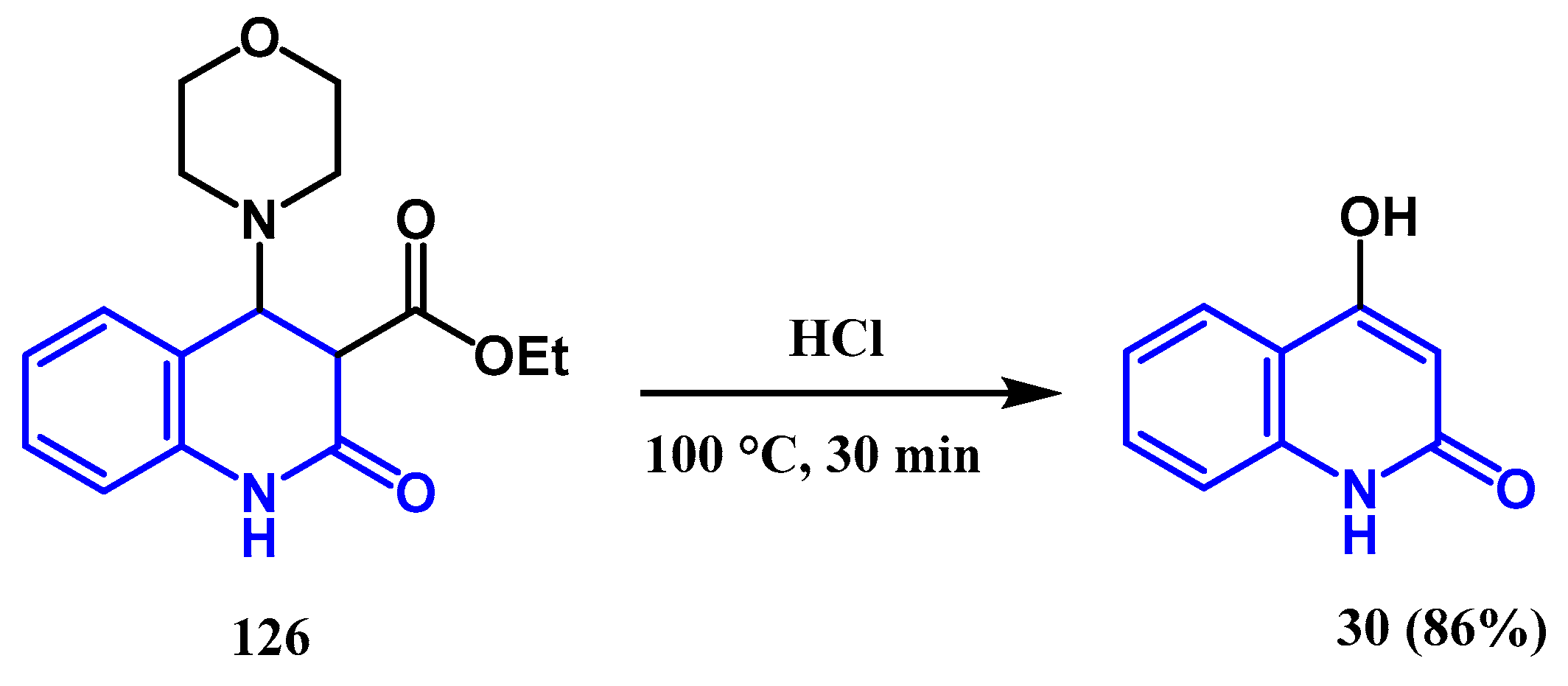
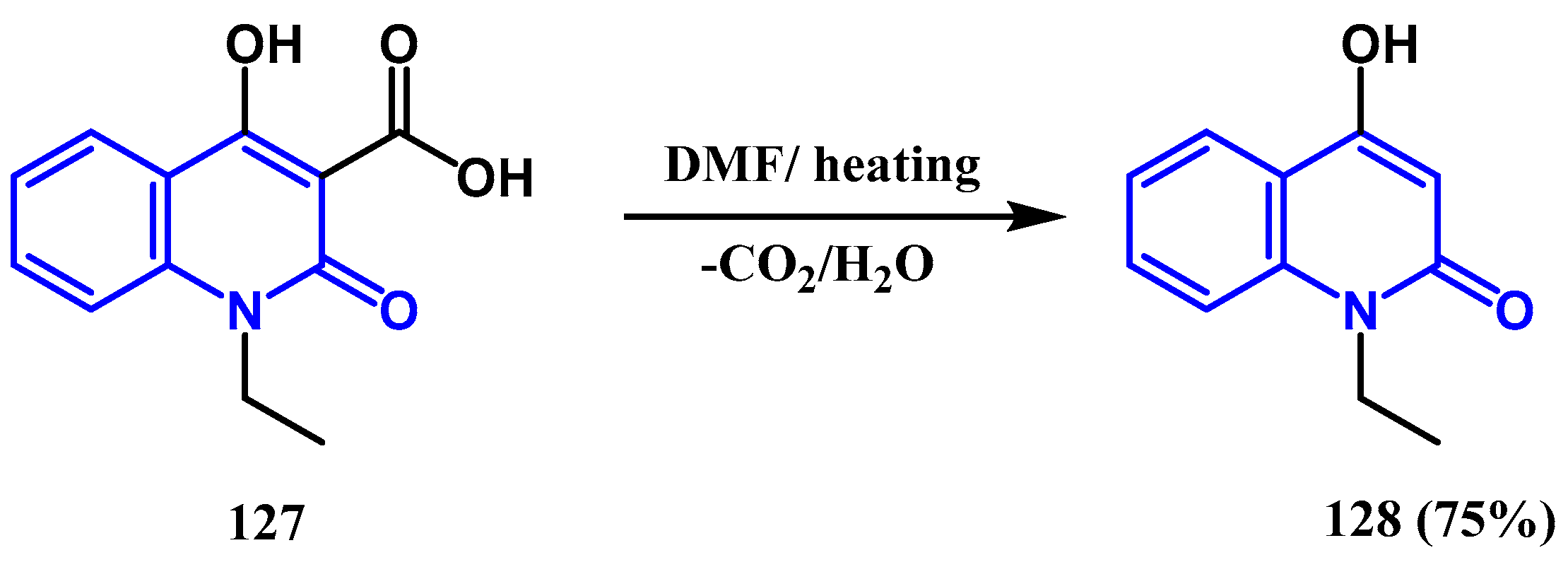
3.6. Hydrolyse and Decarboxylation
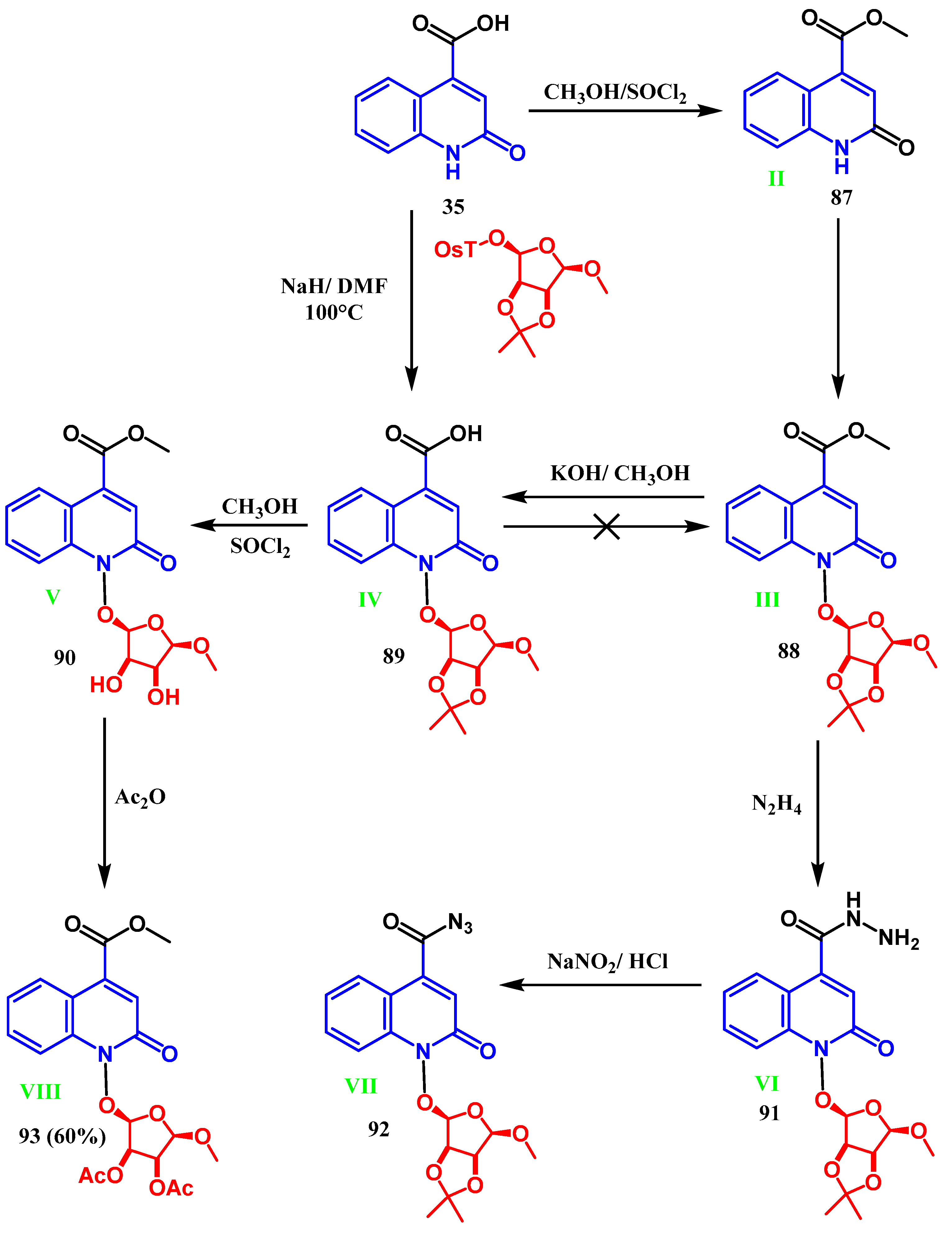
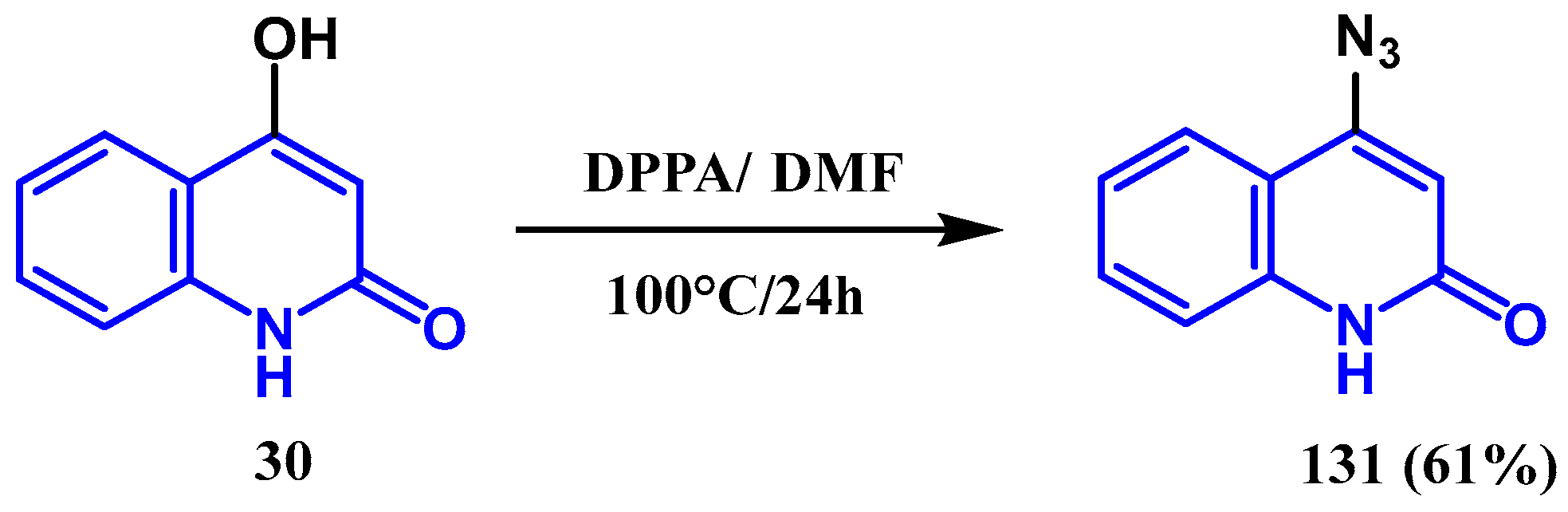
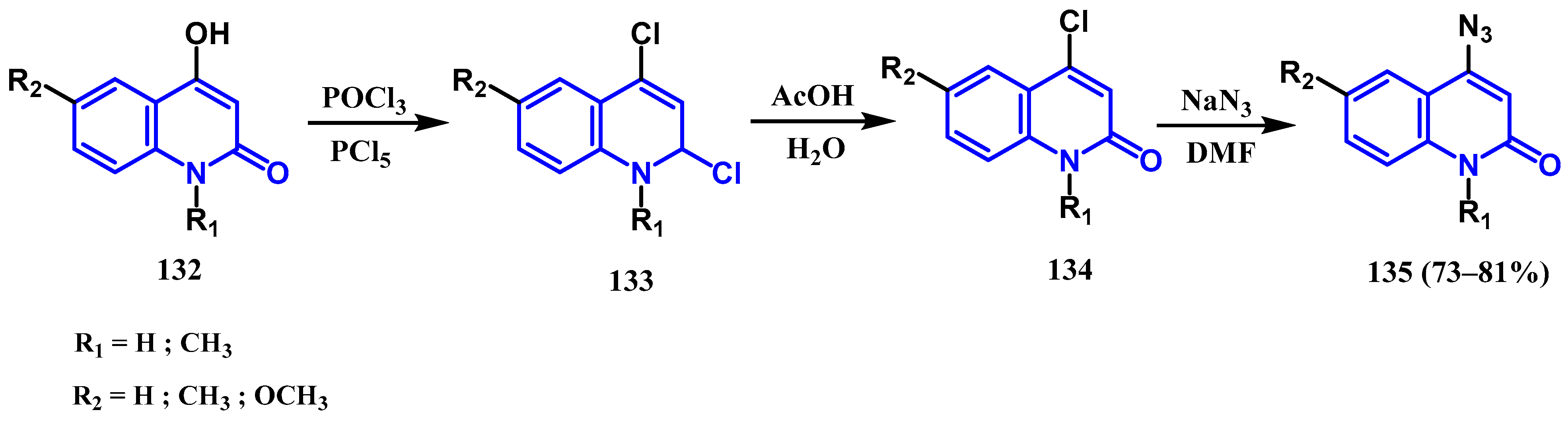
3.7. Azidation
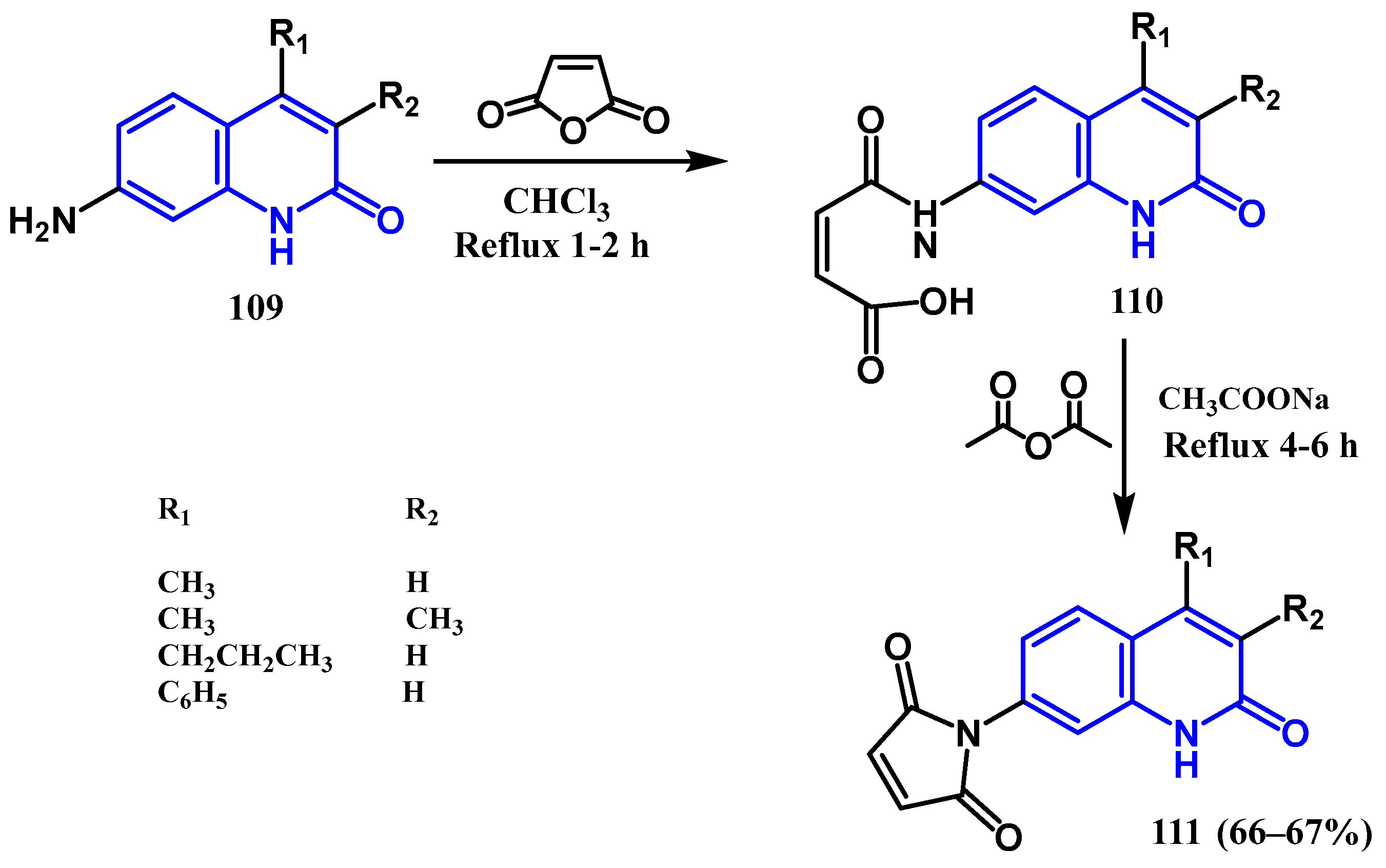
3.8. Cycloaddition
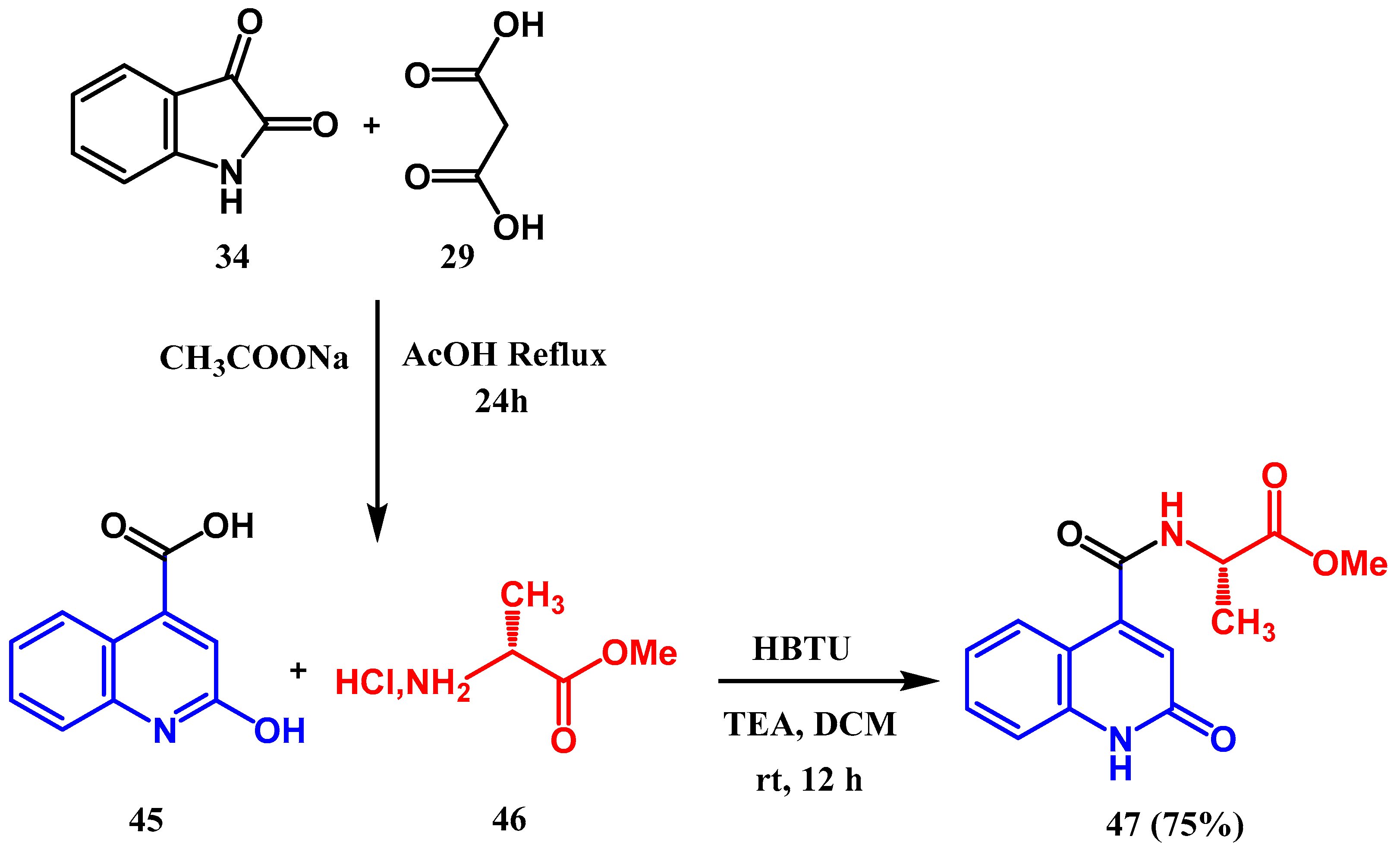
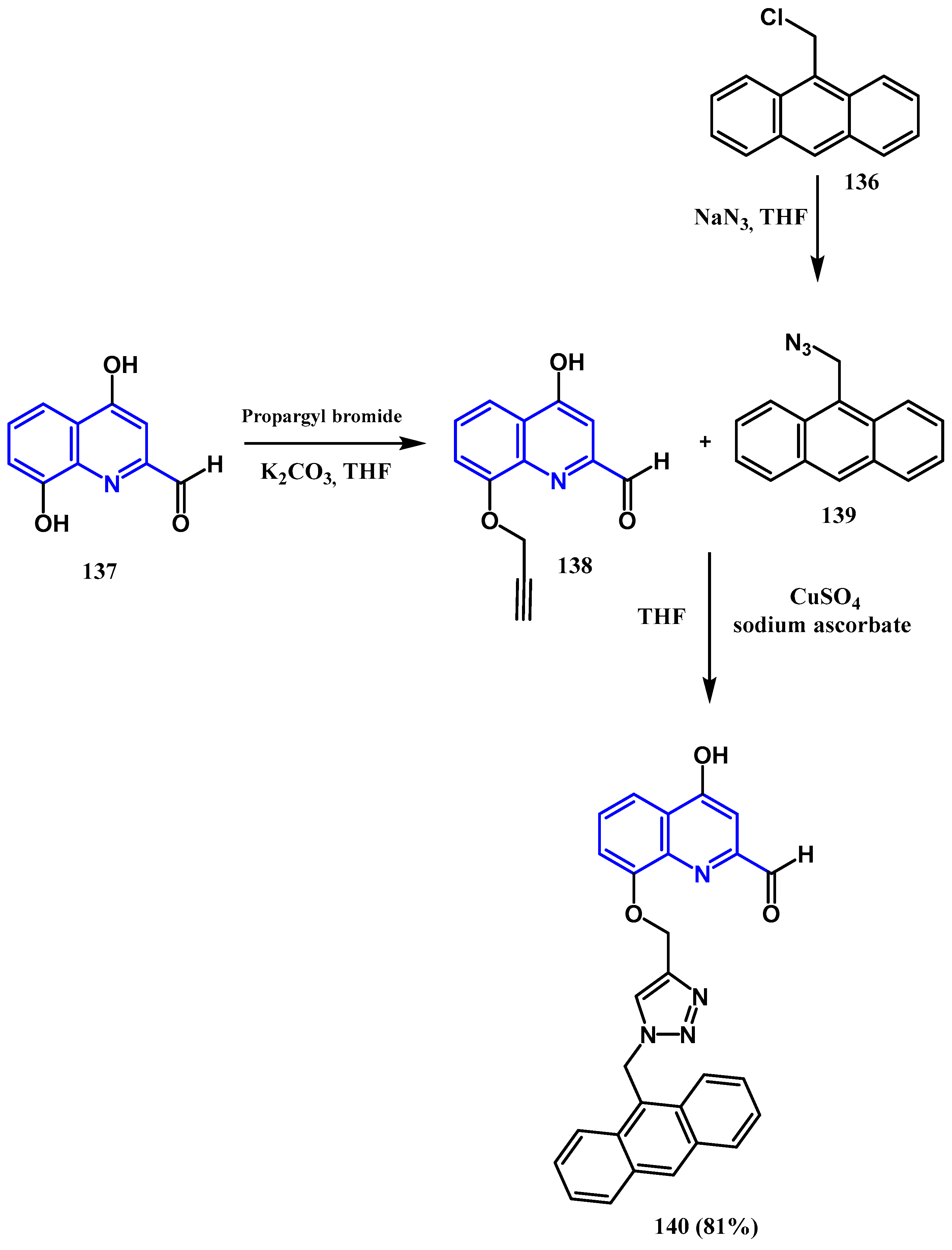
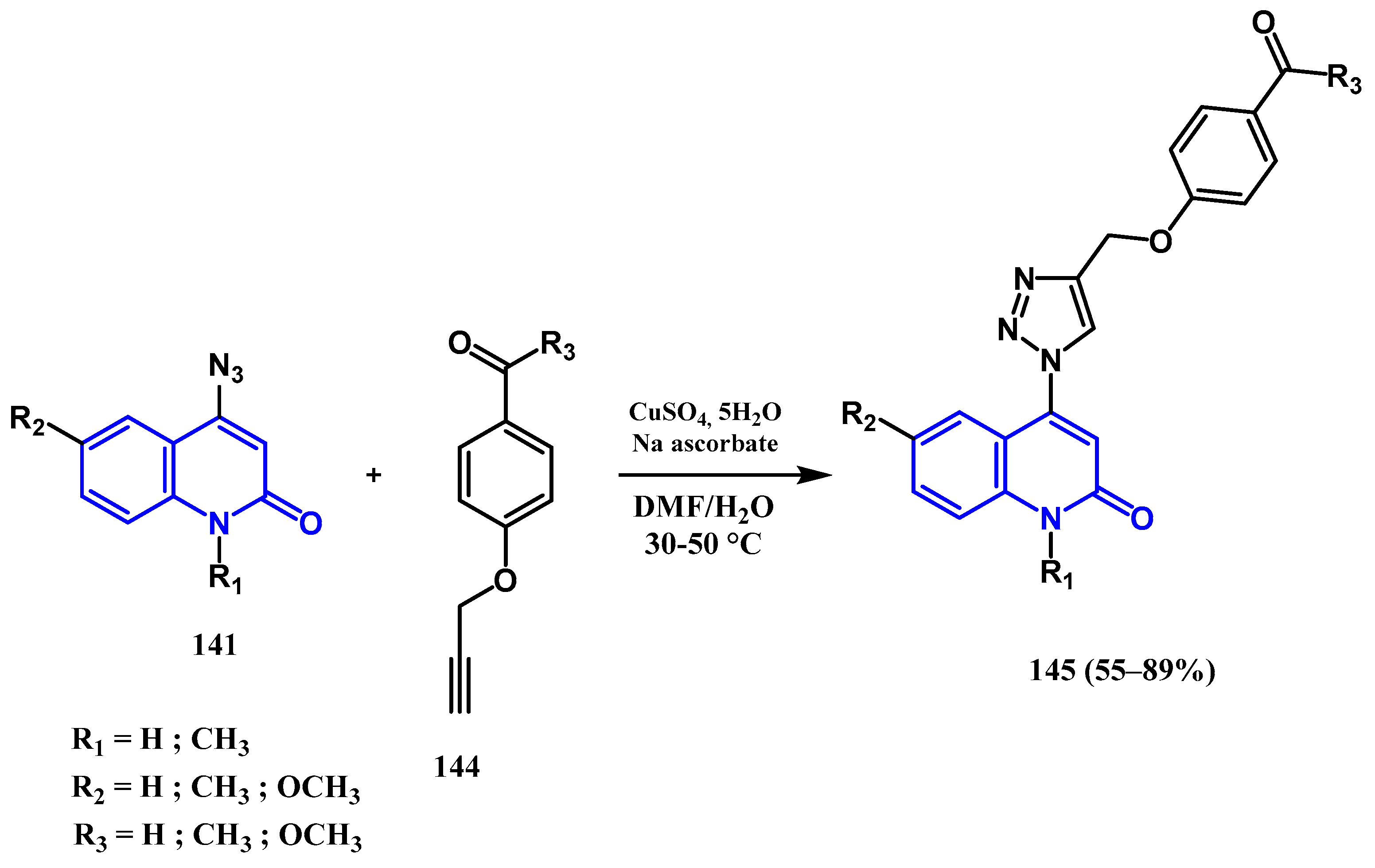
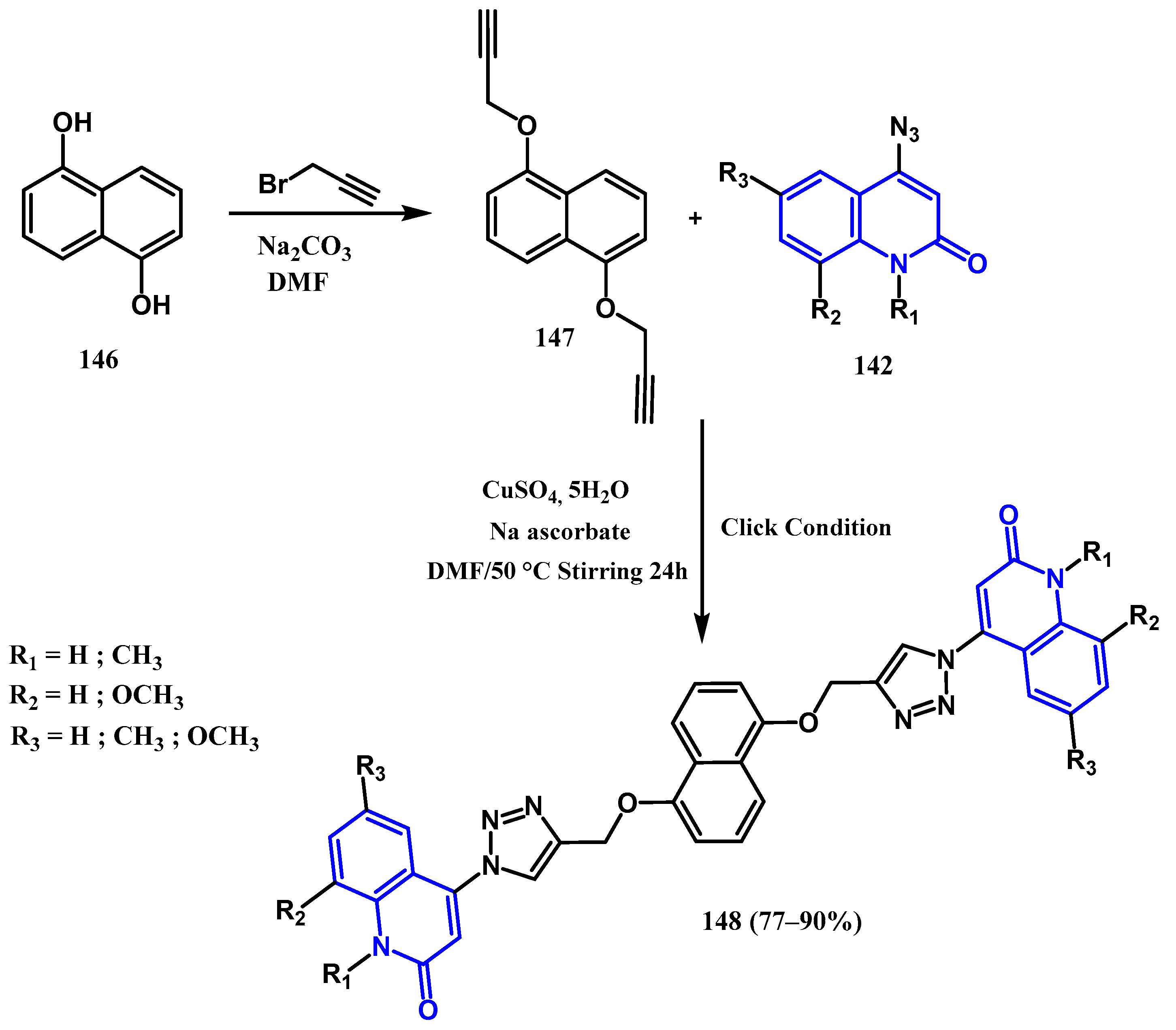
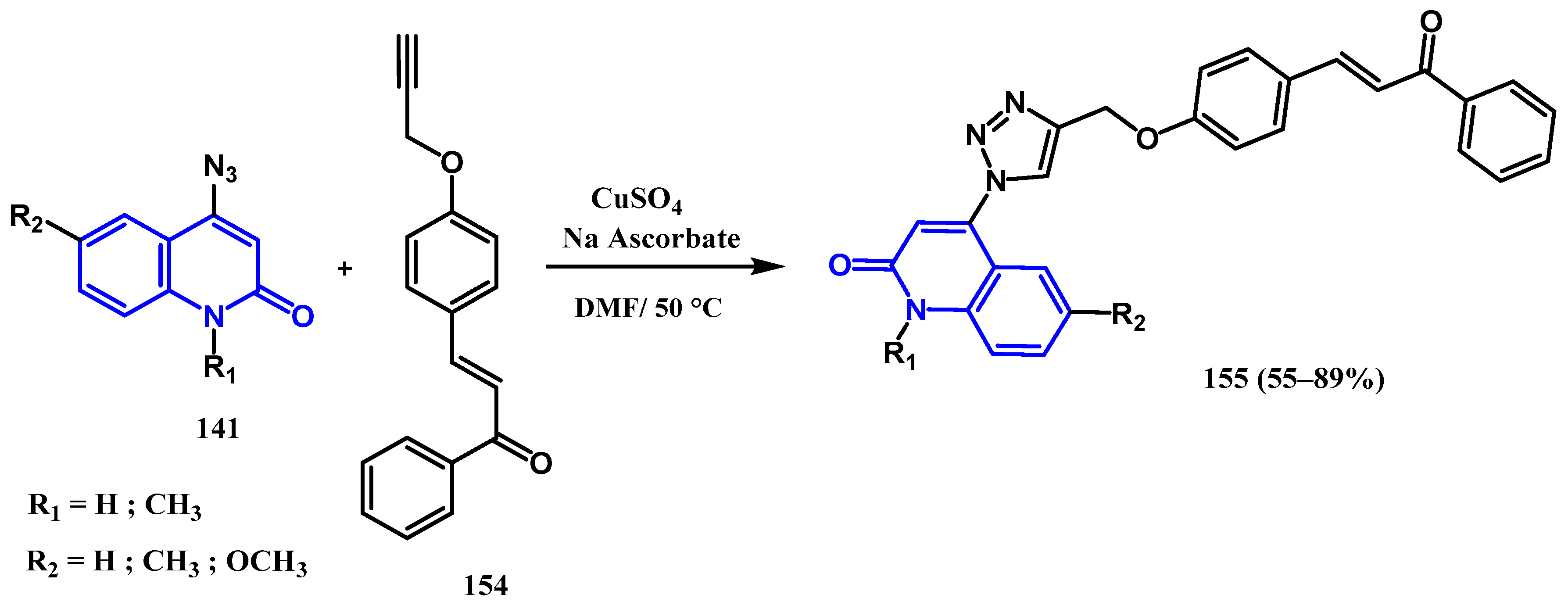
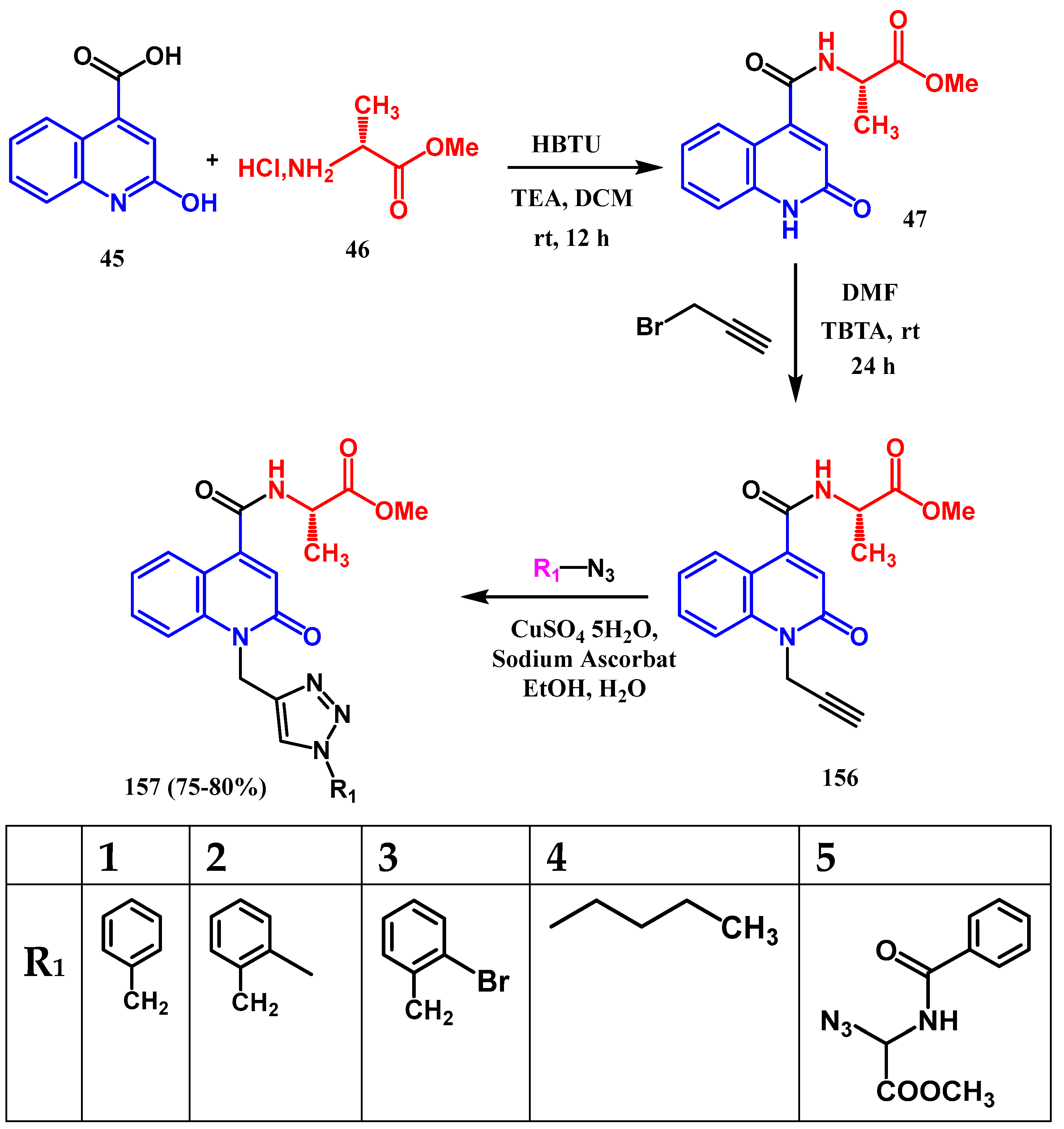
3.8.1. Preparation of Triazole-Quinolone
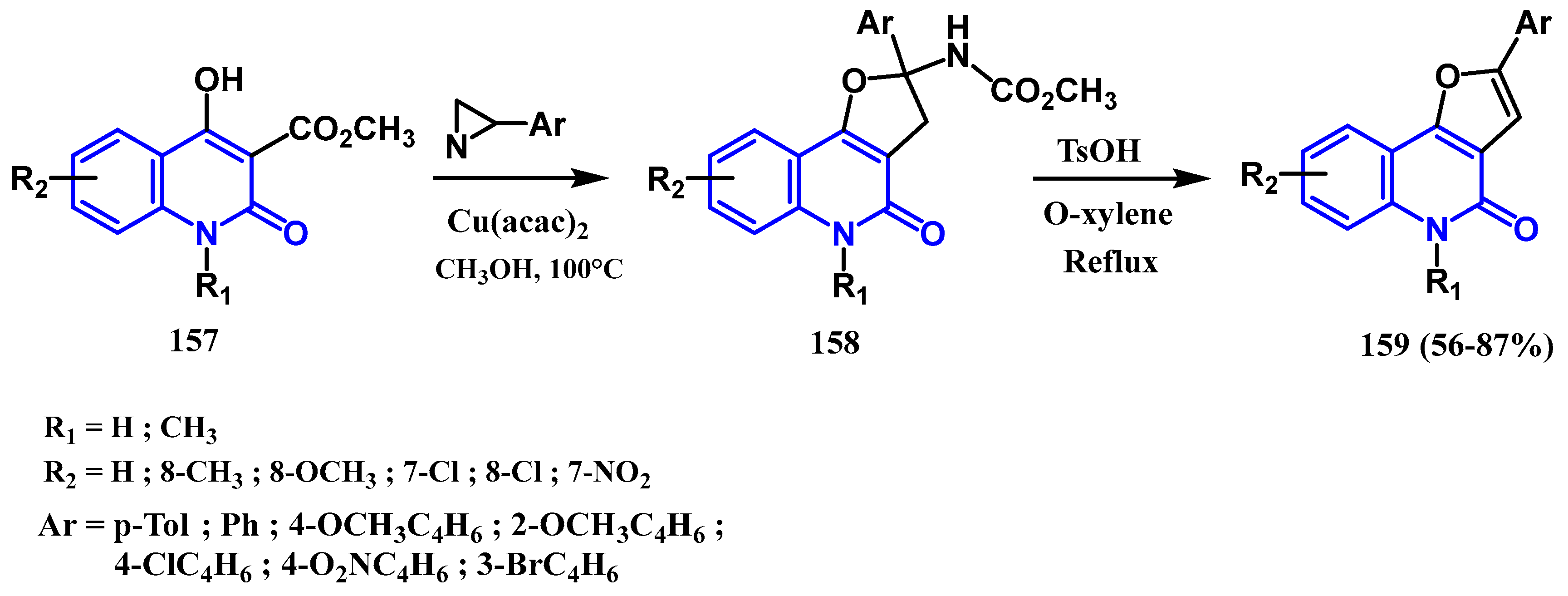
3.8.2. Preparation of Furanoquinolones and Pyranoquinolones
3.8.3. Preparation of Imidazole Quinolones
3.8.4. Preparation of Thiazolidin-4-one Quinolones
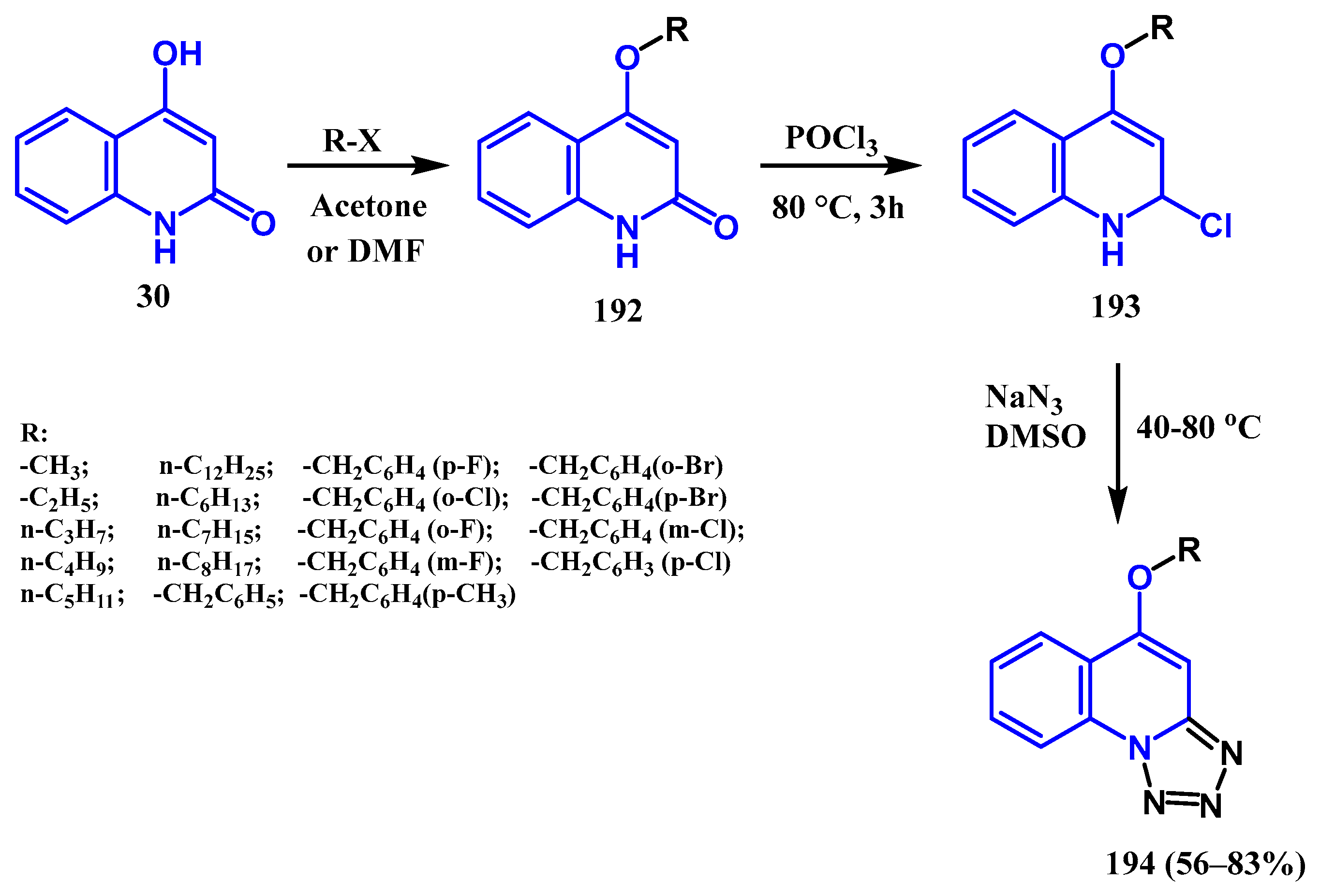
3.8.5. Preparation of Tetrazole Quinolones
4. Biological Activities of Quinolone Derivatives
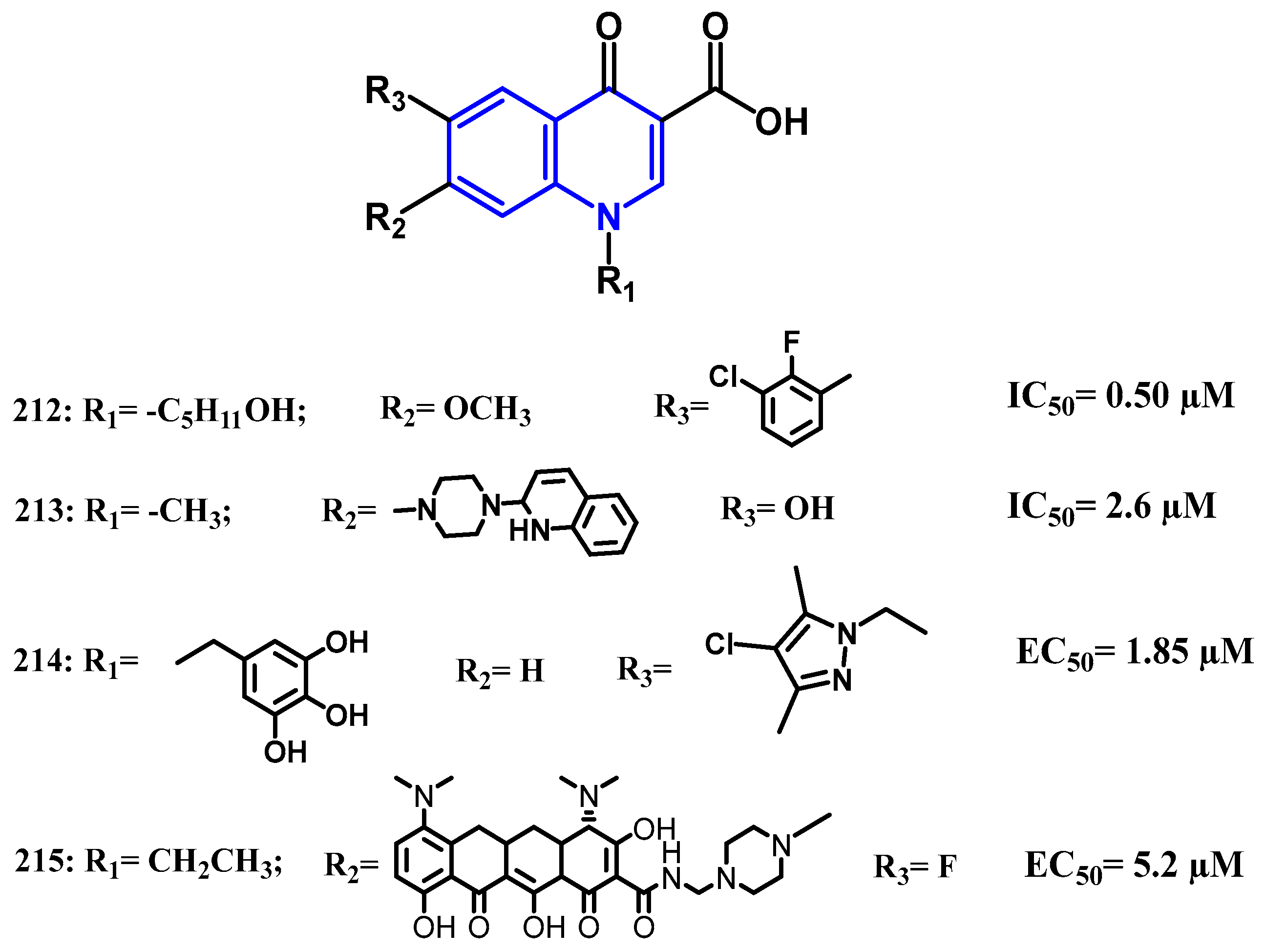
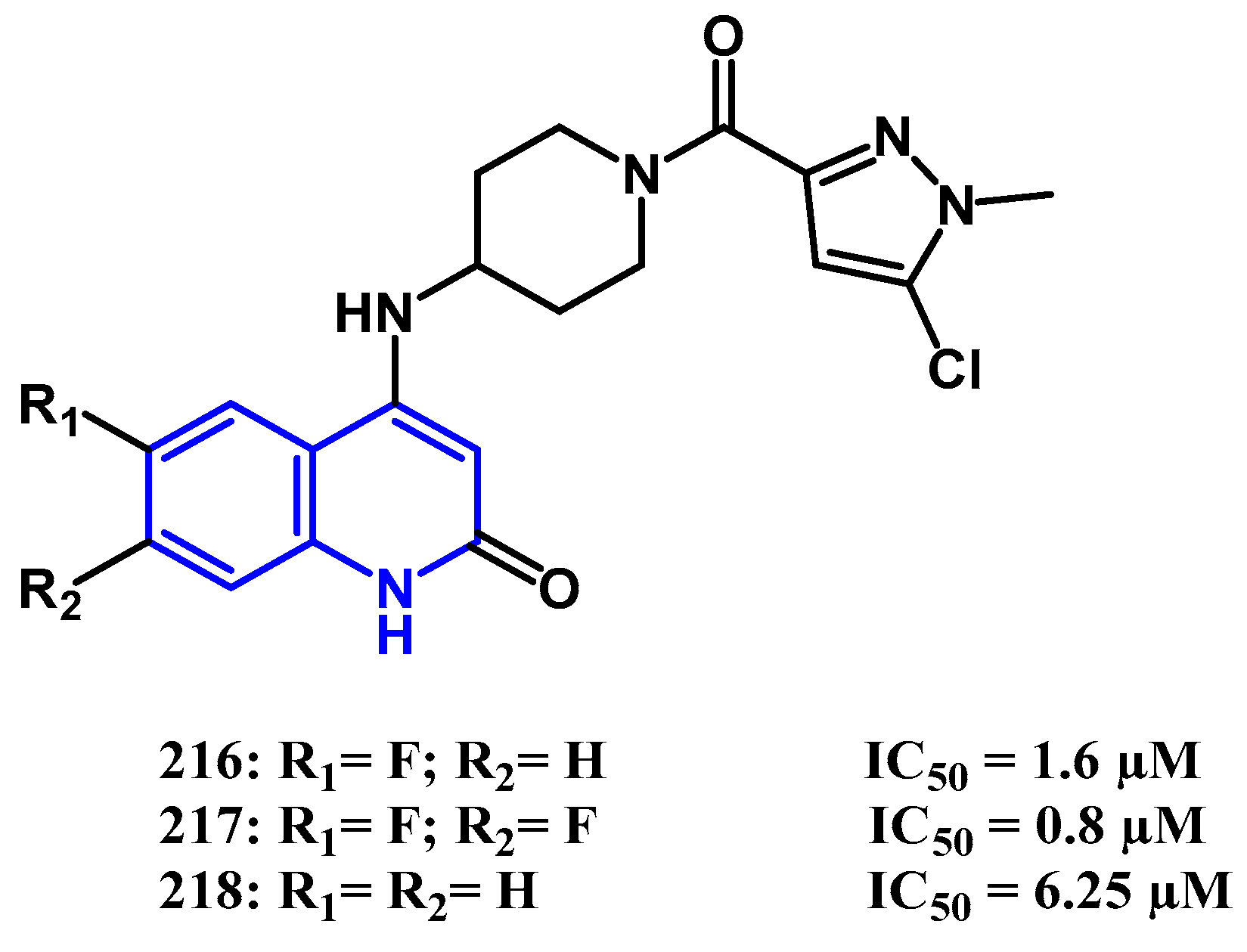
4.1. Antibacterial Activity
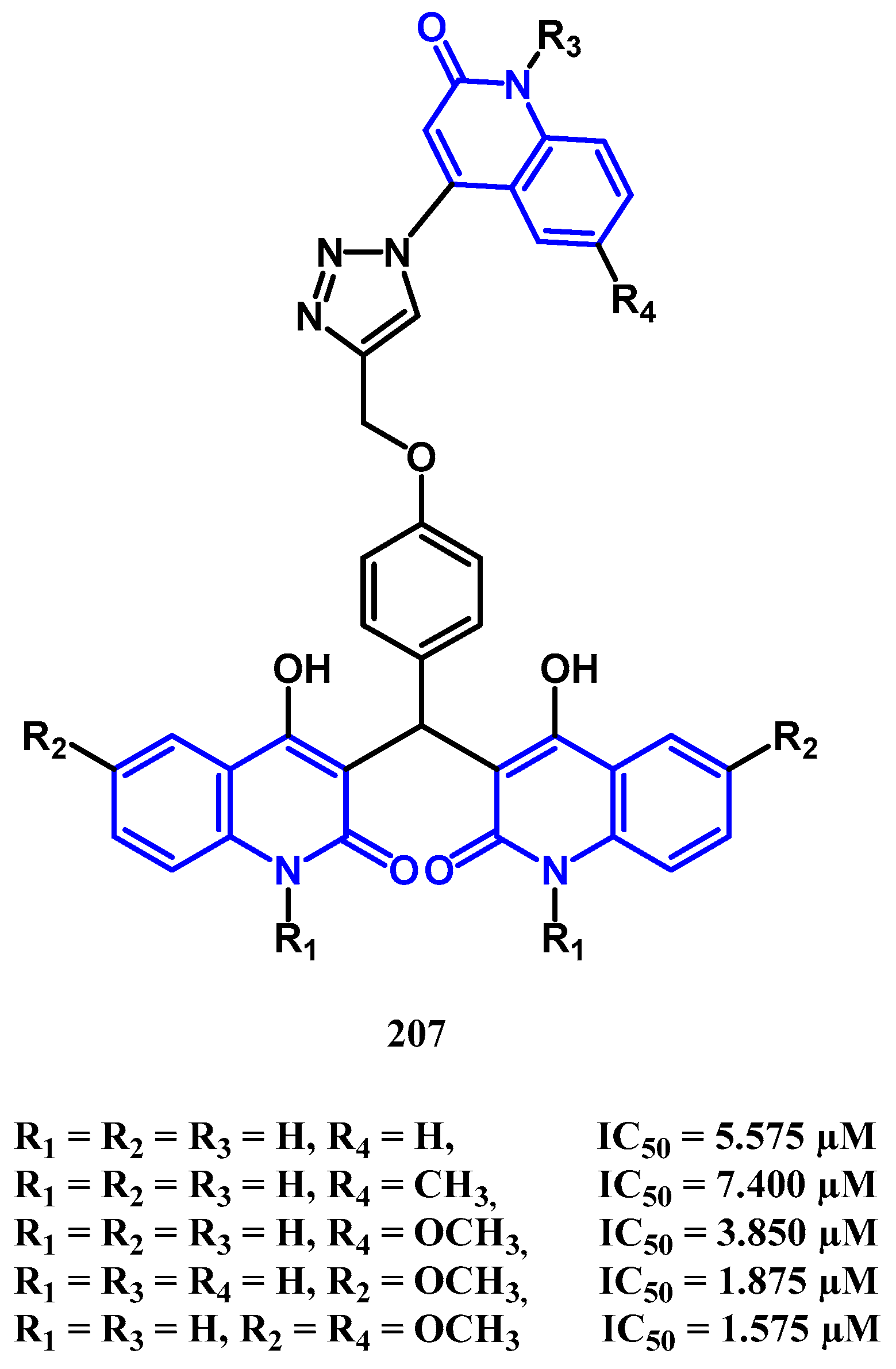
4.2. Antiproliferative Activity
4.3. Antiviral Activity
4.4. Antitrypanosomal and Antileishmaniasis Activity
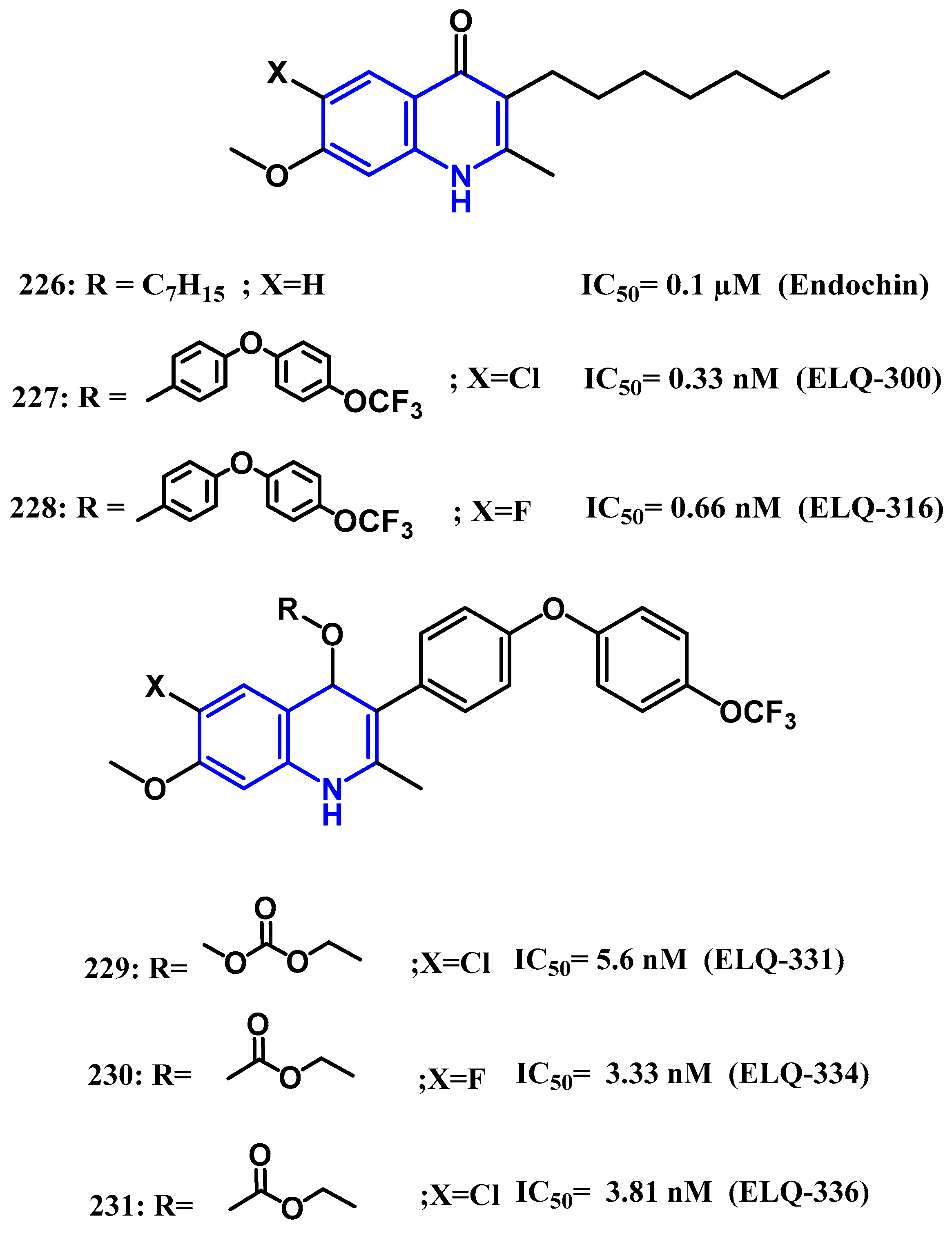
4.5. Anti-Malaria Activity
5. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barros, A.I.; Nunes, F.M.; Gonçalves, B.; Bennett, R.N.; Silva, A.P. Effect of cooking on total vitamin C contents and antioxidant activity of sweet chestnuts (Castanea sativa Mill.). Food Chem. 2011, 128, 165–172. [Google Scholar] [PubMed]
- Gothelf, K.V.; Jørgensen, K.A. Asymmetric 1, 3-dipolar cycloaddition reactions. Chem. Rev. 1998, 98, 863–910. [Google Scholar] [CrossRef]
- Ashley, E.A.; Dhorda, M.; Fairhurst, R.M.; Amaratunga, C.; Lim, P.; Suon, S.; Sreng, S.; Anderson, J.M.; Mao, S.; Sam, B.; et al. Spread of Artemisinin Resistance in Plasmodium falciparum Malaria. N. Engl. J. Med. 2014, 371, 411–423. [Google Scholar] [CrossRef] [PubMed]
- Xing, G.; Pan, L.; Yi, C.; Li, X.; Ge, X.; Zhao, Y.; Liu, Y.; Li, J.; Woo, A.; Lin, B.; et al. Design, synthesis and biological evaluation of 5-(2-amino-1-hydroxyethyl)-8-hydroxyquinolin-2(1H)-one derivatives as potent β2-adrenoceptor agonists. Bioorg. Med. Chem. 2019, 27, 2306–2314. [Google Scholar]
- Batista, V.F.; Pinto, D.C.G.A.; Silva, A.M.S. Synthesis of Quinolines: A Green Perspective. ACS Sustain. Chem. Eng. 2016, 4, 4064–4078. [Google Scholar] [CrossRef]
- Diaz, G.; Miranda, I.L.; Diaz, M.A.N. Quinolines, isoquinolines, angustureine, and congeneric alkaloids—Occurrence, chemistry, and biological activity. In Phytochemicals: Isolation, Characterisation and Role in Human Health; IntechOpen: Rijeka, Croatia, 2015; pp. 141–162. [Google Scholar]
- Pranger, A.D.; Van Der Werf, T.S.; Kosterink, J.G.W.; Alffenaar, J.W.C. The Role of Fluoroquinolones in the Treatment of Tuberculosis in 2019. Drugs 2019, 79, 161–171. [Google Scholar] [CrossRef]
- Fan, Y.-L.; Cheng, X.-W.; Wu, J.-B.; Liu, M.; Zhang, F.-Z.; Xu, Z.; Feng, L.-S. Antiplasmodial and antimalarial activities of quinolone derivatives: An overview. Eur. J. Med. Chem. 2018, 146, 1–14. [Google Scholar]
- Sood, D.; Kumar, N.; Singh, A.; Sakharkar, M.K.; Tomar, V.; Chandra, R. Antibacterial and pharmacological evaluation of fluoroquinolones: A chemoinformatics approach. Genom. Inform. 2018, 16, 44–51. [Google Scholar] [CrossRef]
- Chokkar, N.; Kalra, S.; Chauhan, M.; Kumar, R. A review on quinoline derived scaffolds as anti-HIV agents. Mini Rev. Med. Chem. 2019, 19, 510–526. [Google Scholar] [CrossRef]
- Kojima, H.; Kaita, K.D.E.; Hawkins, K.; Uhanova, J.; Minuk, G.Y. Use of Fluoroquinolones in Patients with Chronic Hepatitis C Virus-Induced Liver Failure. Antimicrob. Agents Chemother. 2002, 46, 3280–3282. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xia, Y.; Yang, Z.-Y.; Morris-Natschke, S.L.; Lee, K.-H. Recent advances in the discovery and development of quinolones and analogs as antitumor agents. Curr. Med. Chem. 1999, 6, 179–194. [Google Scholar] [CrossRef]
- Li, J.; Zheng, T.; Jin, Y.; Xu, J.; Yu, J.; Lv, Y. Synthesis, molecular docking and biological evaluation of quinolone derivatives as novel anticancer agents. Chem. Pharm. Bull. 2018, 66, 55–60. [Google Scholar] [CrossRef]
- Sharma, V.; Das, R.; Mehta, D.K.; Gupta, S.; Venugopala, K.N.; Mailavaram, R.; Nair, A.B.; Shakya, A.K.; Deb, P.K. Recent insight into the biological activities and SAR of quinolone derivatives as multifunctional scaffold. Bioorg. Med. Chem. 2022, 59, 116674. [Google Scholar] [CrossRef]
- Daneshtalab, M.; Ahmed, A. Nonclassical biological activities of quinolone derivatives. J. Pharm. Pharm. Sci. 2012, 15, 52–72. [Google Scholar] [CrossRef]
- Dhiman, P.; Arora, N.; Thanikachalam, P.V.; Monga, V. Recent advances in the synthetic and medicinal perspective of quinolones: A review. Bioorg. Chem. 2019, 92, 103291. [Google Scholar] [CrossRef]
- Sales, E.M.; Figueroa-Villar, J.D. Recent studies about synthesis and biological activity of quinolones and derivatives: A Review. World J. Pharm. Pharm. Sci. 2016, 5, 253–268. [Google Scholar]
- Lungu, I.-A.; Moldovan, O.-L.; Biriș, V.; Rusu, A. Fluoroquinolones hybrid molecules as promising antibacterial agents in the fight against antibacterial resistance. Pharmaceutics 2022, 14, 1749. [Google Scholar] [CrossRef]
- Dube, P.S.; Legoabe, L.J.; Beteck, R.M. Quinolone: A versatile therapeutic compound class. Mol. Divers. 2023, 27, 1501–1526. [Google Scholar] [CrossRef]
- Zaman, S.B.; Hussain, M.A.; Nye, R.; Mehta, V.; Mamun, K.T.; Hossain, N. A review on antibiotic resistance: Alarm bells are ringing. Cureus 2017, 9, e1403. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5573035/ (accessed on 24 January 2025).
- Terreni, M.; Taccani, M.; Pregnolato, M. New antibiotics for multidrug-resistant bacterial strains: Latest research developments and future perspectives. Molecules 2021, 26, 2671. [Google Scholar] [CrossRef]
- Uddin, T.M.; Chakraborty, A.J.; Khusro, A.; Zidan, B.R.M.; Mitra, S.; Emran, T.B.; Dhama, K.; Ripon, M.K.H.; Gajdács, M.; Sahibzada, M.U.K.; et al. Antibiotic resistance in microbes: History, mechanisms, therapeutic strategies and future prospects. J. Infect. Public Health 2021, 14, 1750–1766. [Google Scholar]
- Liu, X.; Deng, J.; Xu, Z.; Lv, Z.-S. Recent advances of 2-Quinolone-based derivatives as anti-tubercular agents. Anti-Infect. Agents 2018, 16, 4–10. [Google Scholar] [CrossRef]
- Gao, F.; Zhang, X.; Wang, T.; Xiao, J. Quinolone hybrids and their anti-cancer activities: An overview. Eur. J. Med. Chem. 2019, 165, 59–79. [Google Scholar]
- Naeem, A.; Badshah, S.L.; Muska, M.; Ahmad, N.; Khan, K. The current case of quinolones: Synthetic approaches and antibacterial activity. Molecules. 2016, 21, 268. [Google Scholar] [CrossRef]
- Ezelarab, H.A.A.; Abbas, S.H.; Hassan, H.A.; Abuo-Rahma, G.E.A. Recent updates of fluoroquinolones as antibacterial agents. Arch. Pharm. 2018, 351, e1800141. [Google Scholar] [CrossRef]
- Fan, Y.-L.; Wu, J.-B.; Cheng, X.-W.; Zhang, F.-Z.; Feng, L.-S. Fluoroquinolone derivatives and their anti-tubercular activities. Eur. J. Med. Chem. 2018, 146, 554–563. [Google Scholar]
- Conrad, M.; Limpach, L. Ueber das γ-Oxychinaldin und dessen Derivate. Berichte Dtsch. Chem. Ges. 1887, 20, 948–959. [Google Scholar]
- Weyesa, A.; Mulugeta, E. Recent advances in the synthesis of biologically and pharmaceutically active quinoline and its analogues: A review. RSC Adv. 2020, 10, 20784–20793. [Google Scholar]
- Zhou, W.; Zhang, L.; Jiao, N. The tandem reaction combining radical and ionic processes: An efficient approach to substituted 3,4-dihydroquinolin-2-ones. Tetrahedron 2009, 65, 1982–1987. [Google Scholar]
- Kostopoulou, I.; Tzani, A.; Chronaki, K.; Prousis, K.C.; Pontiki, E.; Hadjiplavlou-Litina, D.; Detsi, A. Novel Multi-Target Agents Based on the Privileged Structure of 4-Hydroxy-2-quinolinone. Molecules 2023, 29, 190. [Google Scholar] [CrossRef]
- Li, J.J. Name Reactions: A Collection of Detailed Mechanisms and Synthetic Applications; Springer: Berlin/Heidelberg, Germany, 2009; ISBN 978-3-642-01052-1. [Google Scholar]
- Wang, H. Comprehensive Organic Name Reactions; Wiley: New York, NY, USA, 2010; Volume 2. [Google Scholar]
- Jampilek, J.; Musiol, R.; Pesko, M.; Kralova, K.; Vejsova, M.; Carroll, J.; Coffey, A.; Finster, J.; Tabak, D.; Niedbala, H.; et al. Ring-substituted 4-hydroxy-1H-quinolin-2-ones: Preparation and biological activity. Molecules 2009, 14, 1145–1159. [Google Scholar] [CrossRef] [PubMed]
- Aly, A.A.; Ramadan, M.; Abuo-Rahma, G.E.-D.A.; Elshaier, Y.A.; Elbastawesy, M.A.; Brown, A.B.; Braese, S. Quinolones as prospective drugs: Their syntheses and biological applications. In Advances in Heterocyclic Chemistry; Elsevier: Amsterdam, The Netherlands, 2021; Volume 135, pp. 147–196. [Google Scholar]
- Abdou, M.M. Chemistry of 4-Hydroxy-2(1H)-quinolone. Part 1: Synthesis and reactions. Arab. J. Chem. 2017, 10, S3324–S3337. [Google Scholar]
- Ahmed, N.; Brahmbhatt, K.G.; Singh, I.P.; Bhutani, K.K. Efficient chemoselective alkylation of quinoline 2,4-diol derivatives in water. J. Heterocycl. Chem. 2011, 48, 237–240. [Google Scholar] [CrossRef]
- Ahmed, N.; Brahmbhatt, K.G.; Sabde, S.; Mitra, D.; Singh, I.P.; Bhutani, K.K. Synthesis and anti-HIV activity of alkylated quinoline 2,4-diols. Bioorg. Med. Chem. 2010, 18, 2872–2879. [Google Scholar] [PubMed]
- Arya, K.; Agarwal, M. Microwave prompted multigram synthesis, structural determination, and photo-antiproliferative activity of fluorinated 4-hydroxyquinolinones. Bioorg. Med. Chem. Lett. 2007, 17, 86–93. [Google Scholar]
- Cheng, P.; Gu, Q.; Liu, W.; Zou, J.-F.; Ou, Y.-Y.; Luo, Z.-Y.; Zeng, J.-G. Synthesis of quinolin-2-one alkaloid derivatives and their inhibitory activities against HIV-1 reverse transcriptase. Molecules 2011, 16, 7649–7661. [Google Scholar] [CrossRef]
- El Ashry, E.S.H.; Ramadan, E.S.; Abdel Hamid, H.; Hagar, M. Microwave-Assisted Synthesis of Quinoline Derivatives from Isatin. Synth. Commun. 2005, 35, 2243–2250. [Google Scholar] [CrossRef]
- Baba, Y.F.; Gökce, H.; Rodi, Y.K.; Hayani, S.; Chahdi, F.O.; Boukir, A.; Jasinski, J.P.; Kaur, M.; Hökelek, T.; Sebbar, N.K.; et al. Syntheses of novel 2-oxo-1,2-dihydroquinoline derivatives: Molecular and crystal structures, spectroscopic characterizations, Hirshfeld surface analyses, molecular docking studies and density functional theory calculations. J. Mol. Struct. 2020, 1217, 128461. [Google Scholar]
- Baba, Y.F.; Misbahi, K.; Chahdi, F.O.; Kerbal, A. Synthese et Reactivite de Nouveaux Systemes Heterocyliques Derives de la Quinoleine. Moroc. J. Heterocycl. Chem. 2014, 13. [Google Scholar]
- Moussaoui, O.; Bhadane, R.; Sghyar, R.; Ilaš, J.; El Hadrami, E.M.; Chakroune, S.; Salo-Ahen, O.M.H. Design, Synthesis, in vitro and in silico Characterization of 2-Quinolone-L-alaninate-1,2,3-triazoles as Antimicrobial Agents. ChemMedChem 2022, 17, e202100714. [Google Scholar] [CrossRef]
- Pashaei, H.; Rouhani, A.; Nejabat, M.; Hadizadeh, F.; Mirzaei, S.; Nadri, H.; Maleki, M.F.; Ghodsi, R. Synthesis and molecular dynamic simulation studies of novel N-(1-benzylpiperidin-4-yl) quinoline-4-carboxamides as potential acetylcholinesterase inhibitors. J. Mol. Struct. 2021, 1244, 130919. [Google Scholar] [CrossRef]
- Slania, S.L.; Das, D.; Lisok, A.; Du, Y.; Jiang, Z.; Mease, R.C.; Rowe, S.P.; Nimmagadda, S.; Yang, X.; Pomper, M.G. Imaging of Fibroblast Activation Protein in Cancer Xenografts Using Novel (4-Quinolinoyl)-glycyl-2-cyanopyrrolidine-Based Small Molecules. J. Med. Chem. 2021, 64, 4059–4070. [Google Scholar] [CrossRef] [PubMed]
- Baragaña, B.; Norcross, N.R.; Wilson, C.; Porzelle, A.; Hallyburton, I.; Grimaldi, R.; Osuna-Cabello, M.; Norval, S.; Riley, J.; Stojanovski, L.; et al. Discovery of a Quinoline-4-carboxamide Derivative with a Novel Mechanism of Action, Multistage Antimalarial Activity, and Potent in Vivo Efficacy. J. Med. Chem. 2016, 59, 9672–9685. [Google Scholar] [CrossRef] [PubMed]
- Jansen, K.; Heirbaut, L.; Cheng, J.D.; Joossens, J.; Ryabtsova, O.; Cos, P.; Maes, L.; Lambeir, A.-M.; De Meester, I.; Augustyns, K.; et al. Selective Inhibitors of Fibroblast Activation Protein (FAP) with a (4-Quinolinoyl)-glycyl-2-cyanopyrrolidine Scaffold. ACS Med. Chem. Lett. 2013, 4, 491–496. [Google Scholar] [CrossRef]
- Ma, Y.; Zhu, Y.; Zhang, D.; Meng, Y.; Tang, T.; Wang, K.; Ma, J.; Wang, J.; Sun, P. Eco-friendly decarboxylative cyclization in water: Practical access to the anti-malarial 4-quinolones. Green Chem. 2019, 21, 478–482. [Google Scholar]
- Bernini, R.; Cacchi, S.; Fabrizi, G.; Sferrazza, A. 4-Aryl-2-quinolones via a domino Heck reaction/cyclization process. Heterocycles 2006, 69, 99. [Google Scholar] [CrossRef]
- Cortese, N.A.; Ziegler, C.B.; Hrnjez, B.J.; Heck, R.F. Palladium-catalyzed synthesis of 2-quinolone derivatives from 2-iodoanilines. J. Org. Chem. 1978, 43, 2952–2958. [Google Scholar] [CrossRef]
- Cho, C.S.; Kim, J.U. An approach for quinolines via palladium-catalyzed Heck coupling followed by cyclization. Tetrahedron Lett. 2007, 48, 3775–3778. [Google Scholar] [CrossRef]
- Åkerbladh, L.; Nordeman, P.; Wejdemar, M.; Odell, L.R.; Larhed, M. Synthesis of 4-Quinolones via a Carbonylative Sonogashira Cross-Coupling Using Molybdenum Hexacarbonyl as a CO Source. J. Org. Chem. 2015, 80, 1464–1471. [Google Scholar] [CrossRef]
- Bunce, R.A.; Nammalwar, B. 4(1H)-Quinolinones by a Tandem Reduction-Addition-Elimination Reaction. Org. Prep. Proced. Int. 2010, 42, 557–563. [Google Scholar] [CrossRef]
- Flipo, M.; Beghyn, T.; Leroux, V.; Florent, I.; Deprez, B.P.; Deprez-Poulain, R.F. Novel Selective Inhibitors of the Zinc Plasmodial Aminopeptidase PfA-M1 as Potential Antimalarial Agents. J. Med. Chem. 2007, 50, 1322–1334. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.M.; Kamel, M.M.; Mohamed, L.W.; Faggal, S.I. Synthesis of new indole derivatives structurally related to donepezil and their biological evaluation as acetylcholinesterase inhibitors. Molecules 2012, 17, 4811–4823. [Google Scholar] [CrossRef]
- Kikuchi, S.; Yamada, T. Carbon Dioxide Incorporation into Alkyne Compounds Mediated by Silver Catalysts. Chem. Rec. 2014, 14, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Harayama, T. A Concise and Versatile Synthesis of Viridicatin Alkaloids from Cyanoacetanilides. Org. Lett. 2009, 11, 1603–1606. [Google Scholar] [CrossRef]
- Ribeiro, N.; Tabaka, H.; Peluso, J.; Fetzer, L.; Nebigil, C.; Dumont, S.; Muller, C.D.; Désaubry, L. Synthesis of 3-O-methylviridicatin analogues with improved anti-TNF-α properties. Bioorg. Med. Chem. Lett. 2007, 17, 5523–5524. [Google Scholar]
- Liu, L.; Lu, H.; Wang, H.; Yang, C.; Zhang, X.; Zhang-Negrerie, D.; Du, Y.; Zhao, K. PhI(OCOCF3)2-Mediated C–C Bond Formation Concomitant with a 1,2-Aryl Shift in a Metal-Free Synthesis of 3-Arylquinolin-2-ones. Org. Lett. 2013, 15, 2906–2909. [Google Scholar] [CrossRef]
- Schlummer, B.; Scholz, U. Palladium-Catalyzed C—N and C—O Coupling–A Practical Guide from an Industrial Vantage Point†. Adv. Synth. Catal. 2004, 346, 1599–1626. [Google Scholar] [CrossRef]
- Dubrovin, A.N.; Mikhalev, A.I.; Ukhov, S.V.; Goldshtein, A.G.; Novikova, V.V.; Odegova, T.F.; Makhmudov, R.R. Synthesis, Properties, and Biological Activities of 2-Methyl- and 2-Styrylquinoline-4-Carboxylic Acids. Pharm. Chem. J. 2015, 49, 309–312. [Google Scholar] [CrossRef]
- Kumar, A.; Fernandes, J.; Kumar, P. Synthesis, antimicrobial and antitubercular activities of some novel carboxamide derivatives of 2-quinolones. Orient. J. Chem. 2014, 30, 1993–1997. [Google Scholar]
- Shivaraj, Y.; Naveen, M.H.; Vijayakumar, G.R.; Kumar, D.B.A. Design, synthesis and antibacterial activity studies of novel quinoline carboxamide derivatives. J. Korean Chem. Soc. 2013, 57, 241–245. [Google Scholar]
- Suthar, S.K.; Jaiswal, V.; Lohan, S.; Bansal, S.; Chaudhary, A.; Tiwari, A.; Alex, A.T.; Joesph, A. Novel quinolone substituted thiazolidin-4-ones as anti-inflammatory, anticancer agents: Design, synthesis and biological screening. Eur. J. Med. Chem. 2013, 63, 589–602. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.A.; Rizk, H.F.; El-Borai, M.A.; Sadek, M.E. Green routes for the synthesis of new pyrazole bearing biologically active imidiazolyl, pyridine and quinoxaline derivatives as promising antimicrobial and antioxidant agents. J. Iran. Chem. Soc. 2021, 18, 1391–1404. [Google Scholar] [CrossRef]
- Ibrahim, S.R.; Mohamed, G.A.; Al Haidari, R.A.; El-Kholy, A.A.; Zayed, M.F.; Khayat, M.T. Biologically active fungal depsidones: Chemistry, biosynthesis, structural characterization, and bioactivities. Fitoterapia 2018, 129, 317–365. [Google Scholar]
- Donaire-Arias, A.; Montagut, A.M.; Puig de la Bellacasa, R.; Estrada-Tejedor, R.; Teixidó, J.; Borrell, J.I. 1H-Pyrazolo [3,4-b] pyridines: Synthesis and biomedical applications. Molecules 2022, 27, 2237. [Google Scholar] [CrossRef]
- Polo-Cuadrado, E.; Rojas-Peña, C.; Acosta-Quiroga, K.; Camargo-Ayala, L.; Brito, I.; Cisterna, J.; Moncada, F.; Trilleras, J.; Rodríguez-Núñez, Y.A.; Gutierrez, M. Design, synthesis, theoretical study, antioxidant, and anticholinesterase activities of new pyrazolo-fused phenanthrolines. RSC Adv. 2022, 12, 33032–33048. [Google Scholar]
- Noser, A.A.; Baren, M.H.; Ibrahim, S.A.; Rekaby, M.; Salem, M.M. New Pyrazolothiazole as Potential Wnt/β-Catenin Inhibitors: Green Synthesis, Characterization, Antimicrobial, Antioxidant, Antineoplastic Evaluation, and Molecular Docking Study. ChemistrySelect 2023, 8, e202204670. [Google Scholar] [CrossRef]
- Aljaar, N.; Ibrahim, M.M.; Younes, E.A.; Al-Noaimi, M.; Abu-Safieh, K.A.; Ali, B.F.; Kant, K.; Al-Zaqri, N.; Sengupta, R.; Malakar, C.C. Strategies towards the Synthesis of 2-Ketoaryl Azole Derivatives using C-H Functionalization Approach and 1,2-Bis-Nucleophile Precursors. Asian J. Org. Chem. 2023, 12, e202300036. [Google Scholar] [CrossRef]
- Okada, K.; Sakuma, H.; Kondo, M.; Inoue, S. Diels–Alder Reaction of 3-Methoxycarbonylmethylene-2-Oxoindoline Derivatives with Unsymmetrical Butadienes. Chem. Lett. 1979, 8, 213–216. [Google Scholar] [CrossRef]
- Abdel-Rahman, A.A.-H.; Abdel-Megied, A.E.-S.; Goda, A.E.-S.; Zeid, I.F.; El Ashry, E.S.H. Synthesis and Anti-HBV Activity of Thiouracils Linked via S and N-1 to the 5-Position of Methyl β-D-Ribofuranoside. Nucleosides Nucleotides Nucleic Acids 2003, 22, 2027–2038. [Google Scholar] [CrossRef]
- Kuş, N.S. The synthesis of ribose and nucleoside derivatives. Madridge J. Nov. Drug Res. 2018, 2, 37–56. [Google Scholar]
- Khwaza, V.; Mlala, S.; Aderibigbe, B.A. Advancements in Synthetic Strategies and Biological Effects of Ciprofloxacin Derivatives: A Review. Int. J. Mol. Sci. 2024, 25, 4919. [Google Scholar] [CrossRef] [PubMed]
- Alasadi, G.M.; Al-Obaidi, Z. Synthesis of novel acylated and esterified ciprofloxacin derivatives as efficient anticancer and antimicrobial agents. Front. Mater. 2023, 10, 1255955. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, N.; Wang, J.; Guo, Y.; Sun, B.; Liu, W.; Zhou, H.; Yang, C. Synthesis and Biological Evaluation of Quinolinone Compounds as SARS CoV 3CL pro Inhibitors. Chin. J. Chem. 2013, 31, 1199–1206. [Google Scholar] [CrossRef]
- Priya, N.; Gupta, A.; Chand, K.; Singh, P.; Kathuria, A.; Raj, H.G.; Parmar, V.S.; Sharma, S.K. Characterization of 4-methyl-2-oxo-1,2-dihydroquinolin-6-yl acetate as an effective antiplatelet agent. Bioorg. Med. Chem. 2010, 18, 4085–4094. [Google Scholar] [CrossRef]
- El-Mrabet, A.; Haoudi, A.; Dalbouha, S.; Skalli, M.K.; Hökelek, T.; Capet, F.; Kandri Rodi, Y.; Mazzah, A.; Sebbar, N.K. Crystal structure, Hirshfeld surface analysis, interaction energy and energy framework calculations, as well as density functional theory (DFT) computation, of methyl 2-oxo-1-(prop-2-ynyl)-1,2-dihydroquinoline-4-carboxylate. Acta Crystallogr. Sect. E Crystallogr. Commun. 2023, 79, 883–889. [Google Scholar] [CrossRef]
- Deng, X.-Q.; Wei, C.-X.; Song, M.-X.; Chai, K.-Y.; Sun, Z.-G.; Quan, Z.-S. Synthesis and studies on anticonvulsant and antidepressant activities of 5-alkoxy-tetrazolo [1,5-a] quinolines. Bull. Korean Chem. Soc. 2010, 31, 447–452. [Google Scholar] [CrossRef]
- Morel, A.F.; Larghi, E.L.; Selvero, M.M. Mild, Efficient and Selective Silver Carbonate Mediated O-Alkylation of 4-Hydroxy-2-quinolones: Synthesis of 2,4-Dialkoxyquinolines. Synlett 2005, 2005, 2755–2758. [Google Scholar] [CrossRef]
- Chen, C.; Chen, I.-L.; Chen, J.; Wei, D.; Hsieh, H.; Chang, K.-M.; Tzeng, C.-C.; Wang, T.-C. Studies on the alkylation of quinolin-2(1H)-one derivatives. J. Chil. Chem. Soc. 2015, 60, 2812–2816. [Google Scholar] [CrossRef]
- Grzelakowska, A.; Kolińska, J.; Mąkiewicz, M. The synthesis and spectroscopic characterisation of 3-formyl-2-quinolones in the presence of biothiols. Color. Technol. 2018, 134, 440–449. [Google Scholar] [CrossRef]
- Kolińska, J.; Grzelakowska, A.; Sokołowska, J. Novel 7-maleimido-2(1H)-quinolones as potential fluorescent sensors for the detection of sulphydryl groups. Color. Technol. 2018, 134, 148–155. [Google Scholar] [CrossRef]
- Selvero, M.M.; Ledesma, G.N.; Abram, U.; Schulz-Lang, E.; Morel, A.F.; Larghi, E.L. 2,2,2-trifluoroethanol-promoted access to symmetrically 3,3-disubstituted quinoline-2,4-diones. J. Fluor. Chem. 2020, 234, 109520. [Google Scholar] [CrossRef]
- Refouvelet, B.; Guyon, C.; Jacquot, Y.; Girard, C.; Fein, H.; Bévalot, F.; Robert, J.-F.; Heyd, B.; Mantion, G.; Richert, L.; et al. Synthesis of 4-hydroxycoumarin and 2,4-quinolinediol derivatives and evaluation of their effects on the viability of HepG2 cells and human hepatocytes culture. Eur. J. Med. Chem. 2004, 39, 931–937. [Google Scholar] [CrossRef] [PubMed]
- Hao, F.; Asahara, H.; Nishiwaki, N. A direct and vicinal functionalization of the 1-methyl-2-quinolone framework: 4-alkoxylation and 3-chlorination. Org. Biomol. Chem. 2016, 14, 5128–5135. [Google Scholar]
- Bergman, J.; Pettersson, B.; Hasimbegovic, V.; Svensson, P.H. Thionations Using a P4S10−Pyridine Complex in Solvents Such as Acetonitrile and Dimethyl Sulfone. J. Org. Chem. 2011, 76, 1546–1553. [Google Scholar] [CrossRef]
- de Macedo, M.B.; Kimmel, R.; Urankar, D.; Gazvoda, M.; Peixoto, A.; Cools, F.; Torfs, E.; Verschaeve, L.; Lima, E.S.; Lyčka, A.; et al. Design, synthesis and antitubercular potency of 4-hydroxyquinolin-2(1H)-ones. Eur. J. Med. Chem. 2017, 138, 491–500. [Google Scholar]
- Ukrainets, I.V.; Sidorenko, L.V.; Gorokhova, O.V.; Slobodzyan, S.V. 4-Hydroxy-2-quinolones. 97. Simple synthesis of the esters of 4-halo-substituted 2-oxo-1,2-dihydroquinoline-3-carboxylic acids. Chem. Heterocycl. Compd. 2006, 42, 882–885. [Google Scholar] [CrossRef]
- Ukrainets, I.V.; Sidorenko, L.V.; Slobodzyan, S.V.; Rybakov, V.B.; Chernyshev, V.V. 4-Hydroxyquinol-2-ones. 87. Unusual Synthesis of 1-R-4-Hydroxy-2-oxo-1,2-dihydroquinoline-3-carboxylic Acid Pyridylamides. Chem. Heterocycl. Compd. 2005, 41, 1158–1166. [Google Scholar] [CrossRef]
- Täubl, A.E.; Langhans, K.; Kappe, T.; Stadlbauer, W. Thermolytic ring closure reactions of 4-azido-3-phenylsulfanyl- and 4-azido-3-phenylsulfonyl-2-quinolones to 12H-quinolino-[3,4-b][1,4]benzothiazin-6(5H)-ones. J. Heterocycl. Chem. 2002, 39, 1259–1264. [Google Scholar] [CrossRef]
- Aizikovich, A.; Kuznetsov, V.; Gorohovsky, S.; Levy, A.; Meir, S.; Byk, G.; Gellerman, G. A new application of diphenylphosphorylazide (DPPA) reagent: Convenient transformations of quinolin-4-one, pyridin-4-one and quinazolin-4-one derivatives into the 4-azido and 4-amino counterparts. Tetrahedron Lett. 2004, 45, 4241–4243. [Google Scholar]
- Alshammari, M.B.; Aly, A.A.; Brown, A.B.; Bakht, M.A.; Shawky, A.M.; Abdelhakem, A.M.; El-Sheref, E.M. An efficient click synthesis of chalcones derivatized with two 1-(2-quinolon-4-yl)-1,2,3-triazoles. Z. Naturforsch. B 2021, 76, 395–403. [Google Scholar] [CrossRef]
- Chung, P.-Y.; Bian, Z.-X.; Pun, H.-Y.; Chan, D.; Chan, A.S.-C.; Chui, C.-H.; Tang, J.C.-O.; Lam, K.-H. Recent Advances in Research of Natural and Synthetic Bioactive Quinolines. Future Med. Chem. 2015, 7, 947–967. [Google Scholar] [CrossRef] [PubMed]
- Faraz, K.M.; Garima, V.; Wasim, A.; Akranth, M.; Mumtaz, A.M.; Mymoona, A.; Asif, H.; Misbahul, H.S.; Mohammad, S.; Rashiduddin, H.S. Synthetic trends followed for the development of 1,2,3-triazole derivatives. Int. J. Drug Dev. 2017, 9, 22–25. [Google Scholar]
- Agalave, S.G.; Maujan, S.R.; Pore, V.S. Click Chemistry: 1,2,3-Triazoles as Pharmacophores. Chem. Asian J. 2011, 6, 2696–2718. [Google Scholar] [CrossRef]
- Chen, Y.-L.; Fang, K.-C.; Sheu, J.-Y.; Hsu, S.-L.; Tzeng, C.-C. Synthesis and Antibacterial Evaluation of Certain Quinolone Derivatives. J. Med. Chem. 2001, 44, 2374–2377. [Google Scholar] [CrossRef]
- Abass, M.; Hassanin, H.M.; Allimony, H.A.; Hassan, H. Substituted quinolinones 27.* Regioselective synthesis of pyrazolo-, oxazolo-, and triazepinoquinoline derivatives. Chem. Heterocycl. Compd. 2015, 51, 1023–1029. [Google Scholar] [CrossRef]
- Abass, M.; Mostafa, B.B. Synthesis and evaluation of molluscicidal and larvicidal activities of some novel enaminones derived from 4-hydroxyquinolinones: Part IX. Bioorg. Med. Chem. 2005, 13, 6133–6144. [Google Scholar] [CrossRef]
- Ganesh, I. Conversion of carbon dioxide into several potential chemical commodities following different pathways—A review. In Materials Science Forum; Trans Tech Publications Ltd.: Bäch, Switzerland, 2013; Volume 764, pp. 1–82. [Google Scholar]
- Rostovtsev, V.V.; Green, L.G.; Fokin, V.V.; Sharpless, K.B. A Stepwise Huisgen Cycloaddition Process: Copper(I)-Catalyzed Regioselective “Ligation” of Azides and Terminal Alkynes. Angew. Chem. 2002, 114, 2708–2711. [Google Scholar] [CrossRef]
- Tornøe, C.W.; Christensen, C.; Meldal, M. Peptidotriazoles on Solid Phase: [1,2,3]-Triazoles by Regiospecific Copper(I)-Catalyzed 1,3-Dipolar Cycloadditions of Terminal Alkynes to Azides. J. Org. Chem. 2002, 67, 3057–3064. [Google Scholar] [CrossRef]
- Goddard-Borger, E.D.; Stick, R.V. An Efficient, Inexpensive, and Shelf-Stable Diazotransfer Reagent: Imidazole-1-sulfonyl Azide Hydrochloride. Org. Lett. 2007, 9, 3797–3800. [Google Scholar] [CrossRef]
- Bock, V.D.; Hiemstra, H.; Van Maarseveen, J.H. CuI-Catalyzed Alkyne–Azide “Click” Cycloadditions from a Mechanistic and Synthetic Perspective. Eur. J. Org. Chem. 2006, 2006, 51–68. [Google Scholar] [CrossRef]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Gumus, A.; Gumus, S. Synthesis of Triazole-Coupled Quinoline-Based Fluorescent Sensor. Eurasia Proc. Sci. Technol. Eng. Math. 2019, 7, 58–62. [Google Scholar]
- El-Sheref, E.M.; Aly, A.A.; Ameen, M.A.; Brown, A.B. Synthesis of new 4-(1,2,3-triazolo)quinolin-2(1H)-ones via Cu-catalyzed [3 + 2] cycloaddition. Monatshefte Chem. Chem. Mon. 2019, 150, 747–756. [Google Scholar] [CrossRef]
- El-Sheref, E.M.; Ameen, M.A.; El-Shaieb, K.M.; Abdel-Latif, F.F.; Abdel-Naser, A.I.; Brown, A.B.; Bräse, S.; Fathy, H.M.; Ahmad, I.; Patel, H.; et al. Design, synthesis and biological evaluation of syn and anti-like double warhead quinolinones bearing dihydroxy naphthalene moiety as epidermal growth factor receptor inhibitors with potential apoptotic antiproliferative action. Molecules 2022, 27, 8765. [Google Scholar] [CrossRef]
- El-Sheref, E.M.; Elbastawesy, M.A.; Brown, A.B.; Shawky, A.M.; Gomaa, H.A.; Bräse, S.; Youssif, B.G. Design and synthesis of (2-oxo-1,2-dihydroquinolin-4-yl)-1,2,3-triazole derivatives via click reaction: Potential apoptotic antiproliferative agents. Molecules 2021, 26, 6798. [Google Scholar] [CrossRef]
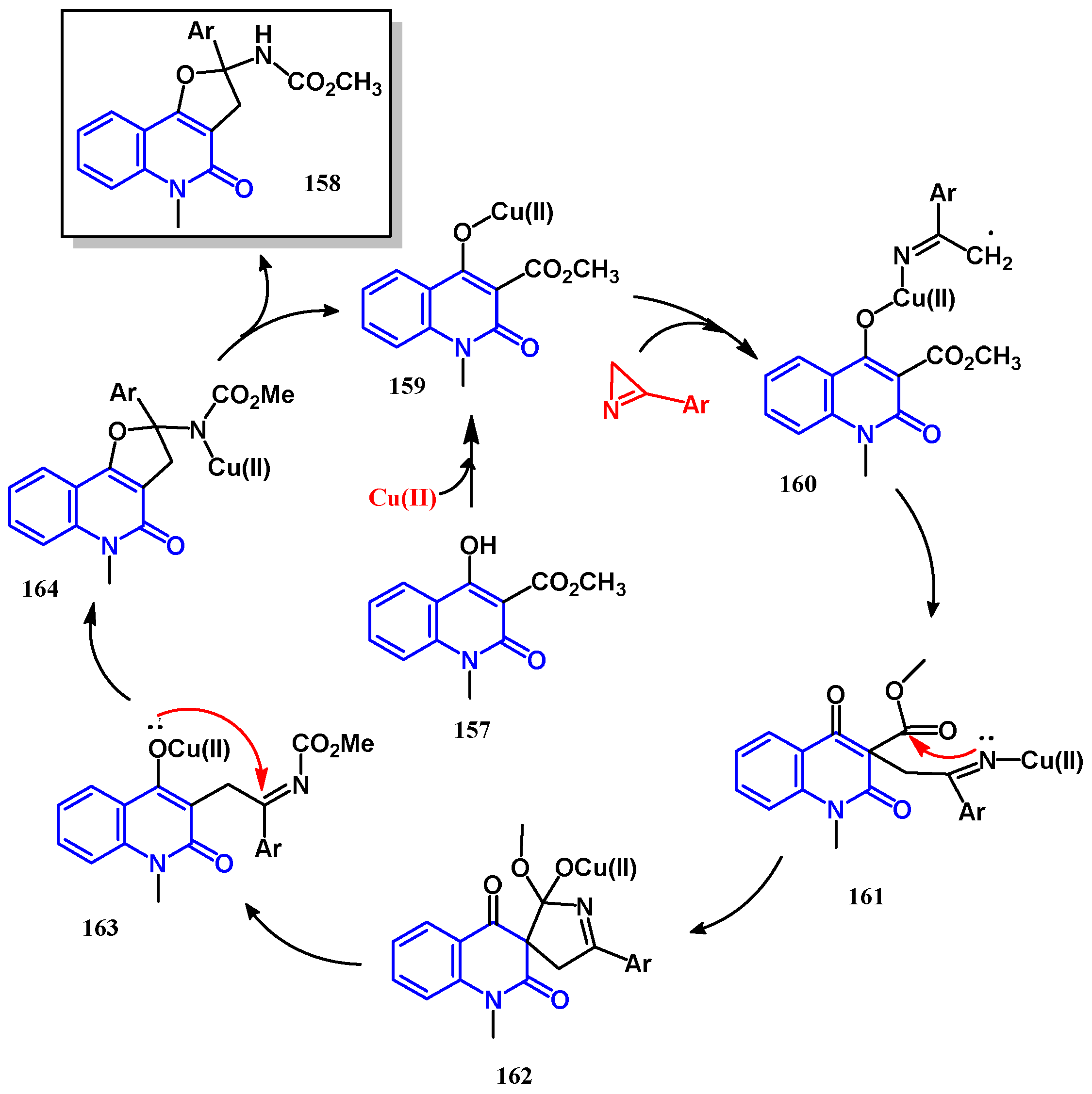
- Sakharov, P.A.; Rostovskii, N.V.; Khlebnikov, A.F.; Panikorovskii, T.L.; Novikov, M.S. 2H-Azirines as C–C Annulation Reagents in Cu-Catalyzed Synthesis of Furo[3,2-c]quinolone Derivatives. Org. Lett. 2019, 21, 3615–3619. [Google Scholar] [CrossRef]
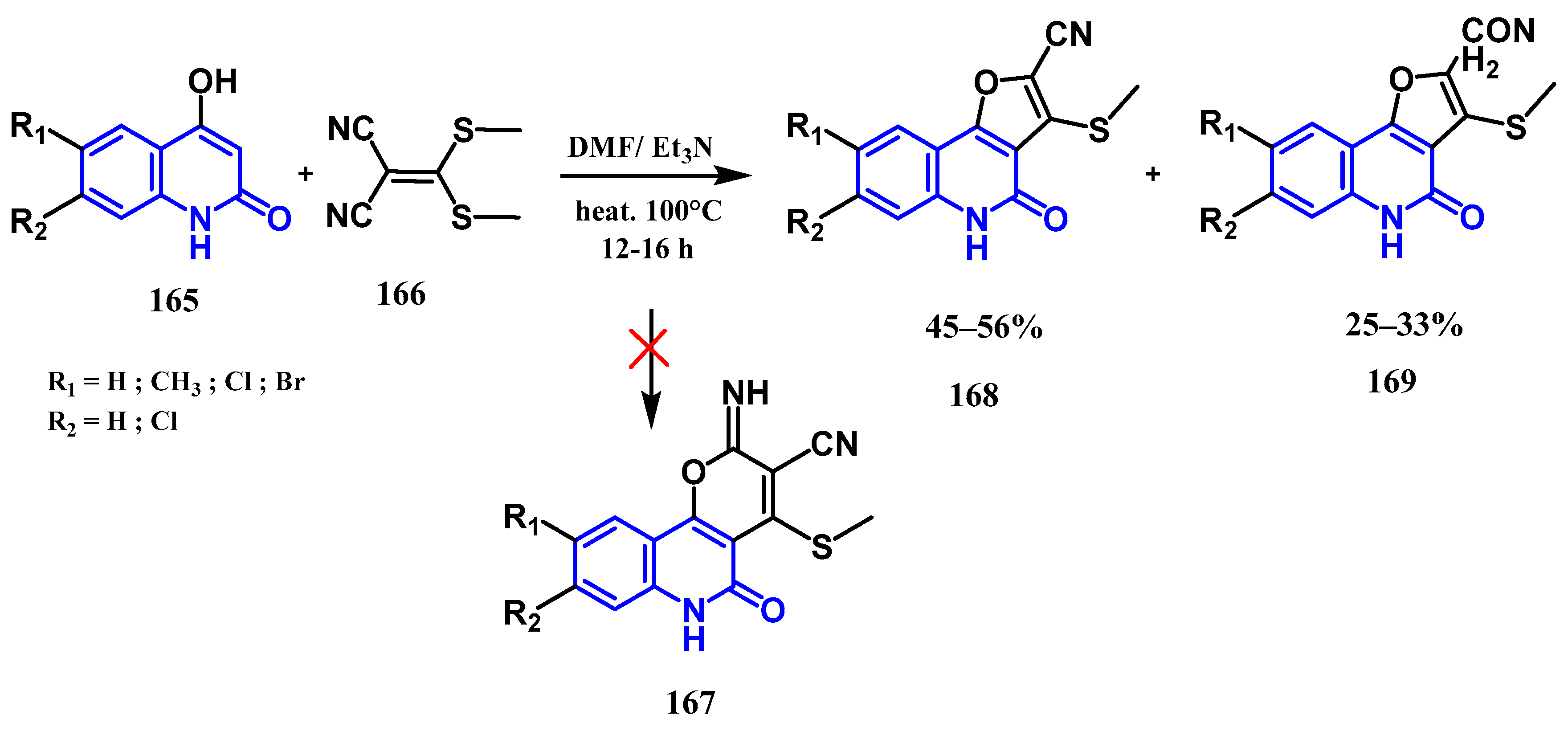
- Aly, A.A.; Ishak, E.A.; Shwaky, A.M.; Mohamed, A.H. Formation of furo[3,2-c]quinolone-2-carbonitriles and 4-oxo-4,5-dihydrofuro[3,2-c]quinolone-2-carboxamides from reaction of quinoline-2,4-diones with 2-[bis(methylthio)methylene]malononitrile. Monatshefte Chem. Chem. Mon. 2020, 151, 223–229. [Google Scholar] [CrossRef]
- Michael, J.P. Quinoline, quinazoline and acridone alkaloids. Nat. Prod. Rep. 2002, 19, 742–760. [Google Scholar] [CrossRef]
- Michael, J.P. Quinoline, quinazoline and acridone alkaloids. Nat. Prod. Rep. 2007, 24, 223–246. [Google Scholar] [CrossRef]
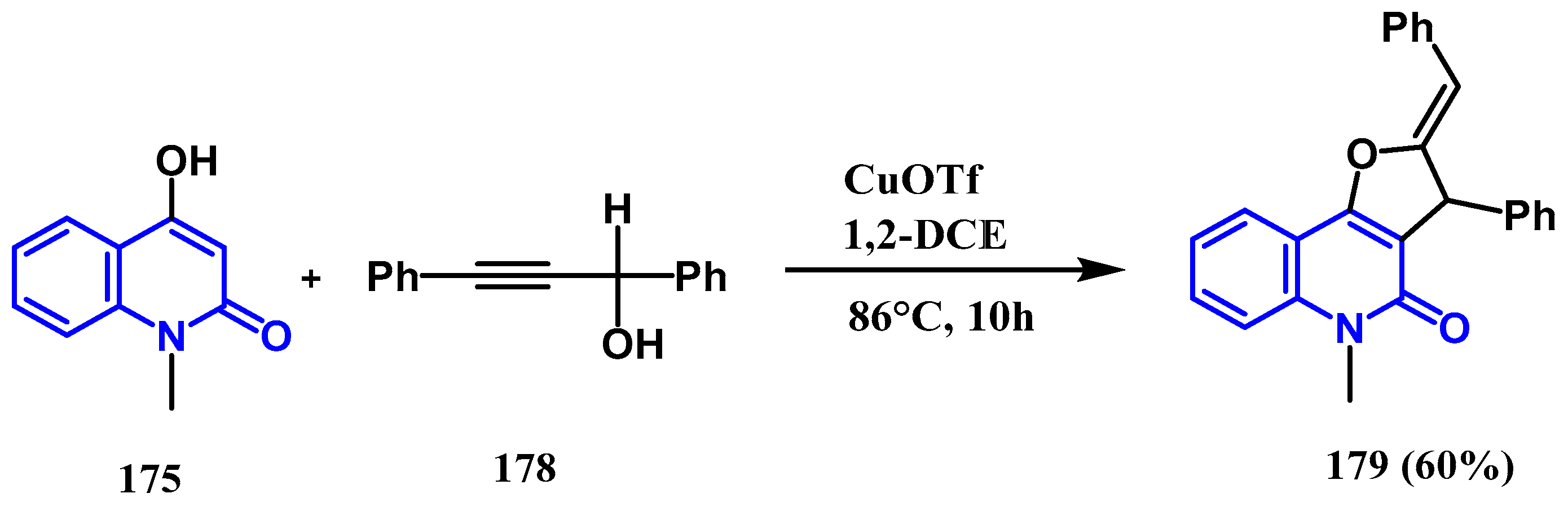
- Yin, H.; Wu, Y.; Gu, X.; Feng, Z.; Wang, M.; Feng, D.; Wang, M.; Cheng, Z.; Wang, S. Synthesis of pyrano [3,2-c] quinolones and furo [3,2-c] quinolones via acid-catalyzed tandem reaction of 4-hydroxy-1-methylquinolin-2(1H)-one and propargylic alcohols. RSC Adv. 2022, 12, 21066–21078. [Google Scholar] [CrossRef]
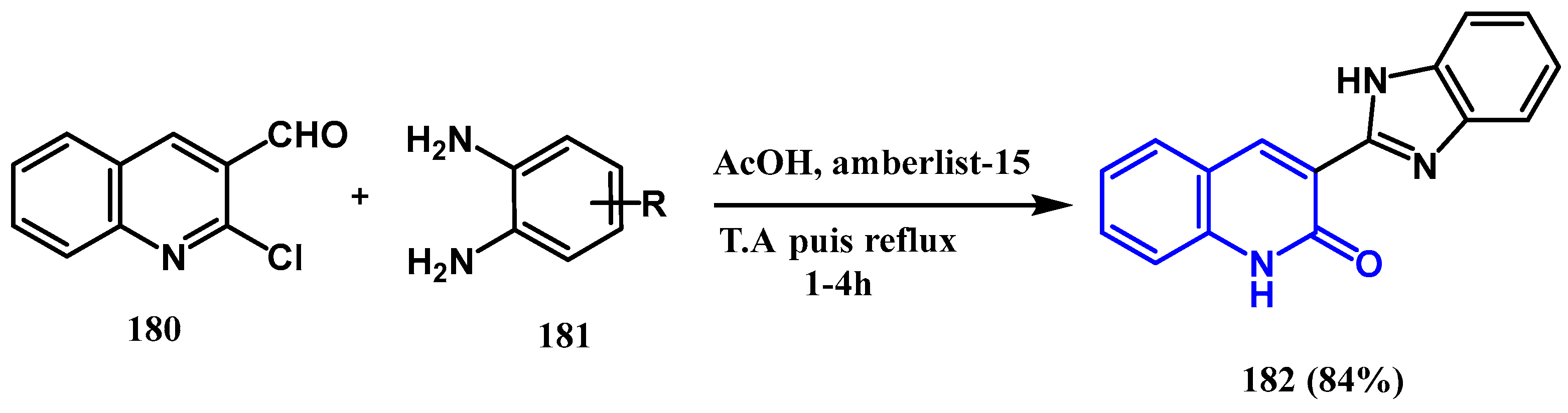
- Abonia, R.; Castillo, J.; Cuervo, P.; Insuasty, B.; Quiroga, J.; Ortíz, A.; Nogueras, M.; Cobo, J. A Simple One-Pot Synthesis of New Imidazol-2-yl-1H-quinolin-2-ones from the Direct Reaction of 2-Chloroquinolin-3-carbaldehyde with Aromatic o-Diamines. Eur. J. Org. Chem. 2010, 2010, 317–325. [Google Scholar] [CrossRef]
- Al-Wahaibi, L.H.; El-Sheref, E.M.; Hammouda, M.M.; Youssif, B.G. One-pot synthesis of 1-Thia-4-azaspiro [4.4/5] alkan-3-ones via schiff base: Design, synthesis, and apoptotic antiproliferative properties of dual EGFR/BRAFV600E inhibitors. Pharmaceuticals 2023, 16, 467. [Google Scholar] [CrossRef]
- Pais, J.P.; Policarpo, M.; Pires, D.; Francisco, A.P.; Madureira, A.M.; Testa, B.; Anes, E.; Constantino, L. Fluoroquinolone derivatives in the treatment of mycobacterium tuberculosis infection. Pharmaceuticals 2022, 15, 1213. [Google Scholar] [CrossRef]
- Shang, X.F.; Morris-Natschke, S.L.; Liu, Y.Q.; Guo, X.; Xu, X.-S.; Goto, M.; Li, J.-C.; Yang, G.-Z.; Lee, K.-H. Biologically active quinoline and quinazoline alkaloids part I. Med. Res. Rev. 2018, 38, 775–828. [Google Scholar]
- Xue, W.; Li, X.; Ma, G.; Zhang, H.; Chen, Y.; Kirchmair, J.; Xia, J.; Wu, S. N-thiadiazole-4-hydroxy-2-quinolone-3-carboxamides bearing heteroaromatic rings as novel antibacterial agents: Design, synthesis, biological evaluation and target identification. Eur. J. Med. Chem. 2020, 188, 112022. [Google Scholar]
- Berhan, A.; Bayleyegn, B.; Getaneh, Z. HIV/AIDS Associated Lymphoma: Review. Blood Lymphat. Cancer Targets Ther. 2022, 12, 31–45. [Google Scholar] [CrossRef]
- He, N. Research progress in the epidemiology of HIV/AIDS in China. China CDC Wkly. 2021, 3, 1022–1030. [Google Scholar]
- Obeagu, E.I.; Amekpor, F.; Scott, G.Y. An update of human immunodeficiency virus infection: Bleeding disorders. J. Public Health Nutr. 2023, 6, 139. [Google Scholar]
- Wang, R.; Xu, K.; Shi, W. Quinolone derivatives: Potential anti-HIV agent—Development and application. Arch. Pharm. 2019, 352, 1900045. [Google Scholar] [CrossRef]
- Hu, L.; Yan, S.; Luo, Z.; Han, X.; Wang, Y.; Wang, Z.; Zeng, C. Design, practical synthesis, and biological evaluation of novel 6-(pyrazolylmethyl)-4-quinoline-3-carboxylic acid derivatives as HIV-1 integrase inhibitors. Molecules 2012, 17, 10652–10666. [Google Scholar] [CrossRef]
- Senerovic, L.; Opsenica, D.; Moric, I.; Aleksic, I.; Spasić, M.; Vasiljevic, B. Quinolines and quinolones as antibacterial, antifungal, anti-virulence, antiviral and anti-parasitic agents. Adv. Microbiol. Infect. Dis. Public Health 2000, 14, 37–69. [Google Scholar]
- Papagni, R.; Novara, R.; Minardi, M.L.; Frallonardo, L.; Panico, G.G.; Pallara, E.; Cotugno, S.; Ascoli Bartoli, T.; Guido, G.; De Vita, E.; et al. Human African Trypanosomiasis (sleeping sickness): Current knowledge and future challenges. Front. Trop. Dis. 2023, 4, 1087003. [Google Scholar]
- Ortiz-Martínez, Y.; Kouamé, M.G.; Bongomin, F.; Lakoh, S.; Henao-Martínez, A.F. Human African Trypanosomiasis (Sleeping Sickness)—Epidemiology, Clinical Manifestations, Diagnosis, Treatment, and Prevention. Curr. Trop. Med. Rep. 2023, 10, 222–234. [Google Scholar] [CrossRef]
- Jamabo, M.; Mahlalela, M.; Edkins, A.L.; Boshoff, A. Tackling sleeping sickness: Current and promising therapeutics and treatment strategies. Int. J. Mol. Sci. 2023, 24, 12529. [Google Scholar] [CrossRef]
- Pham, T.D.; Ziora, Z.M.; Blaskovich, M.A. Quinolone antibiotics. Medchemcomm 2019, 10, 1719–1739. [Google Scholar]
- Dine, I.; Mulugeta, E.; Melaku, Y.; Belete, M. Recent advances in the synthesis of pharmaceutically active 4-quinolone and its analogues: A review. RSC Adv. 2023, 13, 8657–8682. [Google Scholar] [CrossRef]
- Pedron, J.; Boudot, C.; Brossas, J.-Y.; Pinault, E.; Bourgeade-Delmas, S.; Sournia-Saquet, A.; Boutet-Robinet, E.; Destere, A.; Tronnet, A.; Bergé, J.; et al. New 8-Nitroquinolinone Derivative Displaying Submicromolar in Vitro Activities against Both Trypanosoma brucei and cruzi. ACS Med. Chem. Lett. 2020, 11, 464–472. [Google Scholar] [CrossRef]
- Hiltensperger, G.; Jones, N.G.; Niedermeier, S.; Stich, A.; Kaiser, M.; Jung, J.; Puhl, S.; Damme, A.; Braunschweig, H.; Meinel, L.; et al. Synthesis and Structure–Activity Relationships of New Quinolone-Type Molecules against Trypanosoma brucei. J. Med. Chem. 2012, 55, 2538–2548. [Google Scholar] [CrossRef]
- Beteck, R.M.; Isaacs, M.; Hoppe, H.C.; Khanye, S.D. Synthesis, in vitro cytotoxicity and trypanocidal evaluation of novel 1,3,6-substituted non-fluoroquinolones. S. Afr. J. Chem. 2018, 71, 188–195. [Google Scholar]
- Angula, K.T.; Legoabe, L.J.; Swart, T.; Hoppe, H.C.; Beteck, R.M. Synthesis and in vitro antitrypanosomal evaluation of novel 6-heteroarylidene-substituted quinolone derivatives. Eur. J. Med. Chem. 2022, 227, 113913. [Google Scholar]
- Chanquia, S.N.; Larregui, F.; Puente, V.; Labriola, C.; Lombardo, E.; Liñares, G.G. Synthesis and biological evaluation of new quinoline derivatives as antileishmanial and antitrypanosomal agents. Bioorg. Chem. 2019, 83, 526–534. [Google Scholar] [PubMed]
- Arora, G.; Chuang, Y.-M.; Sinnis, P.; Dimopoulos, G.; Fikrig, E. Malaria: Influence of Anopheles mosquito saliva on Plasmodium infection. Trends Immunol. 2023, 44, 256–265. [Google Scholar] [PubMed]
- Patel, P.; Bagada, A.; Vadia, N. Epidemiology and Current Trends in Malaria. In Rising Contagious Diseases; Amponsah, S.K., Shegokar, R., Pathak, Y.V., Eds.; Wiley: Hoboken, NJ, USA, 2024; pp. 261–282. ISBN 978-1-394-18871-0. [Google Scholar]
- Shaw, W.R.; Marcenac, P.; Catteruccia, F. Plasmodium development in Anopheles: A tale of shared resources. Trends Parasitol. 2022, 38, 124–135. [Google Scholar] [CrossRef] [PubMed]
- Balogun, T.A.; Omoboyowa, D.A.; Saibu, O.A. In silico Anti-malaria Activity of Quinolone Compounds against Plasmodium falciparum Dihydrofolate Reductase (pfDHFR). Int. J. Biochem. Res. Rev. 2020, 29, 10–17. [Google Scholar] [CrossRef]
- Van Schalkwyk, D.A.; Riscoe, M.K.; Pou, S.; Winter, R.W.; Nilsen, A.; Duffey, M.; Moon, R.W.; Sutherland, C.J. Novel Endochin-Like Quinolones Exhibit Potent In Vitro Activity against Plasmodium knowlesi but Do Not Synergize with Proguanil. Antimicrob. Agents Chemother. 2020, 64, e02549-19. [Google Scholar] [CrossRef]
- Pou, S.; Dodean, R.A.; Frueh, L.; Liebman, K.M.; Gallagher, R.T.; Jin, H.; Jacobs, R.T.; Nilsen, A.; Stuart, D.R.; Doggett, J.S.; et al. New Scalable Synthetic Routes to ELQ-300, ELQ-316, and Other Antiparasitic Quinolones. Org. Process Res. Dev. 2021, 25, 1841–1852. [Google Scholar] [CrossRef]
- Doggett, J.S.; Schultz, T.; Miller, A.J.; Bruzual, I.; Pou, S.; Winter, R.; Dodean, R.; Zakharov, L.N.; Nilsen, A.; Riscoe, M.K.; et al. Orally Bioavailable Endochin-Like Quinolone Carbonate Ester Prodrug Reduces Toxoplasma gondii Brain Cysts. Antimicrob. Agents Chemother. 2020, 64, e00535-20. [Google Scholar] [CrossRef]
- Miley, G.P.; Pou, S.; Winter, R.; Nilsen, A.; Li, Y.; Kelly, J.X.; Stickles, A.M.; Mather, M.W.; Forquer, I.P.; Pershing, A.M.; et al. ELQ-300 Prodrugs for Enhanced Delivery and Single-Dose Cure of Malaria. Antimicrob. Agents Chemother. 2015, 59, 5555–5560. [Google Scholar] [CrossRef]
- Frueh, L.; Li, Y.; Mather, M.W.; Li, Q.; Pou, S.; Nilsen, A.; Winter, R.W.; Forquer, I.P.; Pershing, A.M.; Xie, L.H.; et al. Alkoxycarbonate Ester Prodrugs of Preclinical Drug Candidate ELQ-300 for Prophylaxis and Treatment of Malaria. ACS Infect. Dis. 2017, 3, 728–735. [Google Scholar] [CrossRef]
- Balbino, L.S.; Bernardes, J.C.; Ladeia, W.A.; Martins, F.D.C.; Nino, B.D.S.L.; Mitsuka-Breganó, R.; Navarro, I.T.; Pinto-Ferreira, F. Epidemiological study of toxoplasmosis outbreaks in Brazil. Transbound. Emerg. Dis. 2022, 69, 2021–2028. [Google Scholar] [CrossRef]
- Garrido-Cardenas, J.A.; Mesa-Valle, C.; Manzano-Agugliaro, F. Human parasitology worldwide research. Parasitology 2018, 145, 699–712. [Google Scholar] [CrossRef]
- Eberhard, N.; Balmer, V.; Müller, J.; Müller, N.; Winter, R.; Pou, S.; Nilsen, A.; Riscoe, M.; Francisco, S.; Leitao, A.; et al. Activities of Endochin-Like Quinolones Against in vitro Cultured Besnoitia besnoiti Tachyzoites. Front. Vet. Sci. 2020, 7, 96. [Google Scholar] [CrossRef]










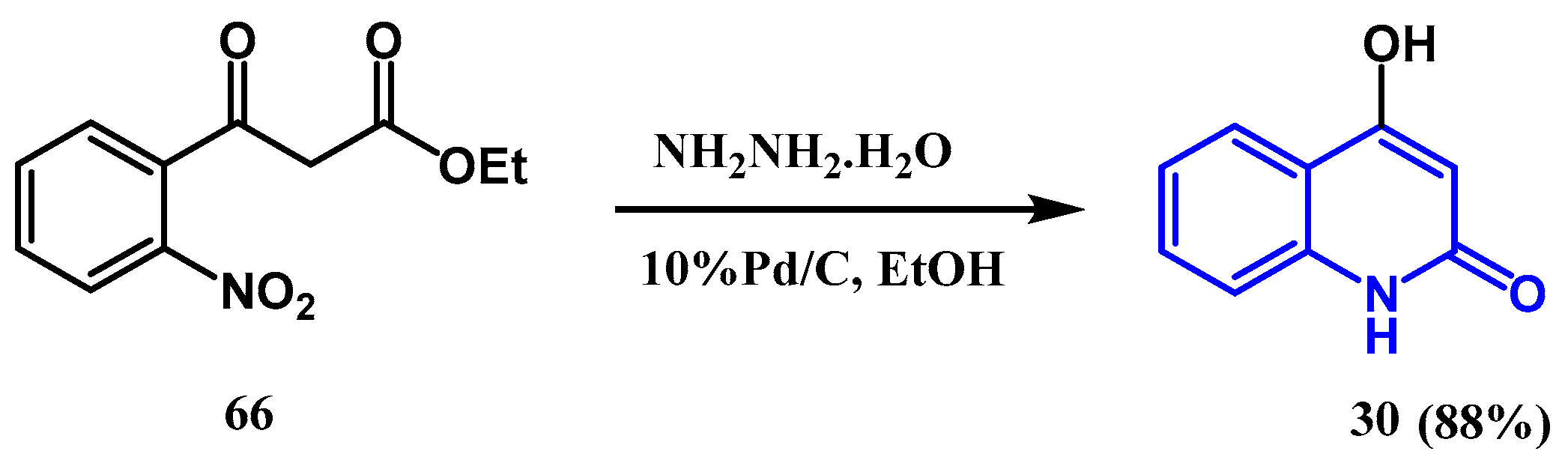



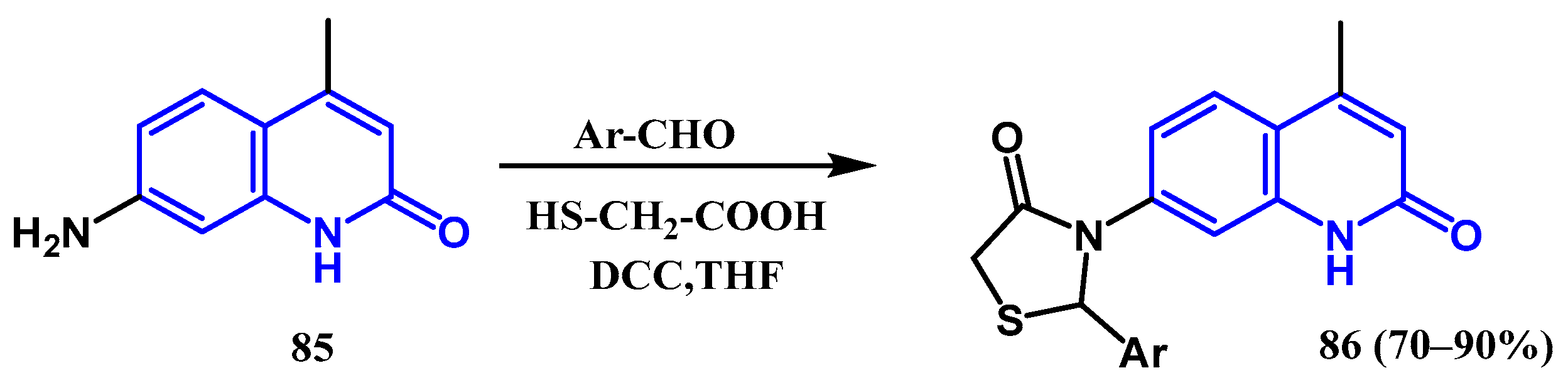




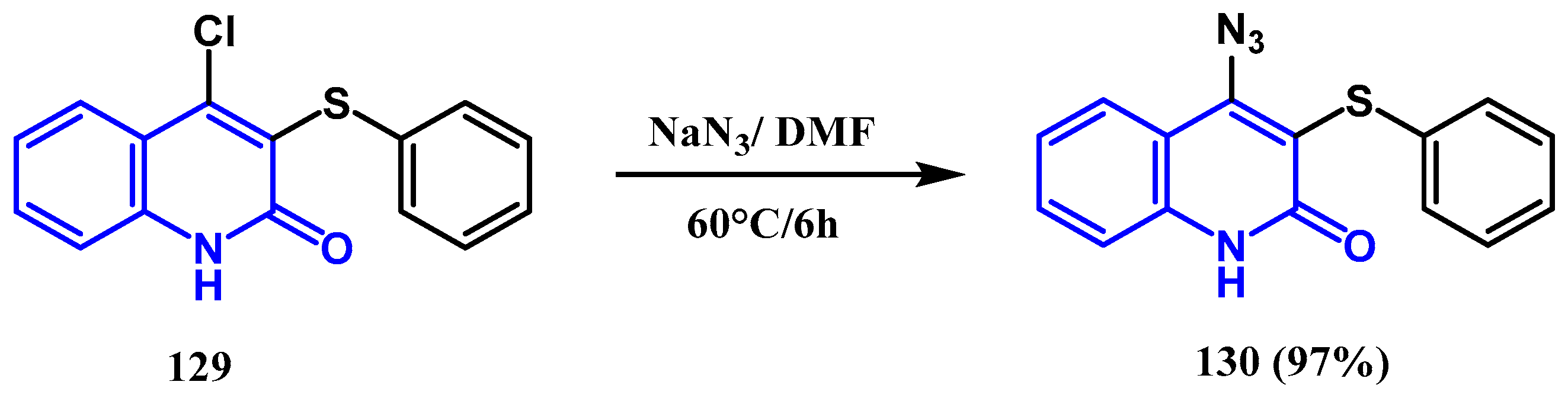












Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-mrabet, A.; Haoudi, A.; Kandri-Rodi, Y.; Mazzah, A. An Overview of Quinolones as Potential Drugs: Synthesis, Reactivity and Biological Activities. Organics 2025, 6, 16. https://doi.org/10.3390/org6020016
El-mrabet A, Haoudi A, Kandri-Rodi Y, Mazzah A. An Overview of Quinolones as Potential Drugs: Synthesis, Reactivity and Biological Activities. Organics. 2025; 6(2):16. https://doi.org/10.3390/org6020016
Chicago/Turabian StyleEl-mrabet, Ayoub, Amal Haoudi, Youssef Kandri-Rodi, and Ahmed Mazzah. 2025. "An Overview of Quinolones as Potential Drugs: Synthesis, Reactivity and Biological Activities" Organics 6, no. 2: 16. https://doi.org/10.3390/org6020016
APA StyleEl-mrabet, A., Haoudi, A., Kandri-Rodi, Y., & Mazzah, A. (2025). An Overview of Quinolones as Potential Drugs: Synthesis, Reactivity and Biological Activities. Organics, 6(2), 16. https://doi.org/10.3390/org6020016