Review on Hydrometallurgical Recovery of Metals with Deep Eutectic Solvents
Abstract
:1. Introduction
2. Discussion
2.1. DESs for the Leaching of Metals
2.2. DESs for the Electrochemical Recovery of Metals
2.3. Hydrophobic DESs for the Solvent Extraction of Metals
3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| LIBs | Lithium-ion batteries |
| DESs | Deep eutectic solvents |
| LTTMs | Low-temperature transition mixtures |
| HBA | Hydrogen bond acceptor |
| HBD | Hydrogen bond donor |
| ChCl | Choline chloride |
| MA | Malonic acid |
| EG | Ethylene glycol |
| [N4444][Cl] | Tetrabutylammonium chloride |
| [P4444][Cl] | Tetrabutylphosphonium chloride |
| [N7777][Cl] | Tetraheptylammonium chloride |
| TOPO | Trioctylphosphine oxide |
References
- Halada, K.; Shimada, M.; Ijima, K. Forecasting of the Consumption of Metals up to 2050. Mater. Trans. 2008, 49, 402–410. [Google Scholar] [CrossRef] [Green Version]
- Henckens, M.L.C.M.; Driessen, P.P.J.; Worrell, E. Metal scarcity and sustainability, analyzing the necessity to reduce the extraction of scarce metals. Resour. Conserv. Recycl. 2014, 93, 1–8. [Google Scholar] [CrossRef]
- Sverdrup, H.U. Modelling global extraction, supply, price and depletion of the extractable geological resources with the LITHIUM model. Resour. Conserv. Recycl. 2016, 114, 112–129. [Google Scholar] [CrossRef]
- Sverdrup, H.U.; Ragnarsdottir, K.V.; Koca, D. An assessment of metal supply sustainability as an input to policy: Security of supply extraction rates, stocks-in-use, recycling, and risk of scarcity. J. Clean. Prod. 2017, 140, 359–372. [Google Scholar] [CrossRef]
- Legarth, J.B. Sustainable metal resource management—The need for industrial development: Efficiency improvement demands on metal resource management to enable a (sustainable) supply until 2050. J. Clean. Prod. 1996, 4, 97–104. [Google Scholar] [CrossRef]
- Belchí Lorente, D.; Mandil, G.; Svecova, L.; Thivel, X.-P.; Zwolinski, P. Life Cycle and Sustainability. In Lithium Process Chemistry; Elsevier: Amsterdam, The Netherlands, 2015; pp. 269–288. [Google Scholar] [CrossRef]
- Zheng, X.; Zhu, Z.; Lin, X.; Zhang, Y.; He, Y.; Cao, H.; Sun, Z. A Mini-Review on Metal Recycling from Spent Lithium Ion Batteries. Engineering 2018, 4, 361–370. [Google Scholar] [CrossRef]
- Mossali, E.; Picone, N.; Gentilini, L.; Rodrìguez, O.; Pérez, J.M.; Colledani, M. Lithium-ion batteries towards circular economy: A literature review of opportunities and issues of recycling treatments. J. Environ. Manag. 2020, 264, 110500. [Google Scholar] [CrossRef]
- Boyden, A.; Soo, V.K.; Doolan, M. The Environmental Impacts of Recycling Portable Lithium-Ion Batteries. Procedia CIRP 2016, 48, 188–193. [Google Scholar] [CrossRef] [Green Version]
- Qi, C.; Ye, L.; Ma, X.; Yang, D.; Hong, J. Life cycle assessment of the hydrometallurgical zinc production chain in China. J. Clean. Prod. 2017, 156, 451–458. [Google Scholar] [CrossRef]
- Norgate, T.E.; Jahanshahi, S.; Rankin, W.J. Assessing the environmental impact of metal production processes. J. Clean. Prod. 2007, 15, 838–848. [Google Scholar] [CrossRef]
- Vieceli, N.; Nogueira, C.A.; Guimarães, C.; Pereira, M.F.C.; Durão, F.O.; Margarido, F. Hydrometallurgical recycling of lithium-ion batteries by reductive leaching with sodium metabisulphite. Waste Manag. 2018, 71, 350–361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, T.; He, Y.; Wang, F.; Ge, L.; Zhu, X.; Li, H. Chemical and process mineralogical characterizations of spent lithium-ion batteries: An approach by multi-analytical techniques. Waste Manag. 2014, 34, 1051–1058. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Diaz, L.A.; Yang, Z.; Jin, H.; Lister, T.E.; Vahidi, E.; Zhao, F. Comparative life cycle analysis for value recovery of precious metals and rare earth elements from electronic waste. Resour. Conserv. Recycl. 2019, 149, 20–30. [Google Scholar] [CrossRef]
- Iannicelli-Zubiani, E.M.; Giani, M.I.; Recanati, F.; Dotelli, G.; Puricelli, S.; Cristiani, C. Environmental impacts of a hydrometallurgical process for electronic waste treatment: A life cycle assessment case study. J. Clean. Prod. 2017, 140, 1204–1216. [Google Scholar] [CrossRef]
- Rydberg, J.; Cox, M.; Musikas, C.; Choppin, G.R. Solvent Extraction Principles and Practice, Revised and Expanded; CRC press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Pearson, R.G. Hard and Soft Acids and Bases. J. Am. Chem. Soc. 1963, 85, 3533–3539. [Google Scholar] [CrossRef]
- Vahidi, E.; Zhao, F. Life Cycle Analysis for Solvent Extraction of Rare Earth Elements from Aqueous Solutions. In REWAS 2016: Towards Materials Resource Sustainability; Kirchain, R.E., Blanpain, B., Meskers, C., Olivetti, E., Apelian, D., Howarter, J., Kvithyld, A., Mishra, B., Neelameggham, N.R., Spangenberger, J., Eds.; Springer International Publishing: Cham, Swizerlands, 2016; pp. 113–120. [Google Scholar] [CrossRef]
- Abbott, A.P.; Boothby, D.; Capper, G.; Davies, D.L.; Rasheed, R.K. Deep Eutectic Solvents Formed between Choline Chloride and Carboxylic Acids: Versatile Alternatives to Ionic Liquids. J. Am. Chem. Soc. 2004, 126, 9142–9147. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. 2003, 70–71. [Google Scholar] [CrossRef] [Green Version]
- Francisco, M.; van den Bruinhorst, A.; Kroon, M.C. Low-Transition-Temperature Mixtures (LTTMs): A New Generation of Designer Solvents, Angew. Chem. Int. Ed. 2013, 52, 3074–3085. [Google Scholar] [CrossRef]
- Florindo, C.; Branco, L.C.; Marrucho, I.M. Quest for Green--Solvent Design: From Hydrophilic to Hydrophobic (Deep) Eutectic Solvents. ChemSusChem 2019, 12, 1549–1559. [Google Scholar] [CrossRef] [PubMed]
- Rogers, R.D. CHEMISTRY: Ionic Liquids—Solvents of the Future? Science 2003, 302, 792–793. [Google Scholar] [CrossRef]
- Paiva, A.; Craveiro, R.; Aroso, I.; Martins, M.; Reis, R.L.; Duarte, A.R.C. Natural Deep Eutectic Solvents – Solvents for the 21st Century. ACS Sustain. Chem. Eng. 2014, 2, 1063–1071. [Google Scholar] [CrossRef]
- De los Fernández, M.Á.; Boiteux, J.; Espino, M.; Gomez, F.J.V.; Silva, M.F. Natural deep eutectic solvents-mediated extractions: The way forward for sustainable analytical developments. Anal. Chim. Acta 2018, 1038, 1–10. [Google Scholar] [CrossRef]
- Li, X.; Row, K.H. Development of deep eutectic solvents applied in extraction and separation: Liquid Chromatography. J. Sep. Sci. 2016, 39, 3505–3520. [Google Scholar] [CrossRef] [PubMed]
- Makoś, P.; Słupek, E.; Gębicki, J. Hydrophobic deep eutectic solvents in microextraction techniques–A review. Microchem. J. 2020, 152, 104384. [Google Scholar] [CrossRef]
- Perna, F.M.; Vitale, P.; Capriati, V. Deep eutectic solvents and their applications as green solvents. Curr. Opin. Green Sustain. Chem. 2020, 21, 27–33. [Google Scholar] [CrossRef]
- Shishov, A.; Bulatov, A.; Locatelli, M.; Carradori, S.; Andruch, V. Application of deep eutectic solvents in analytical chemistry. A Review. Microchem. J. 2017, 135, 33–38. [Google Scholar] [CrossRef]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef] [Green Version]
- Tang, B.; Zhang, H.; Row, K.H. Application of deep eutectic solvents in the extraction and separation of target compounds from various samples: Other Techniques. J. Sep. Sci. 2015, 38, 1053–1064. [Google Scholar] [CrossRef]
- Zainal-Abidin, M.H.; Hayyan, M.; Hayyan, A.; Jayakumar, N.S. New horizons in the extraction of bioactive compounds using deep eutectic solvents: A review. Anal. Chim. Acta 2017, 979, 1–23. [Google Scholar] [CrossRef]
- Silva, J.M.; Reis, R.L.; Paiva, A.; Duarte, A.R.C. Design of Functional Therapeutic Deep Eutectic Solvents Based on Choline Chloride and Ascorbic Acid. ACS Sustain. Chem. Eng. 2018, 6, 10355–10363. [Google Scholar] [CrossRef]
- Richter, J.; Ruck, M. Synthesis and Dissolution of Metal Oxides in Ionic Liquids and Deep Eutectic Solvents. Molecules 2019, 25, 78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tran, M.K.; Rodrigues, M.-T.F.; Kato, K.; Babu, G.; Ajayan, P.M. Deep eutectic solvents for cathode recycling of Li-ion batteries. Nat. Energy 2019, 4, 339–345. [Google Scholar] [CrossRef]
- Cojocaru, P.; Magagnin, L.; Gomez, E.; Vallés, E. Using deep eutectic solvents to electrodeposit CoSm films and nanowires. Mater. Lett. 2011, 65, 3597–3600. [Google Scholar] [CrossRef]
- Pollet, B.G.; Hihn, J.-Y.; Mason, T.J. Sono-electrodeposition (20 and 850 kHz) of copper in aqueous and deep eutectic solvents. Electrochim. Acta 2008, 53, 4248–4256. [Google Scholar] [CrossRef]
- Bahadori, L.; Chakrabarti, M.H.; Mjalli, F.S.; AlNashef, I.M.; Manan, N.S.A.; Hashim, M.A. Physicochemical properties of ammonium-based deep eutectic solvents and their electrochemical evaluation using organometallic reference redox systems. Electrochim. Acta 2013, 113, 205–211. [Google Scholar] [CrossRef]
- Tereshatov, E.E.; Boltoeva, Y.M.; Folden, C.M. First evidence of metal transfer into hydrophobic deep eutectic and low-transition-temperature mixtures: Indium extraction from hydrochloric and oxalic acids. Green Chem. 2016, 18, 4616–4622. [Google Scholar] [CrossRef]
- Dwamena, A.K. Recent Advances in Hydrophobic Deep Eutectic Solvents for Extraction. Separations 2019, 6, 9. [Google Scholar] [CrossRef] [Green Version]
- Abbott, A.P.; Capper, G.; Davies, D.L.; McKenzie, K.J.; Obi, S.U. Solubility of Metal Oxides in Deep Eutectic Solvents Based on Choline Chloride. J. Chem. Eng. Data 2006, 51, 1280–1282. [Google Scholar] [CrossRef]
- Binnemans, K.; Jones, P.T. Solvometallurgy: An Emerging Branch of Extractive Metallurgy. J. Sustain. Metall. 2017, 3, 570–600. [Google Scholar] [CrossRef] [Green Version]
- Abbott, A.P.; Collins, J.; Dalrymple, I.; Harris, R.C.; Mistry, R.; Qiu, F.; Scheirer, J.; Wise, W.R. Processing of Electric Arc Furnace Dust using Deep Eutectic Solvents. Aust. J. Chem. 2009, 62, 341. [Google Scholar] [CrossRef]
- Rodriguez Rodriguez, N.; Machiels, L.; Binnemans, K. Toluenesulfonic Acid-Based Deep-Eutectic Solvents for Solubilizing Metal Oxides. ACS Sustain. Chem. Eng. 2019, 7, 3940–3948. [Google Scholar] [CrossRef]
- Entezari-Zarandi, A.; Larachi, F. Selective dissolution of rare-earth element carbonates in deep eutectic solvents. J. Rare Earths 2019, 37, 528–533. [Google Scholar] [CrossRef]
- Riaño, S.; Petranikova, M.; Onghena, B.; Vander Hoogerstraete, T.; Banerjee, D.; StForeman, M.R.J.; Ekberg, C.; Binnemans, K. Separation of rare earths and other valuable metals from deep-eutectic solvents: A new alternative for the recycling of used NdFeB magnets. RSC Adv. 2017, 7, 32100–32113. [Google Scholar] [CrossRef] [Green Version]
- Bakkar, A. Recycling of electric arc furnace dust through dissolution in deep eutectic ionic liquids and electrowinning. J. Hazard. Mater. 2014, 280, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Bakkar, A.; Neubert, V. Recycling of cupola furnace dust: Extraction and electrodeposition of zinc in deep eutectic solvents. J. Alloys Compd. 2019, 771, 424–432. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Mukherjee, S.; Adnan, N.F.; Hayyan, A.; Hayyan, M.; Hashim, M.A.; Sen Gupta, B. Ammonium-based deep eutectic solvents as novel soil washing agent for lead removal. Chem. Eng. J. 2016, 294, 316–322. [Google Scholar] [CrossRef]
- Petračić, A.; Sander, A.; Cvetnić, M. A novel approach for the removal of trace elements from waste fats and oils. Sep. Sci. Technol. 2019, 1–15. [Google Scholar] [CrossRef]
- Zürner, P.; Frisch, G. Leaching and Selective Extraction of Indium and Tin from Zinc Flue Dust Using an Oxalic Acid-Based Deep Eutectic Solvent. ACS Sustain. Chem. Eng. 2019, 7, 5300–5308. [Google Scholar] [CrossRef]
- Jenkin, G.R.T.; Al-Bassam, Z.M.A.; Harris, R.C.; Abbott, A.P.; Smith, D.J.; Holwell, D.A.; Chapman, R.J.; Stanley, C.J. The application of deep eutectic solvent ionic liquids for environmentally-friendly dissolution and recovery of precious metals. Miner. Eng. 2016, 87, 18–24. [Google Scholar] [CrossRef] [Green Version]
- Peeters, N.; Binnemans, K.; Riaño, S. Solvometallurgical recovery of cobalt from lithium-ion battery cathode materials using deep-eutectic solvents. Green Chem. 2020, 22, 4210–4221. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Z.; Lu, Z.; Xu, Z. A novel method for screening deep eutectic solvent to recycle the cathode of Li-ion batteries. Green Chem. 2020, 22, 4473–4482. [Google Scholar] [CrossRef]
- Zeng, X.; Li, J.; Shen, B. Novel approach to recover cobalt and lithium from spent lithium-ion battery using oxalic acid. J. Hazard. Mater. 2015, 295, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.K.; Rhee, K.-I. Preparation of LiCoO2 from spent lithium-ion batteries. J. Power Sources 2002, 109, 17–21. [Google Scholar] [CrossRef]
- Cui, Y.; Li, C.; Yin, J.; Li, S.; Jia, Y.; Bao, M. Design, synthesis and properties of acidic deep eutectic solvents based on choline chloride. J. Mol. Liq. 2017, 236, 338–343. [Google Scholar] [CrossRef]
- Albler, F.-J.; Bica, K.; StForeman, M.R.J.; Holgersson, S.; Tyumentsev, M.S. A comparison of two methods of recovering cobalt from a deep eutectic solvent: Implications for battery recycling. J. Clean. Prod. 2017, 167, 806–814. [Google Scholar] [CrossRef]
- Ghareh Bagh, F.S.; Mjalli, F.S.; Hashim, M.A.; Hadj-Kali, M.K.O.; AlNashef, I.M. Solubility of Sodium Salts in Ammonium-Based Deep Eutectic Solvents. J. Chem. Eng. Data 2013, 58, 2154–2162. [Google Scholar] [CrossRef]
- Gupta, R.; Vats, B.; Pandey, A.K.; Sharma, M.K.; Sahu, P.; Yadav, A.K.; Ali, M.; Kannan, S. Insight into Speciation and Electrochemistry of Uranyl Ions in Deep Eutectic Solvents. J. Phys. Chem. B 2020, 124, 181–189. [Google Scholar] [CrossRef]
- Poll, C.G.; Nelson, G.W.; Pickup, D.M.; Chadwick, A.V.; Riley, D.J.; Payne, D.J. Electrochemical recycling of lead from hybrid organic–inorganic perovskites using deep eutectic solvents. Green Chem. 2016, 18, 2946–2955. [Google Scholar] [CrossRef] [Green Version]
- Almeida, J.; Craveiro, R.; Faria, P.; Silva, A.S.; Mateus, E.P.; Barreiros, S.; Paiva, A.; Ribeiro, A.B. Electrodialytic removal of tungsten and arsenic from secondary mine resources—Deep eutectic solvents enhancement. Sci. Total Environ. 2020, 710, 136364. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Reddy, R.G. Electrochemical deposition of zinc from zinc oxide in 2:1 urea/choline chloride ionic liquid. Electrochim. Acta 2014, 147, 513–519. [Google Scholar] [CrossRef]
- Mandroyan, A.; Mourad-Mahmoud, M.; Doche, M.-L.; Hihn, J.-Y. Effects of ultrasound and temperature on copper electro reduction in Deep Eutectic Solvents (DES). Ultrason. Sonochem. 2014, 21, 2010–2019. [Google Scholar] [CrossRef] [PubMed]
- Florindo, C.; Romero, L.; Rintoul, I.; Branco, L.C.; Marrucho, I.M. From Phase Change Materials to Green Solvents: Hydrophobic Low Viscous Fatty Acid–Based Deep Eutectic Solvents. ACS Sustain. Chem. Eng. 2018, 6, 3888–3895. [Google Scholar] [CrossRef]
- Shi, Y.; Xiong, D.; Zhao, Y.; Li, T.; Zhang, K.; Fan, J. Highly efficient extraction/separation of Cr (VI) by a new family of hydrophobic deep eutectic solvents. Chemosphere 2020, 241, 125082. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, S.; Poletti, F.; Zanardi, C.; Pigani, L.; Zanfrognini, B.; Corsi, E.; Dossi, N.; Salomäki, M.; Kivelä, H.; Lukkari, J.; et al. Chemical and electrochemical properties of a hydrophobic deep eutectic solvent. Electrochim. Acta 2019, 295, 124–129. [Google Scholar] [CrossRef]
- Phelps, T.E.; Bhawawet, N.; Jurisson, S.S.; Baker, G.A. Efficient and Selective Extraction of 99mTcO4- from Aqueous Media Using Hydrophobic Deep Eutectic Solvents. ACS Sustain. Chem. Eng. 2018, 6, 13656–13661. [Google Scholar] [CrossRef]
- Van Osch, D.J.G.P.; Parmentier, D.; Dietz, C.H.J.T.; van den Bruinhorst, A.; Tuinier, R.; Kroon, M.C. Removal of alkali and transition metal ions from water with hydrophobic deep eutectic solvents. Chem. Commun. 2016, 52, 11987–11990. [Google Scholar] [CrossRef] [Green Version]
- Ola, P.D.; Matsumoto, M. Use of deep eutectic solvent as extractant for separation of Fe (III) and Mn (II) from aqueous solution. Sep. Sci. Technol. 2019, 54, 759–765. [Google Scholar] [CrossRef]
- Schaeffer, N.; Conceição, J.H.F.; Martins, M.A.R.; Neves, M.C.; Pérez-Sánchez, G.; Gomes, J.R.B.; Papaiconomou, N.; Coutinho, J.A.P. Non-ionic hydrophobic eutectics—Versatile solvents for tailored metal separation and valorisation. Green Chem. 2020. [Google Scholar] [CrossRef]
- Schaeffer, N.; Martins, M.A.R.; Neves, C.M.S.S.; Pinho, S.P.; Coutinho, J.A.P. Sustainable hydrophobic terpene-based eutectic solvents for the extraction and separation of metals. Chem. Commun. 2018, 54, 8104–8107. [Google Scholar] [CrossRef] [Green Version]
- Gilmore, M.; McCourt, É.N.; Connolly, F.; Nockemann, P.; Swadźba-Kwaśny, M.; Holbrey, J.D. Hydrophobic Deep Eutectic Solvents Incorporating Trioctylphosphine Oxide: Advanced Liquid Extractants. ACS Sustain. Chem. Eng. 2018, 6, 17323–17332. [Google Scholar] [CrossRef] [Green Version]
- Arain, M.B.; Yilmaz, E.; Soylak, M. Deep eutectic solvent based ultrasonic assisted liquid phase microextraction for the FAAS determination of cobalt. J. Mol. Liq. 2016, 224, 538–543. [Google Scholar] [CrossRef]
- Preston, J.S. Solvent extraction of metals by carboxylic acids. Hydrometallurgy 1985, 14, 171–188. [Google Scholar] [CrossRef]
- Cartoni, G.P.; Liberti, A.; Palombari, R. Metal complexes of β-diketones as liquid phases in gas—Liquid chromatography. J. Chromatogr. A 1965, 20, 278–282. [Google Scholar] [CrossRef]
- Van den Bruinhorst, A.; Raes, S.; Atika Maesara, S.; Kroon, M.C.; Esteves, A.C.C.; Meuldijk, J. Hydrophobic Eutectic Mixtures as Volatile Fatty Acid Extractants. Sep. Purif. Technol. 2019, 216, 147–157. [Google Scholar] [CrossRef]
- Van Osch, D.J.G.P.; Zubeir, L.F.; van den Bruinhorst, A.; Rocha, M.A.A.; Kroon, M.C. Hydrophobic deep eutectic solvents as water-immiscible extractants. Green Chem. 2015, 17, 4518–4521. [Google Scholar] [CrossRef] [Green Version]
- Dupont, D.; Depuydt, D.; Binnemans, K. Overview of the Effect of Salts on Biphasic Ionic Liquid/Water Solvent Extraction Systems: Anion Exchange, Mutual Solubility, and Thermomorphic Properties. J. Phys. Chem. B 2015, 119, 6747–6757. [Google Scholar] [CrossRef]
- Kudłak, B.; Owczarek, K.; Namieśnik, J. Selected issues related to the toxicity of ionic liquids and deep eutectic solvents—A review. Environ. Sci. Pollut. Res. 2015, 22, 11975–11992. [Google Scholar] [CrossRef] [PubMed]
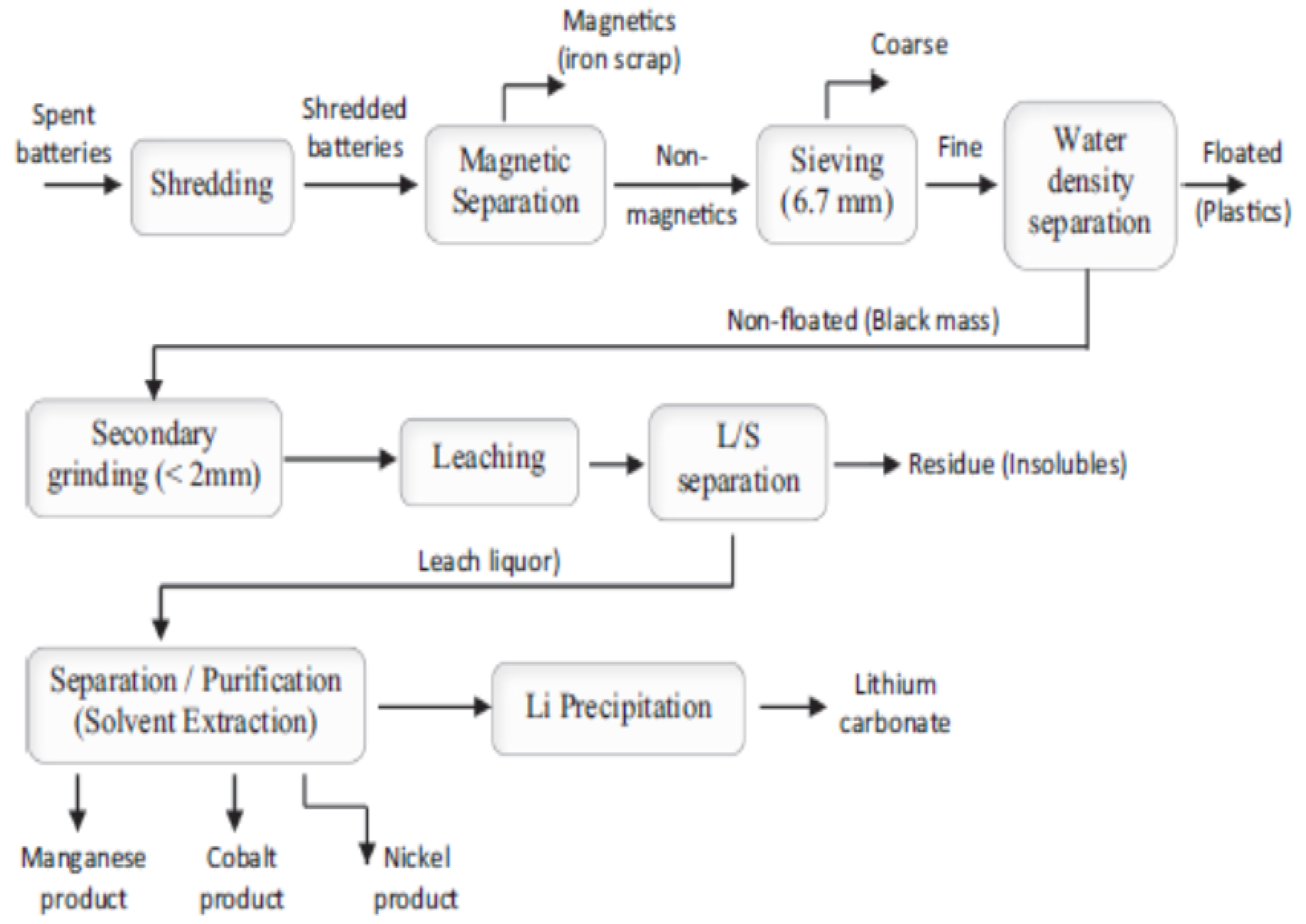
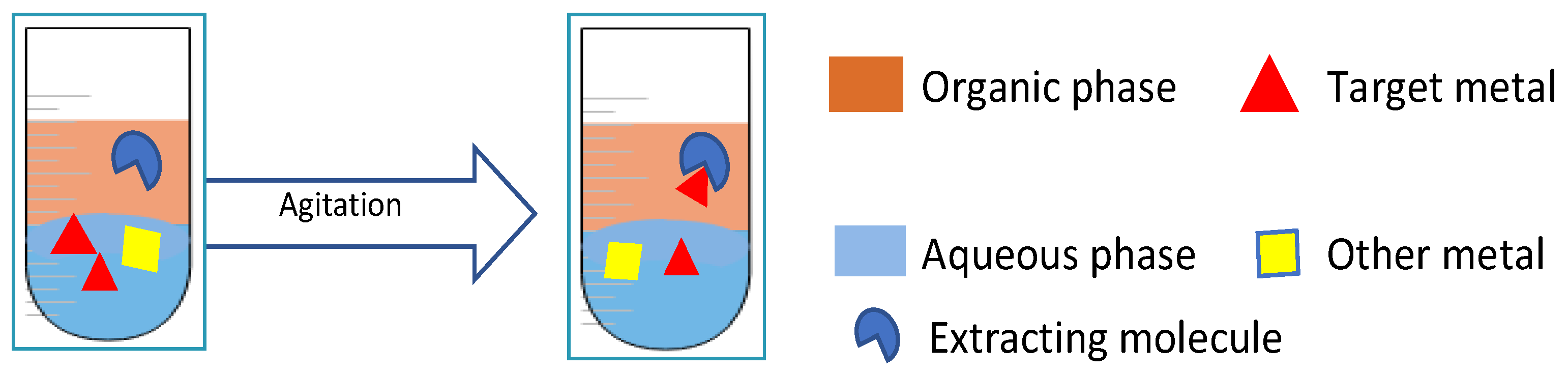
| Materials Leached | Metals Leached/Oxides Dissolved | DES | Noticeable Results | Ref. | |
|---|---|---|---|---|---|
| HBA | HBD | ||||
| Metal Oxides | CuO; Fe3O4; ZnO | ChCl | Carboxylic acids | Up to 0.5 mol⋅L−1 of metal oxides solubilized | [19] |
| Metal Oxides | TiO2; V2O5; Cr2O3 | ChCl | Urea MA EG | Solubility highly dependent on the temperature, the metal oxide and the DES used | [41] |
| Electric Arc Furnace Dust | ZnO; PbO | ChCl | Urea EG | High extraction efficiency for Zn (>70%) but poor lead extraction | [43] |
| Metal Oxides | Cu2O In2O3 Fe2O3 | ChCl [P4444][Cl] [N4444][Cl] | p-toluenesulfonic acid | Solubility of Cu2O and In2O3 higher than 120 g⋅L−1 | [44] |
| Rare-Earth Carbonates | Y; La; Ce; Nd; Sm | ChCl | MA Urea Citric acid | Selective dissolution of the heavy rare earth elements | [45] |
| Used NdFeB Magnets | Fe; Nd; Dy; Pr; B; Gd; Co | ChCl | Lactic acid | Leaching rates >80% (24 h; 70 °C) | [46] |
| Spent LIBs | Li; Co | ChCl | EG | >90% of Li and Co leached; (≥24 h; >150 °C) | [35] |
| Electric Arc Furnace Dust | ZnO; PbO | ChCl | Urea | 60% Zn and 39% Pb leached | [47] |
| Cupola Furnace Dust | ZnO; PbO | ChCl | Urea EG | 38 wt.% of Zn leached (48 h; 60 °C) | [48] |
| Soil | Pb | ChCl | Fructose Sucrose EG Glycerol | Up to 72% of Pb removed | [49] |
| Waste Fats and Oils | Na; K; Mg; Ca; P; B; Fe | ChCl K2CO3 | EG Glycerol | Up to 90% of metal removed (3 h; 60 °C) | [50] |
| Zinc Flue Dust | Fe; Zn; Pb; Cu; In; Sn | ChCl | Urea EG Oxalic acid | Leaching rates > 80% for all the metals after 48 h leaching at 50 °C (oxalic acid as an HBD) | [51] |
| Sulfides and Tellurides | Bi; Te; Ag; Au | ChCl | Urea | Dissolution rates similar to the cyanide processes currently applied | [52] |
| Spent LIBs | Co | ChCl | Citric acid | >85% of Co and Li leached; 1 h leaching at 40 °C | [53] |
| Spent LIBs | Li; Co | ChCl | Urea | 95% of Li and Co 12 h leaching at 180 °C | [54] |
| HBA | |
 tetraalkylammonium chloride | 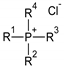 tetraalkylphosphonium chloride |
 Choline chloride (ChCl) | |
| HBD | |
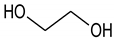 Ethylene glycol (EG) | 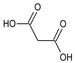 Malonic acid (MA) |
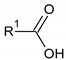 Carboxylic acids | 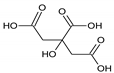 Citric acid |
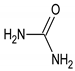 Urea | 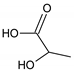 Lactic acid |
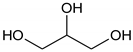 Glycerol |  p-toluenesulfonic acid |
 Oxalic acid | 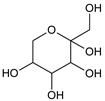 Fructose |
 Sucrose | |
| Purpose of the Study | Metals Investigated | DES | Method Employed | Reference | |
|---|---|---|---|---|---|
| HBA | HBD | ||||
| Understand the behavior of actinide ions in DES media | UO22+ | ChCl | Urea | Voltammetry | [60] |
| Recovery of lead from hybrid organic–inorganic perovskites | Pb | ChCl | EG | Electrodeposition | [61] |
| Removal of Zn from electric arc furnace dust | ZnO; PbO | ChCl | Urea | Electrodeposition | [47] |
| Removal of Zn and Pb from electric arc furnace dust | ZnO; PbO | ChCl | Urea EG | Electrodeposition | [48] |
| Recovery of tungsten and arsenic from secondary mine resource | W; As | ChCl | MA Oxalic acid | Electrodialysis | [62] |
| Target Metals | DES | Reference | |
|---|---|---|---|
| HBA | HBD | ||
| In(III) | Tetraheptylammonium chloride DL-menthol | Dodecanoic acid Decanoic acid Oleic acid Ibuprofen | [39] |
| Cr(VI) | Trioctylmethylammonium chloride | methyl 4-hydroxybenzoate butyl 4-hydroxybenzoate isobutyl 4-hydroxybenzoate n-octyl 4-hydroxybenzoate 2-ethylhexyl 4-hydroxybenzoate | [66] |
| Cr(VI) | Tetrabutylammonium chloride | Decanoic acid | [67] |
| Tc(VII) Re(VII) | Tetra-alkyl ammonium bromides Tetra-alkyl phosphonium chloride | Decanoic acid Hexanoic acid | [68] |
| Alkali and transition metal ions | Lidocaine | Decanoic acid | [69] |
| Fe(III); Mn(II) | Lidocaine | Decanoic acid | [70] |
| Pt(IV); Pd(II); Fe(III) | Trioctylphosphine oxide Thymol Hydrocinnamic acid | Capric acid | [71] |
| Cu(II) | Thymol Menthol | Carboxylic acids | [72] |
| U(VI) | Trioctylphosphine oxide | Phenol | [73] |
| Co(II) | Tetrabutylammonium chloride Trioctylmethylammonium chloride ChCl | Phenol | [74] |
| HBA | |
 DL-menthol | 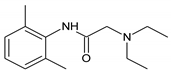 Lidocaine |
 Thymol | 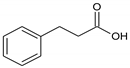 Hydrocinnamic acid |
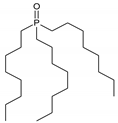 Trioctylphosphine oxide (TOPO) | |
| HBD | |
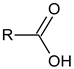 Carboxylic acids (with R = CnH2n+1 and n ≥ 5) 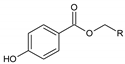 |  Phenol 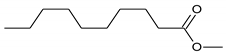 |
| alkyl 4-hydroxybenzoate (with R = alkyl chain) | Capric acid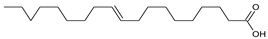 |
| Oleic acid | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zante, G.; Boltoeva, M. Review on Hydrometallurgical Recovery of Metals with Deep Eutectic Solvents. Sustain. Chem. 2020, 1, 238-255. https://doi.org/10.3390/suschem1030016
Zante G, Boltoeva M. Review on Hydrometallurgical Recovery of Metals with Deep Eutectic Solvents. Sustainable Chemistry. 2020; 1(3):238-255. https://doi.org/10.3390/suschem1030016
Chicago/Turabian StyleZante, Guillaume, and Maria Boltoeva. 2020. "Review on Hydrometallurgical Recovery of Metals with Deep Eutectic Solvents" Sustainable Chemistry 1, no. 3: 238-255. https://doi.org/10.3390/suschem1030016
APA StyleZante, G., & Boltoeva, M. (2020). Review on Hydrometallurgical Recovery of Metals with Deep Eutectic Solvents. Sustainable Chemistry, 1(3), 238-255. https://doi.org/10.3390/suschem1030016





