Synthesis of Silica-Based Materials Using Bio-Residues through the Sol-Gel Technique
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Digital Photographs
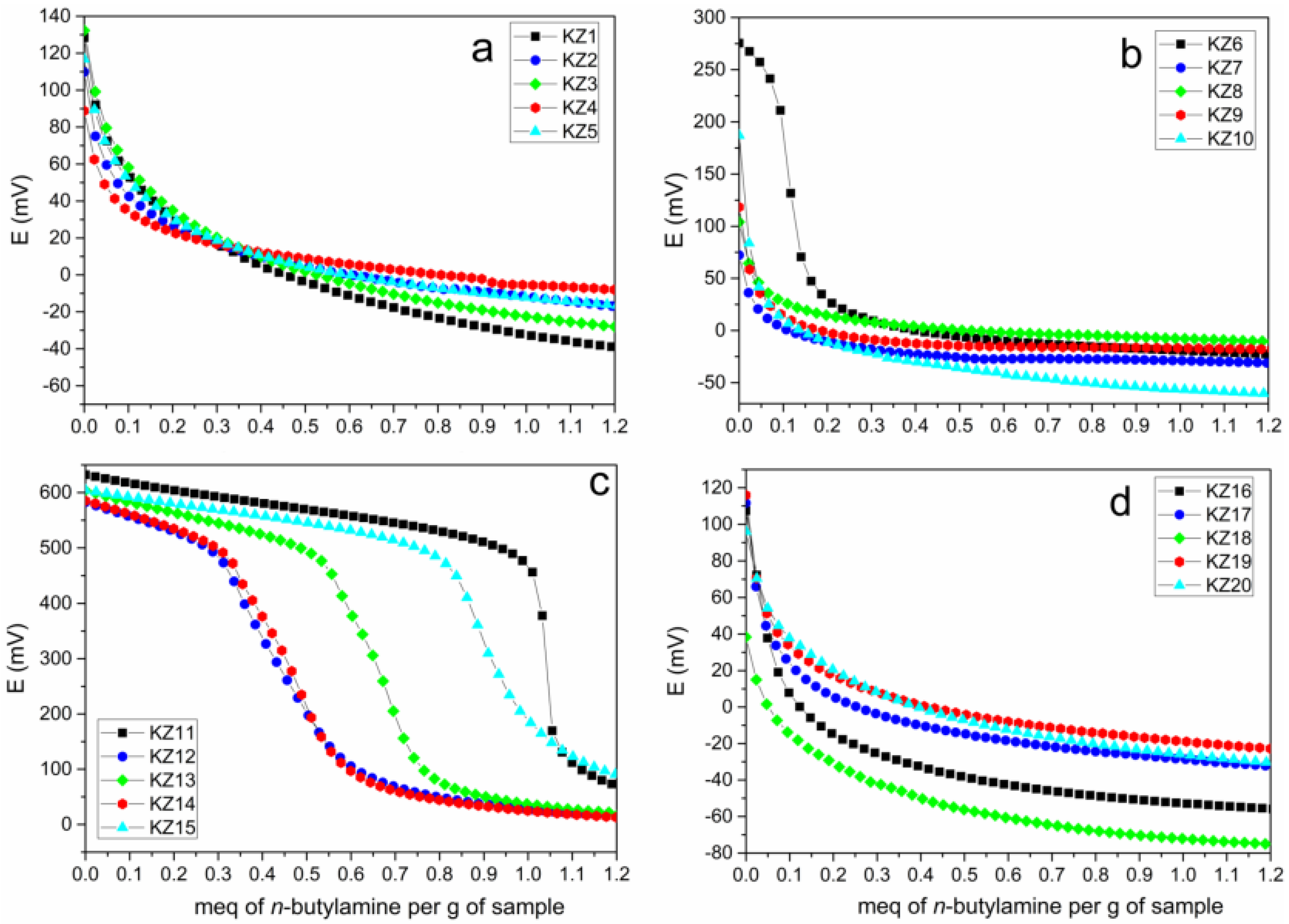
3.2. Potentiometric Titration Characterization
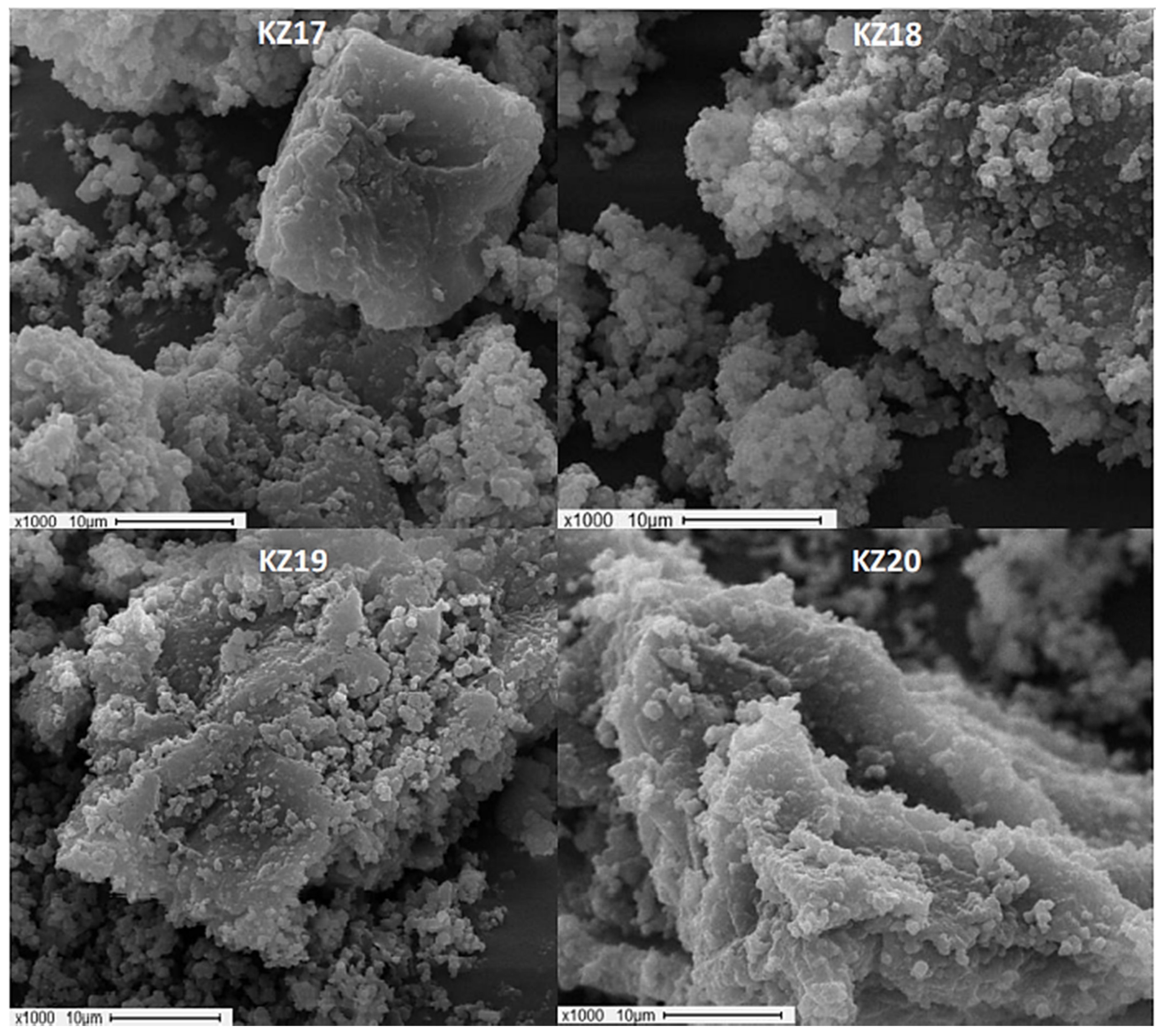
3.3. SEM Characterization
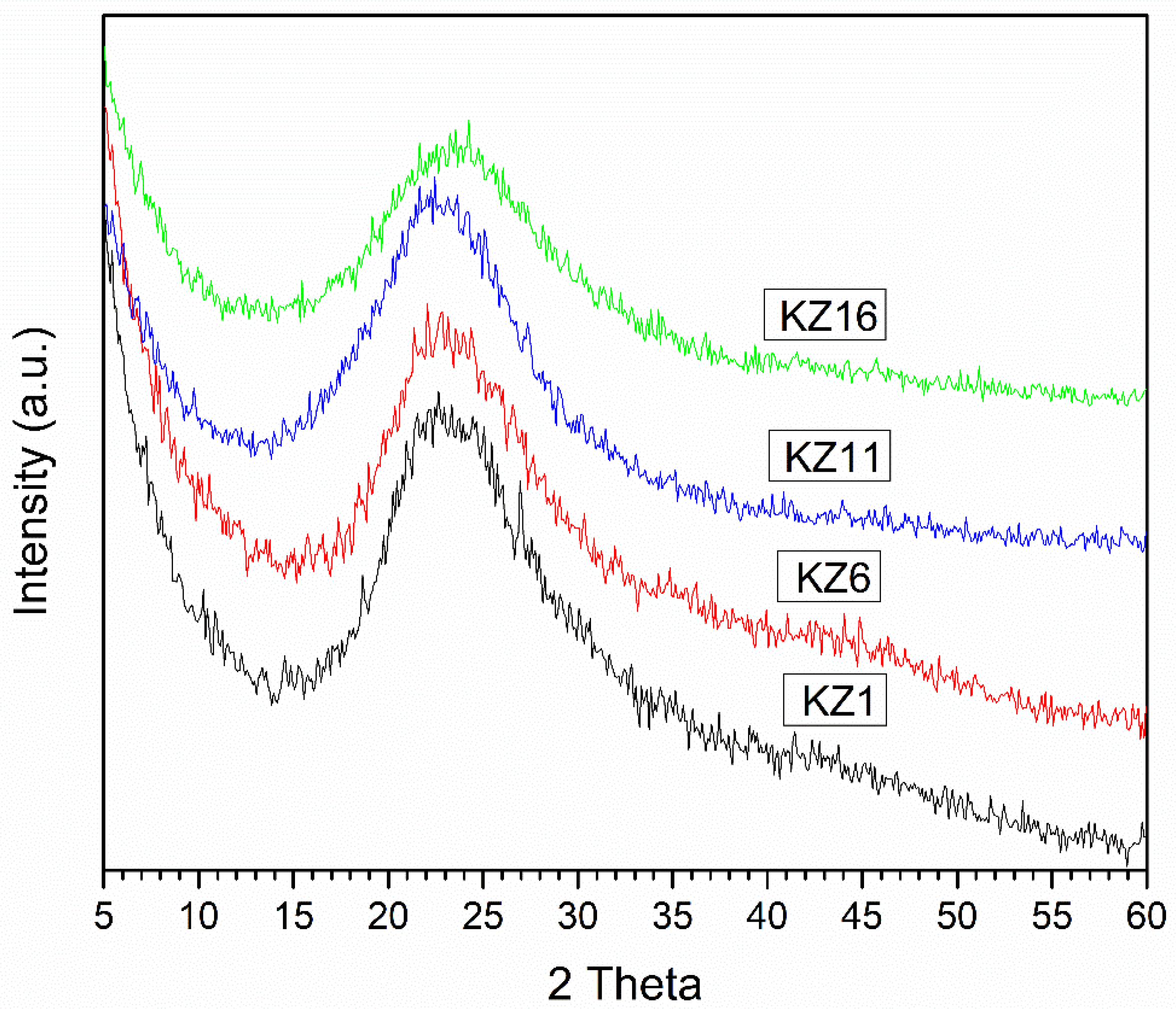
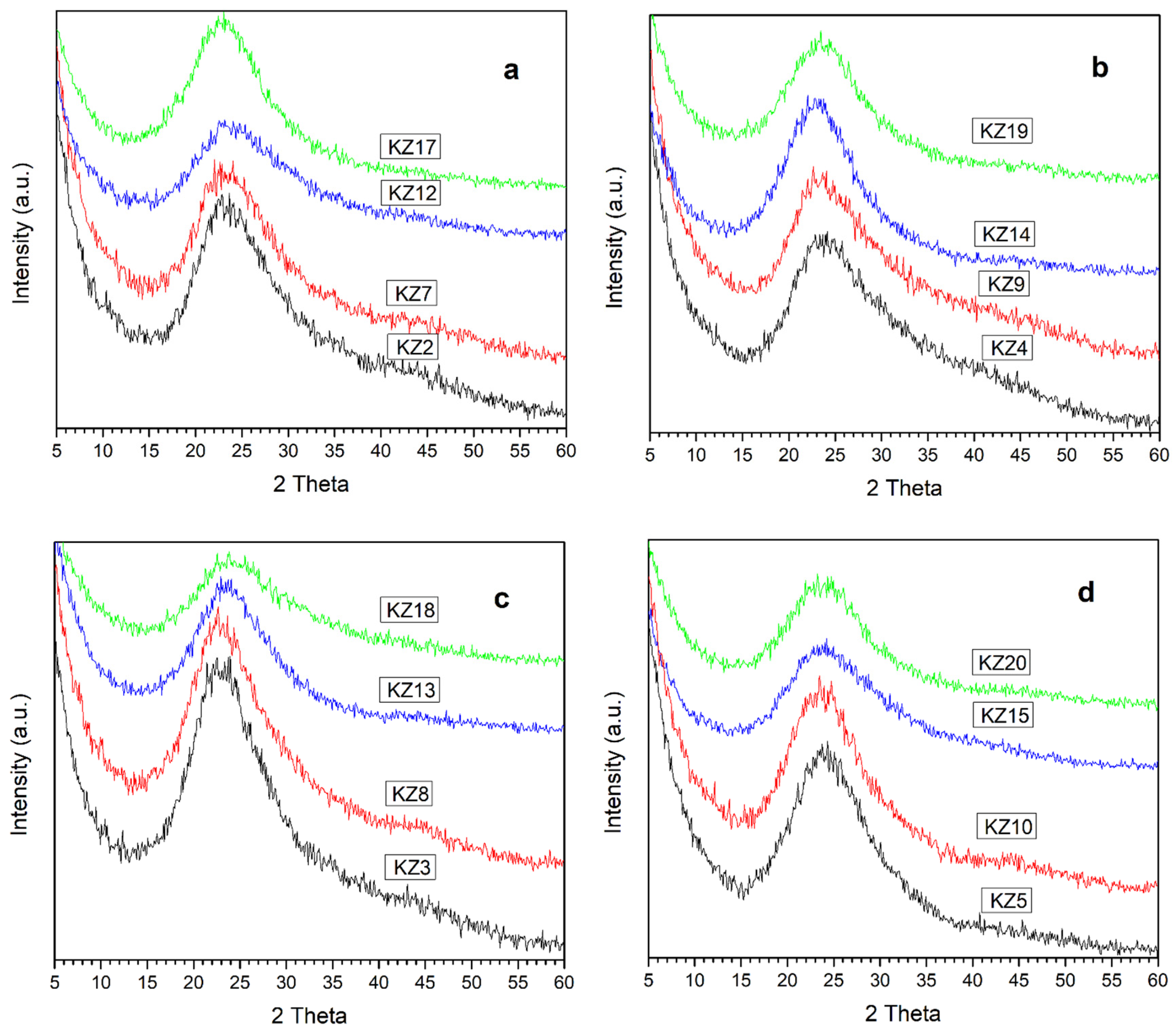
3.4. XRD Characterization
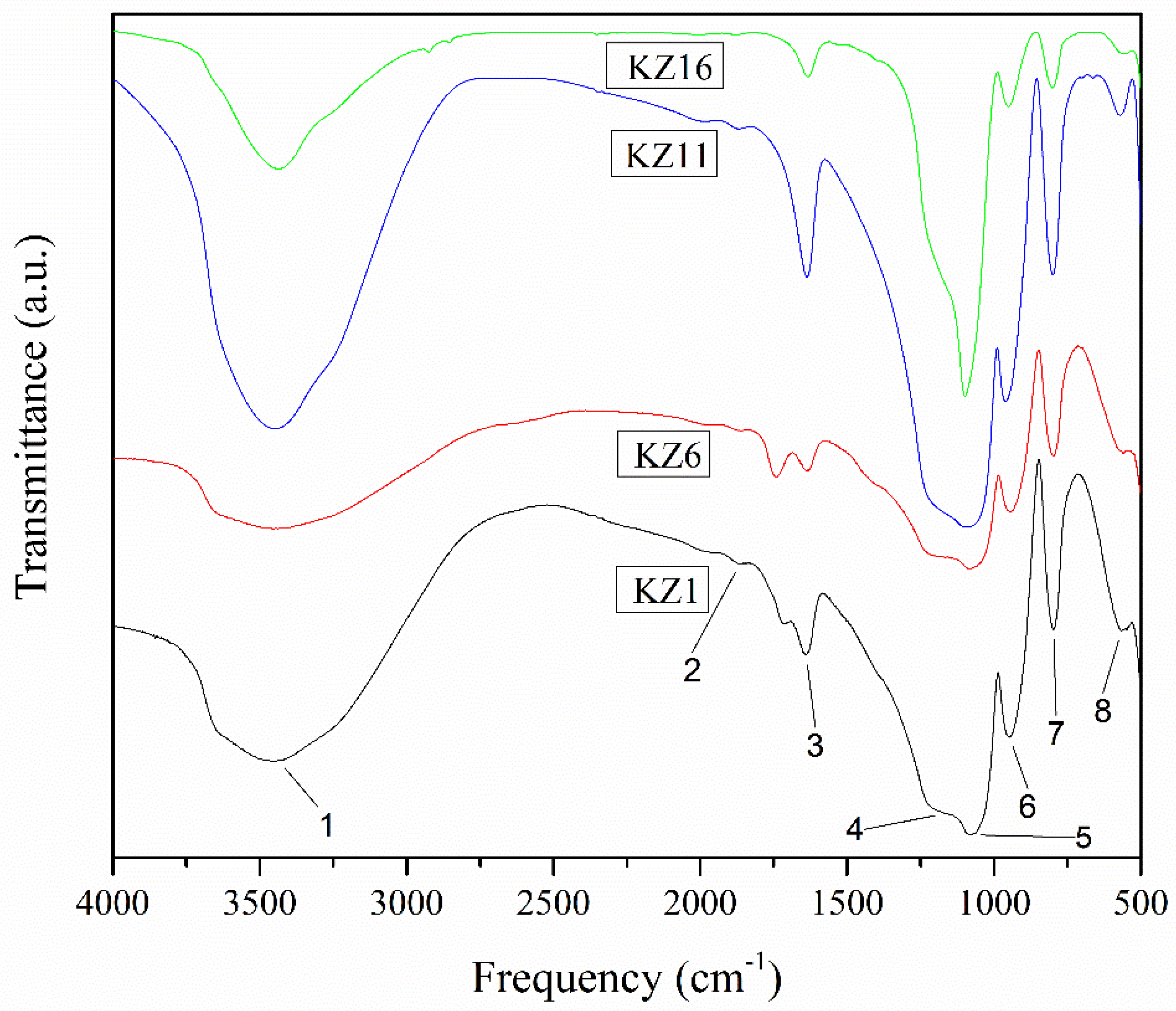
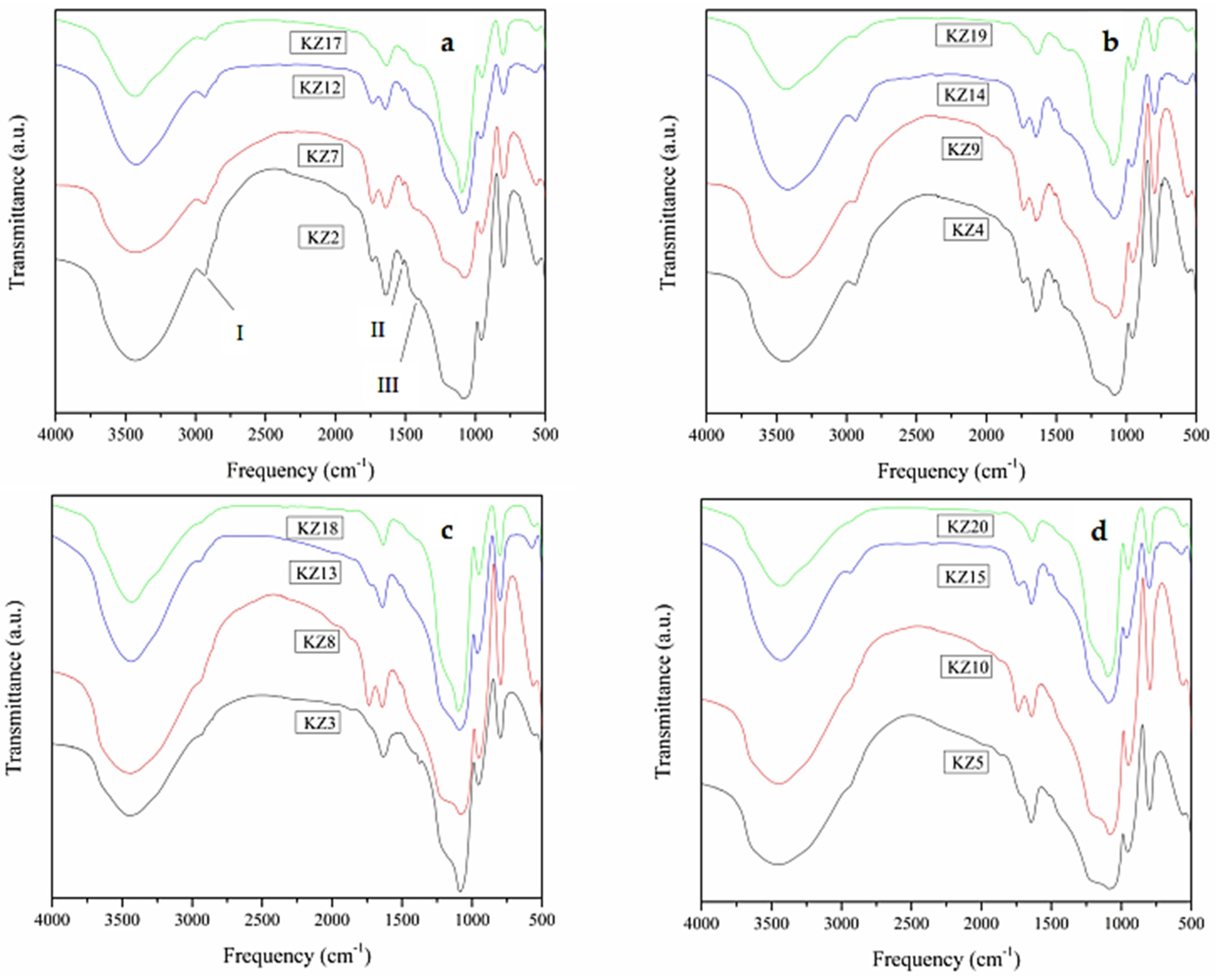
3.5. FT₋IR Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kümmerer, K. Sustainable Chemistry: A Future Guiding Principle. Angew. Chem. Int. Ed. 2017, 56, 16420–16421. [Google Scholar] [CrossRef]
- Kümmerer, K.; Clark, J.H.; Zuin, V.G. Rethinking Chemistry for a Circular Economy. Science 2020, 367, 369–370. [Google Scholar] [CrossRef]
- Zuin, V.G. Circularity in Green Chemical Products, Processes and Services: Innovative Routes Based on Integrated Eco-Design and Solution Systems. Curr. Opin. Green Sustain. Chem. 2016, 2, 40–44. [Google Scholar] [CrossRef]
- Hamilton-Smith, E. Terrestrial Ecoregions of the Indo-Pacific: A Conservation Assessment. Electron. Green J. 2002, 1. [Google Scholar] [CrossRef]
- Satari, B.; Karimi, K. Citrus Processing Wastes: Environmental Impacts, Recent Advances, and Future Perspectives in Total Valorization. Resour. Conserv. Recycl. 2018, 129, 153–167. [Google Scholar] [CrossRef]
- García, R.M.L. La agricultura ecológica en el quehacer científico. Tema incipiente en la geografía. An. Geogr. Univ. Complut. 1999, 19, 351. [Google Scholar]
- DeLind, L.B. Transforming Organic Agriculture into Industrial Organic Products: Reconsidering National Organic Standards. Hum. Organ. 2000, 59, 198–208. [Google Scholar] [CrossRef]
- Planella, J.; Pagès Santacana, A.; Darnell i Vianya, M. Antropologia de l’Educació; Editorial UOC: Barcelona, Spain, 2007; ISBN 978-84-9788-576-8. [Google Scholar]
- Willer, E.H.; Lernoud, J. The World of Organic Agriculture-Statistics and Emerging Trends 2019; Research Institute of Organic Agriculture (FiBL) and IFOAM—Organics International: Frick, Switzerland, 2019; 356p. [Google Scholar]
- Willer, E.H.; Schlatter, B.; Trávní, J.; Kemper, L.; Lernoud, J. The World of Organic Agriculture Statistics and Emerging Trends 2020; Research Institute of Organic Agriculture (FiBL) and IFOAM—Organics International: Frick, Switzerland, 2020; 337p. [Google Scholar]
- Paull, J.; Hennig, B. Atlas of Organics: Four Maps of the World of Organic Agriculture. J. Org. 2016, 3, 25–32. [Google Scholar]
- Sarris, A. Medium-Term Prospects for Agricultural Commodities: Projections to the Year 2010; Food and Agriculture Organization of the United Nations, Ed.; Food and Agriculture Organization of the United Nations: Rome, Italy, 2003; ISBN 978-92-5-105077-4. [Google Scholar]
- Sharma, K.; Mahato, N.; Cho, M.H.; Lee, Y.R. Converting Citrus Wastes into Value-Added Products: Economic and Environmently Friendly Approaches. Nutrition 2017, 34, 29–46. [Google Scholar] [CrossRef]
- Ananthi, A.; Ramesh, D. Preparation and Characterization of Silica Material from Rice Husk Ash-An Economically Viable Method. Chem. Mater. Res. 2016, 8, 1–7. [Google Scholar]
- Shreya, M.K.; Indhumathi, C.; Rajarajeswari, G.R.; Ashokkumar, V.; Preethi, T. Facile Green Route Sol–Gel Synthesis of Nano-Titania Using Bio-Waste Materials as Templates. Clean Technol. Environ. Policy 2021, 23, 163–171. [Google Scholar] [CrossRef]
- Boffa, V.; Perrone, D.G.; Magnacca, G.; Montoneri, E. Role of a Waste-Derived Polymeric Biosurfactant in the Sol–Gel Synthesis of Nanocrystalline Titanium Dioxide. Ceram. Int. 2014, 40, 12161–12169. [Google Scholar] [CrossRef]
- Patel, K.G.; Shettigar, R.R.; Misra, N.M. Recent Advance in Silica Production Technologies from Agricultural Waste Stream–Review. J. Adv. Agric. Technol. 2017, 4, 274–279. [Google Scholar] [CrossRef] [Green Version]
- Srinath, P.; Azeem, P.A.; Reddy, K.V. Sol-Gel Synthesis of SiO2-CaO-Na2O Bio-Ceramics Using Bio-Waste. AIP Conf. Proc. 2020, 2265, 030042. [Google Scholar]
- Silvestri, B.; Vitiello, G.; Luciani, G.; Calcagno, V.; Costantini, A.; Gallo, M.; Parisi, S.; Paladino, S.; Iacomino, M.; D’Errico, G.; et al. Probing the Eumelanin–Silica Interface in Chemically Engineered Bulk Hybrid Nanoparticles for Targeted Subcellular Antioxidant Protection. ACS Appl. Mater. Interfaces 2017, 9, 37615–37622. [Google Scholar] [CrossRef]
- Gonzalo-Juan, I.; Riedel, R. Ceramic Synthesis from Condensed Phases. Chem. Texts 2016, 2, 6. [Google Scholar] [CrossRef] [Green Version]
- Igal, K.; Vázquez, P. Antimicrobial Fabrics Impregnated with Ag Particles Included in Silica Matrices. In Waste in Textile and Leather Sectors; Körlü, A., Ed.; IntechOpen: London, UK, 2020; ISBN 978-1-78985-243-1. [Google Scholar]
- Igal, K.; Arreche, R.A.; Sambeth, J.E.; Bellotti, N.; Vega-Baudrit, J.R.; Redondo-Gómez, C.; Vázquez, P.G. Antifungal Activity of Cotton Fabrics Finished Modified Silica-Silver-Carbon-Based Hybrid Nanoparticles. Text. Res. J. 2019, 89, 825–833. [Google Scholar] [CrossRef]
- Pota, G.; Venezia, V.; Vitiello, G.; Di Donato, P.; Mollo, V.; Costantini, A.; Avossa, J.; Nuzzo, A.; Piccolo, A.; Silvestri, B.; et al. Tuning Functional Behavior of Humic Acids through Interactions with Stöber Silica Nanoparticles. Polymers 2020, 12, 982. [Google Scholar] [CrossRef] [Green Version]
- Migliorero, M.B.C.; Palermo, V.; Vázquez, P.G.; Romanelli, G.P. Valorization of Citrus Waste: Use in Catalysis for the Oxidation of Sulfides. J. Renew. Mater. 2017, 5, 167–173. [Google Scholar] [CrossRef]
- Palermo, V.; Igal, K.; Colombo Migliorero, M.B.; Sathicq, A.G.; Quaranta, N.; Vazquez, P.G.; Romanelli, G.P. Valorization of Different Wastes and Their Use for the Design of Multifunctional Eco-Catalysts. Waste Biomass Valorization 2017, 8, 69–83. [Google Scholar] [CrossRef]
- Yilmaz, E.; Soylak, M. Functionalized Nanomaterials for Sample Preparation Methods. In Handbook of Nanomaterials in Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2020; pp. 375–413. ISBN 978-0-12-816699-4. [Google Scholar]
- Zanella, R. Metodologías Para La Síntesis de Nanopartículas Controlando Forma y Tamaño. Mundo Nano Rev. Interdiscip. En Nanociencia Nanotecnol. 2014, 5, 69–81. [Google Scholar] [CrossRef]
- Brinker, C.J.; Scherer, G.W. Sol.-Gel Science: The Physics and Chemistry of Sol.-Gel Processing; Academic Press, INC: San Diego, CA, USA, 2013; ISBN 978-0-08-057103-4. [Google Scholar]
- Fidalgo, A.; Ilharco, L.M. Correlation between Physical Properties and Structure of Silica Xerogels. J. Non-Cryst. Solids 2004, 347, 128–137. [Google Scholar] [CrossRef]
- Jiménez-González, C.; Curzons, A.D.; Constable, D.J.C.; Cunningham, V.L. Expanding GSK’s Solvent Selection Guide?Application of Life Cycle Assessment to Enhance Solvent Selections. Clean Technol. Environ. Policy 2004, 7, 42–50. [Google Scholar] [CrossRef]
- Cordero Castaño, A.F. Nuevos Materiales Utilizados Para la Obtención de Ecocatalizadores a Partir de Bio-Residuos. Ph.D. Thesis, Universidad Nacional de La Plata, La Plata, Argentina, 2020. [Google Scholar]
- Schmidt, H. New Type of Non-Crystalline Solids between Inorganic and Organic Materials. J. Non-Cryst. Solids 1985, 73, 681–691. [Google Scholar] [CrossRef] [Green Version]
- Mendoza, E.; García, C. Recubrimientos por Sol-Gel Sobre Sustratos de Acero Inoxidable, Revisión del Estado del Arte. Dyna 2007, 74, 101–110. [Google Scholar]
- Matlack, A.S. Introduction to Green Chemistry, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2010; ISBN 978-1-4200-7811-4. [Google Scholar]
- Byrne, F.P.; Jin, S.; Paggiola, G.; Petchey, T.H.M.; Clark, J.H.; Farmer, T.J.; Hunt, A.J.; Robert McElroy, C.; Sherwood, J. Tools and Techniques for Solvent Selection: Green Solvent Selection Guides. Sustain. Chem. Process. 2016, 4, 7. [Google Scholar] [CrossRef] [Green Version]
- Milea, C.A.; Bogatu, C.; Du, A. The Influence of Parameters in Silica Sol-Gel Process. Bull. Transilv. Univ. Brasov. Eng. Sci. Ser. I 2011, 4, 59. [Google Scholar]
- Arreche, R. Inclusión de Ag en Materiales Basados en Sílice y Circonia, Sintetizados Por el Método Sol-Gel, Para Su aplicación Como Aditivos Antimicrobianos en Pinturas. Ph.D. Thesis, Universidad Nacional de La Plata, La Plata, Argentina, 2016. [Google Scholar]
- Igal, K. Telas Antimicrobianas Impregnadas Con Partículas de Ag o Zn Reciclado Incluidas en Matrices Silíceas Modificadas. Ph.D. Thesis, Universidad Nacional de La Plata, La Plata, Argentina, 2019. [Google Scholar]
- Meixner, D.L.; Dyer, P.N. Influence of Sol-Gel Synthesis Parameters on the Microstructure of Particulate Silica Xerogels. J. Sol.-Gel Sci. Technol. 1999, 14, 223–232. [Google Scholar] [CrossRef]
- de Gennes, P.-G. Scaling Concepts in Polymer Physics; Cornell University Press: New York, NY, USA, 1979; ISBN 978-0-8014-1203-5. [Google Scholar]
- Kirkbir, F.; Murata, H.; Meyers, D.; Chaudhuri, S.R.; Sarkar, A. Drying and Sintering of Sol-Gel Derived Large SiO2 Monoliths. J. Sol.-Gel Sci. Technol. 1996, 6, 203–217. [Google Scholar] [CrossRef]
- Soleimani Dorcheh, A.; Abbasi, M.H. Silica Aerogel; Synthesis, Properties and Characterization. J. Mater. Process. Technol. 2008, 199, 10–26. [Google Scholar] [CrossRef]
- Carman, P.C. Constitution of Colloidal Silica. Trans. Faraday Soc. 1940, 36, 964–973. [Google Scholar] [CrossRef]
- Romanelli, G.; Vázquez, P.; Pizzio, L.; Quaranta, N.; Autino, J.; Blanco, M.; Cáceres, C. Phenol Tetrahydropyranylation Catalyzed by Silica-Alumina Supported Heteropolyacids with Keggin Structure. Appl. Catal. Gen. 2004, 261, 163–170. [Google Scholar] [CrossRef]
- Curran, M.D.; Stiegman, A.E. Morphology and Pore Structure of Silica Xerogels Made at Low PH. J. Non-Cryst. Solids 1999, 249, 62–68. [Google Scholar] [CrossRef]
- Zhao, S.; Xu, D.; Ma, H.; Sun, Z.; Guan, J. Controllable Preparation and Formation Mechanism of Monodispersed Silica Particles with Binary Sizes. J. Colloid. Interface Sci. 2012, 388, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Tadanaga, K.; Morita, K.; Mori, K.; Tatsumisago, M. Synthesis of Monodispersed Silica Nanoparticles with High Concentration by the Stöber Process. J. Sol.-Gel Sci. Technol. 2013, 68, 341–345. [Google Scholar] [CrossRef]
- Zarib, N.S.M.; Abdullah, S. Effect of C6H8O7 Concentration on Silica Extraction of Rice Husk, Rice Husk Ash and Mixture of Rice Husk With Rice Husk Ash Via Acid Leaching Process. Int. J. Eng. 2018, 7, 190–195. [Google Scholar]
- Saini, J.; Garg, V.K.; Gupta, R.K. Green Synthesized SiO2@OPW Nanocomposites for Enhanced Lead (II) Removal from Water. Arab. J. Chem. 2020, 13, 2496–2507. [Google Scholar] [CrossRef]
- Cui, J.; Sun, H.; Luo, Z.; Sun, J.; Wen, Z. Preparation of Low Surface Area SiO2 Microsphere from Wheat Husk Ash with a Facile Precipitation Process. Mater. Lett. 2015, 156, 42–45. [Google Scholar] [CrossRef]
- Worathanakul, P.; Trisuwan, D.; Phatruk, A.; Kongkachuichay, P. Effect of Sol–Gel Synthesis Parameters and Cu Loading on the Physicochemical Properties of a New SUZ-4 Zeolite. Colloids Surf. Physicochem. Eng. Asp. 2011, 377, 187–194. [Google Scholar] [CrossRef]
- Czarnobaj, K. Preparation and Characterization of Silica Xerogels as Carriers for Drugs. Drug Deliv. 2008, 15, 485–492. [Google Scholar] [CrossRef]
- Ng, E.-P.; Mohammad, A.G.S.; Rigolet, S.; Daou, T.J.; Mintova, S.; Ling, T.C. Micro-and Macroscopic Observations of the Nucleation Process and Crystal Growth of Nanosized Cs-Pollucite in an Organotemplate-Free Hydrosol. New J. Chem. 2019, 43, 17433–17440. [Google Scholar] [CrossRef]
- Pakizeh, M.; Omidkhah, M.R.; Zarringhalam, A. Synthesis and Characterization of New Silica Membranes Using Template–Sol–Gel Technology. Int. J. Hydrogen Energy 2007, 32, 1825–1836. [Google Scholar] [CrossRef]
- Hu, S.; Hsieh, Y.-L. Preparation of Activated Carbon and Silica Particles from Rice Straw. ACS Sustain. Chem. Eng. 2014, 2, 726–734. [Google Scholar] [CrossRef] [Green Version]
- Kirk, C.T. Quantitative Analysis of the Effect of Disorder-Induced Mode Coupling on Infrared Absorption in Silica. Phys. Rev. B 1988, 38, 1255–1273. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.-F.; Deekomwong, K.; Wittayakun, J.; Ling, T.C.; Muraza, O.; Adam, F.; Ng, E.-P. Crystal Growth Study of K-F Nanozeolite and Its Catalytic Behavior in Aldol Condensation of Benzaldehyde and Heptanal Enhanced by Microwave Heating. Mater. Chem. Phys. 2017, 196, 295–301. [Google Scholar] [CrossRef]
- Araujo-Andrade, C.; Ortega-Zarzosa, G.; Selina, P.; Martinez, J.R.; Villegas-Aguirre, F.; Ruiz, F. Análisis de Las Reacciones de Hidrólisis y Condensación En Muestras de Sílica Xerogeles Usando Espectroscopía Infrarroja. Rev. Mex. Fis. 2000, 46, 593–597. [Google Scholar]
- Orcel, G.; Phalippou, J.; Hench, L.L. Structural Changes of Silica Xerogels during Low Temperature Dehydration. J. Non-Cryst. Solids 1986, 88, 114–130. [Google Scholar] [CrossRef]
- Martınez, J.R.; Ruiz, F. Mapeo estructural de sílica xerogel utilizando espectroscopía infrarroja. Rev. Mex. Fis. 2002, 48, 142–149. [Google Scholar]
- Duran, A.; Serna, C.; Fornes, V.; Fernandez Navarro, J.M. Structural Considerations about SiO2 Glasses Prepared by Sol-Gel. J. Non-Cryst. Solids 1986, 82, 69–77. [Google Scholar] [CrossRef]




| Sample | Precursor | Solvent | Acid | Water | Bio-Waste |
|---|---|---|---|---|---|
| KZ1 | TEOS | EtOH | CH₃COOH | H2O | Pure silica |
| 17.0 mL | 21.8 mL | 5.0 mL | 5.0 mL | 5.0 g | |
| KZ2 | TEOS | EtOH | CH₃COOH | H2O | 50% orange peel |
| 8.5 ml | 10.9 mL | 2.5 mL | 2.5 mL | 2.5 g | |
| KZ3 | TEOS | EtOH | CH₃COOH | H2O | 25% orange peel |
| 12.8 mL | 16.3 mL | 3.75 mL | 3.75 mL | 1.25 g | |
| KZ4 | TEOS | EtOH | CH₃COOH | H2O | 50% lemon peel |
| 8.5 mL | 10.9 mL | 2.5 mL | 2.5 mL | 2.5 g | |
| KZ5 | TEOS | EtOH | CH₃COOH | H2O | 25% lemon peel |
| 12.8 mL | 16.3 mL | 3.75 mL | 3.75 mL | 1.25 g |
| Sample | Precursor | Solvent | Acid | Water | Bio-Waste |
|---|---|---|---|---|---|
| KZ6 | TEOS | EtOH | C6H8O7 | H2O | Pure silica |
| 17.0 mL | 21.8 mL | 0.43 mL | 5.0 mL | 5.0 g | |
| KZ7 | TEOS | EtOH | C6H8O7 | H2O | 50% orange peel |
| 8.5 ml | 10.9 mL | 0.20 mL | 2.5 mL | 2.5 g | |
| KZ8 | TEOS | EtOH | C6H8O7 | H2O | 25% orange peel |
| 12.8 mL | 16.3 mL | 0.30 mL | 3.75 mL | 1.25 g | |
| KZ9 | TEOS | EtOH | C6H8O7 | H2O | 50% lemon peel |
| 8.5 mL | 10.9 mL | 0.20 mL | 2.5 mL | 2.5 g | |
| KZ10 | TEOS | EtOH | C6H8O7 | H2O | 25% lemon peel |
| 12.8 mL | 16.3 mL | 0.30 mL | 3.75 mL | 1.25 g |
| Sample | Precursor | Solvent | Acid | Water | Bio-Waste |
|---|---|---|---|---|---|
| KZ11 | TEOS | EtOH | HCl | H2O | Pure silica |
| 17.0 mL | 21.8 ml | 7.5 mL | 5.0 mL | 5.0 g | |
| KZ12 | TEOS | EtOH | HCl | H2O | 50% orange peel |
| 8.5 ml | 10.9 mL | 3.8 mL | 2.5 mL | 2.5 g | |
| KZ13 | TEOS | EtOH | HCl | H2O | 25% orange peel |
| 12.8 mL | 16.3 mL | 5.6 mL | 3.75 mL | 1.25 g | |
| KZ14 | TEOS | EtOH | HCl | H2O | 50% lemon peel |
| 8.5 mL | 10.9 mL | 3.8 mL | 2.5 mL | 2.5 g | |
| KZ15 | TEOS | EtOH | HCl | H2O | 25% lemon peel |
| 12.8 mL | 16.3 mL | 5.6 mL | 3.75 mL | 1.25 g |
| Sample | Precursor | Solvent | Alkali | Water | Bio-Waste |
|---|---|---|---|---|---|
| KZ16 | TEOS | EtOH | NH4OH | H2O | Pure silica |
| 17.0 mL | 21.8 ml | 11.8 mL | 5.0 mL | 5.0 g | |
| KZ17 | TEOS | EtOH | NH4OH | H2O | 50% orange peel |
| 8.5 ml | 10.9 mL | 5.9 mL | 2.5 mL | 2.5 g | |
| KZ18 | TEOS | EtOH | NH4OH | H2O | 25% orange peel |
| 12.8 mL | 16.3 mL | 8.8 mL | 3.75 mL | 1.25 g | |
| KZ19 | TEOS | EtOH | NH4OH | H2O | 50% lemon peel |
| 8.5 mL | 10.9 mL | 5.9 mL | 2.5 mL | 2.5 g | |
| KZ20 | TEOS | EtOH | NH4OH | H2O | 25% lemon peel |
| 12.8 mL | 16.3 mL | 8.8 mL | 3.75 mL | 1.25 g |
| Sample | Ei (mV) | Sample | Ei (mV) |
|---|---|---|---|
| KZ1 | 128 | KZ11 | 632 |
| KZ2 | 110 | KZ12 | 582 |
| KZ3 | 132 | KZ13 | 604 |
| KZ4 | 89 | KZ14 | 585 |
| KZ5 | 117 | KZ15 | 604 |
| KZ6 | 275 | KZ16 | 107 |
| KZ7 | 72 | KZ17 | 112 |
| KZ8 | 104 | KZ18 | 38 |
| KZ9 | 118 | KZ19 | 116 |
| KZ10 | 187 | KZ20 | 96 |
| Bands | Wavenumber (cm−1) | Band Assignment | Functional Group |
|---|---|---|---|
| 1 | 3437 | ν OH 1 | O-H bonded |
| 2 | 1864 | νβ (Si-O) 2 | Si-OH |
| 3 | 1639 | δ H-O-H 3 | H-O-H |
| 4 | 1166 | νS (Si-O-Si) 4 | Si-O-Si |
| 5 | 1079 | νa (Si-O-Si) 5 | Si-O-Si |
| 6 | 948 | νβ (Si-O) 2 | Si-OH |
| 7 | 797 | νs (Si-O) 6 | Si-O-Si |
| 8 | 566 | ν (Si-O) 1 | SiO2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zanotti, K.; Igal, K.; Colombo Migliorero, M.B.; Gomes Zuin, V.; Vázquez, P.G. Synthesis of Silica-Based Materials Using Bio-Residues through the Sol-Gel Technique. Sustain. Chem. 2021, 2, 670-685. https://doi.org/10.3390/suschem2040037
Zanotti K, Igal K, Colombo Migliorero MB, Gomes Zuin V, Vázquez PG. Synthesis of Silica-Based Materials Using Bio-Residues through the Sol-Gel Technique. Sustainable Chemistry. 2021; 2(4):670-685. https://doi.org/10.3390/suschem2040037
Chicago/Turabian StyleZanotti, Karine, Katerine Igal, María Belen Colombo Migliorero, Vânia Gomes Zuin, and Patricia Graciela Vázquez. 2021. "Synthesis of Silica-Based Materials Using Bio-Residues through the Sol-Gel Technique" Sustainable Chemistry 2, no. 4: 670-685. https://doi.org/10.3390/suschem2040037
APA StyleZanotti, K., Igal, K., Colombo Migliorero, M. B., Gomes Zuin, V., & Vázquez, P. G. (2021). Synthesis of Silica-Based Materials Using Bio-Residues through the Sol-Gel Technique. Sustainable Chemistry, 2(4), 670-685. https://doi.org/10.3390/suschem2040037







