Technospheric Mining of Mine Wastes: A Review of Applications and Challenges
Abstract
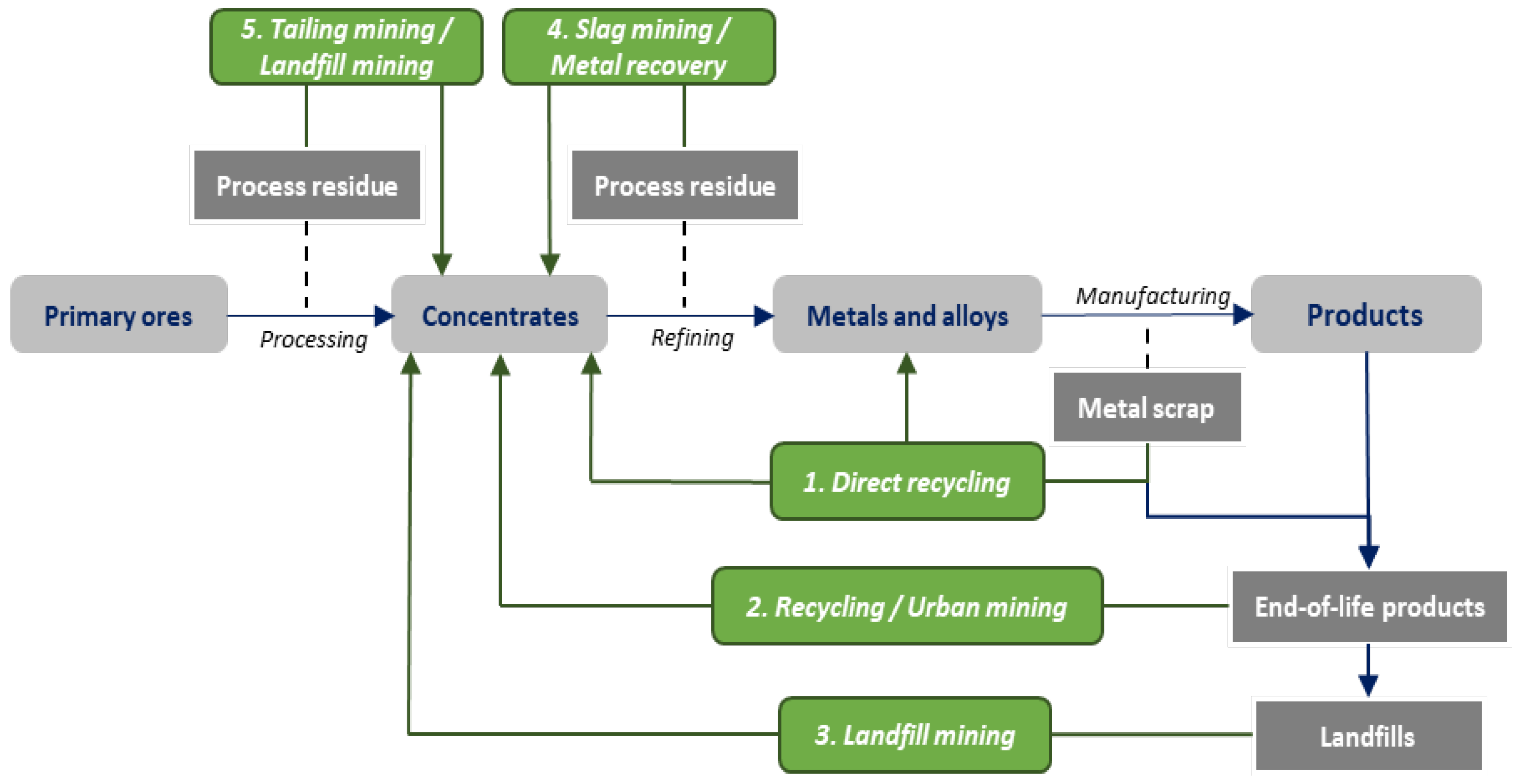
:1. Introduction
2. Technosphere
3. Technospheric Mining
3.1. Metal Recovery
3.1.1. Hydrometallurgical Residue
3.1.2. Pyrometallurgical By-Products
3.1.3. Mine Tailings
3.2. Mineral Recovery
4. Challenges and Future Perspectives
4.1. Technological Development and Data Management
4.2. Eco-Efficiency
4.3. Governance
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Johansson, N.; Krook, J.; Eklund, M.; Berglund, B. An integrated review of concepts and initiatives for mining the technosphere: Towards a new taxonomy. J. Clean. Prod. 2013, 55, 35–44. [Google Scholar] [CrossRef] [Green Version]
- Guo, R.; Lv, S.; Liao, T.; Xi, F.; Zhang, J.; Zuo, X.; Cao, X.; Feng, Z.; Zhang, Y. Classifying green technologies for sustainable innovation and investment. Resour. Conserv. Recycl. 2020, 153, 104580. [Google Scholar] [CrossRef]
- OECD. Mining and Green Growth in the EECCA Region. 2019. Available online: https://www.oecd-ilibrary.org/environment/mining-and-green-growth-in-the-eecca-region_1926a45a-en (accessed on 14 February 2020).
- Hudson-Edwards, K.A.; Jamieson, H.E.; Lottermoser, B.G. Mine wastes: Past, present, future. Elements 2011, 7, 375–380. [Google Scholar] [CrossRef]
- Lèbre, É.; Corder, G.D.; Golev, A. Sustainable practices in the management of mining waste: A focus on the mineral resource. Miner. Eng. 2017, 107, 34–42. [Google Scholar] [CrossRef]
- Tayebi-Khorami, M.; Edraki, M.; Corder, G.; Golev, A. Re-thinking mining waste through an integrative approach led by circular economy aspirations. Minerals 2019, 9, 286. [Google Scholar] [CrossRef] [Green Version]
- Worrall, R.; Neil, D.; Brereton, D.; Mulligan, D. Towards a sustainability criteria and indicators framework for legacy mine land. J. Clean. Prod. 2009, 17, 1426–1434. [Google Scholar] [CrossRef]
- Lottermoser, B.G. Recycling, reuse and rehabilitation of mine wastes. Elements 2011, 7, 405–410. [Google Scholar] [CrossRef]
- Binnemans, K.; Jones, P.T.; Blanpain, B.; Van Gerven, T.; Pontikes, Y. Towards zero-waste valorisation of rare-earth-containing industrial process residues: A critical review. J. Clean. Prod. 2015, 99, 17–38. [Google Scholar] [CrossRef] [Green Version]
- Alkan, G.; Yagmurlu, B.; Cakmakoglu, S.; Hertel, T.; Kaya, Ş.; Gronen, L.; Stopic, S.; Friedrich, B. Novel approach for enhanced scandium and titanium leaching efficiency from bauxite residue with suppressed silica gel formation. Sci. Rep. 2018, 8, 5676. [Google Scholar] [CrossRef] [PubMed]
- Araya, N.; Kraslawski, A.; Cisternas, L.A. Towards mine tailings valorization: Recovery of critical materials from Chilean mine tailings. J. Clean. Prod. 2020, 263, 121555. [Google Scholar] [CrossRef]
- Borra, C.R.; Blanpain, B.; Pontikes, Y.; Binnemans, K.; Van Gerven, T. Recovery of rare earths and other valuable metals from bauxite residue (Red Mud): A Review. J. Sustain. Metall. 2016, 2, 365–386. [Google Scholar] [CrossRef]
- Falagán, C.; Grail, B.M.; Johnson, D.B. New approaches for extracting and recovering metals from mine tailings. Miner. Eng. 2017, 106, 71–78. [Google Scholar] [CrossRef]
- Gaballah, I.; Allain, E.; Djona, M. Extraction of tantalum and niobium from tin slags by chlorination and carbochlorination. Metall. Mater. Trans. B 1997, 28, 359–369. [Google Scholar] [CrossRef]
- Etxeberria, M.; Pacheco, C.; Meneses, J.M.; Berridi, I. Properties of concrete using metallurgical industrial by-products as aggregates. Constr. Build. Mater. 2010, 24, 1594–1600. [Google Scholar] [CrossRef]
- Kresta, F. Utilisation of metallurgical by-products in road construction in the Czech Republic. IOP Conf. Ser. Mater. Sci. Eng. 2017, 236, 012090. [Google Scholar] [CrossRef] [Green Version]
- Kiventerä, J.; Golek, L.; Yliniemi, J.; Ferreira, V.; Deja, J.; Illikainen, M. Utilization of sulphidic tailings from gold mine as a raw material in geopolymerization. Int. J. Miner. Process. 2016, 149, 104–110. [Google Scholar] [CrossRef]
- Yang, T.; Yao, X.; Zhang, Z. Geopolymer prepared with high-magnesium nickel slag: Characterization of properties and microstructure. Constr. Build. Mater. 2014, 59, 188–194. [Google Scholar] [CrossRef]
- Barca, C.; Scanu, D.; Podda, N.; Miche, H.; Poizat, L.; Hennebert, P. Phosphorus removal from wastewater by carbonated bauxite residue under aerobic and anoxic conditions. J. Water Process. Eng. 2021, 39, 101757. [Google Scholar] [CrossRef]
- Liu, L.; Du, T.; Li, G.; Yang, F.; Che, S. Using one waste to tackle another: Preparation of a CO2 capture material zeolite X from laterite residue and bauxite. J. Hazard. Mater. 2014, 278, 551–558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hitch, M.; Ballantyne, S.M.; Hindle, S.R. Revaluing mine waste rock for carbon capture and storage. Int. J. Min. Reclam. Environ. 2010, 24, 64–79. [Google Scholar] [CrossRef] [Green Version]
- Palm, V.; Östlund, C. Lead and zinc flows from technosphere to biosphere in a city region. Sci. Total Environ. 1996, 192, 95–109. [Google Scholar] [CrossRef]
- Hofstetter, P. Perspectives in Life Cycle Impact Assessment: A Structured Approach to Combine Models of the Technosphere, Ecosphere, and Valuesphere; Springer Science & Business Media: Zurich, Switzerland, 1998. [Google Scholar]
- Karlsson, S. Closing the technospheric flows of toxic metals: Modeling lead losses from a lead-acid battery system for Sweden. J. Ind. Ecol. 1999, 3, 23–40. [Google Scholar] [CrossRef]
- Haff, P.K. Technology as a geological phenomenon: Implications for human well-being. Geol. Soc. Lond. Spec. Publ. 2014, 395, 301–309. [Google Scholar] [CrossRef]
- Mendes, J.R. Does the sustainability of the anthropocene technosphere imply an existential risk for our species? Thinking with Peter Haff. Soc. Sci. 2021, 10, 314. [Google Scholar] [CrossRef]
- Zalasiewicz, J.; Williams, M.; Waters, C.N.; Barnosky, A.D.; Palmesino, J.; Rönnskog, A.-S.; Edgeworth, M.; Neal, C.; Cearreta, A.; Ellis, E.C. Scale and diversity of the physical technosphere: A geological perspective. Anthr. Rev. 2017, 4, 9–22. [Google Scholar] [CrossRef]
- Sonderegger, T.; Berger, M.; Alvarenga, R.; Bach, V.; Cimprich, A.; Dewulf, J.; Frischknecht, R.; Guinée, J.; Helbig, C.; Huppertz, T.; et al. Mineral resources in life cycle impact assessment—Part I: A critical review of existing methods. Int. J. Life Cycle Assess. 2020, 25, 784–797. [Google Scholar] [CrossRef]
- Jones, R.; Denton, G.; Reynolds, Q.G.; Parker, J.A.L.; Van Tonder, G.J.J. Recovery of cobalt from slag in a DC arc furnace at Chambishi, Zambia. J. S. Afr. Inst. Min. Metall. 2002, 102, 5–9. [Google Scholar]
- Shen, H.; Forssberg, E. An overview of recovery of metals from slags. Waste Manag. 2003, 23, 933–949. [Google Scholar] [CrossRef]
- Gbor, P.K.; Mokri, V.; Jia, C.Q. Characterization of smelter slags. J. Environ. Sci. Health Part A 2000, 35, 147–167. [Google Scholar] [CrossRef]
- Rozendaal, A.; Horn, R. Textural, mineralogical and chemical characteristics of copper reverb furnace smelter slag of the Okiep Copper District, South Africa. Miner. Eng. 2013, 52, 184–190. [Google Scholar] [CrossRef]
- Krook, J.; Baas, L. Getting serious about mining the technosphere: A review of recent landfill mining and urban mining research. J. Clean. Prod. 2013, 55, 1–9. [Google Scholar] [CrossRef]
- Reid, S.; Tam, J.; Yang, M.; Azimi, G. Technospheric mining of rare earth elements from bauxite residue (red mud): Process optimization, kinetic investigation, and microwave pretreatment. Sci. Rep. 2017, 7, 15252. [Google Scholar] [CrossRef] [Green Version]
- Bonomi, C.; Cardenia, C.; Yin, P.T.W.; Panias, D. Review of technologies in the recovery of iron, aluminium, titanium and rare earth elements from bauxite residue (red mud). In Proceedings of the International Symposium on Enhanced Landfill Mining, Lisboa, Portugal, 8–10 February 2016. [Google Scholar]
- Sapsford, D.; Cleall, P.; Harbottle, M. In situ resource recovery from waste repositories: Exploring the potential for mobilization and capture of metals from anthropogenic ores. J. Sustain. Metall. 2017, 3, 375–392. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Azimi, G. Technospheric mining of niobium and titanium from electric arc furnace slag. Hydrometallurgy 2020, 191, 105203. [Google Scholar] [CrossRef]
- Kapur, A.; Graedel, T.E. Copper Mines above and below the Ground; ACS Publications Environmental Science & Technology: Washington, DC, USA, 2006. [Google Scholar]
- Suppes, R.; Heuss-Aßbichler, S. Resource potential of mine wastes: A conventional and sustainable perspective on a case study tailings mining project. J. Clean. Prod. 2021, 297, 126446. [Google Scholar] [CrossRef]
- Tost, M.; Hitch, M.; Chandurkar, V.; Moser, P.; Feiel, S. The state of environmental sustainability considerations in mining. J. Clean. Prod. 2018, 182, 969–977. [Google Scholar] [CrossRef]
- Tunsu, C.; Menard, Y.; Eriksen, D.Ø.; Ekberg, C.; Petranikova, M. Recovery of critical materials from mine tailings: A comparative study of the solvent extraction of rare earths using acidic, solvating and mixed extractant systems. J. Clean. Prod. 2019, 218, 425–437. [Google Scholar] [CrossRef]
- Ujaczki, É.; Feigl, V.; Molnár, M.; Cusack, P.; Curtin, T.; Courtney, R.; O’Donoghue, L.; Davris, P.; Hugi, C.; Evangelou, M.W. Re-using bauxite residues: Benefits beyond (critical raw) material recovery. J. Chem. Technol. Biotechnol. 2018, 93, 2498–2510. [Google Scholar] [CrossRef] [Green Version]
- Gaballah, I.; Allain, E.; Meyer-Joly, M.-C.; Malau, K. A possible method for the characterization of amorphous slags: Recovery of refractory metal oxides from tin slags. Metall. Mater. Trans. B 1992, 23, 249–259. [Google Scholar] [CrossRef]
- Gbor, P.K.; Ahmed, I.B.; Jia, C.Q. Behaviour of Co and Ni during aqueous sulphur dioxide leaching of nickel smelter slag. Hydrometallurgy 2000, 57, 13–22. [Google Scholar] [CrossRef]
- Kossoff, D.; Dubbin, W.; Alfredsson, M.; Edwards, S.; Macklin, M.; Hudson-Edwards, K.A. Mine tailings dams: Characteristics, failure, environmental impacts, and remediation. Appl. Geochem. 2014, 51, 229–245. [Google Scholar] [CrossRef] [Green Version]
- Kinnunen, P.H.-M.; Kaksonen, A.H. Towards circular economy in mining: Opportunities and bottlenecks for tailings valorization. J. Clean. Prod. 2019, 228, 153–160. [Google Scholar] [CrossRef]
- Bellenfant, G.; Guezennec, A.-G.; Bodénan, F.; d’Hugues, P.; Cassard, D. Reprocessing of Mining Waste: Combining Environmental Management and Metal Recovery? Proceedings of the Eighth International Seminar on Mine Closure, Cornwall, UK, 18–20 September 2013; The Australian Centre for Geomechanics: Crawley, WA, Australia, 2013. [Google Scholar]
- Petrunic, B.M.; Al, T.A.; Weaver, L.; Hall, D. Identification and characterization of secondary minerals formed in tungsten mine tailings using transmission electron microscopy. Appl. Geochem. 2009, 24, 2222–2233. [Google Scholar] [CrossRef]
- Li, C.; Sun, H.; Bai, J.; Li, L. Innovative methodology for comprehensive utilization of iron ore tailings: Part 2: The residues after iron recovery from iron ore tailings to prepare cementitious material. J. Hazard. Mater. 2010, 174, 78–83. [Google Scholar] [CrossRef]
- Peelman, S.; Kooijman, D.; Sietsma, J.; Yang, Y. Hydrometallurgical recovery of rare earth elements from mine tailings and WEEE. J. Sustain. Metall. 2018, 4, 367–377. [Google Scholar] [CrossRef] [Green Version]
- Peiravi, M.; Dehghani, F.; Ackah, L.; Baharlouei, A.; Godbold, J.; Liu, J.; Mohanty, M.; Ghosh, T. A review of rare-earth elements extraction with emphasis on non-conventional sources: Coal and coal byproducts, iron ore tailings, apatite, and phosphate byproducts. Min. Metall. Explor. 2020, 38, 1–26. [Google Scholar] [CrossRef]
- Lei, C.; Yan, B.; Chen, T.; Xiao, X.-M. Recovery of metals from the roasted lead-zinc tailings by magnetizing roasting followed by magnetic separation. J. Clean. Prod. 2017, 158, 73–80. [Google Scholar] [CrossRef]
- Stamboliadis, E.; Alevizos, G.; Zafiratos, J. Leaching residue of nickeliferous laterites as a source of iron concentrate. Miner. Eng. 2004, 17, 245–252. [Google Scholar] [CrossRef]
- Ettler, V.; Kvapil, J.; Šebek, O.; Johan, Z.; Mihaljevič, M.; Ratié, G.; Garnier, J.; Quantin, C. Leaching behaviour of slag and fly ash from laterite nickel ore smelting (Niquelândia, Brazil). Appl. Geochem. 2016, 64, 118–127. [Google Scholar] [CrossRef]
- Bertocchi, A.F.; Ghiani, M.; Peretti, R.; Zucca, A. Red mud and fly ash for remediation of mine sites contaminated with As, Cd, Cu, Pb and Zn. J. Hazard. Mater. 2006, 134, 112–119. [Google Scholar] [CrossRef]
- Gentzmann, M.C.; Schraut, K.; Vogel, C.; Gäbler, H.-E.; Huthwelker, T.; Adam, C. Investigation of scandium in bauxite residues of different origin. Appl. Geochem. 2021, 126, 104898. [Google Scholar] [CrossRef]
- Li, G.; Ye, Q.; Deng, B.; Luo, J.; Rao, M.; Peng, Z.; Jiang, T. Extraction of scandium from scandium-rich material derived from bauxite ore residues. Hydrometallurgy 2018, 176, 62–68. [Google Scholar] [CrossRef]
- Gräfe, M.; Power, G.; Klauber, C. Bauxite residue issues: III. Alkalinity and associated chemistry. Hydrometallurgy 2011, 108, 60–79. [Google Scholar] [CrossRef]
- Meyer, F. Availability of bauxite reserves. Nat. Resour. Res. 2004, 13, 161–172. [Google Scholar] [CrossRef] [Green Version]
- Zarasvandi, A.; Charchi, A.; Carranza, E.J.M.; Alizadeh, B. Karst bauxite deposits in the Zagros Mountain Belt, Iran. Ore Geol. Rev. 2008, 34, 521–532. [Google Scholar] [CrossRef]
- Gu, J.; Huang, Z.; Fan, H.; Jin, Z.; Yan, Z.; Zhang, J. Mineralogy, geochemistry, and genesis of lateritic bauxite deposits in the Wuchuan–Zheng’an–Daozhen area, Northern Guizhou Province, China. J. Geochem. Explor. 2013, 130, 44–59. [Google Scholar] [CrossRef]
- Ochsenkühn-Petropulu, M.; Lyberopulu, T.; Ochsenkühn, K.M.; Parissakis, G. Recovery of lanthanides and yttrium from red mud by selective leaching. Anal. Chim. Acta 1996, 319, 249–254. [Google Scholar] [CrossRef]
- Liu, Z.; Zong, Y.; Li, H.; Jia, D.; Zhao, Z. Selectively recovering scandium from high alkali Bayer red mud without impurities of iron, titanium and gallium. J. Rare Earths 2017, 35, 896–905. [Google Scholar] [CrossRef]
- Rychkov, V.; Botalov, M.; Kirillov, E.; Kirillov, S.; Semenishchev, V.; Bunkov, G.; Smyshlyaev, D. Intensification of carbonate scandium leaching from red mud (bauxite residue). Hydrometallurgy 2021, 199, 105524. [Google Scholar] [CrossRef]
- Ghiat, I.; Al-Ansari, T. A review of carbon capture and utilisation as a CO2 abatement opportunity within the EWF nexus. J. CO2 Util. 2021, 45, 101432. [Google Scholar] [CrossRef]
- Miganei, L.; Gock, E.; Achimovičová, M.; Koch, L.; Zobel, H.; Kähler, J. New residue-free processing of copper slag from smelter. J. Clean. Prod. 2017, 164, 534–542. [Google Scholar] [CrossRef]
- Gorai, B.; Jana, R.K. Characteristics and utilisation of copper slag—A review. Resour. Conserv. Recycl. 2003, 39, 299–313. [Google Scholar] [CrossRef]
- Wang, G.C. 3-Nonferrous metal extraction and nonferrous slags. In The Utilization of Slag in Civil Infrastructure Construction; Wang, G.C., Ed.; Woodhead Publishing: Sawston, UK, 2016; pp. 35–61. [Google Scholar]
- Dutta, S.K.; Lodhari, D.R. Extraction of Nuclear and Non-Ferrous Metals; Springer: Singapore, 2018; pp. 149–154. [Google Scholar] [CrossRef]
- Allain, E.; Kanari, N.; Diot, F.; Yvon, J. Development of a process for the concentration of the strategic tantalum and niobium oxides from tin slags. Miner. Eng. 2019, 134, 97–103. [Google Scholar] [CrossRef] [Green Version]
- Gaballah, I.; Allain, E. Recycling of strategic metals from industrial slag by a hydro-and pyrometallurgical process. Resour. Conserv. Recycl. 1994, 10, 75–85. [Google Scholar] [CrossRef]
- Fetherston, J.M. Tantalum in western Australia. In Mineral Resources Bulletin; Geological Survey of Western Australia: Perth, WA, Australia, 2004; p. 162. [Google Scholar]
- Li, H.; Peng, J.; Long, H.; Li, S.; Zhang, L. Cleaner process: Efficacy of chlorine in the recycling of gold from gold-containing tailings. J. Clean. Prod. 2021, 287, 125066. [Google Scholar] [CrossRef]
- Borra, C.R.; Mermans, J.; Blanpain, B.; Pontikes, Y.; Binnemans, K.; Van Gerven, T. Selective recovery of rare earths from bauxite residue by combination of sulfation, roasting and leaching. Miner. Eng. 2016, 92, 151–159. [Google Scholar] [CrossRef]
- Carvalho, J.; Diamantino, C.; Rosa, C.; Carvalho, E. Potential recovery of mineral resources from mining tailing of abandoned mines in Portugal. In Proceedings of the 3rd International Symposium on Enhanced Landfill Mining, Lisbon, Portugal, 8–10 February 2016; pp. 8–10. [Google Scholar]
- Li, L.; Ge, J.; Wu, F.; Chen, R.; Chen, S.; Wu, B. Recovery of cobalt and lithium from spent lithium ion batteries using organic citric acid as leachant. J. Hazard. Mater. 2010, 176, 288–293. [Google Scholar] [CrossRef]
- Yang, H.; Jing, L.; Zhang, B. Recovery of iron from vanadium tailings with coal-based direct reduction followed by magnetic separation. J. Hazard. Mater. 2011, 185, 1405–1411. [Google Scholar] [CrossRef]
- Guo-dong, Z.; Qing, L. Leaching of copper from tailings using ammonia/ammonium chloride solution and its dynamics. In Proceedings of the International Conference on Chemistry and Chemical Engineering (ICCCE), Kyoto, Japan, 1–3 August 2010. [Google Scholar]
- Klauber, C.; Gräfe, M.; Power, G. Bauxite residue issues: II. options for residue utilization. Hydrometallurgy 2011, 108, 11–32. [Google Scholar] [CrossRef]
- Dhir, R.K.; de Brito, J.; Mangabhai, R.; Lye, C.Q. Sustainable Construction Materials: Copper Slag; Woodhead Publishing: Sawston, UK, 2017. [Google Scholar]
- Xu, D.; Yang, S.; Su, Y.; Xiong, Y.; Zhang, S. Catalytic conversion of plastic wastes using cost-effective bauxite residue as catalyst into H2-rich syngas and magnetic nanocomposites for chrome (VI) detoxification. J. Hazard. Mater. 2021, 413, 125289. [Google Scholar] [CrossRef] [PubMed]
- Pacheco-Torgal, F.; Castro-Gomes, J.P.; Jalali, S. Investigations of tungsten mine waste geopolymeric binder: Strength and microstructure. Constr. Build. Mater. 2008, 22, 2212–2219. [Google Scholar] [CrossRef] [Green Version]
- Fang, Y.; Gu, Y.; Kang, Q.; Wen, Q.; Dai, P. Utilization of copper tailing for autoclaved sand–lime brick. Constr. Build. Mater. 2011, 25, 867–872. [Google Scholar] [CrossRef]
- Thomas, B.S.; Damare, A.; Gupta, R.C. Strength and durability characteristics of copper tailing concrete. Constr. Build. Mater. 2013, 48, 894–900. [Google Scholar] [CrossRef]
- Peng, K.; Yang, H.; Ouyang, J. Tungsten tailing powders activated for use as cementitious material. Powder Technol. 2015, 286, 678–683. [Google Scholar] [CrossRef]
- Zhang, S.; Xue, X.; Liu, X.; Duan, P.; Yang, H.; Jiang, T.; Wang, D.; Liu, R. Current situation and comprehensive utilization of iron ore tailing resources. J. Min. Sci. 2006, 42, 403–408. [Google Scholar] [CrossRef]
- Pan, J.; Zheng, G.-L.; Zhu, D.-Q.; Zhou, X.-L. Utilization of nickel slag using selective reduction followed by magnetic separation. Trans. Nonferrous Met. Soc. China 2013, 23, 3421–3427. [Google Scholar] [CrossRef]
- Yusof, M.A.W. Investigating the Potential for Incorporating Tin Slag in Road Pavements. Ph.D. Thesis, University of Nottingham, Nottinghan, UK, 2005. [Google Scholar]
- Ndlovu, S.; Simate, G.S.; Matinde, E. Waste Production and Utilization in the Metal Extraction Industry; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Saha, A.K.; Saker, P.K. Sustainable use of ferronickel slag fine aggregate and fly ash in structural concrete: Mechanical properties and leaching study. J. Clean. Prod. 2017, 162, 438–448. [Google Scholar] [CrossRef]
- Javadian, H.; Ghorbani, F.; Tayebi, H.-A.; Asl, S.H. Study of the adsorption of Cd (II) from aqueous solution using zeolite-based geopolymer, synthesized from coal fly ash; kinetic, isotherm and thermodynamic studies. Arab. J. Chem. 2015, 8, 837–849. [Google Scholar] [CrossRef] [Green Version]
- Duxson, P.; Fernández-Jiménez, A.; Provis, J.L.; Lukey, G.C.; Palomo, A.; van Deventer, J.S. Geopolymer technology: The current state of the art. J. Mater. Sci. 2007, 42, 2917–2933. [Google Scholar] [CrossRef]
- Rao, F.; Liu, Q. Geopolymerization and its potential application in mine tailings consolidation: A review. Miner. Process. Extr. Metall. Rev. 2015, 36, 399–409. [Google Scholar] [CrossRef]
- Du, T.; Liu, L.-Y.; Xiao, P.; Che, S.; Wang, H.-M. Preparation of zeolite NaA for CO2 capture from nickel laterite residue. Int. J. Miner. Metall. Mater. 2014, 21, 820–825. [Google Scholar] [CrossRef]
- ICMM; UNEP; PRI. Global Industry Standard on Tailings Management; International Council on Mining and Metals (ICMM), United Nationals Environment Programme (UNEP), Principles for Responsible Investment (PRI): London, UK, 2020. [Google Scholar]
- ICMM; UNEP; PRI. Global Tailings Review—Consultation on the Draft Global Tailings Standard; International Council (ICMM), United Nations Environment Programme (UNEP), Principles for Responsible Investment (PRI): London, UK, 2020. [Google Scholar]
- Spooren, J.; Binnemans, K.; Björkmalm, J.; Breemersch, K.; Dams, Y.; Folens, K.; González-Moya, M.; Horckmans, L.; Komnitsas, K.; Kurylak, W. Near-zero-waste processing of low-grade, complex primary ores and secondary raw materials in Europe: Technology development trends. Resour. Conserv. Recycl. 2020, 160, 104919. [Google Scholar] [CrossRef]
- Nikolić, I.P.; Milošević, I.M.; Milijić, N.N.; Mihajlović, I.N. Cleaner production and technical effectiveness: Multi-criteria analysis of copper smelting facilities. J. Clean. Prod. 2019, 215, 423–432. [Google Scholar] [CrossRef]
- Jakob, L.; Michal, Š.; Franz-Georg, S.; Margarida, Q.; Jiri, H.; Florian, H.; Valerio, F.; Johann, F.; Roberto, B.; Elza, B. What waste management can learn from the traditional mining sector: Towards an integrated assessment and reporting of anthropogenic resources. Waste Manag. 2020, 113, 154–156. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, F.; Hartlieb, P. Innovation in the mining industry: Technological trends and a case study of the challenges of disruptive innovation. Min. Metall. Explor. 2020, 37, 1385–1399. [Google Scholar] [CrossRef]
- Ghimire, H.; Ariya, P.A. E-wastes: Bridging the knowledge gaps in global production budgets, composition, recycling and sustainability implications. Sustain. Chem. 2020, 1, 154–182. [Google Scholar] [CrossRef]
- Imoisili, P.E.; Ukoba, K.O.; Jen, T.-C. Green technology extraction and characterisation of silica nanoparticles from palm kernel shell ash via sol–gel. J. Mater. Res. Technol. 2020, 9, 307–313. [Google Scholar] [CrossRef]
- Yousef, S.; Tatariants, M.; Tichonovas, M.; Kliucininkas, L.; Lukošiūtė, S.-I.; Yan, L. Sustainable green technology for recovery of cotton fibers and polyester from textile waste. J. Clean. Prod. 2020, 254, 120078. [Google Scholar] [CrossRef]
- Zante, G.; Boltoeva, M. Review on hydrometallurgical recovery of metals with deep eutectic solvents. Sustain. Chem. 2020, 1, 238–255. [Google Scholar] [CrossRef]
- Periyapperuma, K.; Sanchez-Cupido, L.; Pringle, J.M.; Pozo-Gonzalo, C. Analysis of sustainable methods to recover neodymium. Sustain. Chem. 2021, 2, 550–563. [Google Scholar] [CrossRef]
- IEA. Net Zero by 2050: A Roadmap for the Global Energy Sector; International Energy Agency: Paris, France, 2021. [Google Scholar]
- Horowitz, C.A. Paris agreement. Int. Leg. Mater. 2016, 55, 740–755. [Google Scholar] [CrossRef]
- Ruokonen, E. Preconditions for successful implementation of the Finnish standard for sustainable mining. Extr. Ind. Soc. 2020, 7, 611–620. [Google Scholar] [CrossRef]
- Zhao, Y.; Pohl, O.; Bhatt, A.I.; Collis, G.E.; Mahon, P.J.; Rüther, T.; Hollenkamp, A.F. A review on battery market trends, second-life reuse, and recycling. Sustain. Chem. 2021, 2, 167–205. [Google Scholar] [CrossRef]
- Islam, K.; Vilaysouk, X.; Murakami, S. Integrating remote sensing and life cycle assessment to quantify the environmental impacts of copper-silver-gold mining: A case study from Laos. Resour. Conserv. Recycl. 2020, 154, 104630. [Google Scholar] [CrossRef]
- Liu, Y.G.; Zhou, M.; Zeng, G.M.; Li, X.; Xu, W.H.; Fan, T. Effect of solids concentration on removal of heavy metals from mine tailings via bioleaching. J. Hazard. Mater. 2007, 141, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Vestola, E.A.; Kuusenaho, M.K.; Närhi, H.M.; Tuovinen, O.H.; Puhakka, J.A.; Plumb, J.J.; Kaksonen, A.H. Acid bioleaching of solid waste materials from copper, steel and recycling industries. Hydrometallurgy 2010, 103, 74–79. [Google Scholar] [CrossRef]
- Potysz, A.; van Hullebusch, E.D.; Kierczak, J. Perspectives regarding the use of metallurgical slags as secondary metal resources—A review of bioleaching approaches. J. Environ. Manag. 2018, 219, 138–152. [Google Scholar] [CrossRef]
- Williamson, A.J.; Folens, K.; Van Damme, K.; Olaoye, O.; Atia, T.A.; Mees, B.; Nicomel, N.R.; Verbruggen, F.; Spooren, J.; Boon, N. Conjoint bioleaching and zinc recovery from an iron oxide mineral residue by a continuous electrodialysis system. Hydrometallurgy 2020, 195, 105409. [Google Scholar] [CrossRef]
- Oraby, E.A.; Eksteen, J.J. Gold leaching in cyanide-starved copper solutions in the presence of glycine. Hydrometallurgy 2015, 156, 81–88. [Google Scholar] [CrossRef]
- Czaplicka-Kolarz, K.; Burchart-Korol, D.; Turek, M.; Borkowski, W. Model of eco-efficiency assessment of mining production processes. Arch. Min. Sci. 2015, 60, 477–486. [Google Scholar] [CrossRef]
- Oliveira, R.; Camanho, A.S.; Zanella, A. Expanded eco-efficiency assessment of large mining firms. J. Clean. Prod. 2017, 142, 2364–2373. [Google Scholar] [CrossRef]
- Liu, X.; Guo, P.; Guo, S. Assessing the eco-efficiency of a circular economy system in China’s coal mining areas: Emergy and data envelopment analysis. J. Clean. Prod. 2019, 206, 1101–1109. [Google Scholar] [CrossRef]
- Saling, P.; Kicherer, A.; Dittrich-Krämer, B.; Wittlinger, R.; Zombik, W.; Schmidt, I.; Schrott, W.; Schmidt, S. Eco-efficiency analysis by BASF: The method. Int. J. Life Cycle Assess. 2002, 7, 203–218. [Google Scholar] [CrossRef]
- Grosse-Sommer, A.P.; Grünenwald, T.H.; Paczkowski, N.S.; van Gelder, R.N.; Saling, P.R. Applied sustainability in industry: The BASF Eco-efficiency toolbox. J. Clean. Prod. 2020, 258, 120792. [Google Scholar] [CrossRef]
- Singh, S.; Sukla, L.; Goyal, S. Mine waste & circular economy. Mater. Today Proc. 2020, 30, 332–339. [Google Scholar]
- Singh, R.K.; Kumar, A.; Garza-Reyes, J.A.; de Sá, M.M. Managing operations for circular economy in the mining sector: An analysis of barriers intensity. Resour. Policy 2020, 69, 101752. [Google Scholar] [CrossRef]
- Sousa, R.; Ramos, V.; Guedes, A.; Noronha, F.; Botelho de Sousa, A.; Machado Leite, M.; Seltmann, R.; Dolgopolova, A. The Alvarrões-Gonçalo Li project: An example of sustainable lithium mining. Adv. Geosci. 2018, 45, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Woźniak, J.; Pactwa, K. Overview of polish mining wastes with circular economy model and its comparison with other wastes. Sustainability 2018, 10, 3994. [Google Scholar] [CrossRef] [Green Version]
- Geissler, B.; Hermann, L.; Mew, M.C.; Steiner, G. Striving toward a circular economy for phosphorus: The role of phosphate rock mining. Minerals 2018, 8, 395. [Google Scholar] [CrossRef] [Green Version]
- van Berkel, R. Eco-efficiency in the Australian minerals processing sector. J. Clean. Prod. 2007, 15, 772–781. [Google Scholar] [CrossRef]
- Norgate, T.; Haque, N. Energy and greenhouse gas impacts of mining and mineral processing operations. J. Clean. Prod. 2010, 18, 266–274. [Google Scholar] [CrossRef]
- Zhilina, V.; Akhmetzyanova, M.; Zhilina, E. Technosphere thinking in the transformations of earth sciences. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Vladivostok, Russia, 2021. [Google Scholar]

| Technosphere | Location | Relative Size | Concentration | Management | State |
|---|---|---|---|---|---|
| In-use | Urban | Large | High | - | Active |
| Hibernation | Urban | Small | High | Uncontrolled | Inactive |
| Dissipation | Rural | Small | Low | Uncontrolled | Inactive |
| Tailings | Rural | Medium | Average | Controlled | Inactive |
| Slag | Rural | Small | Average | Controlled | Inactive |
| Landfill | Fringe | Medium | Average | Controlled | Inactive |
| Stocks | Samples | Metals | Sources |
|---|---|---|---|
| Tailing | Copper tailing | Cu, REEs, Co, Ni, and manganese (Mn) | [41,45] |
| Tungsten tailing | Tungsten (W), molybdenum (Mo), and Mn | [48] | |
| Iron tailing | REEs and Fe | [49,50,51] | |
| Lead-zinc tailing | Fe, silver (Ag), gallium (Ga), lead (Pb), and Mn | [52] | |
| Slag | Copper slag | Cu, Co, Ni, Ti, vanadium (V), and chromium (Cr) | [32] |
| Nickel slag | Ni, Co, and Ti | [31] | |
| Tin slag | Nb, Ta, Ti, and Sn | [43] | |
| Residue | Nickel laterite | Ni, Co, Cr, and Mn | [53] |
| Bauxite residue | REEs, Ti, and Sc | [10,34] | |
| Fly ash | Nickel laterite | Ni, Co, Cr, and Mn | [54] |
| Coal powder | Ti, Mn, and magnesium (Mg) | [55] |
| Stocks | Samples | Applications | Sources |
|---|---|---|---|
| Overburden | Waste rocks dumps | Backfill and construction | [6] |
| Tungsten mine waste mud | Geopolymeric binder | [82] | |
| Tailing | Gold tailing | Geopolymer | [17] |
| Copper tailing | Concrete and brick | [83,84] | |
| Tungsten tailing | Cement | [85] | |
| Iron tailing | Sand substitute, production of cement, glass, brick, ceramic, and tile | [86] | |
| Slag | Copper slag | Construction, cement additive, blasting agent, fertiliser, and metal salt | [66,80] |
| Nickel slag | Ceramic, cement, and geopolymer | [18,87] | |
| Tin slag | Road pavement | [88] | |
| Residue | Nickel laterite | Zeolite X (CO2 capture material) | [20] |
| Bauxite residue | Construction, catalysts, adsorbents, and ceramics | [79,81] | |
| Jarosite residue | Construction | [89] | |
| Fly ash | Nickel laterite | Concrete | [90] |
| Coal powder | Geopolymer | [91] |
| Aims (Resources Productive Themes) | Means (Prevention Practices) |
|---|---|
| • Resource efficiency | • Process design |
| • Energy use and greenhouse gas emissions | • Input substitution |
| • Water use and impacts | • Plant improvement |
| • Control of minor elements and toxics | • Good housekeeping |
| • By-product creation | • Reuse, recycling, and recovery |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, B.; Alorro, R.D. Technospheric Mining of Mine Wastes: A Review of Applications and Challenges. Sustain. Chem. 2021, 2, 686-706. https://doi.org/10.3390/suschem2040038
Lim B, Alorro RD. Technospheric Mining of Mine Wastes: A Review of Applications and Challenges. Sustainable Chemistry. 2021; 2(4):686-706. https://doi.org/10.3390/suschem2040038
Chicago/Turabian StyleLim, Bona, and Richard Diaz Alorro. 2021. "Technospheric Mining of Mine Wastes: A Review of Applications and Challenges" Sustainable Chemistry 2, no. 4: 686-706. https://doi.org/10.3390/suschem2040038
APA StyleLim, B., & Alorro, R. D. (2021). Technospheric Mining of Mine Wastes: A Review of Applications and Challenges. Sustainable Chemistry, 2(4), 686-706. https://doi.org/10.3390/suschem2040038









