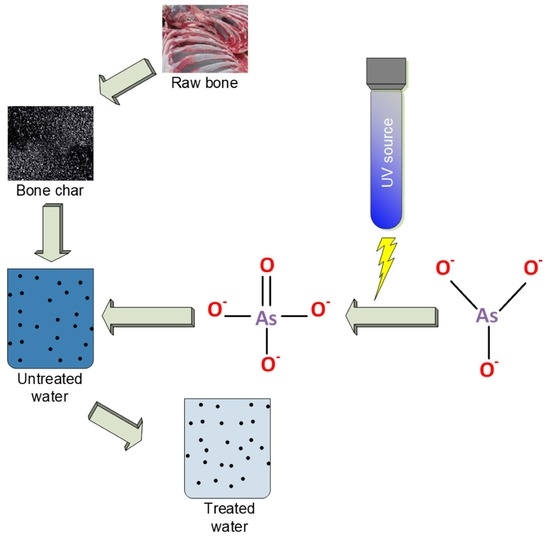
Evaluating the Ability of Bone Char/nTiO2 Composite and UV Radiation for Simultaneous Oxidation and Adsorption of Arsenite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Equipment
2.2. Adsorbent Preparation
2.3. Adsorbent Experiments
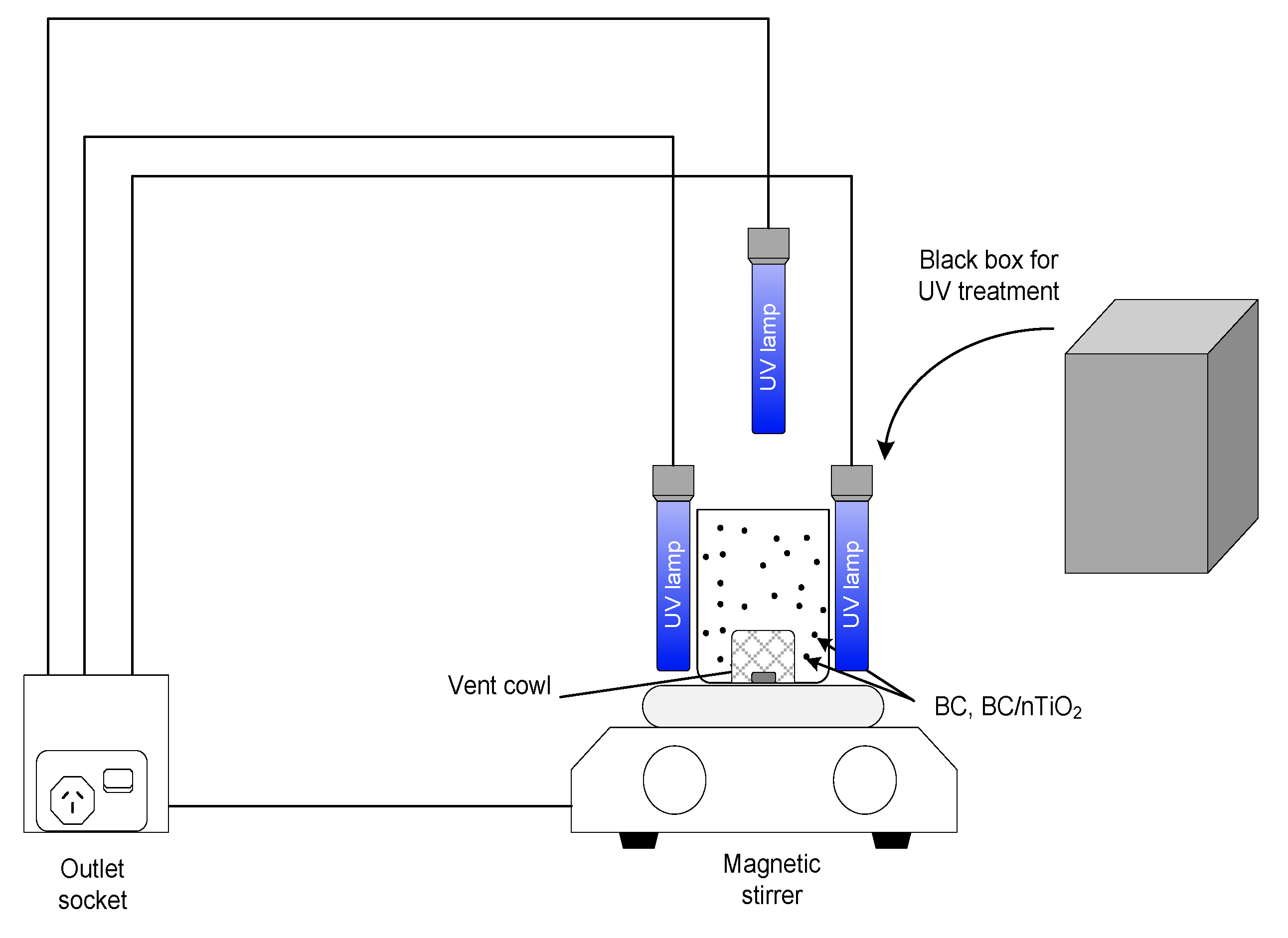
2.3.1. Experimental Setup
2.3.2. Experimental Procedure
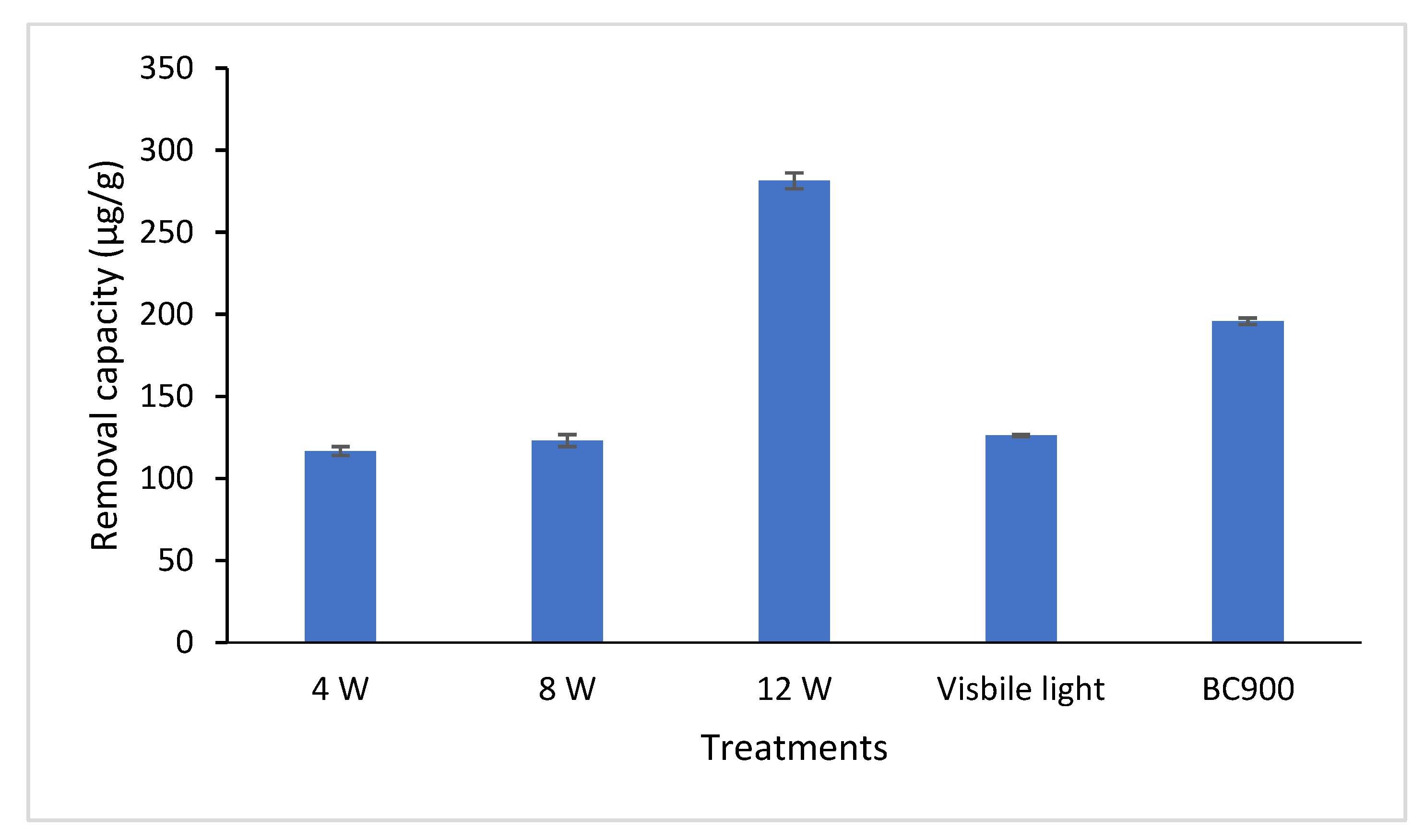
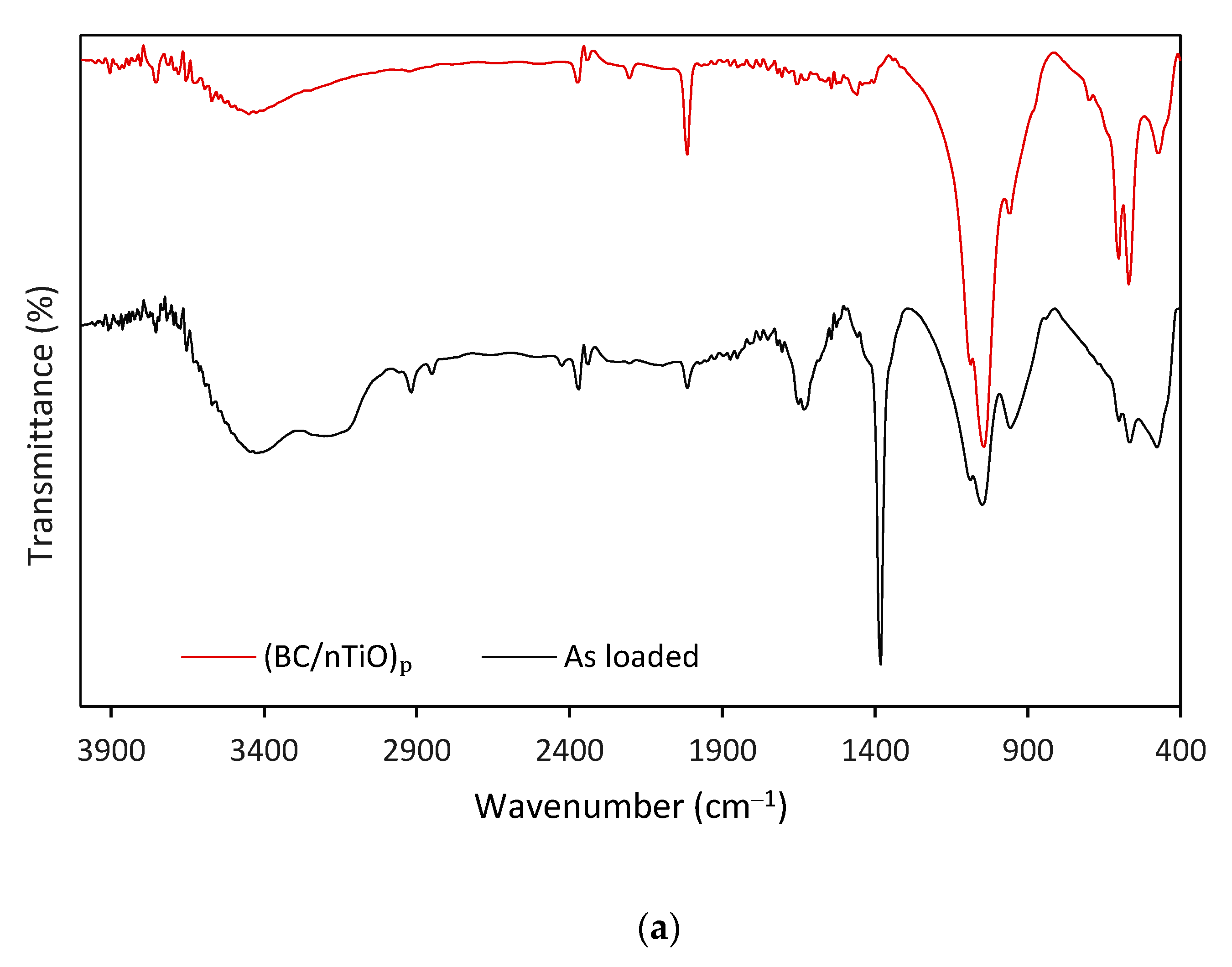
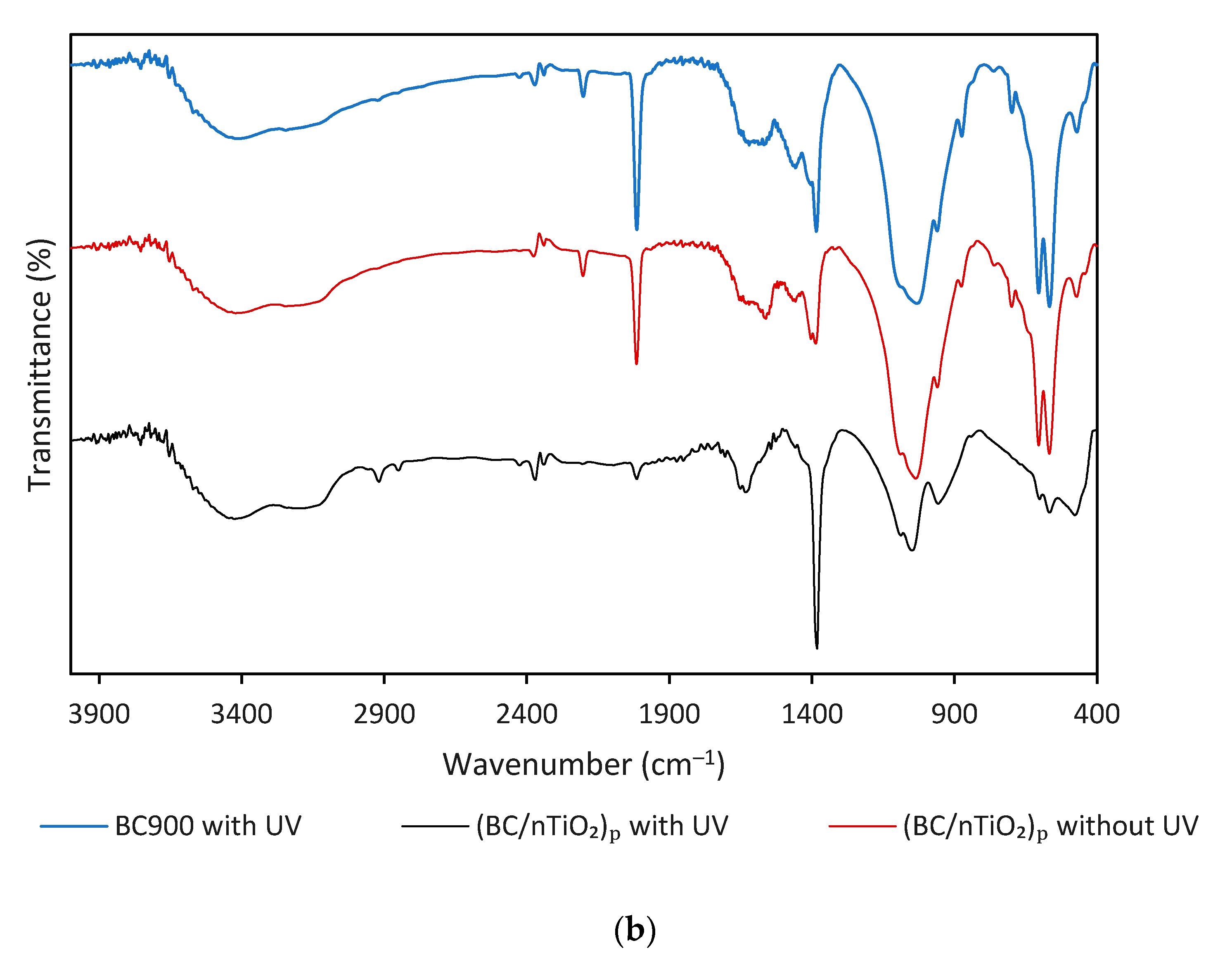
3. Results and Discussion
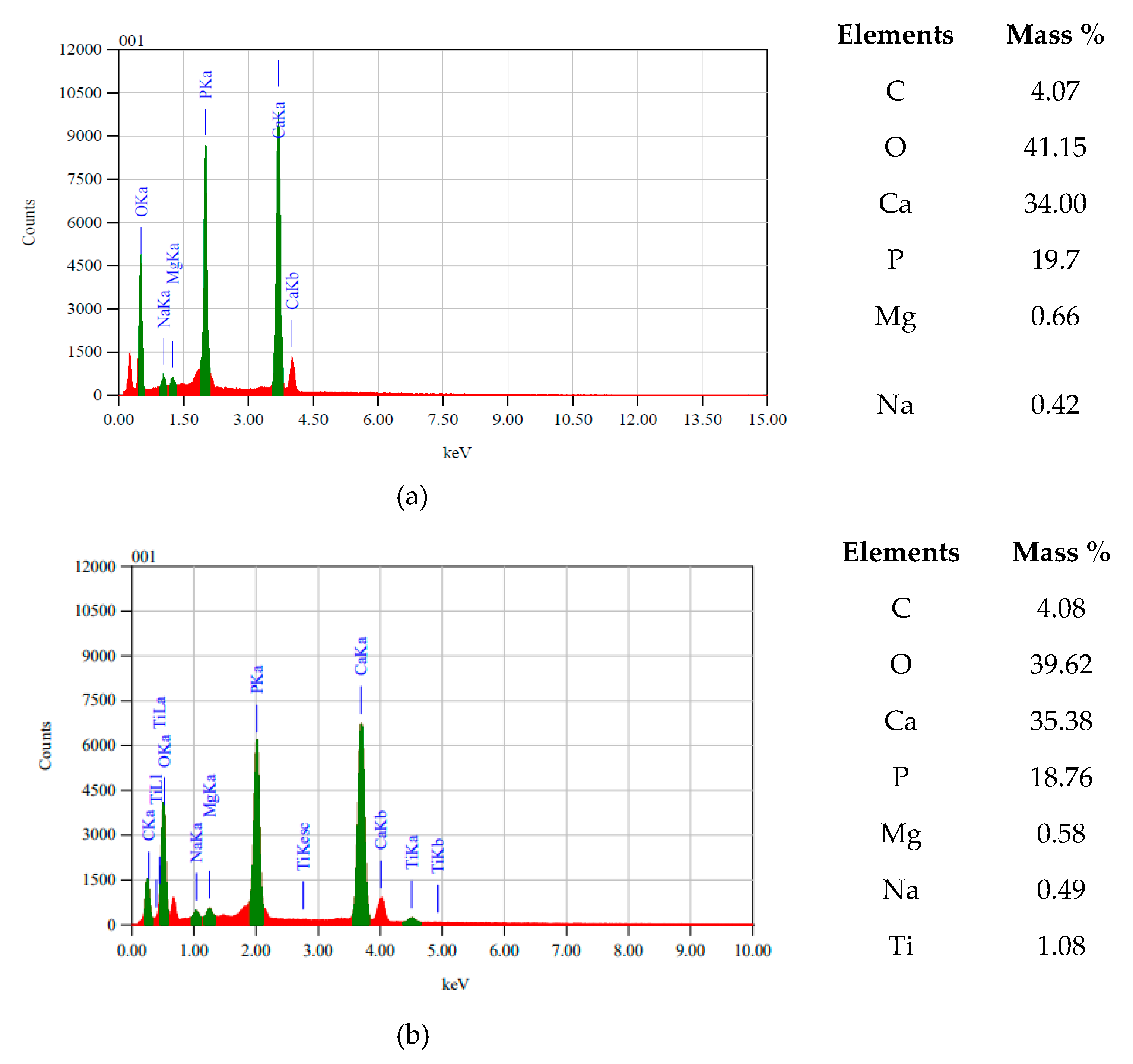
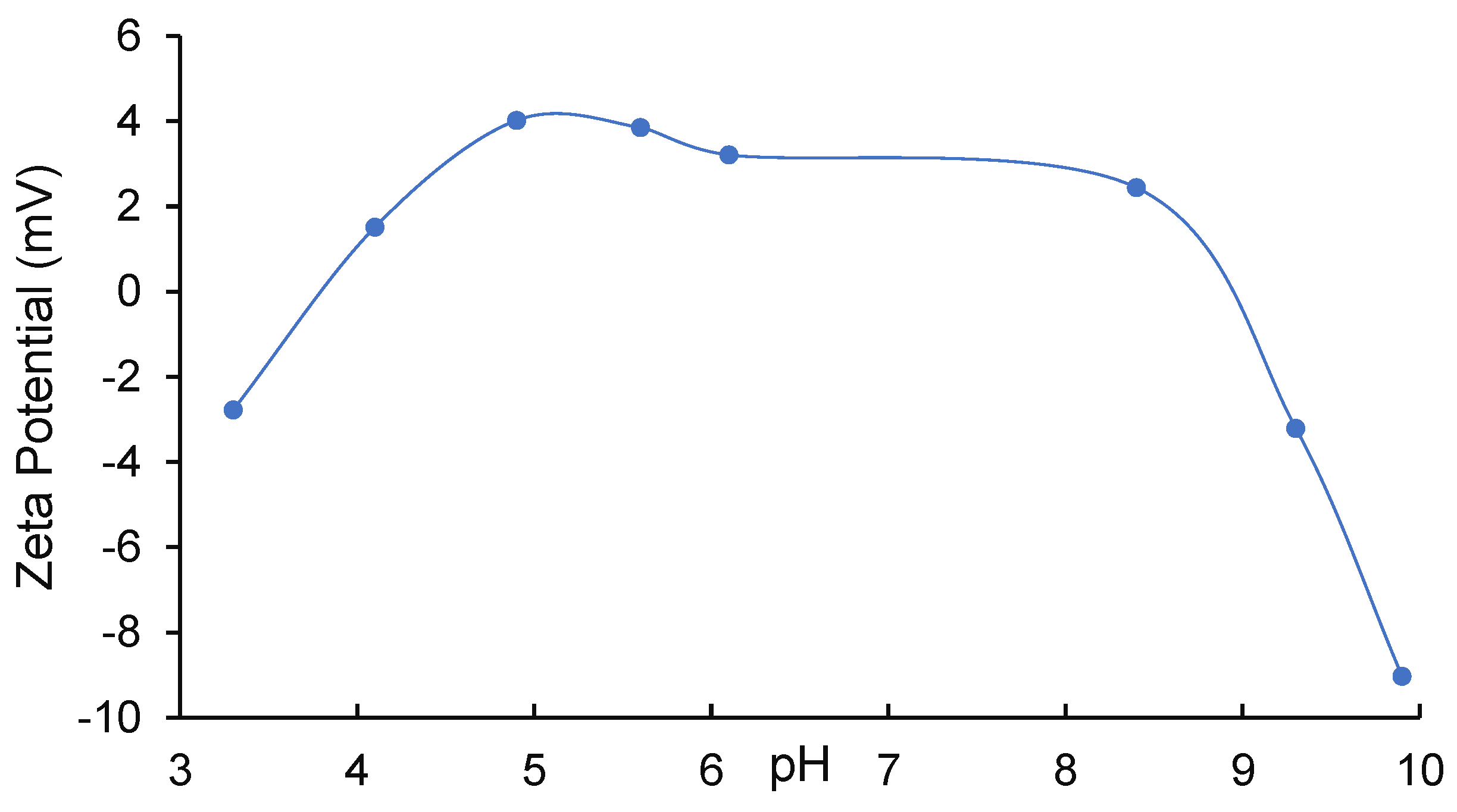
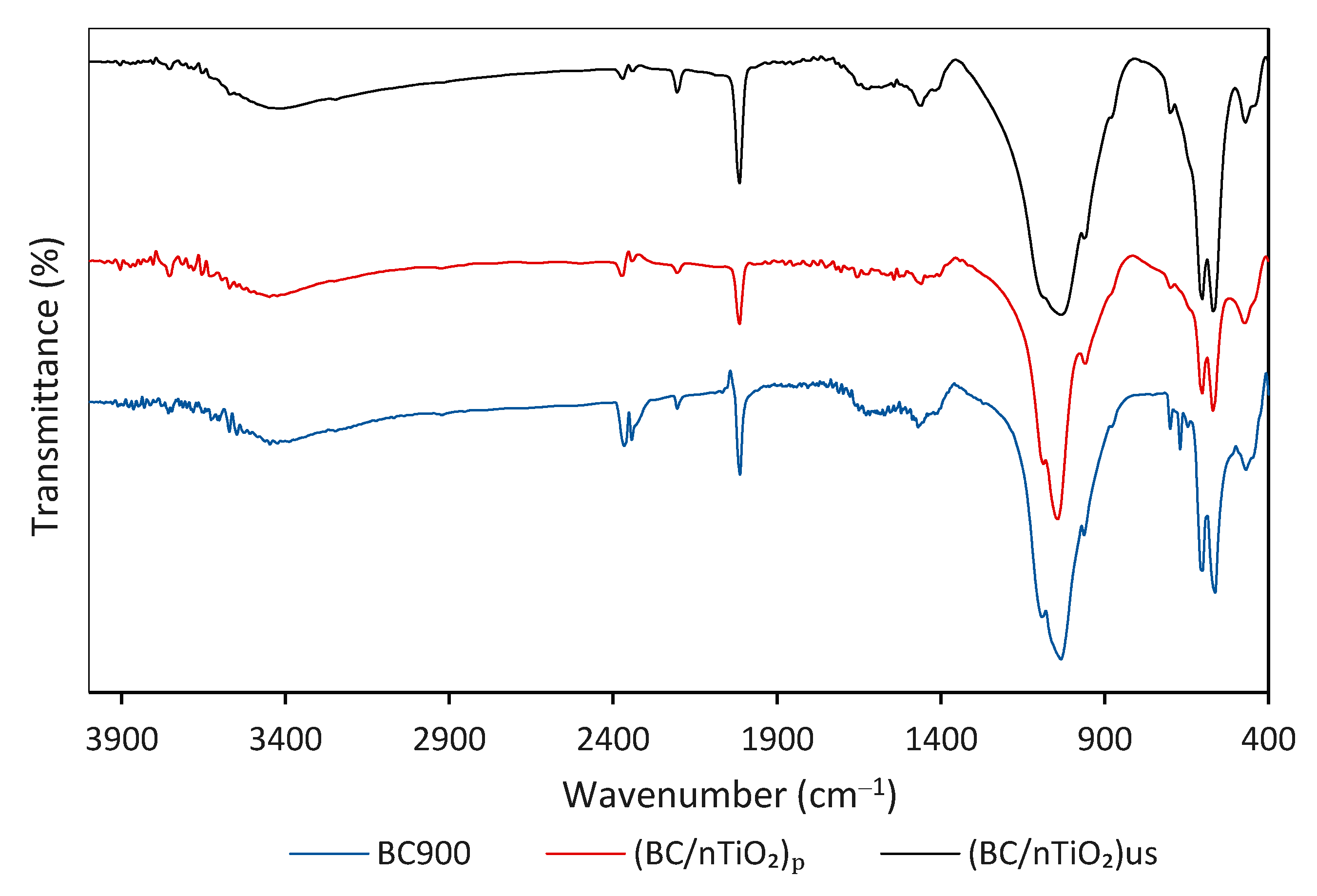
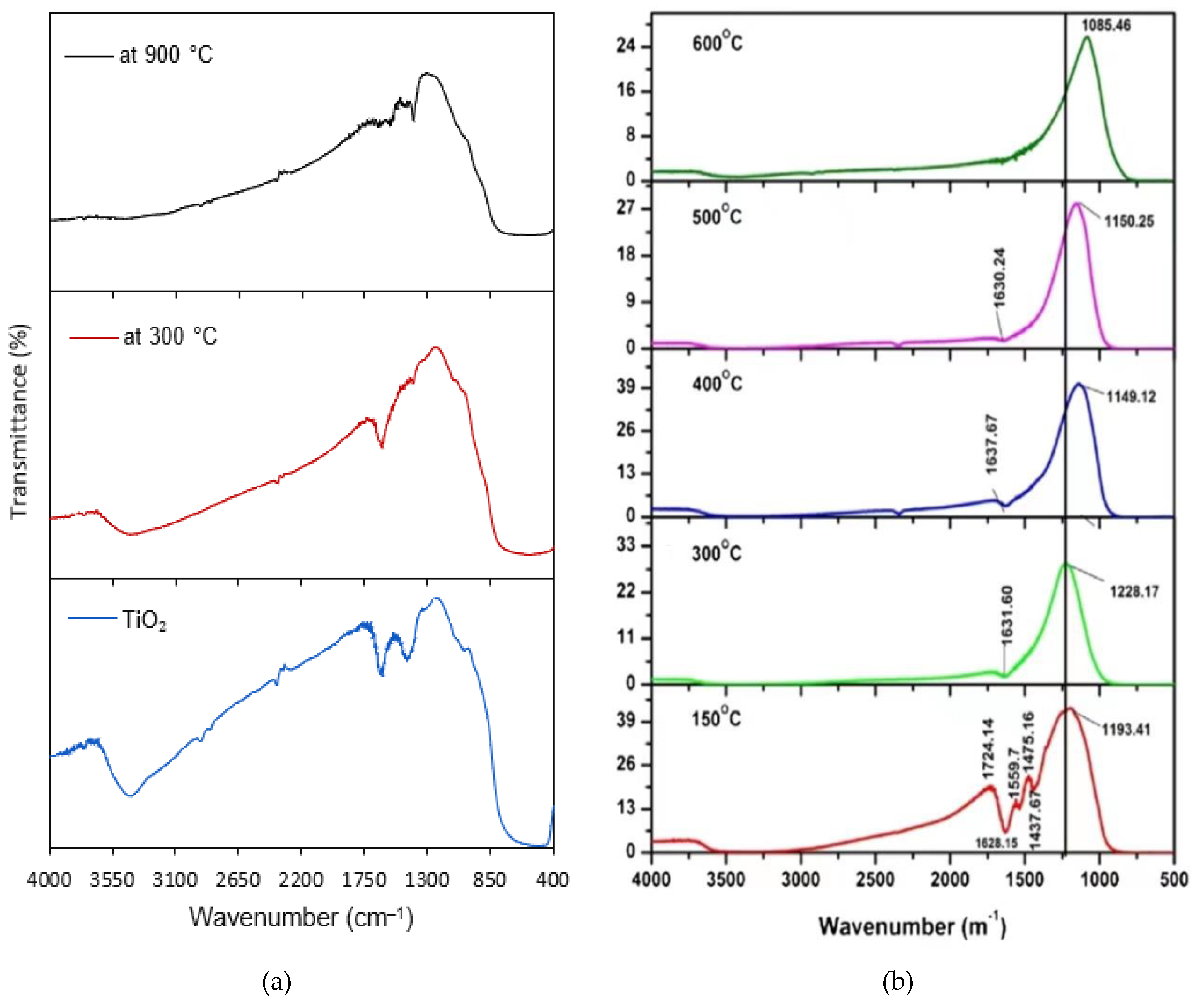
3.1. BC/nTiO2 Characteristics
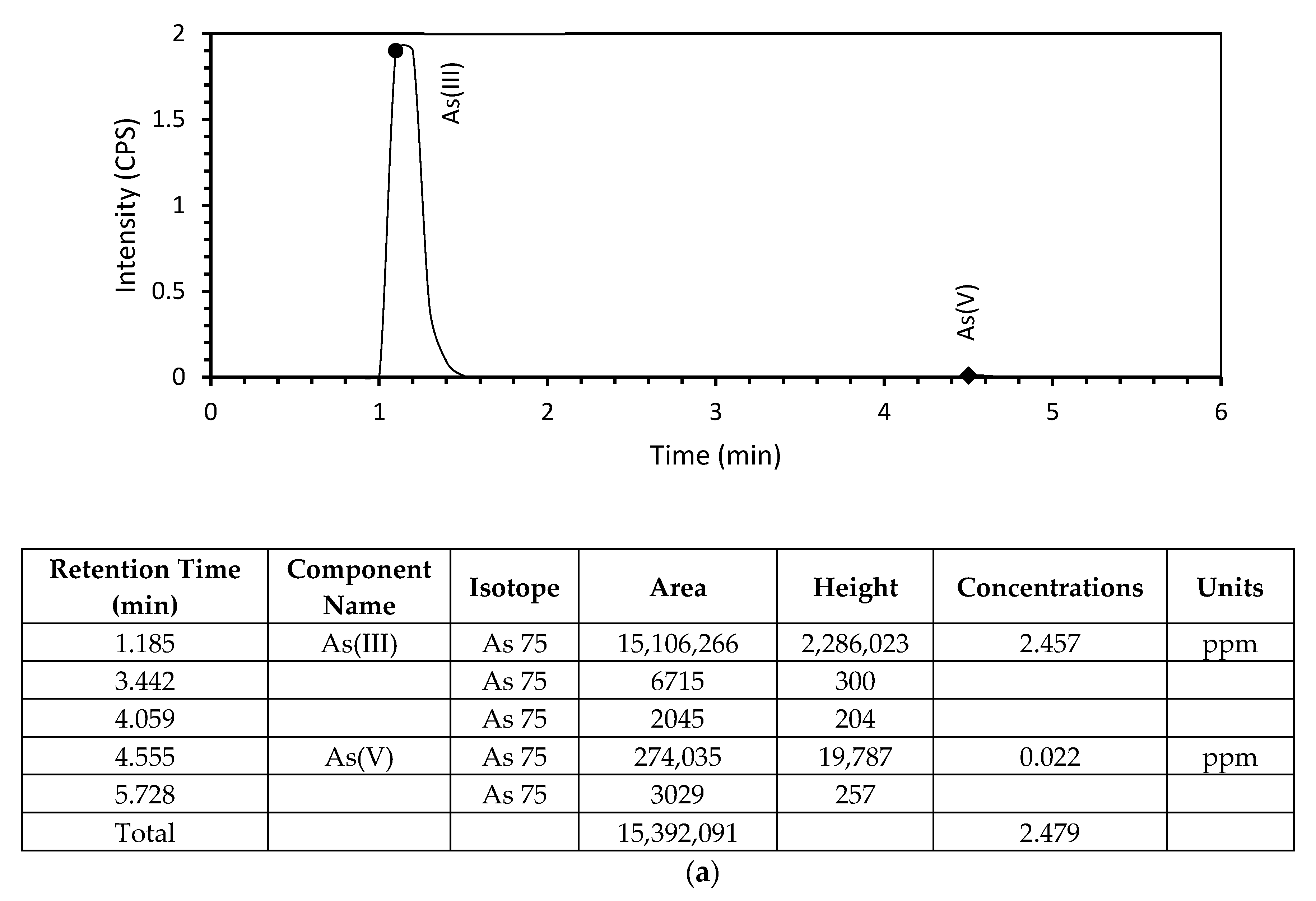
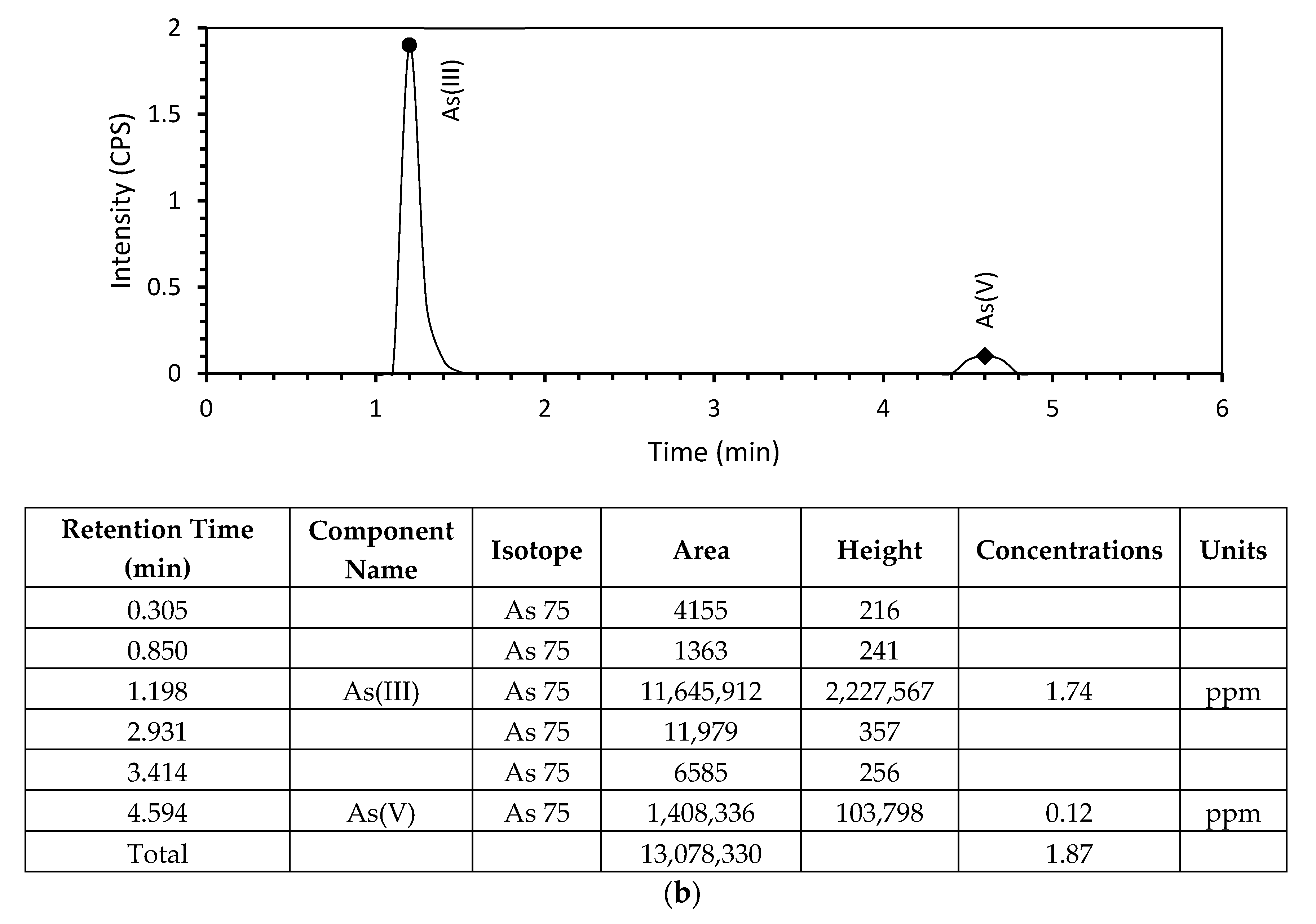
3.2. Arsenic Adsorption
4. Conclusions and Recommendations for Future Actions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumarathilaka, P.; Seneweera, S.; Ok, Y.-S.; Meharg, A.A.; Bundschuh, J. Mitigation of arsenic accumulation in rice: An agronomical, physico-chemical, and biological approach—A critical review. Crit. Rev. Environ. Sci. Technol. 2020, 50, 31–71. [Google Scholar] [CrossRef]
- Clifford, D.; Ghurye, G. Oxidizing Arsenic III to Arsenic V for Better Removal. Water Quality Product. 2001, pp. 28–29. Available online: https://www.wwdmag.com/groundwater/oxidizing-arsenic-iii-arsenic-v-better-removal (accessed on 27 October 2021).
- Zeltner, W.A. Simultaneous Removal of Arsenite As (III) and Arsenate As (V) From Drinking Water Using a Novel Photoactive Adsorbent; EPA: Washington, DC, USA, 2002. [Google Scholar]
- Weiss, S.; Carapito, C.; Cleiss, J.; Koechler, S.; Turlin, E.; Coppee, J.-Y.; Heymann, M.; Kugler, V.; Stauffert, M.; Cruveiller, S.; et al. Enhanced structural and functional genome elucidation of the arsenite-oxidizing strain Herminiimonas arsenicoxydans by proteomics data. Biochim. 2009, 91, 192–203. [Google Scholar] [CrossRef]
- Bissen, M.; Frimmel, F.H. Arsenic—A Review. Part II: Oxidation of arsenic and its removal in water treatment. Acta Hydrochim. Hydrobiol. 2003, 31, 97–107. [Google Scholar] [CrossRef]
- Alkurdi, S.S.A.; Al-Juboori, R.A.; Bundschuh, J.; Hamawand, I. Bone char as a green sorbent for removing health threatening fluoride from drinking water. Environ. Int. 2019, 127, 704–719. [Google Scholar] [CrossRef] [PubMed]
- OECD/FAO. OECD-FAO Agricultural Outlook 2018–2027; OECD: Paris, France; FAO: Rome, Italy, 2018. [Google Scholar]
- Minja, R. Practical approach for removal of natural organic matter and defluoridation of Maji ya Chai river water: Use of acid pre-treated bone char and coagulants. Tanzan. J. Eng. Technol. 2019, 38, 204–206. [Google Scholar] [CrossRef]
- Rojas-Mayorga, C.K.; Albero, J.S.; Aguayo, I.; Castillo, D.I.M.; Bonilla-Petriciolet, A. A new synthesis route for bone chars using CO2 atmosphere and their application as fluoride adsorbents. Microporous Mesoporous Mater. 2015, 209, 38–44. [Google Scholar] [CrossRef]
- Alkurdi, S.S.; Al-Juboori, R.A.; Bundschuh, J.; Bowtell, L.; McKnight, S. Effect of pyrolysis conditions on bone char characterization and its ability for arsenic and fluoride removal. Environ. Pollut. 2020, 262, 114221. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Su, G.; Zhang, B.; Jiang, G.; Yan, B. Nanoparticle-based strategies for detection and remediation of environmental pollutants. Anal. 2011, 136, 872–877. [Google Scholar] [CrossRef]
- Savage, N.; Diallo, M.S. Nanomaterials and water purification: Opportunities and challenges. J. Nanoparticle Res. 2005, 7, 331–342. [Google Scholar] [CrossRef]
- Hashimoto, K.; Irie, H.; Fujishima, A. TiO2 Photocatalysis: A historical overview and future prospects. Jpn. J. Appl. Phys. 2005, 44, 8269. [Google Scholar] [CrossRef]
- Woan, K.; Pyrgiotakis, G.; Sigmund, W. Photocatalytic carbon-nanotube-TiO2 composites. Adv. Mater. 2009, 21, 2233–2239. [Google Scholar] [CrossRef]
- Xu, T.; Cai, A.Y.; O’Shea, K.E. Adsorption and photocatalyzed oxidation of methylated arsenic species in TiO2 suspensions. Environ. Sci. Technol. 2007, 41, 5471–5477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mancardi, G.; Tamargo, C.H.; Terranova, U.; de Leeuw, N.H. Calcium phosphate deposition on planar and stepped (101) surfaces of anatase TiO2: Introducing an interatomic potential for the TiO2/Ca-PO4/water interface. Langmuir 2018, 34, 10144–10152. [Google Scholar] [CrossRef] [Green Version]
- Wei, Z.; Fang, Y.; Wang, Z.; Liu, Y.; Wu, Y.; Liang, K.; Yan, J.; Pan, Z.; Hu, G. pH effects of the arsenite photocatalytic oxidation reaction on different anatase TiO2 facets. Chemosphere 2019, 225, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Du, J.; Meng, X.; Sun, Y.; Sun, B.; Hu, Q. Application of titanium dioxide in arsenic removal from water: A review. J. Hazard. Mater. 2012, 215–216, 1–16. [Google Scholar] [CrossRef]
- Ren, H.-T.; Han, J.; Li, T.-T.; Sun, F.; Lin, J.-H.; Lou, C.-W. Visible light-induced oxidation of aqueous arsenite using facile Ag2O/TiO2 composites: Performance and mechanism. J. Photochem. Photobiol. A Chem. 2019, 377, 260–267. [Google Scholar] [CrossRef]
- Deng, M.; Wu, X.; Zhu, A.; Zhang, Q.; Liu, Q. Well-dispersed TiO2 nanoparticles anchored on Fe3O4 magnetic nanosheets for efficient arsenic removal. J. Environ. Manag. 2019, 237, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Herath, I.; Kumarathilaka, P.; Bundschuh, J.; Marchuk, A.; Rinklebe, J. A fast analytical protocol for simultaneous speciation of arsenic by Ultra-High Performance Liquid Chromatography (UHPLC) hyphenated to Inductively Coupled Plasma Mass Spectrometry (ICP-MS) as a modern advancement in liquid chromatography approaches. Talanta 2020, 208, 120457. [Google Scholar] [CrossRef]
- Li, J.; Tian, B.; Li, T.; Dai, S.; Weng, Y.; Lu, J.; Xu, X.; Jin, Y.; Pang, R.; Hua, Y. Biosynthesis of Au, Ag and Au-Ag bimetallic nanoparticles using protein extracts of Deinococcus radiodurans and evaluation of their cytotoxicity. Int. J. Nanomed. 2018, 13, 1411–1424. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Liao, Y.; Chen, Y.; Wu, J.; Ma, X. Performance of Ce-modified VW-Ti type catalyst on simultaneous control of NO and typical VOCS. Fuel Process. Technol. 2020, 207, 106483. [Google Scholar] [CrossRef]
- Motlochová, M.; Slovák, V.; Pližingrová, E.; Szatmáry, L.; Bezdička, P.; Šubrt, J. The influence of annealing temperature on properties of TiO2 based materials as adsorbents of radionuclides. Thermochim. Acta 2019, 673, 34–39. [Google Scholar] [CrossRef]
- Jia, B.-Y.; Duan, L.-Y.; Ma, C.-L.; Wang, C.-M. Characterization of TiO2 loaded on activated carbon fibers and its photocatalytic reactivity. Chin. J. Chem. 2007, 25, 553–557. [Google Scholar] [CrossRef]
- Yoon, S.-H.; Lee, J.H. Oxidation mechanism of As(III) in the UV/TiO2 system: Evidence for a direct hole oxidation mechanism. Environ. Sci. Technol. 2005, 39, 9695–9701. [Google Scholar] [CrossRef] [PubMed]
- Ghurye, G.; Clifford, D. Laboratory study on the oxidation of As (III) to As (V). National Risk Management Research Laboratory, Office of Research and Development, US Environmental Protection Agency. In Proceedings of the AWWA Water Quality Technology Conference, Houston, TX, USA, 1 March 2001; Volume 1, pp. 12–20. [Google Scholar]
- Zhao, X.; Zhang, A.; Zhang, J.; Wang, Q.; Huang, X.; Wu, Y.; Tang, C. Enhanced selenate removal in aqueous phase by copper-coated activated carbon. Mater. 2020, 13, 468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, F.; Min, L.; Luo, X.; Wu, S.; Luo, S. Visible-light photocatalytic degradation performances and thermal stability due to the synergetic effect of TiO2 with conductive copolymers of polyaniline and polypyrrole. Nanoscale 2013, 5, 8703–8710. [Google Scholar] [CrossRef] [PubMed]
- Kaya, A.; Yukselen, Y. Zeta potential of clay minerals and quartz contaminated by heavy metals. Can. Geotech. J. 2005, 42, 1280–1289. [Google Scholar] [CrossRef]
- Hsu, J.-P.; Nacu, A. Behavior of soybean oil-in-water emulsion stabilized by nonionic surfactant. J. Colloid Interface Sci. 2003, 259, 374–381. [Google Scholar] [CrossRef]
- Roguska, A.; Pisarek, M.; Andrzejczuk, M.; Dolata, M.; Lewandowska, M.; Janik-Czachor, M. Characterization of a calcium phosphate-TiO2 nanotube composite layer for biomedical applications. Mater. Sci. Eng. C 2011, 31, 906–914. [Google Scholar] [CrossRef]
- Kannaiyan, D.; Kochuveedu, S.T.; Jang, Y.H.; Jang, Y.J.; Lee, J.Y.; Lee, J.; Lee, J.; Kim, J.; Kim, D.H. Enhanced photophysical properties of nanopatterned titania nanodots/nanowires upon hybridization with silica via bock copolymer templated sol-gel process. Polym. 2010, 2, 490–504. [Google Scholar] [CrossRef] [Green Version]
- Thamir, A.D.; Haider, A.J.; Ali, G.A. Preparation of nanostructureTiO2 at different temperatures by pulsed laser deposition as solar cell. Eng. Technol. J. 2016, 34, 193–204. [Google Scholar]
- Hanaor, D.A.H.; Sorrell, C.C. Review of the anatase to rutile phase transformation. J. Mater. Sci. 2011, 46, 855–874. [Google Scholar] [CrossRef] [Green Version]
- Kalaivani, T.; Anilkumar, P. Role of temperature on the phase modification of TiO2 nanoparticles synthesized by the precipitation method. Silicon 2018, 10, 1679–1686. [Google Scholar] [CrossRef]
- Gupta, K.K.; Singh, N.L.; Pandey, A.; Shukla, S.K.; Upadayay, S.N.; Mishra, V.; Srivastava, P.; Lalla, N.P.; Mishra, P.K. Effect of anatase/rutile TiO2 phase composition on arsenic adsorption. J. Dispers. Sci. Technol. 2013, 34, 1043–1052. [Google Scholar] [CrossRef]
- Ma, L.; Tu, S.X. Removal of arsenic from aqueous solution by two types of nano TiO2 crystals. Environ. Chem. Lett. 2011, 9, 465–472. [Google Scholar] [CrossRef]
- Driehaus, W. Arsenic removal—experience with the GEH® process in Germany. Water Supply 2002, 2, 275–280. [Google Scholar] [CrossRef]
- Katsoyiannis, I.A.; Mitrakas, M.; Zouboulis, A. Arsenic occurrence in Europe: Emphasis in Greece and description of the applied full-scale treatment plants. Desal. Water Treat. 2014, 54, 2100–2107. [Google Scholar] [CrossRef]
- Janakiraman, N.; Johnson, M. Functional groups of tree ferns (Cyathea) using FTIR: Chemotaxonomic implications. Rom. J. Biophys. 2015, 25, 131–141. [Google Scholar]
- Li, Z.; Hong, H.; Jean, J.-S.; Koski, A.J.; Liu, C.-C.; Reza, S.; Randolph, J.J.; Kurdas, S.R.; Friend, J.H.; Antinucci, S.J. Characterization on arsenic sorption and mobility of the sediments of Chia-Nan Plain, where Blackfoot disease occurred. Environ. Earth Sci. 2011, 64, 823–831. [Google Scholar] [CrossRef]
- Rytwo, G.; Zakai, R.; Wicklein, B. The use of ATR-FTIR spectroscopy for quantification of adsorbed compounds. J. Spectrosc. 2015, 2015, 727595. [Google Scholar] [CrossRef]
- Cowen, S.; Duggal, M.; Hoang, T.; Al-Abadleh, H.A. Vibrational spectroscopic characterization of some environmentally important organoarsenicals—A guide for understanding the nature of their surface complexes. Can. J. Chem. 2008, 86, 942–950. [Google Scholar] [CrossRef]
- Min, X.; Li, Y.; Ke, Y.; Shi, M.; Chai, L.; Xue, K. Fe-FeS2 adsorbent prepared with iron powder and pyrite by facile ball milling and its application for arsenic removal. Water Sci. Technol. 2017, 76, 192–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pena, M.; Meng, X.; Korfiatis, G.P.; Jing, C. Adsorption mechanism of arsenic on nanocrystalline titanium dioxide. Environ. Sci. Technol. 2006, 40, 1257–1262. [Google Scholar] [CrossRef] [PubMed]


| Experimental Condition | Range | As Species | |
|---|---|---|---|
| As(III) Optimum | As(V) Optimum | ||
| Initial concentration (mg/L) | 0.5–50 | Maximum removal of 45.99% at 2.5 mg/L | Maximum removal of 55.29% at 1 mg/L |
| pH | 4–10 | 8.6 | 7.5 |
| Contact time (h) | 0–12 | 4 | 4 |
| Bone char dose (g/L) | 2.5, 5 and 7.5 | 5 | 5 |
| Pyrolysis Temperature (°C) | 900 |
| Surface pH | 9.9 |
| pHpzc | 3.82 and 8.79 |
| BET Surface Area (m2/g) | 38 |
| Pore Volume (cm3/g) | 0.16 |
| Pore Size (nm) | 17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alkurdi, S.; Al-Juboori, R.; Bundschuh, J.; Marchuk, A. Evaluating the Ability of Bone Char/nTiO2 Composite and UV Radiation for Simultaneous Oxidation and Adsorption of Arsenite. Sustain. Chem. 2022, 3, 19-34. https://doi.org/10.3390/suschem3010002
Alkurdi S, Al-Juboori R, Bundschuh J, Marchuk A. Evaluating the Ability of Bone Char/nTiO2 Composite and UV Radiation for Simultaneous Oxidation and Adsorption of Arsenite. Sustainable Chemistry. 2022; 3(1):19-34. https://doi.org/10.3390/suschem3010002
Chicago/Turabian StyleAlkurdi, Susan, Raed Al-Juboori, Jochen Bundschuh, and Alla Marchuk. 2022. "Evaluating the Ability of Bone Char/nTiO2 Composite and UV Radiation for Simultaneous Oxidation and Adsorption of Arsenite" Sustainable Chemistry 3, no. 1: 19-34. https://doi.org/10.3390/suschem3010002
APA StyleAlkurdi, S., Al-Juboori, R., Bundschuh, J., & Marchuk, A. (2022). Evaluating the Ability of Bone Char/nTiO2 Composite and UV Radiation for Simultaneous Oxidation and Adsorption of Arsenite. Sustainable Chemistry, 3(1), 19-34. https://doi.org/10.3390/suschem3010002