Formulation of the Polymeric Double Networks (DNs) for Biomedical Applications with Physicochemical Properties to Resemble a Biological Tissue
Abstract
:1. Introduction
2. Materials and Methods
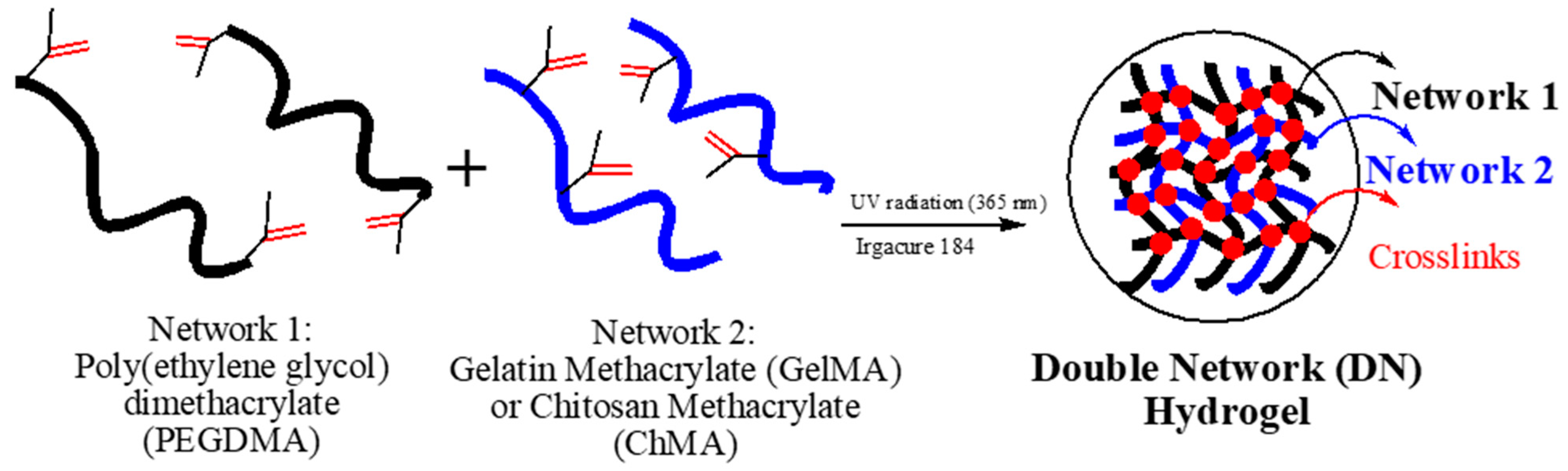
2.1. Preparation of Hydrogel Based on Double Networks (DN)
2.2. Characterization of Double Networks
2.3. Fibroblast Cell Attachment and Proliferation on the Hydrogel Scaffolds
3. Results and Discussion
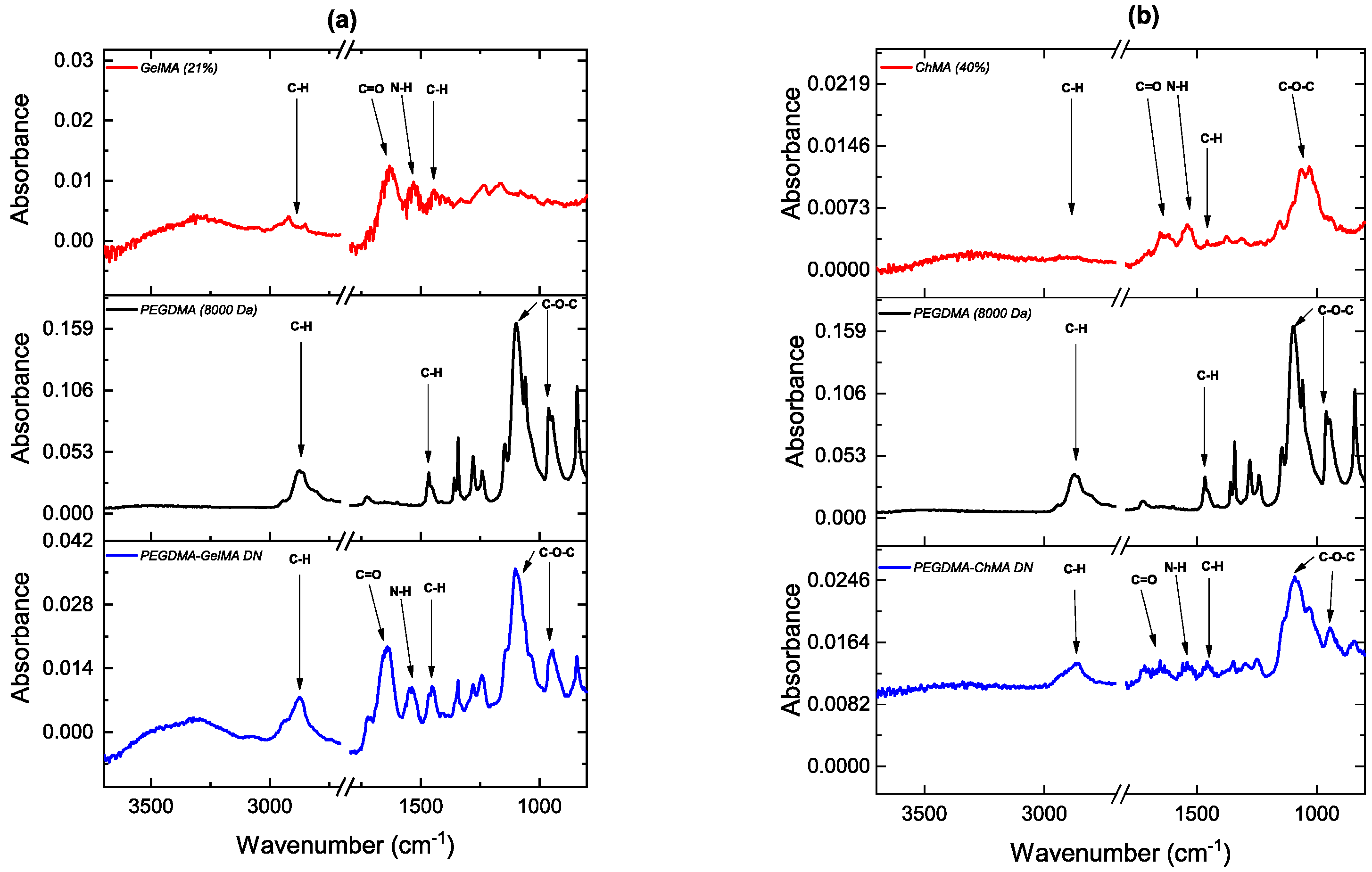
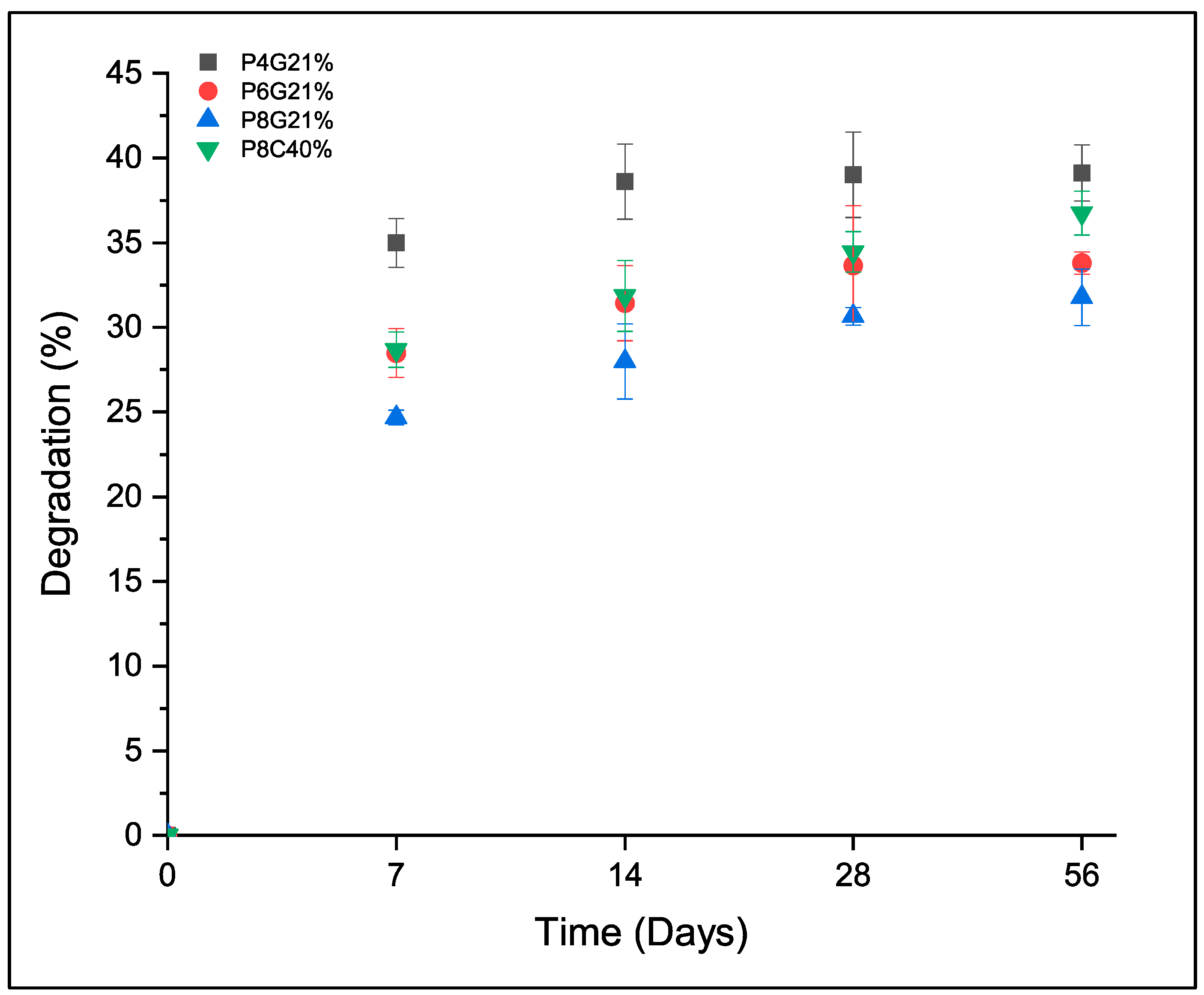
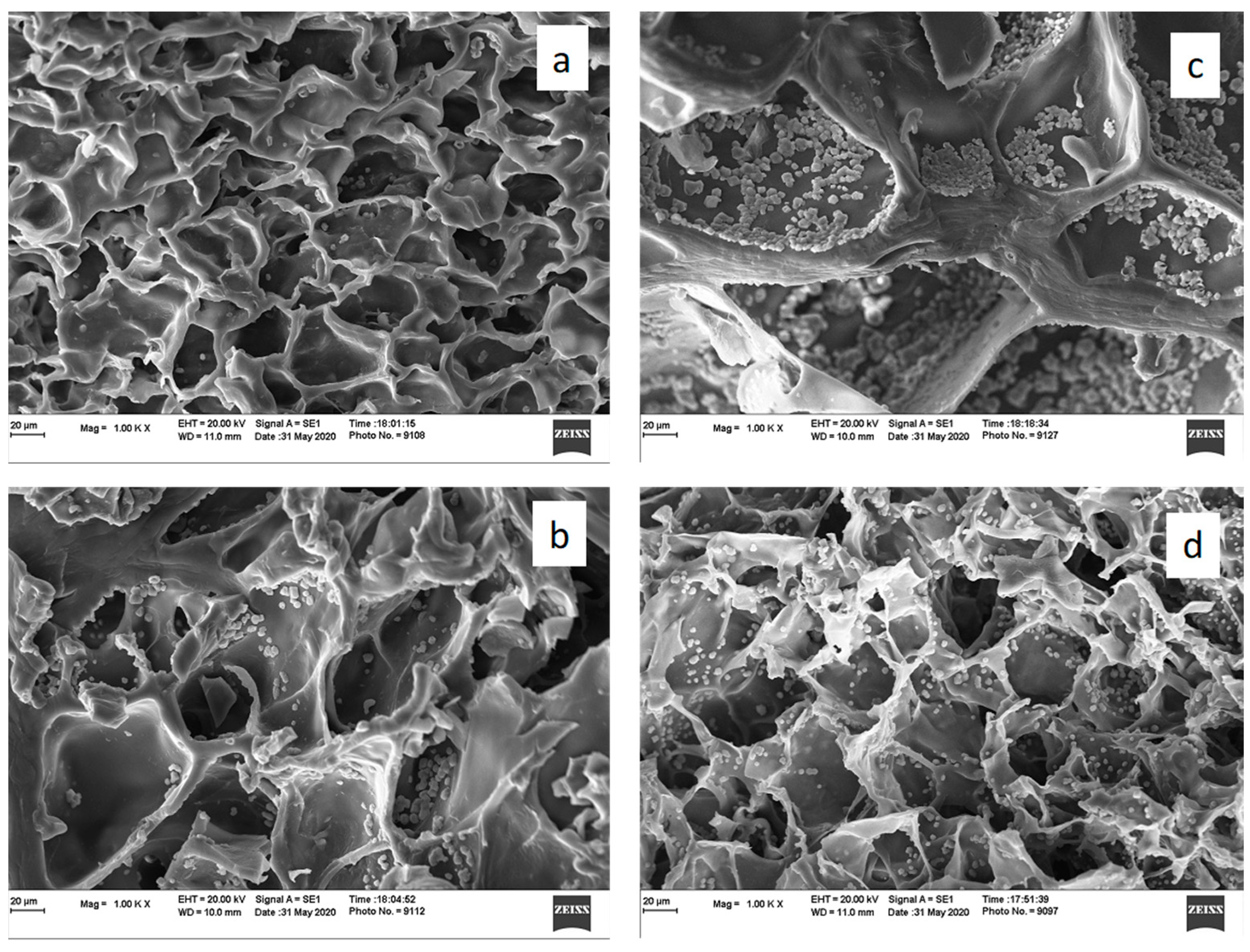
3.1. Characterization of Double Networks
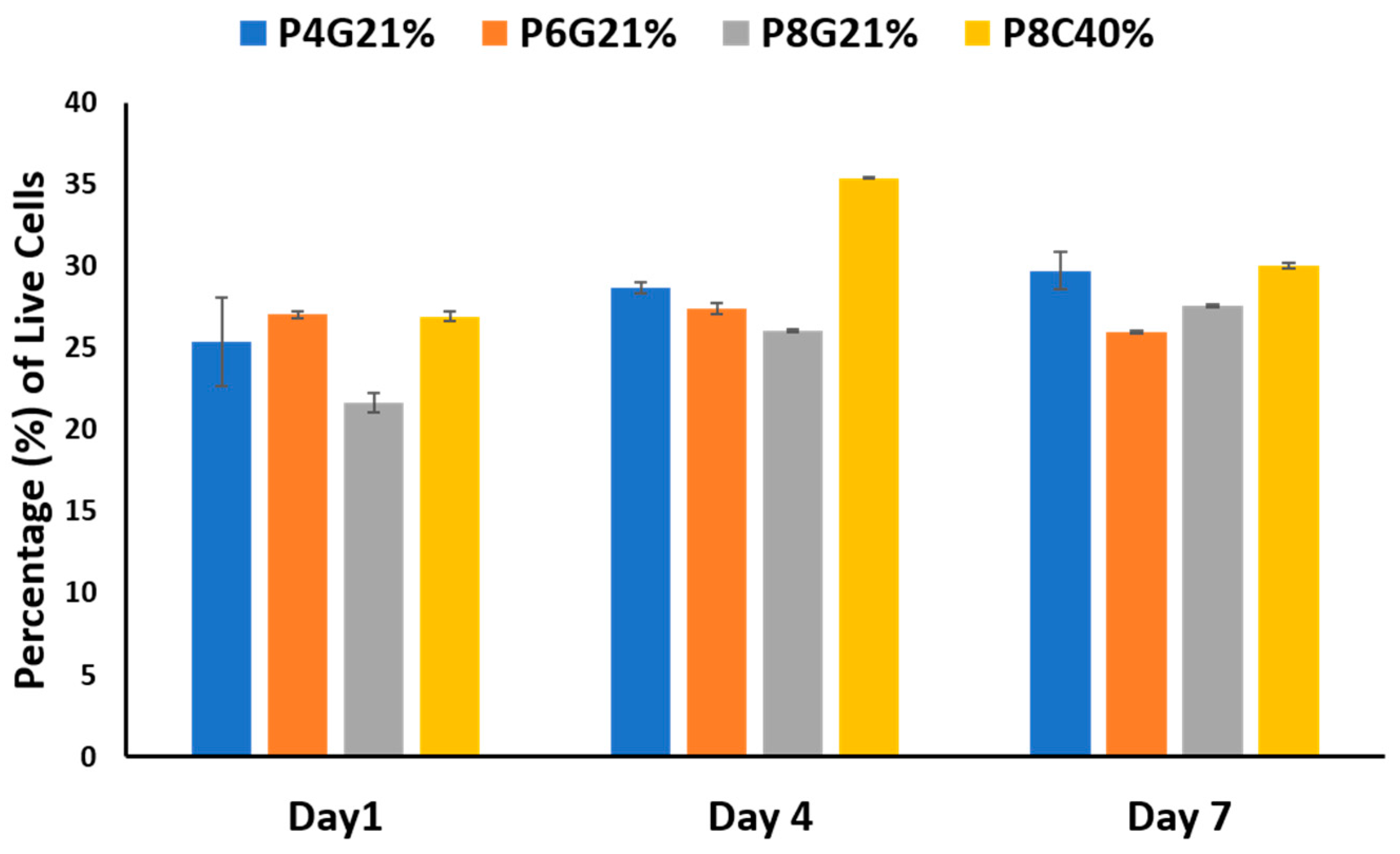
3.2. Cell Growth and Proliferation on the Hydrogel Scaffolds
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kousar, F.; Malana, M.A.; Chughtai, A.H.; Khan, M.S. Synthesis and characterization of methacrylamide-acrylic acid-N-isopropylacrylamide polymeric hydrogel: Degradation kinetics and rheological studies. Biomed. Mater. 2018, 155, 1275–1298. [Google Scholar] [CrossRef]
- Caló, E.; Khutoryanskiy, V.V. Biomedical applications of hydrogels: A review of patents and commercial products. Eur. Polym. J. 2015, 65, 252–267. [Google Scholar] [CrossRef] [Green Version]
- Hoare, T.R.; Kohane, D.S. Hydrogels in drug delivery: Progress and challenges. Polymer 2008, 49, 1993–2007. [Google Scholar] [CrossRef] [Green Version]
- Fu, Y.; Xu, K.; Zheng, X.; Giacomin, A.J.; Mix, A.W.; Kao, W.J. 3D cell entrapment in crosslinked thiolated gelatin-poly (ethylene glycol) diacrylate hydrogels. Biomaterials 2012, 33, 48–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gopinathan, J.; Noh, I. Recent trends in bioinks for 3D printing. Biomater. Res. 2018, 22, 11. [Google Scholar] [CrossRef] [Green Version]
- Khademhosseini, A.; Langer, R. Microengineered hydrogels for tissue engineering. Biomaterials 2007, 28, 5087–5092. [Google Scholar] [CrossRef]
- Peppas, N.A.; Hilt, J.Z.; Khademhosseini, A.; Langer, R. Hydrogels in biology and medicine: From molecular principles to bionanotechnology. Adv. Mater. 2006, 18, 1345–1360. [Google Scholar] [CrossRef]
- Baolin, G.; Ma, P.X. Synthetic biodegradable functional polymers for tissue engineering: A brief review. Sci. China Chem. 2014, 57, 490–500. [Google Scholar]
- Cheng, Y.; Lu, J.; Liu, S.; Zhao, P.; Lu, G.; Chen, J. The preparation, characterization and evaluation of regenerated cellulose/collagen composite hydrogel films. Carbohydr. Polym. 2014, 107, 57–64. [Google Scholar] [CrossRef]
- Matricardi, P.; Di Meo, C.; Coviello, T.; Hennink, W.E.; Alhaique, F. Interpenetrating polymer networks polysaccharide hydrogels for drug delivery and tissue engineering. Adv. Drug Deliv. Rev. 2013, 65, 1172–1187. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.P.; Daniels, A.U.; Ronken, S.; Ardura García, H.; Friederich, N.F.; Kurokawa, T.; Gong, J.P.; Wirz, D. Acrylamide Polymer Double-Network Hydrogels: Candidate Cartilage Repair Materials with Cartilage-Like Dynamic Stiffness and Attractive Surgery-Related Attachment Mechanics. Cartilage 2011, 2, 374–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Wang, H.; Zhang, D.; Xu, Z.; Liu, W. A highly tough and stiff supramolecular polymer double network hydrogel. Polymer 2018, 153, 193–200. [Google Scholar] [CrossRef]
- Li, Z.; Wu, C.; Liu, Z.; Li, Z.; Peng, X.; Huang, J.; Ren, J.; Wang, P. A polypropylene mesh coated with interpenetrating double network hydrogel for local drug delivery in temporary closure of open abdomen. RSC Adv. 2020, 10, 1331–1340. [Google Scholar] [CrossRef] [Green Version]
- Haque, M.A.; Kurokawa, T.; Gong, J.P. Super tough double network hydrogels and their application as biomaterials. Polymer 2012, 53, 1805–1822. [Google Scholar] [CrossRef]
- Lei, K.; Li, Z.; Zhu, D.; Sun, C.; Sun, Y.; Yang, C.; Zheng, Z.; Wang, X. Polysaccharide-based recoverable double-network hydrogel with high strength and self-healing properties. J. Mater. Chem. B 2020, 8, 794–802. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, N.; Thrivikraman, G.; Athirasala, A.; Tahayeri, A.; França, C.M.; Ferracane, J.L.; Bertassoni, L.E. Photopolymerization of cell-laden gelatin methacryloyl hydrogels using a dental curing light for regenerative dentistry. Dent. Mater. 2018, 34, 389–399. [Google Scholar] [CrossRef]
- Joshi, P.; Ahmed, M.S.U.; Vig, K.; Vega Erramuspe, I.B.; Auad, M.L. Synthesis and characterization of chemically crosslinked gelatin and chitosan to produce hydrogels for biomedical applications. Polym. Adv. Technol. 2021, 32, 2229–2239. [Google Scholar] [CrossRef]
- Joshi, P.; Breaux, S.; Naro, J.; Al, E. Synthesis and characterization of photopolymerizable hydrogels based on poly (ethylene glycol) for biomedical applications. J. Appl. Polym. Sci. 2021, 138, e50489. [Google Scholar] [CrossRef]
- Blaine, R.L. Determination of Polymer Crystallinity by DSC; TA Instruments: New Castle, DE, USA, 2010; pp. 1–3. [Google Scholar]
- Pielichowski, K.; Flejtuch, K. Differential Scanning Calorimetry Studies on Poly (ethylene Glycol) with Different Molecular Weights for Thermal Energy Storage Materials. Polym. Adv. Technol. 2002, 13, 690–696. [Google Scholar] [CrossRef]
- ASTMD695-15; Standard Test Method for Compressive Properties of Rigid Plastics. Astm International: West Conshohocken, PE, USA, 2015.
- ASTMD1708-93; Standard Test Method for Tensile Peroperties of Plastics by Use of Microtensile Specimens. Astm International: West Conshohocken, PE, USA, 1993.
- Lončarević, A.; Ivanković, M.; Rogina, A. Lysozyme-Induced Degradation of Chitosan: The Characterisation of Degraded Chitosan Scaffolds. J. Tissue Repair Regen. 2017, 19, 177. Available online: www.openaccesspub.org (accessed on 15 November 2019). [CrossRef] [Green Version]
- Thakur, V.K.; Thakur, M.K. (Eds.) Handbook of Polymers for Pharmaceutical Technologies, Biodegradable Polymers; Scrivener Publishing: Beverly, MA, USA, 2015. [Google Scholar]
- Sarem, M.; Moztarzadeh, F.; Mozafari, M.; Shastri, V.P. Optimization strategies on the structural modeling of gelatin/chitosan scaffolds to mimic human meniscus tissue. Mater. Sci Eng. C. 2013, 33, 4777–4785. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, S.M.; Miguel, S.P.; Ribeiro, M.P.; Coutinho, P.; Correia, I.J. Synthesis and characterization of a photocrosslinkable chitosan-gelatin hydrogel aimed for tissue regeneration. RSC Adv. 2015, 5, 63478–63488. [Google Scholar] [CrossRef]
- Yang, C.; Xu, L.; Zhou, Y.; Zhang, X.; Huang, X.; Wang, M.; Han, Y.K.; Zhai, M.; Wei, S.L.J. A green fabrication approach of gelatin/CM-chitosan hybrid hydrogel for wound healing. Carbohydr. Polym. 2010, 82, 1297–1305. [Google Scholar] [CrossRef]
- Monier, M.; Wei, Y.; Sarhan, A.A.; Ayad, D.M. No Title. Polymer 2010, 51, 1002–1009. [Google Scholar] [CrossRef]
- Escudero-Castellanos, A.; Ocampo-García, B.E.; Domínguez-García, M.V.; Flores-Estrada, J.; Flores-Merino, M.V. Hydrogels based on poly (ethylene glycol) as scaffolds for tissue engineering application: Biocompatibility assessment and effect of the sterilization process. J. Mater. Sci Mater. Med. 2016, 27, 176. [Google Scholar] [CrossRef]
- Guo, X.; Wang, W.; Wu, G.; Zhang, J.; Mao, C.; Deng, Y.; Xia, H. Controlled synthesis of hydroxyapatite crystals templated by novel surfactants and their enhanced bioactivity. New J. Chem. 2011, 35, 663–671. [Google Scholar] [CrossRef]
- Cheing, B.W.; Ibrahim, N.A.; Yunus, W.M.Z.W.; Hussein, M.Z. Poly (lactic acid)/poly (ethylene glycol) polymer nanocomposites: Effects of graphene nanoplatelets. Polymers 2013, 6, 93–104. [Google Scholar] [CrossRef] [Green Version]
- Yasmin, M.; Gupta, M. Thermodynamical Study of Alcoholic Solutions of Poly (ethylene glycol) Diacrylate and Poly (ethylene glycol) Dimethacrylate. Int. J. Thermodyn. 2012, 15, 111–117. [Google Scholar] [CrossRef] [Green Version]


| Double Network Hydrogels | PEGDMA 4000 | PEGDMA 6000 | PEGDMA 8000 |
|---|---|---|---|
| GelMA (7%) | P4G7% | P6G7% | P8G7% |
| GelMA (16%) | P4G16% | P6G16% | P8G16% |
| GelMA (21%) | P4G21% | P6G21% | P8G21% |
| ChMA (40%) | NA | NA | P8C40% |
| Pore Size (µm) | Swelling Ratio (%) | |
|---|---|---|
| PEGDMA 4000 | 61.0 ± 6.7 | 1400 ± 230 |
| PEGDMA 6000 | 72.0 ± 12.7 | 1500 ± 64 |
| PEGDMA 8000 | 87.0 ± 12.7 | 1900 ± 140 |
| GelMA (21%) | 27.4 ± 10.7 | 475 ± 122 |
| ChMA (40%) | 68.1 ± 14.4 | 2208 ± 240 |
| P4G21% | 74.1 ± 20.3 | 708 ± 39 |
| P6G21% | 64.0 ± 30.6 | 588 ± 20 |
| P8G21% | 79.5 ± 5.9 | 678 ± 30 |
| P8C40% | 88.2 ± 18.9 | 2033 ± 463 |
| Tm (°C) | ΔHm (J/g) | Crystallinity (%) | Compressive Modulus (kPa) | Shear Modulus (kPa) | Tensile Modulus (kPa) | Degradative Weight Loss (%) | |
|---|---|---|---|---|---|---|---|
| P4G21% | 22.83 | 17.93 | 18.2 | 8.41 ± 1.1 | 3.67 ± 0.05 | 8.4 ± 2.3 | 39 ± 1.6 |
| P6G21% | 29.57 | 25.37 | 25.8 | 10.8 ± 2.3 | 6.42 ± 0.86 | 13.9 ± 2.6 | 34 ± 1.2 |
| P8G21% | 44.23 | 27.79 | 28.2 | 9.97 ± 1.2 | 4.04 ± 0.85 | 16.1 ± 3.7 | 32 ± 1.1 |
| P8C40% | 27.33 | 7.15 | 7.3 | 77.6 ± 11.0 | 13.8 ± 1.1 | 21.2 ± 6.7 | 37 ± 1.3 |
| GelMA (21%) | NA | NA | NA | 30.4 ± 7.2 | 4.0 ± 0.6 | NA | 16 ± 3.8 |
| ChMA (40%) | NA | NA | NA | 33.0 ± 9.2 | 5.6 ± 0.9 | NA | 35 ± 5.5 |
| PEGDMA 4000 | 48.42 | 70.57 | 35.9 | 17 ± 7 | 2.7 ± 0.73 | 13.1 ± 6.5 | 23 ± 1.1 |
| PEGDMA 6000 | 56.31 | 109.3 | 55.5 | 18.8 ± 6 | 4.31 ± 0.68 | 20.7 ± 6.1 | 24 ± 1.2 |
| PEGDMA 8000 | 57.93 | 151.6 | 77.0 | 16.5 ± 4 | 1.9 ± 0.43 | 31.3 ± 9.7 | 28 ± 1.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joshi, P.; Uddin Ahmed, M.S.; Vig, K.; Auad, M.L. Formulation of the Polymeric Double Networks (DNs) for Biomedical Applications with Physicochemical Properties to Resemble a Biological Tissue. Sustain. Chem. 2022, 3, 248-258. https://doi.org/10.3390/suschem3020016
Joshi P, Uddin Ahmed MS, Vig K, Auad ML. Formulation of the Polymeric Double Networks (DNs) for Biomedical Applications with Physicochemical Properties to Resemble a Biological Tissue. Sustainable Chemistry. 2022; 3(2):248-258. https://doi.org/10.3390/suschem3020016
Chicago/Turabian StyleJoshi, Prutha, Md Shakir Uddin Ahmed, Komal Vig, and Maria L. Auad. 2022. "Formulation of the Polymeric Double Networks (DNs) for Biomedical Applications with Physicochemical Properties to Resemble a Biological Tissue" Sustainable Chemistry 3, no. 2: 248-258. https://doi.org/10.3390/suschem3020016
APA StyleJoshi, P., Uddin Ahmed, M. S., Vig, K., & Auad, M. L. (2022). Formulation of the Polymeric Double Networks (DNs) for Biomedical Applications with Physicochemical Properties to Resemble a Biological Tissue. Sustainable Chemistry, 3(2), 248-258. https://doi.org/10.3390/suschem3020016







