Exploring Neuroprotection against Radiation-Induced Brain Injury: A Review of Key Compounds
Abstract
1. Introduction
2. Principles of Radiotherapy in Normal and Cancerous Cells
2.1. Radiation-Induced DNA Damage
2.2. Double-Strand Break Repair Mechanisms (DSB)
2.3. Cell Death Mechanisms
2.3.1. Apoptosis
2.3.2. Mitotic Catastrophe (MC)
2.3.3. Senescence
3. Oxidative Stress and Antioxidant Defenses in the CNS: Implications for Radiation-Induced Brain Injury
4. Hallmarks of Brain Injury Induced by Radiation
4.1. Inflammation
4.2. Brain Edema
4.3. Astrogliosis
5. Use of Other Pharmacological Approaches against RIBI in Clinical Setting
5.1. Memantine
5.2. Vitamin C or Ascorbic Acid
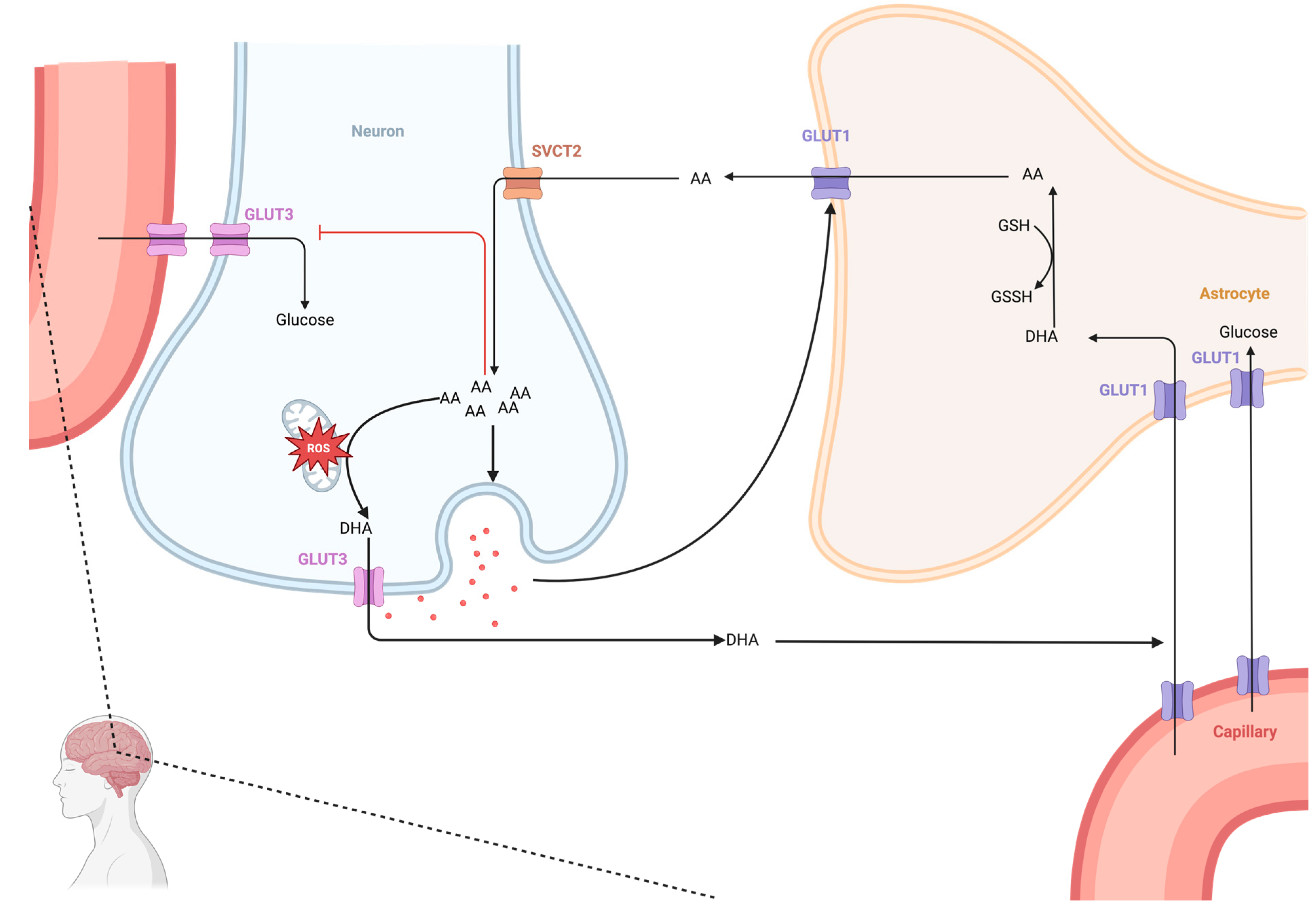
5.2.1. Dynamics of Ascorbate in the CNS
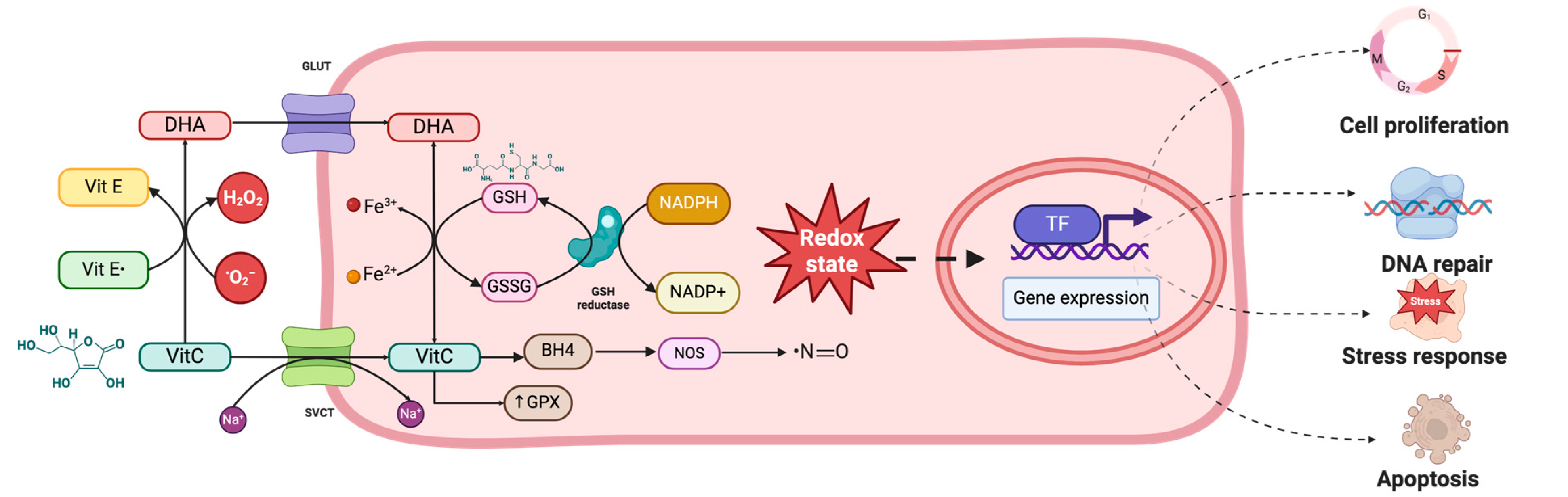
5.2.2. Antioxidant Effects of Ascorbic Acid
5.3. The Role of Ascorbic Acid in Preventing Radiation-Induced Brain Injury (RIBI)
5.4. The Dual Effect of Ascorbic Acid in Cancer
| Details of the Study | Model | Groups | Irradiation Procedure | Drug Tested | Cognitive Testing | Other Evaluations | Results | Ref. |
|---|---|---|---|---|---|---|---|---|
| MitoQ | KM | Divided into four groups, 10 mice each: 1. G1: ip PSS 0.9% for 3 days 2. G2: ip MitoQ 3. G3: WBI 4. G4: ip MitoQ + WBI | WBI of mice was performed using a high-LET 56-Fe ions beams at the energy of 160 MeV/μ. Each mouse received 2 Gy doses at a dose rate of 0.5 Gy/min, and the mice were placed in the plateau region. | MitoQ groups received MitoQ (5 mg/kg/day) for 3 days | (-) | -Determination of oxidative stress parameters (PCO, MDA, SOD, CAT) -Mitochondrial respiration measurements (O2 consumption and RCR) -Measurement of mitochondria-generated ROS -mtDNA damage assay -mitochondrial dynamics protein (Mfn2, Drp1, bcl-2, bax, cyto c) -Gene expression analysis (BA; Casp3; SOD2; Opa1) | MitoQ reduced radiation-induced oxidative stress with decreased lipid peroxidation and reduced protein and DNA oxidation. MitoQ protected mitochondrial respiration after RT. MitoQ increased Mfn2 and OPA1 and decreased Drp1. MitoQ also suppressed mitochondrial DNA damage, cyto c release, and caspase-3 activity in RT-treated mice compared to the control group [152]. | [155] |
| Quercetin | WAR | Divided into 4 groups (n = 8/each): 1. control group 2. G QUER: quercetin 3. G RAD was given only irradiation 4. G RAD + QUER: quercetin + irradiation | RAD groups were subjected to cranium irradiation with a single dose of 20 Gy of photons using a 6 MV LINAC at a dose rate of ~1 Gy/min, with the source–axis distance technique, with 1.0 cm of bolus material on the surface. | QUER groups received Quercetin 50 mg/kg body weight (BW) daily in distilled water and 0.25 mL PS for 15 days. | (-) | -Total antioxidant status and MDA -Brain histopathological evaluation | Tissue samples and biochemical levels of tissue-injury markers in the four groups were compared. In all measured parameters of oxidative stress, administration of quercetin significantly demonstrated favorable effects. Both plasma and tissue levels of MDA and total antioxidant status significantly changed in favor of antioxidant activity. Histopathological evaluation of the tissues also demonstrated a significant decrease in cellular degeneration and infiltration parameters after quercetin administration. Quercetin demonstrated significant neuroprotection after radiation-induced brain injury. | [156] |
| Date syrup | WAR | Divided into 4 groups, 15 rats each. 1. G1 (Control); received 1 mL 0.9% saline solution orally for 4 weeks and served as control; 2. G2 (Irradiated); was exposed to radiation at a dose level of 6 Gy and sacrificed after 48 h. 3. G3 (Date syrup); 4. G4 (Irradiated + Date syrup) | Whole-body gamma-irradiation. Animals were irradiated at an acute Single-dose level of 6 Gy delivered at a dose rate of 0.713 rad/s. | Date syrup group received daily date syrup by stomach intubation at a dose of 4 mL/kg body weight for 4 weeks. | (-) | -Serum biochemical analysis. -Assessment of oxidant/antioxidant biomarkers (lipid peroxidation, DNA damage, GSH, CAT activity -Assessment of MMP-9 -q RT-PCR evaluation for TNF-α gene expression -Liver histopathological examination | Pretreatment of rats with Date syrup ameliorated the tissue damage induced by radiation as evidenced by the improvement in liver function, antioxidant status and reduction in DNA damage. Moreover, liver TNF-α expression and serum MMP-9 activity were reduced. | [157] |
| NSI-189 | LER | Divided into 3 groups (n = 15–16/each): 1. controls receiving oral gavage (vehicle only) and sham irradiation 2. cohorts receiving oral gavage (vehicle only) and 27 Gy head-only fractionated exposure 3. cohorts receiving oral gavage (NSI-189, 30 mg/kg) and 27 Gy head-only fractionated exposure | For CI, animals were positioned under a collimated (1 cm2 diameter) beam for head-only irradiation delivered at a dose rate of 1 Gy/min. Fractionation of 27 Gy was delivered over 3 separate doses of 8.67 Gy, which were administered 48 h apart. | NSI 189 The drug was administered by daily oral gavage at a concentration adjusted to the weight of the animals. The daily dosing was set at 2 mL/kg, setting the target daily dose of 30 mg/kg. Thus, the daily volume of the drug typically varied between 0.6 and 1.0 mL/rat. | cognitive testing 1 week after termination of oral gavage (5 weeks post-RT). Cognitive testing was performed over the course of three weeks and included four different spontaneous exploration tasks (novel place recognition, novel object recognition, object in place and temporal order) followed by contextual and cued fear conditioning | -Assessment of neurogenesis -Determination of hippocampal volume -Assessment of activated microglia | NSI-189 treatment resulted in significantly improved performance in four of these tasks: novel-place recognition, novel-object recognition, object in place and temporal order. In addition, there was a trend for improved performance in the contextual phase of the fear-conditioning task. Importantly, enhanced cognition in the NSI-189-treated cohort was found to persist one month after the cessation of drug treatment. These neurocognitive benefits of NSI-189 coincided with a significant increase in neurogenesis and a significant decrease in the numbers of activated microglia compared to the irradiated cohort that was given the vehicle alone. | [158] |
| Fingolimod | CM | Divided into 4 groups: 1. G1: methylcellulose vehicle alone 2. G2: vehicle + radiation 3. G3: FTY720 4. G4: FTY720 + radiation | For irradiation, a Gammacell 40 irradiator with a dose rate of 95. cGy/minute was used. A single dose of 7 Gy was administered to each animal. | FTY720 groups received three ip of 0.5 mg/kg FTY720 in the week prior to irradiation. They then received 3 ip/week of vehicle or 0.5 mg/kg FTY720 for 6 weeks. | Fear conditioning and MWM were then employed to test learning and memory. | -IF and IHC of brain tissue (antibodies: anti S1PR1, nestin, GFAP, doublecortin, NeuN, Tubulin III/Tuj1) -qRT-PCR of BDNF vs. B2 | The learning deficits were fully restored by FTY720. In irradiated brains, FTY720 maintained the cytoarchitecture of the dentate gyrus granular cell layer and partially restored the pool of NPC. In mice harboring BTSC xenografts, FTY720 delayed tumor growth and improved survival. | [159] |
| mNGF | SDR | Divided into 3 groups: G1: control (n = 15) G2: mNGF + CI (n = 20) G3: PSS + CI (n = 20) | CI at a single dose of 12 Gy by X-ray. | ? | MWM experiment | EB leakage of the brain, and expressions of neuN, vWF, ZO-1 in hippocampus by immunofluorescence, and expressions of neuN, vWF, ZO-1, VEGF and GFAP in hippocampus by WB | mNGF decreases the damage by RT, improving the latency time of escape in the Morris water maze, and decreases the EB leakage. In the IF, mNGF increases the expression of neunN, vWF abd ZO-1. In WB, mNGF increases the expression of neuN, vWF and ZO-1. | [160] |
| Kukoamine (KuA) | WAR | Divided into 5 groups (n = 5–8/group): 1. G1: sham irradiation 2. G2: CI 3. G3: CI + KuA low dose 4. G4: CI + KuA middle dose 5. G5: CI + KuA high dose | CI was performed with 6-MeV electron beams delivered by a LINAC. Irradiated rats received a single dose of 30 Gy X-rays at a dose rate of 250 cGy/min. | KuA was administered at a dose of 5, (G3) 10 (G4) and 20 mg/kg (G5) body weight. | (-) | -MDA, GSH level and SOD, CAT activity assays -Nissl Staining and TUNEL staining -WB, using antibodies anti: BDNF, Casp3, CytC, Bax, Bcl2, GAPDH, BA | Whole brain irradiation led to the neuronal abnormality and it was alleviated by KuA. KuA decreased MDA level, increased GSH level, SOD and CAT activities, as well as alleviated neuronal apoptosis by regulating the expression of cleaved caspase-3, cytochrome C, Bax and Bcl2. Additionally, KuA increased the expression of BDNF. | [15] |
| Acanthopanax | KM | Divided into 3 large groups (n = 32 each) G1: behavioral test G2: pathological sections G3: metabolomics analysis. Each large group was divided into 4 small groups for the experiments (n = 8 per group) g1: normal control g2: model set (CI) g3: treatment group AS + CI g4: treatment group V + CI | Irradiated by 60 Co-γ ray irradiation with the mean LET of 62.2 KeV/μm at a dose of 4 Gy and a dose rate of 0.1 Gy/min. | AS was administered at a dose of 235.7 mg/kg/day. V was administered at a dose of 13.75 mg/kg/day. | MWM and sucrose preference test | -Production of pathological sections for brain tissues (PFC) -Metabolomics analysis based on 1H NMR | AS significantly improved the decline of low LET-induced learning ability and spatial memory capacity, increased the sensitivity of the nervous system and, to a certain degree, prevented brain tissue lesions caused by radiation. In our study, we also observed that AS had a better effect on brain tissue development and brain–glutamate-cycle balance compared with a chemical drug (Venlafaxine). | [161] |
6. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AA | ascorbic acid |
| AQP4 | aquaporin-4 |
| BMs | brain metastases |
| CAT | catalase |
| CD | cell death |
| CNS | central nervous system |
| CSF | cerebrospinal fluid |
| DDR | DNA damage response |
| DHA | dehydroascorbic acid |
| DSB | double-strand break |
| ERK | extracellular-signal-regulated kinase |
| FRs | free radicals |
| GPx | glutathione peroxidase |
| GSH | reduced glutathione |
| HR | homologous recombination |
| IAP | intrinsic apoptotic pathway |
| JNK | c-Jun N-terminal kinase |
| LQ | linear quadratic |
| MAPK | mitogen-activated protein kinase |
| NHJE | non-homologous end-joining |
| NMDAR | N-methyl-D-aspartate receptor |
| RIBI | radiation-induced brain injury |
| ROS | reactive oxygen species |
| RT | radiotherapy |
| SOD | superoxide dismutase |
| SSB | single-strand break |
| TME | tumor microenvironment |
| WBRT | whole brain radiation therapy |
References
- Rahman, R.; Sulman, E.; Haas-Kogan, D.; Cagney, D.N. Update on Radiation Therapy for Central Nervous System Tumors. Hematol. Oncol. Clin. N. Am. 2022, 36, 77–93. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, W.; Cao, W.-D.; Cheng, G.; Liu, B.; Cheng, J. A Review of Current Management of Brain Metastases. Ann. Surg. Oncol. 2012, 19, 1043–1050. [Google Scholar] [CrossRef] [PubMed]
- Wefel, J.S.; Parsons, M.W.; Gondi, V.; Brown, P.D. Neurocognitive Aspects of Brain Metastasis. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2018; pp. 155–165. [Google Scholar]
- Khuntia, D.; Brown, P.; Li, J.; Mehta, M.P. Whole-Brain Radiotherapy in the Management of Brain Metastasis. J. Clin. Oncol. 2006, 24, 1295–1304. [Google Scholar] [CrossRef]
- Gondi, V.; Bauman, G.; Bradfield, L.; Burri, S.H.; Cabrera, A.R.; Cunningham, D.A.; Eaton, B.R.; Hattangadi-Gluth, J.A.; Kim, M.M.; Kotecha, R.; et al. Radiation Therapy for Brain Metastases: An ASTRO Clinical Practice Guideline. Pract. Radiat. Oncol. 2022, 12, 265–282. [Google Scholar] [CrossRef]
- Rades, D.; Bohlen, G.; Lohynska, R.; Veninga, T.; Stalpers, L.J.A.; Schild, S.E.; Dunst, J. Whole-Brain Radiotherapy with 20 Gy in 5 Fractions for Brain Metastases in Patients with Cancer of Unknown Primary (CUP). Strahlenther. Onkol. 2007, 183, 631–636. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Bentzen, S.M.; Renschler, M.; Mehta, M.P. Regression after Whole-Brain Radiation Therapy for Brain Metastases Correlates with Survival and Improved Neurocognitive Function. J. Clin. Oncol. 2007, 25, 1260–1266. [Google Scholar] [CrossRef]
- Achrol, A.S.; Rennert, R.C.; Anders, C.; Soffietti, R.; Ahluwalia, M.S.; Nayak, L.; Peters, S.; Arvold, N.D.; Harsh, G.R.; Steeg, P.S.; et al. Brain Metastases. Nat. Rev. Dis. Primer 2019, 5, 5. [Google Scholar] [CrossRef]
- Gondi, V.; Tomé, W.A.; Mehta, M.P. Why Avoid the Hippocampus? A Comprehensive Review. Radiother. Oncol. 2010, 97, 370–376. [Google Scholar] [CrossRef]
- Brown, P.D.; Jaeckle, K.; Ballman, K.V.; Farace, E.; Cerhan, J.H.; Anderson, S.K.; Carrero, X.W.; Barker, F.G.; Deming, R.; Burri, S.H.; et al. Effect of Radiosurgery Alone vs Radiosurgery With Whole Brain Radiation Therapy on Cognitive Function in Patients With 1 to 3 Brain Metastases: A Randomized Clinical Trial. JAMA 2016, 316, 401–409. [Google Scholar] [CrossRef]
- Brown, P.D.; Ballman, K.V.; Cerhan, J.H.; Anderson, S.K.; Carrero, X.W.; Whitton, A.C.; Greenspoon, J.; Parney, I.F.; Laack, N.N.I.; Ashman, J.B.; et al. Postoperative Stereotactic Radiosurgery Compared with Whole Brain Radiotherapy for Resected Metastatic Brain Disease (NCCTG N107C/CEC·3): A Multicentre, Randomised, Controlled, Phase 3 Trial. Lancet Oncol. 2017, 18, 1049–1060. [Google Scholar] [CrossRef]
- Chang, E.L.; Wefel, J.S.; Hess, K.R.; Allen, P.K.; Lang, F.F.; Kornguth, D.G.; Arbuckle, R.B.; Swint, J.M.; Shiu, A.S.; Maor, M.H.; et al. Neurocognition in Patients with Brain Metastases Treated with Radiosurgery or Radiosurgery plus Whole-Brain Irradiation: A Randomised Controlled Trial. Lancet Oncol. 2009, 10, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- Gondi, V.; Pugh, S.L.; Tome, W.A.; Caine, C.; Corn, B.; Kanner, A.; Rowley, H.; Kundapur, V.; DeNittis, A.; Greenspoon, J.N.; et al. Preservation of Memory with Conformal Avoidance of the Hippocampal Neural Stem-Cell Compartment during Whole-Brain Radiotherapy for Brain Metastases (RTOG 0933): A Phase II Multi-Institutional Trial. J. Clin. Oncol. 2014, 32, 3810–3816. [Google Scholar] [CrossRef] [PubMed]
- Tsao, M.N.; Xu, W.; Wong, R.K.; Lloyd, N.; Laperriere, N.; Sahgal, A.; Rakovitch, E.; Chow, E. Whole Brain Radiotherapy for the Treatment of Newly Diagnosed Multiple Brain Metastases. Cochrane Database Syst. Rev. 2018, 1, CD003869. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheng, Z.; Wang, C.; Ma, H.; Meng, W.; Zhao, Q. Neuroprotective Effects of Kukoamine a against Radiation-Induced Rat Brain Injury through Inhibition of Oxidative Stress and Neuronal Apoptosis. Neurochem. Res. 2016, 41, 2549–2558. [Google Scholar] [CrossRef]
- Warrington, J.P.; Ashpole, N.; Csiszar, A.; Lee, Y.W.; Ungvari, Z.; Sonntag, W.E. Whole Brain Radiation-Induced Vascular Cognitive Impairment: Mechanisms and Implications. J. Vasc. Res. 2013, 50, 445–457. [Google Scholar] [CrossRef]
- Yang, L.; Yang, J.; Li, G.; Li, Y.; Wu, R.; Cheng, J.; Tang, Y. Pathophysiological Responses in Rat and Mouse Models of Radiation-Induced Brain Injury. Mol. Neurobiol. 2017, 54, 1022–1032. [Google Scholar] [CrossRef] [PubMed]
- Barker, H.E.; Paget, J.T.E.; Khan, A.A.; Harrington, K.J. The Tumour Microenvironment after Radiotherapy: Mechanisms of Resistance and Recurrence. Nat. Rev. Cancer 2015, 15, 409–425. [Google Scholar] [CrossRef]
- Steel, G.G.; McMillan, T.J.; Peacock, J.H. The 5Rs of Radiobiology. Int. J. Radiat. Biol. 1989, 56, 1045–1048. [Google Scholar] [CrossRef]
- Fowler, J.F. Review: Total Doses in Fractionated Radiotherapy--Implications of New Radiobiological Data. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1984, 46, 103–120. [Google Scholar] [CrossRef]
- Santacroce, A.; Kamp, M.A.; Budach, W.; Hänggi, D. Radiobiology of Radiosurgery for the Central Nervous System. BioMed Res. Int. 2013, 2013, 362761. [Google Scholar] [CrossRef]
- Sminia, P.; Guipaud, O.; Viktorsson, K.; Ahire, V.; Baatout, S.; Boterberg, T.; Cizkova, J.; Dostál, M.; Fernandez-Palomo, C.; Filipova, A.; et al. Clinical Radiobiology for Radiation Oncology. In Radiobiology Textbook; Baatout, S., Ed.; Springer International Publishing: Cham, Switzerland, 2023; pp. 237–309. ISBN 978-3-031-18810-7. [Google Scholar]
- Shiloh, Y. ATM and Related Protein Kinases: Safeguarding Genome Integrity. Nat. Rev. Cancer 2003, 3, 155–168. [Google Scholar] [CrossRef] [PubMed]
- Abraham, R.T. Cell Cycle Checkpoint Signaling through the ATM and ATR Kinases. Genes Dev. 2001, 15, 2177–2196. [Google Scholar] [CrossRef] [PubMed]
- Maier, P.; Hartmann, L.; Wenz, F.; Herskind, C. Cellular Pathways in Response to Ionizing Radiation and Their Targetability for Tumor Radiosensitization. Int. J. Mol. Sci. 2016, 17, 102. [Google Scholar] [CrossRef] [PubMed]
- Lukas, J.; Lukas, C.; Bartek, J. Mammalian Cell Cycle Checkpoints: Signalling Pathways and Their Organization in Space and Time. DNA Repair 2004, 3, 997–1007. [Google Scholar] [CrossRef] [PubMed]
- Sia, J.; Szmyd, R.; Hau, E.; Gee, H.E. Molecular Mechanisms of Radiation-Induced Cancer Cell Death: A Primer. Front. Cell Dev. Biol. 2020, 8, 41. [Google Scholar] [CrossRef]
- Helton, E.S.; Chen, X. P53 Modulation of the DNA Damage Response. J. Cell. Biochem. 2007, 100, 883–896. [Google Scholar] [CrossRef]
- Abend, M. Reasons to Reconsider the Significance of Apoptosis for Cancer Therapy. Int. J. Radiat. Biol. 2003, 79, 927–941. [Google Scholar] [CrossRef]
- Shinomiya, N. New Concepts in Radiation-Induced Apoptosis: “premitotic Apoptosis” and “Postmitotic Apoptosis. J. Cell. Mol. Med. 2001, 5, 240–253. [Google Scholar] [CrossRef]
- Eriksson, D.; Stigbrand, T. Radiation-Induced Cell Death Mechanisms. Tumour Biol. 2010, 31, 363–372. [Google Scholar] [CrossRef]
- Igney, F.H.; Krammer, P.H. Death and Anti-Death: Tumour Resistance to Apoptosis. Nat. Rev. Cancer 2002, 2, 277–288. [Google Scholar] [CrossRef]
- Scaffidi, C.; Fulda, S.; Srinivasan, A.; Friesen, C.; Li, F.; Tomaselli, K.J.; Debatin, K.M.; Krammer, P.H.; Peter, M.E. Two CD95 (APO-1/Fas) Signaling Pathways. EMBO J. 1998, 17, 1675–1687. [Google Scholar] [CrossRef] [PubMed]
- Kastan, M. On the TRAIL from P53 to Apoptosis? Nat. Genet. 1997, 17, 130–131. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; El-Deiry, W.S. Overview of Cell Death Signaling Pathways. Cancer Biol. Ther. 2005, 4, 147–171. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Maiuri, M.C.; Vitale, I.; Zischka, H.; Castedo, M.; Zitvogel, L.; Kroemer, G. Cell Death Modalities: Classification and Pathophysiological Implications. Cell Death Differ. 2007, 14, 1237–1243. [Google Scholar] [CrossRef]
- Roninson, I.B.; Broude, E.V.; Chang, B.D. If Not Apoptosis, Then What? Treatment-Induced Senescence and Mitotic Catastrophe in Tumor Cells. Drug Resist. Updat. Rev. Comment. Antimicrob. Anticancer Chemother. 2001, 4, 303–313. [Google Scholar] [CrossRef]
- Ruth, A.C.; Roninson, I.B. Effects of the Multidrug Transporter P-Glycoprotein on Cellular Responses to Ionizing Radiation. Cancer Res. 2000, 60, 2576–2578. [Google Scholar]
- Weaver, B.A.A.; Cleveland, D.W. Decoding the Links between Mitosis, Cancer, and Chemotherapy: The Mitotic Checkpoint, Adaptation, and Cell Death. Cancer Cell 2005, 8, 7–12. [Google Scholar] [CrossRef]
- Yamada, H.Y.; Gorbsky, G.J. Spindle Checkpoint Function and Cellular Sensitivity to Antimitotic Drugs. Mol. Cancer Ther. 2006, 5, 2963–2969. [Google Scholar] [CrossRef]
- Ianzini, F.; Bertoldo, A.; Kosmacek, E.A.; Phillips, S.L.; Mackey, M.A. Lack of P53 Function Promotes Radiation-Induced Mitotic Catastrophe in Mouse Embryonic Fibroblast Cells. Cancer Cell Int. 2006, 6, 11. [Google Scholar] [CrossRef][Green Version]
- Eriksson, D.; Löfroth, P.-O.; Johansson, L.; Riklund, K.A.; Stigbrand, T. Cell Cycle Disturbances and Mitotic Catastrophes in HeLa Hep2 Cells Following 2.5 to 10 Gy of Ionizing Radiation. Clin. Cancer Res. 2007, 13, 5501s–5508s. [Google Scholar] [CrossRef][Green Version]
- Somosy, Z. Radiation Response of Cell Organelles. Micron 2000, 31, 165–181. [Google Scholar] [CrossRef] [PubMed]
- Castedo, M.; Kroemer, G. Mitotic catastrophe: A special case of apoptosis. J. Soc. Biol. 2004, 198, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Hayflick, L.; Moorhead, P.S. The Serial Cultivation of Human Diploid Cell Strains. Exp. Cell Res. 1961, 25, 585–621. [Google Scholar] [CrossRef]
- Stein, G.H.; Dulić, V. Origins of G1 Arrest in Senescent Human Fibroblasts. BioEssays News Rev. Mol. Cell. Dev. Biol. 1995, 17, 537–543. [Google Scholar] [CrossRef]
- Coppé, J.-P.; Desprez, P.-Y.; Krtolica, A.; Campisi, J. The Senescence-Associated Secretory Phenotype: The Dark Side of Tumor Suppression. Annu. Rev. Pathol. 2010, 5, 99–118. [Google Scholar] [CrossRef]
- Novakova, Z.; Hubackova, S.; Kosar, M.; Janderova-Rossmeislova, L.; Dobrovolna, J.; Vasicova, P.; Vancurova, M.; Horejsi, Z.; Hozak, P.; Bartek, J.; et al. Cytokine Expression and Signaling in Drug-Induced Cellular Senescence. Oncogene 2010, 29, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Fuchs-Tarlovsky, V. Role of Antioxidants in Cancer Therapy. Nutr. Burbank Los Angel. Cty. Calif 2013, 29, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Shirazi, A.; Mihandoost, E.; Ghobadi, G.; Mohseni, M.; Ghazi-khansari, M. Evaluation of Radio-Protective Effect of Melatonin on Whole Body Irradiation Induced Liver Tissue Damage. Cell J. Yakhteh 2013, 14, 292–297. [Google Scholar]
- Rani, V.; Deep, G.; Singh, R.K.; Palle, K.; Yadav, U.C.S. Oxidative Stress and Metabolic Disorders: Pathogenesis and Therapeutic Strategies. Life Sci. 2016, 148, 183–193. [Google Scholar] [CrossRef]
- Pinilla-González, V.; Montecinos-Barrientos, B.; Martin-Kommer, C.; Chichiarelli, S.; Saso, L.; Rodrigo, R. Exploring Antioxidant Strategies in the Pathogenesis of ALS. Open Life Sci. 2024, 19, 20220842. [Google Scholar] [CrossRef]
- Maldonado, E.; Morales-Pison, S.; Urbina, F.; Solari, A. Aging Hallmarks and the Role of Oxidative Stress. Antioxidants 2023, 12, 651. [Google Scholar] [CrossRef] [PubMed]
- Ionescu-Tucker, A.; Cotman, C.W. Emerging Roles of Oxidative Stress in Brain Aging and Alzheimer’s Disease. Neurobiol. Aging 2021, 107, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Kirkman, H.N.; Gaetani, G.F. Mammalian Catalase: A Venerable Enzyme with New Mysteries. Trends Biochem. Sci. 2007, 32, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Baxter, P.S.; Hardingham, G.E. Adaptive Regulation of the Brain’s Antioxidant Defences by Neurons and Astrocytes. Free Radic. Biol. Med. 2016, 100, 147–152. [Google Scholar] [CrossRef]
- Cobley, J.N.; Fiorello, M.L.; Bailey, D.M. 13 Reasons Why the Brain Is Susceptible to Oxidative Stress. Redox Biol. 2018, 15, 490–503. [Google Scholar] [CrossRef]
- Magistretti, P.J.; Allaman, I. A Cellular Perspective on Brain Energy Metabolism and Functional Imaging. Neuron 2015, 86, 883–901. [Google Scholar] [CrossRef]
- Goyal, M.S.; Hawrylycz, M.; Miller, J.A.; Snyder, A.Z.; Raichle, M.E. Aerobic Glycolysis in the Human Brain Is Associated with Development and Neotenous Gene Expression. Cell Metab. 2014, 19, 49–57. [Google Scholar] [CrossRef]
- Ren, X.; Zou, L.; Zhang, X.; Branco, V.; Wang, J.; Carvalho, C.; Holmgren, A.; Lu, J. Redox Signaling Mediated by Thioredoxin and Glutathione Systems in the Central Nervous System. Antioxid. Redox Signal. 2017, 27, 989–1010. [Google Scholar] [CrossRef]
- Que, E.L.; Domaille, D.W.; Chang, C.J. Metals in Neurobiology: Probing Their Chemistry and Biology with Molecular Imaging. Chem. Rev. 2008, 108, 1517–1549. [Google Scholar] [CrossRef]
- Edmondson, D.E. Hydrogen Peroxide Produced by Mitochondrial Monoamine Oxidase Catalysis: Biological Implications. Curr. Pharm. Des. 2014, 20, 155–160. [Google Scholar] [CrossRef]
- Bazinet, R.P.; Layé, S. Polyunsaturated Fatty Acids and Their Metabolites in Brain Function and Disease. Nat. Rev. Neurosci. 2014, 15, 771–785. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An Iron-Dependent Form of Nonapoptotic Cell Death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Burns, T.C.; Awad, A.J.; Li, M.D.; Grant, G.A. Radiation-Induced Brain Injury: Low-Hanging Fruit for Neuroregeneration. Neurosurg. Focus 2016, 40, E3. [Google Scholar] [CrossRef] [PubMed]
- Sotomayor, C.G.; González, C.; Soto, M.; Moreno-Bertero, N.; Opazo, C.; Ramos, B.; Espinoza, G.; Sanhueza, Á.; Cárdenas, G.; Yévenes, S.; et al. Ionizing Radiation-Induced Oxidative Stress in Computed Tomography-Effect of Vitamin C on Prevention of DNA Damage: PREVIR-C Randomized Controlled Trial Study Protocol. J. Clin. Med. 2024, 13, 3866. [Google Scholar] [CrossRef]
- Azzam, E.I.; Jay-Gerin, J.-P.; Pain, D. Ionizing Radiation-Induced Metabolic Oxidative Stress and Prolonged Cell Injury. Cancer Lett. 2012, 327, 48–60. [Google Scholar] [CrossRef]
- Benhar, M.; Engelberg, D.; Levitzki, A. ROS, Stress-Activated Kinases and Stress Signaling in Cancer. EMBO Rep. 2002, 3, 420–425. [Google Scholar] [CrossRef] [PubMed]
- Son, Y.; Cheong, Y.-K.; Kim, N.-H.; Chung, H.-T.; Kang, D.G.; Pae, H.-O. Mitogen-Activated Protein Kinases and Reactive Oxygen Species: How Can ROS Activate MAPK Pathways? J. Signal Transduct. 2011, 2011, 792639. [Google Scholar] [CrossRef]
- Selim, K.A.; Abdelrasoul, H.; Aboelmagd, M.; Tawila, A.M. The Role of the MAPK Signaling, Topoisomerase and Dietary Bioactives in Controlling Cancer Incidence. Diseases 2017, 5, 13. [Google Scholar] [CrossRef]
- Dai, H.-L.; Hu, W.-Y.; Jiang, L.-H.; Li, L.; Gaung, X.-F.; Xiao, Z.-C. P38 MAPK Inhibition Improves Synaptic Plasticity and Memory in Angiotensin II-Dependent Hypertensive Mice. Sci. Rep. 2016, 6, 27600. [Google Scholar] [CrossRef]
- He, L.; Deng, Y.; Gao, J.; Zeng, L.; Gong, Q. Icariside II Ameliorates Ibotenic Acid-Induced Cognitive Impairment and Apoptotic Response via Modulation of MAPK Pathway in Rats. Phytomed. Int. J. Phytother. Phytopharm. 2018, 41, 74–81. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, H.T. MAPK Signal Pathways in the Regulation of Cell Proliferation in Mammalian Cells. Cell Res. 2002, 12, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Gaitanaki, C.; Papatriantafyllou, M.; Stathopoulou, K.; Beis, I. Effects of Various Oxidants and Antioxidants on the P38-MAPK Signalling Pathway in the Perfused Amphibian Heart. Mol. Cell. Biochem. 2006, 291, 107–117. [Google Scholar] [CrossRef]
- Chiarini, A.; Dal Pra, I.; Marconi, M.; Chakravarthy, B.; Whitfield, J.F.; Armato, U. Calcium-Sensing Receptor (CaSR) in Human Brain’s Pathophysiology: Roles in Late-Onset Alzheimer’s Disease (LOAD). Curr. Pharm. Biotechnol. 2009, 10, 317–326. [Google Scholar] [CrossRef]
- Dent, P.; Yacoub, A.; Fisher, P.B.; Hagan, M.P.; Grant, S. MAPK Pathways in Radiation Responses. Oncogene 2003, 22, 5885–5896. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.K.; Choi, E.-J. Pathological Roles of MAPK Signaling Pathways in Human Diseases. Biochim. Biophys. Acta 2010, 1802, 396–405. [Google Scholar] [CrossRef]
- Deng, Z.; Sui, G.; Rosa, P.M.; Zhao, W. Radiation-Induced c-Jun Activation Depends on MEK1-ERK1/2 Signaling Pathway in Microglial Cells. PLoS ONE 2012, 7, e36739. [Google Scholar] [CrossRef] [PubMed]
- Ruffels, J.; Griffin, M.; Dickenson, J.M. Activation of ERK1/2, JNK and PKB by Hydrogen Peroxide in Human SH-SY5Y Neuroblastoma Cells: Role of ERK1/2 in H2O2-Induced Cell Death. Eur. J. Pharmacol. 2004, 483, 163–173. [Google Scholar] [CrossRef]
- Dabrowski, A.; Boguslowicz, C.; Dabrowska, M.; Tribillo, I.; Gabryelewicz, A. Reactive Oxygen Species Activate Mitogen-Activated Protein Kinases in Pancreatic Acinar Cells. Pancreas 2000, 21, 376–384. [Google Scholar] [CrossRef]
- Zomosa, G.; Lühr, C.; Bova, F.; González-Johnson, L.; Rojas-Solé, C.; Troncoso, L.; Miranda, G.; Lorenzoni, J.; Zomosa, G.; Lühr, C.; et al. Radiosurgery for Intracranial Meningiomas. In Meningioma—The Essentials From Bench to Bedside; IntechOpen: London, UK, 2024; ISBN 978-0-85466-145-9. [Google Scholar]
- Wang, Y.; Tian, J.; Liu, D.; Li, T.; Mao, Y.; Zhu, C. Microglia in Radiation-Induced Brain Injury: Cellular and Molecular Mechanisms and Therapeutic Potential. CNS Neurosci. Ther. 2024, 30, e14794. [Google Scholar] [CrossRef]
- Boyd, A.; Byrne, S.; Middleton, R.J.; Banati, R.B.; Liu, G.-J. Control of Neuroinflammation through Radiation-Induced Microglial Changes. Cells 2021, 10, 2381. [Google Scholar] [CrossRef]
- Schmal, Z.; Rübe, C.E. Region-Specific Effects of Fractionated Low-Dose Versus Single-Dose Radiation on Hippocampal Neurogenesis and Neuroinflammation. Cancers 2022, 14, 5477. [Google Scholar] [CrossRef] [PubMed]
- Markou, A.; Kitchen, P.; Aldabbagh, A.; Repici, M.; Salman, M.M.; Bill, R.M.; Balklava, Z. Mechanisms of Aquaporin-4 Vesicular Trafficking in Mammalian Cells. J. Neurochem. 2024, 168, 100–114. [Google Scholar] [CrossRef] [PubMed]
- Mader, S.; Brimberg, L. Aquaporin-4 Water Channel in the Brain and Its Implication for Health and Disease. Cells 2019, 8, 90. [Google Scholar] [CrossRef]
- Kitchen, P.; Salman, M.M.; Halsey, A.M.; Clarke-Bland, C.; MacDonald, J.A.; Ishida, H.; Vogel, H.J.; Almutiri, S.; Logan, A.; Kreida, S.; et al. Targeting Aquaporin-4 Subcellular Localization to Treat Central Nervous System Edema. Cell 2020, 181, 784–799.e19. [Google Scholar] [CrossRef]
- Sylvain, N.J.; Salman, M.M.; Pushie, M.J.; Hou, H.; Meher, V.; Herlo, R.; Peeling, L.; Kelly, M.E. The Effects of Trifluoperazine on Brain Edema, Aquaporin-4 Expression and Metabolic Markers during the Acute Phase of Stroke Using Photothrombotic Mouse Model. Biochim. Biophys. Acta Biomembr. 2021, 1863, 183573. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Tan, C.; Liu, Y.; Liu, X.; Wang, X.; Gui, Y.; Qin, L.; Deng, F.; Yu, Z.; Hu, C.; et al. Resveratrol Ameliorates Oxidative Stress and Inhibits Aquaporin 4 Expression Following Rat Cerebral Ischemia-Reperfusion Injury. Mol. Med. Rep. 2015, 12, 7756–7762. [Google Scholar] [CrossRef]
- Alhadidi, Q.M.; Bahader, G.A.; Arvola, O.; Kitchen, P.; Shah, Z.A.; Salman, M.M. Astrocytes in Functional Recovery Following Central Nervous System Injuries. J. Physiol. 2024, 602, 3069–3096. [Google Scholar] [CrossRef]
- Burda, J.E.; Bernstein, A.M.; Sofroniew, M.V. Astrocyte Roles in Traumatic Brain Injury. Exp. Neurol. 2016, 275, 305–315. [Google Scholar] [CrossRef]
- Linnerbauer, M.; Rothhammer, V. Protective Functions of Reactive Astrocytes Following Central Nervous System Insult. Front. Immunol. 2020, 11, 573256. [Google Scholar] [CrossRef]
- Zhou, Y.; Shao, A.; Yao, Y.; Tu, S.; Deng, Y.; Zhang, J. Dual Roles of Astrocytes in Plasticity and Reconstruction after Traumatic Brain Injury. Cell Commun. Signal. CCS 2020, 18, 62. [Google Scholar] [CrossRef]
- Pekny, M.; Pekna, M. Astrocyte Reactivity and Reactive Astrogliosis: Costs and Benefits. Physiol. Rev. 2014, 94, 1077–1098. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Lin, L.; Yin, L.; Hao, X.; Tian, J.; Zhang, X.; Ren, Y.; Li, C.; Yang, Y. Acutely Inhibiting AQP4 With TGN-020 Improves Functional Outcome by Attenuating Edema and Peri-Infarct Astrogliosis After Cerebral Ischemia. Front. Immunol. 2022, 13, 870029. [Google Scholar] [CrossRef] [PubMed]
- Xin, W.; Pan, Y.; Wei, W.; Tatenhorst, L.; Graf, I.; Popa-Wagner, A.; Gerner, S.T.; Huber, S.; Kilic, E.; Hermann, D.M.; et al. Preconditioned Extracellular Vesicles from Hypoxic Microglia Reduce Poststroke AQP4 Depolarization, Disturbed Cerebrospinal Fluid Flow, Astrogliosis, and Neuroinflammation. Theranostics 2023, 13, 4197–4216. [Google Scholar] [CrossRef] [PubMed]
- Bin Alamer, O.; Palmisciano, P.; Mallela, A.N.; Labib, M.A.; Gardner, P.A.; Couldwell, W.T.; Lunsford, L.D.; Abou-Al-Shaar, H. Stereotactic Radiosurgery in the Management of Petroclival Meningiomas: A Systematic Review and Meta-Analysis of Treatment Outcomes of Primary and Adjuvant Radiosurgery. J. Neurooncol. 2022, 157, 207–219. [Google Scholar] [CrossRef]
- Kozin, S.V. Vascular Damage in Tumors: A Key Player in Stereotactic Radiation Therapy? Trends Cancer 2022, 8, 806–819. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.K. A Link between Vascular Damage and Cognitive Deficits after Whole-Brain Radiation Therapy for Cancer: A Clue to Other Types of Dementia? Drug Discov. Ther. 2016, 10, 79–81. [Google Scholar] [CrossRef]
- Lee, D.; Riestenberg, R.A.; Haskell-Mendoza, A.; Bloch, O. Brain Metastasis Recurrence Versus Radiation Necrosis: Evaluation and Treatment. Neurosurg. Clin. N. Am. 2020, 31, 575–587. [Google Scholar] [CrossRef]
- Pazzaglia, S.; Briganti, G.; Mancuso, M.; Saran, A. Neurocognitive Decline Following Radiotherapy: Mechanisms and Therapeutic Implications. Cancers 2020, 12, 146. [Google Scholar] [CrossRef]
- Xiang, J.; Lu, Y.; Quan, C.; Gao, Y.; Zhou, G. Metformin Protects Radiation-Induced Early Brain Injury by Reducing Inflammation and DNA Damage. Brain Sci. 2023, 13, 645. [Google Scholar] [CrossRef]
- Hladik, D.; Tapio, S. Effects of Ionizing Radiation on the Mammalian Brain. Mutat. Res. Mutat. Res. 2016, 770, 219–230. [Google Scholar] [CrossRef]
- Xu, K.; Sun, G.; Wang, Y.; Luo, H.; Wang, Y.; Liu, M.; Liu, H.; Lu, X.; Qin, X. Mitigating Radiation-Induced Brain Injury via NLRP3/NLRC4/Caspase-1 Pyroptosis Pathway: Efficacy of Memantine and Hydrogen-Rich Water. Biomed. Pharmacother. 2024, 177, 116978. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Bai, S.; Gu, B.; Peng, S.; Liao, W.; Liu, J. Radiation-Induced Brain Injury After Radiotherapy for Brain Tumor. In Molecular Considerations and Evolving Surgical Management Issues in the Treatment of Patients with a Brain Tumor; IntechOpen: London, UK, 2015; ISBN 978-953-51-2031-5. [Google Scholar]
- Dye, N.B.; Gondi, V.; Mehta, M.P. Strategies for Preservation of Memory Function in Patients with Brain Metastases. Chin. Clin. Oncol. 2015, 4, 24. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.S.; Pellegrini, J.W.; Aggarwal, S.K.; Lei, S.Z.; Warach, S.; Jensen, F.E.; Lipton, S.A. Open-Channel Block of N-Methyl-D-Aspartate (NMDA) Responses by Memantine: Therapeutic Advantage against NMDA Receptor-Mediated Neurotoxicity. J. Neurosci. 1992, 12, 4427–4436. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.S.; Lipton, S.A. Mechanism of Memantine Block of NMDA-Activated Channels in Rat Retinal Ganglion Cells: Uncompetitive Antagonism. J. Physiol. 1997, 499, 27–46. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, J.W.; Lipton, S.A. Delayed Administration of Memantine Prevents N-Methyl-D-Aspartate Receptor-Mediated Neurotoxicity. Ann. Neurol. 1993, 33, 403–407. [Google Scholar] [CrossRef]
- Orgogozo, J.-M.; Rigaud, A.-S.; Stöffler, A.; Möbius, H.-J.; Forette, F. Efficacy and Safety of Memantine in Patients with Mild to Moderate Vascular Dementia: A Randomized, Placebo-Controlled Trial (MMM 300). Stroke 2002, 33, 1834–1839. [Google Scholar] [CrossRef]
- Wilcock, G.; Möbius, H.J.; Stöffler, A. MMM 500 group A Double-Blind, Placebo-Controlled Multicentre Study of Memantine in Mild to Moderate Vascular Dementia (MMM500). Int. Clin. Psychopharmacol. 2002, 17, 297–305. [Google Scholar] [CrossRef]
- Brown, P.D.; Pugh, S.; Laack, N.N.; Wefel, J.S.; Khuntia, D.; Meyers, C.; Choucair, A.; Fox, S.; Suh, J.H.; Roberge, D.; et al. Memantine for the Prevention of Cognitive Dysfunction in Patients Receiving Whole-Brain Radiotherapy: A Randomized, Double-Blind, Placebo-Controlled Trial. Neuro-Oncology 2013, 15, 1429–1437. [Google Scholar] [CrossRef]
- Greene-Schloesser, D.; Robbins, M.E.; Peiffer, A.M.; Shaw, E.G.; Wheeler, K.T.; Chan, M.D. Radiation-Induced Brain Injury: A Review. Front. Oncol. 2012, 2, 73. [Google Scholar] [CrossRef]
- Brown, P.D.; Gondi, V.; Pugh, S.; Tome, W.A.; Wefel, J.S.; Armstrong, T.S.; Bovi, J.A.; Robinson, C.; Konski, A.; Khuntia, D.; et al. Hippocampal Avoidance During Whole-Brain Radiotherapy Plus Memantine for Patients With Brain Metastases: Phase III Trial NRG Oncology CC001. J. Clin. Oncol. 2020, 38, 1019–1029. [Google Scholar] [CrossRef]
- Chambial, S.; Dwivedi, S.; Shukla, K.K.; John, P.J.; Sharma, P. Vitamin C in Disease Prevention and Cure: An Overview. Indian J. Clin. Biochem. IJCB 2013, 28, 314–328. [Google Scholar] [CrossRef] [PubMed]
- Castro, M.A.; Beltrán, F.A.; Brauchi, S.; Concha, I.I. A Metabolic Switch in Brain: Glucose and Lactate Metabolism Modulation by Ascorbic Acid. J. Neurochem. 2009, 110, 423–440. [Google Scholar] [CrossRef] [PubMed]
- Grünewald, R.A. Ascorbic Acid in the Brain. Brain Res. Brain Res. Rev. 1993, 18, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Nishikimi, M.; Yagi, K. Biochemistry and Molecular Biology of Ascorbic Acid Biosynthesis. In Subcellular Biochemistry: Ascorbic Acid: Biochemistry and Biomedical Cell Biology; Harris, J.R., Ed.; Springer: Boston, MA, USA, 1996; pp. 17–39. ISBN 978-1-4613-0325-1. [Google Scholar]
- Hammarström, L. Autoradiographic Studies on the Distribution of C14-Labelled Ascorbic Acid and Dehydroascorbic Acid. Acta Physiol. Scand. 1966, 70, 1–83. [Google Scholar] [CrossRef]
- May, J.M. Vitamin C Transport and Its Role in the Central Nervous System. In Water Soluble Vitamins: Clinical Research and Future Application; Subcellular Biochemistry; Springer: Dordrecht, The Netherlands, 2012; Volume 56, pp. 85–103. [Google Scholar] [CrossRef]
- Rice, M.E. Ascorbate Regulation and Its Neuroprotective Role in the Brain. Trends Neurosci. 2000, 23, 209–216. [Google Scholar] [CrossRef]
- Bürzle, M.; Suzuki, Y.; Ackermann, D.; Miyazaki, H.; Maeda, N.; Clémençon, B.; Burrier, R.; Hediger, M.A. The Sodium-Dependent Ascorbic Acid Transporter Family SLC23. Mol. Aspects Med. 2013, 34, 436–454. [Google Scholar] [CrossRef] [PubMed]
- Stamford, J.A.; Kruk, Z.L.; Millar, J. Regional Differences in Extracellular Ascorbic Acid Levels in the Rat Brain Determined by High Speed Cyclic Voltammetry. Brain Res. 1984, 299, 289–295. [Google Scholar] [CrossRef]
- Schenk, J.O.; Miller, E.; Gaddis, R.; Adams, R.N. Homeostatic Control of Ascorbate Concentration in CNS Extracellular Fluid. Brain Res. 1982, 253, 353–356. [Google Scholar] [CrossRef]
- Miele, M.; Fillenz, M. In Vivo Determination of Extracellular Brain Ascorbate. J. Neurosci. Methods 1996, 70, 15–19. [Google Scholar] [CrossRef]
- Huang, J.; Agus, D.B.; Winfree, C.J.; Kiss, S.; Mack, W.J.; McTaggart, R.A.; Choudhri, T.F.; Kim, L.J.; Mocco, J.; Pinsky, D.J.; et al. Dehydroascorbic Acid, a Blood-Brain Barrier Transportable Form of Vitamin C, Mediates Potent Cerebroprotection in Experimental Stroke. Proc. Natl. Acad. Sci. USA 2001, 98, 11720–11724. [Google Scholar] [CrossRef]
- Astuya, A.; Caprile, T.; Castro, M.; Salazar, K.; García, M.D.L.A.; Reinicke, K.; Rodríguez, F.; Vera, J.C.; Millán, C.; Ulloa, V.; et al. Vitamin C Uptake and Recycling among Normal and Tumor Cells from the Central Nervous System. J. Neurosci. Res. 2005, 79, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Diliberto, E.J.; Dean, G.; Carter, C.; Allen, P.L. Tissue, Subcellular, and Submitochondrial Distributions of Semidehydroascorbate Reductase: Possible Role of Semidehydroascorbate Reductase in Cofactor Regeneration. J. Neurochem. 1982, 39, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Rice, M.E.; Russo-Menna, I. Differential Compartmentalization of Brain Ascorbate and Glutathione between Neurons and Glia. Neuroscience 1998, 82, 1213–1223. [Google Scholar] [CrossRef] [PubMed]
- Castro, M.A.; Pozo, M.; Cortés, C.; García, M.D.L.A.; Concha, I.I.; Nualart, F. Intracellular Ascorbic Acid Inhibits Transport of Glucose by Neurons, but Not by Astrocytes. J. Neurochem. 2007, 102, 773–782. [Google Scholar] [CrossRef] [PubMed]
- Castro, M.; Caprile, T.; Astuya, A.; Millán, C.; Reinicke, K.; Vera, J.C.; Vásquez, O.; Aguayo, L.G.; Nualart, F. High-Affinity Sodium-Vitamin C Co-Transporters (SVCT) Expression in Embryonic Mouse Neurons. J. Neurochem. 2001, 78, 815–823. [Google Scholar] [CrossRef]
- Padayatty, S.J.; Katz, A.; Wang, Y.; Eck, P.; Kwon, O.; Lee, J.-H.; Chen, S.; Corpe, C.; Dutta, A.; Dutta, S.K.; et al. Vitamin C as an Antioxidant: Evaluation of Its Role in Disease Prevention. J. Am. Coll. Nutr. 2003, 22, 18–35. [Google Scholar] [CrossRef]
- Majewska, M.D.; Bell, J.A. Ascorbic Acid Protects Neurons from Injury Induced by Glutamate and NMDA. Neuroreport 1990, 1, 194–196. [Google Scholar] [CrossRef]
- Niki, E. Action of Ascorbic Acid as a Scavenger of Active and Stable Oxygen Radicals. Am. J. Clin. Nutr. 1991, 54, 1119S–1124S. [Google Scholar] [CrossRef]
- Rebec, G.V.; Pierce, R.C. A Vitamin as Neuromodulator: Ascorbate Release into the Extracellular Fluid of the Brain Regulates Dopaminergic and Glutamatergic Transmission. Prog. Neurobiol. 1994, 43, 537–565. [Google Scholar] [CrossRef]
- Dizdaroglu, M.; Jaruga, P.; Birincioglu, M.; Rodriguez, H. Free Radical-Induced Damage to DNA: Mechanisms and Measurement. Free Radic. Biol. Med. 2002, 32, 1102–1115. [Google Scholar] [CrossRef]
- Milne, G.L.; Musiek, E.S.; Morrow, J.D. F2-Isoprostanes as Markers of Oxidative Stress in Vivo: An Overview. Biomark. Biochem. Indic. Expo. Response Susceptibility Chem. 2005, 10 (Suppl. 1), S10–S23. [Google Scholar] [CrossRef]
- Reznick, A.Z.; Packer, L. Oxidative Damage to Proteins: Spectrophotometric Method for Carbonyl Assay. Methods Enzymol. 1994, 233, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Galley, H.F.; Davies, M.J.; Webster, N.R. Ascorbyl Radical Formation in Patients with Sepsis: Effect of Ascorbate Loading. Free Radic. Biol. Med. 1996, 20, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, R.; Guichard, C.; Charles, R. Clinical Pharmacology and Therapeutic Use of Antioxidant Vitamins. Fundam. Clin. Pharmacol. 2007, 21, 111–127. [Google Scholar] [CrossRef]
- Padh, H. Cellular Functions of Ascorbic Acid. Biochem. Cell Biol. Biochim. Biol. Cell. 1990, 68, 1166–1173. [Google Scholar] [CrossRef]
- Tomé, A.D.R.; Ferreira, P.M.P.; Freitas, R.M. de Inhibitory Action of Antioxidants (Ascorbic Acid or Alpha-Tocopherol) on Seizures and Brain Damage Induced by Pilocarpine in Rats. Arq. Neuropsiquiatr. 2010, 68, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Tsutsui, T.; Miwa, N. The Lipophilic Vitamin C Derivative, 6-o-Palmitoylascorbate, Protects Human Lymphocytes, Preferentially over Ascorbate, against X-Ray-Induced DNA Damage, Lipid Peroxidation, and Protein Carbonylation. Mol. Cell. Biochem. 2014, 394, 247–259. [Google Scholar] [CrossRef]
- Stehli, J.; Fuchs, T.A.; Ghadri, J.R.; Gaemperli, O.; Fiechter, M.; Kaufmann, P.A. Antioxidants Prevent DNA Double-Strand Breaks from X-Ray-Based Cardiac Examinations: A Randomized, Double-Blinded, Placebo-Controlled Trial. J. Am. Coll. Cardiol. 2014, 64, 117–118. [Google Scholar] [CrossRef]
- Tao, S.M.; Zhou, F.; Joseph Schoepf, U.; Fischer, A.M.; Giovagnoli, D.; Lin, Z.X.; Zhou, C.S.; Lu, G.M.; Zhang, L.J. The Effect of Prophylactic Oral Vitamin C Use on DNA Double-Strand Breaks after Abdominal Contrast-Enhanced CT: A Preliminary Study. Eur. J. Radiol. 2019, 117, 69–74. [Google Scholar] [CrossRef]
- Rostami, A.; Moosavi, S.A.; Dianat Moghadam, H.; Bolookat, E.R. Micronuclei Assessment of The Radioprotective Effects of Melatonin and Vitamin C in Human Lymphocytes. Cell J. 2016, 18, 46–51. [Google Scholar] [CrossRef]
- Du, J.; Cieslak, J.A.; Welsh, J.L.; Sibenaller, Z.A.; Allen, B.G.; Wagner, B.A.; Kalen, A.L.; Doskey, C.M.; Strother, R.K.; Button, A.M.; et al. Pharmacological Ascorbate Radiosensitizes Pancreatic Cancer. Cancer Res. 2015, 75, 3314–3326. [Google Scholar] [CrossRef] [PubMed]
- Didier, A.J.; Stiene, J.; Fang, L.; Watkins, D.; Dworkin, L.D.; Creeden, J.F. Antioxidant and Anti-Tumor Effects of Dietary Vitamins A, C, and E. Antioxidants 2023, 12, 632. [Google Scholar] [CrossRef] [PubMed]
- Kaffash Farkhad, N.; Asadi-Samani, M.; Asadi-Samani, F.; Asadi-Samani, H. The Role of Natural Antioxidants in Reducing Oxidative Stress in Cancer. In Plant Antioxidants and Health; Ekiert, H.M., Ramawat, K.G., Arora, J., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 1–16. ISBN 978-3-030-45299-5. [Google Scholar]
- Asadi-Samani, M.; Farkhad, N.K.; Mahmoudian-Sani, M.R.; Shirzad, H.; Asadi-Samani, M.; Farkhad, N.K.; Mahmoudian-Sani, M.R.; Shirzad, H. Antioxidants as a Double-Edged Sword in the Treatment of Cancer. In Antioxidants; IntechOpen: London, UK, 2019; ISBN 978-1-78923-920-1. [Google Scholar]
- Zahra, K.F.; Lefter, R.; Ali, A.; Abdellah, E.-C.; Trus, C.; Ciobica, A.; Timofte, D. The Involvement of the Oxidative Stress Status in Cancer Pathology: A Double View on the Role of the Antioxidants. Oxid. Med. Cell. Longev. 2021, 2021, 9965916. [Google Scholar] [CrossRef]
- Cockfield, J.A.; Schafer, Z.T. Antioxidant Defenses: A Context-Specific Vulnerability of Cancer Cells. Cancers 2019, 11, 1208. [Google Scholar] [CrossRef]
- Zaher, A.; Petronek, M.S.; Allen, B.G.; Mapuskar, K.A. Balanced Duality: H2O2-Based Therapy in Cancer and Its Protective Effects on Non-Malignant Tissues. Int. J. Mol. Sci. 2024, 25, 8885. [Google Scholar] [CrossRef] [PubMed]
- Petronek, M.S.; Monga, V.; Bodeker, K.L.; Kwofie, M.; Lee, C.-Y.; Mapuskar, K.A.; Stolwijk, J.M.; Zaher, A.; Wagner, B.A.; Smith, M.C.; et al. Magnetic Resonance Imaging of Iron Metabolism with T2* Mapping Predicts an Enhanced Clinical Response to Pharmacologic Ascorbate in Patients with GBM. Clin. Cancer Res. 2024, 30, 283–293. [Google Scholar] [CrossRef]
- Gan, L.; Wang, Z.; Si, J.; Zhou, R.; Sun, C.; Liu, Y.; Ye, Y.; Zhang, Y.; Liu, Z.; Zhang, H. Protective Effect of Mitochondrial-Targeted Antioxidant MitoQ against Iron Ion 56Fe Radiation Induced Brain Injury in Mice. Toxicol. Appl. Pharmacol. 2018, 341, 1–7. [Google Scholar] [CrossRef]
- Kale, A.; Piskin, Ö.; Bas, Y.; Aydin, B.G.; Can, M.; Elmas, Ö.; Büyükuysal, Ç. Neuroprotective Effects of Quercetin on Radiation-Induced Brain Injury in Rats. J. Radiat. Res. 2018, 59, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Abou-Zeid, S.M.; El-Bialy, B.E.; El-Borai, N.B.; AbuBakr, H.O.; Elhadary, A.M.A. Radioprotective Effect of Date Syrup on Radiation- Induced Damage in Rats. Sci. Rep. 2018, 8, 7423. [Google Scholar] [CrossRef]
- Allen, B.D.; Acharya, M.M.; Lu, C.; Giedzinski, E.; Chmielewski, N.N.; Quach, D.; Hefferan, M.; Johe, K.K.; Limoli, C.L. Remediation of Radiation-Induced Cognitive Dysfunction through Oral Administration of the Neuroprotective Compound NSI-189. Radiat. Res. 2018, 189, 345–353. [Google Scholar] [CrossRef]
- Stessin, A.M.; Banu, M.A.; Clausi, M.G.; Berry, N.; Boockvar, J.A.; Ryu, S. FTY720/Fingolimod, an Oral S1PR Modulator, Mitigates Radiation Induced Cognitive Deficits. Neurosci. Lett. 2017, 658, 1–5. [Google Scholar] [CrossRef] [PubMed]
- He, G.Y.; Huang, H.W.; Deng, Z.Z.; Guo, J.J. Effect of mouse nerve growth factor on cognitive impairment in whole brain irradiation rats. Zhonghua Yi Xue Za Zhi 2016, 96, 1530–1534. [Google Scholar] [CrossRef] [PubMed]
- Zhou, A.Y.; Song, B.W.; Fu, C.Y.; Baranenko, D.D.; Wang, E.J.; Li, F.Y.; Lu, G.W. Acanthopanax Senticosus Reduces Brain Injury in Mice Exposed to Low Linear Energy Transfer Radiation. Biomed. Pharmacother. Biomedecine Pharmacother. 2018, 99, 781–790. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Johnson, L.; Fariña, A.; Farías, G.; Zomosa, G.; Pinilla-González, V.; Rojas-Solé, C. Exploring Neuroprotection against Radiation-Induced Brain Injury: A Review of Key Compounds. NeuroSci 2024, 5, 462-484. https://doi.org/10.3390/neurosci5040034
González-Johnson L, Fariña A, Farías G, Zomosa G, Pinilla-González V, Rojas-Solé C. Exploring Neuroprotection against Radiation-Induced Brain Injury: A Review of Key Compounds. NeuroSci. 2024; 5(4):462-484. https://doi.org/10.3390/neurosci5040034
Chicago/Turabian StyleGonzález-Johnson, Lucas, Ariel Fariña, Gonzalo Farías, Gustavo Zomosa, Víctor Pinilla-González, and Catalina Rojas-Solé. 2024. "Exploring Neuroprotection against Radiation-Induced Brain Injury: A Review of Key Compounds" NeuroSci 5, no. 4: 462-484. https://doi.org/10.3390/neurosci5040034
APA StyleGonzález-Johnson, L., Fariña, A., Farías, G., Zomosa, G., Pinilla-González, V., & Rojas-Solé, C. (2024). Exploring Neuroprotection against Radiation-Induced Brain Injury: A Review of Key Compounds. NeuroSci, 5(4), 462-484. https://doi.org/10.3390/neurosci5040034







