Immediate Effects of Distinct Intensities of Transcutaneous Spinal Direct Current Stimulation on Chronic Pain: A Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
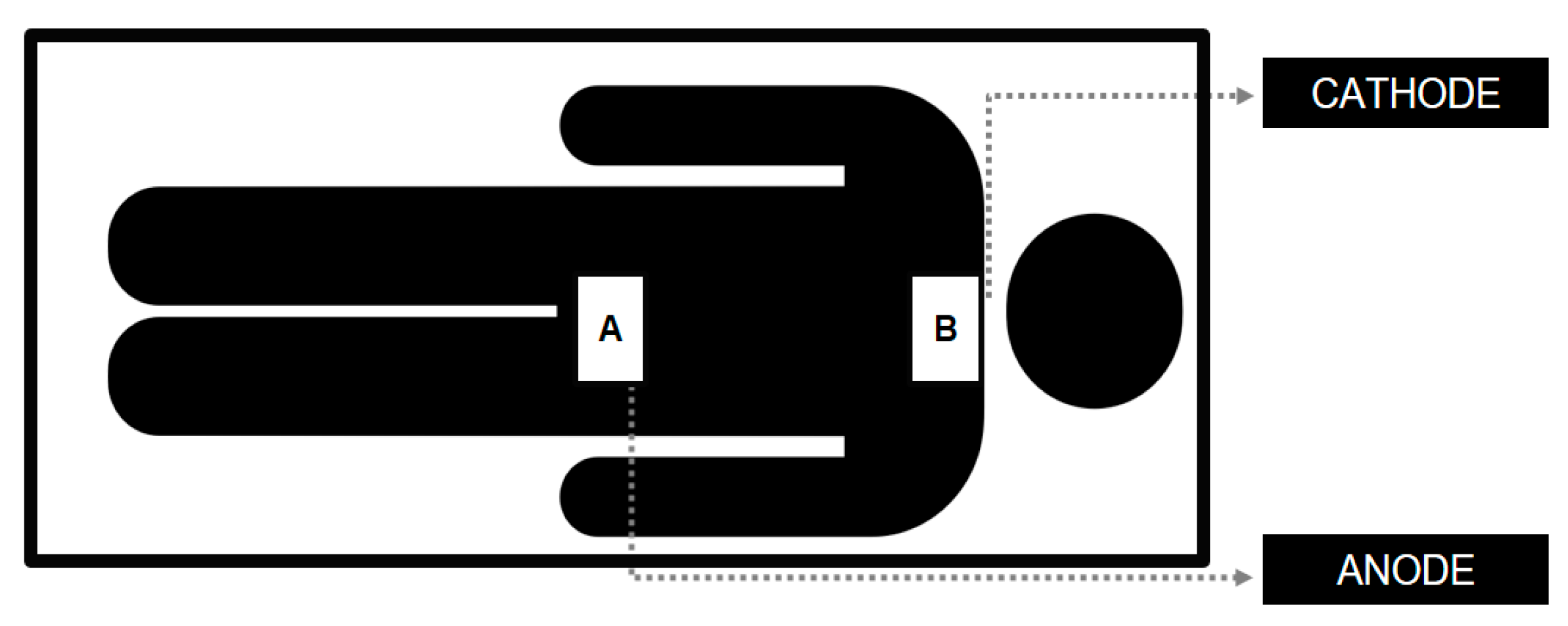
2.2. Transcutaneous Spinal Direct Current Stimulation (tsDCS)
2.3. Experimental Protocol
2.4. Data Extraction
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- St. John Smith, E. Advances in understanding nociception and neuropathic pain. J. Neurol. 2018, 265, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Dubin, A.E.; Patapoutian, A. Nociceptors: The sensors of the pain pathway Review series Nociceptors: The sensors of the pain pathway. J. Clin. Investig. 2010, 120, 3760–3772. [Google Scholar] [CrossRef] [PubMed]
- Rikard, S.M.; Strahan, A.E.; Schmit, K.M.; Guy, G.P. Chronic Pain Among Adults—United States, 2019–2021. MMWR Morb. Mortal. Wkly. Rep. 2023, 72, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Yong, R.J.; Mullins, P.M.; Bhattacharyya, N. Prevalence of chronic pain among adults in the United States. Pain 2022, 163, e328–e332. [Google Scholar] [CrossRef]
- Santiago, B.V.M.; de Oliveira, A.B.G.; da Silva, G.M.R.; da Silva, M.d.F.; Bergamo, P.E.; Parise, M.; Andrade, F.G.; Salgado, J.V.; Santos, J.F.; Amorim, M.C.; et al. Prevalence of chronic pain in Brazil: A systematic review and meta-analysis. Clinics 2023, 78, 100209. [Google Scholar] [CrossRef]
- Dahlhamer, J.; Lucas, J.; Zelaya, C.; Nahin, R.; Mackey, S.; DeBar, L.; Kerns, R.; Von Korff, M.; Porter, L.; Helmick, C. Prevalence of Chronic Pain and High-Impact Chronic Pain Among Adults—United States, 2016. MMWR Morb. Mortal. Wkly. Rep. 2018, 67, 1001–1006. [Google Scholar] [CrossRef]
- Mills, S.E.E.; Nicolson, K.P.; Smith, B.H. Chronic pain: A review of its epidemiology and associated factors in population-based studies. Br. J. Anaesth. 2019, 123, e273–e283. [Google Scholar] [CrossRef]
- Aguiar, D.P.; Souza, C.P.d.Q.; Barbosa, W.J.M.; Santos-Júnior, F.F.U.; de Oliveira, A.S. Prevalence of chronic pain in Brazil: Systematic review. Braz. J. Pain 2021, 4, 257–267. [Google Scholar] [CrossRef]
- Kosminsky, M.; do Nascimento, M.G.; de Oliveira, G.N.S. Financial stress and pain, what follows an economic crisis? Integrative review. Braz. J. Pain 2020, 3, 280–284. [Google Scholar] [CrossRef]
- Lam, C.M.; Sanderson, M.; Vu, D.T.; Sayed, D.; Latif, U.; Chadwick, A.L.; Hussain, S.A.; Rehman, M.J.; Singh, K.; Ghani, K.R.; et al. Musculoskeletal and Neuropathic Pain in COVID-19. Diagnostics 2024, 14, 332. [Google Scholar] [CrossRef]
- Dieleman, J.L.; Cao, J.; Chapin, A.; Chen, C.; Li, Z.; Liu, A.; Horst, C.; Gao, W.; Zhang, S.; Schneider, M.T.; et al. US Health Care Spending by Payer and Health Condition, 1996–2016. JAMA 2020, 323, 863–884. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.P.; Vase, L.; Hooten, W.M. Chronic pain: An update on burden, best practices, and new advances. Lancet 2021, 397, 2082–2097. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Tharu, N.S.; Gustin, S.M.; Zheng, Y.P.; Alam, M. Trans-Spinal Electrical Stimulation Therapy for Functional Rehabilitation after Spinal Cord Injury: Review. J. Clin. Med. 2022, 11, 1550. [Google Scholar] [CrossRef] [PubMed]
- Guidetti, M.; Giannoni-Luza, S.; Bocci, T.; Pacheco-Barrios, K.; Bianchi, A.M.; Parazzini, M.; Bellini, F.; Rocca, M.A.; Filippi, M.; Fregni, F.; et al. Modeling Electric Fields in Transcutaneous Spinal Direct Current Stimulation: A Clinical Perspective. Biomedicines 2023, 11, 1283. [Google Scholar] [CrossRef]
- Mekhail, N.A.; Mathews, M.; Nageeb, F.; Guirguis, M.; Mekhail, M.N.; Cheng, J. Retrospective Review of 707 Cases of Spinal Cord Stimulation: Indications and Complications. Pain Pract. 2011, 11, 148–153. [Google Scholar] [CrossRef]
- Lenoir, C.; Jankovski, A.; Mouraux, A. Anodal Transcutaneous Spinal Direct Current Stimulation (tsDCS) Selectively Inhibits the Synaptic Efficacy of Nociceptive Transmission at Spinal Cord Level. Neuroscience 2018, 393, 150–163. [Google Scholar] [CrossRef]
- Guidetti, M.; Ferrucci, R.; Vergari, M.; Aglieco, G.; Naci, A.; Versace, S.; Bianchi, A.M.; Parazzini, M.; Rocca, M.A.; Filippi, M.; et al. Effects of Transcutaneous Spinal Direct Current Stimulation (tsDCS) in Patients With Chronic Pain: A Clinical and Neurophysiological Study. Front. Neurol. 2021, 12, 695910. [Google Scholar] [CrossRef]
- Bocci, T.; Barloscio, D.; Vergari, M.; Di Rollo, A.; Rossi, S.; Priori, A.; Parazzini, M.; Avanzini, G.; Minonzio, M.; Mariotti, C.; et al. Spinal Direct Current Stimulation Modulates Short Intracortical Inhibition. Neuromodulation 2015, 18, 686–693. [Google Scholar] [CrossRef]
- Vergara, F.; Sardi, N.F.; Pescador, A.C.; Guaita, G.O.; Jark Stern, C.A.; Chichorro, J.G.; Oliveira, M.C.; Santos, J.G.; Takahashi, R.N.; Morato, G.S.; et al. Contribution of mesolimbic dopamine and kappa opioid systems to the transition from acute to chronic pain. Neuropharmacology 2020, 178, 108226. [Google Scholar] [CrossRef]
- Thordstein, M.; Svantesson, M.; Rahin, H. Effect of transspinal direct current stimulation on afferent pain signalling in humans. J. Clin. Neurosci. 2020, 77, 163–167. [Google Scholar] [CrossRef]
- Akcay, G.; Nemutlu Samur, D.; Derin, N. Transcranial direct current stimulation alleviates nociceptive behavior in male rats with neuropathic pain by regulating oxidative stress and reducing neuroinflammation. J. Neurosci. Res. 2023, 101, 1457–1470. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.A.; Kim, Y.; Shin, H.I. Pilot study of feasibility and effect of anodal transcutaneous spinal direct current stimulation on chronic neuropathic pain after spinal cord injury. Spinal Cord 2019, 57, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Boonstra, A.M.; Schiphorst Preuper, H.R.; Reneman, M.F.; Posthumus, J.B.; Stewart, R.E. Reliability and validity of the visual analogue scale for disability in patients with chronic musculoskeletal pain. Int. J. Rehabil. Res. 2008, 31, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Jerez-Mayorga, D.; dos Anjos, C.F.; de Cássia Macedo, M.; Fernandes, I.G.; Aedo-Muñoz, E.; Intelangelo, L.; Espinoza-Suarez, N.R.; Rodriguez-Fernandez, A.; García-Cifuentes, C.A.; da Silva-Grigoletto, M.E.; et al. Instrumental validity and intra/inter-rater reliability of a novel low-cost digital pressure algometer. PeerJ 2020, 8, e10162. [Google Scholar] [CrossRef]
- Antal, A.; Alekseichuk, I.; Bikson, M.; Brockmöller, J.; Brunoni, A.R.; Chen, R.; Cohen, L.G.; Dowthwaite, G.; Ellrich, J.; Flöel, A.; et al. Low intensity transcranial electric stimulation: Safety, ethical, legal regulatory and application guidelines. Clin. Neurophysiol. 2017, 128, 1774–1809. [Google Scholar] [CrossRef]

| Characteristics | tsDCS 2.0 mA Median (Min–Max) | tsDCS 0.5 mA Median (Min–Max) | p-Value |
|---|---|---|---|
| Age (years) | 46.5 (21–84) | 52 (20–80) | 0.54 |
| Weight (kg) | 71.5 (51–45) | 72 (51–45) | 0.06 |
| Height (cm) | 165 (140–194) | 165 (151–176) | 0.41 |
| Group | VASPRE Mean (SD) | VASPOST Mean (SD) | PPTPRE Mean (SD) | PPTPOST Mean (SD) |
|---|---|---|---|---|
| tsDCS 2 mA | 4.83 (2.74) | 3.68 (2.55) | 2.31 (1.46) | 2.56 (1.56) |
| tsDCS 0.5 mA | 4.79 (2.17) | 3.97 (2.47) | 2.56 (1.48) | 2.88 (1.82) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, K.R.d.R.; Macedo, M.d.C.S.; Alves, A.L.G.; Esquírio, A.F.; Botim, B.R.; Jacob, G.S.; dos Santos, M.E.C.; Gama, G.L.; Barbosa, M.C.S.A.; Barbosa, A.W.C. Immediate Effects of Distinct Intensities of Transcutaneous Spinal Direct Current Stimulation on Chronic Pain: A Randomized Controlled Trial. NeuroSci 2024, 5, 614-622. https://doi.org/10.3390/neurosci5040043
Ferreira KRdR, Macedo MdCS, Alves ALG, Esquírio AF, Botim BR, Jacob GS, dos Santos MEC, Gama GL, Barbosa MCSA, Barbosa AWC. Immediate Effects of Distinct Intensities of Transcutaneous Spinal Direct Current Stimulation on Chronic Pain: A Randomized Controlled Trial. NeuroSci. 2024; 5(4):614-622. https://doi.org/10.3390/neurosci5040043
Chicago/Turabian StyleFerreira, Kariny Realino do Rosário, Maria de Cássia Souza Macedo, Ana Luiza Guimarães Alves, Arthur Ferreira Esquírio, Bianca Rossi Botim, Gabrielly Souza Jacob, Mayra Evelise Cunha dos Santos, Gabriela Lopes Gama, Michelle Cristina Sales Almeida Barbosa, and Alexandre Wesley Carvalho Barbosa. 2024. "Immediate Effects of Distinct Intensities of Transcutaneous Spinal Direct Current Stimulation on Chronic Pain: A Randomized Controlled Trial" NeuroSci 5, no. 4: 614-622. https://doi.org/10.3390/neurosci5040043
APA StyleFerreira, K. R. d. R., Macedo, M. d. C. S., Alves, A. L. G., Esquírio, A. F., Botim, B. R., Jacob, G. S., dos Santos, M. E. C., Gama, G. L., Barbosa, M. C. S. A., & Barbosa, A. W. C. (2024). Immediate Effects of Distinct Intensities of Transcutaneous Spinal Direct Current Stimulation on Chronic Pain: A Randomized Controlled Trial. NeuroSci, 5(4), 614-622. https://doi.org/10.3390/neurosci5040043





