Development of Spatial Memory: A Behavioral Study
Abstract
:1. Introduction
1.1. Spatial Memory
1.2. Spatial Memory as a Cognitive Skill
1.3. The Development of Spatial Memory
1.4. Aim of This Study
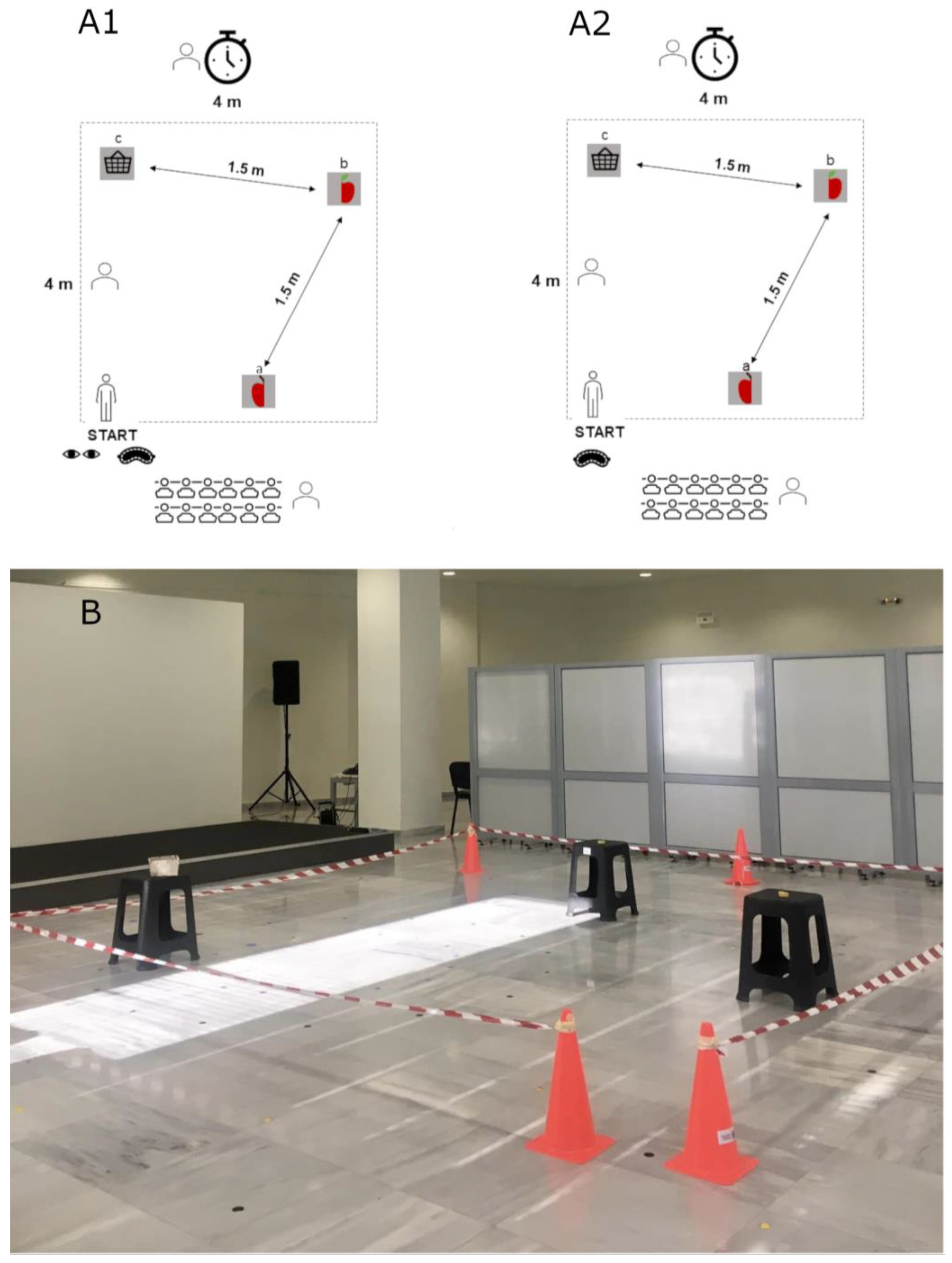
2. Materials and Methods
2.1. General Experimental Procedure
2.2. Data Collection
2.3. Testing Rooms
2.4. Data Analysis
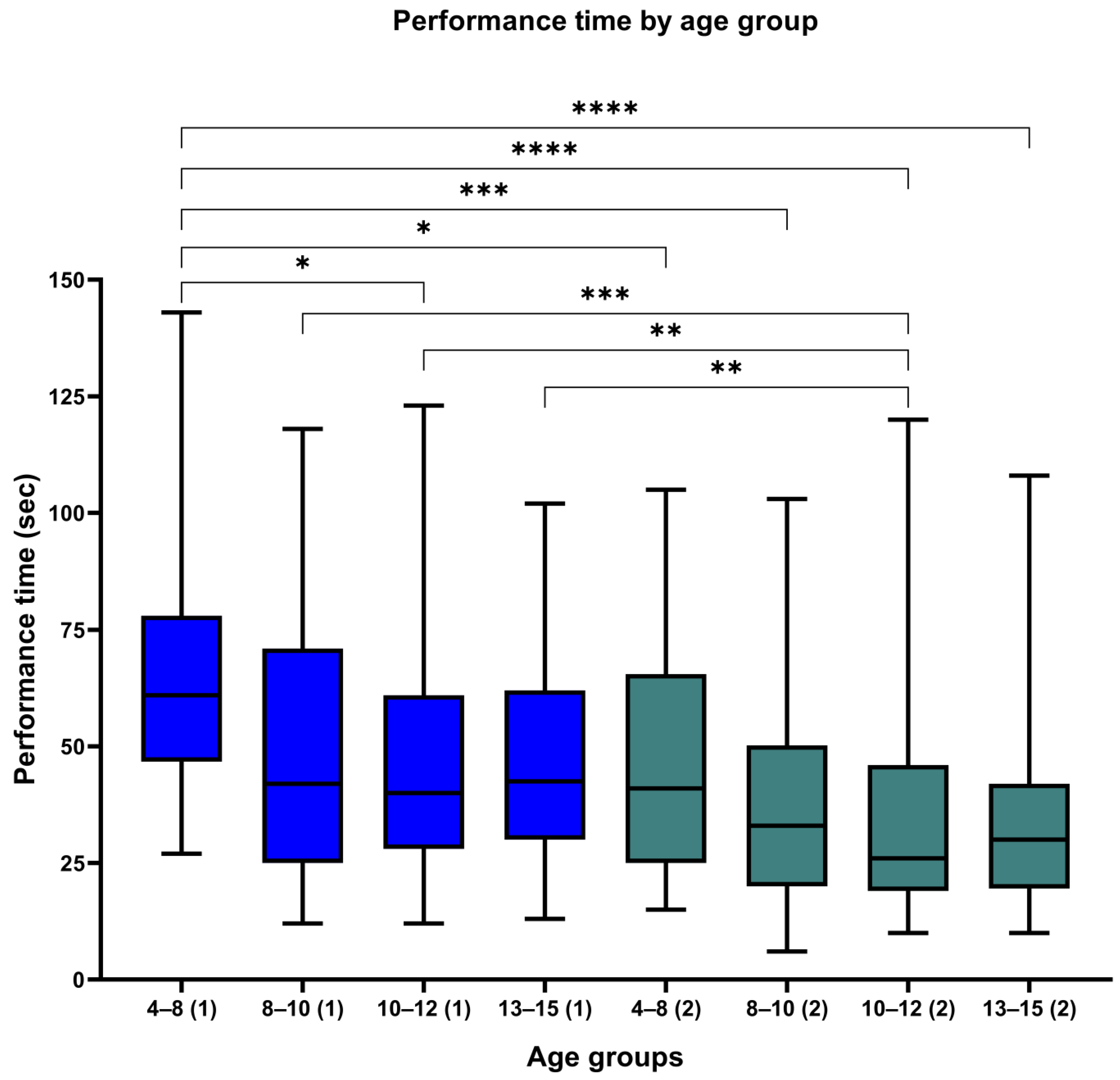
3. Results
3.1. Test 1
3.2. Test 2
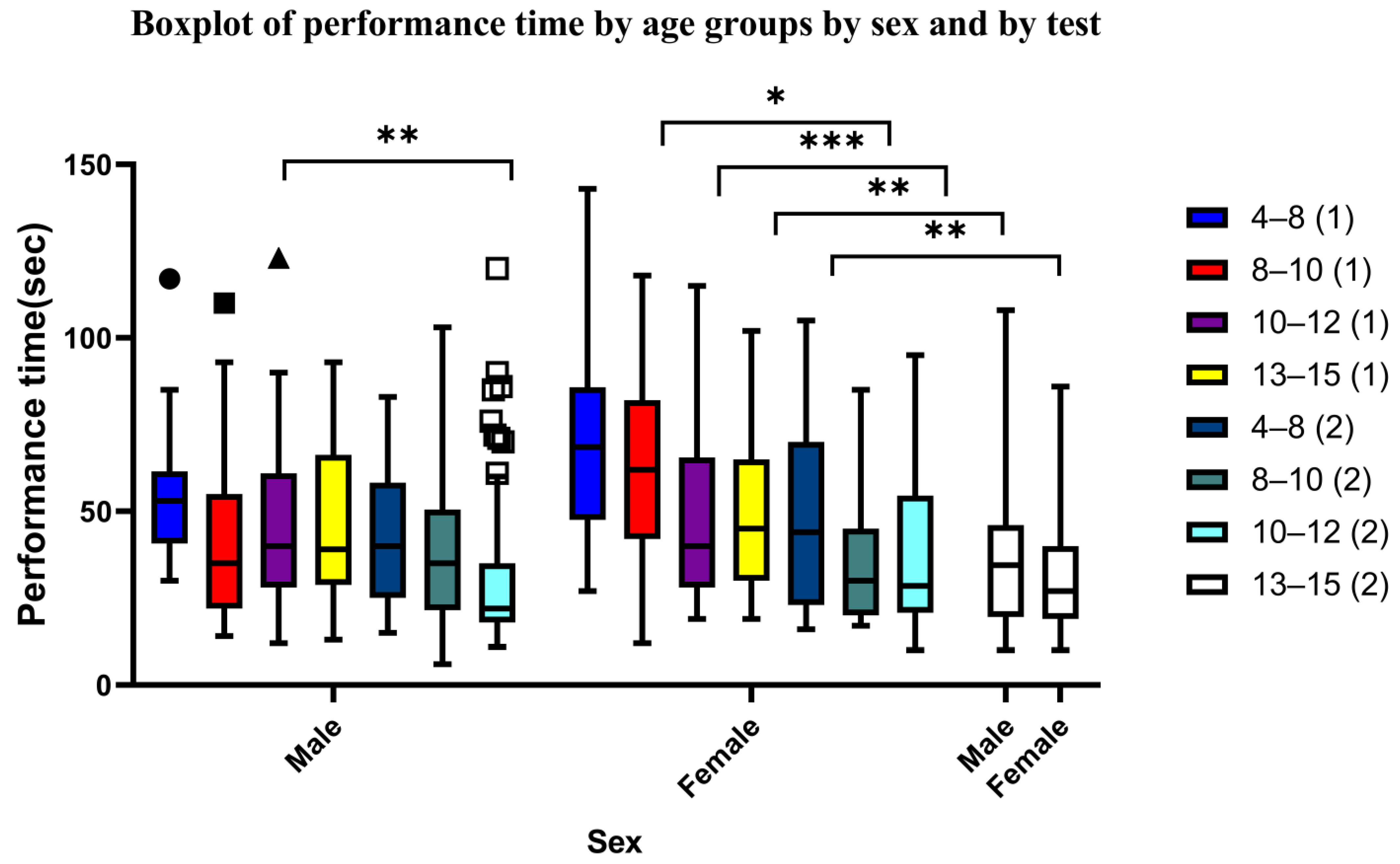
3.3. Sex Differences
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- O’Keefe, J.; Dostrovsky, J. The Hippocampus as a Spatial Map. Preliminary Evidence from Unit Activity in the Freely-Moving Rat. Brain Res. 1971, 34, 171–175. [Google Scholar] [CrossRef]
- Scoville, W.B.; Milner, B. Loss of recent memory after bilateral hippocampal lesions. J. Neurol. Neurosurg. Psychiatry 1957, 20, 11–21. [Google Scholar] [CrossRef]
- Witter, M.P. Hippocampal Formation. In Encyclopedia of Neuroscience; Springer: Berlin/Heidelberg, Germany, 2009; pp. 1840–1845. [Google Scholar]
- Moser, E.I.; Roudi, Y.; Witter, M.P.; Kentros, C.; Bonhoeffer, T.; Moser, M.-B. Grid Cells and Cortical Representation. Nat. Rev. Neurosci. 2014, 15, 466–481. [Google Scholar] [CrossRef] [PubMed]
- Moser, M.-B.; Rowland, D.C.; Moser, E.I. Place Cells, Grid Cells, and Memory. Cold Spring Harb. Perspect. Biol. 2015, 7, a021808. [Google Scholar] [CrossRef]
- Muller, R.U.; Ranck, J.B.; Taube, J.S. Head Direction Cells: Properties and Functional Significance. Curr. Opin. Neurobiol. 1996, 6, 196–206. [Google Scholar] [CrossRef] [PubMed]
- O’Keefe, J.; Nade, L. The Hippocampus as a Cognitive Map; Oxford University Press: Oxford, UK, 1978. [Google Scholar]
- Buzsáki, G.; Moser, E.I. Memory, Navigation and Theta Rhythm in the Hippocampal-Entorhinal System. Nat. Neurosci. 2013, 16, 130–138. [Google Scholar] [CrossRef]
- Hafting, T.; Fyhn, M.; Molden, S.; Moser, M.-B.; Moser, E.I. Microstructure of a Spatial Map in the Entorhinal Cortex. Nature 2005, 436, 801–806. [Google Scholar] [CrossRef]
- Moser, E.I.; Kropff, E.; Moser, M.-B. Place Cells, Grid Cells, and the Brain’s Spatial Representation System. Annu. Rev. Neurosci. 2008, 31, 69–89. [Google Scholar] [CrossRef] [PubMed]
- Awh, E.; Jonides, J. Overlapping Mechanisms of Attention and Spatial Working Memory. Trends Cogn. Sci. 2001, 5, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.E.; Jonides, J. Working Memory: A View from Neuroimaging. Cogn. Psychol. 1997, 33, 5–42. [Google Scholar] [CrossRef] [PubMed]
- Courtney, S.M.; Ungerleider, L.G.; Keil, K.; Haxby, J.V. Transient and Sustained Activity in a Distributed Neural System for Human Working Memory. Nature 1997, 386, 608–611. [Google Scholar] [CrossRef]
- Epstein, R.A.; Vass, L.K. Neural Systems for Landmark-Based Wayfinding in Humans. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20120533. [Google Scholar] [CrossRef]
- Burgess, N.; Maguire, E.A.; O’Keefe, J. The Human Hippocampus and Spatial and Episodic Memory. Neuron 2002, 35, 625–641. [Google Scholar] [CrossRef] [PubMed]
- Wolbers, T.; Hegarty, M. What Determines Our Navigational Abilities? Trends Cogn. Sci. 2010, 14, 138–146. [Google Scholar] [CrossRef]
- Billig, A.J.; Lad, M.; Sedley, W.; Griffiths, T.D. The Hearing Hippocampus. Prog. Neurobiol. 2022, 218, 102326. [Google Scholar] [CrossRef]
- Baeta-Corral, R.; Giménez-Llort, L. Persistent Hyperactivity and Distinctive Strategy Features in the Morris Water Maze in 3xTg-AD Mice at Advanced Stages of Disease. Behav. Neurosci. 2015, 129, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Burgess, N. Spatial Memory: How Egocentric and Allocentric Combine. Trends Cogn. Sci. 2006, 10, 551–557. [Google Scholar] [CrossRef]
- Smith, P.F. The Vestibular System and Cognition. Curr. Opin. Neurol. 2017, 30, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.F. The Growing Evidence for the Importance of the Otoliths in Spatial Memory. Front. Neural Circuits 2019, 13, 66. [Google Scholar] [CrossRef] [PubMed]
- Baddeley, A.D.; Hitch, G. Working Memory. Psychol. Learn. Motiv. 1974, 8, 47–89. [Google Scholar]
- Smith, E.E.; Jonides, J. Storage and Executive Processes in the Frontal Lobes. Science (1979) 1999, 283, 1657–1661. [Google Scholar] [CrossRef] [PubMed]
- Courtney, S.M.; Petit, L.; Maisog, J.M.; Ungerleider, L.G.; Haxby, J.V. An Area Specialized for Spatial Working Memory in Human Frontal Cortex. Science (1979) 1998, 279, 1347–1351. [Google Scholar] [CrossRef]
- Postle, B.R.; Awh, E.; Jonides, J.; Smith, E.E.; D’Esposito, M. The Where and How of Attention-Based Rehearsal in Spatial Working Memory. Cogn. Brain Res. 2004, 20, 194–205. [Google Scholar] [CrossRef]
- Grill-Spector, K.; Malach, R. The human visual cortex. Annu. Rev. Neurosci. 2004, 27, 649–677. [Google Scholar] [CrossRef] [PubMed]
- Horner, A.J.; Bisby, J.A.; Bush, D.; Lin, W.-J.; Burgess, N. Evidence for Holistic Episodic Recollection via Hippocampal Pattern Completion. Nat. Commun. 2015, 6, 7462. [Google Scholar] [CrossRef] [PubMed]
- Eichenbaum, H. The Role of the Hippocampus in Navigation Is Memory. J. Neurophysiol. 2017, 117, 1785–1796. [Google Scholar] [CrossRef] [PubMed]
- Committeri, G.; Galati, G.; Paradis, A.-L.; Pizzamiglio, L.; Berthoz, A.; LeBihan, D. Reference Frames for Spatial Cognition: Different Brain Areas Are Involved in Viewer-, Object-, and Landmark-Centered Judgments About Object Location. J. Cogn. Neurosci. 2004, 16, 1517–1535. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.K.; Cohen, J.D. An Integrative Theory of Prefrontal Cortex Function. Annu. Rev. Neurosci. 2001, 24, 167–202. [Google Scholar] [CrossRef]
- Postma, A.; Kessels, R.; Vanasselen, M. How the Brain Remembers and Forgets Where Things Are: The Neurocognition of Object–Location Memory. Neurosci. Biobehav. Rev. 2008, 32, 1339–1345. [Google Scholar] [CrossRef]
- Galati, G.; Pelle, G.; Berthoz, A.; Committeri, G. Multiple Reference Frames Used by the Human Brain for Spatial Perception and Memory. Exp. Brain Res. 2010, 206, 109–120. [Google Scholar] [CrossRef]
- Maguire, E.A.; Frith, C.D.; Burgess, N.; Donnett, J.G.; O’Keefe, J. Knowing Where Things Are: Parahippocampal Involvement in Encoding Object Locations in Virtual Large-Scale Space. J. Cogn. Neurosci. 1998, 10, 61–76. [Google Scholar] [CrossRef] [PubMed]
- Caffò, A.O.; Lopez, A.; Spano, G.; Serino, S.; Cipresso, P.; Stasolla, F.; Savino, M.; Lancioni, G.E.; Riva, G.; Bosco, A. Spatial Reorientation Decline in Aging: The Combination of Geometry and Landmarks. Aging Ment. Health 2018, 22, 1372–1383. [Google Scholar] [CrossRef]
- Traficante, S.; Tinella, L.; Lopez, A.; Koppel, S.; Ricciardi, E.; Napoletano, R.; Spano, G.; Bosco, A.; Caffò, A.O. “Regulating My Anxiety Worsens the Safety of My Driving”: The Synergistic Influence of Spatial Anxiety and Self-Regulation on Driving Behavior. Accid. Anal. Prev. 2024, 208, 107768. [Google Scholar] [CrossRef] [PubMed]
- Acredolo, L.P.; Evans, D. Developmental Changes in the Effects of Landmarks on Infant Spatial Behavior. Dev. Psychol. 1980, 16, 312–318. [Google Scholar] [CrossRef]
- Acredolo, L.P. Development of Spatial Orientation in Infancy. Dev. Psychol. 1978, 14, 224–234. [Google Scholar] [CrossRef]
- Bushnell, E.W.; McKenzie, B.E.; Lawrence, D.A.; Connell, S. The Spatial Coding Strategies of One-Year-Old Infants in a Locomotor Search Task. Child Dev. 1995, 66, 937–958. [Google Scholar] [CrossRef] [PubMed]
- Lew, A.R.; Bremner, J.G.; Lefkovitch, L.P. The Development of Relational Landmark Use in Six- to Twelve-Month-Old Infants in a Spatial Orientation Task. Child Dev. 2000, 71, 1179–1190. [Google Scholar] [CrossRef]
- Newcombe, N.S.; Lloyd, M.E.; Ratliff, K.R. Development of episodic and autobiographical memory: A cognitive neuroscience perspective. Adv. Child Dev. Behav. 2007, 35, 37–85. [Google Scholar] [PubMed]
- Newcombe, N.; Huttenlocher, J.; Drummey, A.B.; Wiley, J.G. The Development of Spatial Location Coding: Place Learning and Dead Reckoning in the Second and Third Years. Cogn. Dev. 1998, 13, 185–200. [Google Scholar] [CrossRef]
- Ribordy, F.; Jabès, A.; Banta Lavenex, P.; Lavenex, P. Development of Allocentric Spatial Memory Abilities in Children from 18 Months to 5 Years of Age. Cogn. Psychol. 2013, 66, 1–29. [Google Scholar] [CrossRef]
- Landau, B. Spatial Knowledge in a Young Blind Child. Cognition 1984, 16, 225–260. [Google Scholar] [CrossRef]
- Bostelmann, M.; Lavenex, P.; Banta Lavenex, P. Children Five-to-Nine Years Old Can Use Path Integration to Build a Cognitive Map without Vision. Cogn. Psychol. 2020, 121, 101307. [Google Scholar] [CrossRef]
- Nardini, M.; Jones, P.; Bedford, R.; Braddick, O. Development of Cue Integration in Human Navigation. Curr. Biol. 2008, 18, 689–693. [Google Scholar] [CrossRef] [PubMed]
- Nardini, M.; Thomas, R.L.; Knowland, V.C.P.; Braddick, O.J.; Atkinson, J. A Viewpoint-Independent Process for Spatial Reorientation. Cognition 2009, 112, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Newcombe, N.S. Navigation and the Developing Brain. J. Exp. Biol. 2019, 222, jeb186460. [Google Scholar] [CrossRef]
- Martín-Pozuelo, N.; Carballo-Costa, L.; Solís-García, M.; Giancola, M.; Piccardi, L.; De las Cuevas-Terán, I.; Robles-García, V. Measuring Spatial Navigation during Locomotion in Children: A Systematic Review. Heliyon 2024, 10, e33817. [Google Scholar] [CrossRef] [PubMed]
- Østby, Y.; Tamnes, C.K.; Fjell, A.M.; Westlye, L.T.; Due-Tønnessen, P.; Walhovd, K.B. Heterogeneity in Subcortical Brain Development: A Structural Magnetic Resonance Imaging Study of Brain Maturation from 8 to 30 Years. J. Neurosci. 2009, 29, 11772–11782. [Google Scholar] [CrossRef]
- Pavlova, M.; Sokolov, A.; Krageloh-Mann, I. Visual Navigation in Adolescents with Early Periventricular Lesions: Knowing Where, but Not Getting There. Cereb. Cortex 2006, 17, 363–369. [Google Scholar] [CrossRef]
- Bartonek, A.; Lidbeck, C.M.; Gutierrez-Farewik, E.M. Influence of External Visual Focus on Gait in Children with Bilateral Cerebral Palsy. Pediatr. Phys. Ther. 2016, 28, 393–399. [Google Scholar] [CrossRef]
- Uematsu, A.; Matsui, M.; Tanaka, C.; Takahashi, T.; Noguchi, K.; Suzuki, M.; Nishijo, H. Developmental Trajectories of Amygdala and Hippocampus from Infancy to Early Adulthood in Healthy Individuals. PLoS ONE 2012, 7, e46970. [Google Scholar] [CrossRef] [PubMed]
- Blankenship, S.L.; Redcay, E.; Dougherty, L.R.; Riggins, T. Development of Hippocampal Functional Connectivity during Childhood. Hum. Brain Mapp. 2017, 38, 182–201. [Google Scholar] [CrossRef]
- Calabro, F.J.; Murty, V.P.; Jalbrzikowski, M.; Tervo-Clemmens, B.; Luna, B. Development of Hippocampal–Prefrontal Cortex Interactions through Adolescence. Cereb. Cortex 2020, 30, 1548–1558. [Google Scholar] [CrossRef]
- Bartonek, A.; Lidbeck, C.; Hellgren, K.; Gutierrez-Farewik, E. Head and Trunk Movements During Turning Gait in Children with Cerebral Palsy. J. Mot. Behav. 2019, 51, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Bartonek, Å.; Guariglia, C.; Piccardi, L. Topographical Working Memory in Children and Adolescents with Motor Disabilities. Cogent Psychol. 2020, 7, 1757855. [Google Scholar] [CrossRef]
- Belmonti, V.; Berthoz, A.; Cioni, G.; Fiori, S.; Guzzetta, A. Navigation Strategies as Revealed by Error Patterns on the Magic Carpet Test in Children with Cerebral Palsy. Front. Psychol. 2015, 6, 880. [Google Scholar] [CrossRef]
- Mürner-Lavanchy, I.; Ritter, B.C.; Spencer-Smith, M.M.; Perrig, W.J.; Schroth, G.; Steinlin, M.; Everts, R. Visuospatial Working Memory in Very Preterm and Term Born Children—Impact of Age and Performance. Dev. Cogn. Neurosci. 2014, 9, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Noble, K.G.; Houston, S.M.; Brito, N.H.; Bartsch, H.; Kan, E.; Kuperman, J.M.; Akshoomoff, N.; Amaral, D.G.; Bloss, C.S.; Libiger, O.; et al. Family Income, Parental Education and Brain Structure in Children and Adolescents. Nat. Neurosci. 2015, 18, 773–778. [Google Scholar] [CrossRef] [PubMed]
- Piccardi, L.; Nori, R.; Cimadevilla, J.M.; Kozhevnikov, M. The Contribution of Internal and External Factors to Human Spatial Navigation. Brain Sci. 2024, 14, 585. [Google Scholar] [CrossRef]
- Levine, S.C.; Huttenlocher, J.; Taylor, A.; Langrock, A. Early Sex Differences in Spatial Skill. Dev. Psychol. 1999, 35, 940–949. [Google Scholar] [CrossRef] [PubMed]
- Safari, S.; Ahmadi, N.; Mohammadkhani, R.; Ghahremani, R.; Khajvand-Abedeni, M.; Shahidi, S.; Komaki, A.; Salehi, I.; Karimi, S.A. Sex Differences in Spatial Learning and Memory and Hippocampal Long-Term Potentiation at Perforant Pathway-Dentate Gyrus (PP-DG) Synapses in Wistar Rats. Behav. Brain Funct. 2021, 17, 9. [Google Scholar] [CrossRef]
- Hamson, D.K.; Roes, M.M.; Galea, L.A.M. Sex Hormones and Cognition: Neuroendocrine Influences on Memory and Learning. In Comprehensive Physiology; Wiley: Hoboken, NJ, USA, 2016; pp. 1295–1337. [Google Scholar]
| Test 1 | Test 2 | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | n (%) | M | SD | CI (95%) | n (%) | M | SD | CI (95%) |
| Total | 247 (100) | 49.48 | 26.0 | 249 (100) | 36.73 | 22.9 | ||
| 4–8 | 26 (10.5) | 64.88 | 28.7 | 53.27–76.50 | 45 (18.1) | 45.96 | 23.4 | 38.90–53.01 |
| 8–10 | 58 (23.5) | 49.59 | 28.1 | 42.19–56.98 | 46 (18.5) | 36.87 | 22.1 | 30.20–43.53 |
| 10–12 | 107 (43.3) | 46.31 | 24.0 | 41.70–50.92 | 117 (47.0) | 33.43 | 21.8 | 29.43–37.42 |
| 13–15 | 56 (22.7) | 48.27 | 24.1 | 41.81–54.73 | 41 (16.5) | 35.51 | 24.3 | 27.83–43.19 |
| One-Way ANOVA Test 1 | One-Way ANOVA Test 2 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Performance Time (s) | Performance Time | ||||||||||||
| Sum of Squares | df | Mean Square | F | Sig. | Sum of Squares | df | Mean Square | F | Sig. | ||||
| Between Groups | 7329.10 | 3.00 | 2443.03 | 3.73 | 0.01 | Between Groups | 5176.03 | 3.00 | 1725.35 | 3.38 | 0.02 | ||
| Within Groups | 159,036.53 | 243.00 | 654.47 | Within Groups | 125,021.40 | 245.00 | 510.29 | ||||||
| Total | 166,365.63 | 246.00 | Total | 130,197.43 | 248.00 | ||||||||
| Multiple Comparisons | Multiple Comparisons | ||||||||||||
| Dependent Variable: | Dependent Variable: | ||||||||||||
| Tukey HSD | Tukey HSD | ||||||||||||
| (I) Age Groups | Mean Difference (I-J) | Std. Error | Sig. | 95% Confidence Interval | (I) Age Groups | Mean Difference (I-J) | Std. Error | Sig. | 95% Confidence Interval | ||||
| Lower Bound | Upper Bound | Lower Bound | Upper Bound | ||||||||||
| 4–8 | 8–10 | 15.30 | 6.04 | 0.06 | −0.32 | 30.92 | 4–8 | 8–10 | 8.78 | 4.74 | 0.25 | −3.47 | 21.03 |
| 10–12 | 18,576 * | 5.59 | 0.01 | 4.11 | 33.05 | 45,636.00 | 12,528 * | 3.96 | 0.01 | 2.28 | 22.78 | ||
| 12–15 | 16,617 * | 6.07 | 0.03 | 0.91 | 32.32 | 12–15 | 10.44 | 4.88 | 0.14 | −2.17 | 23.06 | ||
| 8–10 | 4–8 | −15.30 | 6.04 | 0.06 | −30.92 | 0.32 | 8–10 | 4–8 | −8.78 | 4.74 | 0.25 | −21.03 | 3.47 |
| 10–12 | 3.28 | 4.17 | 0.86 | −7.51 | 14.07 | 10–12 | 3.75 | 3.93 | 0.78 | −6.42 | 13.92 | ||
| 12–15 | 1.32 | 4.79 | 0.99 | −11.08 | 13.72 | 12–15 | 1.66 | 4.85 | 0.99 | −10.89 | 14.21 | ||
| 10–12 | 4–8 | −18,576 * | 5.59 | 0.01 | −33.05 | −4.11 | 10–12 | 4–8 | −12,528 * | 3.96 | 0.01 | −22.78 | −2.28 |
| 8–10 | −3.28 | 4.17 | 0.86 | −14.07 | 7.51 | 8–10 | −3.75 | 3.93 | 0.78 | −13.92 | 6.42 | ||
| 12–15 | −1.96 | 4.22 | 0.97 | −12.87 | 8.96 | 12–15 | −2.08 | 4.10 | 0.96 | −12.69 | 8.52 | ||
| 12–15 | 4–8 | −16,617 * | 6.07 | 0.03 | −32.32 | −0.91 | 12–15 | 4–8 | −10.44 | 4.88 | 0.14 | −23.06 | 2.17 |
| 8–10 | −1.32 | 4.79 | 0.99 | −13.72 | 11.08 | 8–10 | −1.66 | 4.85 | 0.99 | −14.21 | 10.89 | ||
| 10–12 | 1.96 | 4.22 | 0.97 | −8.96 | 12.87 | 10–12 | 2.08 | 4.10 | 0.96 | −8.52 | 12.69 | ||
| Independent Samples Test Between Performance Time in the Same Age Groups of Each Test | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age Groups 4–8 | Levene’s Test * | t-test for Equality of Means | ||||||||
| F | Sig. | t | df | Significance | Mean Difference | Std. Error Difference | 95% Confidence Interval of the Difference | |||
| One-Sided p | Two-Sided p | Lower | Upper | |||||||
| Equal Variances Assumed | 0.383 | 0.538 | 3.011 | 69 | 0.002 | 0.004 | 18.92906 | 6.28626 | 6.38832 | 31.4698 |
| Equal Variances not Assumed | 2.851 | 44.226 | 0.003 | 0.007 | 18.92906 | 6.63829 | 5.5524 | 32.30572 | ||
| Age Groups 8–10 | Levene’s Test * | t-test for Equality of Means | ||||||||
| F | Sig. | t | df | Significance | Mean Difference | Std. Error Difference | 95% Confidence Interval of the Difference | |||
| One-Sided p | Two-Sided p | Lower | Upper | |||||||
| Equal Variances Assumed | 7.445 | 0.007 | 2.455 | 102 | 0.008 | 0.016 | 12.41229 | 5.05652 | 2.38271 | 22.44188 |
| Equal Variances not Assumed | 2.524 | 101.991 | 0.007 | 0.013 | 12.41229 | 4.91778 | 2.65789 | 22.16669 | ||
| Age Groups 10–12 | Levene’s Test * | t-test for Equality of Means | ||||||||
| F | Sig. | t | df | Significance | Mean Difference | Std. Error Difference | 95% Confidence Interval of the Difference | |||
| One-Sided p | Two-Sided p | Lower | Upper | |||||||
| Equal Variances Assumed | 1.222 | 0.27 | 4.204 | 222 | <0.001 | <0.001 | 12.88106 | 3.06415 | 6.84252 | 18.9196 |
| Equal Variances not Assumed | 4.186 | 214.572 | <0.001 | <0.001 | 12.88106 | 3.07748 | 6.8151 | 18.94702 | ||
| Age Groups 13–15 | Levene’s Test * | t-test for Equality of Means | ||||||||
| F | Sig. | t | df | Significance | Mean Difference | Std. Error Difference | 95% Confidence Interval of the Difference | |||
| One-Sided p | Two-Sided p | Lower | Upper | |||||||
| Equal Variances Assumed | 0.761 | 0.385 | 2.562 | 95 | 0.006 | 0.012 | 12.75566 | 4.97828 | 2.87252 | 22.6388 |
| Equal Variances not Assumed | 2.559 | 85.926 | 0.006 | 0.012 | 12.75566 | 4.98496 | 2.84577 | 22.66556 | ||
| N | Mean | Std. Deviation | Std. Error | 95% Confidence Interval for Mean | Minimum | Maximum | ||
|---|---|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | |||||||
| Test 1 | ||||||||
| Male 4–8 (1) | 13 | 58.4615 | 23.10705 | 6.40874 | 44.4981 | 72.425 | 30 | 117 |
| Female 4–8 (1) | 13 | 71.3077 | 33.16973 | 9.19963 | 51.2634 | 91.352 | 27 | 143 |
| Male 8–10 (1) | 35 | 42.8286 | 26.56234 | 4.48986 | 33.7041 | 51.9531 | 14 | 110 |
| Female 8–10 (1) | 23 | 59.8696 | 27.82299 | 5.80149 | 47.838 | 71.9011 | 12 | 118 |
| Male 10–12 (1) | 59 | 45.1017 | 23.33393 | 3.03782 | 39.0208 | 51.1825 | 12 | 123 |
| Female 10–12 (1) | 48 | 47.7917 | 25.05395 | 3.61623 | 40.5168 | 55.0666 | 19 | 115 |
| Male 13–15 (1) | 26 | 46.4615 | 24.77455 | 4.85869 | 36.4549 | 56.4682 | 13 | 93 |
| Female 13–15 (1) | 30 | 49.8333 | 23.87335 | 4.35866 | 40.9189 | 58.7478 | 19 | 102 |
| Test 2 | ||||||||
| Male 4–8 (2) | 22 | 44 | 20.00714 | 4.26554 | 35.1293 | 52.8707 | 15 | 83 |
| Female 4–8 (2) | 23 | 47.8261 | 26.69636 | 5.56658 | 36.2817 | 59.3705 | 16 | 105 |
| Male 8–10 (2) | 25 | 39.28 | 25.35008 | 5.07002 | 28.816 | 49.744 | 6 | 103 |
| Female 8–10 (2) | 21 | 34.6667 | 17.59072 | 3.83861 | 26.6595 | 42.6739 | 17 | 85 |
| Male 10–12 (2) | 71 | 32.2113 | 22.97944 | 2.72716 | 26.7721 | 37.6504 | 11 | 120 |
| Female 10–12 (2) | 46 | 35.3043 | 19.99319 | 2.94783 | 29.3671 | 41.2416 | 10 | 95 |
| Male 13–15 (2) | 18 | 40.5556 | 29.5699 | 6.96969 | 25.8508 | 55.2603 | 10 | 108 |
| Female 13–15 (2) | 23 | 31.5652 | 19.09028 | 3.9806 | 23.31 | 39.8205 | 10 | 86 |
| Total | 496 | 43.0766 | 25.29515 | 1.13579 | 40.8451 | 45.3082 | 6 | 143 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kostakos, K.; Pliakopanou, A.; Meimaridis, V.; Galanou, O.-N.; Anagnostou, A.A.; Sertidou, D.; Katis, P.; Anastasiou, P.; Katsoulidis, K.; Lykogiorgos, Y.; et al. Development of Spatial Memory: A Behavioral Study. NeuroSci 2024, 5, 713-728. https://doi.org/10.3390/neurosci5040050
Kostakos K, Pliakopanou A, Meimaridis V, Galanou O-N, Anagnostou AA, Sertidou D, Katis P, Anastasiou P, Katsoulidis K, Lykogiorgos Y, et al. Development of Spatial Memory: A Behavioral Study. NeuroSci. 2024; 5(4):713-728. https://doi.org/10.3390/neurosci5040050
Chicago/Turabian StyleKostakos, Konstantinos, Alexandra Pliakopanou, Vasileios Meimaridis, Ourania-Natalia (Oriana) Galanou, Aikaterini Argyro Anagnostou, Dimitra Sertidou, Panagiotis Katis, Periklis Anastasiou, Konstantinos Katsoulidis, Yannis Lykogiorgos, and et al. 2024. "Development of Spatial Memory: A Behavioral Study" NeuroSci 5, no. 4: 713-728. https://doi.org/10.3390/neurosci5040050
APA StyleKostakos, K., Pliakopanou, A., Meimaridis, V., Galanou, O.-N., Anagnostou, A. A., Sertidou, D., Katis, P., Anastasiou, P., Katsoulidis, K., Lykogiorgos, Y., Mytilinaios, D., Katsenos, A. P., Simos, Y. V., Bellos, S., Konitsiotis, S., Peschos, D., & Tsamis, K. I. (2024). Development of Spatial Memory: A Behavioral Study. NeuroSci, 5(4), 713-728. https://doi.org/10.3390/neurosci5040050







