Metastatic Renal-Cell Carcinoma of the Oro-Facial Tissues: A Comprehensive Review of the Literature with a Focus on Clinico–Pathological Findings
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
4.1. General Considerations
4.2. Diagnostic Challenges and Clinical Work-Up
4.3. Pathological Differential Diagnosis and Imaging
4.4. Summary of Clinico-Epidemiologic Aspects
5. Conclusions and Future Directions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hirshberg, A.; Buchner, A. Metastatic tumours to the oral region. An overview. Eur. J. Cancer Part B Oral Oncol. 1995, 31, 355–360. [Google Scholar] [CrossRef]
- Hirshberg, A.; Berger, R.; Allon, I.; Kaplan, I. Metastatic Tumors to the Jaws and Mouth. Head Neck Pathol. 2014, 8, 463–474. [Google Scholar] [CrossRef] [PubMed]
- McClure, S.A.; Movahed, R.; Salama, A.; Ord, R.A. Maxillofacial Metastases: A Retrospective Review of One Institution’s 15-Year Experience. J. Oral Maxillofac. Surg. 2013, 71, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.-L.; Kang, J.; Wen, Y.-L.; Ying, W.-M.; Yi, J.; Hua, C.-G.; Tang, X.-F.; Wen, Y.-M. Metastatic Tumors to the Oral and Maxillofacial Region: A Retrospective Study of 19 Cases in West China and Review of the Chinese and English Literature. J. Oral Maxillofac. Surg. 2009, 67, 718–737. [Google Scholar] [CrossRef] [PubMed]
- Pastremoli, A. Gingival metastasis, the first clinical sign of a silent kidney carcinoma. A case report. Minerva Stomatol. 1991, 40, 825–828. [Google Scholar]
- Raiss, H.; Duplomb, S.; Tartas, S.; Layachi, M.; Errihani, H. Lingual metastasis as an initial presentation of renal cell carcinoma: A case report. J. Med. Case Rep. 2017, 11, 314. [Google Scholar] [CrossRef]
- Vallalta Morales, M.; Todolí Parra, J.; Cervera Miguel, J.I.; Calabuig Alborch, J.R. Hemiparesia derecha como forma de presentación de carcinoma renal de células claras. Ann. Intern. Med. 2004, 21, 359–360. [Google Scholar] [CrossRef]
- Hirshberg, A.; Leibovich, P.; Buchner, A. Metastatic tumors to the jawbones: Analysis of 390 cases. J. Oral Pathol. Med. 1994, 23, 337–341. [Google Scholar] [CrossRef]
- Hirshberg, A.; Leibovich, P.; Buchner, A. Metastases to the oral mucosa: Analysis of 157 cases. J. Oral Pathol. Med. 1993, 22, 385–390. [Google Scholar] [CrossRef]
- Hirshberg, A.; Leibovich, P.; Horowitz, I.; Buchner, A. Metastatic tumors to postextraction sites. J. Oral Maxillofac. Surg. 1993, 51, 1334–1337. [Google Scholar] [CrossRef]
- Ljungberg, B.; Campbell, S.C.; Cho, H.Y.; Jacqmin, D.; Lee, J.E.; Weikert, S.; Kiemeney, L.A. The Epidemiology of Renal Cell Carcinoma. Eur. Urol. 2011, 60, 615–621. [Google Scholar] [CrossRef]
- Unverzagt, S.; Moldenhauer, I.; Nothacker, M.; Roßmeißl, D.; Hadjinicolaou, A.V.; Peinemann, F.; Greco, F.; Seliger, B. Immunotherapy for metastatic renal cell carcinoma. Cochrane Urology Group, curatore. Cochrane Database Syst. Rev. 2017, CD011673. [Google Scholar] [CrossRef]
- Zerdes, I.; Tolia, M.; Tsoukalas, N.; Mitsis, M.; Kardamakis, D.; Pistevou-Gombaki, K.; Tsekeris, P.; Kyrgias, G. Systemic therapy of metastatic renal cell carcinoma: Review of the current literature. Urol. J. 2019, 86, 3–8. [Google Scholar] [CrossRef]
- Nazha, S.; Tanguay, S.; Kapoor, A.; Jewett, M.; Kollmannsberger, C.; Wood, L.; Bjarnason, G.; Heng, D.; Soulières, D.; Reaume, N.; et al. Use of Targeted Therapy in Patients with Metastatic Renal Cell Carcinoma: Clinical and Economic Impact in a Canadian Real-Life Setting. Curr. Oncol. 2018, 25, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Goebell, P.J.; Staehler, M.; Müller, L.; Nusch, A.; Scheffler, M.; Sauer, A.; Von Verschuer, U.; Tech, S.; Kruggel, L.; Jänicke, M.; et al. Changes in Treatment Reality and Survival of Patients with Advanced Clear Cell Renal Cell Carcinoma—Analyses from the German Clinical RCC-Registry. Clin. Genitourin. Cancer 2018, 16, e1101–e1115. [Google Scholar] [CrossRef] [PubMed]
- De Groot, S.; Redekop, W.K.; Versteegh, M.M.; Sleijfer, S.; Oosterwijk, E.; Kiemeney, L.A.L.M.; Uyl-de Groot, C.A. Health-related quality of life and its determinants in patients with metastatic renal cell carcinoma. Qual. Life Res. 2018, 27, 115–124. [Google Scholar] [CrossRef]
- Atkins, M.B.; Tannir, N.M. Current and emerging therapies for first-line treatment of metastatic clear cell renal cell carcinoma. Cancer Treat Rev. 2018, 70, 127–137. [Google Scholar] [CrossRef]
- Macleod, L.C.; Hotaling, J.M.; Wright, J.L.; Davenport, M.T.; Gore, J.L.; Harper, J.; White, E. Risk Factors for Renal Cell Carcinoma in the VITAL Study. J. Urol. 2013, 190, 1657–1661. [Google Scholar] [CrossRef]
- Cheungpasitporn, W.; Thongprayoon, C.; O’Corragain, O.A.; Edmonds, P.J.; Ungprasert, P.; Kittanamongkolchai, W.; Erickson, S.B. The risk of kidney cancer in patients with kidney stones: A systematic review and meta-analysis. QJM Int. J. Med. 2015, 108, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Joh, H.K.; Willett, W.C.; Cho, E. Type 2 Diabetes and the Risk of Renal Cell Cancer in Women. Diabetes Care 2011, 34, 1552–1556. [Google Scholar] [CrossRef]
- Christensson, A.; Savage, C.; Sjoberg, D.D.; Cronin, A.M.; Frank O’Brien, M.; Lowrance, W.; Nilsson, P.M.; Vickers, A.J.; Russo, P.; Lilja, H. Association of cancer with moderately impaired renal function at baseline in a large, representative, population-based cohort followed for up to 30 years: Cancer. Int. J. Cancer 2013, 133, 1452–1458. [Google Scholar] [CrossRef]
- Choueiri, T.K.; Je, Y.; Cho, E. Analgesic use and the risk of kidney cancer: A meta-analysis of epidemiologic studies. Int. J. Cancer 2014, 134, 384–396. [Google Scholar] [CrossRef]
- Lambe, M.; Lindblad, P.; Wuu, J.; Remler, R.; Hsieh, C.-C. Pregnancy and risk of renal cell cancer: A population-based study in Sweden. Br. J. Cancer 2002, 86, 1425–1429. [Google Scholar] [CrossRef]
- Kabat, G.C.; Silvera, S.A.N.; Miller, A.B.; Rohan, T.E. A cohort study of reproductive and hormonal factors and renal cell cancer risk in women. Br. J. Cancer 2007, 96, 845–849. [Google Scholar] [CrossRef] [PubMed]
- McKay, R.R.; Kroeger, N.; Xie, W.; Lee, J.L.; Knox, J.J.; Bjarnason, G.A.; MacKenzie, M.J.; Wood, L.; Srinivas, S.; Vaishampayan, U.N.; et al. Impact of Bone and Liver Metastases on Patients with Renal Cell Carcinoma Treated with Targeted Therapy. Eur. Urol. 2014, 65, 577–584. [Google Scholar] [CrossRef]
- Ðanić, P.; Ðanić, D.; Macan, D. Tongue metastasis as an initial presentation of renal cell carcinoma. Med. Glas. 2018, 15, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Makos, C.P.; Psomaderis, K. A literature review in renal carcinoma metastasis to the oral mucosa and a new report of an epulis-like metastasis. J. Oral Maxillofac. Surg. 2009, 67, 653–660. [Google Scholar] [CrossRef]
- Pires, F.R.; Azevedo, R.S.; Ficarra, G.; Cardoso, A.S.; Carlos, R.; Kowalski, L.P.; de Almeida, O.P. Metastatic renal cell carcinoma to the oral cavity and clear cell mucoepidermoid carcinoma: Comparative clinicopathologic and immunohistochemical study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2010, 109, e22–e27. [Google Scholar] [CrossRef] [PubMed]
- Maiorano, E.; Altini, M.; Favia, G. Clear cell tumors of the salivary glands, jaws, and oral mucosa. Semin. Diagn. Pathol. 1997, 14, 203–212. [Google Scholar]
- Lopez-Beltran, A.; Carrasco, J.C.; Cheng, L.; Scarpelli, M.; Kirkali, Z.; Montironi, R. 2009 update on the classification of renal epithelial tumors in adults. Int. J. Urol. 2009, 16, 432–443. [Google Scholar] [CrossRef]
- Eble, J.N.; Sauter, G.; Epstein, L.I.; Sesterhenn, I.A. World Health Organization Classification of Tumours. In Pathology and Genetics of Tumours of the Urinary System and Male Genital Organs; ARC Press: Lyon, France, 2004. [Google Scholar]
- Gobbo, S.; Eble, J.N.; Grignon, D.J.; Martignoni, G.; MacLennan, G.T.; Shah, R.B.; Zhang, S.; Brunelli, M.; Cheng, L. Clear Cell Papillary Renal Cell Carcinoma: A Distinct Histopathologic and Molecular Genetic Entity. Am. J. Surg. Pathol. 2008, 32, 1239–1245. [Google Scholar] [CrossRef] [PubMed]
- Sangoi, A.R.; Fujiwara, M.; West, R.B.; Montgomery, K.D.; Bonventre, J.V.; Higgins, J.P.; Rouse, R.V.; Gokden, N.; McKenney, J.K. Immunohistochemical Distinction of Primary Adrenal Cortical Lesions From Metastatic Clear Cell Renal Cell Carcinoma: A Study of 248 Cases. Am. J. Surg. Pathol. 2011, 35, 678–686. [Google Scholar] [CrossRef] [PubMed]
- Griffin, N.; Gore, M.E.; Sohaib, S.A. Imaging in Metastatic Renal Cell Carcinoma. Am. J. Roentgenol. 2007, 189, 360–370. [Google Scholar] [CrossRef] [PubMed]
- Corsi, A.; Guerra, F.; Grippaudo, G.; Bosman, C. Oral metastasis of renal cell carcinoma. Report of case and critical evaluation of morphologic features for differential diagnosis. Pathologica 1994, 86, 665–669. [Google Scholar] [PubMed]
- Kumamoto, H.; Yamazaki, S.; Sato, A.; Yamaguchi, T.; Tezuka, F.; Ooya, K. Clear cell odontogenic tumor in the mandible: Report of a case with duct-like appearances and dentinoid induction. J. Oral Pathol. Med. 2000, 29, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Nair, M.K.; Burkes, E.J.; Chai-U-Dom, O. Radiographic manifestation of clear cell odontogenic tumor. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2000, 89, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Pastore, A.; Ciorba, A.; Soliani, M.; Di Laora, A.; Valpiani, G.; Bianchini, C.; Stomeo, F.; Merlo, R.; Pelucchi, S. Secondary malignant tumors of the parotid gland: Not a secondary problem! J. Buon 2017, 22, 513–518. [Google Scholar] [PubMed]
- Franzen, A.; Buchali, A.; Lieder, A. The rising incidence of parotid metastases: Our experience from four decades of parotid gland surgery. Acta Otorhinolaryngol. Ital. 2017, 37, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Majewska, H.; Skálová, A.; Radecka, K.; Stodulski, D.; Hyrcza, M.; Stankiewicz, C.; Biernat, W. Renal clear cell carcinoma metastasis to salivary glands—A series of 9 cases: Clinico-pathological study. Pol. J. Pathol. 2016, 1, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Ray, A.; Bhattacharya, J.; Ganguly, S. Renal cell carcinoma presenting with oral tongue metastasis: A rare case presentation. J. Cancer Res. Ther. 2013, 9, 117. [Google Scholar] [CrossRef]
- Kalinin, Y.; Correia-Neto, I.J.; Do Nascimento, S.V.; De Branco Gonçaves, V.C.; De Andrade, B.A.B.; Nonaka, C.F.W.; Alves, P.M.; Cunha, J.L.S. Lingual metastasis as the first presentation of clear cell renal cell carcinoma: Report of a rare case clinically mimicking a benign lesion. Oral Oncol. 2023, 137, 106293. [Google Scholar] [CrossRef] [PubMed]
- Nishii, N.; Shimamoto, H.; Ohsako, T.; Yokokawa, M.; Sato, Y.; Ohata, Y.; Kayamori, K.; Ikeda, T.; Harada, H. Renal cell carcinoma metastasis to the maxillary bone successfully treated with surgery after vascular embolization: A case report. J. Med. Case Rep. 2020, 14, 193. [Google Scholar] [CrossRef]
- Zhang, R.; Lee, C.W.; Basyuni, S.; Santhanam, V. Mandibular swelling as the initial presentation for renal cell carcinoma: A case report. Int. J. Surg. Case Rep. 2020, 70, 96–100. [Google Scholar] [CrossRef]
- Jung, S.Y.; Maeng, J.Y.; Lee, H.; Han, J.J.; Kim, S.M.; Myoung, H. Metastasis of Renal Cell Carcinoma to the Mandible. J. Craniofac. Surg. 2023, 34, e334–e336. [Google Scholar] [CrossRef]
- Stojanovic, M.; Krasic, D.; Trajkovic, M.; Petrovic, V. Rare renal cell carcinoma metastasis to mandibular gingiva: A case report and literature review. Niger. J. Clin. Pract. 2020, 23, 1483. [Google Scholar]
- Li, L.; Friedrich, R.E.; Schmelzle, R.; Donath, K. Metachronous bilateral metastases of renal cell carcinoma to the parotid region. J. Oral Maxillofac. Surg. 2001, 59, 434–438. [Google Scholar] [CrossRef] [PubMed]
- Kundu, S.; Eynon-Lewis, N.J.; Radcliffe, G.J. Extensive metastatic renal cell carcinoma presenting as facial nerve palsy. J. Laryngol. Otol. 2001, 115, 488–490. [Google Scholar] [CrossRef]
- Park, Y.W.; Hlivko, T.J. Parotid gland metastasis from renal cell carcinoma. Laryngoscope 2002, 112, 453–456. [Google Scholar] [CrossRef]
- Pritchyk, K.M.; Schiff, B.A.; Newkirk, K.A.; Krowiak, E.; Deeb, Z.E. Metastatic Renal Cell Carcinoma to the Head and Neck. Laryngoscope 2002, 112, 1598–1602. [Google Scholar] [CrossRef]
- Göğüş, Ç.; Kiliç, Ö.; Tulunay, Ö.; Tulunay, Ö.; Bedük, Y. Solitary metastasis of renal cell carcinoma to the parotid gland 10 years after radical nephrectomy. Int. J. Urol. 2004, 11, 894–896. [Google Scholar] [CrossRef]
- Torres-Carranza, E.; Garcia-Perla, A.; Infante-Cossio, P.; Belmonte-Caro, R.; Loizaga-Iriondo, J.M.; Gutierrez-Perez, J.L. Airway obstruction due to metastatic renal cell carcinoma to the tongue. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2006, 101, e76–e78. [Google Scholar] [CrossRef]
- Newton, J.R.; O’Donnell, M.; Samuel, P.R. A case of renal cell carcinoma metastasizing to the parotid gland. Otolaryngol. Head Neck Surg. 2007, 136 (Suppl. S4), S65–S67. [Google Scholar] [CrossRef]
- Yoshitomi, I.; Kawasaki, G.; Mizuno, A.; Nishikido, M.; Hayashi, T.; Fujita, S.; Ikeda, T. Lingual metastasis as an initial presentation of renal cell carcinoma. Med. Oncol. 2011, 28, 1389–1394. [Google Scholar] [CrossRef][Green Version]
- Morvan, J.B.; Veyrières, J.B.; Mimouni, O.; Cathelinaud, O.; Allali, L.; Verdalle, P. Clear-cell renal carcinoma metastasis to the base of the tongue and sphenoid sinus: Two very rare atypical ENT locations. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2011, 128, 91–94. [Google Scholar] [CrossRef]
- Balliram, S.; Goetz, L.; Ramsoobhag, K.; Narinesingh, D.; Medford, S.; Naraynsingh, V. Renal Cell Carcinoma Presenting as a Tongue Lesion. J. Oral Maxillofac. Surg. 2012, 70, 1605–1608. [Google Scholar] [CrossRef]
- Serouya, S.M.; Dultz, L.A.; Concors, S.J.; Wang, B.; Patel, K.N. Late Solitary Metastasis of Renal Cell Carcinoma to the Submandibular Gland. J. Oral Maxillofac. Surg. 2012, 70, 2356–2359. [Google Scholar] [CrossRef]
- Wadasadawala, T.; Kumar, P.; Agarwal, J.; Ghosh-Laskar, S. Palliation of dysphagia with radiotherapy for exophytic base tongue metastases in a case of renal cell carcinoma. Indian J. Urol. 2011, 27, 550. [Google Scholar]
- Deeb, R.; Zhang, Z.; Ghanem, T. Metastatic Renal Cell Carcinoma to the Parotid Gland in the Setting of Chronic Lymphocytic Leukemia. Case Rep. Med. 2012, 2012, 265708. [Google Scholar] [CrossRef]
- Özkiris, M.; Kubilay, U.; Sezen, O. Cervical lymph node metastasis in renal cell carcinoma. J. Oral Maxillofac. Pathol. 2011, 15, 211. [Google Scholar] [CrossRef] [PubMed]
- Ghazali, N.; Davis, C.; Barrett, A.W.; Tighe, J.V. Bilateral Asynchronous Renal Cell Carcinoma with Metastatic Involvement of the Tongue. Case Rep. Pathol. 2012, 2012, 729642. [Google Scholar] [CrossRef] [PubMed]
- Lau, S.Y.C.; Chittleborough, T.J.; McCracken, J.A.; Wijeratne, S. Metastatic clear-cell renal carcinoma to the parotid. ANZ J. Surg. 2012, 82, 760–761. [Google Scholar] [CrossRef]
- Mazeron, R.; Fenoll, L.; Mathieu, M.-C.; Dumas, I.; Haie-Meder, C. Brachytherapy for isolated tongue metastasis of renal clear cell carcinoma. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2013, 130, 149–151. [Google Scholar] [CrossRef][Green Version]
- Yanlan, C.; Liping, S.; Shaomin, C.; Zi, L. Metastasis to the parotid region as an initial presentation of renal cell carcinoma: A case report. Oncol. Lett. 2013, 5, 997–999. [Google Scholar] [CrossRef]
- Udager, A.M.; Rungta, S.A. Metastatic renal cell carcinoma, clear cell type, of the parotid gland: A case report, review of literature, and proposed algorithmic approach to salivary gland clear cell neoplasms in fine-needle aspiration biopsies. Diagn. Cytopathol. 2014, 42, 974–983. [Google Scholar] [CrossRef]
- Abbaszadeh-Bidokhty, H.; Motallebnejad, M.; Rajabi-Moghaddam, M. Metastatic Renal cell Carcinoma Presenting as a clear-cell Tumor in Tongue: A Case Report. Iran. J. Otorhinolaryngol. 2014, 26, 185–190. [Google Scholar]
- Kotak, A.; Merrick, G. Presentation of metastatic renal cell carcinoma as a lip lesion. J. Surg. Case Rep. 2014, 2014, rju083. [Google Scholar] [CrossRef]
- Suojanen, J.; Färkkilä, E.; Helkamaa, T.; Loimu, V.; Törnwall, J.; Lindqvist, C.; Hagström, J.; Mesimäki, K. Rapidly growing and ulcerating metastatic renal cell carcinoma of the lower lip: A case report and review of the literature. Oncol. Lett. 2014, 8, 2175–2178. [Google Scholar] [CrossRef]
- Kudva, R.; Nayal, B.; Kantipudi, S.; Ray, S. Metastatic renal cell carcinoma of the buccal mucosa masquerading as a salivary gland neoplasm. J. Oral Maxillofac. Pathol. 2016, 20, 547. [Google Scholar]
- Georgy, J.T.; Mathuram, A.J.; George, A.A.; Chandramohan, J. Renal cell carcinoma presenting as a cutaneous horn and nodules on the gingiva and scalp. BMJ Case Rep. 2017, 2017, bcr-2017-220913. [Google Scholar] [CrossRef] [PubMed]
- Nifosì, G.; Bressand, H.; Nifosì, A.F.; Nifosì, L.; Damseaux, P. Epulis-Like Presentation of Gingival Renal Cancer Metastasis. Case Rep. Oncol. 2017, 10, 758–763. [Google Scholar] [CrossRef] [PubMed]
- Yoon, A.; Vasilyeva, D.; Peters, S.; Philipone, E. Renal cell carcinoma metastatic to the maxillary gingiva: A case report and review of the literature. J. Oral Maxillofac. Pathol. 2018, 22, 102. [Google Scholar] [CrossRef]
- McNattin, R.F.; Dean, J.; Archie, L. Clinical Reports from Memorial Hospital, New York City: A Case of Renal Adenocarcinoma with Unusual Manifestations. Am. J. Cancer 1931, 15, 1570–1576. [Google Scholar]
- Altinel, D.; Etit, D.; Tan, A.; Bayol, Ü.; Bulut, V.; Erdogan, I.G.; Beyhan, R.; Yalçin, Y. Metastatic Renal Cell Carcinoma Initially Presented as a Tongue Mass. Turk. J. Pathol. 2010, 26, 261–263. [Google Scholar]
- Syryło, T.; Syryło, A.; Jurkiewicz, D.; Zieliński, H.; Piętka, T. An upper lip tumour as the presenting symptom of metastatic renal cancer. Otolaryngol. Pol. 2010, 64, 318–319. [Google Scholar] [CrossRef]
- Gil-Julio, H.; Vázquez-Alonso, F.; Fernández-Sánchez, A.J.; Puche-Sanz, I.; Flores-Martín, J.F.; Cózar, J.M. Metastasis of Renal Cell Carcinoma to the Buccal Mucosa 19 Years after Radical Nephrectomy. Case Rep. Oncol. Med. 2012, 2012, 823042. [Google Scholar] [CrossRef]
- Shirazian, S.; Bahrami, N. An oral metastatic carcinoma guiding to discovery of a renal carcinoma: A case report. J. Craniomaxillofacial Res. 2016, 3, 230–234. [Google Scholar]
- Schrag, A.R.; Jordan, F.B. Unusual metastasis from primary hypernephroma. Can. Med. Assoc. J. 1945, 53, 168. [Google Scholar]
- Carmen, B.V.D.; Korbitz, B.C. Oral Metastasis from Hypernephroma. J. Am. Geriatr. Soc. 1970, 18, 743–746. [Google Scholar] [CrossRef]
- Friedlander, A.H.; Singer, R. Renal adenocarcinoma of the kidney with metastasis to the tongue. J. Am. Dent. Assoc. 1978, 97, 989–991. [Google Scholar] [CrossRef]
- Fitzgerald, R.H.; McInnes, B.K.; Manry, H.C. Renal cell carcinoma involving oral soft tissues. J. Oral Maxillofac. Surg. 1982, 40, 604–606. [Google Scholar] [CrossRef]
- Inai, T.; Kagawa, S.; Aga, Y.; Akiyama, K. A renal cell carcinoma with metastasis to the tongue. Hinyokika Kiyo Acta Urol. Jpn. 1987, 33, 1240–1243. [Google Scholar]
- Ishikawa, J.; Morisue, K.; Imanishi, O.; Kamidono, S. Renal cell carcinoma metastatic to the tongue: A case report. Hinyokika Kiyo Acta Urol. Jpn. 1991, 37, 263–265. [Google Scholar]
- Okabe, Y.; Ohoka, H.; Miwa, T.; Nagayama, I.; Furukawa, M. Renal cell carcinoma metastasis to the tongue. J. Laryngol. Otol. 1992, 106, 282–284. [Google Scholar] [CrossRef] [PubMed]
- Shibayama, T.; Hasegawa, S.; Nakamura, S.; Tachibana, M.; Jitsukawa, S.; Shiotani, A.; Morinaga, S. Disappearance of Metastatic Renal Cell Carcinoma to the Base of the Tongue after Systemic Administration of Interferon-Alpha. Eur. Urol. 1993, 24, 297–299. [Google Scholar] [CrossRef]
- Ziyada, W.F.; Brookes, J.D.; Penman, H.G. Expectorated tissue leading to diagnosis of renal adenocarcinoma. J. Laryngol. Otol. 1994, 108, 1108–1110. [Google Scholar] [CrossRef] [PubMed]
- Airoldi, M.; Succo, G.; Valente, G.; Cavalot, A.; Gabriele, P.; Bumma, C. Head and Neck Metastases of Renal Cancer after Nephrectomy: A Report of 2 Cases. Tumori J. 1995, 81, 213–214. [Google Scholar] [CrossRef]
- Aguirre, A.; Rinaggio, J.; Diaz-Ordaz, E. Lingual metastasis of renal cell carcinoma. J. Oral Maxillofac. Surg. 1996, 54, 344–347. [Google Scholar] [CrossRef]
- Konya, E.; Hara, Y.; Umekawa, T.; Uejima, S.; Sugiyama, T.; Kurita, T. Two cases of renal cell carcinoma detected by metastasis to another organ. Hinyokika Kiyo Acta Urol. Jpn. 1997, 43, 647–650. [Google Scholar]
- Tomita, T.; Inouye, T.; Shinden, S.; Mukai, M. Palliative radiotherapy for lingual metastasis of renal cell carcinoma. Auris Nasus Larynx 1998, 25, 209–214. [Google Scholar] [CrossRef]
- Navarro, F.; Vicente, J.; Villanueva, M.J.; Sánchez, A.; Provencio, M.; España, P. Metastatic Renal Cell Carcinoma to the Head and Neck Area. Tumori J. 2000, 86, 88–90. [Google Scholar] [CrossRef]
- Mekni, A.; Bouraoui, S.; Touati, S.; el Ouertani, L.; el May, A. Linguinal metastasis from clear cell carcinoma of the kidney. La Tunis. Med. 2002, 80, 570–573. [Google Scholar]
- Kyan, A.; Kato, S.N. Renal cell carcinoma metastatic to the base of tongue: A case report. Hinyokika Kiyo Acta Urol. Jpn. 2004, 50, 791–793. [Google Scholar]
- Huang, H.C.; Chang, K.P.; Chen, T.M.; Wu, K.F.; Ueng, S.H. Renal cell carcinoma metastases in the head and neck. Chang Gung Med. J. 2006, 29, 59–65. [Google Scholar]
- Cochrane, T.; Cheng, L.; Crean, J. Renal Cell Carcinoma: A Rare Metastasis to the Tongue—A Case Report. Dent. Update 2006, 33, 186–187. [Google Scholar] [CrossRef] [PubMed]
- Del Rosario Regalado, R.; Gallana Álvarez, S.; Creo Martínez, T.; Herce López, J.; Pereira Gallardo, S. Lingual metastasis from renal carcinoma. Rev. Esp. Cir. Oral Maxilofac. 2007, 29, 179–181. [Google Scholar]
- Longo, R.; Baldini, D.; Gasparini, G. An atypical tongue metastasis of renal cell carcinoma in a patient with metachronous hepatocellular carcinoma. Cancer Ther. 2008, 6, 707. [Google Scholar]
- Kella, V.K.N.; Cosgrove, J.M.; Krishnamoorthy, V. Synchronous lingual and thyroid metastasis from renal cell carcinoma. Am. J. Case Rep. 2009, 10, 88–92. [Google Scholar]
- Friedmann, I.; Osborn, D.A. Metastatic Tumours in the Ear, Nose and Throat Region. J. Laryngol. Otol. 1965, 79, 576–591. [Google Scholar] [CrossRef] [PubMed]
- Trinca, A.J.; Willis, R.A. Primary Carcinoma Unsuspected by the Clinician. Med. J. Aust. 1936, 2, 222–227. [Google Scholar]
- Branch, C.; Norton, R. Metastatic hypernephroma of the jaw. N. Engl. J. Med. 1928, 198, 559–561. [Google Scholar] [CrossRef]
- Salman, I.; Langel, I. Metastatic tumors of the oral cavity. Oral Surg. Oral Med. Oral Pathol. 1954, 7, 1141–1149. [Google Scholar] [CrossRef] [PubMed]
- Persson, P.A.; Wallenius, K. Metastatic Renal Carcinoma (Hypernephroma) in the Gingiva of the Lower Jaw. Acta Odontol. Scand. 1961, 19, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Cranin, A.N.; Berman, S.; Tucker, N. Renal-cell carcinoma of the mandibular periodontium. Oral Surg. Oral Med. Oral Pathol. 1966, 21, 626–631. [Google Scholar] [CrossRef]
- Buchner, A.; Begleiter, A. Metastatic Renal Cell Carcinoma in the Gingiva Mimicking a Hyperplastic Lesion: Case Report. J. Periodontol. 1980, 51, 413–415. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, Y.; Yakata, H.; Kawasaki, T.; Nakajima, T. Metastatic tumours of the mouth and jaws. J. Maxillofac. Surg. 1982, 10, 253–258. [Google Scholar] [CrossRef]
- Fay, J.T.; Weir, G.T. Metastatic renal cell carcinoma from a primary tumor removed 14 years previously. J. Oral Maxillofac. Surg. 1983, 41, 129–132. [Google Scholar] [CrossRef]
- Zohar, Y.; Ben-Tovim, R.; Gal, R.; Laurian, N. Metastatic carcinoma of oral soft tissue. Head Neck Surg. 1985, 7, 484–486. [Google Scholar] [CrossRef]
- Tsianos, E.B.; Karentzos, C.; Papadopoulos, N.E. Metastatic renal cell carcinoma in the gingiva of the maxilla and mandible: Report of a case. J. Oral Maxillofac. Surg. 1987, 45, 975–977. [Google Scholar] [CrossRef] [PubMed]
- Müller-Mattheis, V.; Hagen, M.; Frenzel, H.; Ackermann, R. A rare form of metastasis of renal cell cancer. A case report of intra-oral soft tissue metastasis. Urol. Ausg. A 1989, 28, 355–358. [Google Scholar]
- Hagen, M.; Müller-Mattheis, V.; Frenzel, H.; Fritzemeier, C.U. Intraoral soft tissue metastases of a renal cell carcinoma. Dtsch. Z. Mund- Kiefer- Gesichts-Chir. 1989, 13, 155–160. [Google Scholar]
- Salman, I.; Darlington, C. Rare (unusual) malignant tumors of the jaws. Am. J. Orthod. Oral Surg. 1944, 30, 725. [Google Scholar] [CrossRef]
- Mallett, S.P. A renal-cell metastatic carcinoma involving the mandible and submaxillary gland. Oral Surg. Oral Med. Oral Pathol. 1961, 14, 4–7. [Google Scholar] [CrossRef]
- Meyer, I.; Shklar, G. Malignant tumors metastatic to mouth and jaws. Oral Surg. Oral Med. Oral Pathol. 1965, 20, 350–362. [Google Scholar] [CrossRef]
- Godby, A.F.; Sonntag, R.W.; Cosentino, B.J. Hypernephroma with metastasis to the mandibular gingiva. Oral Surg. Oral Med. Oral Pathol. 1967, 23, 696–700. [Google Scholar] [CrossRef]
- Milobsky, S.A.; Milobsky, L.; Epstein, L.I. Metastatic renal adenocarcinoma presenting as periapical pathosis in the maxilla. Oral Surg. Oral Med. Oral Pathol. 1975, 39, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Nagayama, M.; Oka, T. Two cases of clear cell carcinoma found in the jaws. Nagoya J. Med. Sci. 1979, 42, 1–6. [Google Scholar] [PubMed]
- Susan, L.P.; Daughtry, J.D.; Stewart, B.H.; Straffon, R.A. Palatal metastases in renal cell carcinoma. Urology 1979, 13, 304–305. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, Y.; Yanagihara, N. Renal clear cell carcinoma metastatic to the nose and paranasal sinuses. Laryngoscope 1982, 92, 1190–1193. [Google Scholar] [CrossRef] [PubMed]
- Pick, J.B.; Wagner, R.M.; Indresano, A.T. Initial appearance of renal cell carcinoma as a metastatic mass in the mandible. J. Am. Dent. Assoc. 1986, 113, 759–761. [Google Scholar] [CrossRef]
- Zachariades, N.; Koumoura, F.; Vairaktaris, E.; Mezitis, M. Metastatic tumors to the jaws: A report of seven cases. J. Oral Maxillofac. Surg. 1989, 47, 991–996. [Google Scholar] [CrossRef]
- Jones, G.M.; Telfer, M.R.; Eveson, J.W. Metastatic renal clear cell carcinoma of the jaws. Two cases illustrating clinical and pathological diagnostic problems. Br. J. Oral Maxillofac. Surg. 1990, 28, 172–175. [Google Scholar] [CrossRef]
- Fandella, A.; Anselmo, G.; Maccatrozzo, L.; Frezza, D.; Marchiori, C. Epistaxis in Renal Carcinoma: Case Report. Scand. J. Urol. Nephrol. 1992, 26, 89. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; Sharma, S.D.; Bullock, K.N. An Unusual Case of Renal Cell Carcinoma with Two Rare Metastases. Scand. J. Urol. Nephrol. 1998, 32, 239–240. [Google Scholar] [PubMed]
- Guyot, L.; Sauvant, J.; Menasse, F.; Garcia, S.; Portier, F.; Gola, R. Hemorrhagic Mandibular Metastasis of Renal Origin: Usefulness of Therapeutic Embolization; Presse Medicale: Paris, France, 1983; Volume 28, pp. 1066–1068. [Google Scholar]
- Hönig, J.F. Inheritance of Hippel-Lindau Disease: A Rare Case of Maxillary Bone Metastasis. J. Craniofac. Surg. 2000, 11, 71–72. [Google Scholar] [CrossRef] [PubMed]
- Shetty, S.C.; Gupta, S.; Nagsubramanium, S.; Hasan, S.; Cherry, G. Mandibular metastasis from renal cell carcinoma. A case report. Indian J. Dent. Res. Off. Publ. Indian Soc. Dent. Res. 2001, 12, 77–80. [Google Scholar]
- Heinroth, S.; Bilkenroth, U.; Eckert, A.W.; Maurer, P. Die ossäre Metastase im Oberkiefer als Erstmanifestation eines Nierenzellkarzinoms: Ein Fallbericht. Oral Maxillofac. Surg. 2006, 10, 42–45. [Google Scholar]
- Madison, J.F.; Frierson, H.F. Pathologic quiz case 2. Clear cell carcinoma, consistent with metastatic renal cell carcinoma. Arch. Otolaryngol. Head Neck Surg. 1988, 114, 570–571, 573. [Google Scholar]
- Kishore, M.; Chauhan, D.S.; Dogra, S. Unusual presentation of renal cell carcinoma: A rare case report. J. Lab Physicians 2018, 10, 241–244. [Google Scholar] [CrossRef]
- Abro, C.; Sedhom, R.; Soni, A.; Markowski, M. Cutaneous finger and tongue metastases in renal cell carcinoma. BMJ Case Rep. 2019, 12, e230516. [Google Scholar] [CrossRef]
- Netto, R.; De Freitas Filho, S.A.J.; Cortezzi, W.; Merly, F.; De Andrade, V.M.; Pires, F.R. Metastasis of Renal Cell Carcinoma Causing Significant Facial Asymmetry. Case Rep. Surg. 2019, 2019, 6840873. [Google Scholar] [CrossRef]
- Walsh, M.A.; Quinn, A.J.; Mahesh, B. Case report: Renal cell carcinoma metastasis to the tongue. J. Surg. Case Rep. 2022, 2022, rjac565. [Google Scholar] [CrossRef]
- Mrena, R.; Leivo, I.; Passador-Santos, F.; Hagström, J.; Mäkitie, A.A. Histopathological findings in parotid gland metastases from renal cell carcinoma. Eur. Arch. Otorhinolaryngol. 2008, 265, 1005–1009. [Google Scholar] [CrossRef]
- Aljawad, M.; Alharbi, M.K.; Algahtani, S.M.; Mughallis, H.M.; Almhna, S.M. Metastasis of Clear Cell Renal Cell Carcinoma to the Parotid Gland: A Case Report. Cureus 2023, 15, e43676. [Google Scholar] [CrossRef]
- Migliorelli, A.; Caranti, A.; Manuelli, M.; Bianchini, C.; Ciorba, A.; Pelucchi, S. Clear-Cell Renal Cell Carcinoma Metastasis into Pterygomaxillary Fossa—A Case Report. Ann. Maxillofac. Surg. 2023, 13, 95–97. [Google Scholar] [CrossRef] [PubMed]
- Maschino, F.; Guillet, J.; Curien, R.; Dolivet, G.; Bravetti, P. Oral metastasis: A report of 23 cases. Int. J. Oral Maxillofac. Surg. 2013, 42, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Wallace, J.; Abelardo, E.; Ramachandran, K.; Prabhu, V. Renal cell carcinoma uvula metastasis leading to airway compromise: An unusual site. BMJ Case Rep. 2022, 15, e248098. [Google Scholar] [CrossRef]
- Ludwig, D.C.; Garcia, J.; Chang, O.H.; Closmann, J.J. Metastatic renal cell carcinoma to the mandible: A case report with clinical and histologic findings. Gen. Dent. 2020, 68, 41–44. [Google Scholar]
- Melnick, S.J.; Amazon, K.; Dembrow, V. Metastatic renal cell carcinoma presenting as a parotid tumor: A case report with immunohistochemical findings and a review of the literature. Hum. Pathol. 1989, 20, 195–197. [Google Scholar] [CrossRef] [PubMed]
- Borghi, L.; Bianchini, E.; Ballotta, M.R.; Reale, D. Metastatic renal cell carcinoma presenting as a parotid tumor: A case report. Pathologica 1995, 87, 168–170. [Google Scholar]
- Seijas, B.P.; Franco, F.L.; Sastre, R.M.; García, A.A.; López-Cedrún Cembranos, J.L. Metastatic renal cell carcinoma presenting as a parotid tumor. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2005, 99, 554–557. [Google Scholar] [CrossRef]
- Goel, M.C.; Williams, D.W.; Evans, H.; Roberts, J.G. Lingual metastasis from renal cell carcinoma management and review of the literature. Urol. Int. 2003, 71, 418–421. [Google Scholar] [CrossRef]
- Lenkeit, C.; Bank, J.; Shirazi, M. Renal Cell Carcinoma in the Head and Neck: Case Presentation of a Patient with a Rare Metastatic Pattern. Cureus 2020, 12, e11894. [Google Scholar] [CrossRef]
- Ruiz-Oslé, S.; Prol, C.; Lardies, R.; Gaafar, A.; Barbier, L.; Arruza, A. Renal Cell Carcinoma metastases in the maxillofacial area: Case series. Arch. Esp. Urol. 2017, 70, 732–735. [Google Scholar]
- Schwab, B.; Lee, W.T. Bilateral renal cell carcinoma metastasis in the oral cavity. Am. J. Otolaryngol. 2012, 33, 154–155. [Google Scholar] [CrossRef]
- Erkilic, S.; Keskinruzgar, A.; Bozdag, Z.; Gunhan, O. Metastasis of a Renal Collecting Duct Adenocarcinoma to the Oral Cavity After Tooth Extraction. J. Craniofac. Surg. 2017, 28, e398–e399. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Lee, J.I. Metastatic carcinoma of the oral region: An analysis of 21 cases. Med. Oral Patol. Oral Cir. Bucal. 2017, 22, e359. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, D.M.; Pontes, F.S.C.; Miyahara, L.A.N.; Guerreiro, M.Y.R.; de Almeida, M.C.L.; Pontes, H.A.R.; Pinto, D.D.S. Metastatic Renal Cell Carcinoma to the Oral Cavity. J. Craniofac. Surg. 2016, 27, e533–e534. [Google Scholar] [CrossRef] [PubMed]
- Owosho, A.A.; Xu, B.; Kadempour, A.; Yom, S.K.; Randazzo, J.; Ghossein, R.A.; Huryn, J.M.; Estilo, C.L. Metastatic solid tumors to the jaw and oral soft tissue: A retrospective clinical analysis of 44 patients from a single institution. J. Cranio-Maxillofac. Surg. 2016, 44, 1047–1053. [Google Scholar] [CrossRef]
- Nisi, M.; Izzetti, R.; Graziani, F.; Gabriele, M. Renal Cell Carcinoma Metastases to the Oral Cavity: Report of 2 Cases and Review of Literature. J. Oral Maxillofac. Surg. 2020, 78, 1557–1571. [Google Scholar] [CrossRef]
- Lang, E.E.; Patil, N.; Walsh, R.M.; Leader, M.; Walsh, M.A. A case of renal cell carcinoma metastatic to the nose and tongue. Ear Nose Throat J. 2003, 82, 382–383. [Google Scholar] [CrossRef]
- Bućin, E.; Andréasson, L.; Björlin, G. Metastases in the oral cavity. Case reports. Int. J. Oral Surg. 1982, 11, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Marioni, G.; Gaio, E.; Poletti, A.; Derosas, F.; Staffieri, A. Uncommon metastatic site of renal adenocarcinoma: The oral tongue. Acta Otolaryngol. 2004, 124, 197–201. [Google Scholar] [CrossRef] [PubMed]
- van der Waal, R.I.F.; Buter, J.; van der Waal, I. Oral metastases: Report of 24 cases. Br. J. Oral Maxillofac. Surg. 2003, 41, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, M.; Miyata, M.; Okabe, K.; Sakashita, H. A case series of 9 tumors metastatic to the oral and maxillofacial region. J. Oral Maxillofac. Surg. 2002, 60, 942–944. [Google Scholar] [CrossRef] [PubMed]
- Morii, T. A case report of metastatic clear cell carcinoma of the oral cavity. Jpn. J. Oral Maxillofac. Surg. 1975, 21, 213–216. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sidhu, S.S.; Parkash, H.; Chopra, P. Renal metastatic carcinoma of the mandible. J. Dent. 1982, 10, 103–106. [Google Scholar] [CrossRef]
- Sánchez Aniceto, G.; García Peñín, A.; de la Mata Pages, R.; Montalvo Moreno, J.J. Tumors metastatic to the mandible: Analysis of nine cases and review of the literature. J. Oral Maxillofac. Surg. 1990, 48, 246–251. [Google Scholar] [CrossRef]
- Maestre-Rodríguez, O.; González-García, R.; Mateo-Arias, J.; Moreno-García, C.; Serrano-Gil, H.; Villanueva-Alcojol, L.; Campos-de-Orellana, A.M.; Monje-Gil, F. Metastasis of renal clear-cell carcinoma to the oral mucosa, an atypical location. Med. Oral Patol. Oral Cir. Bucal. 2009, 14, e601–e604. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Will, T.A.; Agarwal, N.; Petruzzelli, G.J. Oral cavity metastasis of renal cell carcinoma: A case report. J. Med. Case Rep. 2008, 2, 313. [Google Scholar] [CrossRef]
- Nesbitt, A.L.; Lim, Z.L.T.; Chan, K.J.; Zardawi, I.; Pridgeon, S.W. Metastatic renal cell carcinoma presenting with both acute stroke and an oral lesion. Urol. Case Rep. 2019, 23, 75–77. [Google Scholar] [CrossRef]
- Patel, S.; Barros, J.; Nwizu, N.N.; Ogbureke, K.U.E. Metastatic renal cell carcinoma to the oral cavity as first sign of disease: A case report. Clin. Case Rep. 2020, 8, 1517–1521. [Google Scholar] [CrossRef] [PubMed]
- Narea-Matamala, G.; Fernández-Toro, M.d.l.A.; Villalabeitía-Ugarte, E.; Landaeta-Mendoza, M.; Rojas-Alcayaga, G. Oral metastasis of renal cell carcinoma, presentation of a case. Med. Oral Patol. Oral Cir. Bucal. 2008, 13, E742–E744. [Google Scholar] [PubMed]
- Massaccesi, M.; Morganti, A.G.; Serafini, G.; Di Lallo, A.; Deodato, F.; Picardi, V.; Scambia, G. Late tonsil metastases from renal cell cancer: A case report. Tumori J. 2009, 95, 521–524. [Google Scholar] [CrossRef] [PubMed]
- Shinozaki, Y.; Ito, H.; Nakayama, R.; Noguchi, T.; Jinbu, Y.; Kusama, M.; Ichimura, K. Metastatic Clear Cell Carcinoma of the Mandible in a Patient with Renal Cancer undergoing Haemodialysis. Asian J. Oral Maxillofac. Surg. 2009, 21, 43–47. [Google Scholar] [CrossRef]
- Ohmura, S.; Kitagawa, T.; Kida, Y.; Fujita, K.; Masuda, M.; Ohtani, T. Renal cell carcinoma metastatic to the mandibular angle. Jpn. J. Oral Maxillofac. Surg. 1981, 27, 662–667. [Google Scholar] [CrossRef]
- Nakano, H.; Naito, K.; Suzuki, S.; Naito, K.; Kubota, T.; Takizawa, S. Metastatic renal cell carcinoma in the cheek: Report of a case. J. Oral Maxillofac. Surg. Med. Pathol. 2013, 25, 291–293. [Google Scholar] [CrossRef]
- Ficarra, G.; Pierleoni, L.; Panzoni, E. Metastatic renal cell carcinoma involving Wharton’s duct. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1996, 81, 580–583. [Google Scholar] [CrossRef] [PubMed]
- Tunio, M.A.; Al Asiri, M.; Ahmad, S.; Fareed, M.; Bayoumi, Y. Tongue metastasis as an initial manifestation of metastasis in renal cell carcinoma: A case report. J. Solid Tumors 2012, 2, 39. [Google Scholar] [CrossRef][Green Version]
- Milner, P.; Janas, A.; Grzesiak-Janas, G. Clear cell renal carcinoma metastasis in the oral cavity—Case report. J. Pre-Clin. Clin. Res. 2015, 8, 127–129. [Google Scholar] [CrossRef]
- Santana, L.N.; Ribeiro, J.T.; Domingues, M.; De Oliveira, M.G.; Rivero, L.F.; Carrard, V.C.; Trevizani, M.A. A rare case of oral metastasis of renal clear cell carcinoma: Case report and review of literature. J. Oral Diagn. 2000, 5, e20200006. [Google Scholar]
- Kizaekka, A.; Chengot, P.; Mannion, C. Recurrent oral metastatic lesion of renal cell carcinoma—A case report. Int. J. Oral Craniofacial Sci. 2019, 5, 024–026. [Google Scholar] [CrossRef]
- Paraskevopoulos, K.; Vahtsevanos, K.; Ntomouchtsis, A.; Kalaitsidou, I.; Patrikidou, A.; Andreadis, C.; Antoniades, K. Metastatic tumors to the oral cavity—A retrospective analysis. Int. Res. J. Otolaryngol. 2021, 4, 10. [Google Scholar]
- Morita, Y.; Iwagami, T.; Kawakita, C.; Kusuyama, Y.; Niki-Yonekawa, A.; Morita, N. Oral metastasis of renal cell carcinoma mimicking recurrence of excised malignant myoepithelioma: A case report. Mol. Clin. Oncol. 2018, 9, 66–69. [Google Scholar] [CrossRef]
- Prol, C.; Ruiz-Oslé, S.; Malaxetxebarría, S.; Dolado, A.; Del Hoyo, O.M.; Barbier, L. Oral and Maxillary Metastases: Retrospective Clinical Analysis of 21 Cases. Rev. Española Cirugía Oral Maxilofac. Publicación Soc. Española Cirugía Oral Maxilofac. 2019, 41, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Shimono, H.; Hirai, H.; Oikawa, Y.; Mochizuki, Y.; Kuroshima, T.; Tomioka, H.; Kayamori, K.; Ikeda, T.; Harada, H. Metastatic tumors in the oral region: A retrospective chart review of clinical characteristics and prognosis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2021, 132, 648–652. [Google Scholar] [CrossRef]
- Ali, R.A.E.; Mohamed, K.E.H. Metastatic Clear Cell Renal Cell Carcinoma Presenting with a Gingival Metastasis. Clin. Pract. 2016, 6, 847. [Google Scholar] [CrossRef][Green Version]
- Selvi, F.; Faquin, W.C.; Michaelson, M.D.; August, M. Three Synchronous Atypical Metastases of Clear Cell Renal Carcinoma to the Maxillary Gingiva, Scalp and the Distal Phalanx of the Fifth Digit: A Case Report. J. Oral Maxillofac. Surg. 2016, 74, 1286.e1–1286.e9. [Google Scholar] [CrossRef]
- Jatti, D.; Puri, G.; Aravinda, K.; Dheer, D.S. An atypical metastasis of renal clear cell carcinoma to the upper lip: A case report. J. Oral Maxillofac. Surg. 2015, 73, 371.e1–371.e6. [Google Scholar] [CrossRef]
- Sikka, S.; Sikka, P.; Kaur, G.; Shetty, D.C. A review of histopathological and immunohistochemical parameters in diagnosis of metastatic renal cell carcinoma with a case of gingival metastasis. J. Cancer Res. Ther. 2013, 9, 105–107. [Google Scholar] [CrossRef] [PubMed]
- Ganini, C.; Lasagna, A.; Ferraris, E.; Gatti, P.; Paglino, C.; Imarisio, I.; Morbini, P.; Benazzo, M.; Porta, C. Lingual metastasis from renal cell carcinoma: A case report and literature review. Rare Tumors 2012, 4, e41. [Google Scholar] [CrossRef]
- Lutcavage, G.J.; Branham, G.B.; Winterholler, B.W.; Wood, D.A. Renal cell carcinoma metastasis to the hard palate. J. Oral Maxillofac. Surg. 1984, 42, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Azam, F.; Abubakerr, M.; Gollins, S. Tongue metastasis as an initial presentation of renal cell carcinoma: A case report and literature review. J. Med. Case Rep. 2008, 2, 249. [Google Scholar] [CrossRef]
- Basely, M.; Bonnel, S.; Maszelin, P.; Verdalle, P.; Bussy, E.; de Jaureguiberry, J.P. A rare presentation of metastatic renal clear cell carcinoma to the tongue seen on FDG PET. Clin. Nucl. Med. 2009, 34, 566–569. [Google Scholar] [CrossRef]
- Mansourian, E.; Ahmadnia, H.; Amirmajdi, N. Renal cell carcinoma presenting as mandibular metastasis. Saudi J. Kidney Dis. Transplant. 2013, 24, 789. [Google Scholar] [CrossRef]
- Ord, R.A.; Malins, T.; Ward-Booth, P.R. Vascular metastatic renal carcinoma of the maxilla. Report of two cases. Int. J. Oral Maxillofac. Surg. 1990, 19, 106–109. [Google Scholar] [CrossRef] [PubMed]
- Capodiferro, S.; Limongelli, L.; Mastropasqua, M.G.; Favia, G.; Lajolo, C.; Colella, G.; Tempesta, A.; Maiorano, E. Metastatic Tumors of the Oro-Facial Tissues: Clear Cell Renal Cell Carcinoma: A Clinico-Pathological and Immunohistochemical Study of Seven Cases. J. Clin. Med. 2020, 9, 1151. [Google Scholar] [CrossRef]
- Andabak Rogulj, A.; Tomasovic Loncaric, C.; Muller, D.; Blivajs, I.; Andabak, M.; Vucicevic Boras, V.; Sekerija, M. Solid malignant metastases in the jaw bones. Br. J. Oral Maxillofac. Surg. 2018, 56, 705–708. [Google Scholar] [CrossRef]
- Derakhshan, S.; Rahrotaban, S.; Mahdavi, N.; Mirjalili, F. Metastatic renal cell carcinoma presenting as maxillary lesion: Report of two rare cases. J. Oral Maxillofac. Pathol. 2018, 22 (Suppl. S1), S39–S43. [Google Scholar] [PubMed]
- Altuntaş, O.; Petekkaya, İ.; Süslü, N.; Güllü, İ. Renal cell carcinoma metastatic to the tongue: A case report and review of the literature. J. Oral Maxillofac. Surg. 2015, 73, 1227–1230. [Google Scholar] [CrossRef]
- Amiruddin, S.; Yunus, M.R.M. Tongue mass in post nephrectomy patient. Egypt. J. Ear Nose Throat Allied Sci. 2013, 14, 147–149. [Google Scholar] [CrossRef][Green Version]
- Lieder, A.; Guenzel, T.; Lebentrau, S.; Schneider, C.; Franzen, A. Diagnostic relevance of metastatic renal cell carcinoma in the head and neck: An evaluation of 22 cases in 671 patients. Int. Braz J. Urol. 2017, 43, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Kase, A.M.; George, D.J.; Ramalingam, S. Clear Cell Renal Cell Carcinoma: From Biology to Treatment. Cancers 2023, 15, 665. [Google Scholar] [CrossRef] [PubMed]
- Schütz, V.; Lin, H.; Kaczorowski, A.; Zschäbitz, S.; Jäger, D.; Stenzinger, A.; Duensing, A.; Debus, J.; Hohenfellner, M.; Duensing, S. Long-Term Survival of Patients with Stage T1N0M1 Renal Cell Carcinoma. Cancers 2023, 15, 5715. [Google Scholar] [CrossRef] [PubMed]
- Semenescu, L.E.; Kamel, A.; Ciubotaru, V.; Baez-Rodriguez, S.M.; Furtos, M.; Costachi, A.; Dricu, A.; Tătăranu, L.G. An Overview of Systemic Targeted Therapy in Renal Cell Carcinoma, with a Focus on Metastatic Renal Cell Carcinoma and Brain Metastases. Curr. Issues Mol. Biol. 2023, 45, 7680–7704. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Albiges, L.; McGregor, B.A.; Heng, D.Y.C.; Procopio, G.; de Velasco, G.; Taguieva-Pioger, N.; Martín-Couce, L.; Tannir, N.M.; Powles, T. Vascular endothelial growth factor-targeted therapy in patients with renal cell carcinoma pretreated with immune checkpoint inhibitors: A systematic literature review. Cancer Treat. Rev. 2024, 122, 102652. [Google Scholar] [CrossRef] [PubMed]
- Kaddissi, A.E.; Ducleon, G.G.; Lefort, F.; Mezepo, G.; Frontczak, A.; Goujon, M.; Mouillet, G.; Almotlak, H.; Gross-Goupil, M.; Thiery-Vuillemin, A. Metastatic renal cell cancer and first-line combinations: For which patients? (focus on tolerance and health-related quality of life). Bull. Cancer 2022, 109 (Suppl. S2), 2S19–2S30. [Google Scholar] [CrossRef] [PubMed]
- Heng, D.Y.; Xie, W.; Regan, M.M.; Harshman, L.C.; Bjarnason, G.A.; Vaishampayan, U.N.; Mackenzie, M.; Wood, L.; Donskov, F.; Tan, M.H.; et al. External validation and comparison with other models of the International Metastatic Renal-Cell Carcinoma Database Consortium prognostic model: A population-based study. Lancet Oncol. 2013, 14, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Brierley, D.J.; Crane, H.; Hunter, K.D. Lumps and Bumps of the Gingiva: A Pathological Miscellany. Head Neck Pathol. 2019, 13, 103–113. [Google Scholar] [CrossRef]
- Ide, F.; Obara, K.; Mishima, K.; Saito, I.; Horie, N.; Shimoyama, T.; Kusama, K. Peripheral odontogenic tumor: A clinicopathologic study of 30 cases: General features and hamartomatous lesions. J. Oral Pathol. Med. 2005, 34, 552–557. [Google Scholar] [CrossRef]
- Wulfrank, D.; Speelman, T.; Pauwels, C.; Roels, H.; De Schryver, A. Extranodal non-Hodgkin’s lymphoma of the head and neck. Radiother. Oncol. 1987, 8, 199–207. [Google Scholar] [CrossRef]
- Epstein, J.B.; Epstein, J.D.; Le, N.D.; Gorsky, M. Characteristics of oral and paraoral malignant lymphoma: A population-based review of 361 cases. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2001, 96, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Fantasia, J.E.; Kaplan, R. Oral manifestations of acute myelomonocytic leukemia: A case report and review of the classification of leukemias. J. Periodontol. 2002, 73, 664–668. [Google Scholar] [CrossRef]
- Irani, S. Metastasis to the Jawbones: A review of 453 cases. J. Int. Soc. Prev. Community Dent. 2017, 7, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Bellin, M.F.; Valente, C.; Bekdache, O.; Maxwell, F.; Balasa, C.; Savignac, A.; Meyrignac, O. Update on Renal Cell Carcinoma Diagnosis with Novel Imaging Approaches. Cancers 2024, 16, 1926. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]

| Authors | Year | Site | Histotype | Gender | Age | First Sign of Disease |
|---|---|---|---|---|---|---|
| Ray et al. [41] | 2013 | Tongue | RCC | M | 65 | Yes |
| Kalinin et al. [42] | 2023 | Tongue | ccRCC | F | 58 | yes |
| Nishii et al. [43] | 2020 | Maxillary bone | ccRCC | M | 89 | No |
| Zhang et al. [44] | 2020 | Mandibular bone | RCC | F | 56 | Yes |
| Jung et al. [45] | 2023 | Mandibular bone | RCC | F | 22 | Yes |
| Stojanovic et al. [46] | 2020 | Gingiva | RCC | M | 53 | Yes |
| Li et al. [47] | 2001 | Parotid | RCC | M | 63 | No |
| Kundu et al. [48] | 2001 | Parotid | ccRCC | M | 61 | Yes |
| Park and Hlivko [49] | 2002 | Parotid | ccRCC | F | 83 | No |
| Pritchyk et al. [50] | 2002 | Lip Maxillary bone Tongue | RCC RCC RCC | M F M | 70 53 60 | Yes |
| Göğüş et al. [51] | 2004 | Parotid | ccRCC | F | 59 | No |
| Torres-Carranza et al. [52] | 2006 | Tongue | ccRCC | F | 49 | No |
| Newton et al. [53] | 2007 | Parotid | ccRCC | F | 74 | No |
| Yoshitomi et al. [54] | 2011 | Tongue | ccRCC | M | 47 | Yes |
| Morvan et al. [55] | 2011 | Tongue | ccRCC | F | 48 | No |
| Balliram et al. [56] | 2012 | Tongue | pRCC | M | 72 | Yes |
| Serouya et al. [57] | 2012 | Submandibular gland | ccRCC | M | 60 | No |
| Wadasadawala et al. [58] | 2011 | Tongue | RCC | M | 48 | No |
| Deeb et al. [59] | 2012 | Parotid | RCC | M | 82 | No |
| Özkiriş et al. [60] | 2011 | Cervical lymph nodes | ccRCC | F | 56 | No |
| Ghazali et al. [61] | 2012 | Tongue | ccRCC | F | 64 | No |
| Lau et al. [62] | 2012 | Parotid | ccRCC | F | 79 | No |
| Mazeron et al. [63] | 2013 | Tongue | ccRCC | M | 66 | Yes |
| Yanlan et al. [64] | 2013 | Parotid | ccRCC | F | 44 | Yes |
| Udager and Rungta [65] | 2014 | Parotid | ccRCC | M | 64 | No |
| Abbaszadeh-Bidokhty et al. [66] | 2014 | Tongue | ccRCC | M | 80 | No |
| Kotak and Merrick [67] | 2014 | Lip | ccRCC | M | 64 | No |
| Suojanen et al. [68] | 2014 | Lip | ccRCC | M | 71 | No |
| Kudva et al. [69] | 2016 | Buccal mucosa | ccRCC | F | 36 | Yes |
| Georgy et al. [70] | 2017 | Gingiva | ccRCC | M | 63 | Yes |
| Nifosì et al. [71] | 2017 | Gingiva | ccRCC | M | 58 | No |
| Raiss et al. [6] | 2017 | Tongue | RCC | M | 55 | Yes |
| Vasilyeva et al. [72] | 2018 | Gingiva | RCC | F | 78 | Yes |
| McNattin and Dean [73] | 1931 | Tongue | Tubular Adenocarcinoma | M | 58 | Yes |
| Altinel et al. [74] | 2010 | Tongue | ccRCC | M | 67 | Yes |
| Syryło et al. [75] | 2010 | Lip | ccRCC | M | 59 | Yes |
| Gil-Julio et al. [76] | 2012 | Buccal mucosa | ccRCC | M | 65 | No |
| Shirazian and Bahrami [77] | 2016 | Gingiva | ccRCC | M | 45 | Yes |
| Schrag and Jordan [78] | 1945 | Tongue | RCC | M | 34 | No |
| Carmen and Korbitz [79] | 1970 | Tongue | ccRCC | M | 77 | No |
| Friedlander et al. [80] | 1978 | Tongue | RCC | M | 84 | No |
| Fitzgerald et al. [81] | 1982 | Gingiva and Tongue | RCC | M | 63 | No |
| Inai et al. [82] | 1987 | Tongue | RCC | M | 42 | No |
| Ishikawa et al. [83] | 1991 | Tongue | RCC | F | 58 | No |
| Okabe et al. [84] | 1992 | Tongue | ccRCC | M | 58 | No |
| Shibayama et al. [85] | 1993 | Tongue | RCC | M | 41 | No |
| Ziyada et al. [86] | 1994 | Tongue | ccRCC | M | 59 | Yes |
| Airoldi et al. [87] | 1995 | Tongue | RCC | M | 51 | No |
| Aguirre et al. [88] | 1996 | Tongue | ccRCC | F | 82 | Yes |
| Konya et al. [89] | 1997 | Tongue | RCC | M | 59 | Yes |
| Tomita et al. [90] | 1998 | Tongue | ccRCC | M | 50 | No |
| Navarro et al. [91] | 2000 | Tongue | ccRCC | M | 62 | No |
| Mekni et al. [92] | 2002 | Tongue | ccRCC | M | 63 | No |
| Kyan and Kato [93] | 2004 | Tongue | ccRCC | M | 66 | No |
| Huang et al. [94] | 2006 | Tongue Parotid | RCC ccRCC | F F | 76 56 | No No |
| Cochrane et al. [95] | 2006 | Tongue | RCC | M | 41 | No |
| Del Rosario Regalado et al. [96] | 2007 | Tongue | RCC | M | 81 | No |
| Longo et al. [97] | 2008 | Tongue | RCC | M | 68 | No |
| Kella et al. [98] | 2009 | Tongue | ccRCC | F | 67 | Yes |
| Friedmann and Osborn [99] | 1965 | Maxillary bone | RCC | M | 63 | No |
| Trinca and Willis [100] | 1936 | Tongue | RCC | M | 57 | Yes |
| Branch and Norton [101] | 1928 | Gingiva | ccRCC | F | 64 | Yes |
| Salman and Langel [102] | 1954 | Gingiva | RCC | F | 62 | No |
| Persson and Wallenius [103] | 1961 | Gingiva | ccRCC | F | 60 | No |
| Cranin et al. [104] | 1966 | Gingiva | RCC | M | 72 | No |
| Buchner and Begleiter [105] | 1980 | Gingiva | ccRCC | M | 46 | No |
| Nishimura et al. [106] | 1982 | Mandibular bone Gingiva Mandibular bone | RCC RCC RCC | F M F | 61 72 36 | yes yes yes |
| Fay and Weir [107] | 1983 | Gingiva | ccRCC | F | 18 | No |
| Zohar et al. [108] | 1985 | Gingiva | ccRCC | F | 54 | Yes |
| Tsianos et al. [109] | 1987 | Gingiva | RCC | M | 78 | No |
| Müller-Mattheis et al. [110] | 1989 | Gingiva | RCC | F | 47 | No |
| Hagen et al. [111] | 1989 | Gingiva | RCC | F | 46 | No |
| Corsi et al. [35] | 1994 | Lip | ccRCC | M | 44 | No |
| Salman and Darlington [112] | 1944 | Hard palate | ccRCC | F | 54 | No |
| Mallet [113] | 1961 | Mandibular bone | ccRCC | F | 72 | Yes |
| Meyer and Shklar [114] | 1965 | Parotid Maxillary bone Mandibular bone Mandibular bone | RCC RCC Reticulum cell RCC | M F M M | 48 73 43 57 | No No No No |
| Godby et al. [115] | 1967 | Gingiva | ccRCC | M | 45 | No |
| Milobsky et al. [116] | 1975 | Maxillary bone | RCC | F | 66 | Yes |
| Nagayama and Oka [117] | 1979 | Mandibular bone Hard palate | ccRCC ccRCC | F F | 61 43 | yes |
| Susan et al. [118] | 1979 | Hard palate Hard palate | ccRCC ccRCC | M M | 53 62 | yes yes |
| Matsumoto and Yanagihara [119] | 1982 | Maxillary bone Maxillary bone | ccRCC ccRCC | M M | 73 48 | yes yes |
| Pick et al. [120] | 1986 | Mandibular bone | ccRCC | M | 71 | Yes |
| Zachariades et al. [121] | 1989 | Mandibular bone | RCC | M | 78 | No |
| Jones and al [122] | 1990 | Mandibular bone Mandibular bone | ccRCC ccRCC | F F | 62 52 | yes yes |
| Fandella et al. [123] | 1992 | Maxillary bone | ccRCC | M | 62 | Yes |
| Lee et al. [124] | 1998 | Maxillary bone | RCC | M | 76 | Yes |
| Guyot et al. [125] | 1999 | Mandibular bone | RCC | M | 83 | No |
| Hönig [126] | 2000 | Maxillary bone | RCC | M | 46 | No |
| Shetty et al. [127] | 2001 | Mandibular bone | RCC | M | 62 | Yes |
| Heinroth et al. [128] | 2006 | Maxillary bone | ccRCC | F | 53 | yes |
| Ðanić et al. [26] | 2018 | Tongue | RCC | M | 51 | yes |
| Madison and Frierson [129] | 1988 | Tongue Tongue | ccRCC ccRCC | M M | 29 63 | No No |
| Kishore et al. [130] | 2018 | Lip | ccRCC | M | 54 | No |
| Abro et al. [131] | 2019 | Tongue | RCC | M | 54 | No |
| Netto et al. [132] | 2019 | Gingiva | RCC | M | 68 | Yes |
| Walsh et al. [133] | 2022 | Tongue | ccRCC | M | 63 | No |
| Mrena et al. [134] | 2008 | Parotid Parotid Parotid | ccRCC RCC RCC | F F F | 58 76 62 | Yes No No |
| Aljawad et al. [135] | 2023 | Parotid | ccRCC | M | 65 | No |
| Migliorelli et al. [136] | 2023 | Maxillary bone | ccRCC | F | 54 | Yes |
| Maschino et al. [137] | 2013 | Maxillary bone Maxillary bone Parotid Tongue | ccRCC ccRCC ccRCC RCC | M F M M | 73 84 78 66 | No No No No |
| Wallace et al. [138] | 2022 | Soft palate | ccRCC | M | 50 | No |
| Ludwig et al. [139] | 2020 | Mandibular bone | ccRCC | M | 78 | Yes |
| Melnick et al. [140] | 1989 | Parotid | ccRCC | M | 72 | Yes |
| Borghi et al. [141] | 1995 | Parotid | ccRCC | M | 68 | No |
| Seijas et al. [142] | 2005 | Parotid | ccRCC | M | 67 | Yes |
| Goel et al. [143] | 2003 | Tongue | ccRCC | M | 62 | Yes |
| Lenkeit et al. [144] | 2020 | Tongue | RCC | M | 71 | No |
| Ruiz-Oslé et al. [145] | 2017 | Parotid Mandibular bone Gingiva Masticatory space | RCC RCC RCC RCC | M M M F | 72 55 62 52 | yes yes yes no |
| Schwab and Lee [146] | 2012 | Maxillary bone | ccRCC | M | 63 | No |
| Erkilic et al. [147] | 2017 | Gingiva | Collecting duct carcinoma | F | 54 | Yes |
| Lee and Lee [148] | 2017 | Mandibular bone | RCC | M | 62 | No |
| Guimarães et al. [149] | 2016 | Gingiva | ccRCC | F | 31 | No |
| Owosho et al. [150] | 2016 | Mandibular bone Mandibular bone Gingiva Buccal mucosa Buccal mucosa Gingiva Buccal mucosa | RCC RCC RCC RCC RCC RCC RCC | F F F M M M M | 61 63 18 75 70 59 66 | No No No No No No No |
| Nisi et al. [151] | 2020 | Tongue Buccal mucosa | ccRCC ccRCC | M M | 61 71 | yes yes |
| Lang et al. [152] | 2003 | Tongue | ccRCC | M | 45 | No |
| Bucín et al. [153] | 1982 | Gingiva | RCC | M | 65 | No |
| Marioni et al. [154] | 2004 | Tongue | ccRCC | F | 87 | No |
| Van der Wall et al. [155] | 2003 | Soft palate Maxillary bone Mandibular bone Buccal mucosa | ccRCC ccRCC ccRCC ccRCC | F F M M | 62 64 48 67 | No No No No |
| Fukuda et al. [156] | 2002 | Mandibular bone | RCC | M | 76 | No |
| Makos and Psomaderis [27] | 2009 | Gingiva | ccRCC | M | 63 | No |
| Morii [157] | 1975 | Buccal mucosa | ccRCC | M | 63 | No |
| Sidhu [158] | 1982 | Mandibular bone | RCC | F | 32 | Yes |
| Sánchez Aniceto et al. [159] | 1990 | Mandibular bone | RCC | M | 54 | Yes |
| Maestre-Rodríguez et al. [160] | 2009 | Gingiva | ccRCC | M | 52 | Yes |
| Will et al. [161] | 2008 | Floor of mouth | ccRCC | M | 63 | no |
| Nesbitt et al. [162] | 2019 | Gingiva | Sarcomatoid RCC | M | 59 | Yes |
| Patel et al. [163] | 2020 | Gingiva | ccRCC | F | 59 | yes |
| Narea-Matamala et al. [164] | 2008 | Gingiva | RCC | M | 74 | yes |
| Massaccesi et al. [165] | 2009 | Tonsil | ccRCC | M | 76 | yes |
| Shinozaki et al. [166] | 2009 | Mandibular bone | ccRCC | F | 76 | No |
| Ohmura et al. [167] | 1981 | Mandibular bone | ccRCC | M | 53 | No |
| Nakano et al. [168] | 2013 | Gingiva | ccRCC | M | 72 | No |
| Ficarra et al. [169] | 1996 | Wharton’s duct | ccRCC | M | 73 | No |
| Tunio et al. [170] | 2012 | Tongue | ccRCC | M | 35 | No |
| Milner et al. [171] | 2014 | Hard palate | ccRCC | M | 67 | Yes |
| Santana et al. [172] | 2000 | Gingiva | ccRCC | M | 63 | Yes |
| Kizaekka et al. [173] | 2019 | Tongue | ccRCC | M | 77 | No |
| Paraskevopoulos et al. [174] | 2021 | Mandibular bone | ccRCC | M | 72 | Yes |
| Morita et al. [175] | 2018 | Buccal mucosa | ccRCC | M | 75 | No |
| Prol et al. [176] | 2019 | Mandibular bone Gingiva Gingiva Mandibular bone Masticatory space | ccRCC ccRCC ccRCC chRCC ccRCC | M M F M M | 55 62 52 56 65 | No No No No No |
| Shimono et al. [177] | 2021 | Mandibular bone Maxillary bone Tongue | RCC RCC RCC | M M M | 62 89 63 | Yes No no |
| Ali and Mohamed [178] | 2016 | Gingiva | ccRCC | M | 60 | Yes |
| Selvi et al. [179] | 2016 | Gingiva | ccRCC | M | 51 | No |
| Jatti et al. [180] | 2015 | Lip | ccRCC | M | 60 | No |
| Sikka et al. [181] | 2013 | Gingiva | ccRCC | M | 73 | Yes |
| Ganini et al. [182] | 2012 | Tongue | ccRCC | M | 70 | No |
| Lutcavage et al. [183] | 1984 | Hard palate | RCC | M | 55 | No |
| Azam et al. [184] | 2008 | Tongue | ccRCC | M | 78 | Yes |
| Basely et al. [185] | 2009 | Tongue | ccRCC | F | 46 | No |
| Ahmadnia et al. [186] | 2013 | Mandibular bone | ccRCC | M | 57 | Yes |
| Ord et al. [187] | 1990 | Maxillary bone Maxillary bone | RCC RCC | M M | 58 73 | yes |
| Capodiferro et al. [188] | 2020 | Gingiva Tongue Mandibular bone Mandibular bone Parotid Parotid Mandibular bone | ccRCC ccRCC ccRCC ccRCC ccRCC ccRCC ccRCC | F M M M M F M | 69 56 45 63 55 55 60 | No No No No No No No |
| Andabak Rogulj et al. [189] | 2018 | Maxillary bone Maxillary bone Mandibular bone Maxillary bone Mandibular bone | ccRCC ccRCC RCC RCC RCC | M M F M F | 65 58 64 61 68 | No No No No No |
| Derakhshan et al. [190] | 2018 | Maxillary bone Maxillary bone | ccRCC ccRCC | M M | 54 51 | yes yes |
| Altuntaş et al. [191] | 2014 | Tongue | pRCC | M | 70 | No |
| Amiruddin and Yunus [192] | 2013 | Tongue | ccRCC | M | 66 | No |
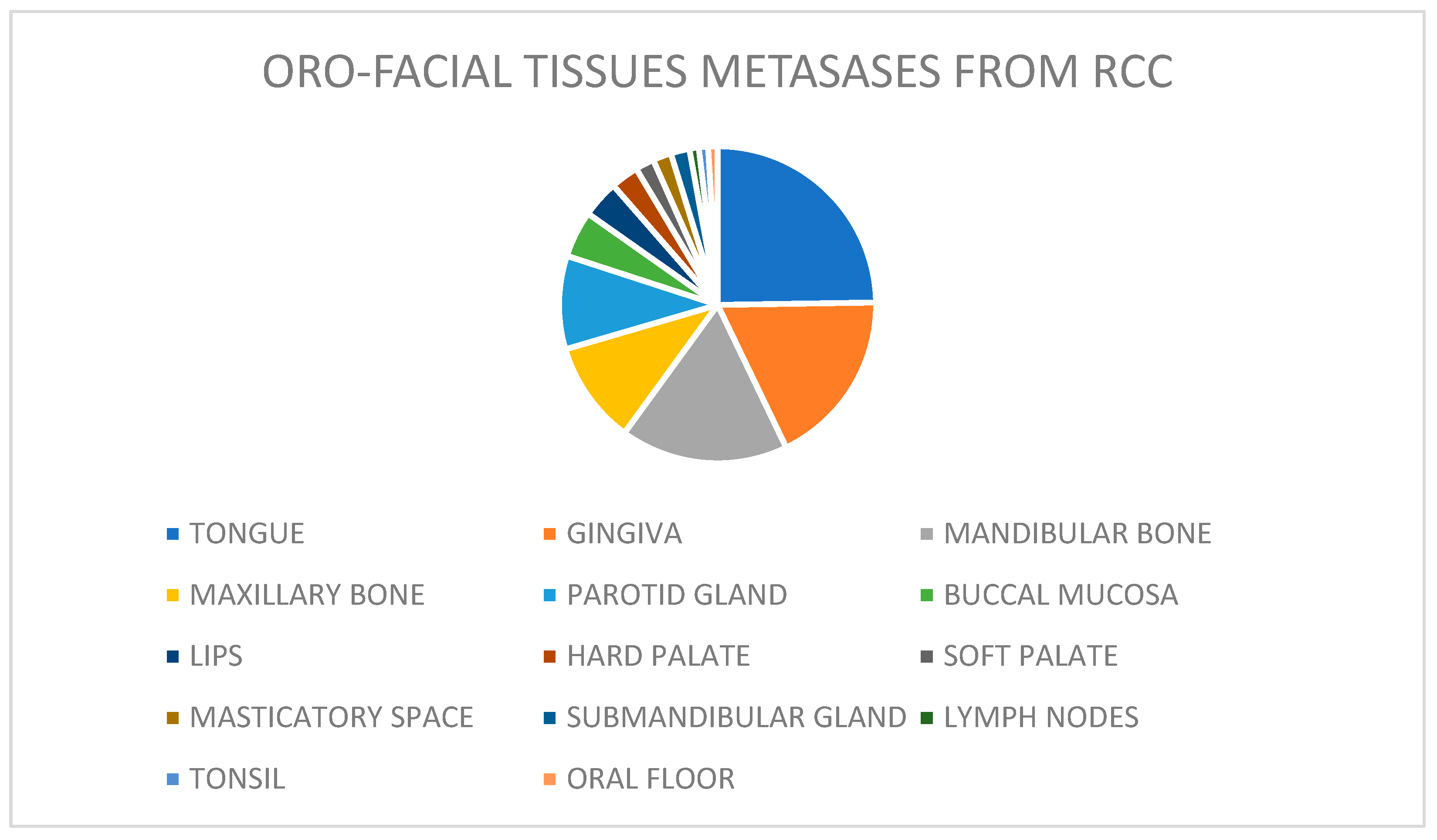
| SITE | CASES | |
| Tongue | 55 | 26.9% |
| Gingiva | 39 | 18.9% |
| Mandibular bone | 35 | 16.9% |
| Maxillary bone | 23 | 11.1% |
| Parotid gland | 22 | 10.6% |
| Buccal mucosa | 11 | 5.3% |
| Lips | 7 | 3.3% |
| Hard palate | 6 | 2.8% |
| Soft palate | 2 | 0.9% |
| Masticatory space | 2 | 0.9% |
| Submandibular gland | 2 | 0.9% |
| Lymph nodes | 1 | 0.4% |
| Tonsil | 1 | 0.4% |
| Oral floor | 1 | 0.4% |
| GENDER | CASES | |
| Male | 145 | 70.3% |
| Female | 61 | 29.6% |
| Authors | Site | Gender | Age | First Sign of Disease | Clinical Presentation | Radiological Aspect |
|---|---|---|---|---|---|---|
| Kalinin et al. [42] | Tongue | F | 58 | yes | Painless nodule | - |
| Nishii et al. [43] | Maxillary bone | M | 89 | No | Swelling of the left maxillary ginguva | Osteolytic area |
| Kundu et al. [48] | Parotid | M | 61 | Yes | Facial weakness and post-auricular pain | - |
| Park and Hlivko [49] | Parotid | F | 83 | No | infra-auricular swelling | - |
| Göğüş et al. [51] | Parotid | F | 59 | No | pre-auricular swelling | - |
| Torres-Carranza et al. [52] | Tongue | F | 49 | No | Pedunculated painless mass | - |
| Newton et al. [53] | Parotid | F | 74 | No | Pre- auricular swelling | - |
| Yoshitomi et al. [54] | Tongue | M | 47 | Yes | mass | - |
| Morvan et al. [55] | Tongue | F | 48 | No | Painful mass | - |
| Serouya et al. [57] | Submandibular gland | M | 60 | No | Submandibular mass | - |
| Özkiriş et al. [60] | Cervical lymph nodes | F | 56 | No | Multiple mass in neck region | - |
| Ghazali et al. [61] | Tongue | F | 64 | No | Painless mass | - |
| Lau et al. [62] | Parotid | F | 79 | No | Parotid mass | - |
| Mazeron et al. [63] | Tongue | M | 66 | Yes | Exophytic mass | - |
| Yanlan et al. [64] | Parotid | F | 44 | Yes | Painless mass in parotid region | - |
| Udager and Rungta [65] | Parotid | M | 64 | No | Painless mass in parotid region | - |
| Abbaszadeh-Bidokhty et al. [66] | Tongue | M | 80 | No | Swelling | - |
| Kotak and Merrick [67] | Lip | M | 64 | No | Asymptomatic swelling | - |
| Suojanen et al. [68] | Lip | M | 71 | No | Spontaneously bleeding mass | - |
| Kudva et al. [69] | Buccal mucosa | F | 36 | Yes | Painful ulcer | Bone erosion |
| Georgy et al. [70] | Gingiva | M | 63 | Yes | Gingival nodule | - |
| Nifosì et al. [71] | Gingiva | M | 58 | No | small painful reddish indurated swelling | - |
| Altinel et al. [74] | Tongue | M | 67 | Yes | Tongue mass | - |
| Syryło et al. [75] | Lip | M | 59 | Yes | Upper lip nodule | - |
| Gil-Julio et al. [76] | Buccal mucosa | M | 65 | No | Discomfort in left cheek | - |
| Shirazian and Bahrami [77] | Gingiva | M | 45 | Yes | red-purple rubbery, sessile exophytic lesion with smooth surface | Saucer shape resorption of the crestal bone |
| Carmen and Korbitz [79] | Tongue | M | 77 | No | Painful mass | - |
| Okabe et al. [84] | Tongue | M | 58 | No | Painless mass | - |
| Ziyada et al. [86] | Tongue | M | 59 | Yes | Tongue mass | - |
| Aguirre et al. [88] | Tongue | F | 82 | Yes | swelling | - |
| Tomita et al. [90] | Tongue | M | 50 | No | Hemorragic mass | - |
| Navarro et al. [91] | Tongue | M | 62 | No | Exophytic lesion | - |
| Mekni et al. [92] | Tongue | M | 63 | No | NA | - |
| Kyan and Kato [93] | Tongue | M | 66 | No | Tongue mass | - |
| Huang et al. [94] | Parotid | F | 56 | No | Bilateral enlarging mass in parotid region | - |
| Kella et al. [98] | Tongue | F | 67 | Yes | NA | - |
| Branch and Norton [101] | Gingiva | F | 64 | Yes | Epulis-like mass | - |
| Persson and Wallenius [103] | Gingiva | F | 60 | No | Rapidly growing swelling | - |
| Buchner and Begleiter [105] | Gingiva | M | 46 | No | Rapidly growing mass | - |
| Fay and Weir [107] | Gingiva | F | 18 | No | Soft, fluctuant mass | Demarcated radiolucency |
| Zohar et al. [108] | Gingiva | F | 54 | Yes | Soft, friable red mass | - |
| Corsi et al. [35] | Lip | M | 44 | No | NA | - |
| Salman and Darlington [112] | Hard palate | F | 54 | No | Ulcerated nodule | NA |
| Mallett [113] | Mandibular bone | F | 72 | Yes | Pain and swelling | Osteolytic area |
| Godby et al. [115] | Gingiva | M | 45 | No | Gingival mass | Bone resorption |
| Nagayama and Oka [117] | Mandibular bone Hard palate | F F | 61 43 | yes | Swelling Palate’s perforation | Osteolytic area NA |
| Susan et al. [118] | Hard palate Hard palate | M M | 53 62 | yes yes | Swelling Pedunculated lesion | NA NA |
| Matsumoto and Yanagihara [119] | Maxillary bone Maxillary bone | M M | 73 48 | yes yes | Cheek’s swelling epistaxis | Osteolytic area NA |
| Pick et al. [120] | Mandibular bone | M | 71 | Yes | Swelling | mixed radiolucent and radiopaque lesion |
| Jones and al [122] | Mandibular bone Mandibular bone | F F | 62 52 | yes yes | Swelling Swelling | osteolytic area osteolytic area |
| Fandella et al. [123] | Maxillary bone | M | 62 | Yes | epistaxis | NA |
| Heinroth et al. [128] | Maxillary bone | F | 53 | yes | Painful swelling | opacity in the maxillary sinus |
| Madison and Frierson [129] | Tongue Tongue | M M | 29 63 | No No | NA NA | - - |
| Kishore et al. [130] | Lip | M | 54 | No | swelling | - |
| Walsh et al. [133] | Tongue | M | 63 | No | Pedunculated lesion | - |
| Mrena et al. [134] | Parotid | F | 58 | Yes | Non-tender nodule | - |
| Aljawad et al. [135] | Parotid | M | 65 | No | Non-tender mass | - |
| Migliorelli et al. [136] | Maxillary bone | F | 54 | Yes | Facial pain | Bone erosion |
| Maschino et al. [137] | Maxillary bone Maxillary bone Parotid | M F M | 73 84 78 | No No No | Exophytic mass Pain, discomfort Rapid growth mass | Osteolytic lesion NA |
| Wallace et al. [138] | Soft palate | M | 50 | No | Globular lesion | - |
| Ludwig et al. [139] | Mandibular bone | M | 78 | Yes | Painful swelling and paresthesia | NA |
| Melnick et al. [140] | Parotid | M | 72 | Yes | Parotid mass | - |
| Borghi et al. [141] | Parotid | M | 68 | No | Painless swelling | - |
| Seijas et al. [142] | Parotid | M | 67 | Yes | Painless mass | - |
| Goel et al. [143] | Tongue | M | 62 | Yes | Swelling | - |
| Schwab and Lee [146] | Maxillary bone | M | 63 | No | Bilateral, friable masses with a foul odor | NA |
| Guimarães et al. [149] | Gingiva | F | 31 | No | Painful growth | Enlargement of the periodontal ligament |
| Nisi et al. [151] | Tongue Buccal mucosa | M M | 61 71 | yes yes | Swelling Large mass | - - |
| Lang et al. [152] | Tongue | M | 45 | No | Pedunculated mass | - |
| Marioni et al. [154] | Tongue | F | 87 | No | Exophytic, ulcerated mass | - |
| Van der Wall et al. [155] | Soft palate Maxillary bone Mandibular bone Buccal mucosa | F F M M | 62 64 48 67 | No No No No | NA NA NA NA | - - - - |
| Makos and Psomaderis [27] | Gingiva | M | 63 | No | Epulis-like mass | - |
| Morii [157] | Buccal mucosa | M | 63 | No | NA | - |
| Maestre-Rodríguez et al. [160] | Gingiva | M | 52 | Yes | Granulomatous gingival lesion | - |
| Will et al. [161] | Floor of mouth | M | 63 | no | Indurated mass | - |
| Patel et al. [163] | Gingiva | F | 59 | yes | pink-red, oval, ulcerated lesion with a white pseudomembranous surface | - |
| Massaccesi et al. [165] | Tonsil | M | 76 | yes | dysphagia | - |
| Shinozaki et al. [166] | Mandibular bone | F | 76 | No | swelling | Multilocular bone destruction |
| Ohmura et al. [167] | Mandibular bone | M | 53 | No | NA | NA |
| Nakano et al. [168] | Gingiva | M | 72 | No | swelling | - |
| Ficarra et al. [169] | Wharton’s duct | M | 73 | No | Movable mass in the floor of the mouth | - |
| Tunio et al. [170] | Tongue | M | 35 | No | Painless swelling | - |
| Milner et al. [171] | Hard palate | M | 67 | Yes | Irregularly shaped lump | none |
| Santana et al. [172] | Gingiva | M | 63 | Yes | Double lobe nodule | Radiolucent lesion |
| Kizaekka et al. [173] | Tongue | M | 77 | No | Pedunculated lesion | - |
| Paraskevopoulos et al. [174] | Mandibular bone | M | 72 | Yes | NA | - |
| Morita et al. [175] | Buccal mucosa | M | 75 | No | Swelling and facial asymmetry | - |
| Prol et al. [176] | Mandibular bone Gingiva Gingiva Masticatory space | M M F M | 55 62 52 65 | No No No No | Mass Mass NA Mass | NA - - NA |
| Ali and Mohamed [178] | Gingiva | M | 60 | Yes | Gingival mass | Erosive bone changes |
| Selvi et al. [179] | Gingiva | M | 51 | No | Rapidly progressive, painless exophytic lesion | Destruction of the alveolar bone |
| Jatti et al. [180] | Lip | M | 60 | No | Ulcerated nodule | - |
| Sikka et al. [181] | Gingiva | M | 73 | Yes | Multiple painless swelling | - |
| Ganini et al. [182] | Tongue | M | 70 | No | Ulcerated lesion | - |
| Azam et al. [184] | Tongue | M | 78 | Yes | Pedunculated lesion, difficulty in swallowing solid | - |
| Basely et al. [185] | Tongue | F | 46 | No | Swelling on the left side of the neck | - |
| Ahmadnia et al. [186] | Mandibular bone | M | 57 | Yes | Swelling, trismus | Radiolucent lesion |
| Capodiferro et al. [188] | Gingiva Tongue Mandibular bone Mandibular bone Parotid Parotid Mandibular bone | F M M M M F M | 69 56 45 63 55 55 60 | No No No No No No No | Large fungating mass Large fungating mass - - growing mass growing mass - | Bone rarefaction - Osteolytic area Osteolytic area - - Osteolytic area |
| Andabak Rogulj et al. [189] | Maxillary bone Maxillary bone | M M | 65 58 | No No | Mobility of tooth Exophytic lesion | NA NA |
| Derakhshan et al. [190] | Maxillary bone Maxillary bone | M M | 54 51 | yes yes | Pain and swelling Polypoid mass | intraosseous radiolucency |
| Amiruddin and Yunus [192] | Tongue | M | 66 | No | Painless mass | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Granberg, V.; Laforgia, A.; Forte, M.; Di Venere, D.; Favia, G.; Copelli, C.; Manfuso, A.; Ingravallo, G.; d’Amati, A.; Capodiferro, S. Metastatic Renal-Cell Carcinoma of the Oro-Facial Tissues: A Comprehensive Review of the Literature with a Focus on Clinico–Pathological Findings. Surgeries 2024, 5, 694-718. https://doi.org/10.3390/surgeries5030055
Granberg V, Laforgia A, Forte M, Di Venere D, Favia G, Copelli C, Manfuso A, Ingravallo G, d’Amati A, Capodiferro S. Metastatic Renal-Cell Carcinoma of the Oro-Facial Tissues: A Comprehensive Review of the Literature with a Focus on Clinico–Pathological Findings. Surgeries. 2024; 5(3):694-718. https://doi.org/10.3390/surgeries5030055
Chicago/Turabian StyleGranberg, Vanja, Alessandra Laforgia, Marta Forte, Daniela Di Venere, Gianfranco Favia, Chiara Copelli, Alfonso Manfuso, Giuseppe Ingravallo, Antonio d’Amati, and Saverio Capodiferro. 2024. "Metastatic Renal-Cell Carcinoma of the Oro-Facial Tissues: A Comprehensive Review of the Literature with a Focus on Clinico–Pathological Findings" Surgeries 5, no. 3: 694-718. https://doi.org/10.3390/surgeries5030055
APA StyleGranberg, V., Laforgia, A., Forte, M., Di Venere, D., Favia, G., Copelli, C., Manfuso, A., Ingravallo, G., d’Amati, A., & Capodiferro, S. (2024). Metastatic Renal-Cell Carcinoma of the Oro-Facial Tissues: A Comprehensive Review of the Literature with a Focus on Clinico–Pathological Findings. Surgeries, 5(3), 694-718. https://doi.org/10.3390/surgeries5030055











