Negative Impacts of Trace Metal Contamination on the Macrobenthic Communities along the Santos Port Complex—Brazil
Abstract
1. Introduction
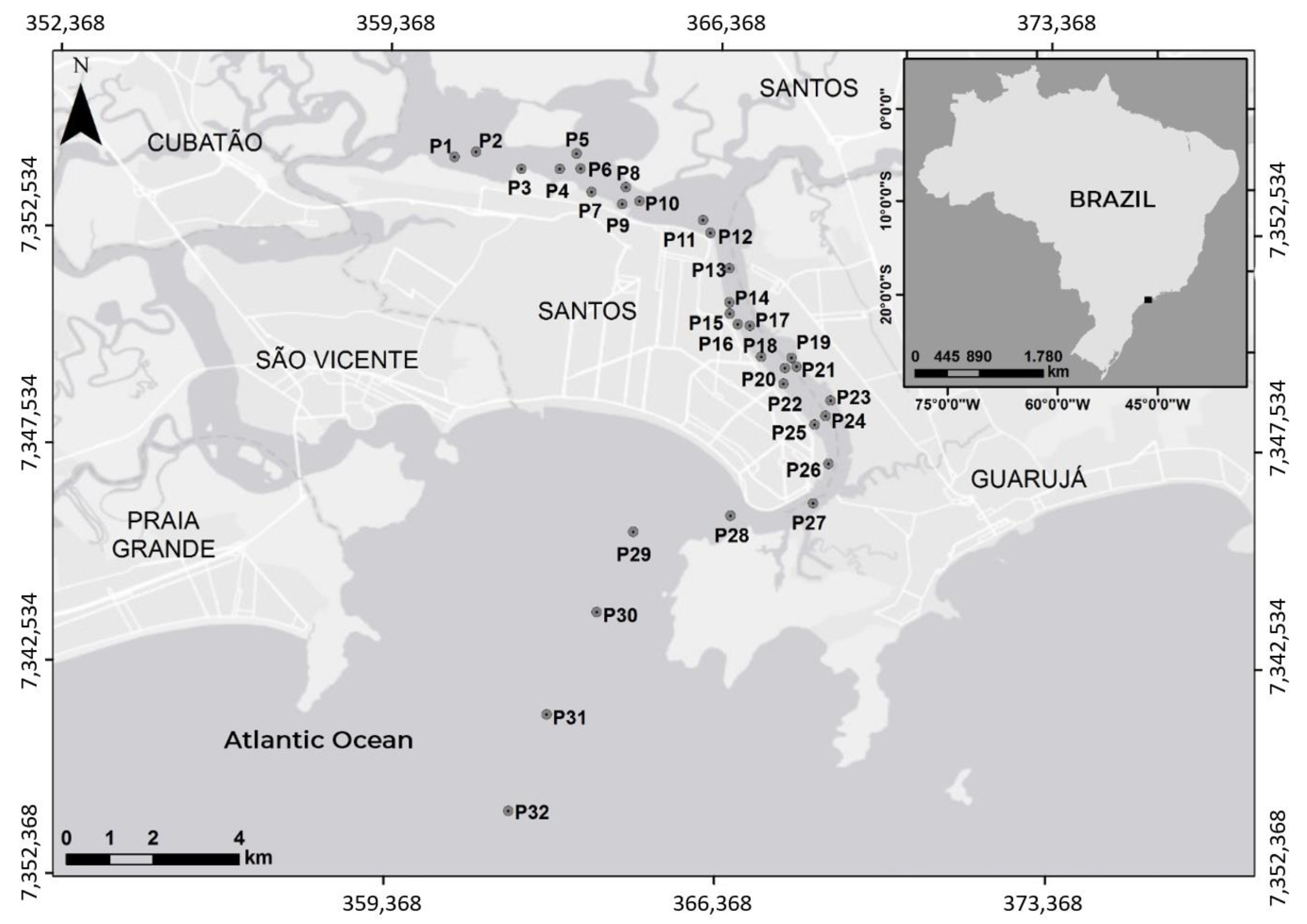
2. Study Site
3. Methodology
3.1. Field Campaign
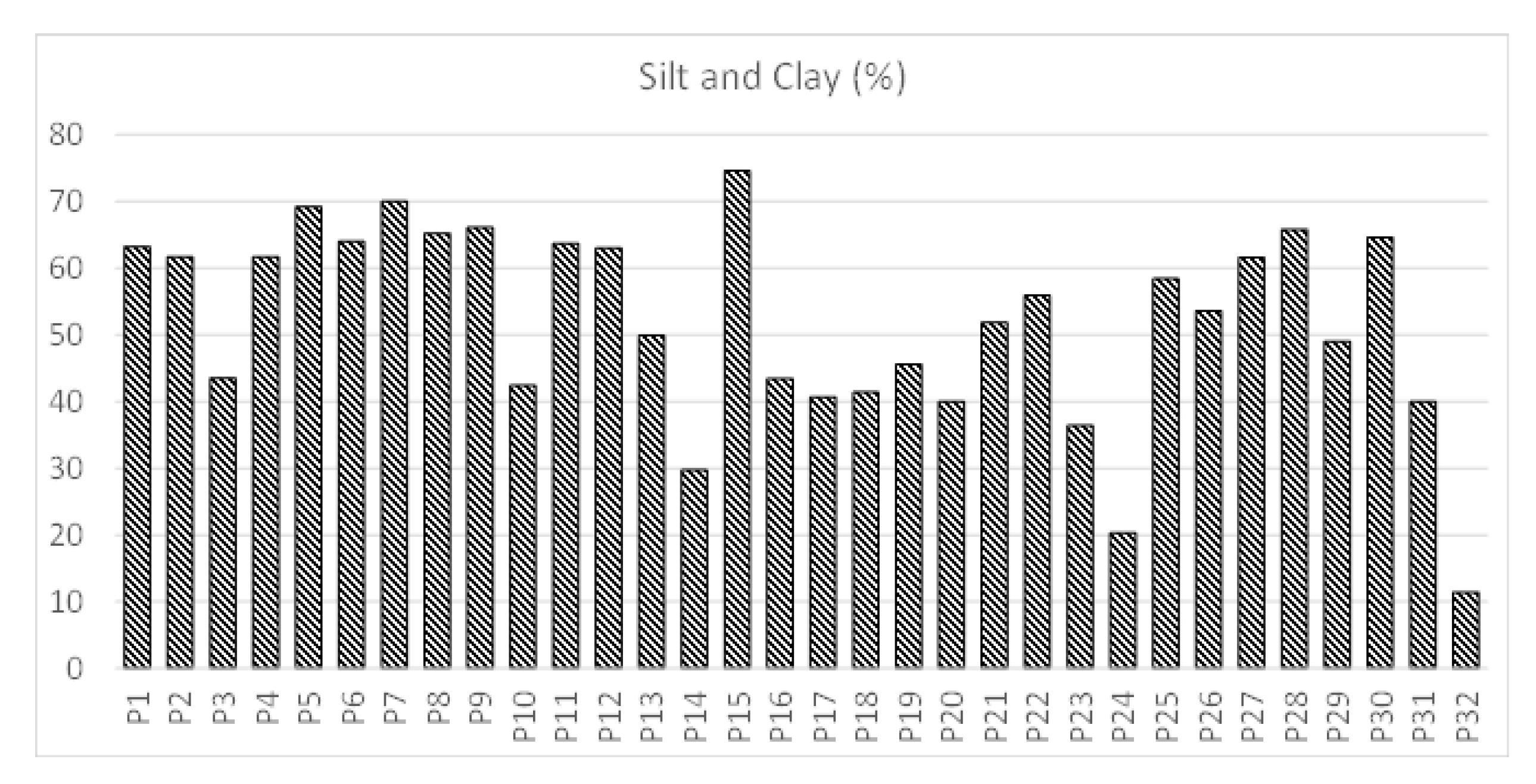
3.2. Laboratory Analysis
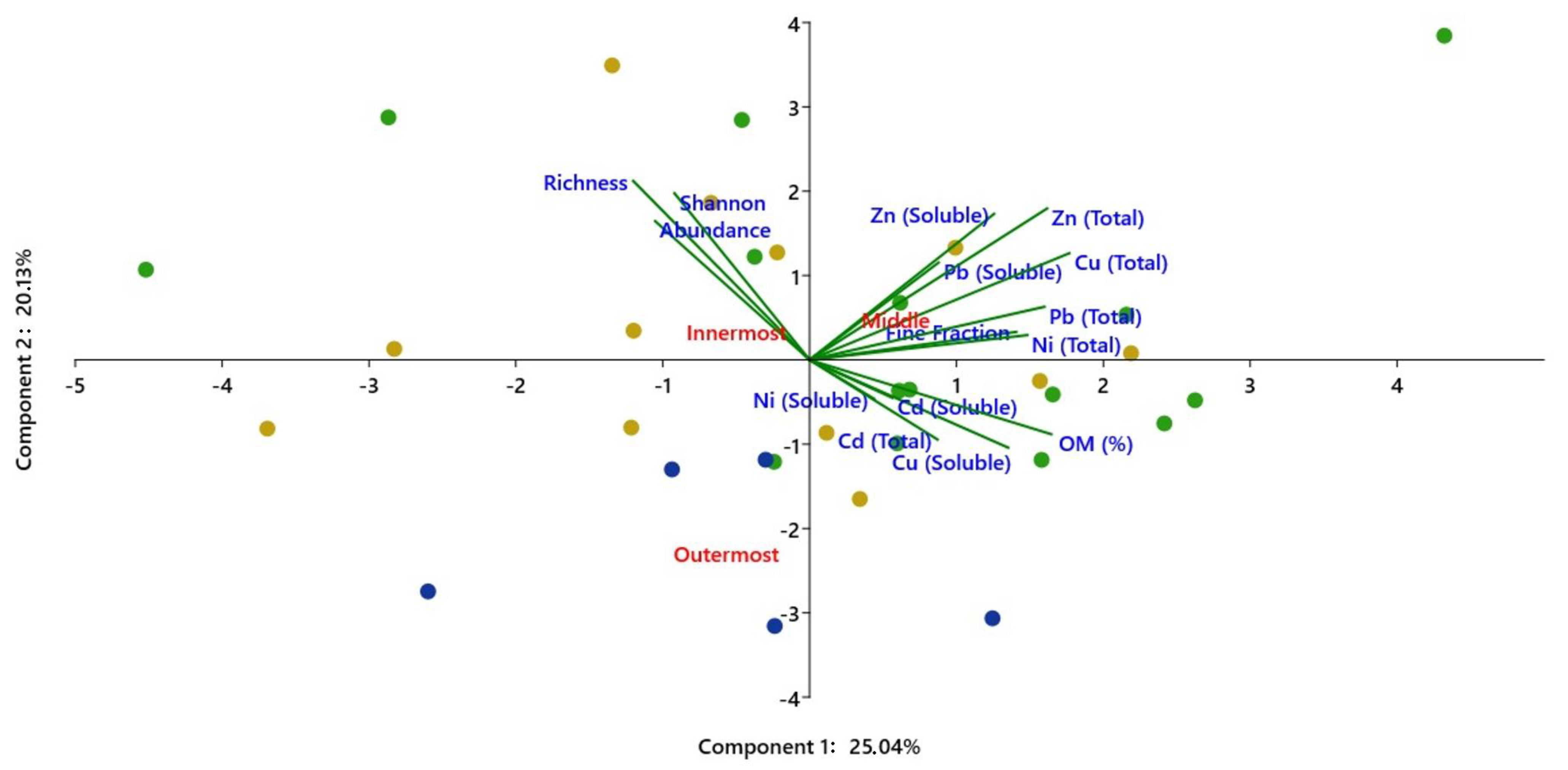
3.3. Statistical Analysis
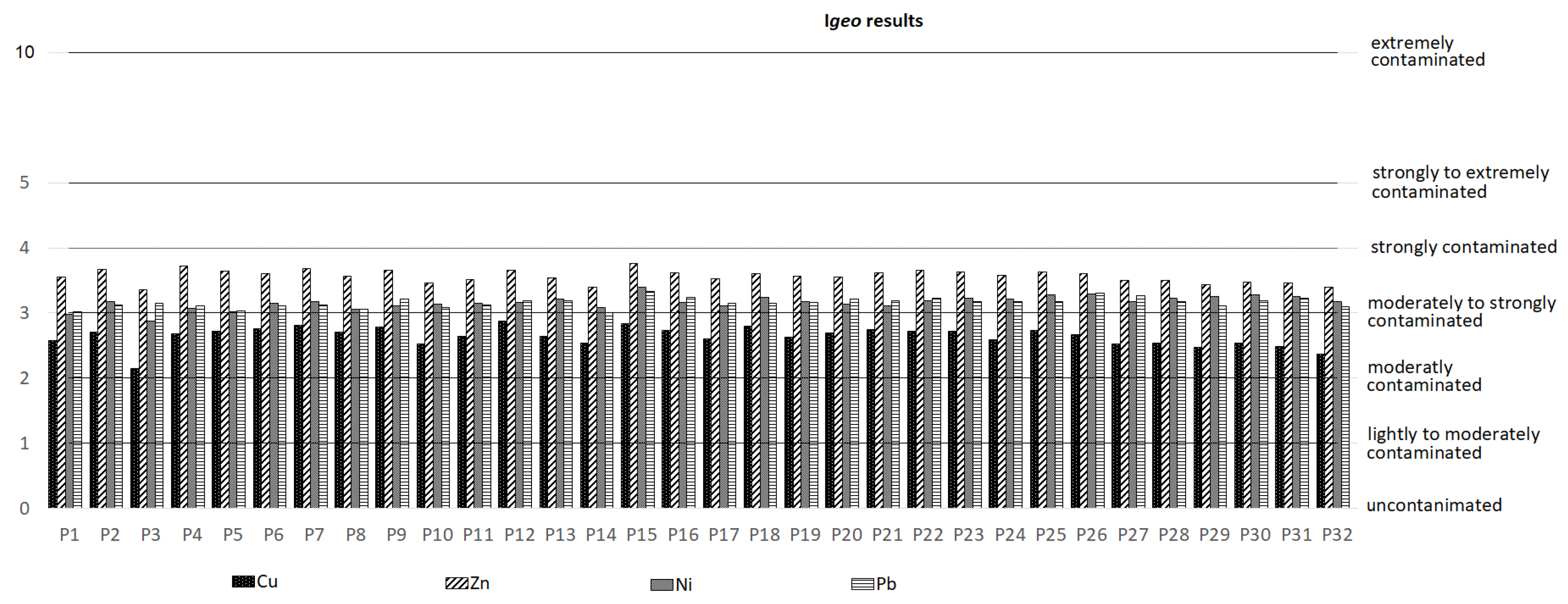
4. Results and Discussion
5. Conclusions and Recommendations
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Guerra-García, J.M.; García-Góme, J.C. Polychaete assemblages and sediment pollution in a harbour with two opposing entrances. Helgol. Mar. Res. 2004, 58, 183–191. [Google Scholar] [CrossRef]
- Galkus, A.; Joksas, K.; Stakeniene, R.; Lagunaviciene, L. Heavy Metal Contamination of Harbor Bottom Sediments. Pol. J. Environ. Stud. 2012, 21, 1583–1594. [Google Scholar]
- Zaaboub, N.; Oueslati, W.; Helali, M.A.; Abdeljaouad, S.; Huertas, J.F.; Galindo, A.L. Trace elements in different marine sediment fractions of the Gulf of Tunis (Central Mediterranean Sea). Chem. Speciat. Bioavailab. 2014, 26, 1–12. [Google Scholar] [CrossRef]
- Chang, C.Y.; Chen, S.Y.; Klipkhayai, P.; Chiemchaisri, C. Bioleaching of heavy metals from harbor sediment using sulfur-oxidizing microflora acclimated from native sediment and exogenous soil. Environ. Sci. Pollut. Res. 2019, 26, 6818–6828. [Google Scholar] [CrossRef] [PubMed]
- Rajeshkumar, S.; Liu, Y.; Zhang, X.; Ravikumar, B.; Bai, G.; Li, X. Studies on seasonal pollution of heavy metals in water, sediment, fish and oyster from the Meiliang Bay of Taihu Lake in China. Chemosphere 2018, 191, 626–638. [Google Scholar] [CrossRef] [PubMed]
- Bussan, D.; Harris, A.; Douvris, C. Monitoring of selected trace elements in sediments of heavily industrialized areas in Calcasieu Parish, Louisiana, United States by inductively coupled plasma-optical emission spectroscopy (ICP-OES). Microchem. J. 2019, 144, 51–55. [Google Scholar] [CrossRef]
- Alomary, A.A.; Belhadj, S. Determination of heavy metals (Cd, Cr, Cu, Fe, Ni, Pb, Zn) by ICP-OES and their speciation in Algerian Mediterranean Sea sediments after a five-stage sequential extraction procedure. Environ. Monit. Assess. 2007, 135, 265. [Google Scholar] [CrossRef]
- Guo, R.; He, X. Spatial variations and ecological risk assessment of heavy metals in surface sediments on the upper reaches of Hun River, Northeast China. Environ. Earth Sci. 2013, 70, 1083–1090. [Google Scholar] [CrossRef]
- Nriagu, J.O. A history of global metal pollution. Science 1996, 272, 223–224. [Google Scholar] [CrossRef]
- Tylmann, W.; Lysek, K.; Kinder, M.; Pempkowiak, J. Regional pattern of heavy metal content in Lake sediments in Northern Poland. Water Air Soil Pollut. 2011, 216, 217–228. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, B.; Peng, X.; Wang, H.; Li, Z.; Cai, W.; Fang, H. Sediment quality assessment for heavy metal contamination in the Dongzhai Harbor (Hainan Island, China) with pollution indice approach. Open Chem. Eng. J. 2014, 8, 32–37. [Google Scholar] [CrossRef]
- Liu, Q.; Sheng, Y.; Jiang, M.; Zhao, G.; Li, C. Attempt of basin-scale sediment quality standard establishment for heavy metals in coastal rivers. Chemosphere 2019, 245, 125596. [Google Scholar] [CrossRef]
- Chen, K.; Tian, S.; Jiao, J.J. Macrobenthic Community in Tolo Harbour, Hong Kong and its Relations with Heavy Metals. Estuaries Coasts 2010, 33, 600–608. [Google Scholar] [CrossRef][Green Version]
- Rosenberg, D.M.; Resh, V.H. Introduction to freshwater biomonitoring and benthic macroinvertebrates. In Freshwater Biomonitoring and Benthic Macroinvertebrates; Rosenberg, D.M., Resh, V.H., Eds.; Chapman & Hall: New York, NY, USA, 1993; 488p. [Google Scholar]
- Armah, F.A.; Ason, B.; Luginaah, I.; Essandoh, P.K. Characterization of macro-benthic fauna for ecological health status of the Fosu and Benya lagoons in coastal Ghana. Journal of Ecology and Environment. Ecol. Soc. Korea 2012. [Google Scholar] [CrossRef]
- Dar, M.; Belal, A.; El-Sawy, M. The effect of water quality on the distribution of macro-benthic fauna in Western Lagoon and Timsah Lake, Egypt. I. Egypt. J. Aquat. Res. 2017, 42, 437–448. [Google Scholar] [CrossRef]
- Amorim, R.M.; Delgado, J.D.F.; Neto, J.A.B.; Crapez, M.A.C.C.; Fernandez, C.S.; Negrello Filho, O.A.; Fonseca, E.M. The benthic macrofauna along the estuarine gradient of the Paranaguá estuary. Reg. Stud. Mar. Sci. 2020, 39, 101459. [Google Scholar] [CrossRef]
- Li, F.; Ma, Y.; Song, X.; Li, S.; Zhang, X.; Wang, X.; Wang, T.; Sun, Z. Community structure and ecological quality assessment of macrobenthos in the coastal sea areas of Northern Yantai, China. Front. Mar. Sci. 2022, 9, 989034. [Google Scholar] [CrossRef]
- Ryu, J.; Kang, S.-G.; Kang, D.; Lee, C.H.; Koh, C.H. The impact of heavy metal pollution gradients in sediments on benthic macrofauna at population and community levels. Environ. Pollut. 2011, 159, 2622–2629. [Google Scholar] [CrossRef]
- Desrosiers, M.; Usseglio-Polatera, P.; Archaimbault, V.; Larras, F.; Méthot, G.; Pinel-Alloul, B. Assessing anthropogenic pressure in the St. Lawrence River using traits of benthic macroinvertebrates. Sci. Total. Environ. 2019, 1, 233–246. [Google Scholar] [CrossRef]
- Oresanz, J.M.; Gianuca, N.M. Contribuição ao conhecimento dos anelídeos poliquetas do Rio Grande do Sul. Lista sistemática preliminar e descrição de três novas espécies. Comun. Do Mus. Ciências PUCRS 1974, 4, 1–37. [Google Scholar]
- Pamplin, P.A.Z.; Almeida, T.C.M.; Silva-Filho, J.P. New record of Laeonereisacuta (Treadwell, 1923) (Nereididae: Polychaeta) in Northeast coast of Brazil. Biota Neotrop. 2007, 7, 353–355. [Google Scholar] [CrossRef]
- Netto, S.A.; Lana, P.C. Effects of sediment disturbance on the structure of benthic fauna in a subtropical tidal creek of southeastern Brazil. Mar. Ecol. Prog. Ser. 1994, 106, 239–247. [Google Scholar] [CrossRef]
- Müller, G. Index of geo accumulation in sediments of the Rhine River. Geo. J. 1969, 2, 108–118. [Google Scholar]
- De-la-Ossa-Carretero, J.A.; Del-Pilar-Ruso, Y.; Gimenez-Casalduero, F.; Sanchez-Lizaso, J.L. Assessing reliable indicators to sewage pollution in coastal soft-bottom communities. Environ. Monit. Assess. 2012, 184, 2133–2149. [Google Scholar] [CrossRef]
- Ellis, J.I.; Clark, D.; Atalah, J.; Jiang, W.; Taiapa, C.; Patterson, M.; Sinner, J.; Hewitt, J. Multiple stressor effects on marine infauna: Responses of estuarine taxa and functional traits to sedimentation, nutrient and metal loading. Sci. Rep. 2017, 7, 12013. [Google Scholar] [CrossRef] [PubMed]
- Azevedo Netto, A.; de Souza, P.F.; Lima, L.S.; Vieira, K.S.; Delgado, J.F.; Menezes, C.R.; Neves, C.V.; Baptista Neto, J.A.; Fonseca, E.M. Dinâmica de distribuição e fontes potenciais de hidrocarbonetos aromáticos policíclicos para sedimentos de superfície e bivalves de um estuário altamente antropizado. Sist. Gestão 2022, 17, 104–117. [Google Scholar] [CrossRef]
- Hortellani, M.A.; Sarkis, J.E.S.; Abessa, D.M.S.; Sousa, E.C.P.M. Avaliação da contaminação por elementos metálicos dos sedimentos do estuário Santos—São Vicente. Química Nova 2008, 31, 10–19. [Google Scholar] [CrossRef]
- Courrat, A.; Lobry, J.; Nicolas, D.; Laffargue, P.; Amara, R.; Lepage, M.; Girardin, M.; Lepape, O. Anthropogenic disturbance on nursery function of estuarine areas for marine species. Estuar. Coast. Shelf Sci. 2009, 81, 179–190. [Google Scholar] [CrossRef]
- Schwartzkopf, B.; Heppell, S. A Feeding-Ecology-Based Approach to Evaluating Nursery Potential of Estuaries for Black Rockfish. Mar. Coast. Fish. Dyn. Manag. Ecosyst. Sci. 2020, 12, 124–141. [Google Scholar] [CrossRef]
- Guerreiro, M.A.; Martinho, F.; Baptista, J.; Costa, F.; Pardal, M.Â.; Primo, A.L. Function of estuaries and coastal areas as nursery grounds for marine fish early life stages. Mar. Environ. Res. 2021, 1, 70105408. [Google Scholar] [CrossRef]
- Denis, J.; Rabhi, K.; Le Loc’hm, F.; Lasram, F.B.R.; Boutin, K.; Kazour, M.; Diop, M.; Gruselle, M.-C.; Amara, R. Role of estuarine habitats for the feeding ecology of the European eel (Anguilla anguilla L.). PLoS ONE 2022, 17, e0270348. [Google Scholar] [CrossRef]
- Santos, M.; Amorim, A.; Brotas, V.V.; Cruz, J.P.C.; Palma, C.; Borges, C.; Favareto, L.R.; Veloso, V.; Dâmaso-Rodrigues, M.L.; Chainho, P.P.; et al. Spatio-temporal dynamics of phytoplankton community in a well-mixed temperate estuary (Sado Estuary, Portugal). Sci. Rep. 2022, 12, 16423. [Google Scholar] [CrossRef] [PubMed]
- Duarte, B.; Silva, G.; Costa, J.L.; Medeiros, J.P.; Azeda, C.; Sá, E.; Metelo, I.; Costa, M.J.; Caçador, I. Heavy metal distribution and partitioning in the vicinity of the discharge areas of Lisbon drainage basins (Tagus Estuary, Portugal). J. Sea Res. 2014, 93, 101–111. [Google Scholar] [CrossRef]
- Mosley, L.; Liss, P. Particle aggregation, pH changes and metal behaviour during estuarine mixing; review and integration. Mar. Freshw. Res. 2019, 71, 300–310. [Google Scholar] [CrossRef]
- Onabule, O.A.; Mitchell, S.B.; Couceiro, F. The effects of freshwater flow and salinity on turbidity and dissolved oxygen in a shallow Macrotidal estuary: A case study of Portsmouth Harbour. Ocean. Coast. Manag. 2020, 191, 105179. [Google Scholar] [CrossRef]
- Euler, S.; Jeffrey, L.; Maher, D.; Mackenzie, D.; Tait, D. Shifts in methanogenic archaea communities and methane dynamics along a subtropical estuarine land use gradient. PLoS ONE 2020, 15, e0242339. [Google Scholar] [CrossRef] [PubMed]
- Karbassi, A.; Marefat, A. The impact of increased oxygen conditions on heavy metal flocculation in the Sefidrud estuary. Mar. Poll. Bull. 2017, 121, 168–175. [Google Scholar] [CrossRef]
- Jiang, L.Q.; Carter, B.R.; Feely, R.A.; Lauvset, S.K.; Olsen, A. Surface ocean pH and buffer capacity: Past, present and future. Sci. Rep. 2019, 9, 18624. [Google Scholar] [CrossRef] [PubMed]
- Gray, J.S. Species richness of marine soft sediments. Mar. Ecol. Prog. Ser. 2002, 244, 285–297. Available online: http://www.jstor.org/stable/24866382 (accessed on 1 March 2023). [CrossRef]
- Vaquer-Sunyer, R.; Duarte, C.M. Thresholds of hypoxia for marine biodiversity. Proc. Natl. Acad. Sci. USA 2008, 105, 15452–15457. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, H.C.; Rosenberg, R. Hypoxic response of two marine benthic communities. Mar. Ecol. Progr. Seri. 1994, 115, 209–217. [Google Scholar] [CrossRef]
- Crain, C.M. Shifting nutrient limitation and eutrophication effects in marsh vegetation across estuarine salinity gradients. Estuaries Coasts 2007, 30, 26–34. [Google Scholar] [CrossRef]
- Sturdivant, S.K.; Brush, M.J.; Diaz, R.J. Correction: Modeling the Effect of Hypoxia on Macrobenthos Production in the Lower Rappahannock River, Chesapeake Bay, USA. PLoS ONE 2014, 9, 1–13. [Google Scholar] [CrossRef]
- Díaz-Asencio, L.; Helguera, Y.; Fernández-Garcés, R.; Batista, M.; Rosell, G.; Hernández, Y.; Pulido, A.; Armenteros, M. Two-year temporal response of benthic macrofauna and sediments to hypoxia in a tropical semi-enclosed bay (Cienfuegos, Cuba). Rev. De Biol. Trop. 2015, 64, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Diaz, R.J. Overview of hypoxia around the world. J. Environ. Qual. 2001, 30, 275–281. [Google Scholar] [CrossRef]
- Keeling, R.F.; Körtzinger, A.; Gruber, N. Ocean deoxygenation in a warming world. Annu. Rev. Mar. Sci. 2010, 2, 199–229. [Google Scholar] [CrossRef] [PubMed]
- Semlali, R.M.; van Oort, F.; Denaix, L.M.L. Estimating distributions of endogenous and exogenous Pb in soils by using Pb isotopic ratios. Environ. Sci. Technol. 2001, 35, 4180–4188. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Cabrera, J.C.; Georgiadis, M.; Jayachandran, K. Assessment of arsenic mobility in the soils of some golf courses in South Florida. Sci. Total Environ. 2002, 291, 123–134. [Google Scholar] [CrossRef]
- Ljung, K.; Selinus, O.; Otabbong, E.; Berglund, M. Metal and arsenic distribution in soil particle sizes relevant to soil ingestion by children. Appl. Geochem. 2006, 21, 1613–1624. [Google Scholar] [CrossRef]
- Ugwu, I.M.; Igbokwe, O.A. Sorption of Heavy Metals on Clay Minerals and Oxides: A Review. In Advanced Sorption Process Applications; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Hu, J.; Peng, P.; Jia, G.; Mai, B.; Zhang, G. Distribution and sources of organic carbon, nitrogen and their isotopes in sediments of the subtropical Pearl river estuary and adjacent shelf, South China. Mar. Chem. 2006, 98, 274–285. [Google Scholar] [CrossRef]
- Huang, J.; Wang, S.; Li, X.; Xie, R.; Sun, J.; Shi, B.; Liu, F.; Cai, H.; Yang, Q.; Zheng, Z. Effects of Shear Stress and Salinity Stratification on Floc Size Distribution During the Dry Season in the Modaomen Estuary of the Pearl River. Front. Mar. Sci. 2022, 9, 836927. [Google Scholar] [CrossRef]
- Colombo, J.C.; Silverberg, N.; Gearing, J.N. Biochemistry of organic matter in the Laurentian Trough, II. Bulk composition of the sediments and relative reactivity of major components during early diagenesis. Mar. Chem. 1996, 51, 295–314. [Google Scholar] [CrossRef]
- Yamamuro, M. Chemical tracers of sediment organic matter origins in two coastal lagoons. J. Mar. Syst. 2000, 26, 127–134. [Google Scholar] [CrossRef]
- Liu, S.M.; Li, X.N.; Zhang, J.; Wei, H. Nutrient dynamics in Jiaozhou Bay. Water Air Soil Pollut Focus. 2007, 7, 625–643. [Google Scholar] [CrossRef]
- Wu, Y.; Bao, H.; Unger, D.; Herbeck, L.S.; Zhu, Z.; Zhang, J.; Jennerjahn, T.C. Biogeochemical behaviour of organic carbon in a small tropical river and estuary, Hainan, China. Cont. Shelf. Res. 2013, 57, 32–43. [Google Scholar] [CrossRef]
- Van Leussen, W. Aggregation of particles, settling velocity of mud flocs A review. In Physical Processes in Estuaries; Dronkers, J., van Leussen, W., Eds.; Springer: Berlin/Heidelberg, Germany, 1988; pp. 347–403. [Google Scholar] [CrossRef]
- Gregory, J. Particles in Water: Properties and Processes; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar] [CrossRef]
- Hang, X.; Wang, H.; Zhou, J.; Du, C.; Chen, X. Characteristics and accumulation of heavy metals in sediments originated from an electroplating plant. J. Hard. Mater. 2009, 163, 922–930. [Google Scholar] [CrossRef] [PubMed]
- Ramos e Silva, C.A.; Silva, A.P.; Oliveira, S.R. Concentration, stock and transport rate Concentration, stock and transport rate of heavy metals in a tropical red mangrove, Natal, Brazil. Mar. Chem. 2006, 99, 2–11. [Google Scholar] [CrossRef]
- Davutluoglu, O.I.; Galip, S.; Cagatayhan, B.E.; Turan, Y.; Bulent, S. Heavy metal content and distribution in surface sediments of the Seyhan River, Turkey. J. Environ. Manag. 2011, 92, 2250–2259. [Google Scholar] [CrossRef] [PubMed]
- Salomons, W.; Stigliani, W. Biogeodynamics of Pollutants in Soils and Sediments; Springer: Berlin/Heidelberg, Germany, 1995; 352p. [Google Scholar]
- Passos, E.A.; Alves, J.P.H.; Garcia, C.A.B.; Costa, A.C.S. Metal Fractionation in Sediments of the Sergipe River, Northeast, Brazil. J. Braz. Chem. Soc. 2011, 22, 828–835. [Google Scholar] [CrossRef]
- Bighiu, M.A.; Gorokhova, E.; Almroth, B.C.; Wiklund, A.K. Metal contamination in harbours impacts life-history traits and metallothionein levels in snails. PLoS ONE 2017, 12, e0180157. [Google Scholar] [CrossRef]
- Díaz-Castañeda, V.; Valenzuela-Solano, S. Polychaete fauna in thevicinity of bluefin tuna sea-cages in Ensenada, Baja California, Mexico. Zoosymposia 2009, 2, 505–526. [Google Scholar] [CrossRef][Green Version]
- Olsgard, F.; Brattegard, T.; Holthe, T. Polychaetes as surrogates formarine biodiversity: Lower taxonomic resolution and indicatorgroups. Biodivers. Conser. 2003, 12, 1033–1049. [Google Scholar] [CrossRef]
- Gray, J.S.; Elliot, M. Ecology of marine sediments. In From Science to Management, 2nd ed.; Oxford University Press: Oxford, UK, 2009; 225p. [Google Scholar]
- Nayak, A.; Equbal, J.; Rout, S.S.; Dash, B.; Thiruchitrambalam, G.; Bhadury, P.; Satyanarayana, B.; Raut, D. Macrobenthic community of an anthropogenically influenced mangrove associated estuary on the East coast of India: An approach for ecological assessment. Front. Mar. Sci. 2022, 9, 1008912. [Google Scholar] [CrossRef]
- Lima, S.F.B.; Lucena, R.A.; Santos, G.M.; Souza, J.W.; Christoffersen, M.L.; Guimarães, C.R.; Oliveira, G.S. Inventory of mollusks from the estuary of the Paraíba River in northeastern Brazil. Biota. Neotrop. 2017, 17, e20160239. [Google Scholar] [CrossRef]
- Rygg, B. Distribution of species along pollution-induced diversity gradient in benthic communities in Norwegian Fjords. Mar. Pollut. Bull. 1985, 16, 469–474. [Google Scholar] [CrossRef]
- Hall, J.A.; Frid, C.L.J.; Proudfoot, R.K. Effects of metal contamination on macrobenthos of two North Sea estuaries. ICES J. Mar. Sci. 1996, 53, 1014–1023. [Google Scholar] [CrossRef]
- Warwick, R.M. Evidence for the effects of metal contamination on the intertidal macrobenthic assemblages of the Fal Estuary. Mar. Pollut. Bull. 2001, 42, 145–148. [Google Scholar] [CrossRef]
- Harriague, A.C.; Misic, C.; Petrillo, M.; Albertelli, G. Stressors affecting the macrobenthic community in Rapallo Harbour (Ligurian Sea, Italy). Sci. Mar. 2007, 71, 705–714. [Google Scholar] [CrossRef]
- Dauvin, J.C. Effects of heavy metal contamination on the macrobenthic fauna in estuaries: The case of the Seine estuary. Mar. Poll. Bull. 2008, 57, 22–40. [Google Scholar] [CrossRef]
- Seiderer, L.J.; Newell, R.C. Analysis of the relationship between sediment composition and benthic community structure in coastal deposits: Implications for marine aggregate dredging. ICES J. Mar. Sci. 1999, 56, 757–765. [Google Scholar] [CrossRef]
- Van Dalfsen, J.A.; Essink, K.; Toxvig, M.H.; Birklund, J.; Romero, J.; Manzanera, M. Differential response of macrozoobenthos to marine sand extraction in the North Sea and the western Mediterranean. ICES J. Mar. Sci. 2000, 57, 1439–1445. [Google Scholar] [CrossRef]
- Etter, R.J.; Grassle, J.F. Patterns of species-diversity in the deep sea as a function of sediment particle size diversity. Nature 1992, 360, 576–578. [Google Scholar] [CrossRef]
- Moreira, J.; Quintas, P.; Troncoso, J. Spatial distribution of soft-bottom polychaete annelids in the Ensenada de Baiona (Ría de Vigo, Galicia, north-west Spain). Sci. Mar. 2006, 70, 217–224. [Google Scholar] [CrossRef]
- Mwakisunga, B.; Machiwa, J.F.; Pratap, H.B. Assessment of Sediment and Benthic Macrofauna Distribution at Dar es Salaam Harbour Channel. J. Geosci. Environ. Prot. 2020, 8, 133. [Google Scholar] [CrossRef]
- Mannino, A.; Montagna, P.A. Small-scale spatial variation of macrobenthic community structure. Estuaries 1997, 20, 159–173. [Google Scholar] [CrossRef]
- Gray, J.S.; Wu, R.S.-S.; Or, W.Y. Effects of hypoxia and organic enrichment in the coastal marine environment. Mar. Ecol. Prog. Ser. 2002, 238, 249–279. [Google Scholar] [CrossRef]
- Gutiérrez, D.; Gallardo, V.A.; Mayor, S.; Neira, C.; Vasquez, C.; Sellanes, J.; Rivas, M.; Soto, A.; Carrasco, F.D.; Baltazar, M. Effects of dissolved oxygen and fresh organic matter on macrofaunal bioturbation potential in sublittoral bottoms off central Chile, during the 1997-1998 El Niño. Mar. Ecol. Prog. Ser. 2000, 202, 81–99. [Google Scholar] [CrossRef]
- Cooksey, C.; Hyland, J. Sediment quality of the Lower St. Johns River, Florida: An integrative assessment of benthic fauna, sediment-associated stressors, and general habitat characteristics. Mar. Pollut. Bull. 2007, 54, 9–21. [Google Scholar] [CrossRef]


| Taxon | P1 | P2 | P3 | P4 | P5 | P6 | P7 | P8 | P9 | P10 | P11 | P12 | P13 | P14 | P15 | P16 | P17 | P18 | P19 | P20 | P21 | P22 | P23 | P24 | P25 | P26 | P27 | P28 | P29 | P30 | P31 | P32 | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phylum Mollusca | 0 | ||||||||||||||||||||||||||||||||
| Class Bivalvia | 0 | ||||||||||||||||||||||||||||||||
| Order Venerida | 0 | ||||||||||||||||||||||||||||||||
| Family Veneridae | 0 | ||||||||||||||||||||||||||||||||
| Anomalocardia brasiliana | 0 | 7 | 2 | 2 | 0 | 1 | 3 | 1 | 0 | 2 | 0 | 0 | 2 | 2 | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 2 | 2 | 4 | 0 | 0 | 1 | 0 | 38 |
| Chione subrostrata | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 49 |
| Family Mesodesmatidae | 11 | ||||||||||||||||||||||||||||||||
| Mesodesma mactroides | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 1 | 0 | 0 | 1 | 0 | 12 |
| Order Pectinida | 12 | ||||||||||||||||||||||||||||||||
| Family Pectinidae | 0 | ||||||||||||||||||||||||||||||||
| Chlamys tehuelchus | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 |
| Order Cardiida | 4 | ||||||||||||||||||||||||||||||||
| Family Cardiidae | 0 | ||||||||||||||||||||||||||||||||
| Laevicardium brasilianum | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 2 | 0 | 2 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 13 |
| Order Mytilida | 13 | ||||||||||||||||||||||||||||||||
| Family Mytilidae | 0 | ||||||||||||||||||||||||||||||||
| Mytella charruana | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 6 |
| Class Gastropoda | 6 | ||||||||||||||||||||||||||||||||
| Order Neogastropoda | 0 | ||||||||||||||||||||||||||||||||
| Family Columbellidae | 0 | ||||||||||||||||||||||||||||||||
| Costoanachis sparsa | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 10 |
| Family Pyramidellidae | 10 | ||||||||||||||||||||||||||||||||
| Turbonilla brasiliensis | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 7 |
| Turbonilla fasciata | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 20 |
| Phylum Echinodermata | 13 | ||||||||||||||||||||||||||||||||
| Class Ophiuroidea | 0 | ||||||||||||||||||||||||||||||||
| Order Amphilepidida | 0 | ||||||||||||||||||||||||||||||||
| Family Amphiuridae | 0 | ||||||||||||||||||||||||||||||||
| Amphiodia sp. | 0 | 3 | 0 | 0 | 0 | 2 | 2 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 18 |
| Phylum Crustacea | 18 | ||||||||||||||||||||||||||||||||
| Class Malacostraca | 0 | ||||||||||||||||||||||||||||||||
| Order Decapoda | 0 | ||||||||||||||||||||||||||||||||
| Family Diogenidae | 0 | ||||||||||||||||||||||||||||||||
| Clibanarius sp. | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 |
| Phylum Annelida | 4 | ||||||||||||||||||||||||||||||||
| Class Polychaeta | 0 | ||||||||||||||||||||||||||||||||
| Order Phyllodocida | 0 | ||||||||||||||||||||||||||||||||
| Family Nereididae | 0 | ||||||||||||||||||||||||||||||||
| Ceratonereis sp. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 9 |
| Order Eunicida | 9 | ||||||||||||||||||||||||||||||||
| Family Onuphidae | 0 | ||||||||||||||||||||||||||||||||
| Diopatra sp. | 0 | 2 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 10 |
| Others | 2 | 0 | 4 | 14 | 0 | 2 | 5 | 3 | 0 | 2 | 0 | 3 | 3 | 8 | 4 | 4 | 3 | 2 | 1 | 0 | 0 | 0 | 2 | 52 | 0 | 4 | 6 | 8 | 0 | 0 | 3 | 4 | 149 |
| Sampling Station | Pielou Equability | Shannon Test | Diversity |
|---|---|---|---|
| J′ | H′ | ||
| P1 | 0.00000 | 0.00000 | Very low |
| P2 | 0.43841 | 1.15699 | Low |
| P3 | 0.54075 | 1.42706 | Low |
| P4 | 0.57713 | 1.52308 | Low |
| P5 | 0.00000 | 0.00000 | Very low |
| P6 | 0.59003 | 1.55711 | Low |
| P7 | 0.53964 | 1.42413 | Low |
| P8 | 0.36008 | 0.95027 | Very low |
| P9 | 0.00000 | 0.00000 | Very low |
| P10 | 0.64256 | 1.69574 | Low |
| P11 | 0.00000 | 0.00000 | Very low |
| P12 | 0.00000 | 0.00000 | Very low |
| P13 | 0.75463 | 1.99151 | Low |
| P14 | 0.69372 | 1.83077 | Low |
| P15 | 0.48497 | 1.27985 | Low |
| P16 | 0.81901 | 2.16142 | Low |
| P17 | 0.00000 | 0.00000 | Very low |
| P18 | 0.00000 | 0.00000 | Very low |
| P19 | 0.26265 | 0.69315 | Very low |
| P20 | 0.00000 | 0.00000 | Very low |
| P21 | 0.00000 | 0.00000 | Very low |
| P22 | 0.00000 | 0.00000 | Very low |
| P23 | 0.39397 | 1.03972 | Low |
| P24 | 0.50673 | 1.33729 | Low |
| P25 | 0.24119 | 0.63651 | Low |
| P26 | 0.50384 | 1.32966 | Low |
| P27 | 0.51457 | 1.35798 | Low |
| P28 | 0.39966 | 1.05472 | Low |
| P29 | 0.00000 | 0.00000 | Very low |
| P30 | 0.00000 | 0.00000 | Very low |
| P31 | 0.36008 | 0.95027 | Very low |
| P32 | 0.00000 | 0.00000 | Very low |
| Variation Source | SQ | gl | MQ | F | Value-p | F |
|---|---|---|---|---|---|---|
| Inter group test | 464.9196429 | 31 | 14.99740783 | 2.0878927 | 0.00073392 | - |
| Intra group test | 2988.142857 | 416 | 7.183035714 | - | ||
| Total | 3453.0625 | 447 |
| Cu (F1) | Zn (F1) | Ni (F1) | Pb (F1) | Cd (F1) | Fine Grainsize | Richness | Abundance | Shannon | Mo (%) | Cu (Total) | Zn (Total) | Ni (Total) | Pb (Total) | Cd (Total) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cu (Soluble) | −0.172 | 0.343 | 0.050 | −0.198 | 0.133 | −0.387 | −0.337 | −0.354 | 0.356 | 0.174 | 0.060 | 0.625 | 0.555 | 0.182 | |
| Zn (Soluble) | −0.172 | −0.114 | 0.201 | 0.144 | 0.340 | 0.012 | −0.017 | −0.017 | −0.038 | 0.723 | 0.790 | −0.099 | 0.044 | −0.170 | |
| Ni (Soluble) | 0.343 | −0.114 | 0.064 | 0.059 | −0.197 | −0.062 | −0.093 | −0.073 | 0.002 | −0.110 | −0.156 | 0.488 | 0.181 | 0.156 | |
| Pb (Soluble) | 0.050 | 0.201 | 0.064 | 0.203 | −0.265 | 0.170 | 0.100 | 0.203 | 0.091 | 0.309 | 0.279 | 0.164 | 0.617 | 0.456 | |
| Cd (Soluble) | −0.198 | 0.144 | 0.059 | 0.203 | 0.089 | −0.155 | −0.221 | −0.157 | 0.222 | 0.174 | 0.072 | −0.019 | 0.026 | 0.549 | |
| Fine Grainsize | 0.133 | 0.340 | −0.197 | −0.265 | 0.089 | −0.171 | −0.127 | −0.091 | 0.392 | 0.453 | 0.470 | −0.062 | 0.004 | −0.213 | |
| Richness | −0.387 | 0.012 | −0.062 | 0.170 | −0.155 | −0.171 | 0.946 | 0.953 | −0.444 | −0.135 | −0.009 | 0.004 | −0.024 | −0.163 | |
| Abundance | −0.337 | −0.017 | −0.093 | 0.100 | −0.221 | −0.127 | 0.946 | 0.865 | −0.403 | −0.132 | 0.029 | 0.089 | −0.008 | −0.208 | |
| Shannon | −0.354 | −0.017 | −0.073 | 0.203 | −0.157 | −0.091 | 0.953 | 0.865 | −0.375 | −0.112 | −0.013 | −0.011 | 0.017 | −0.106 | |
| Mo (%) | 0.356 | −0.038 | 0.002 | 0.091 | 0.222 | 0.392 | −0.444 | −0.403 | −0.375 | 0.217 | 0.160 | 0.288 | 0.361 | 0.399 | |
| Cu (Total) | 0.174 | 0.723 | −0.110 | 0.309 | 0.174 | 0.453 | −0.135 | −0.132 | −0.112 | 0.217 | 0.868 | 0.051 | 0.262 | −0.020 | |
| Zn (Total) | 0.060 | 0.790 | −0.156 | 0.279 | 0.072 | 0.470 | −0.009 | 0.029 | −0.013 | 0.160 | 0.868 | 0.051 | 0.257 | −0.117 | |
| Ni (Total) | 0.625 | −0.099 | 0.488 | 0.164 | −0.019 | −0.062 | 0.004 | 0.089 | −0.011 | 0.288 | 0.051 | 0.051 | 0.546 | 0.384 | |
| Pb (Total) | 0.555 | 0.044 | 0.181 | 0.617 | 0.026 | 0.004 | −0.024 | −0.008 | 0.017 | 0.361 | 0.262 | 0.257 | 0.546 | 0.407 | |
| Cd (Total) | 0.182 | −0.170 | 0.156 | 0.456 | 0.549 | −0.213 | −0.163 | −0.208 | −0.106 | 0.399 | −0.020 | −0.117 | 0.384 | 0.407 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delgado, J.d.F.; Amorim, R.M.; Lima, L.d.S.; Gaylarde, C.C.; Neto, J.A.B.; Pinto, S.C.d.S.; Gonçalves, B.F.d.S.; Fonseca, E.M.d. Negative Impacts of Trace Metal Contamination on the Macrobenthic Communities along the Santos Port Complex—Brazil. Eng 2023, 4, 1210-1224. https://doi.org/10.3390/eng4020071
Delgado JdF, Amorim RM, Lima LdS, Gaylarde CC, Neto JAB, Pinto SCdS, Gonçalves BFdS, Fonseca EMd. Negative Impacts of Trace Metal Contamination on the Macrobenthic Communities along the Santos Port Complex—Brazil. Eng. 2023; 4(2):1210-1224. https://doi.org/10.3390/eng4020071
Chicago/Turabian StyleDelgado, Jéssica de F., Renan M. Amorim, Leonardo da S. Lima, Christine C. Gaylarde, José Antônio Baptista Neto, Samira C. de S. Pinto, Beatriz F. dos S. Gonçalves, and Estefan M. da Fonseca. 2023. "Negative Impacts of Trace Metal Contamination on the Macrobenthic Communities along the Santos Port Complex—Brazil" Eng 4, no. 2: 1210-1224. https://doi.org/10.3390/eng4020071
APA StyleDelgado, J. d. F., Amorim, R. M., Lima, L. d. S., Gaylarde, C. C., Neto, J. A. B., Pinto, S. C. d. S., Gonçalves, B. F. d. S., & Fonseca, E. M. d. (2023). Negative Impacts of Trace Metal Contamination on the Macrobenthic Communities along the Santos Port Complex—Brazil. Eng, 4(2), 1210-1224. https://doi.org/10.3390/eng4020071








