Performance of Thermal-, Acid-, and Mechanochemical-Activated Montmorillonite for Environmental Protection from Radionuclides U(VI) and Sr(II)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
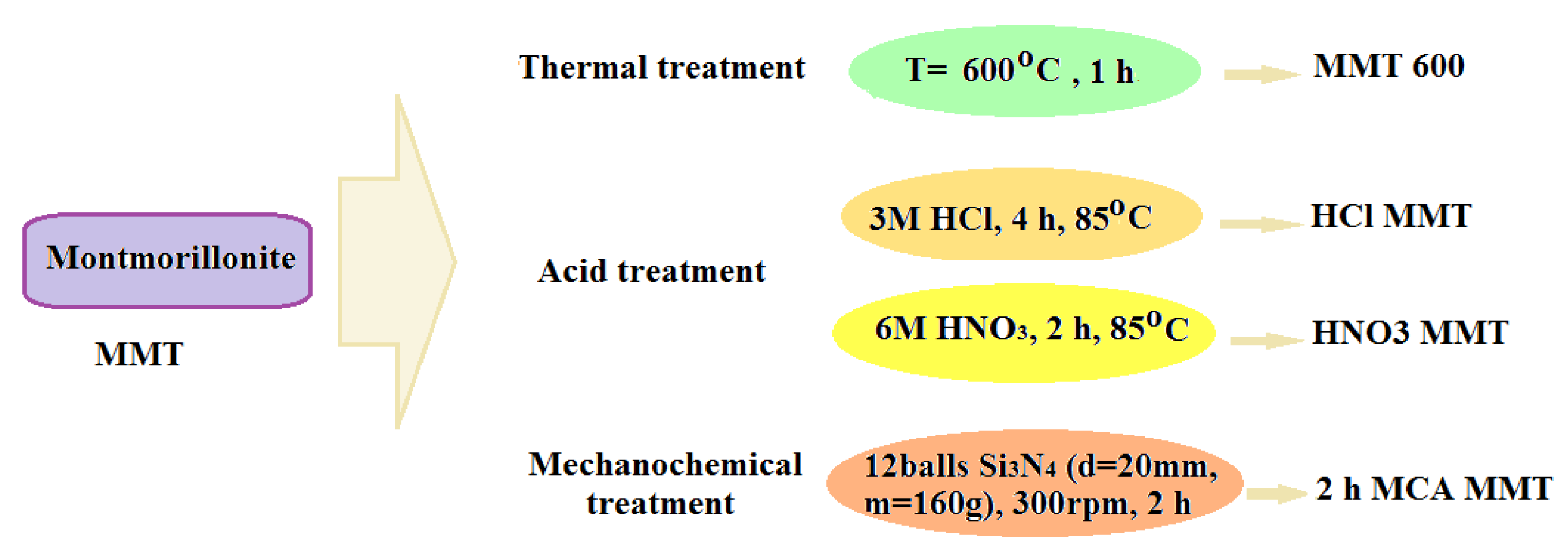
2.2. Preparation of the Samples of Treated Montmorillonite
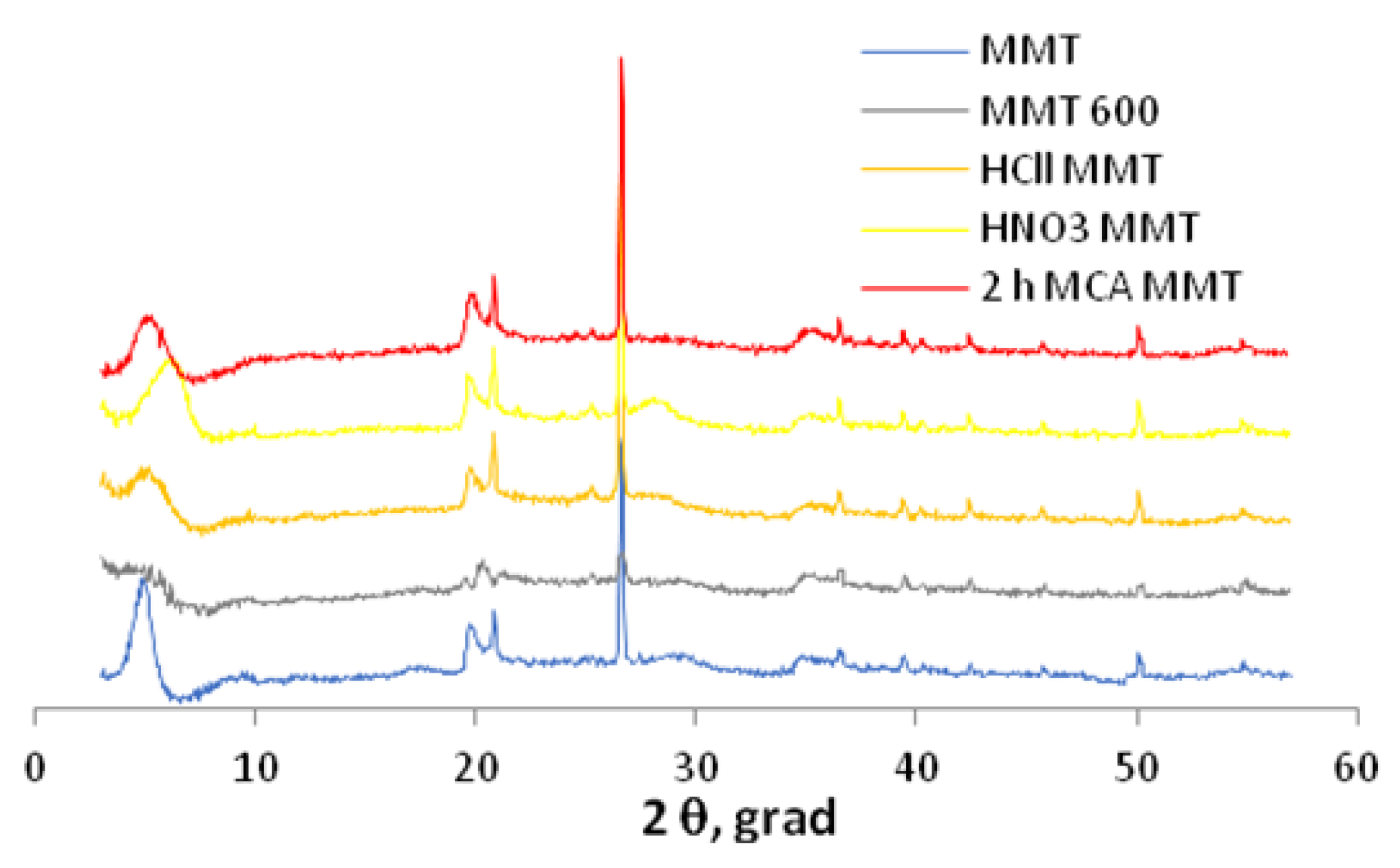
2.3. Characterization of Samples
2.4. Adsorption Experiments
3. Results
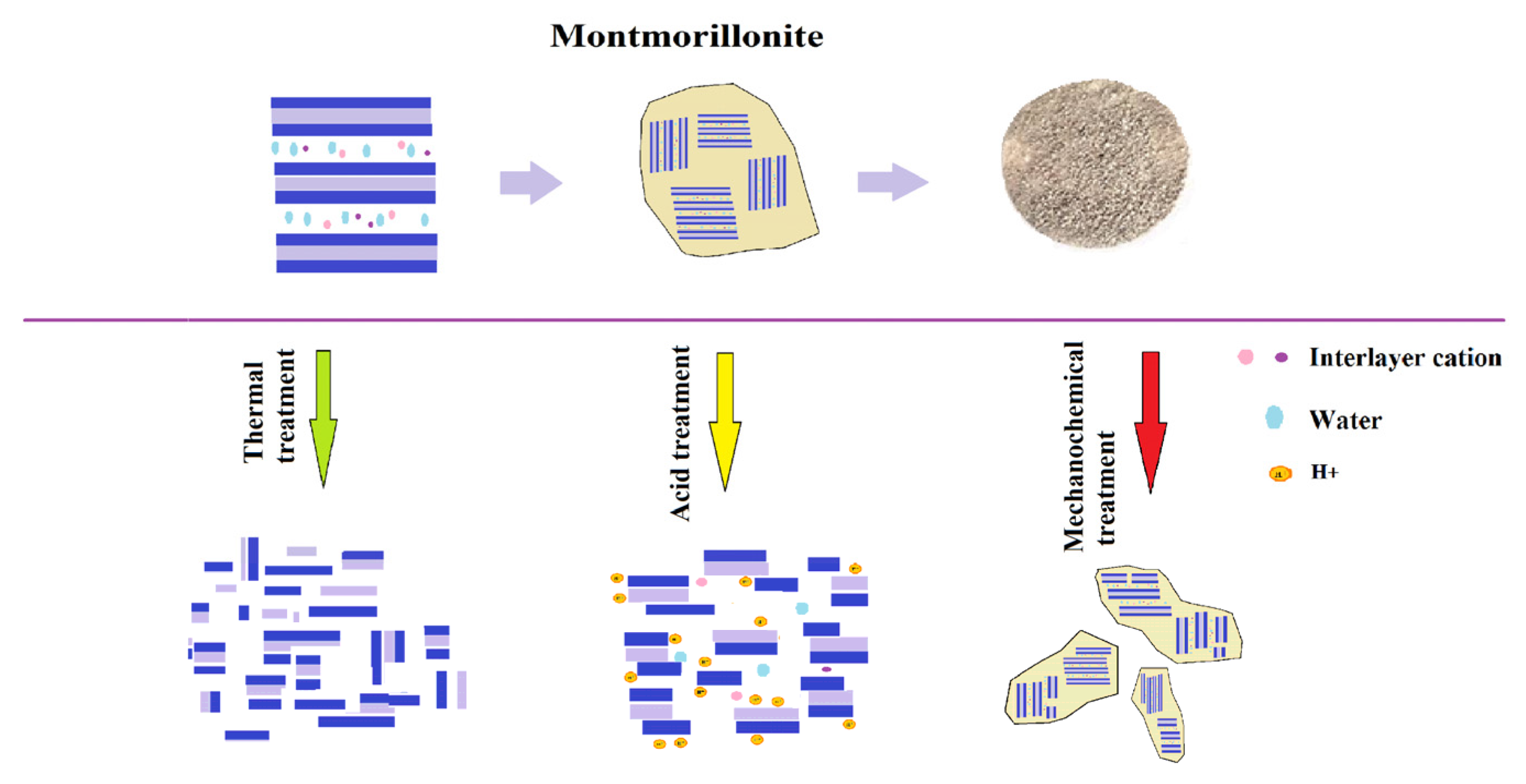
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Landa, E.R. Uranium mine tailings: Nuclear waste and natural laboratory for geochemical and radioecological investigations. J. Environ. Radioact. 2004, 77, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wang, S.; Hou, H.; Chen, K.; Gao, P.; Zhang, Z.; Jin, Q.; Pan, D.; Guo, Z.; Wu, W. China’s progress in radionuclide migration study over the past decade (2010–2021): Sorption, transport and radioactive colloid. Chin. Chem. Lett. 2022, 33, 3405–3412. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for Drinking-Water Quality: Fourth Edition Incorporating the First and Second Addenda; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Kornilovych, B.Y.; Sorokin, O.G.; Pavlenko, V.M.; Koshyk, Y.I. Environmental Protection Technologies in the Uranium Mining and Processing Industry; Norma: Kyiv, Ukraine, 2011; p. 154. (In Ukrainian) [Google Scholar]
- Chakraborty, R.; Asthana, A.; Singh, A.K.; Jain, B.; Susan, A.B.H. Adsorption of heavy metal ions by various low-cost adsorbents: A review. Int. J. Environ. Anal. Chem. 2020, 102, 342–379. [Google Scholar] [CrossRef]
- Jun, B.-M.; Lee, H.-K.; Park, S.; Kim, T.-J. Purification of uranium-contaminated radioactive water by adsorption: A review on adsorbent materials. Sep. Purif. Technol. 2022, 278, 119675. [Google Scholar] [CrossRef]
- Kravchenko, M.V.; Khodakovska, T.A.; Kovtun, M.F.; Romanova, I.V. Inorganic sorbents based on magnesium silicates obtained by two synthetic routes. Environ. Earth Sci. 2022, 81, 549. [Google Scholar] [CrossRef]
- Bergaya, F.; Theng, B.K.; Lagaly, G. (Eds.) Handbook of Clay Science; Elsevier Ltd.: Amsterdam, The Netherlands, 2006; Volume 1. [Google Scholar]
- Komadel, P.; Madejova, J.; Bujdak, J. Preparation and properties of reduced–charge smectites—A review. Clays Clay Miner. 2005, 53, 313–334. [Google Scholar] [CrossRef]
- Bhattacharyya, K.G.; Gupta, S.S. Adsorption of a few heavy metals on natural and modified kaolinite and montmorillonite: A review. Adv. Colloid Interface Sci. 2008, 140, 114–131. [Google Scholar] [CrossRef]
- Novikau, R.; Lujaniene, G. Adsorption behaviour of pollutants: Heavy metals, radionuclides, organic pollutants, on clays and their minerals (raw, modified and treated): A review. J. Environ. Manag. 2022, 309, 114685. [Google Scholar] [CrossRef]
- Yuan, G.D.; Theng, B.K.G.; Churchman, G.J.; Gates, W.P. Clays and Clay Minerals for Pollution Control. In Handbook of Clay Sciences; Elsevier: Amsterdam, The Netherlands, 2013; Volume 5, pp. 587–644. [Google Scholar]
- Misaelides, P. Clay minerals and zeolites for radioactive waste immobilization and containment. In Modified Clay and Zeolite Nanocomposite Materials; Elsevier: Amsterdam, The Netherlands, 2019; pp. 243–274. [Google Scholar]
- Youssef, W.M. Uranium Adsorption from Aqueous Solution Using Sodium Bentonite Activated Clay. J. Chem. Eng. Process Technol. 2017, 8, 157–170. [Google Scholar]
- Tran, E.L.; Teutsch, N.; Klein-BenDavid, O.; Weisbrod, N. Uranium and Cesium sorption to bentonite colloids under carbonate-rich environments: Implications for radionuclide transport. Sci. Total Environ. 2018, 643, 260–269. [Google Scholar] [CrossRef]
- El-Kamash, A.M. Evaluation of zeolite A for the sorptive removal of Cs+ and Sr2+ ions from aqueous solutions using batch and fixed bed column operations. J. Hazard. Mater. 2008, 151, 432–445. [Google Scholar] [CrossRef]
- Tamayo, A.; Kyziol-Komosinska, J.; Sánchez, M.; Calejas, P.; Rubio, J.; Barba, M. Characterization and properties of treated smectites. J. Eur. Ceram. Soc. 2012, 322, 831–2841. [Google Scholar] [CrossRef]
- Kovalchuk, I. Clay-Based Sorbents for Environmental Protection from Inorganic Pollutants. Environ. Sci. Proc. 2023, 25, 34. [Google Scholar] [CrossRef]
- Komadel, P.; Madejova, J. Acid activation of clay minerals. In Handbook of Clay Science; Elsevier: Amsterdam, The Netherlands, 2013; Volume 5, pp. 385–409. [Google Scholar]
- Mucsi, G. A review on mechanical activation and mechanical alloying in stirred media mill. Chem. Eng. Res. Des. 2019, 148, 460–474. [Google Scholar] [CrossRef]
- Heller-Kallai, L. Thermally modified clay minerals. In Handbook of Clay Sciences; Elsevier: Amsterdam, The Netherlands, 2013; Volume 5, pp. 411–433. [Google Scholar]
- Kovalchuk, I.A.; Laguta, A.N.; Kornilovych, B.Y.; Tobilko, V.Y. Organophilic layered silicates for sorption removal of uranium(VI) from mine water. Chem. Phys. Surf. Technol. 2020, 2, 215–227. [Google Scholar] [CrossRef]
- Mnasri-Ghnimi, S.; Frini-Srasra, N. Removal of heavy metals from aqueous solutions by adsorption using single and mixed pillared clays. Appl. Clay Sci. 2019, 179, 105–151. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, W.; Cai, Z.; Han, B.; Qian, T.; Zhao, D. An overview of preparation and applications of stabilized zero-valent iron nanoparticles for soil and groundwater remediation. Water Res. 2016, 100, 245–266. [Google Scholar] [CrossRef] [Green Version]
- Kornilovych, B.; Kovalchuk, I.; Tobilko, V.; Ubaldini, S. Uranium Removal from Groundwater and Wastewater Using Clay-Supported Nanoscale Zero-Valent Iron. Metals 2020, 10, 1421. [Google Scholar] [CrossRef]
- Tobilko, V.Y.; Spasonova, L.M.; Kovalchuk, I.A.; Kornilovych, B.Y.; Kholodko, Y.M. Adsorption of Uranium (VI) from Aqueous Solutions by Amino-functionalized Clay Minerals. Colloids Interfaces 2019, 3, 41. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, C.; Shirai, T.; Fuji, M. Study on intercalation of ionic liquid into montmorillonite and its property evaluation. Mater. Chem. Phys. 2012, 135, 681–686. [Google Scholar] [CrossRef]
- Kovalchuk, I.; Tobilko, V.; Kholodko, Y.; Zahorodniuk, N.; Kornilovych, B. Purification of mineralized waters from U(VI) compounds using bentonite/iron oxide composites. Technol. Audit. Prod. Reserves 2020, 3, 12–18. [Google Scholar] [CrossRef]
- Önal, M.; Sarikaya, Y. Preparation and characterization of acid-activated bentonite powders. Powder Technol. 2007, 172, 14–18. [Google Scholar] [CrossRef]
- Yassin, J.M.; Shiferaw, Y.; Tedla, A. Application of acid activated natural clays for improving quality of Niger (Guizotia abyssinica Cass) oil. Heliyon 2022, 8, e09241. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, K.G.; Gupta, S.S. Adsorptive accumulation of Cd(II), Co(II), Cu(II), Pb(II), and Ni(II) from water on montmorillonite: Influence of acid activation. J. Colloid Interface Sci. 2007, 310, 411–424. [Google Scholar] [CrossRef]
- Gupta, S.S.; Bhattacharyya, K.G. Adsorption of heavy metals on kaolinite and montmorillonite: A review. Phys. Chem. Chem. Phys. 2012, 19, 6698–6723. [Google Scholar] [CrossRef] [PubMed]
- Yariv, S.; Lapides, I. The Effect of Mechanochemical Treatments on Clay Minerals and the Mechanochemical Adsorption of Organic Materials onto Clay Minerals. J. Mater. Synth. Process. 2000, 8, 223–233. [Google Scholar] [CrossRef]
- Xia, M.; Jiang, Y.; Zhao, L.; Li, F.; Xue, B.; Sun, M.; Liu, D.; Zhang, X. Wet grinding of montmorillonite and its effect on the properties of mesoporous montmorillonite. Colloids Surf. A Physicochem. Eng. Asp. 2010, 356, 1–9. [Google Scholar] [CrossRef]
- Vdović, N.; Jurina, I.; Škapin, S.D.; Sondi, I. The surface properties of clay minerals modified by intensive dry milling—Revisited. Appl. Clay Sci. 2010, 48, 575–580. [Google Scholar] [CrossRef] [Green Version]
- Kornilovych, B.Y. Structure and Surface Properties of Mechanochemically Activated Silicates and Carbonates; Naukova Dumka: Kyiv, Ukraine, 1994; p. 127. (In Ukrainian) [Google Scholar]
- Godet-Morand, L.; Chamayou, A.; Dodds, J. Talc grinding in an opposed air jet mill: Start-up, product quality and production rate optimization. Powder Technol. 2002, 128, 306–313. [Google Scholar] [CrossRef] [Green Version]
- Djukić, A.; Jovanović, U.; Tuvić, T.; Andrić, V.; Novaković, J.G.; Ivanović, N.; Matović, L. The potential of ball-milled Serbian natural clay for removal of heavy metal contaminants from wastewaters: Simultaneous sorption of Ni, Cr, Cd and Pb ions. Ceram. Int. 2013, 39, 7173–7178. [Google Scholar] [CrossRef]
- Kumrić, K.R.; Đukić, A.B.; Trtić-Petrović, T.M.; Vukelić, N.S.; Stojanović, Z.; Grbović Novaković, J.D.; Matović, L.L. Simultaneous removal of divalent heavy metals from aqueous solutions using raw and mechanochemically treated interstratified montmorillonite/kaolinite clay. Ind. Eng. Chem. Res. 2013, 52, 7930–7939. [Google Scholar] [CrossRef]
- Hong, W.; Meng, J.; Li, C.; Yan, S.; He, X.; Fu, G. Effects of Temperature on Structural Properties of Hydrated Montmorillonite: Experimental Study and Molecular Dynamics Simulation. Adv. Civil Eng. 2020, 2020, 8885215. [Google Scholar] [CrossRef]
- Aytas, S.; Yurtlu, M.; Donat, R. Adsorption characteristic of U(VI) ion onto thermally activated bentonite. J. Hazard. Mater. 2009, 172, 667–674. [Google Scholar] [CrossRef]
- Tobilko, V.Y.; Kovalchuk, I.A.; Kornilovych, B.Y.; Denisova, T.I.; Kravchenko, O.V. Sorption of uranium and cobalt ions by thermally modified layered silicates. Rep. Natl. Acad. Sci. Ukr. 2010, 5, 150–155. [Google Scholar]
- Espaca, V.A.A.; Sarkar, B.; Biswas, B.; Rusmin, R.; Naidu, R. Environmental applications of thermally modified and acid activated clay minerals: Current status of the art. Environ. Technol. Innov. 2019, 13, 383–397. [Google Scholar] [CrossRef]
- Brindley, G. Crystal Structures of Clay Minerals and Their X-ray Identification; Brown, G., Ed.; The Mineralogical Society of Great Britain and Ireland: London, UK, 1980; p. 496. [Google Scholar]
- Rouquerol, F.; Rouquerol, J.; Sing, K.S.W.; Llewellyn, P.; Maurin, G. Adsorption by Powders and Porous Solids; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef] [Green Version]
- Freundlich, H.; Heller, W. The Adsorption of cis- and trans-Azobenzene. J. Am. Chem. Soc. 1939, 61, 2228–2230. [Google Scholar] [CrossRef]
- Kovalchuk, I.A.; Spasyonova, L.M.; Zakutevskyi, O.I. Sorptive purification of radioactively contaminated waters by acid and mechanical activation montmorillonite. In Proceedings of the Materials of the Seventh International Conference on Nuclear Decommissioning and Environment Recovery INUDECO 22, Slavutych, Ukraine, 27–28 April 2022. [Google Scholar]
- Sun, L.; Liang, X.; Liu, H.; Cao, H.; Liu, X.; Jin, Y.; Li, X.; Chen, S.; Wu, X. Activation of Co-O bond in (110) facet exposed Co3O4 by Cu doping for the boost of propane catalytic oxidation. J. Hazard. Mater. 2023, 452, 131319. [Google Scholar] [CrossRef]
- Feng, X.; Hu, H.; Chen, D. Key nanomaterials for industrial chemical process. Nano Res. 2023, 16, 6212–6219. [Google Scholar] [CrossRef]
- Merkel, B.J.; Hasche-Berger, A. Uranium, Mining and Hydrogeology; Springer: Berlin/Heidelberg, Germany, 2008; p. 955. [Google Scholar]
- Liu, B.; Peng, T.; Hong-Juan, S.; Yue, H. Release behavior of uranium in uranium mill tailings under environmental conditions. J. Environ. Radioact. 2017, 171, 160–168. [Google Scholar] [CrossRef]
- Atwood, D.A. (Ed.) Radionuclides in the Environment; Wiley: Hoboken, NJ, USA, 2010; p. 522. [Google Scholar]
- Kraepiel, A.M.L.; Keller, K.; Morel, F.M.M. A model for metal adsorption on montmorillonite. J. Colloid Interface Sci. 1999, 210, 43–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pradas, E.G.; Sánchez, M.V.; Cruz, F.C.; Viciana, M.S.; Pérez, M.F. Adsorption of cadmium and zinc from aqueous solution on natural and activated bentonite. J. Chem. Technol. Biotechnol. 1994, 59, 289–295. [Google Scholar] [CrossRef]
- Bekri-Abbes, I.; Srasra, E. Effect of mechanochemical treatment on structure and electrical properties of montmorillonite. J. Alloys Compd. 2016, 671, 34–42. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, Y.; Chen, C.; Zhou, J.; Cheng, Y.; Shi, L. Preparation of Montmorillonite Nanosheets with a High Aspect Ratio through Heating/Rehydrating and Gas-Pushing Exfoliation. Langmuir 2022, 38, 10520–10529. [Google Scholar] [CrossRef] [PubMed]


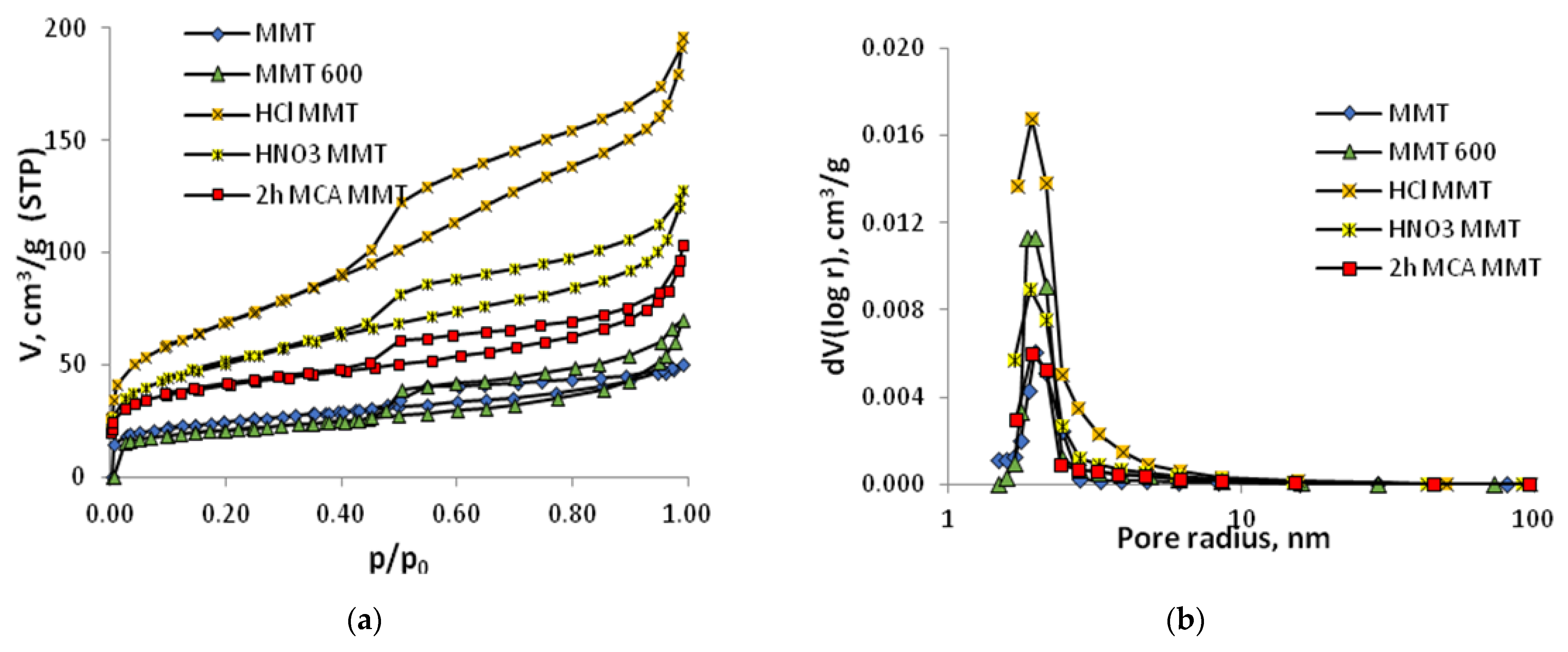

| Sample | S, m2/g | V, cm3/g | r, nm | Distribution of Pore Sizes, nm | ||||
|---|---|---|---|---|---|---|---|---|
| BJH dV(r) | DFT dV(r) | |||||||
| r1 | r2 | r1 | r2 | r3 | ||||
| MMT | 89.1 | 0.078 | 1.753 | 1.98 | - | 1.41 | 2.75 | - |
| MMT 600 | 75.4 | 0.108 | 2.867 | 1.87 | - | 0.83 | 2.59 | - |
| HNO3 MMT | 179.4 | 0.198 | 2.213 | 1.91 | - | 1.25 | 2.54 | 0.69 |
| HCl MMT | 244.0 | 0.304 | 2.500 | 1.93 | - | 0.72 | 2.64 | - |
| 2 h MCA MMT | 146.8 | 0.161 | 2.187 | 1.92 | - | 1.13 | 2.64 | 0.67 |
| Me | Sample | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|---|
| qm, μmol g−1 | KL, L µmol−1 | R2 | n−1 | KF, L µmol−1 | R2 | ||
| U(VI) | MMT | 67.6 | 0.048 | 0.994 | 0.90 | 1.189 | 0.988 |
| MMT 600 | 55.0 | 0.032 | 0.997 | 0.57 | 3.911 | 0.992 | |
| HCl MMT | 161.3 | 0.033 | 0.973 | 0.75 | 1.040 | 0.829 | |
| HNO3 MMT | 140.9 | 0.025 | 0.999 | 0.70 | 0.776 | 0.818 | |
| 2 h MCA MMT | 120.5 | 0.349 | 0.999 | 0.25 | 0.692 | 0.844 | |
| Sr(II) | MMT | 434.8 | 0.024 | 0.999 | 1.19 | 3.786 | 0.914 |
| MMT 600 | 163.9 | 0.018 | 0.990 | 0.51 | 0.630 | 0.931 | |
| HCl MMT | 185.2 | 0.045 | 0.996 | 0.21 | 0.767 | 0.828 | |
| HNO3 MMT | 200.0 | 0.105 | 0.999 | 0.12 | 0.830 | 0.999 | |
| 2 h MCA MMT | 555.6 | 0.038 | 0.982 | 1.47 | 8.335 | 0.878 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovalchuk, I. Performance of Thermal-, Acid-, and Mechanochemical-Activated Montmorillonite for Environmental Protection from Radionuclides U(VI) and Sr(II). Eng 2023, 4, 2141-2152. https://doi.org/10.3390/eng4030122
Kovalchuk I. Performance of Thermal-, Acid-, and Mechanochemical-Activated Montmorillonite for Environmental Protection from Radionuclides U(VI) and Sr(II). Eng. 2023; 4(3):2141-2152. https://doi.org/10.3390/eng4030122
Chicago/Turabian StyleKovalchuk, Iryna. 2023. "Performance of Thermal-, Acid-, and Mechanochemical-Activated Montmorillonite for Environmental Protection from Radionuclides U(VI) and Sr(II)" Eng 4, no. 3: 2141-2152. https://doi.org/10.3390/eng4030122
APA StyleKovalchuk, I. (2023). Performance of Thermal-, Acid-, and Mechanochemical-Activated Montmorillonite for Environmental Protection from Radionuclides U(VI) and Sr(II). Eng, 4(3), 2141-2152. https://doi.org/10.3390/eng4030122






