Comparison between Mullite-Based and Anorthite-Based Porcelain Tiles: A Review
Abstract
:1. Introduction
2. Raw Materials
2.1. Mullite-Based Porcelain Tile
2.2. Anorthite-Based Porcelain Tile
3. Processing
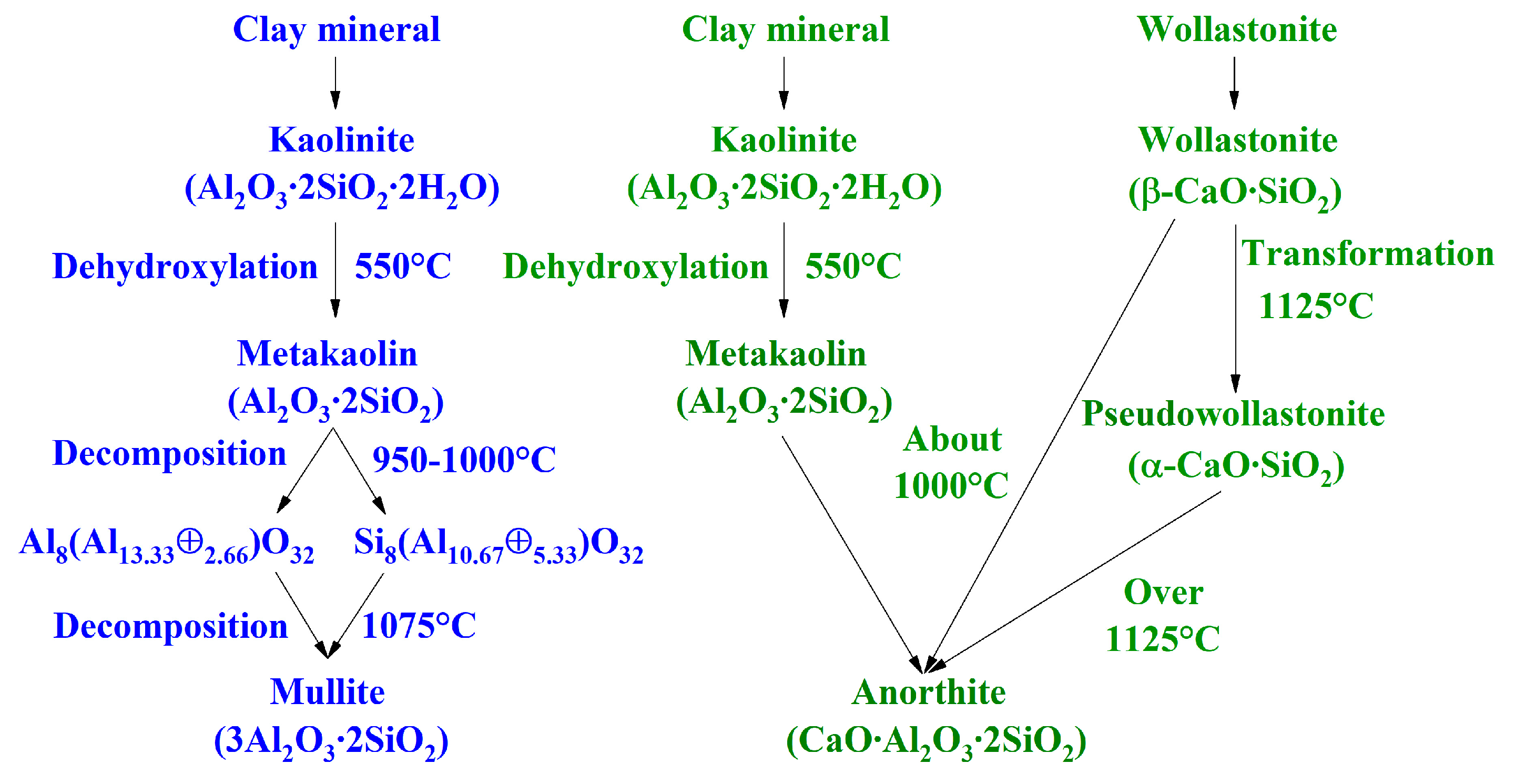
4. Phase Evolution
4.1. Mullite-Based Porcelain Tile
4.2. Anorthite-Based Porcelain Tile
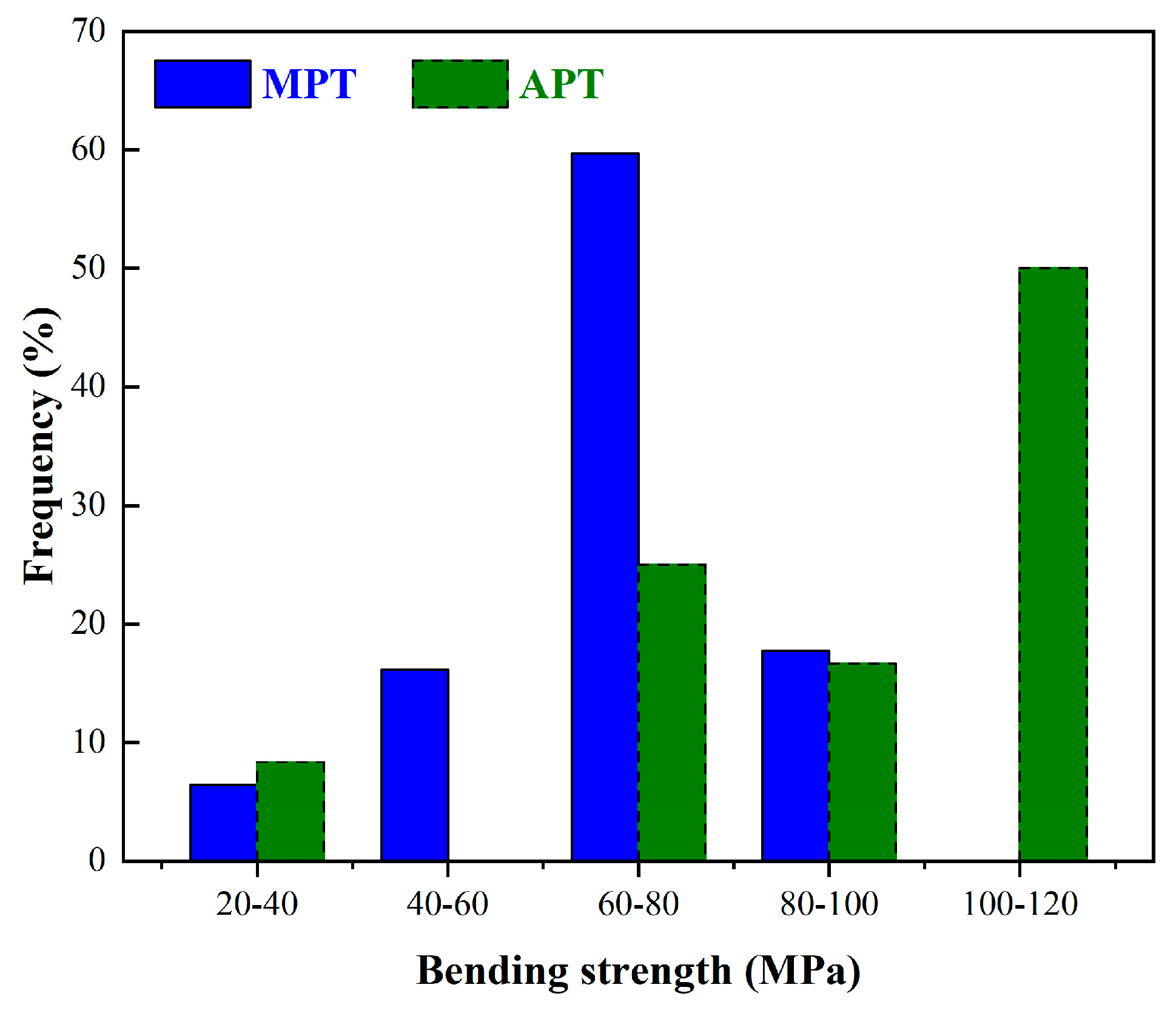
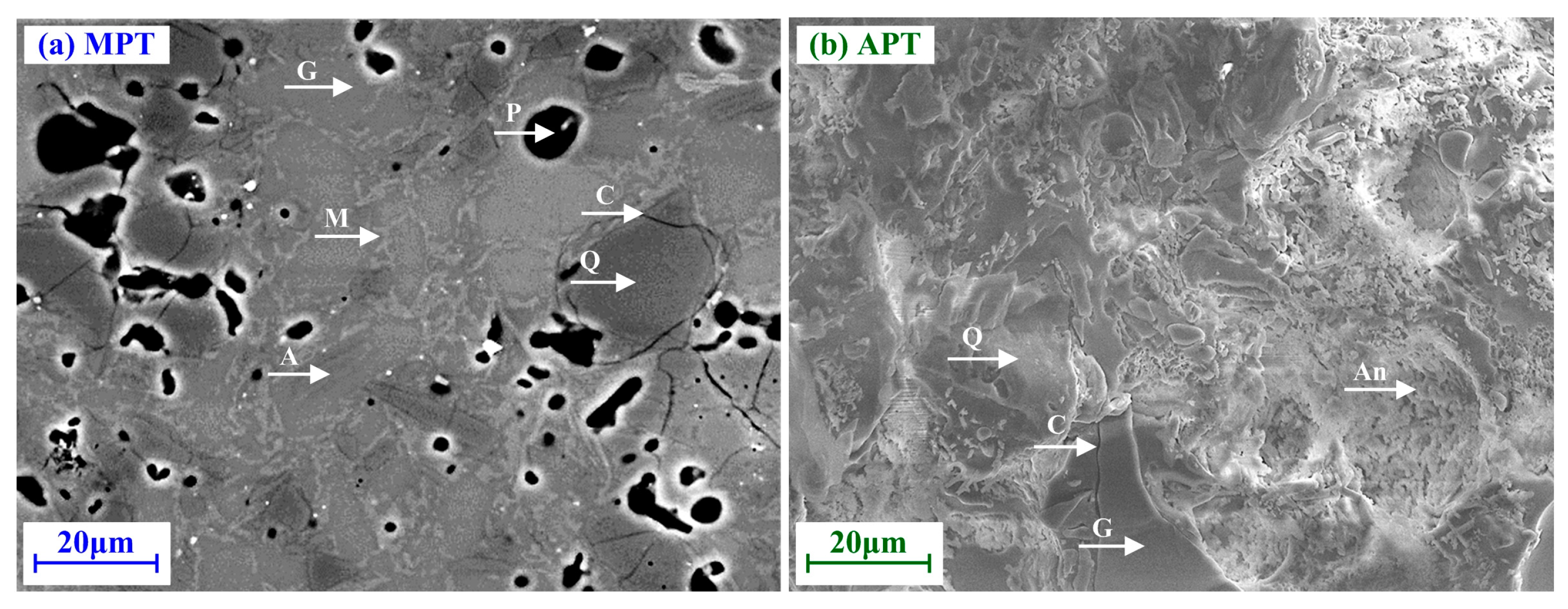
5. Mechanical Behavior
5.1. Mullite-Based Porcelain Tile
5.2. Anorthite-Based Porcelain Tile
6. Prospects and Outlook
- 1.
- Typically, APT is prepared using 50% clay, 40% feldspar, and 10% quartz, and it can be attributed to the SiO2-Al2O3-K2O ternary system; an MPT can be prepared using 20% clay mineral, 25% wollastonite, 30% alumina, 20% quartz, and 5% basic magnesium carbonate, and it can be attributed to the SiO2-Al2O3-CaO ternary system. Variances in the source components and their contents of MPT and APT greatly effects their firing behavior and phase evolution and eventually determines the final microstructure and mechanical properties. The insufficient reserves of wollastonite in major porcelain tile manufacturing countries affects the industrial application of APT.
- 2.
- MPT and APT have no substantial distinctions in their processing routes except the sintering temperature, sintering temperature range, and holding time. The mature similar parameters are a mean powder particle size of 5–6 μm, 10% fine particles and 90%, coarse particles, 5–7% granulating powder moisture, forming pressure of 35–45 MPa, and cold-to-cold time of 35–60 min. The average sintering temperature of APT is 40 °C lower than that of MPT, whereas its sintering temperature range is more than 40 °C narrower than that of MPT. A much narrow sintering temperature range is the main obstacle the industrial application of APT. A combined system of the SiO2-Al2O3-K2O and SiO2-Al2O3-CaO ternary systems, as well as flux consisting of both feldspar and a magnesia-containing component, may improve the firing behavior and further promote the industrial application of APT.
- 3.
- Mullite is the feature phase in MPT, and anorthite is the feature phase in APT. Due to a larger ratio of crystalline to amorphous phase, the crystalline phase type, and a higher CaO content, APT has a mechanical strength two times higher than that of MPT. APT and MPT have comparable whiteness.
- 4.
- MPT has dominated the porcelain tile market to date, and its in-process behavior is better understood compared to that of APT. However, APT represents a promising option for replacing, or for use in combination with, MPT on a large scale, in order to achieve better results.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Amorós, J.L.; Blasco, E.; Moreno, A.; Feliu, C. Kinetics of the transformations occurring during the firing process of an industrial spray—Dried porcelain stoneware body. Ceram. Int. 2022, 48, 17611–17620. [Google Scholar] [CrossRef]
- Alonso De la Garza, D.A.; Guzmán, A.M.; Gómez, C.; Martínez, D.I.; Elizondo, N. Influence of Al2O3 and SiO2 nanoparticles addition on the microstructure and mechano-physical properties of ceramic tiles. Ceram. Int. 2022, 48, 12712–12720. [Google Scholar] [CrossRef]
- Demarch, A.; Waterkemper, A.; Pasini, D.; Ruzza, S.; Montedo, O.R.; Angioletto, E. Effects of roughness parameters on slip resistance for different methods used to determine the coefficient of friction for ceramic floor tiles. Ceram. Int. 2021, 47, 24281–24286. [Google Scholar] [CrossRef]
- ISO 10545-3; Ceramic Tiles—Part 3: Determination of Water Absorption, Apparent Porosity, Apparent Relative Density and Bulk Density. International Organization for Standardization: London, UK, 2018.
- Dana, K.; Das, S.; Das, S.K. Effect of substitution of fly ash for quartz in triaxial kaolin-quartz-feldspar system. J. Eur. Ceram. Soc. 2004, 24, 3169–3175. [Google Scholar] [CrossRef]
- Serra, M.F.; Conconi, M.S.; Suarez, G.; Aglietti, E.F.; Rendtorff, N.M. Volcanic ash as flux in clay based triaxial ceramic materials, effect of the firing temperature in phases and mechanical properties. Ceram. Int. 2015, 41, 6169–6177. [Google Scholar] [CrossRef]
- Bhattarai, J.; Okada, K. Characterization of clay raw materials in Nepal and their applicability for porcelain raw material. Clay Sci. 1992, 8, 393–402. [Google Scholar]
- Selli, N.T. Development of anorthite based white porcelain stoneware tile compositions. Ceram. Int. 2015, 41, 7790–7795. [Google Scholar] [CrossRef]
- Cheng, X.; Ke, S.; Wang, Q.; Wang, H.; Shui, A.; Liu, P. Characterization of transparent glaze for single-crystalline anorthite porcelain. Ceram. Int. 2012, 38, 4901–4908. [Google Scholar] [CrossRef]
- Ozturk, Z.B. Microstructural characterization of mullite and anorthite-based porcelain tile using regional clay. J. Ceram. Process. Res. 2016, 17, 555–559. [Google Scholar]
- Andreola, F.; Barbieri, L.; Corradi, A.; Lancellotti, I.; Manfredini, T. Utilisation of municipal incinerator grate slag for manufacturing porcelainized stoneware tiles manufacturing. J. Eur. Ceram. Soc. 2002, 22, 1457–1462. [Google Scholar] [CrossRef]
- Romero, M.; Martín, J.; Rincón, J.M. Kinetic of mullite formation from a porcelain stoneware body for tiles production. J. Eur. Ceram. Soc. 2006, 26, 1647–1652. [Google Scholar] [CrossRef]
- Martín, J.; Rincón, J.M.; Romero, M. Effect of firing temperature on sintering of porcelain stoneware tiles. Ceram. Int. 2008, 34, 1867–1873. [Google Scholar] [CrossRef] [Green Version]
- Martín, J.; Rincón, J.M.; Romero, M. Effect of microstructure on mechanical properties of porcelain stoneware. J. Eur. Ceram. Soc. 2010, 30, 3063–3069. [Google Scholar] [CrossRef] [Green Version]
- Martín, J.; De la Torre, A.G.; Aranda, M.A.; Rincón, J.M.; Romero, M. Evolution with temperature of crystalline and amorphous phases in porcelain stoneware. J. Am. Ceram. Soc. 2010, 92, 229–234. [Google Scholar] [CrossRef] [Green Version]
- Martín, J.; Rincón, J.M.; Romero, M. Mullite development on firing in porcelain stoneware bodies. J. Eur. Ceram. Soc. 2010, 30, 1599–1607. [Google Scholar] [CrossRef] [Green Version]
- Pérez, J.M.; Rincón, J.M.; Romero, M. Effect of moulding pressure on microstructure and technological properties of porcelain stoneware. Ceram. Int. 2012, 38, 317–332. [Google Scholar] [CrossRef] [Green Version]
- Pérez, J.M.; Romero, M. Microstructure and technological properties of porcelain stoneware tiles moulded at different pressures and thicknesses. Ceram. Int. 2014, 40, 1365–1377. [Google Scholar] [CrossRef] [Green Version]
- Kamseu, E.; Leonelli, C.; Boccaccini, D.N.; Veronesi, P.; Miselli, P.; Pellacani, G.; Melo, U.C. Characterisation of porcelain compositions using two china clays from Cameroon. Ceram. Int. 2007, 33, 851–857. [Google Scholar] [CrossRef]
- Ferrari, S.; Gualtieri, A.F. The use of illitic clays in the production of stoneware tile ceramics. Appl. Clay Sci. 2006, 32, 73–81. [Google Scholar] [CrossRef]
- Mukhopadhyay, T.K.; Ghatak, S.; Maiti, H.S. Effect of pyrophyllite on the mullitization in triaxial porcelain system. Ceram. Int. 2009, 35, 1493–1500. [Google Scholar] [CrossRef]
- Sokolář, R.; Keršnerová, L.; Šveda, M. The effect of different fluxing agents on the sintering of dry pressed porcelain bodies. J. Asian Ceram. Soc. 2017, 5, 290–294. [Google Scholar] [CrossRef] [Green Version]
- Frizzo, G.R.; Zaccaron, A.; de Souza Nandi, V.; Bernardin, A.M. Pyroplasticity on porcelain tiles of the albite-potassium feldspar-kaolin system: A mixture design analysis. J. Build. Eng. 2020, 31, 101432. [Google Scholar] [CrossRef]
- Kamseu, E.; Bakop, T.; Djangang, C.; Melo, U.C.; Hanuskova, M.; Leonelli, C. Porcelain stoneware with pegmatite and nepheline syenite solid solutions: Pore size distribution and descriptive microstructure. J. Eur. Ceram. Soc. 2013, 33, 2775–2784. [Google Scholar] [CrossRef]
- Esposito, L.; Salem, A.; Tucci, A.; Gualtieri, A.; Jazayeri, S.H. The use of nepheline-syenite in a body mix for porcelain stoneware tiles. Ceram. Int. 2005, 31, 233–240. [Google Scholar]
- Bragança, S.R.; Lengler, H.C.M.; Bergmann, C.P. Spodumene-bearing rock as flux for triaxial ceramic bodies. Adv. Appl. Ceram. 2011, 110, 293–300. [Google Scholar]
- Azarov, G.M.; Vlasov, A.S.; Maiorova, E.V.; Oborina, M.A. Diopside: Raw material for porcelain production. Glass Ceram. 1995, 52, 216–218. [Google Scholar]
- Magagnin, D.; Santos, C.M.F.D.; Wanderlind, A.; Jiusti, J.; De Noni, A., Jr. Effect of kaolinite, illite and talc on the processing properties and mullite content of porcelain stoneware tiles. Mater. Sci. Eng. A 2014, 618, 533–539. [Google Scholar]
- Deng, T.; Wang, Y.; Dufresne, A.; Lin, N. Simultaneous enhancement of elasticity and strength of Al2O3-based ceramics body from cellulose nanocrystals via gel-casting process. Carbohydr. Polym. 2018, 181, 111–118. [Google Scholar]
- Lee, H.J.; Park, H.Y.; Kim, E.H.; Choi, H.H.; Jin, J.; Choi, J.; Yang, S.; Jung, Y.G. Relationship between mechanical properties of ceramic green body and structures of photo-cured acrylate polymer for ceramic 3D printing based on photo polymerization. Ceram. Int. 2021, 47, 3867–3875. [Google Scholar] [CrossRef]
- Zhao, L.H.; Wei, W.; Bai, H.; Zhang, X.; Cang, D.Q. Synthesis of steel slag ceramics: Chemical composition and crystalline phases of raw materials. Int. J. Miner. Metall. Mater. 2015, 22, 325–333. [Google Scholar] [CrossRef] [Green Version]
- Tarhan, B.; Tarhan, M.; Aydin, T. Reusing sanitaryware waste products in glazed porcelain tile production. Ceram. Int. 2017, 43, 3107–3112. [Google Scholar] [CrossRef]
- Filho, J.E.S.; Aurich, J.C.; Sousa, F.J.P.; Nascimento, R.M.; Paskocimas, C.A.; Silva, A.H.A. Polishing performance of eco-friendly porcelain stoneware tiles reusing bricks and roof tiles wastes. J. Cleaner Prod. 2020, 256, 120362. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Y.; Chen, T.; Liu, T.; Huang, J. Preparation and characterization of red porcelain tiles with hematite tailings. Constr. Build. Mater. 2013, 38, 1083–1088. [Google Scholar] [CrossRef]
- Yang, H.; Chen, C.; Pan, L.; Lu, H.; Sun, H.; Hu, X. Preparation of double-layer glass-ceramic/ceramic tile from bauxite tailings and red mud. J. Eur. Ceram. Soc. 2009, 29, 1887–1894. [Google Scholar] [CrossRef]
- Dana, K.; Dey, J.; Das, S.K. Synergistic effect of fly ash and blast furnace slag on the mechanical strength of traditional porcelain tiles. Ceram. Int. 2005, 31, 147–152. [Google Scholar] [CrossRef]
- Wei, Q.; Gao, W.; Sui, X. Synthesis of low-temperature, fast, single-firing body for porcelain stoneware tiles with coal gangue. Waste Manage. Res. 2010, 28, 944–950. [Google Scholar]
- Taskiran, M.U.; Demirkol, N.; Capoglu, A. A new porcelainised stoneware material based on anorthite. J. Eur. Ceram. Soc. 2005, 25, 293–300. [Google Scholar] [CrossRef]
- Taskiran, M.U.; Demirkol, N.; Capoglu, A. Influence of mixing/milling on sintering and technological properties of anorthite based porcelainised stoneware. Ceram. Int. 2006, 32, 325–330. [Google Scholar] [CrossRef]
- Capoglu, A. A novel low-clay translucent whiteware based on anorthite. J. Eur. Ceram. Soc. 2011, 31, 321–329. [Google Scholar] [CrossRef]
- Yildirim, H.; Azakli, Y.; Tarakci, M.; Capoglu, A. The effect of surface polishing on the flexural strength of anorthite-based porcelainised stoneware. Acta Phys. Pol. A 2015, 127, 1336–1341. [Google Scholar] [CrossRef]
- Montanaro, L.; Perrot, C.; Esnouf, C.; Thollet, G.; Fantozzi, G.; Negro, A. Sintering of industrial mullites in the presence of magnesia as a sintering aid. J. Am. Ceram. Soc. 2000, 83, 89–96. [Google Scholar] [CrossRef]
- Kurama, S.; Ozel, E. The influence of different CaO source in the production of anorthite ceramics. Ceram. Int. 2009, 35, 827–830. [Google Scholar] [CrossRef]
- Ke, S.; Cheng, X.; Wang, Y.; Wang, Q.; Wang, H. Dolomite, wollastonite and calcite as different CaO sources in anorthite-based porcelain. Ceram. Int. 2013, 39, 4953–4960. [Google Scholar] [CrossRef]
- Ibañez, A.; Sandoval, F. Wollastonite: Properties, synthesis and ceramic uses. Bol. Soc. Esp. Ceram. Vidr. 1993, 32, 349–361. [Google Scholar]
- Sletson, L.C.; Reed, J.S. Microstructure development in a vitrified anorthite porcelain. Am. Ceram. Soc. Bull. 1988, 67, 1403–1408. [Google Scholar]
- Tai, W.P.; Kimura, K.; Jinnai, K. A new approach to anorthite porcelain bodies using nonplastic raw materials. J. Eur. Ceram. Soc. 2002, 22, 463–470. [Google Scholar] [CrossRef]
- Wu, J.F.; Li, K.; Xu, X.H.; Zhang, Y.X.; Xu, X.Y.; Lao, X.B. White porcelain material based on diopside. Int. J. Appl. Ceram. Technol. 2017, 14, 454–560. [Google Scholar]
- Tarhan, M. Whiteness improvement of porcelain tiles incorporated with anorthite and diopside phases. J. Therm. Anal. Calorim. 2019, 138, 929–936. [Google Scholar] [CrossRef]
- Hatch, D.M.; Ghose, S. The α-β phase transition in cristobalite, SiO2. Phys. Chem. Miner. 1991, 17, 554–562. [Google Scholar] [CrossRef]
- Lee, W.E.; Souza, G.P.; McConville, C.J.; Tarvornpanich, T.; Iqbal, Y. Mullite formation in clays and clay-derived vitreous ceramics. J. Eur. Ceram. Soc. 2008, 28, 465–471. [Google Scholar]
- dos Santos Conserva, L.R.; Melchiades, F.G.; Nastri, S.; Boschi, A.O.; Dondi, M.; Guarini, G.; Raimondo, M.; Zanelli, C. Pyroplastic deformation of porcelain stoneware tiles: Wet vs. dry processing. J. Eur. Ceram. Soc. 2017, 37, 333–342. [Google Scholar]
- Melchiades, F.G.; Daros, M.T.; Boschi, A.O. Porcelain tiles by the dry route. Bol. Soc. Esp. Ceram. Vidr. 2010, 49, 221–226. [Google Scholar]
- Mezquita, A.; Monfort, E.; Ferrer, S.; Gabaldón, D. How to reduce energy and water consumption in the preparation of raw materials for ceramic tile manufacturing: Dry versus wet route. J. Cleaner Prod. 2017, 168, 1566–1570. [Google Scholar]
- Soldati, R.; Zanelli, C.; Guarini, G.; Fazio, S.; Bignozzi, M.C.; Dondi, M. Characteristics and rheological behaviour of spray-dried powders for porcelain stoneware slabs. J. Eur. Ceram. Soc. 2018, 38, 4118–4126. [Google Scholar]
- Minlheiro, F.A.C.; Freire, M.N.; Silva, A.G.P.; Holanda, J.N.F. Densification behaviour of red firing Brazilian kaolinitic clay. Ceram. Int. 2005, 31, 757–763. [Google Scholar] [CrossRef]
- Dondi, M.; Ercolani, G.; Melandri, C.; Mingazzini, C.; Marsigli, M. The chemical composition of porcelain stoneware tiles and its influence on microstructural and mechanical properties. Interceram 1999, 48, 75–83. [Google Scholar]
- Abdullayev, A.; Klimm, D.; Kamutzki, F.; Gurlo, A.; Bekheet, M.F. AlF3–assisted flux growth of mullite whiskers and their application in fabrication of porous mullite-alumina monolith. Open Ceram. 2021, 7, 100145. [Google Scholar] [CrossRef]
- Carty, W.M.; Senapati, U. Porcelain—Raw materials, processing, phase evolution, and mechanical behavior. J. Am. Ceram. Soc. 1998, 81, 3–20. [Google Scholar] [CrossRef]
- Iqbal, Y.; Lee, W.E. Microstructural evolution in triaxial porcelain. J. Am. Ceram. Soc. 2000, 832, 3121–3127. [Google Scholar]
- Schuller, K.H. Reactions between mullite and glassy phase in porcelains. Trans. Br. Ceram. Soc. 1964, 64, 103–117. [Google Scholar]
- Lecomte, G.; Pateyron, B.; Blanchart, P. Experimental study and simulation of a vertical section mullite-ternary eutectic (985 °C) in the SiO2–Al2O3–K2O system. Mater. Res. Bull. 2004, 39, 1469–1478. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Kato, E. Low—Temperature fabrication of anorthite ceramics. J. Am. Ceram. Soc. 1994, 77, 833–834. [Google Scholar]
- Kenzour, A.; Belhouchet, H.; Kolli, M.; Djouallah, S.; Ramesh, S. Sintering behavior of anorthite-based composite ceramics produced from natural phosphate and kaolin. Ceram. Int. 2019, 45, 20258–20265. [Google Scholar]
- Qin, J.; Cui, C.; Cui, X.; Hussain, A.; Yang, C.; Yang, S. Recycling of lime mud and fly ash for fabrication of anorthite ceramic at low sintering temperature. Ceram. Int. 2015, 41, 5648–5655. [Google Scholar] [CrossRef]
- Brasileiro, C.T.; Conte, S.; Contartesi, F.; Melchiades, F.G.; Zanelli, C.; Dondi, M.; Boschi, A.O. Effect of strong mineral fluxes on sintering of porcelain stoneware tiles. J. Eur. Ceram. Soc. 2021, 41, 5755–5767. [Google Scholar] [CrossRef]
- Yoon, Y.G.; Car, R.; Srolovitz, D.J.; Scandolo, S. Thermal conductivity of crystalline quartz from classical simulations. Phys. Rev. B 2004, 70, 2199–2208. [Google Scholar] [CrossRef] [Green Version]
- Ackermann, R.J.; Sorrell, C.A. Thermal expansion and the high–low transformation in quartz. I. High-temperature X-ray studies. J. Appl. Crystallogr. 1974, 7, 461–467. [Google Scholar] [CrossRef] [Green Version]
- Schneider, H.; Eberhard, E. Thermal expansion of mullite. J. Am. Ceram. Soc. 1990, 73, 2073–2076. [Google Scholar] [CrossRef]
- Aldebert, P.; Traverse, J.P. αAl2O3: A high-temperature thermal expansion standard. High Temp. High Press. 1984, 16, 127–135. [Google Scholar]
- Horai, K.I.; Simmons, G. Thermal conductivity of rock-forming minerals. Earth Planet. Sci. Lett. 1969, 6, 359–368. [Google Scholar]
- Stewart, D.B.; Von Limbach, D. Thermal expansion of low and high albite. Am. Mineral. 1967, 52, 389–413. [Google Scholar]
- Hovis, G.L.; Medford, A.; Conlon, M.; Tether, A.; Romanoski, A. Principles of thermal expansion in the feldspar system. Am. Mineral. 2010, 95, 1060–1068. [Google Scholar]
- Taylor, D.; Henderson, C.M.B. The thermal expansion of the leucite group of minerals. Am. Mineral. 1968, 53, 1476–1489. [Google Scholar]
- Pal, M.; Das, S.; Das, S.K. Anorthite porcelain: Synthesis, phase and microstructural evolution. Bull. Mater. Sci. 2015, 38, 551–555. [Google Scholar]
- Cukierman, M.; Uhlmann, D.R. Viscosity of liquid anorthite. J. Geophys. Res. 1973, 78, 4920–4923. [Google Scholar]
- Barbieri, L.; Bondioli, F.; Lancellotti, I.; Leonelli, C.; Montorsi, M.; Ferrari, A.M.; Miselli, P. The anorthite-diopside system: Structural and devitrification study. Part II: Crystallinity analysis by the rietveld-RIR method. J. Am. Ceram. Soc. 2005, 88, 3131–3136. [Google Scholar]
- Ikawa, H.; Otagiri, T.; Imai, O.; Suzuki, M.; Urabe, K.; Udagawa, S. Crystal structures and mechanism of thermal expansion of high cordierite and its solid solutions. J. Am. Ceram. Soc. 1986, 69, 492–498. [Google Scholar] [CrossRef]
- Cameron, M.; Sueno, S.; Prewitt, C.T.; Papike, J.J. High-temperature crystal chemistry of acmite, diopside, hedenbergite jadeite, spodumene and ureyite. Am. Mineral. 1973, 58, 594–618. [Google Scholar]
- Sánchez, E.; Ibáñez, M.J.; García-Ten, F.J.; Quereda, M.F.; Xu, Y.M.; Hutchings, I.M. Porcelain tile microstructure: Implications for polished tile properties. J. Eur. Ceram. Soc. 2006, 26, 2533–2540. [Google Scholar]
- Gültekin, E.E.E. The effects of heating rate and sintering temperature on the strength, firing shrinkage, and bulk density of porcelain tiles. J. Aust. Ceram. Soc. 2018, 54, 39–46. [Google Scholar] [CrossRef]
- De Noni, A., Jr.; Hotza, D.; Soler, V.C.; Vilches, E.S. Influence of composition on mechanical behaviour of porcelain tile. Part I: Microstructural characterization and developed phases after firing. Mater. Sci. Eng. A 2010, 527, 1730–1735. [Google Scholar]
- Matthew, G.O.; Fatile, B.O. Characterization of vitrified porcelain tiles using feldspar from three selected deposits in Nigeria. Res. J. Recent Sci. 2014, 3, 67–72. [Google Scholar]
- Cheng, X.S.; Ke, S.J.; Wang, Q.H.; Wang, H.; Shui, A.Z.; Liu, P.A. Fabrication and characterization of anorthite-based ceramic using mineral rawmaterials. Ceram. Int. 2012, 38, 3227–3235. [Google Scholar] [CrossRef]
- Dana, K.; Das, S.K. Partial substitution of feldspar by B.F. slag in triaxial porcelain: Phase andmicrostructural evolution. J. Eur. Ceram. Soc. 2004, 25, 3833–3839. [Google Scholar] [CrossRef]
- Manfredini, T.; Romagnoli, M.; Hanuskova, M. Wollastonite as sintering aid for porcelain tile bodies. Int. Ceram. J. 2000, 61–67. [Google Scholar]
- Wang, S.; Qi, X.; Hu, J.; Tian, X. Characterization of anorthite-based porcelain prepared by using wollastonite as a calcium source. J. Ceram. Process. Res. 2015, 16, 361–365. [Google Scholar]
- Darolt, R.D. Estudo do Efeito da Moagem de Alta Energia no Comportamento Mecânico de Porcelanato. Master’s Thesis, Universidade do Extremo Sul Catarinense, Criciúma, Brazil, 2019. [Google Scholar]
- Bragança, S.R.; Bergmann, C.P. A view of whitewares mechanical strength and microstructure. Ceram. Int. 2003, 29, 801–806. [Google Scholar] [CrossRef]
- De Noni, A., Jr.; Hotza, D.; Soler, V.C.; Vilches, E.S. Analysis of the development of microscopic residual stresses on quartz particles in porcelain tile. J. Eur. Ceram. Soc. 2008, 28, 2629–2637. [Google Scholar] [CrossRef]
- Ece, O.I.; Nakagawa, Z.E. Bending strength of porcelains. Ceram. Int. 2002, 28, 131–140. [Google Scholar] [CrossRef]
- Selsing, J. Internal stresses in ceramics. J. Am. Ceram. Soc. 1961, 44, 419. [Google Scholar] [CrossRef]
- De Noni, A., Jr.; Hotza, D.; Soler, V.C.; Vilches, E.S. Influence of composition on mechanical behaviour of porcelain tile. Part II: Mechanical properties and microscopic residual stress. Mater. Sci. Eng. A 2010, 527, 1736–1743. [Google Scholar] [CrossRef]
- De Oliveira, A.P.N.; Vilches, E.S.; Soler, V.C.; Villegas, F.A.G. Relationship between Young′s modulus and temperature in porcelain tiles. J. Eur. Ceram. Soc. 2012, 32, 2853–2858. [Google Scholar]
- De Noni, A., Jr.; Hotza, D.; Soler, V.C.; Vilches, E.S. Influence of macroscopic residual stresses on the mechanical behavior and microstructure of porcelain tile. J. Eur. Ceram. Soc. 2008, 28, 2463–2469. [Google Scholar]
- De Noni, A., Jr.; Hotza, D.; Soler, V.C.; Vilches, E.S. Influence of composition on mechanical behaviour of porcelain tile. Part III: Effect of the cooling rate of the firing cycle. Mater. Sci. Eng. A 2011, 528, 3330–3336. [Google Scholar] [CrossRef]
| Name | SiO2 | Al2O3 | Fe2O3 | TiO2 | CaO | MgO | K2O | Na2O | I.L | Major Phase |
|---|---|---|---|---|---|---|---|---|---|---|
| MPT | 65.48 | 22.06 | 0.50 | 0.28 | 0.31 | 0.23 | 2.6 | 3.37 | 5.17 | Kaolin, feldspar, Quartz |
| APT | 42.59 | 37.77 | 0.24 | 0.21 | 11.27 | 2.49 | 0.41 | 0.40 | 4.62 | Wollastonite, Kaolin, Corundum, Quartz |
| Phase | Chemical Composition | Density (g/cm3) | Refractive Index | Thermal Conductivity ([W/(m·K)]/K) | Volume Thermal Expansion Coefficient (10−6/°C)/(°C) | Melting Point (°C) |
| α-Quartz | SiO2 | 2.53 | 1.54 | 3.7–14.0/500–800 [67] | 23.8–86.0/298–773 [68] | 1710 |
| β-Quartz | SiO2 | 2.65 | 1.54 | 4.1–4.8/900–1100 [67] | nearly 0/575–1100 [68] | 1710 |
| Mullite | 3Al2O3·2SiO2 | 3.16 | 1.64 [49] | 6.0 | ~16.7/300–900 [69] | 1850 |
| Corundum | Al2O3 | 3.95 | 1.76 | 35 | 22.9–32.4/20–2025 [70] | 2050 |
| Albite | Na2O·Al2O3·6SiO2 | 2.61 | 1.53 | 2.3 [71] | 21.6–37.3/25–1200 [72] | 1118 |
| Microcline | K2O·Al2O3·6SiO2 | 2.54 | 1.52 | 2.4 [71] | ~13/100–1000 [73] | 1290 |
| Leucite | K2O·Al2O3·4SiO2 | 2.45 | 1.51 | 1.1 [71] | 22–30/25–1000 [74] | 1120 |
| Anorthite | CaO·Al2O3·2SiO2 | 2.75 [75] | 1.58 [75] | 1.7 [71] | 14–60/200–900 [76] | 1550 [77] |
| Cordierite | 2MgO·2Al2O3·5SiO2 | 2.61 | 1.54 | 2.7 [71] | 2.6/25–600 [78] | 1460 |
| Diopside | CaO·MgO·2SiO2 | 3.27 | 1.68 | 5.6 [71] | 33.3/24–1000 [79] | 1330 [77] |
| Items | MPT | APT | |
|---|---|---|---|
| Composition | Main mineralogical components | Clay, feldspar, quartz | Wollastonite, clay, alumina, quartz |
| Main oxides components | SiO2, Al2O3, K2O, Na2O | SiO2, Al2O3, CaO, MgO | |
| Phase diagram attribution | SiO2-Al2O3-K2O ternary system | SiO2-Al2O3-CaO ternary system | |
| Processing | Particle size | d50 5–6 μm, Fine/Coarse is 1:10 | |
| Powder moisture | 5–7% | ||
| Forming pressure | 35–45 MPa | ||
| Sintering temperature | 1180–1220 °C | 1120–1230 °C | |
| Sintering temperature range | Wide (40 °C or wider) | Narrow (30 °C or narrower) | |
| Holding time | 4–6 min | >15 min | |
| Cold-to-cold time | 35–60 min | ||
| Final composition and properties | Phase composition | 20–25% α-quartz, 12–16% mullite, balanced by amorphous phase | 52% anorthite, 12% corundum, 8% cristobalite, and 28% glassy phases |
| Microstructure | Glassy matrix embedded with quartz, mullite phase; secondary mullite acts as reinforcing phase | Glassy matrix embedded with anorthite phase; corundum phase acts as reinforcing phase | |
| Ratio crystalline: amorphous phases | ~1:2 | ~3:1 | |
| Mechanical strength | ~55 MPa | ~110 MPa | |
| Whiteness | ~80 | ~92 | |
| Total evaluation | Wider sintering temperature range, medium strength, medium comprehensive performances, small- to large-scale production | Narrower sintering temperature range, good strength: good comprehensive performances, still restricted in large-scale production | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, K.; Cordeiro, E.d.S.; De Noni, A., Jr. Comparison between Mullite-Based and Anorthite-Based Porcelain Tiles: A Review. Eng 2023, 4, 2153-2166. https://doi.org/10.3390/eng4030123
Li K, Cordeiro EdS, De Noni A Jr. Comparison between Mullite-Based and Anorthite-Based Porcelain Tiles: A Review. Eng. 2023; 4(3):2153-2166. https://doi.org/10.3390/eng4030123
Chicago/Turabian StyleLi, Kun, Eloise de Sousa Cordeiro, and Agenor De Noni, Jr. 2023. "Comparison between Mullite-Based and Anorthite-Based Porcelain Tiles: A Review" Eng 4, no. 3: 2153-2166. https://doi.org/10.3390/eng4030123
APA StyleLi, K., Cordeiro, E. d. S., & De Noni, A., Jr. (2023). Comparison between Mullite-Based and Anorthite-Based Porcelain Tiles: A Review. Eng, 4(3), 2153-2166. https://doi.org/10.3390/eng4030123












