SCC Susceptibility of Polystyrene/TiO2 Nanocomposite-Coated Thin-Sheet Aluminum Alloy 2024—T3 in 3.5% NaCl
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimen
2.2. Coating
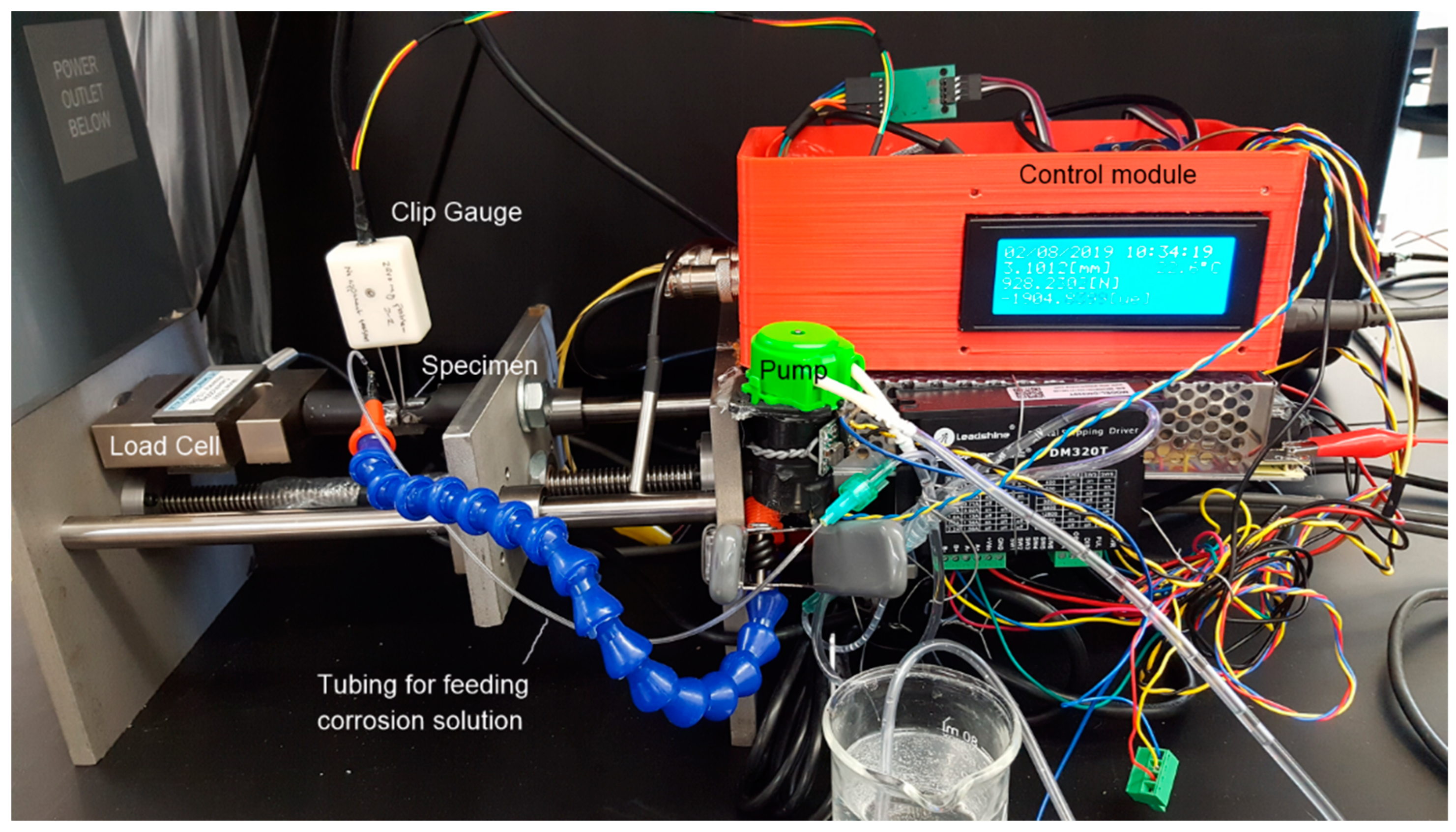
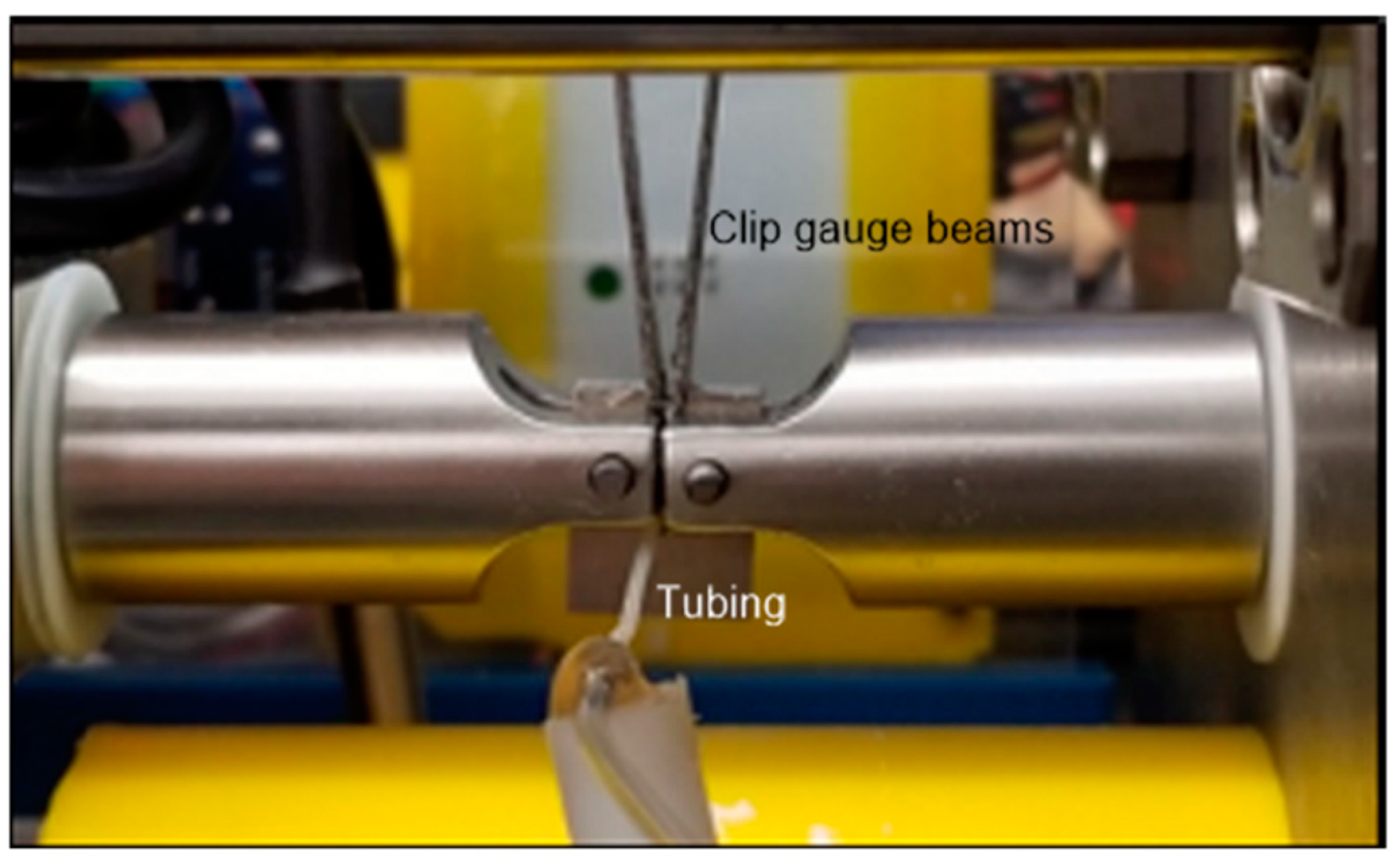
2.3. Instrument for SCC Testing
2.4. Testing Procedure
3. Results
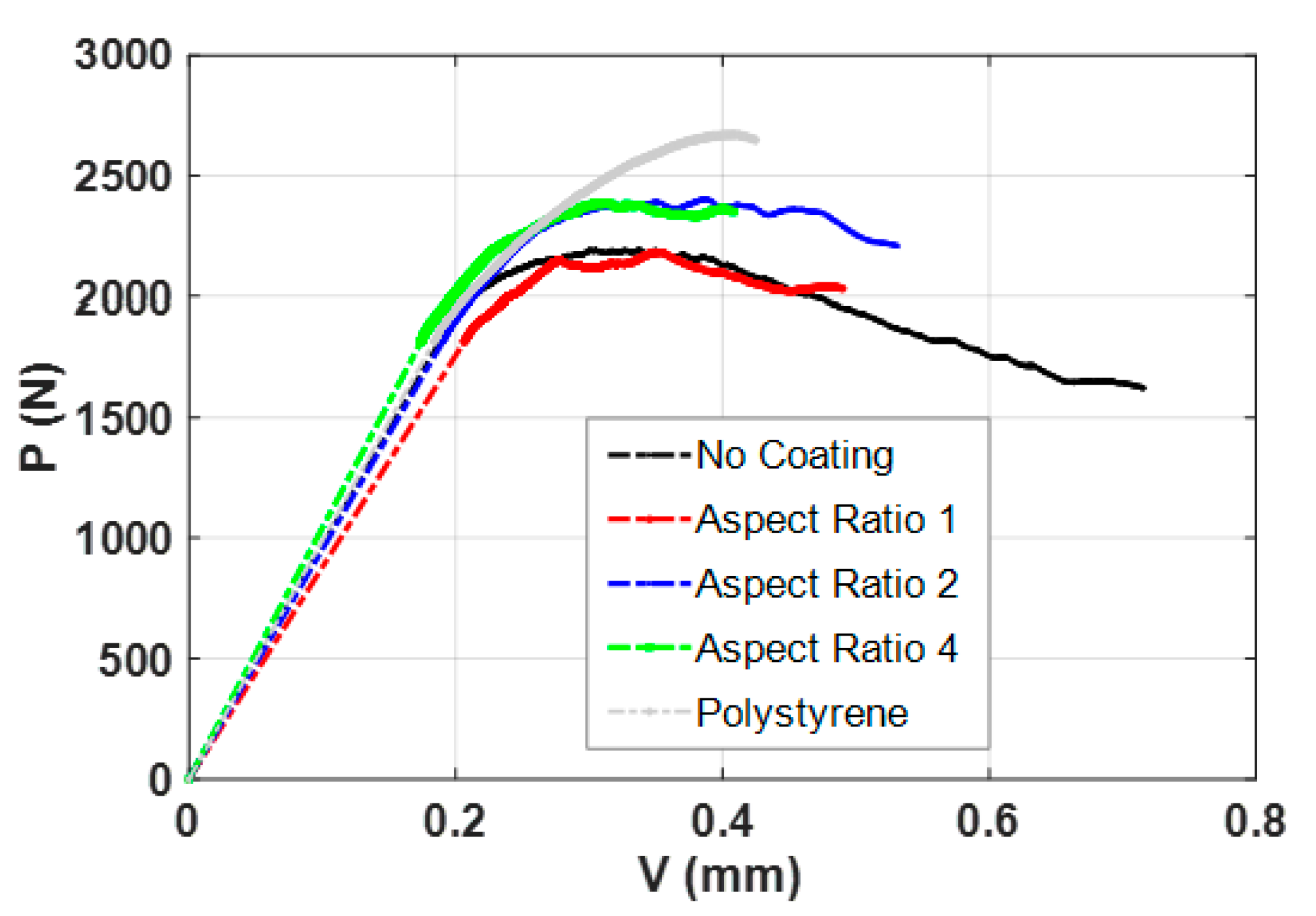
3.1. Mechanical Strength and Ductility
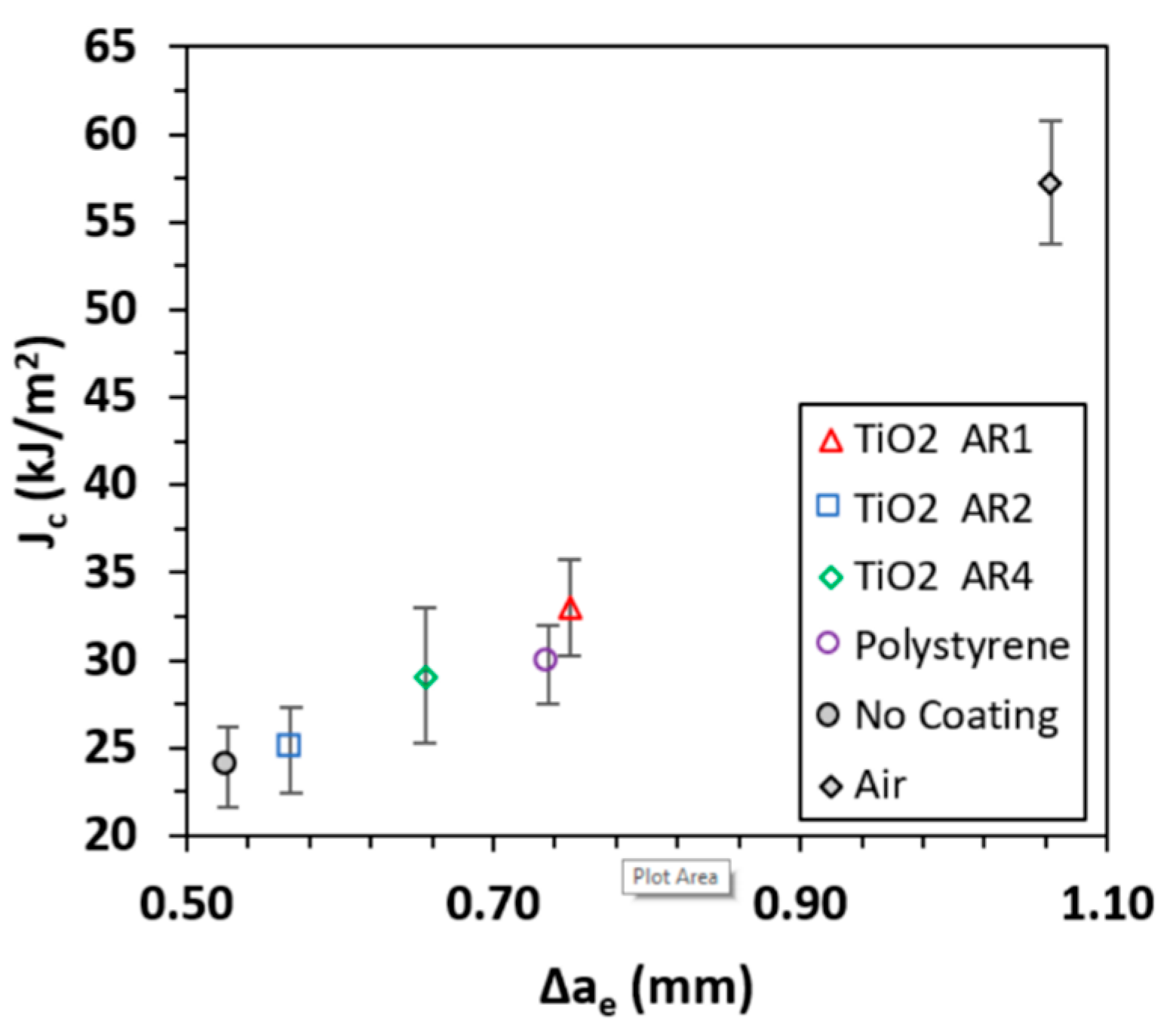
3.2. Critical J-Integral and Critical Fracture Toughness
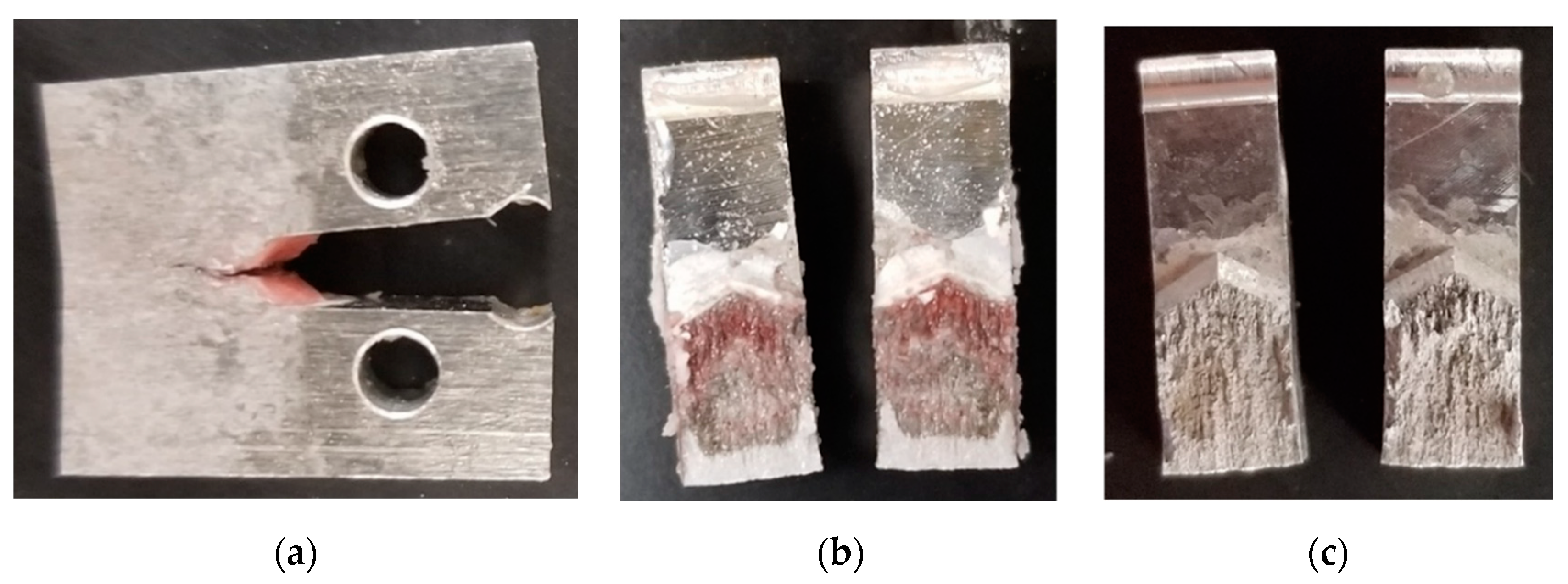
3.3. Characterization of Cracking in SCC
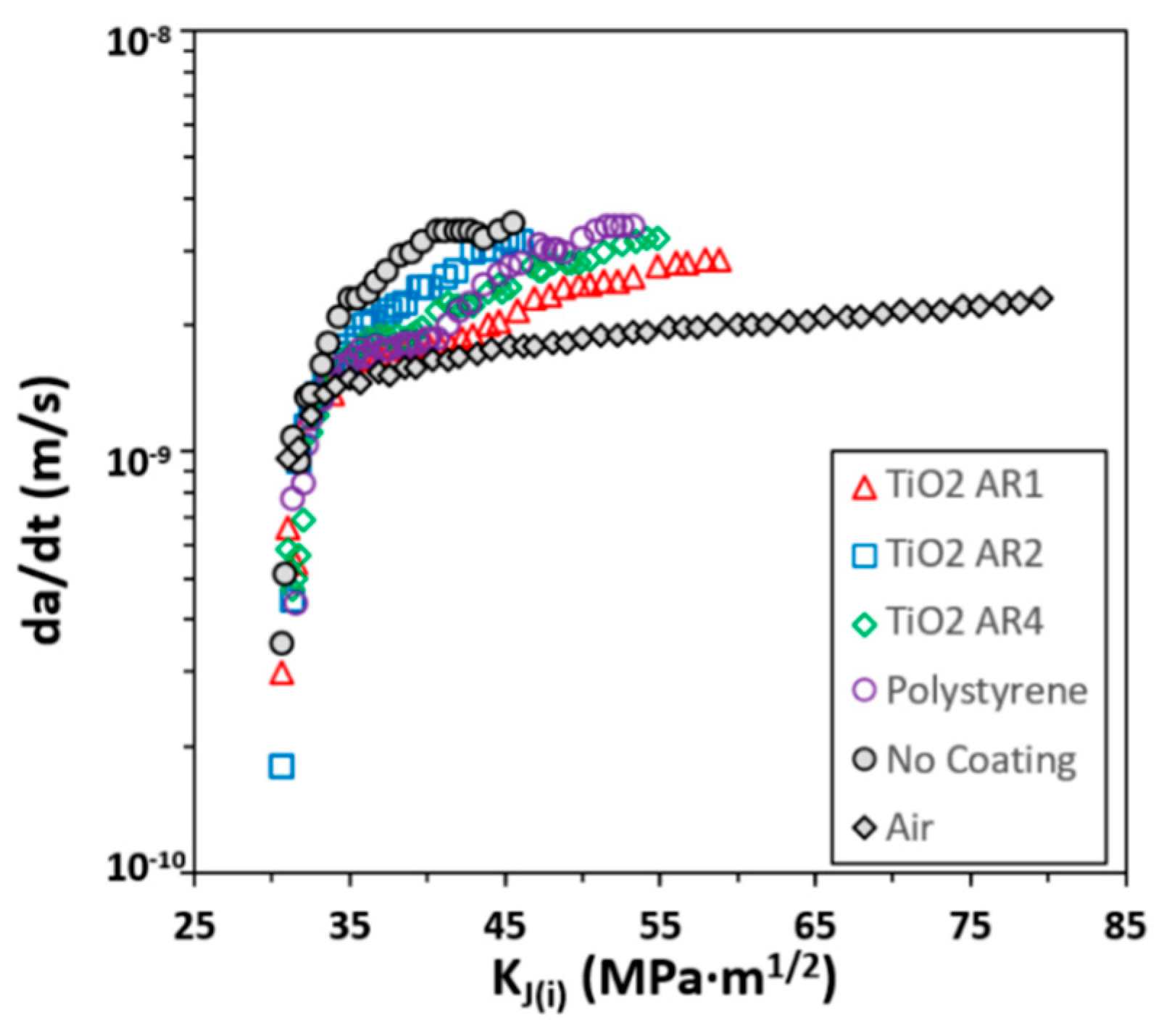
- Crack initiation (Phase I): This phase corresponds to the regime in Figure 8, where the data points are vertically distributed, and the cracking rate is below 10−9 m/s. In this phase, cracking occurs in a presumptively intact specimen only when the stress intensity () is over a critical threshold, which is about 30–32 MPa·m1/2 among the different coatings. The crack growth rate is extremely slow (~10−10 m/s), showing minimal or nearly stagnant crack propagation. However, the cracking rate increases significantly, though still insignificant, as rises to 35 MPa·m1/2. The PS/TiO2 AR2-coated specimens exhibited the smallest value of the critical threshold of , meaning the least resistance to crack initiation.
- Stable cracking (Phase II): This phase occurs between the stress intensity of 35–55 MPa·m1/2, where the cracking rate stabilizes with a less gradual increase over in Figure 8. The plateau-like trend in the cracking rate indicates that the crack propagation is proportional to time, implying stable cracking. The variation among different coatings indicates their different influences on crack resistance in this regime. A steeper rise in in this phase means a quicker transition to unstable fracture. The graph of the PS/TiO2 AR1-coated specimen exhibits the least slope in vs. , showing the most effective in delaying crack growth. A transition from nearly stagnant cracking (Phase I) to stable crack growth (Phase II) suggests a SCC behavior [5].
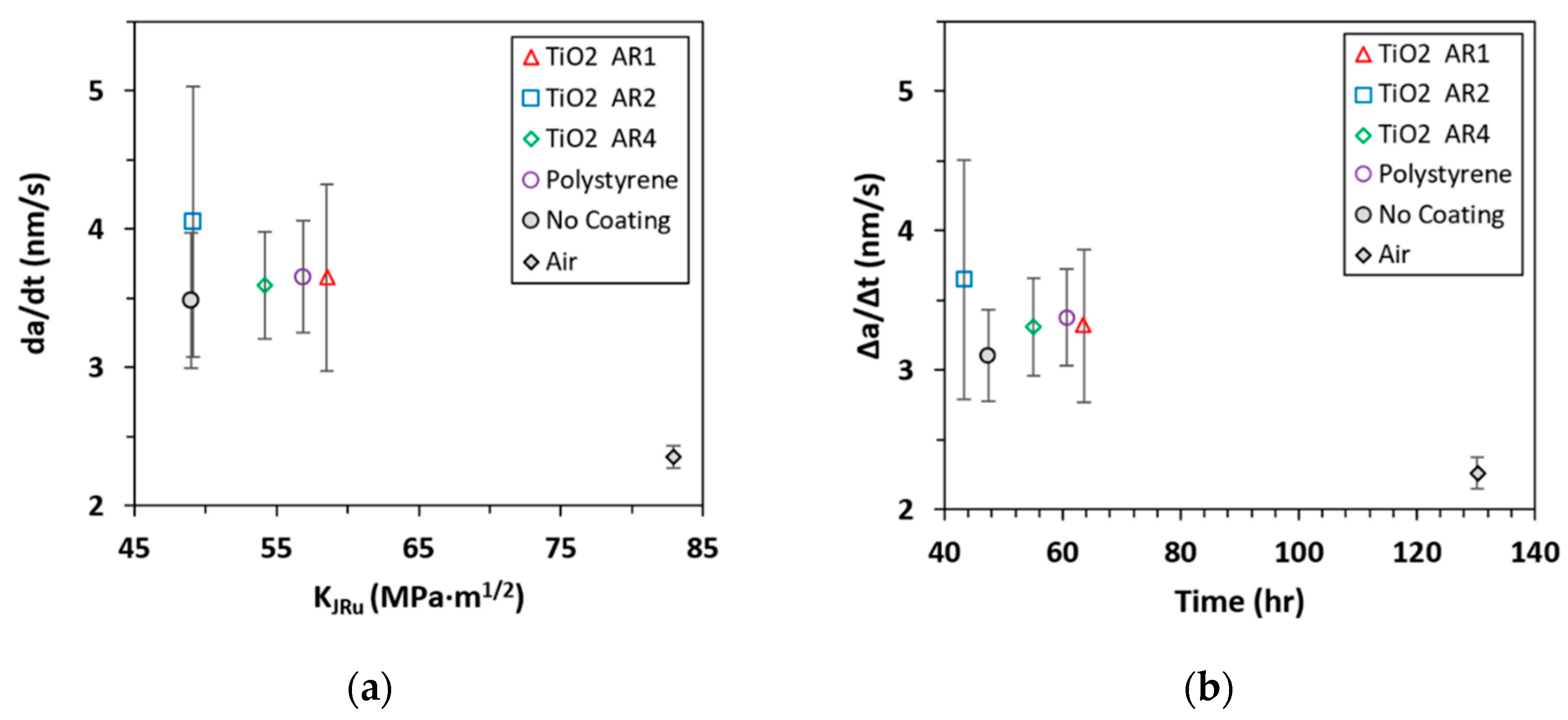
- The largest value in Phase II marks the onset of cracking instability. As increases over 55–60 MPa·m1/2, cracking becomes unstable and rapidly progresses toward rupture. The instantaneous cracking rate at the onset of cracking instability was calculated and plotted against in Figure 9a. It shows that the magnitudes of for all the coatings are statistically close, but the PS/TiO2 AR1-coated has the largest stress intensity (about 58 MPa·m1/2), meaning it is the most resistant to cracking instability. The average cracking rate , which was calculated from the change in the crack length and the total time-to-reach-, was plotted against the total time in Figure 9b. This shows that the PS/TiO2 AR1-coated has the longest deferral of unstable cracking [5].
3.4. SCC Indecies
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. The Basic J-Integral Method
Appendix B. The Incremental J-Integral Method
References
- ASM Handbook Series, Volume 04—Heat Treating. In 63 Heat Treating of Aluminum Alloys; ASM International Handbook Committee: Materials Park, OH, USA, 1991; pp. 841–879.
- Guillaumin, V.; Mankowski, G. Localized Corrosion of 2024 T351 Aluminium Alloy in Chloride Media. Corros. Sci. 1998, 41, 421–438. [Google Scholar] [CrossRef]
- Zhang, W.; Frankel, G.S. Transitions between Pitting and Intergranular Corrosion in AA2024. Electrochim. Acta 2003, 48, 1193–1210. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, X.; Hashimoto, T.; Liu, B. Localized Corrosion in AA2024-T351 Aluminium Alloy: Transition from Intergranular Corrosion to Crystallographic Pitting. Mater. Charact. 2017, 130, 230–236. [Google Scholar] [CrossRef]
- Bobby Kannan, M.; Bala Srinivasan, P. Stress Corrosion Cracking (SCC) of Aluminium Alloys. In Stress Corrosion Cracking: Theory and Practice; Raja, V.S., Shoji, T., Eds.; Elsevier: Cambridge, UK, 2011; pp. 307–340. [Google Scholar]
- Buchheit, R.G.; Grant, R.P.; Hlava, P.F.; Mckenzie, B.; Zender, G.L. Local Dissolution Phenomena Associated with S Phase (Al2CuMg) Particles in Aluminum Alloy 2024-T3. J. Electrochem. Soc. 1997, 144, 2621. [Google Scholar] [CrossRef]
- de Bonfils-Lahovary, M.L.; Laffont, L.; Blanc, C. Characterization of Intergranular Corrosion Defects in a 2024 T351 Aluminium Alloy. Corros. Sci. 2017, 119, 60–67. [Google Scholar] [CrossRef]
- Jorcin, J.-B.; Blanc, C.; Pébère, N.; Tribollet, B.; Vivier, V. Galvanic Coupling Between Pure Copper and Pure Aluminum. J. Electrochem. Soc. 2008, 155, C46. [Google Scholar] [CrossRef]
- Guo, T.; Qiao, L.; Pang, X.; Volinsky, A.A. Brittle Film-Induced Cracking of Ductile Substrates. Acta Mater. 2015, 99, 273–280. [Google Scholar] [CrossRef]
- Becker, M. Chromate-Free Chemical Conversion Coatings for Aluminum Alloys. Corros. Rev. 2019, 37, 321–342. [Google Scholar] [CrossRef]
- Eichinger, E.; Osborne, J.; Van Cleave, T. Hexavalent Chromium Elimination: An Aerospace Industry Progress Report. Met. Finish. 1997, 95, 36–41. [Google Scholar] [CrossRef]
- de Wit, J.H.W.; van der Weijde, D.H.; Ferrari, G. Organic Coatings. In Corrosion Mechanisms in Theory and Practice, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2011; pp. 863–906. ISBN 9781420094633. [Google Scholar]
- Ramezanzadeh, B.; Niroumandrad, S.; Ahmadi, A.; Mahdavian, M.; Moghadam, M.H.M. Enhancement of Barrier and Corrosion Protection Performance of an Epoxy Coating through Wet Transfer of Amino Functionalized Graphene Oxide. Corros. Sci. 2016, 103, 283–304. [Google Scholar] [CrossRef]
- Wang, P. Aggregation of TiO2 Nanoparticles in Aqueous Media: Effects of PH, Ferric Ion and Humic Acid. Int. J. Environ. Sci. Nat. Res. 2017, 1, 157–162. [Google Scholar] [CrossRef]
- Kapridaki, C.; Maravelaki-Kalaitzaki, P. TiO2-SiO2-PDMS Nano-Composite Hydrophobic Coating with Self-Cleaning Properties for Marble Protection. Prog. Org. Coat. 2013, 76, 400–410. [Google Scholar] [CrossRef]
- Cui, X.; Zhu, G.; Pan, Y.; Shao, Q.; Zhao, C.; Dong, M.; Zhang, Y.; Guo, Z. Polydimethylsiloxane-Titania Nanocomposite Coating: Fabrication and Corrosion Resistance. Polymer 2018, 138, 203–210. [Google Scholar] [CrossRef]
- Nahrawy, A.M.E.; Haroun, A.A.; Hammad, A.B.A.; Diab, M.A.; Kamel, S. Uniformly Embedded Cellulose/Polypyrrole-TiO2 Composite in Sol-Gel Sodium Silicate Nanoparticles: Structural and Dielectric Properties. Silicon 2019, 11, 1063–1070. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, L. Polystyrene/TiO2 Nanocomposite Coatings To Inhibit Corrosion of Aluminum Alloy 2024-T3. ACS Appl. Nano Mater. 2019, 2, 6368–6377. [Google Scholar] [CrossRef]
- Doğru Mert, B. Corrosion Protection of Aluminum by Electrochemically Synthesized Composite Organic Coating. Corros. Sci. 2016, 103, 88–94. [Google Scholar] [CrossRef]
- Gonzalez, E.; Solis, R.; Muñoz, L. Sol-Gel Films: Corrosion Protection Coating for Aluminium Alloy. In Sol-Gel Method; Vejar, N., Ed.; IntechOpen: Rijeka, Croatia, 2019; pp. 75–96. ISBN 978-1-78985-334-6. [Google Scholar]
- Deshpande, P.P.; Jadhav, N.G.; Gelling, V.J.; Sazou, D. Conducting Polymers for Corrosion Protection: A Review. J. Coat. Technol. Res. 2014, 11, 473–494. [Google Scholar] [CrossRef]
- Saji, V.S. Review of Rare-Earth-Based Conversion Coatings for Magnesium and Its Alloys. J. Mater. Res. Technol. 2019, 8, 5012–5035. [Google Scholar] [CrossRef]
- Abrahami, S.T.; de Kok, J.M.M.; Gudla, V.C.; Ambat, R.; Terryn, H.; Mol, J.M.C. Interface Strength and Degradation of Adhesively Bonded Porous Aluminum Oxides. Npj Mater. Degrad. 2017, 1, 8. [Google Scholar] [CrossRef]
- Pletincx, S.; Fockaert, L.L.I.; Mol, J.M.C.; Hauffman, T.; Terryn, H. Probing the Formation and Degradation of Chemical Interactions from Model Molecule/Metal Oxide to Buried Polymer/Metal Oxide Interfaces. Npj Mater. Degrad. 2019, 3, 23. [Google Scholar] [CrossRef]
- Djozan, D.; Ebrahimi, B.; Mahkam, M.; Farajzadeh, M.A. Evaluation of a New Method for Chemical Coating of Aluminum Wire with Molecularly Imprinted Polymer Layer. Application for the Fabrication of Triazines Selective Solid-Phase Microextraction Fiber. Anal. Chim. Acta 2010, 674, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Mui, T.S.M.; Silva, L.L.G.; Prysiazhnyi, V.; Kostov, K.G. Surface Modification of Aluminium Alloys by Atmospheric Pressure Plasma Treatments for Enhancement of Their Adhesion Properties. Surf. Coat. Technol. 2017, 312, 32–36. [Google Scholar] [CrossRef]
- Abrahami, S.T.; Hauffman, T.; de Kok, J.M.M.; Mol, J.M.C.; Terryn, H. Effect of Anodic Aluminum Oxide Chemistry on Adhesive Bonding of Epoxy. J. Phys. Chem. C 2016, 120, 19670–19677. [Google Scholar] [CrossRef]
- Venables, J.D.; McNamara, D.K.; Chen, J.M.; Sun, T.S.; Hopping, R.L. Oxide Morphologies on Aluminum Prepared for Adhesive Bonding. Appl. Surf. Sci. 1979, 3, 88–98. [Google Scholar] [CrossRef]
- ASTM Standard E1820-18; Standard Test Method for Measurement of Fracture Toughness. ASTM International: West Conshohocken, PA, USA, 2018; pp. 1–55. [CrossRef]
- Sugimoto, T.; Zhou, X.; Muramatsu, A. Synthesis of Uniform Anatase TiO2 Nanoparticles by Gel–Sol Method: 3. Formation Process and Size Control. J. Colloid Interface Sci. 2003, 259, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.C.; Vasu, V.; Govindaraju, M.; Srinadh, K.V. Stress Corrosion Cracking Behaviour of 7xxx Aluminum Alloys: A Literature Review. Trans. Nonferrous Met. Soc. China 2016, 26, 1447–1471. [Google Scholar] [CrossRef]
- Pineau, A.; Benzerga, A.A.; Pardoen, T. Failure of Metals I: Brittle and Ductile Fracture. Acta Mater. 2016, 107, 424–483. [Google Scholar] [CrossRef]
- Braun, R. Transgranular Environment-Induced Cracking of 7050 Aluminium Alloy under Cyclic Loading Conditions at Low Frequencies. Int. J. Fatigue 2008, 30, 1827–1837. [Google Scholar] [CrossRef]
- Wanhill, R.; Byrnes, R.; Smith, C. Stress Corrosion Cracking (SCC) in Aerospace Vehicles. In Stress Corrosion Cracking Theory and Practice; Raja, V.S., Shoji, T., Eds.; Woodhead Publishing Limited: Cambridge, UK, 2008; pp. 608–650. [Google Scholar]
- Wouterse, A.; Williams, S.R.; Philipse, A.P. Effect of Particle Shape on the Density and Microstructure of Random Packings. J. Phys. Condens. Matter 2007, 19, 406215. [Google Scholar] [CrossRef]
- Chen, C.; Baart, B.V.; Zhang, J.; Zhang, L. Polystyrene/TiO2 Nanocomposite Coating for Strength and Toughness Enhancement of Aluminum Alloy 2024-T3 in Accelerated Stress Corrosion Cracking. Prog. Org. Coat. 2021, 161, 106458. [Google Scholar] [CrossRef]
- Saxena, A.; Hudak, S.J. Review and Extension of Compliance Information for Common Crack Growth Specimens. Int. J. Fract. 1978, 14, 453–468. [Google Scholar] [CrossRef]




| Others | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Al | Cr | Cu | Fe | Mg | Mn | Ni | Pb | Si | Sn | Ti | Zn | Each | Total |
| rest | max 0.1 | 3.8–4.9 | max 0.5 | 1.2–1.8 | 0.3–0.9 | 0.05 | 0.05 | max 0.5 | 0.05 | max 0.15 | max 0.25 | max 0.05 | max 0.15 |
| Coating | Composition (wt. %) | Size Distribution (nm) | |||
|---|---|---|---|---|---|
| PS | TiO2 | Acetone | Minor Axis | Major Axis | |
| PS | 15 | 0 | 85 | - | - |
| PS/TiO2 AR = 1 | 12.9 | 4.3 | 82.8 | 23 ± 3 | 29 ± 4 |
| PS/TiO2 AR = 2 | 12.9 | 4.3 | 82.8 | 29 ± 4 | 62 ± 10 |
| PS/TiO2 AR = 4 | 12.9 | 4.3 | 82.8 | 32 ± 6 | 122 ± 6 |
| Environment | Air | 3.5% wt. NaCl | 3.5% wt. NaCl | 3.5% wt. NaCl | 3.5% wt. NaCl | 3.5% wt. NaCl | |
|---|---|---|---|---|---|---|---|
| Coating | None | None | PS/TiO2 AR = 1 | PS/TiO2 AR = 2 | PS/TiO2 AR = 4 | PS | |
| (N) | Avg | 2813.6 | 2153.2 | 2252.8 | 2115.0 | 2210.3 | 2239.8 |
| 1σ | 6.6 | 68.4 | 115.9 | 44.5 | 46.8 | 59.0 | |
| (mm) | Avg | 0.405 | 0.232 | 0.280 | 0.229 | 0.256 | 0.271 |
| 1σ | 0.038 | 0.018 | 0.017 | 0.012 | 0.018 | 0.020 | |
| (h) | Avg | 130.2 | 47.4 | 63.6 | 43.3 | 55.1 | 60.6 |
| 1σ | 7.0 | 6.9 | 10.0 | 1.4 | 6.7 | 8.2 | |
| (mm) | Avg | 5.66 | 5.12 | 5.40 | 5.14 | 5.26 | 5.35 |
| 1σ | 0.16 | 0.15 | 0.16 | 0.14 | 0.13 | 0.10 | |
| (mm) | Avg | 1.06 | 0.53 | 0.75 | 0.57 | 0.66 | 0.74 |
| 1σ | 0.11 | 0.08 | 0.09 | 0.13 | 0.10 | 0.11 | |
| (nm/s) | Avg | 2.26 | 3.10 | 3.32 | 3.65 | 3.31 | 3.38 |
| 1σ | 0.11 | 0.33 | 0.55 | 0.86 | 0.35 | 0.35 | |
| (nm/s) | Avg | 2.35 | 3.49 | 3.65 | 4.06 | 3.59 | 3.65 |
| 1σ | 0.08 | 0.49 | 0.67 | 0.98 | 0.39 | 0.41 | |
| Stiffness (kN/mm) | Avg | 9.83 | 9.94 | 9.97 | 10.74 | 10.36 | 9.37 |
| 1σ | 0.44 | 1.14 | 0.65 | 0.82 | 0.37 | 0.70 | |
| (kJ/m2) | Avg | 57.25 | 23.93 | 33.02 | 24.88 | 29.12 | 29.77 |
| 1σ | 3.47 | 2.27 | 2.76 | 2.45 | 3.88 | 2.25 | |
| (kJ/m2) | Avg | 94.54 | 32.97 | 47.01 | 33.09 | 40.35 | 44.38 |
| 1σ | 14.82 | 3.99 | 4.81 | 3.56 | 5.72 | 5.79 | |
| (MPa·m1/2) | Avg | 82.91 | 49.00 | 58.53 | 49.10 | 54.18 | 56.84 |
| 1σ | 6.39 | 3.02 | 3.01 | 2.69 | 3.89 | 3.71 | |
| (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Uncoated, tested in air | Tested in 3.5% wt. NaCl | R | Uncoated | −23.5 | −42.6 | −63.6 | −35.4 | −40.9 | −50.4 |
| Uncoated, tested in 3.5% wt. NaCl | 1 | PS/TiO2 AR1 | 4.6 | 20.3 | 34.3 | 17.5 | 19.5 | 42.2 | |
| 2 | PS/TiO2 AR2 | −1.8 | −1.3 | −8.5 | 2.0 | 0.2 | 7.8 | ||
| 3 | PS/TiO2 AR4 | 2.7 | 10.1 | 16.3 | 10.2 | 10.6 | 24.4 | ||
| 4 | PS | 4.0 | 16.6 | 28.0 | 11.6 | 16.0 | 39.6 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.-f.; Baart, B.; Halford, J., IV; Zhang, J. SCC Susceptibility of Polystyrene/TiO2 Nanocomposite-Coated Thin-Sheet Aluminum Alloy 2024—T3 in 3.5% NaCl. Eng 2025, 6, 83. https://doi.org/10.3390/eng6040083
Chen C-f, Baart B, Halford J IV, Zhang J. SCC Susceptibility of Polystyrene/TiO2 Nanocomposite-Coated Thin-Sheet Aluminum Alloy 2024—T3 in 3.5% NaCl. Eng. 2025; 6(4):83. https://doi.org/10.3390/eng6040083
Chicago/Turabian StyleChen, Cheng-fu, Brian Baart, John Halford, IV, and Junqing Zhang. 2025. "SCC Susceptibility of Polystyrene/TiO2 Nanocomposite-Coated Thin-Sheet Aluminum Alloy 2024—T3 in 3.5% NaCl" Eng 6, no. 4: 83. https://doi.org/10.3390/eng6040083
APA StyleChen, C.-f., Baart, B., Halford, J., IV, & Zhang, J. (2025). SCC Susceptibility of Polystyrene/TiO2 Nanocomposite-Coated Thin-Sheet Aluminum Alloy 2024—T3 in 3.5% NaCl. Eng, 6(4), 83. https://doi.org/10.3390/eng6040083







