A Brief Review of FT-IR Spectroscopy Studies of Sphingolipids in Human Cells
Abstract
:1. Introduction
2. General Characteristics of Sphingolipids in Human Cells
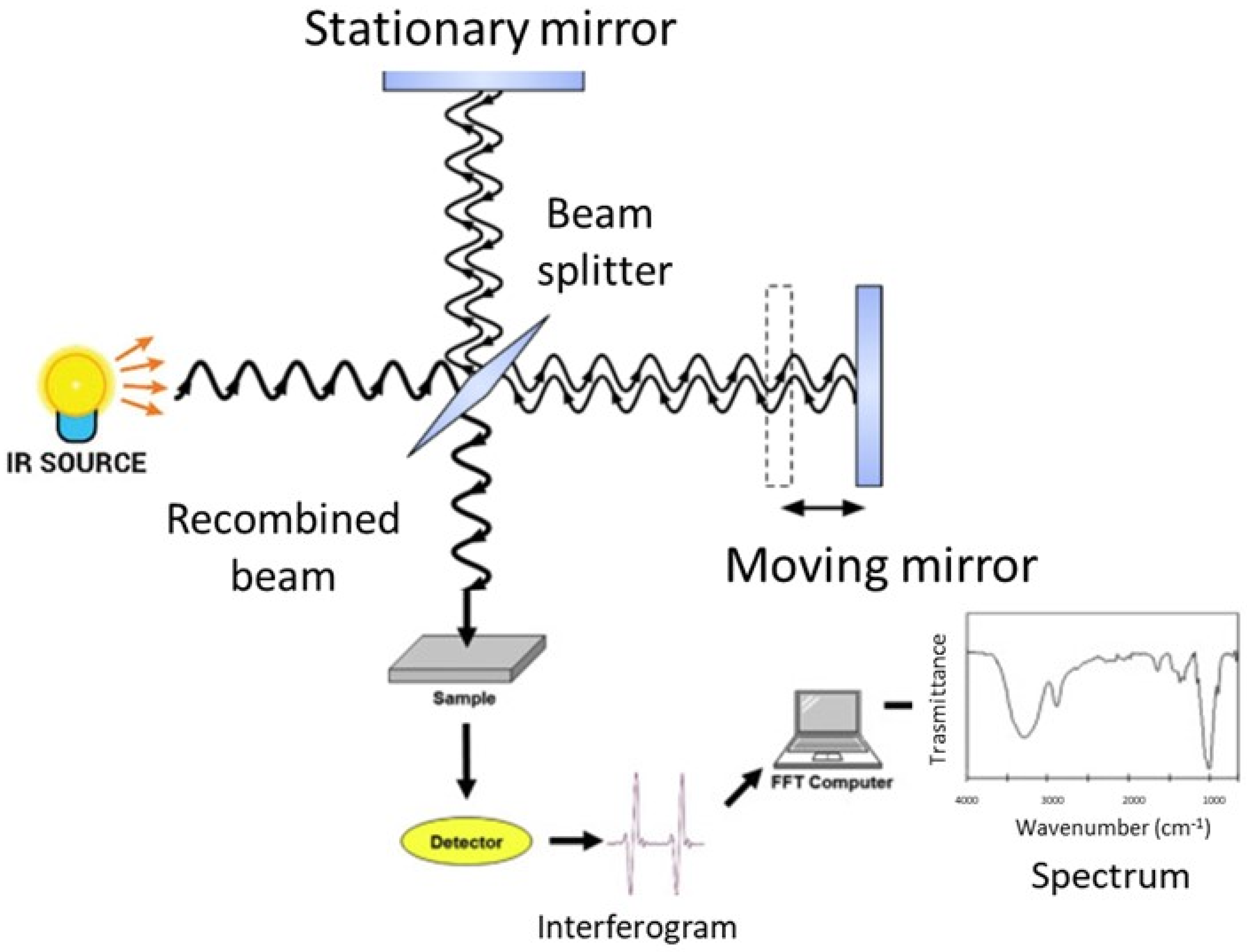
3. Basic Principles of FT-IR Spectroscopy
4. Experimental Aspects of FT-IR Spectroscopy
5. Data Analysis Procedures
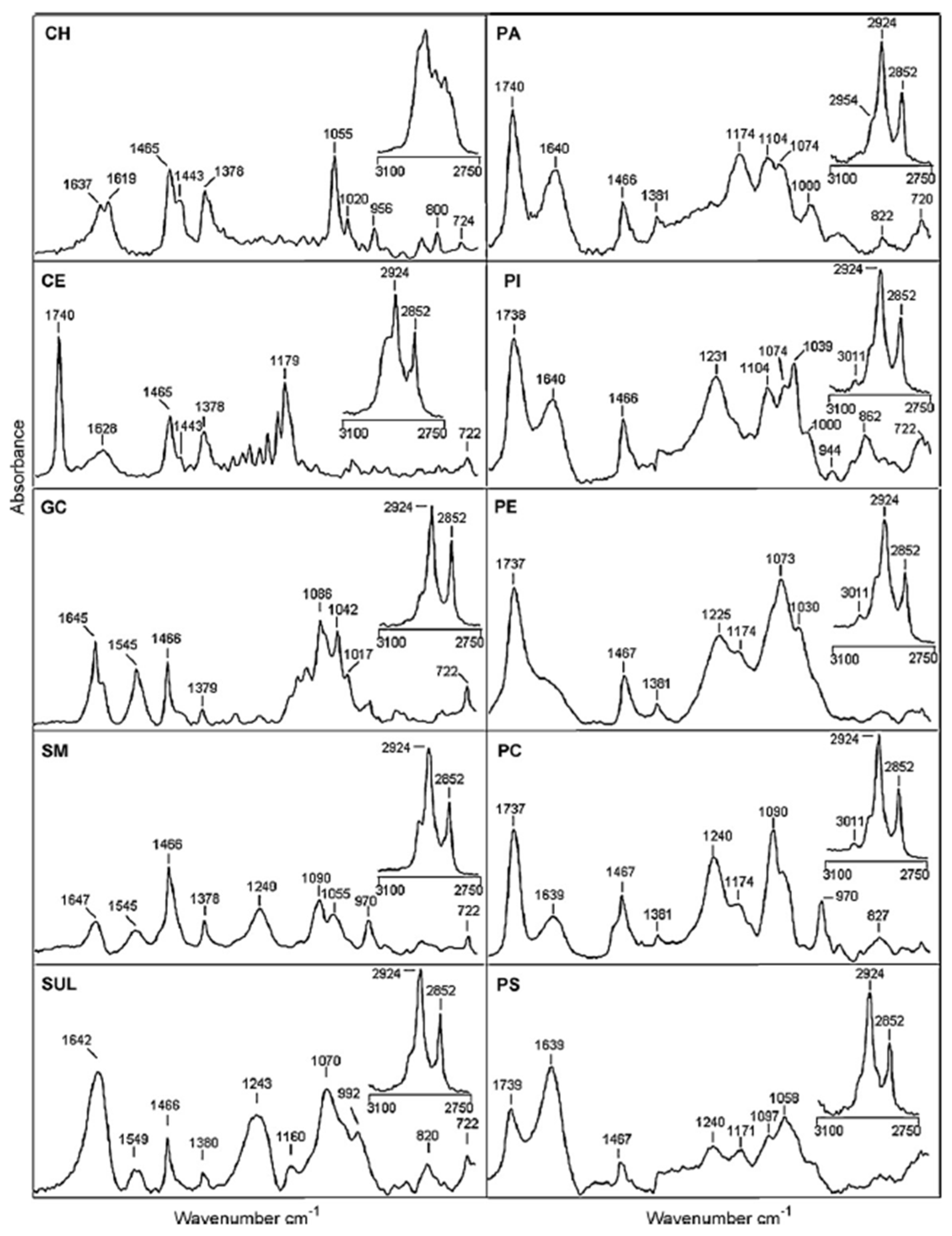
6. FT-IR Characterization of SLs
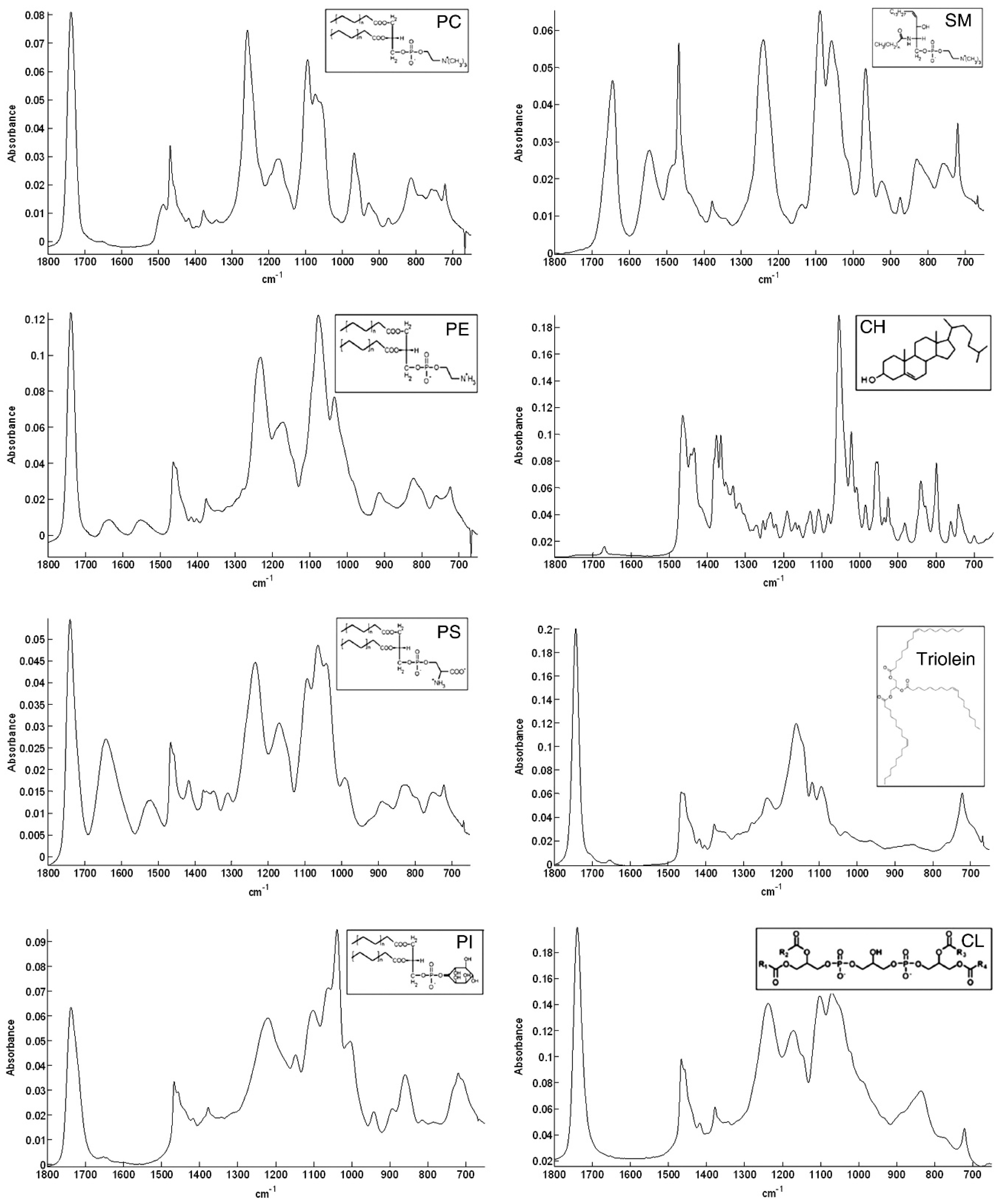
- (a)
- Sphingomyelin (SM)
- (b) Ceramide (Cer)
- (c) Sphingosine (SP) and sphingosine 1-phosphate (S1P)
- (d) FT-IR Lipidomic studies involving sphingolipids
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A. Sample Preparation
References
- Muro, E.; Atilla-Gokcumen, G.E.; Eggert, U.S. Lipids in cell biology: How can we understand them better? Mol. Biol. Cell 2014, 25, 1819–1823. [Google Scholar] [CrossRef] [PubMed]
- van Meer, G. Cellular lipidomics. EMBO J. 2005, 24, 3159–3165. [Google Scholar] [CrossRef] [PubMed]
- Zalba, S.; Ten Hagen, T.L. Cell membrane modulation as adjuvant in cancer therapy. Cancer Treat. Rev. 2017, 52, 48–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breslow, D.K.; Weissman, J.S. Membranes in balance: Mechanisms of sphingolipid homeostasis. Mol. Cell 2010, 40, 267–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haynes, C.A.; Allegood, J.C.; Park, H.; Sullards, M.C. Sphingolipidomics: Methods for the comprehensive analysis of sphingolipids. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2009, 877, 2696–2708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gennis, R.B. Biomembranes: Molecular Structure and Function; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Chapman, D. (Ed.) Biomembrane Structure and Function; Springer: Berlin/Heidelberg, Germany, 1983. [Google Scholar]
- Cullis, P.R.; Hope, M.J. Chapter 1 Physical properties and functional roles of lipids in membranes. In New Comprehensive Biochemistry; Vance, D.E., Vance, J.E., Eds.; Elsevier: Amsterdam, The Netherlands, 1991; Volume 20, pp. 1–41. [Google Scholar]
- Lizardo, D.Y.; Parisi, L.R.; Li, N.; Atilla-Gokcumen, G.E. Noncanonical roles of lipids in different cellular fates. Biochemistry 2018, 57, 22–29. [Google Scholar] [CrossRef]
- Merrill, A.H., Jr.; Sandhoff, K. Sphingolipids: Metabolism and cell signaling. New Compr. Biochem. 2002, 36, 373–407. [Google Scholar]
- Holm, L.J.; Krogvold, L.; Hasselby, J.P.; Kaur, S.; Claessens, L.A.; Russell, M.A.; Mathews, C.E.; Hanssen, K.F.; Morgan, N.G.; Koeleman, B.P. Abnormal islet sphingolipid metabolism in type 1 diabetes. Diabetologia 2018, 61, 1650–1661. [Google Scholar] [CrossRef] [Green Version]
- Bartke, N.; Hannun, Y.A. Bioactive sphingolipids: Metabolism and function. J. Lipid Res. 2009, 50, S91–S96. [Google Scholar] [CrossRef] [Green Version]
- Duan, R.-D.; Nilsson, Å. Metabolism of sphingolipids in the gut and its relation to inflammation and cancer development. Prog. Lipid Res. 2009, 48, 62–72. [Google Scholar] [CrossRef] [Green Version]
- Iessi, E.; Marconi, M.; Manganelli, V.; Sorice, M.; Malorni, W.; Garofalo, T.; Matarrese, P. On the role of sphingolipids in cell survival and death. Int. Rev. Cell Mol. Biol. 2020, 351, 149–195. [Google Scholar] [PubMed]
- Nagahashi, M.; Takabe, K.; Terracina, K.P.; Soma, D.; Hirose, Y.; Kobayashi, T.; Matsuda, Y.; Wakai, T. Sphingosine-1-phosphate transporters as targets for cancer therapy. Biomed. Res. Int. 2014, 2014, 651727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohanian, J.; Ohanian, V. Sphingolipids in mammalian cell signalling. Cell. Mol. Life Sci. 2001, 58, 2053–2068. [Google Scholar] [CrossRef] [PubMed]
- Sassa, T.; Suto, S.; Okayasu, Y.; Kihara, A. A shift in sphingolipid composition from C24 to C16 increases susceptibility to apoptosis in HeLa cells. Biochim. Biophys. Acta 2012, 1821, 1031–1037. [Google Scholar] [CrossRef] [Green Version]
- Mashhadi Akbar Boojar, M.; Mashhadi Akbar Boojar, M.; Golmohammad, S. Ceramide pathway: A novel approach to cancer chemotherapy. Egypt. J. Basic Appl. Sci. 2018, 5, 237–244. [Google Scholar] [CrossRef]
- Carpinteiro, A.; Dumitru, C.; Schenck, M.; Gulbins, E. Ceramide-induced cell death in malignant cells. Cancer Lett. 2008, 264, 1–10. [Google Scholar] [CrossRef]
- Hannun, Y.A.; Obeid, L.M. Principles of bioactive lipid signalling: Lessons from sphingolipids. Nat. Rev. Mol. Cell Biol. 2008, 9, 139–150. [Google Scholar] [CrossRef]
- Hannun, Y.A.; Obeid, L.M. Sphingolipids and their metabolism in physiology and disease. Nat. Rev. Mol. Cell Biol. 2018, 19, 175–191. [Google Scholar] [CrossRef]
- Jurowski, K.; Kochan, K.; Walczak, J.; Barańska, M.; Piekoszewski, W.; Buszewski, B. Analytical techniques in lipidomics: State of the art. Crit. Rev. Anal. Chem. 2017, 47, 418–437. [Google Scholar] [CrossRef]
- Serdyuk, I.N.; Zaccai, N.R.; Zaccai, J.; Zaccai, G. Methods in Molecular Biophysics, 2nd ed.; Cambridge University Press: Cambridge, UK, 2017. [Google Scholar]
- Lee, Y.H.; Tan, C.W.; Venkatratnam, A.; Tan, C.S.; Cui, L.; Loh, S.F.; Griffith, L.; Tannenbaum, S.R.; Chan, J.K.Y. Dysregulated sphingolipid metabolism in endometriosis. J. Clin. Endocrinol. Metab. 2014, 99, E1913–E1921. [Google Scholar] [CrossRef] [Green Version]
- Ramos-Garcia, V.; Ten-Doménech, I.; Moreno-Giménez, A.; Gormaz, M.; Parra-Llorca, A.; Shephard, A.P.; Sepúlveda, P.; Pérez-Guaita, D.; Vento, M.; Lendl, B. ATR-FTIR spectroscopy for the routine quality control of exosome isolations. Chemom. Intell. Lab. Syst. 2021, 217, 104401. [Google Scholar] [CrossRef]
- Guleken, Z.; Bulut, H.; Depciuch, J.; Tarhan, N. Diagnosis of endometriosis using endometrioma volume and vibrational spectroscopy with multivariate methods as a noninvasive method. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 264, 120246. [Google Scholar] [CrossRef] [PubMed]
- Stuart, B.H. Infrared Spectroscopy: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2004. [Google Scholar]
- Raghavachari, R. Near-Infrared Applications in Biotechnology; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar]
- Barth, A.; Haris, P.I. Biological and Biomedical Infrared Spectroscopy; IOS Press: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Abidi, N. FTIR Microspectroscopy: Selected Emerging Applications; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar]
- Baker, M.J.; Trevisan, J.; Bassan, P.; Bhargava, R.; Butler, H.J.; Dorling, K.M.; Fielden, P.R.; Fogarty, S.W.; Fullwood, N.J.; Heys, K.A.; et al. Using Fourier transform IR spectroscopy to analyze biological materials. Nat. Protoc. 2014, 9, 1771–1791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabbatini, S.; Conti, C.; Orilisi, G.; Giorgini, E. Infrared spectroscopy as a new tool for studying single living cells: Is there a niche? Biomed. Spectrosc. Imaging 2017, 6, 85–99. [Google Scholar] [CrossRef] [Green Version]
- Magalhães, S.; Goodfellow, B.J.; Nunes, A. FTIR spectroscopy in biomedical research: How to get the most out of its potential. Appl. Spectrosc. Rev. 2021, 56, 869–907. [Google Scholar] [CrossRef]
- Delfino, I.; Portaccio, M.; Della Ventura, B.; Mita, D.; Lepore, M. Enzyme distribution and secondary structure of sol–gel immobilized glucose oxidase by micro-attenuated total reflection FT-IR spectroscopy. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 304–310. [Google Scholar] [CrossRef]
- Ricciardi, V.; Portaccio, M.; Piccolella, S.; Manti, L.; Pacifico, S.; Lepore, M. Study of SH-SY5Y cancer cell response to treatment with polyphenol extracts using FT-IR spectroscopy. Biosensors 2017, 7, 57. [Google Scholar] [CrossRef] [Green Version]
- Ricciardi, V.; Portaccio, M.; Manti, L.; Lepore, M. An FTIR Microspectroscopy Ratiometric Approach for Monitoring X-ray Irradiation Effects on SH-SY5Y Human Neuroblastoma Cells. Appl. Sci. 2020, 10, 2974. [Google Scholar] [CrossRef]
- d’Apuzzo, F.; Nucci, L.; Delfino, I.; Portaccio, M.; Minervini, G.; Isola, G.; Serino, I.; Camerlingo, C.; Lepore, M. Application of vibrational spectroscopies in the qualitative analysis of gingival crevicular fluid and periodontal ligament during orthodontic tooth movement. J. Clin. Med. 2021, 10, 1405. [Google Scholar] [CrossRef]
- Kallenbach-Thieltges, A.; Großerüschkamp, F.; Mosig, A.; Diem, M.; Tannapfel, A.; Gerwert, K. Immunohistochemistry, histopathology and infrared spectral histopathology of colon cancer tissue sections. J. Biophotonics 2013, 6, 88–100. [Google Scholar] [CrossRef]
- Lasch, P.; Haensch, W.; Naumann, D.; Diem, M. Imaging of colorectal adenocarcinoma using FT-IRmicrospectroscopy and cluster analysis. Biochim. Biophys. Acta 2004, 1688, 176–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bird, B.; Bedrossian, K.; Laver, N.; Miljković, M.; Romeo, M.J.; Diem, M. Detection of breast micro-metastases in axillary lymph nodes by infrared micro-spectral imaging. Analyst 2009, 134, 1067–1076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ooi, G.J.; Fox, J.; Siu, K.; Lewis, R.; Bambery, K.R.; Mcnaughton, D.; Wood, B.R. Fourier transform infrared imaging and small angle x-ray scattering as a combined biomolecular approach to diagnosis of breast cancer. Med. Phys. 2008, 35, 2151–2161. [Google Scholar] [CrossRef] [PubMed]
- Baker, M.J.; Gazi, E.; Brown, M.D.; Shanks, J.H.; Gardner, P.; Clarke, N.W. FTIR-based spectroscopic analysis in the identification of clinically aggressive prostate cancer. Br. J. Cancer 2008, 99, 1859–1866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baker, M.J.; Gazi, E.; Brown, M.D.; Shanks, J.H.; Clarke, N.W.; Gardner, P. Investigating FTIR based histopathology for the diagnosis of prostate cancer. J. Biophotonics 2009, 2, 104–113. [Google Scholar] [CrossRef]
- Lovergne, L.; Lovergne, J.; Bouzy, P.; Untereiner, V.; Offroy, M.; Garnotel, R.; Thiéfin, G.; Baker, M.J.; Sockalingum, G.D. Investigating pre-analytical requirements for serum and plasma based infrared spectro-diagnostic. J. Biophotonics 2019, 12, e201900177. [Google Scholar] [CrossRef] [PubMed]
- Theakstone, A.G.; Rinaldi, C.; Butler, H.J.; Cameron, J.M.; Confield, L.R.; Rutherford, S.H.; Sala, A.; Sangamnerkar, S.; Baker, M.J. Fourier-transform infrared spectroscopy of biofluids: A practical approach. Transl. Biophotonics 2021, 3, e202000025. [Google Scholar] [CrossRef]
- Bruun, S.W.; Kohler, A.; Adt, I.; Sockalingum, G.D.; Manfait, M.; Martens, H. Correcting attenuated total reflection-Fourier transform infrared spectra for water vapor and carbon dioxide. Appl. Spectrosc. 2006, 60, 1029–1039. [Google Scholar] [CrossRef]
- Vaccari, L.; Birarda, G.; Grenci, G.; Pacor, S.; Businaro, L. Synchrotron radiation infrared microspectroscopy of single living cells in microfluidic devices: Advantages, disadvantages and future perspectives. J. Phys. Conf. Ser. 2012, 359, 012007. [Google Scholar] [CrossRef]
- Carrasco-Pancorbo, A.; Navas-Iglesias, N.; Cuadros-Rodrıguez, L. From lipid analysis towards lipidomics, a new challenge for the analytical chemistry of the 21st century. Part I: Modern lipid analysis. Trends Anal. Chem. 2009, 28, 263–278. [Google Scholar] [CrossRef]
- Carrasco-Pancorbo, A.; Navas-Iglesias, N.; Cuadros-Rodrıguez, L. From lipids analysis towards lipidomics, a new challenge for the analytical chemistry of the 21st century. Part II: Analytical lipidomics. Trends Anal. Chem. 2009, 28, 393–403. [Google Scholar] [CrossRef]
- Abdelrazzak, A.B.; Hezma, A.M.; El-Bahy, G.S. ATR-FTIR spectroscopy probing of structural alterations in the cellular membrane of abscopal liver cells. Biochim. Biophys. Acta Biomembr. 2021, 1863, 183726. [Google Scholar] [CrossRef]
- Di Santo, R.; Vaccaro, M.; Romanò, S.; Di Giacinto, F.; Papi, M.; Rapaccini, G.L.; De Spirito, M.; Miele, L.; Basile, U.; Ciasca, G. Machine Learning-Assisted FTIR Analysis of Circulating Extracellular Vesicles for Cancer Liquid Biopsy. J. Pers. Med. 2022, 12, 949. [Google Scholar] [CrossRef] [PubMed]
- Robinson, H.; Molendijk, J.; Shah, A.K.; Rahman, T.; Anderson, G.J.; Hill, M.M. Rapid Assessment of Lipidomics Sample Purity and Quantity Using Fourier-Transform Infrared Spectroscopy. Biomolecules 2022, 12, 1265. [Google Scholar] [CrossRef] [PubMed]
- Murdica, V.; Mancini, G.; Loberto, N.; Bassi, R.; Giussani, P.; Di Muzio, N.; Deantoni, C.; Prinetti, A.; Aureli, M.; Sonnino, S. Abiraterone and ionizing radiation alter the sphingolipid homeostasis in prostate cancer cells. Adv. Exp. Med. Biol. 2018, 1112, 293–307. [Google Scholar] [PubMed]
- Mathew, B.; Jacobson, J.R.; Berdyshev, E.; Huang, Y.; Sun, X.; Zhao, Y.; Gerhold, L.M.; Siegler, J.; Evenoski, C.; Wang, T. Role of sphingolipids in murine radiation-induced lung injury: Protection by sphingosine 1-phosphate analogs. FASEB J. 2011, 25, 3388–3400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez-Lafrasse, C.; Alphonse, G.; Aloy, M.T.; Ardail, D.; Gérard, J.P.; Louisot, P.; Rousson, R. Increasing endogenous ceramide using inhibitors of sphingolipid metabolism maximizes ionizing radiation-induced mitochondrial injury and apoptotic cell killing. Int. J. Cancer 2002, 101, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Srinivasan, A.; Nikolajeff, F. Role of infrared spectroscopy and imaging in cancer diagnosis. Curr. Med. Chem. 2018, 25, 1055–1072. [Google Scholar] [CrossRef] [PubMed]
- Bonnaud, S.; Niaudet, C.; Legoux, F.; Corre, I.; Delpon, G.; Saulquin, X.; Fuks, Z.; Gaugler, M.-H.; Kolesnick, R.; Paris, F. Sphingosine-1-Phosphate Activates the AKT Pathway to Protect Small Intestines from Radiation-Induced Endothelial ApoptosisS1P Protects from Radiation-Induced GI Syndrome. Cancer Res. 2010, 70, 9905–9915. [Google Scholar] [CrossRef] [Green Version]
- Agarwala, P.K.; Aneja, R.; Kapoor, S. Lipidomic landscape in cancer: Actionable insights for membrane-based therapy and diagnoses. Med. Res. Rev. 2022, 42, 983–1018. [Google Scholar] [CrossRef]
- Baenke, F.; Peck, B.; Miess, H.; Schulze, A. Hooked on fat: The role of lipid synthesis in cancer metabolism and tumour development. Dis. Model. Mech. 2013, 6, 1353–1363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woodcock, J. Sphingosine and ceramide signalling in apoptosis. IUBMB Life 2006, 58, 462–466. [Google Scholar] [CrossRef] [PubMed]
- Fernández, L.P.; Gomez de Cedron, M.; Ramírez de Molina, A. Alterations of lipid metabolism in cancer: Implications in prognosis and treatment. Front. Oncol. 2020, 10, 577420. [Google Scholar] [CrossRef] [PubMed]
- Beckham, T.H.; Lu, P.; Jones, E.E.; Marrison, T.; Lewis, C.S.; Cheng, J.C.; Ramshesh, V.K.; Beeson, G.; Beeson, C.C.; Drake, R.R. LCL124, a cationic analog of ceramide, selectively induces pancreatic cancer cell death by accumulating in mitochondria. J. Pharmacol. Exp. Ther. 2013, 344, 167–178. [Google Scholar] [CrossRef] [Green Version]
- Henry, B.; Möller, C.; Dimanche-Boitrel, M.-T.; Gulbins, E.; Becker, K.A. Targeting the ceramide system in cancer. Cancer Lett. 2013, 332, 286–294. [Google Scholar] [CrossRef]
- Aureli, M.; Murdica, V.; Loberto, N.; Samarani, M.; Prinetti, A.; Bassi, R.; Sonnino, S. Exploring the link between ceramide and ionizing radiation. Glycoconj J. 2014, 31, 449–459. [Google Scholar] [CrossRef]
- Modrak, D.E.; Gold, D.V.; Goldenberg, D.M. Sphingolipid targets in cancer therapy. Mol. Cancer Ther. 2006, 5, 200–208. [Google Scholar] [CrossRef] [Green Version]
- Ogretmen, B. Sphingolipid metabolism in cancer signalling and therapy. Nat. Rev. Cancer 2018, 18, 33–50. [Google Scholar] [CrossRef] [Green Version]
- Corre, I.; Niaudet, C.; Paris, F. Plasma membrane signaling induced by ionizing radiation. Mutat. Res. 2010, 704, 61–67. [Google Scholar] [CrossRef]
- Vit, J.P.; Rosselli, F. Role of the ceramide-signaling pathways in ionizing radiation-induced apoptosis. Oncogene 2003, 22, 8645–8652. [Google Scholar] [CrossRef] [Green Version]
- Schramm, C. High temperature ATR-FTIR characterization of the interaction of polycarboxylic acids and organotrialkoxysilanes with cellulosic material. Biochim. Biophys. Acta 2020, 43, 118815. [Google Scholar] [CrossRef]
- Whyman, R.; Hunt, K.; Page, R.; Rigby, S. A high-pressure spectroscopic cell for FTIR measurements. J. Phys. E Sci. Instr. 2000, 17, 559. [Google Scholar] [CrossRef]
- Reffner, J.A. Advances in Infrared Microspectroscopy and Mapping Molecular Chemical Composition at Submicrometer Spatial Resolution. Spectroscopy 2018, 33, 12–17. [Google Scholar]
- Beasley, M.M.; Bartelink, E.J.; Taylor, L.; Miller, R.M. Comparison of transmission FTIR, ATR, and DRIFT spectra: Implications for assessment of bone bioapatite diagenesis. J. Archaeol. Sci. 2014, 46, 16–22. [Google Scholar] [CrossRef]
- Horgnies, M.; Chen, J.; Bouillon, C. Overview about the use of Fourier transform infrared spectroscopy to study cementitious materials. WIT Trans. Eng. Sci 2013, 77, 251–262. [Google Scholar]
- Gendreau, R.M.; Burton, R. The KBr Pellet: A Useful Technique for Obtaining Infrared Spectra of Inorganic Species. Appl. Spectrosc. 1979, 33, 581–584. [Google Scholar] [CrossRef]
- Goormaghtigh, E.; Raussens, V.; Ruysschaert, J.-M. Attenuated total reflection infrared spectroscopy of proteins and lipids in biological membranes. Biochim. Biophys. Acta 1999, 1422, 105–185. [Google Scholar] [CrossRef]
- Grdadolnik, J. ATR-FTIR spectroscopy: Its advantage and limitations. Acta Chim. Slov. 2002, 49, 631–642. [Google Scholar]
- Kazarian, S.; Chan, K. Applications of ATR-FTIR spectroscopic imaging to biomedical samples. Biochim. Biophys. Acta 2006, 1758, 858–867. [Google Scholar] [CrossRef] [Green Version]
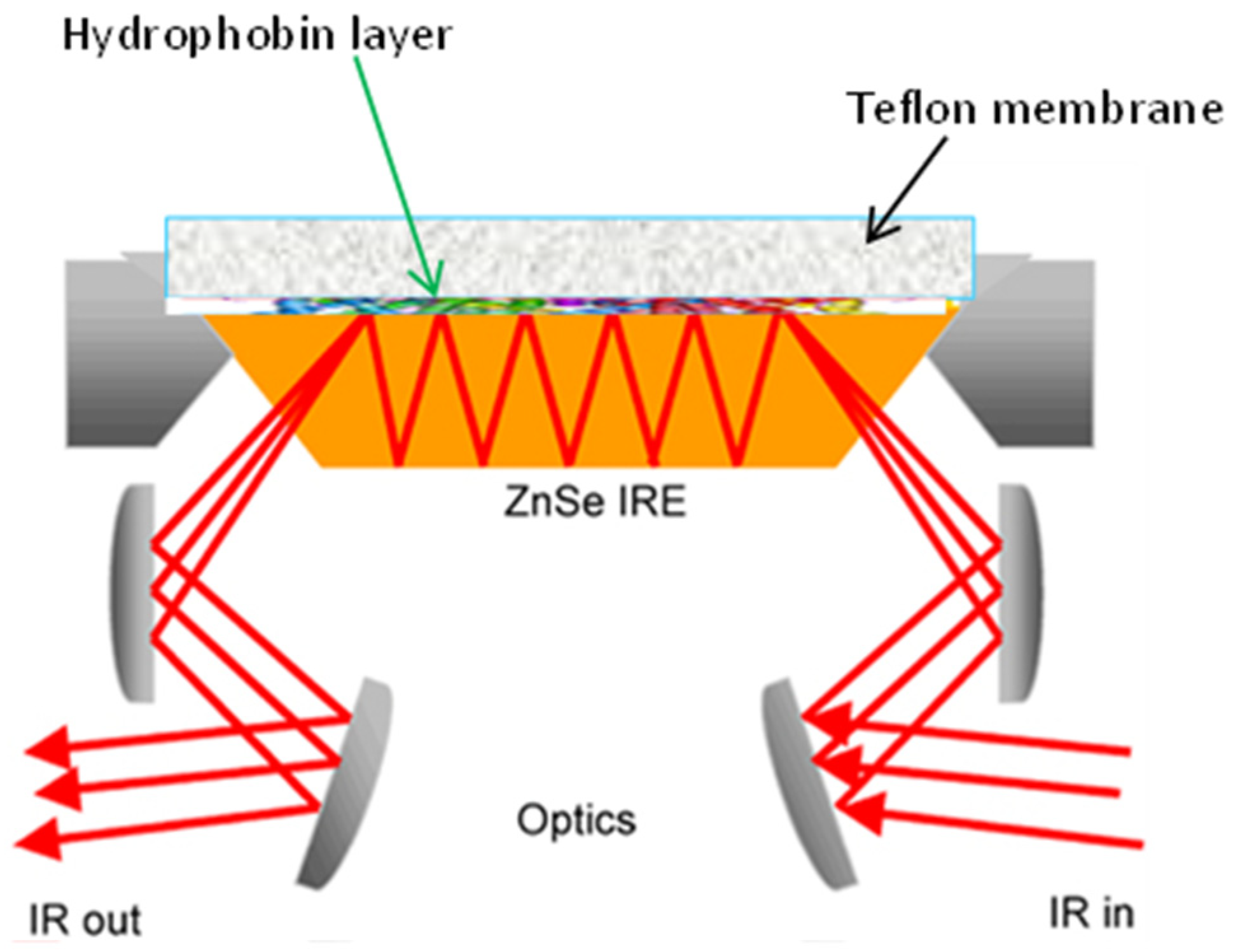
- Portaccio, M.; Gravagnuolo, A.; Longobardi, S.; Giardina, P.; Rea, I.; De Stefano, L.; Cammarota, M.; Lepore, M. ATR FT-IR spectroscopy on Vmh2 hydrophobin self-assembled layers for Teflon membrane bio-functionalization. Appl. Surf. Sci. 2015, 351, 673–680. [Google Scholar] [CrossRef]
- Fringeli, U.P.; Günthard, H.H. Infrared membrane spectroscopy. Mol. Biol. Biochem. Biophys. 1981, 31, 270–332. [Google Scholar] [PubMed]
- Tamm, L.K.; Tatulian, S.A. Infrared spectroscopy of proteins and peptides in lipid bilayers. Q. Rev. Biophys. 1997, 30, 365–429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McIvor, A.M. Background subtraction techniques. Proc. Image Vis. Comput. 2000, 4, 3099–3104. [Google Scholar]
- Bassan, P.; Byrne, H.J.; Bonnier, F.; Lee, J.; Dumas, P.; Gardner, P. Resonant Mie scattering in infrared spectroscopy of biological materials–understanding the ‘dispersion artefact’. Analyst 2009, 134, 1586–1593. [Google Scholar] [CrossRef] [PubMed]
- Bassan, P.; Kohler, A.; Martens, H.; Lee, J.; Byrne, H.J.; Dumas, P.; Gazi, E.; Brown, M.; Clarke, N.; Gardner, P. Resonant Mie scattering (RMieS) correction of infrared spectra from highly scattering biological samples. Analyst 2010, 135, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Vafaei, N.; Ribeiro, R.A.; Camarinha-Matos, L.M. Normalization techniques for multi-criteria decision making: Analytical hierarchy process case study. In Proceedings of the 7th Doctoral Conference on Computing, Electrical and Industrial Systems (DoCEIS), Costa de Caparica, Portugal, 11–13 April 2016; pp. 261–269. [Google Scholar] [CrossRef] [Green Version]
- Byler, D.M.; Wilson, R.M.; Randall, C.S.; Sokoloski, T.D. Second derivative infrared spectroscopy as a non-destructive tool to assess the purity and structural integrity of proteins. Pharm. Res. 1995, 12, 446–450. [Google Scholar] [CrossRef]
- Suresh, S.; Karthikeyan, S.; Jayamoorthy, K. FTIR and multivariate analysis to study the effect of bulk and nano copper oxide on peanut plant leaves. J. Sci. Adv. Mater. Devices 2016, 1, 343–350. [Google Scholar] [CrossRef] [Green Version]
- Elmasry, G.; Kamruzzaman, M.; Sun, D.-W.; Allen, P. Principles and applications of hyperspectral imaging in quality evaluation of agro-food products: A review. Crit. Rev. Food Sci. Nutr. 2012, 52, 999–1023. [Google Scholar] [CrossRef]
- Kumar, S.; Verma, T.; Mukherjee, R.; Ariese, F.; Somasundaram, K.; Umapathy, S. Raman and infra-red microspectroscopy: Towards quantitative evaluation for clinical research by ratiometric analysis. Chem. Soc. Rev. 2016, 45, 1879–1900. [Google Scholar] [CrossRef]
- Cakmak, G.; Miller, L.M.; Zorlu, F.; Severcan, F. Amifostine, a radioprotectant agent, protects rat brain tissue lipids against ionizing radiation induced damage: An FTIR microspectroscopic imaging study. Arch. Biochem. Biophys. 2012, 520, 67–73. [Google Scholar] [CrossRef]
- Severcan, F.; Gorgulu, G.; Gorgulu, S.T.; Guray, T. Rapid monitoring of diabetes-induced lipid peroxidation by Fourier transform infrared spectroscopy: Evidence from rat liver microsomal membranes. Anal. Biochem. 2005, 339, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Garip, S.; Yapici, E.; Ozek, N.S.; Severcan, M.; Severcan, F. Evaluation and discrimination of simvastatin-induced structural alterations in proteins of different rat tissues by FTIR spectroscopy and neural network analysis. Analyst 2010, 135, 3233–3241. [Google Scholar] [CrossRef]
- Yoshida, S.; Koike, K. Lipid and membrane dynamics in biological tissues in nfrared spectroscopic studies. Adv. Planar Lipid Bilayers Liposomes 2011, 13, 1–32. [Google Scholar]
- Movasaghi, Z.; Rehman, S.; ur Rehman, D.I. Fourier transform infrared (FTIR) spectroscopy of biological tissues. Appl. Spectrosc. Rev. 2008, 43, 134–179. [Google Scholar] [CrossRef]
- Talari, A.C.S.; Martinez, M.A.G.; Movasaghi, Z.; Rehman, S.; Rehman, I.U. Advances in Fourier transform infrared (FTIR) spectroscopy of biological tissues. Appl. Spectrosc. Rev. 2017, 52, 456–506. [Google Scholar] [CrossRef]
- Derenne, A.; Claessens, T.; Conus, C.; Goormaghtigh, E. Infrared spectroscopy of membrane lipids. In Encyclopedia of Biophysics; Roberts, G.C.K., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1074–1081. [Google Scholar]
- Derenne, A.; Vandersleyen, O.; Goormaghtigh, E. Lipid quantification method using FTIR spectroscopy applied on cancer cell extracts. Biochim. Biophys. Acta 2014, 1841, 1200–1209. [Google Scholar] [CrossRef]
- Bligh, E.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Saini, R.K.; Prasad, P.; Shang, X.; Keum, Y.S. Advances in Lipid Extraction Methods—A Review. Int. J. Mol. Sci. 2021, 22, 13643. [Google Scholar] [CrossRef]
- Villalaín, J.; Ortiz, A.; Gómez-Fernández, J.C. Molecular interactions between sphingomyelin and phosphatidylcholine in phospholipid vesicles. Biochim. Biophys. Acta 1988, 941, 55–62. [Google Scholar] [CrossRef]
- Nicolini, C.; Kraineva, J.; Khurana, M.; Periasamy, N.; Funari, S.S.; Winter, R. Temperature and pressure effects on structural and conformational properties of POPC/SM/cholesterol model raft mixtures—A FT-IR, SAXS, DSC, PPC and Laurdan fluorescence spectroscopy study. Biochim. Biophys. Acta 2006, 1758, 248–258. [Google Scholar] [CrossRef]
- Beljebbar, A.; Amharref, N.; Lévèques, A.; Dukic, S.; Venteo, L.; Schneider, L.; Pluot, M.; Manfait, M. Modeling and quantifying biochemical changes in C6 tumor gliomas by Fourier transform infrared imaging. Anal. Chem. 2008, 80, 8406–8415. [Google Scholar] [CrossRef] [PubMed]
- Beljebbar, A.; Dukic, S.; Amharref, N.; Bellefqih, S.; Manfait, M. Monitoring of biochemical changes through the C6 gliomas progression and invasion by Fourier transform infrared (FTIR) imaging. Anal. Chem. 2009, 81, 9247–9256. [Google Scholar] [CrossRef] [PubMed]
- Dreissig, I.; Machill, S.; Salzer, R.; Krafft, C. Quantification of brain lipids by FTIR spectroscopy and partial least squares regression. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2009, 71, 2069–2075. [Google Scholar] [CrossRef]
- Gasper, R.; Dewelle, J.; Kiss, R.; Mijatovic, T.; Goormaghtigh, E. IR spectroscopy as a new tool for evidencing antitumor drug signatures. Biochim. Biophys. Acta 2009, 1788, 1263–1270. [Google Scholar] [CrossRef] [PubMed]
- Gasper, R.; Vandenbussche, G.; Goormaghtigh, E. Ouabain-induced modifications of prostate cancer cell lipidome investigated with mass spectrometry and FTIR spectroscopy. Biochim. Biophys. Acta 2011, 1808, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Mereghetti, P.; Corsetto, P.A.; Cremona, A.; Rizzo, A.M.; Doglia, S.M.; Ami, D. A Fourier transform infrared spectroscopy study of cell membrane domain modifications induced by docosahexaenoic acid. Biochim. Biophys. Acta 2014, 1840, 3115–3122. [Google Scholar] [CrossRef]
- Türker-Kaya, S.; Kına, A. Calorimetric and spectroscopic investigation of the interaction of chemotherapeutic agent carboplatin with sphingomyelin lipids. J. Therm. Anal. Calorim. 2021, 146, 2515–2522. [Google Scholar] [CrossRef]
- Moore, D.J.; Rerek, M.E.; Mendelsohn, R. FTIR spectroscopy studies of the conformational order and phase behavior of ceramides. J. Phys. Chem. B 1997, 101, 8933–8940. [Google Scholar] [CrossRef]
- Chen, H.-C.; Mendelsohn, R.; Rerek, M.E.; Moore, D.J. Fourier transform infrared spectroscopy and differential scanning calorimetry studies of fatty acid homogeneous ceramide 2. Biochim. Biophys. Acta 2000, 1468, 293–303. [Google Scholar] [CrossRef] [Green Version]
- Raudenkolb, S.; Hübner, W.; Rettig, W.; Wartewig, S.; Neubert, R.H. Polymorphism of ceramide 3. Part 1: An investigation focused on the head group of N-octadecanoylphytosphingosine. Chem. Phys. Lipids 2003, 123, 9–17. [Google Scholar] [CrossRef]
- Raudenkolb, S.; Wartewig, S.; Neubert, R.H. Polymorphism of ceramide 3. Part 2: A vibrational spectroscopic and X-ray powder diffraction investigation of N-octadecanoyl phytosphingosine and the analogous specifically deuterated d35 derivative. Chem. Phys. Lipids 2003, 124, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Raudenkolb, S.; Wartewig, S.; Neubert, R. Polymorphism of ceramide 6: A vibrational spectroscopic and X-ray powder diffraction investigation of the diastereomers of N-(α-hydroxyoctadecanoyl)-phytosphingosine. Chem. Phys. Lipids 2005, 133, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Raith, K.; Farwanah, H.; Wartewig, S.; Neubert, R.H. Progress in the analysis of stratum corneum ceramides. Eur. J. Lipid Sci. Technol. 2004, 106, 561–571. [Google Scholar] [CrossRef]
- Corbe, E.; Laugel, C.; Yagoubi, N.; Baillet, A. Role of ceramide structure and its microenvironment on the conformational order of model stratum corneum lipids mixtures: An approach by FTIR spectroscopy. Chem. Phys. Lipids 2007, 146, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Kwak, S.; Lafleur, M.; Bloom, M.; Kitson, N.; Thewalt, J. Fatty acids influence “solid” phase formation in models of stratum corneum intercellular membranes. Langmuir 2007, 23, 5548–5556. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-H.; An, E.J.; Kim, J.; Han, S.-H.; Kim, J.-W.; Oh, S.-G.; Suh, K.-D.; Cho, E.C. Fabrication and characterization of pseudo-ceramide-based liposomal membranes. Colloids Surf. B Biointerfaces 2009, 73, 207–211. [Google Scholar] [CrossRef]
- Park, J.-H.; Lee, J.-Y.; Termsarasab, U.; Yoon, I.-S.; Ko, S.-H.; Shim, J.-S.; Cho, H.-J.; Kim, D.-D. Development of poly (lactic-co-glycolic) acid nanoparticles-embedded hyaluronic acid–ceramide-based nanostructure for tumor-targeted drug delivery. Int. J. Pharm. 2014, 473, 426–433. [Google Scholar] [CrossRef]
- De la Arada, I.; González-Ramírez, E.J.; Alonso, A.; Goñi, F.M.; Arrondo, J.-L.R. Exploring polar headgroup interactions between sphingomyelin and ceramide with infrared spectroscopy. Sci. Rep. 2020, 10, 17606. [Google Scholar] [CrossRef]
- López-García, F.; Villaín, J.; Gómez-Fernández, J.C. Effect of sphingosine and stearylamine on the interaction of phosphatidylserine with calcium. A study using DSC, FT-IR and 45Ca2+-binding. Biochim. Biophys. Acta 1995, 1236, 279–288. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Fernández, J.C.; Villalaín, J. The use of FT-IR for quantitative studies of the apparent pKa of lipid carboxyl groups and the dehydration degree of the phosphate group of phospholipids. Chem. Phys. Lipids 1998, 96, 41–52. [Google Scholar] [CrossRef]
- Ahmeda, A.; Zangeneh, M.M.; Zangeneh, A. Green formulation and chemical characterization of Lens culinaris seed aqueous extract conjugated gold nanoparticles for the treatment of acute myeloid leukemia in comparison to mitoxantrone in a leukemic mouse model. Appl. Organomet. Chem. 2020, 34, e5369. [Google Scholar] [CrossRef]
- Hemmati, S.; Joshani, Z.; Zangeneh, A.; Zangeneh, M.M. Biosynthesis and chemical characterization of polydopamine-capped silver nanoparticles for the treatment of acute myeloid leukemia in comparison to doxorubicin in a leukemic mouse model. Appl. Organomet. Chem. 2020, 34, e5277. [Google Scholar] [CrossRef]
- Zangeneh, M.M.; Zangeneh, A. Novel green synthesis of Hibiscus sabdariffa flower extract conjugated gold nanoparticles with excellent anti-acute myeloid leukemia effect in comparison to daunorubicin in a leukemic rodent model. Appl. Organomet. Chem. 2020, 34, e5271. [Google Scholar] [CrossRef]
- Hemmati, S.; Joshani, Z.; Zangeneh, A.; Zangeneh, M.M. Green synthesis and chemical characterization of Thymus vulgaris leaf aqueous extract conjugated gold nanoparticles for the treatment of acute myeloid leukemia in comparison to doxorubicin in a leukemic mouse model. Appl. Organomet. Chem. 2020, 34, e5267. [Google Scholar] [CrossRef]
- Banerjee, A.; Halder, A.; Jadhav, P.; Bankar, R.; Pattarkine, J.; Hole, A.; Shah, A.; Goel, A.; Murali Krishna, C.; Srivastava, S. Metabolomics Profiling of Pituitary Adenomas by Raman Spectroscopy, Attenuated Total Reflection-Fourier Transform Infrared Spectroscopy, and Mass Spectrometry of Serum Samples. Anal. Chem. 2022, 94, 11898–11907. [Google Scholar] [CrossRef] [PubMed]
- Dash, M.K.; Joshi, N.; Dubey, V.S.; Dwivedi, K.N.; Gautam, D.N.S. Screening of anti-cancerous potential of classical Raudra rasa and modified Raudra rasa modified with hiraka bhasma (nanodiamond) through FTIR & LC-MS analysis. J. Complement Integr. Med. 2022, 19, 669–682. [Google Scholar]
- Butler, H.J.; Brennan, P.M.; Cameron, J.M.; Finlayson, D.; Hegarty, M.G.; Jenkinson, M.D.; Palmer, D.S.; Smith, B.R.; Baker, M.J. Development of high-throughput ATR-FTIR technology for rapid triage of brain cancer. Nat. Commun. 2019, 10, 4501. [Google Scholar] [CrossRef] [Green Version]
- De Meutter, J.L.; Goormaghtigh, E. FTIR imaging of protein microarrays for high throughput secondary structure determination. Anal. Chem. 2021, 93, 3733–3741. [Google Scholar] [CrossRef]
- Harrigan, G.G.; LaPlante, R.H.; Cosma, G.N.; Cockerell, G.; Goodacre, R.; Maddox, J.F.; Luyendyk, J.P.; Ganey, P.E.; Roth, R.A. Application of high-throughput Fourier-transform infrared spectroscopy in toxicology studies: Contribution to a study on the development of an animal model for idiosyncratic toxicity. Toxicol. Lett. 2004, 146, 197–205. [Google Scholar] [CrossRef]
- Ellis, D.I.; Dunn, W.B.; Griffin, J.L.; Allwood, J.W.; Goodacre, R. Metabolic fingerprinting as a diagnostic tool. Pharmacogenomics 2007, 8, 1243–1266. [Google Scholar] [CrossRef] [Green Version]
- Pachetti, M.; Zupin, L.; Venturin, I.; Mitri, E.; Boscolo, R.; D’amico, F.; Vaccari, L.; Crovella, S.; Ricci, G.; Pascolo, L. FTIR Spectroscopy to Reveal Lipid and Protein Changes Induced on Sperm by Capacitation: Bases for an Improvement of Sample Selection in ART. Int. J. Mol. Sci. 2020, 21, 8659. [Google Scholar] [CrossRef]
- González-Fernández, M.J.; Manzano-Agugliaro, F.; Zapata-Sierra, A.; Belarbi, E.H.; Guil-Guerrero, J.L. Green argan oil extraction from roasted and unroasted seeds by using various polarity solvents allowed by the EU legislation. J. Clean. Prod. 2020, 276, 123081. [Google Scholar] [CrossRef]
- Lin, J.H.; Liu, L.Y.; Yang, M.H.; Lee, M.H. Ethyl acetate/ethyl alcohol mixtures as an alternative to Folch reagent for extracting animal lipids. J. Agric. Food Chem. 2004, 52, 4984–4986. [Google Scholar] [CrossRef]
- Pati, S.; Nie, B.; Arnold, R.D.; Cummings, B.S. Extraction, chromatographic and mass spectrometric methods for lipid analysis. Biomed. Chromatogr. 2016, 30, 695–709. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Iverson, S.J.; Lang, S.L.C.; Cooper, M.H. Comparison of the Bligh and Dyer and Folch methods for total lipid determination in a broad range of marine tissue. Lipids 2001, 36, 1283–1287. [Google Scholar] [CrossRef] [PubMed]
- Matyash, V.; Liebisch, G.; Kurzchalia, T.V.; Shevchenko, A.; Schwudke, D. Lipid extraction by methyl-tert-butyl ether for high-throughput lipidomics. J. Lipid Res. 2008, 49, 1137–1146. [Google Scholar] [CrossRef] [Green Version]
- Alshehry, Z.H.; Barlow, C.K.; Weir, J.M.; Zhou, Y.; McConville, M.J.; Meikle, P.J. An efficient single-phase method for the extraction of plasma lipids. Metabolites 2015, 5, 389–403. [Google Scholar] [CrossRef]
| Source | Url | Notes |
|---|---|---|
| Lipid Bank—Japanese Conference on the Biochemistry of Lipids (JCBL) | https://lipidbank.jp (accessed on 1 February 2023) | LipidBank is a free database of natural lipids including fatty acids, glycerolipids, SLs, steroids, and various vitamins.The database contains more than 6000 unique molecular structures, their lipid names, and spectral and literature information. |
| NIST Chemistry WebBook | https://webbook.nist.gov (accessed on 1 February 2023) | The NIST Chemistry WebBook provides access to: thermochemical data; IR spectra, mass spectra, UV/Vis spectra, and gas chromatography data. It is possible to search for data on specific compounds based on name, chemical formula, CAS registry number, molecular weight, chemical structure, or selected ion energetics and spectral properties. |
| Spectral Database for Organic Compounds (SDBS) | https://sdbs.db.aist.go.jp (accessed on 1 February 2023) | SDBS is an integrated spectral database system for organic compounds, which includes 6 different types of spectra: an electron impact mass spectrum (EI-MS), a Fourier-transform infrared spectrum (FT-IR), a 1H nuclear magnetic resonance (NMR) spectrum, a 13C NMR spectrum, a laser Raman spectrum, and an electron spin resonance (ESR) spectrum. |
| Sphingolipid Compound | Structure |
|---|---|
| Ceramide (Cer) | 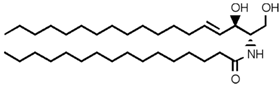 |
| Sphingosine 1-phosphate (S1P) | 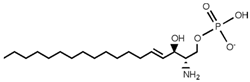 |
| Sphingosine (SP) | 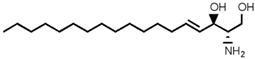 |
| Ceramide-1-phosphate (C1P) |  |
| Dihydroceramide |  |
| Sphingomyelin (SM) | 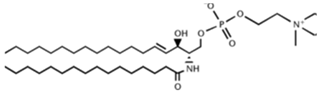 |
| Galactosylceramide | 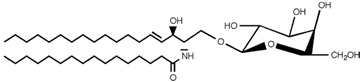 |
| Lactosylceramide | 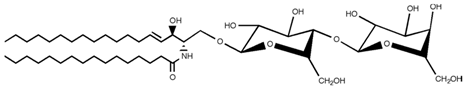 |
| Glucosylceramide | 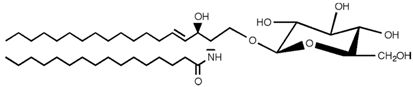 |
| Peaks Position (cm−1) | Assignments |
|---|---|
| 892 | C=C bending (fatty acid) |
| 1050–1070 | C-O-C stretching (nucleic acids and phospholipids) |
| 1085–1090 | PO-2 symmetric stretching (nucleic acids and phospholipid) |
| 1224–1240 | PO-2 asymmetric stretching (nucleic acids and phospholipid) |
| 1343 | CH2 wagging bending (phospholipid, fatty acid, and triglyceride) |
| 1367 | CH3 symmetric bending (lipids) |
| 1392–1400 | CH2 asymmetric bending, COO- stretching (proteins and fatty acids) |
| 1445–1470 | CH2 bending (mainly lipids and phospholipids, with little contribution from proteins) |
| 1456–1467 | CH3 bending (lipids, cholesterol, and proteins) |
| 1545–1549 | N-H bending (lipids) |
| 1660–1670 | C=C stretching (lipids, fatty acids) |
| 1730–1750 | C=O stretching (fatty acid ester, triglycerides, and cholesterol esters) |
| 2850–2865 | CH2 symmetric stretching (lipids, fatty acids) |
| 2870–2874 | CH3 symmetric stretching (protein side chains, lipids, with some contribution from carbohydrates and nucleic acids) |
| 2916–2925 | CH2 asymmetric stretching (mainly lipids, with little contribution from proteins, carbohydrates, and nucleic acids) |
| 2956–2970 | CH3 asymmetric stretching (lipids, fatty acids, protein side chains, with some contribution from carbohydrates and nucleic acids) |
| 3007–3015 | C-H stretching (lipids, unsaturated fatty acids) |
| References | Lipid Extraction Method/Sample Details | Spectra Collection Geometry | Aim | Main Findings |
|---|---|---|---|---|
| [99] | Commercial samples | Transmission geometry using CaF2 windows | To investigate the molecular interactions between SM and PC in phospholipid vesicles. | The changes in the acyl chains and SM, conformation induced by PC are observed. |
| [100] | Commercial samples | Transmission geometry using CaF2 windows | To study the effects of temperature and pressure on structural and conformational properties of PC/SM/cholesterol model raft mixtures. | The conformational properties of the lipid systems are monitored by examining the positions and intensities of infrared absorption bands. |
| [101,102] | Rat brain tissue samples | Transmission geometry using CaF2 windows | To examine the spatial distribution of molecular changes associated with C6 glioma progression. | The concentrations of SM, nucleic acids, PS, and glucocerebroside are significantly affected during C6 glioma development. |
| [103] | Lipids extracted from brain tissues using Folch and Bligh and Dyer methods. | Transmission KBr pellets | To analyze the lipid extracts from the brain to identify their composition. | Lipid content can be evaluated via FT-IR spectroscopy, which may improve the differential diagnosis of brain cancers. |
| [104,105] | Commercial samples and lipids extracted from PC-3 cells using Bligh and Dyer method. | ATR | To analyze the changes in the lipidome of prostate cancer PC-3 cells after exposure to sub-lethal ouabain levels. | Lipid alterations induced by ouabain can be identified by variations in the ester/choline/phosphate ratios in FT-IR spectra. |
| [96] | Commercial samples and lipids extracted from PC-3 cells using Bligh and Dyer method. | Micro-ATR | To develop PLS models based on FT-IR spectra to determine the changes in the amounts of different lipids in extracts from PC-3 cells treated with four antitumor drugs. | After treatments with anticancer drugs, the spectral region of the polar headgroups of samples did not show any noticeable alterations. However, the developed PLS models can be used for high-throughput measurements. |
| [106] | Commercial samples | ATR | To investigate the changes occurring in detergent-resistant membranes (DRM) extracted from human breast cancer cells when treated with the omega 3 fatty acid docosahexaenoic acid. | FT-IR spectroscopy and multivariate analysis enables to monitor the changes in the composition of DRMs. This approach can be useful for label-free characterization of lipid component in cells. |
| [107] | Commercial samples | Transmission geometry using CaF2 windows | To examine the interaction profile of carboplatin at varying concentrations with SM multilamellar vesicles. | Carboplatin affects the phase transition, enthalpy, the cooperativity parameter, the phase transition temperature, the lipid order, the lipid fluidity, and the hydrogen state of specific groups in hydrophilic parts of the examined samples. |
| References | Sphingolipids | Lipid Extraction Method/Sample Details | Spectra Collection Geometry | Aim | Main Findings |
|---|---|---|---|---|---|
| [108] | Cer | Commercial samples | ATR | To investigate the conformational order and phase behavior of Cers. | A thorough investigation of headgroup and intermolecular chain interactions in hydrated non-hydroxy fatty acid (NFA) and hydroxy fatty acid (HFA). Cers is obtained via FT-IR spectroscopy. In addition, information regarding the structure and conformational order of the NFA and HFA Cer chain subcells is derived from the temperature dependency of the methylene stretching, scissoring, and rocking mode frequencies. |
| [109] | Cer | Commercial samples | ATR | To understand the function of the skin barrier organization by means of a detailed characterization of Cer functions and molecular interactions. | FT-IR spectroscopy provides a useful tool for studying domain formation, chain packing, and hydration sites of orthorhombic and hexagonal phases. |
| [110,111,112] | Cer | Commercial samples | Transmission geometry using CaF2 windows | To use FT-IR spectroscopy to investigate the thermotropic phase behavior of the Cers. | FT-IR results show that there are strong intramolecular hydrogen interactions between the hydroxy groups in the Cer headgroup. The amide I and amide II bands are affected by the phase transitions of Cer. |
| [113] | Cer SP | In vivo lipids extraction from tissues by using cyanoacrylate strips | n.a. | To review some methods for analyzing skin lipids and SL structures. | FT-IR spectroscopy is used: to evaluate the state of the order of hydrocarbon chains in terms of population of trans and gauche conformers, the packing behavior, and phase transitions; to elucidate the polymorphism and the hydrogen-bonding network of the headgroups; to characterize the hydration properties of phytosphingosine and SP-Cer. |
| [114] | Cer | Commercial samples | ATR | To study the influence of the Cer headgroup architecture on the lamellar organization of Cers with FT-IR spectroscopy. | The findings revealed that Cer polar structural variations influence their lamellar organization, which may have an impact on the biological evolution of Cers in the stratum corneum. |
| [115] | Cer | Commercial samples | Transmission geometry using CaF2 windows | To determine the influence of free fatty acid (FFA) chain length on the phase behavior of stratum corneum intercellular membranes. | FFA chain length influences the phase behavior, the properties of lipid mixing, and the transition temperatures. FFA chain lengths are present in the stratum corneum and could be necessary for the coexistence of a proportion of solid lipids with some more fluid domains. |
| [116] | Cer | Commercial samples | Transmission geometry using CaF2 windows | To look into the role of pseudo-ceramide in regulating the phase property of lipid membranes. | The results show that the hydrogen bonding interaction between the carboxyl group of stearic acid and the amide group of pseudo-ceramide helps to stabilize the membrane lamellar structure. |
| [102] | Cer SM | Commercial samples | Transmission geometry using CaF2 windows | To identify spectroscopic indicators for early tumor growth diagnosis and examine the spatial distribution of molecular alterations linked to C6 glioma progression. | Cer, DNA, and SM levels are found to be increased in the tumor region and decreased in the invasive and normal brain structures. These compounds can be used as spectroscopic markers to detect tissue abnormalities early and distinguish between normal, invasion, tumor, and necrosis. |
| [117] | Cer | Commercial samples | Transmission geometry using CaF2 windows | To design a hyaluronic acid–ceramide (HACE) nanostructure for tumor-targeted drug delivery that was embedded with docetaxel (DCT)-loaded poly (D,L- lactide-co-glycolide) nanoparticles (NPs). | The appearance of the distinctive HACE peaks in the spectrum of the DCT/PLGA/HACE NPs demonstrated that the DCT/PLGA NPs were successfully embedded into the HACE nanostructure. |
| [118] | Cer SM | Commercial samples | Transmission geometry using CaF2 windows | To investigate polar headgroup interactions between SM and Cer. | The study describes the peculiar properties of the SM and Cer sphingosine-based headgroups that are not found in glycerolipids. |
| References | Sphingolipids | Lipid Extraction Method/Sample Details | Spectra Collection Geometry | Aim | Main Findings |
|---|---|---|---|---|---|
| [119] | SP S1P | Commercial samples | Transmission using CaF2 windows | To study the effects of SP and stearylamine on the interaction of PS with calcium. | Using different experimental techniques information on SP and its interaction with other lipids are obtained. |
| [120] | SP S1P | n.a. | n.a. | To quantitatively determine the apparent pKa of lipid carboxyl groups and the dehydration degree of the phosphate group of phospholipids. | The results indicate the presence of a very strong interaction. |
| [96] | SP | Commercial samples and lipids extracted from PC-3 cells using Bligh and Dyer method | ATR | To develop PLS models based on FT-IR spectra to determine the changes in the amounts of different lipids in extracts from PC-3 cells treated with four antitumor drugs. | A quantitative determination of SP content is obtained. |
| [121,122,123,124] | S1P | Plant extract | KBr pellets | To evaluate the effects of nanoparticles drug delivery. | S1P receptors are identified by quantitative real-time polymerase chain reaction. |
| [125] | SP S1P | Serum samples from pituitary adenomas | ATR-FTIR | To diagnose pituitary adenomas using ATR-FTIR, Raman, and mass spectrometry. | Nucleic acids, lipids, amides, phosphate, and polysaccharides/C-residue helixes can all be identified as being regulated differently via ATR-FTIR; various sphingosine derivatives are also discovered to be expressed differently via mass spectrometry-based analysis. |
| [126] | SP S1P | Ayurvedic medicines | Liquid precursor/gel/solid coatings on KBr pellets | To characterize functional group of different medicines. | SP is identified via LC-MS. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faramarzi, B.; Moggio, M.; Diano, N.; Portaccio, M.; Lepore, M. A Brief Review of FT-IR Spectroscopy Studies of Sphingolipids in Human Cells. Biophysica 2023, 3, 158-180. https://doi.org/10.3390/biophysica3010011
Faramarzi B, Moggio M, Diano N, Portaccio M, Lepore M. A Brief Review of FT-IR Spectroscopy Studies of Sphingolipids in Human Cells. Biophysica. 2023; 3(1):158-180. https://doi.org/10.3390/biophysica3010011
Chicago/Turabian StyleFaramarzi, Bahar, Martina Moggio, Nadia Diano, Marianna Portaccio, and Maria Lepore. 2023. "A Brief Review of FT-IR Spectroscopy Studies of Sphingolipids in Human Cells" Biophysica 3, no. 1: 158-180. https://doi.org/10.3390/biophysica3010011
APA StyleFaramarzi, B., Moggio, M., Diano, N., Portaccio, M., & Lepore, M. (2023). A Brief Review of FT-IR Spectroscopy Studies of Sphingolipids in Human Cells. Biophysica, 3(1), 158-180. https://doi.org/10.3390/biophysica3010011










