Physical Virology in Spain
Abstract
:1. Introduction
2. Theoretical Modeling of the Assembly and Mechanical Properties of Viral Capsids at Different Levels of Coarse Graining
3. Physical Virology with Atomic Force Microscopy
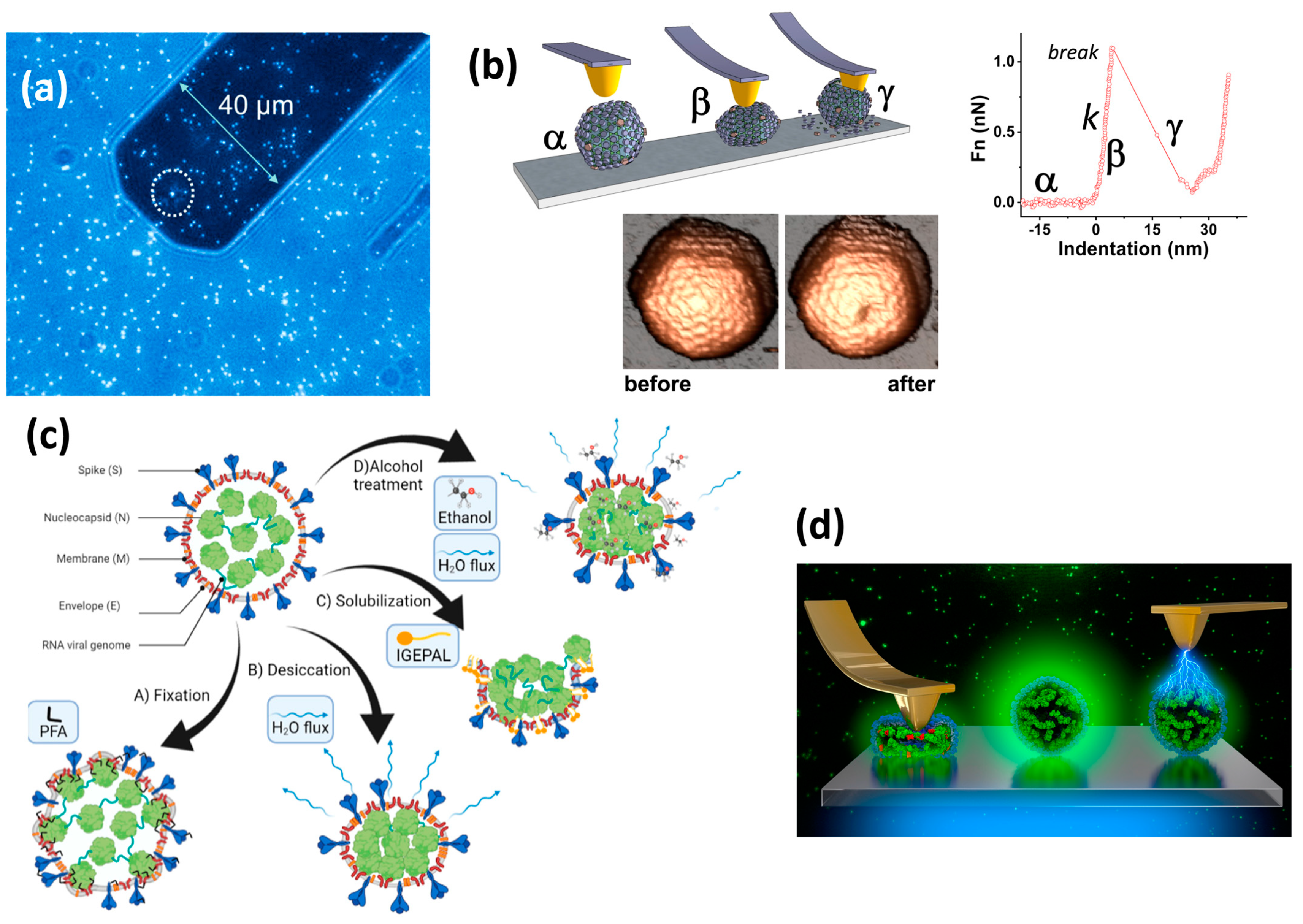
4. Assembly, Stability, Dynamics, and Mechanics of Simple Viruses
5. Structural Studies for the Characterization of Virus-Based Nanocontainers
6. Assembly and Disassembly of Complex Viruses: The Case of Adenovirus
7. Contributions to the Study of Membrane-Containing Bacteriophage PRD1
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Almendral, J.M. Assembly of Simple Icosahedral Viruses. In Structure and Physics of Viruses: An Integrated Textbook; Mateu, M.G., Ed.; Springer: Dordrecht, The Netherlands, 2013; pp. 307–328. [Google Scholar] [CrossRef]
- San Martín, C. Structure and Assembly of Complex Viruses. In Structural and Physical Virology; García Mateu, M., Ed.; Springer: Dordretch, The Netherlands, 2013; pp. 329–360. [Google Scholar] [CrossRef]
- Yamauchi, Y.; Greber, U.F. Principles of Virus Uncoating: Cues and the Snooker Ball. Traffic 2016, 17, 569–592. [Google Scholar] [CrossRef] [PubMed]
- Mateu, M.G. Assembly, stability and dynamics of virus capsids. Arch. Biochem. Biophys. 2013, 531, 65–79. [Google Scholar] [CrossRef] [PubMed]
- Douglas, T.; Young, M. Viruses: Making friends with old foes. Science 2006, 312, 873–875. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Yi, H.; Kim, W.J.; Kang, K.; Yun, D.S.; Strano, M.S.; Ceder, G.; Belcher, A.M. Fabricating genetically engineered high-power lithium-ion batteries using multiple virus genes. Science 2009, 324, 1051–1055. [Google Scholar] [CrossRef] [PubMed]
- de la Escosura, A.; Nolte, R.J.M.; Cornelissen, J.J.L.M. Viruses and protein cages as nanocontainers and nanoreactors. J. Mater. Chem. 2009, 19, 2274–2278. [Google Scholar] [CrossRef]
- Luque, D.; Caston, J.R. Cryo-electron microscopy for the study of virus assembly. Nat. Chem. Biol. 2020, 16, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Kaelber, J.T.; Hryc, C.F.; Chiu, W. Electron Cryomicroscopy of Viruses at Near-Atomic Resolutions. Annu. Rev. Virol. 2017, 4, 287–308. [Google Scholar] [CrossRef]
- Quemin, E.R.J.; Machala, E.A.; Vollmer, B.; Pražák, V.; Vasishtan, D.; Rosch, R.; Grange, M.; Franken, L.E.; Baker, L.A.; Grünewald, K. Cellular Electron Cryo-Tomography to Study Virus-Host Interactions. Annu. Rev. Virol. 2020, 7, 239–262. [Google Scholar] [CrossRef]
- Roos, W.H.; Bruinsma, R.; Wuite, G.J.L. Physical virology. Nat. Phys. 2010, 6, 733–743. [Google Scholar] [CrossRef]
- Bruinsma, R.F.; Wuite, G.J.L.; Roos, W.H. Physics of viral dynamics. Nat. Rev. Phys. 2021, 3, 76–91. [Google Scholar] [CrossRef]
- Schwarz, B.; Uchida, M.; Douglas, T. Chapter One—Biomedical and Catalytic Opportunities of Virus-Like Particles in Nanotechnology. In Advances in Virus Research; Kielian, M., Mettenleiter, T.C., Roossinck, M.J., Eds.; Academic Press: Cambridge, MA, USA, 2017; Volume 97, pp. 1–60. [Google Scholar]
- Buzón, P.; Maity, S.; Roos, W.H. Physical virology: From virus self-assembly to particle mechanics. WIREs Nanomed. Nanobiotechnology 2020, 12, e1613. [Google Scholar] [CrossRef] [PubMed]
- de Pablo, P.J. The application of atomic force microscopy for viruses and protein shells: Imaging and spectroscopy. Adv. Virus Res. 2019, 105, 161–187. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, M.; Wuite, G.; Roos, W.H. Atomic force microscopy observation and characterization of single virions and virus-like particles by nano-indentation. Curr. Opin. Virol. 2016, 18, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsov, Y.G.; McPherson, A. Atomic Force Microscopy in Imaging of Viruses and Virus-Infected Cells. Microbiol. Mol. Biol. Rev. 2011, 75, 268–285. [Google Scholar] [CrossRef] [PubMed]
- Hadden, J.A.; Perilla, J.R. All-atom virus simulations. Curr. Opin. Virol. 2018, 31, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Hagan, M.F.; Zandi, R. Recent advances in coarse-grained modeling of virus assembly. Curr. Opin. Virol. 2016, 18, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Hagan, M.F. Modeling Viral Capsid Assembly. Adv. Chem. Phys. 2014, 155, 1–68. [Google Scholar] [CrossRef]
- Mateu, M.G. (Ed.) Structure and Physics of Viruses, 1st ed; Springer: Dordrecht, The Netherlands, 2013. [Google Scholar] [CrossRef]
- Castón, J.R.; Carrascosa, J.L. The basic architecture of viruses. Sub-Cell. Biochem. 2013, 68, 53–75. [Google Scholar] [CrossRef]
- Baquero, D.P.; Liu, Y.; Wang, F.; Egelman, E.H.; Prangishvili, D.; Krupovic, M. Structure and assembly of archaeal viruses. Adv. Virus Res. 2020, 108, 127–164. [Google Scholar] [CrossRef]
- Sevvana, M.; Klose, T.; Rossmann, M.G. Principles of Virus Structure. In Encyclopedia of Virology, 4th ed.; Bamford, D.H., Zuckerman, M., Eds.; Academic Press: Oxford, UK, 2021; pp. 257–277. [Google Scholar] [CrossRef]
- Zandi, R.; Reguera, D.; Bruinsma, R.F.; Gelbart, W.M.; Rudnick, J. Origin of icosahedral symmetry in viruses. Proc. Natl. Acad. Sci. United States Am. 2004, 101, 15556–15560. [Google Scholar] [CrossRef]
- Caspar, D.L.D.; Klug, A. Physical principles in the construction of regular viruses. Cold Spring Harb. Symp. Quant. Biol. 1962, 27, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Salunke, D.M.; Caspar, D.L.; Garcea, R.L. Polymorphism in the assembly of polyomavirus capsid protein VP1. Biophys. J. 1989, 56, 887–900. [Google Scholar] [CrossRef] [PubMed]
- Luque, A.; Reguera, D. The structure of elongated viral capsids. Biophys. J. 2010, 98, 2993–3003. [Google Scholar] [CrossRef] [PubMed]
- Luque, A.; Zandi, R.; Reguera, D. Optimal architectures of elongated viruses. Proc. Natl. Acad. Sci. USA 2010, 107, 5323–5328. [Google Scholar] [CrossRef] [PubMed]
- Aznar, M.; Reguera, D. Physical Ingredients Controlling Stability and Structural Selection of Empty Viral Capsids. J. Phys. Chem. B 2016, 120, 6147–6159. [Google Scholar] [CrossRef] [PubMed]
- Llorente, J.M.; Hernández-Rojas, J.; Bretón, J. A minimal representation of the self-assembly of virus capsids. Soft Matter 2014, 10, 3560–3569. [Google Scholar] [CrossRef] [PubMed]
- Reguera, D.; Hernández-Rojas, J.; Gómez Llorente, J.M. Kinetics of empty viral capsid assembly in a minimal model. Soft Matter 2019, 15, 7166–7172. [Google Scholar] [CrossRef]
- Mendoza, C.I.; Reguera, D. Shape selection and mis-assembly in viral capsid formation by elastic frustration. Elife 2020, 9, e52525. [Google Scholar] [CrossRef]
- Rochal, S.B.; Konevtsova, O.V.; Myasnikova, A.E.; Lorman, V.L. Hidden symmetry of small spherical viruses and organization principles in “anomalous” and double-shelled capsid nanoassemblies. Nanoscale 2016, 8, 16976–16988. [Google Scholar] [CrossRef]
- Twarock, R.; Luque, A. Structural puzzles in virology solved with an overarching icosahedral design principle. Nat. Commun. 2019, 10, 4414. [Google Scholar] [CrossRef]
- Martín-Bravo, M.; Llorente, J.M.G.; Hernández-Rojas, J.; Wales, D.J. Minimal Design Principles for Icosahedral Virus Capsids. ACS Nano 2021, 15, 14873–14884. [Google Scholar] [CrossRef] [PubMed]
- Zandi, R.; van der Schoot, P.; Reguera, D.; Kegel, W.; Reiss, H. Classical nucleation theory of virus capsids. Biophys. J. 2006, 90, 1939–1948. [Google Scholar] [CrossRef] [PubMed]
- Luque, A.; Reguera, D.; Morozov, A.; Rudnick, J.; Bruinsma, R. Physics of shell assembly: Line tension, hole implosion, and closure catastrophe. J. Chem. Phys. 2012, 136, 184507. [Google Scholar] [CrossRef] [PubMed]
- Martín-Bravo, M.; Gómez Llorente, J.M.; Hernández-Rojas, J. A minimal coarse-grained model for the low-frequency normal mode analysis of icosahedral viral capsids. Soft Matter 2020, 16, 3443–3455. [Google Scholar] [CrossRef] [PubMed]
- Dykeman, E.C.; Sankey, O.F. Low frequency mechanical modes of viral capsids: An atomistic approach. Phys. Rev. Lett. 2008, 100, 028101. [Google Scholar] [CrossRef] [PubMed]
- Llauró, A.; Luque, D.; Edwards, E.; Trus, B.L.; Avera, J.; Reguera, D.; Douglas, T.; de Pablo, P.J.; Castón, J.R. Cargo-shell and cargo-cargo couplings govern the mechanics of artificially loaded virus-derived cages. Nanoscale 2016, 8, 9328–9336. [Google Scholar] [CrossRef] [PubMed]
- Hernando-Pérez, M.; Miranda, R.; Aznar, M.; Carrascosa, J.L.; Schaap, I.A.T.; Reguera, D.; de Pablo, P.J. Direct Measurement of Phage phi29 Stiffness Provides Evidence of Internal Pressure. Small 2012, 8, 2366–2370. [Google Scholar] [CrossRef] [PubMed]
- Hernando-Pérez, M.; Pascual, E.; Aznar, M.; Ionel, A.; Castón, J.R.; Luque, A.; Carrascosa, J.L.; Reguera, D.; de Pablo, P.J. The interplay between mechanics and stability of viral cages. Nanoscale 2014, 6, 2702–2709. [Google Scholar] [CrossRef]
- Martín-González, N.; Hernando-Pérez, M.; Condezo, G.N.; Pérez-Illana, M.; Šiber, A.; Reguera, D.; Ostapchuk, P.; Hearing, P.; San Martín, C.; de Pablo, P.J. Adenovirus major core protein condenses DNA in clusters and bundles, modulating genome release and capsid internal pressure. Nucleic Acids Res. 2019, 47, 9231–9242. [Google Scholar] [CrossRef]
- Ortega-Esteban, A.; Condezo, G.N.; Pérez-Berná, A.J.; Chillón, M.; Flint, S.J.; Reguera, D.; San Martín, C.; de Pablo, P.J. Mechanics of Viral Chromatin Reveals the Pressurization of Human Adenovirus. ACS Nano 2015, 9, 10826–10833. [Google Scholar] [CrossRef]
- Aznar, M.; Luque, A.; Reguera, D. Relevance of capsid structure in the buckling and maturation of spherical viruses. Phys. Biol. 2012, 9, 036003. [Google Scholar] [CrossRef] [PubMed]
- Zandi, R.; Reguera, D. Mechanical properties of viral capsids. Phys. Rev. E Stat Nonlinear Soft Matter Phys. 2005, 72, 021917. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, C.; Luque, A.; Hernando-Pérez, M.; Miranda, R.; Carrascosa, J.L.; Serena, P.A.; de Ridder, M.; Raman, A.; Gómez-Herrero, J.; Schaap, I.A.; et al. Built-in mechanical stress in viral shells. Biophys. J. 2011, 100, 1100–1108. [Google Scholar] [CrossRef] [PubMed]
- Aznar, M.; Roca-Bonet, S.; Reguera, D. Viral nanomechanics with a virtual atomic force microscope. J. Phys. Condens. Matter 2018, 30, 264001. [Google Scholar] [CrossRef] [PubMed]
- Santos-Pérez, I.; Oksanen, H.M.; Bamford, D.H.; Goñi, F.M.; Reguera, D.; Abrescia, N.G.A. Membrane-assisted viral DNA ejection. Biochim. Biophys. Acta 2017, 1861, 664–672. [Google Scholar] [CrossRef] [PubMed]
- de Pablo, P.J.; Colchero, J.; Gómez-Herrero, J.; Baró, A.M. Jumping mode scanning force microscopy. Appl. Phys. Lett. 1998, 73, 3300–3302. [Google Scholar] [CrossRef]
- Ortega-Esteban, A.; Horcas, I.; Hernando-Pérez, M.; Ares, P.; Pérez-Berná, A.J.; San Martín, C.; Carrascosa, J.L.; de Pablo, P.J.; Gómez-Herrero, J. Minimizing tip–sample forces in jumping mode atomic force microscopy in liquid. Ultramicroscopy 2012, 114, 56–61. [Google Scholar] [CrossRef]
- Hernando-Pérez, M.; Lambert, S.; Nakatani-Webster, E.; Catalano, C.E.; de Pablo, P.J. Cementing proteins provide extra mechanical stabilization to viral cages. Nat. Commun. 2014, 5, 4520. [Google Scholar] [CrossRef]
- Ortega-Esteban, A.; Pérez-Berná, A.J.; Menéndez-Conejero, R.; Flint, S.J.; San Martín, C.; de Pablo, P.J. Monitoring dynamics of human adenovirus disassembly induced by mechanical fatigue. Sci. Rep. 2013, 3, 1434. [Google Scholar] [CrossRef]
- Rodríguez-Espinosa, M.; Cantero, M.; Strobl, K.; Ibáñez, P.; Díez-Martínez, A.; Martin Gonzalez, N.; Jiménez-Zaragoza, M.; Ortega-Esteban, A.; Pablo, P. Physical Virology with Atomic Force and Fluorescence Microscopies: Stability, Disassembly and Genome Release. In Physical Virology; Springer: Cham, Switzerland, 2023; pp. 215–236. [Google Scholar]
- Moreno-Madrid, F.; Martín-González, N.; Llauró, A.; Ortega-Esteban, A.; Hernando-Pérez, M.; Douglas, T.; Schaap, I.A.; de Pablo, P.J. Atomic force microscopy of virus shells. Biochem. Soc. Trans. 2017, 45, 499–511. [Google Scholar] [CrossRef]
- Cantero, M.; Carlero, D.; Chichón, F.J.; Martín-Benito, J.; De Pablo, P.J. Monitoring SARS-CoV-2 Surrogate TGEV Individual Virions Structure Survival under Harsh Physicochemical Environments. Cells 2022, 11, 1759. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Berná, A.J.; Marabini, R.; Scheres, S.H.W.; Menéndez-Conejero, R.; Dmitriev, I.P.; Curiel, D.T.; Mangel, W.F.; Flint, S.J.; San Martín, C. Structure and uncoating of immature adenovirus. J. Mol. Biol. 2009, 392, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Berná, A.J.; Ortega-Esteban, A.; Menéndez-Conejero, R.; Winkler, D.C.; Menéndez, M.; Steven, A.C.; Flint, S.J.; de Pablo, P.J.; San Martín, C. The role of capsid maturation on adenovirus priming for sequential uncoating. J. Biol. Chem. 2012, 287, 31582–31595. [Google Scholar] [CrossRef] [PubMed]
- V’Kovski, P.; Kratzel, A.; Steiner, S.; Stalder, H.; Thiel, V. Coronavirus biology and replication: Implications for SARS-CoV-2. Nat. Rev. Microbiol. 2021, 19, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Kiss, B.; Kis, Z.; Pályi, B.; Kellermayer, M.A.-O. Topography, Spike Dynamics, and Nanomechanics of Individual Native SARS-CoV-2 Virions. Biophys. J. 2021, 120, 15a. [Google Scholar] [CrossRef]
- Strobl, K.; Selivanovitch, E.; Ibanez-Freire, P.; Moreno-Madrid, F.; Schaap, I.A.T.; Delgado-Buscalioni, R.; Douglas, T.; de Pablo, P.J. Electromechanical Photophysics of GFP Packed Inside Viral Protein Cages Probed by Force-Fluorescence Hybrid Single-Molecule Microscopy. Small 2022, 18, e2200059. [Google Scholar] [CrossRef] [PubMed]
- Medrano, M.; Fuertes, M.A.; Valbuena, A.; Carrillo, P.J.; Rodríguez-Huete, A.; Mateu, M.G. Imaging and Quantitation of a Succession of Transient Intermediates Reveal the Reversible Self-Assembly Pathway of a Simple Icosahedral Virus Capsid. J. Am. Chem. Soc. 2016, 138, 15385–15396. [Google Scholar] [CrossRef]
- Castellanos, M.; Pérez, R.; Carrillo, P.J.; de Pablo, P.J.; Mateu, M.G. Mechanical disassembly of single virus particles reveals kinetic intermediates predicted by theory. Biophys. J. 2012, 102, 2615–2624. [Google Scholar] [CrossRef]
- Valbuena, A.; Mateu, M.G. Quantification and modification of the equilibrium dynamics and mechanics of a viral capsid lattice self-assembled as a protein nanocoating. Nanoscale 2015, 7, 14953–14964. [Google Scholar] [CrossRef]
- Valbuena, A.; Mateu, M.G. Kinetics of Surface-Driven Self-Assembly and Fatigue-Induced Disassembly of a Virus-Based Nanocoating. Biophys. J. 2017, 112, 663–673. [Google Scholar] [CrossRef]
- Valbuena, A.; Maity, S.; Mateu, M.G.; Roos, W.H. Visualization of Single Molecules Building a Viral Capsid Protein Lattice through Stochastic Pathways. ACS Nano 2020, 14, 8724–8734. [Google Scholar] [CrossRef]
- del Alamo, M.; Mateu, M.G. Electrostatic repulsion, compensatory mutations, and long-range non-additive effects at the dimerization interface of the HIV capsid protein. J. Mol. Biol. 2005, 345, 893–906. [Google Scholar] [CrossRef]
- Bocanegra, R.; Alfonso, C.; Rodríguez-Huete, A.; Fuertes, M.A.; Jiménez, M.; Rivas, G.; Mateu, M.G. Association equilibrium of the HIV-1 capsid protein in a crowded medium reveals that hexamerization during capsid assembly requires a functional C-domain dimerization interface. Biophys. J. 2013, 104, 884–893. [Google Scholar] [CrossRef] [PubMed]
- Rincón, V.; Bocanegra, R.; Rodríguez-Huete, A.; Rivas, G.; Mateu, M.G. Effects of macromolecular crowding on the inhibition of virus assembly and virus-cell receptor recognition. Biophys. J. 2011, 100, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Mateu, M.G. Assembly, Engineering and Applications of Virus-Based Protein Nanoparticles. Adv. Exp. Med. Biol. 2016, 940, 83–120. [Google Scholar] [CrossRef] [PubMed]
- de Pablo, P.J.; Mateu, M.G. Mechanical properties of viruses. Sub-Cell. Biochem. 2013, 68, 519–551. [Google Scholar] [CrossRef]
- Mateu, M.G. Mechanical properties of viruses analyzed by atomic force microscopy: A virological perspective. Virus Res. 2012, 168, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Guerra, P.; Valbuena, A.; Querol-Audi, J.; Silva, C.; Castellanos, M.; Rodríguez-Huete, A.; Garriga, D.; Mateu, M.G.; Verdaguer, N. Structural basis for biologically relevant mechanical stiffening of a virus capsid by cavity-creating or spacefilling mutations. Sci. Rep. 2017, 7, 4101. [Google Scholar] [CrossRef]
- Luque, D.; Ortega-Esteban, A.; Valbuena, A.; Luis Vilas, J.; Rodriguez-Huete, A.; Mateu, M.G.; Caston, J.R. Equilibrium Dynamics of a Biomolecular Complex Analyzed at Single-amino Acid Resolution by Cryo-electron Microscopy. J. Mol. Biol. 2023, 435, 168024. [Google Scholar] [CrossRef]
- Medrano, M.; Valbuena, A.; Rodríguez-Huete, A.; Mateu, M.G. Structural determinants of mechanical resistance against breakage of a virus-based protein nanoparticle at a resolution of single amino acids. Nanoscale 2019, 11, 9369–9383. [Google Scholar] [CrossRef]
- Castellanos, M.; Pérez, R.; Carrasco, C.; Hernando-Pérez, M.; Gómez-Herrero, J.; de Pablo, P.J.; Mateu, M.G. Mechanical elasticity as a physical signature of conformational dynamics in a virus particle. Proc. Natl. Acad. Sci. USA 2012, 109, 12028–12033. [Google Scholar] [CrossRef] [PubMed]
- Castellanos, M.; Carrillo, P.J.; Mateu, M.G. Quantitatively probing propensity for structural transitions in engineered virus nanoparticles by single-molecule mechanical analysis. Nanoscale 2015, 7, 5654–5664. [Google Scholar] [CrossRef] [PubMed]
- Carrillo, P.J.; Medrano, M.; Valbuena, A.; Rodríguez-Huete, A.; Castellanos, M.; Pérez, R.; Mateu, M.G. Amino Acid Side Chains Buried along Intersubunit Interfaces in a Viral Capsid Preserve Low Mechanical Stiffness Associated with Virus Infectivity. ACS Nano 2017, 11, 2194–2208. [Google Scholar] [CrossRef] [PubMed]
- Valbuena, A.; Rodriguez-Huete, A.; Mateu, M.G. Mechanical stiffening of human rhinovirus by cavity-filling antiviral drugs. Nanoscale 2018, 10, 1440–1452. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Zotes, S.; Fuertes, M.A.; Rodríguez-Huete, A.; Valbuena, A.; Mateu, M.G. A Genetically Engineered, Chain Mail-Like Nanostructured Protein Material with Increased Fatigue Resistance and Enhanced Self-Healing. Small 2022, 18, e2105456. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Zotes, S.; Valbuena, A.; Mateu, M.G. Antiviral compounds modulate elasticity, strength and material fatigue of a virus capsid framework. Biophys. J. 2022, 121, 919–931. [Google Scholar] [CrossRef] [PubMed]
- van de Waterbeemd, M.; Llauró, A.; Snijder, J.; Valbuena, A.; Rodríguez-Huete, A.; Fuertes, M.A.; de Pablo, P.J.; Mateu, M.G.; Heck, A.J.R. Structural Analysis of a Temperature-Induced Transition in a Viral Capsid Probed by HDX-MS. Biophys. J. 2017, 112, 1157–1165. [Google Scholar] [CrossRef]
- Carrasco, C.; Carreira, A.; Schaap, I.A.; Serena, P.A.; Gómez-Herrero, J.; Mateu, M.G.; de Pablo, P.J. DNA-mediated anisotropic mechanical reinforcement of a virus. Proc. Natl. Acad. Sci. USA 2006, 103, 13706–13711. [Google Scholar] [CrossRef]
- Carrasco, C.; Castellanos, M.; de Pablo, P.J.; Mateu, M.G. Manipulation of the mechanical properties of a virus by protein engineering. Proc. Natl. Acad. Sci. USA 2008, 105, 4150–4155. [Google Scholar] [CrossRef]
- Mateo, R.; Luna, E.; Rincón, V.; Mateu, M.G. Engineering viable foot-and-mouth disease viruses with increased thermostability as a step in the development of improved vaccines. J. Virol. 2008, 82, 12232–12240. [Google Scholar] [CrossRef]
- Rincón, V.; Rodríguez-Huete, A.; López-Argüello, S.; Ibarra-Molero, B.; Sánchez-Ruiz, J.M.; Harmsen, M.M.; Mateu, M.G. Identification of the structural basis of thermal lability of a virus provides a rationale for improved vaccines. Structure 2014, 22, 1560–1570. [Google Scholar] [CrossRef] [PubMed]
- Bocanegra, R.; Nevot, M.; Domenech, R.; López, I.; Abian, O.; Rodriguez-Huete, A.; Cavasotto, C.N.; Velázquez-Campoy, A.; Gómez, J.; Martínez, M.A.; et al. Rationally designed interfacial peptides are efficient in vitro inhibitors of HIV-1 capsid assembly with antiviral activity. PLoS ONE 2011, 6, e23877. [Google Scholar] [CrossRef] [PubMed]
- de Ruiter, M.V.; Klem, R.; Luque, D.; Cornelissen, J.; Caston, J.R. Structural nanotechnology: Three-dimensional cryo-EM and its use in the development of nanoplatforms for in vitro catalysis. Nanoscale 2019, 11, 4130–4146. [Google Scholar] [CrossRef] [PubMed]
- Merk, A.; Bartesaghi, A.; Banerjee, S.; Falconieri, V.; Rao, P.; Davis, M.I.; Pragani, R.; Boxer, M.B.; Earl, L.A.; Milne, J.L.; et al. Breaking Cryo-EM Resolution Barriers to Facilitate Drug Discovery. Cell 2016, 165, 1698–1707. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, J.M.; Allende-Ballestero, C.; Cornelissen, J.; Castón, J.R. Nanotechnological Applications Based on Bacterial Encapsulins. Nanomaterials 2021, 11, 1467. [Google Scholar] [CrossRef] [PubMed]
- Luque, D.; Goulas, T.; Mata, C.P.; Mendes, S.R.; Gomis-Ruth, F.X.; Caston, J.R. Cryo-EM structures show the mechanistic basis of pan-peptidase inhibition by human alpha(2)-macroglobulin. Proc. Natl. Acad. Sci. USA 2022, 119, e2200102119. [Google Scholar] [CrossRef] [PubMed]
- Heinze, K.; Sasaki, E.; King, N.P.; Baker, D.; Hilvert, D.; Wuite, G.J.L.; Roos, W.H. Protein Nanocontainers from Nonviral Origin: Testing the Mechanics of Artificial and Natural Protein Cages by AFM. J. Phys. Chem. B 2016, 120, 5945–5952. [Google Scholar] [CrossRef] [PubMed]
- Goel, D.; Sinha, S. Naturally occurring protein nano compartments: Basic structure, function, and genetic engineering. Nano Express 2021, 2, 042001. [Google Scholar] [CrossRef]
- Jordan, P.C.; Patterson, D.P.; Saboda, K.N.; Edwards, E.J.; Miettinen, H.M.; Basu, G.; Thielges, M.C.; Douglas, T. Self-assembling biomolecular catalysts for hydrogen production. Nat. Chem. 2016, 8, 179–185. [Google Scholar] [CrossRef]
- Schoonen, L.; van Hest, J.C. Functionalization of protein-based nanocages for drug delivery applications. Nanoscale 2014, 6, 7124–7141. [Google Scholar] [CrossRef]
- Pascual, E.; Mata, C.P.; Gómez-Blanco, J.; Moreno, N.; Bárcena, J.; Blanco, E.; Rodríguez-Frandsen, A.; Nieto, A.; Carrascosa, J.L.; Castón, J.R. Structural basis for the development of avian virus capsids that display influenza virus proteins and induce protective immunity. J. Virol. 2015, 89, 2563–2574. [Google Scholar] [CrossRef]
- Wang, Y.; Douglas, T. Protein nanocage architectures for the delivery of therapeutic proteins. Curr. Opin. Colloid Interface Sci. 2021, 51, 101395. [Google Scholar] [CrossRef]
- Schwarz, B.; Douglas, T. Development of virus-like particles for diagnostic and prophylactic biomedical applications. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2015, 7, 722–735. [Google Scholar] [CrossRef] [PubMed]
- Frey, R.; Mantri, S.; Rocca, M.; Hilvert, D. Bottom-up Construction of a Primordial Carboxysome Mimic. J. Am. Chem. Soc. 2016, 138, 10072–10075. [Google Scholar] [CrossRef] [PubMed]
- McCoy, K.; Selivanovitch, E.; Luque, D.; Lee, B.; Edwards, E.; Caston, J.R.; Douglas, T. Cargo Retention inside P22 Virus-Like Particles. Biomacromolecules 2018, 19, 3738–3746. [Google Scholar] [CrossRef] [PubMed]
- Brasch, M.; Putri, R.M.; de Ruiter, M.V.; Luque, D.; Koay, M.S.; Castón, J.R.; Cornelissen, J.J. Assembling Enzymatic Cascade Pathways inside Virus-Based Nanocages Using Dual-Tasking Nucleic Acid Tags. J. Am. Chem. Soc. 2017, 139, 1512–1519. [Google Scholar] [CrossRef] [PubMed]
- Luque, D.; de la Escosura, A.; Snijder, J.; Brasch, M.; Burnley, R.J.; Koay, M.S.T.; Carrascosa, J.L.; Wuite, G.J.L.; Roos, W.H.; Heck, A.J.R.; et al. Self-assembly and characterization of small and monodisperse dye nanospheres in a protein cage. Chem. Sci. 2014, 5, 575–581. [Google Scholar] [CrossRef]
- Giessen, T.W. Encapsulins. Annu. Rev. Biochem. 2022, 91, 353–380. [Google Scholar] [CrossRef]
- Putri, R.M.; Allende-Ballestero, C.; Luque, D.; Klem, R.; Rousou, K.A.; Liu, A.; Traulsen, C.H.; Rurup, W.F.; Koay, M.S.T.; Castón, J.R.; et al. Structural Characterization of Native and Modified Encapsulins as Nanoplatforms for in Vitro Catalysis and Cellular Uptake. ACS Nano 2017, 11, 12796–12804. [Google Scholar] [CrossRef]
- Goetschius, D.J.; Lee, H.; Hafenstein, S. CryoEM reconstruction approaches to resolve asymmetric features. Adv. Virus Res. 2019, 105, 73–91. [Google Scholar] [CrossRef]
- Ilca, S.L.; Sun, X.; El Omari, K.; Kotecha, A.; de Haas, F.; DiMaio, F.; Grimes, J.M.; Stuart, D.I.; Poranen, M.M.; Huiskonen, J.T. Multiple liquid crystalline geometries of highly compacted nucleic acid in a dsRNA virus. Nature 2019, 570, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Stass, R.; Ilca, S.L.; Huiskonen, J.T. Beyond structures of highly symmetric purified viral capsids by cryo-EM. Curr. Opin. Struct. Biol. 2018, 52, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Rojas, J.M.; Sevilla, N.; Martín, V. Adenovirus as Tools in Animal Health. In Adenoviruses; Desheva, Y., Ed.; IntechOpen: Rijeka, Croatian, 2018; p. 3. [Google Scholar] [CrossRef]
- MacNeil, K.M.; Dodge, M.J.; Evans, A.M.; Tessier, T.M.; Weinberg, J.B.; Mymryk, J.S. Adenoviruses in medicine: Innocuous pathogen, predator, or partner. Trends Mol. Med. 2023, 29, 4–19. [Google Scholar] [CrossRef] [PubMed]
- Marabini, R.; Condezo, G.N.; Krupovic, M.; Menéndez-Conejero, R.; Gómez-Blanco, J.; San Martín, C. Near-atomic structure of an atadenovirus reveals a conserved capsid-binding motif and intergenera variations in cementing proteins. Sci. Adv. 2021, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Illana, M.; Martínez, M.; Condezo, G.N.; Hernando-Pérez, M.; Mangroo, C.; Brown, M.; Marabini, R.; San Martín, C. Cryo-EM structure of enteric adenovirus HAdV-F41 highlights structural variations among human adenoviruses. Sci. Adv. 2021, 7, 9. [Google Scholar] [CrossRef] [PubMed]
- Rafie, K.; Lenman, A.; Fuchs, J.; Rajan, A.; Arnberg, N.; Carlson, L.A. The structure of enteric human adenovirus 41—A leading cause of diarrhea in children. Sci. Adv. 2021, 7, eabe0974. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.S.; Yu, X.; Barry, M.A. Refined Capsid Structure of Human Adenovirus D26 at 3.4 A Resolution. Viruses 2022, 14, 414. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Veesler, D.; Campbell, M.G.; Barry, M.E.; Asturias, F.J.; Barry, M.A.; Reddy, V.S. Cryo-EM structure of human adenovirus D26 reveals the conservation of structural organization among human adenoviruses. Sci. Adv. 2017, 3, e1602670. [Google Scholar] [CrossRef]
- Dai, X.; Wu, L.; Sun, R.; Zhou, Z.H. Atomic Structures of Minor Proteins VI and VII in Human Adenovirus. J. Virol. 2017, 91. [Google Scholar] [CrossRef]
- Liu, H.; Jin, L.; Koh, S.B.; Atanasov, I.; Schein, S.; Wu, L.; Zhou, Z.H. Atomic structure of human adenovirus by cryo-EM reveals interactions among protein networks. Science 2010, 329, 1038–1043. [Google Scholar] [CrossRef]
- Gallardo, J.; Pérez-Illana, M.; Martín-González, N.; San Martín, C. Adenovirus Structure: What Is New? Int. J. Mol. Sci. 2021, 22, 5240. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.S.; Barry, M.A. Structural Organization and Protein-Protein Interactions in Human Adenovirus Capsid. In Macromolecular Protein Complexes III: Structure and Function; Harris, J.R., Marles-Wright, J., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 503–518. [Google Scholar] [CrossRef]
- Pérez-Berná, A.J.; Marion, S.; Chichón, F.J.; Fernández, J.J.; Winkler, D.C.; Carrascosa, J.L.; Steven, A.C.; Šiber, A.; San Martín, C. Distribution of DNA-condensing protein complexes in the adenovirus core. Nucleic Acids Res. 2015, 43, 4274–4283. [Google Scholar] [CrossRef] [PubMed]
- Mangel, W.F.; San Martín, C. Structure, function and dynamics in adenovirus maturation. Viruses 2014, 6, 4536–4570. [Google Scholar] [CrossRef]
- Hearing, P. Adenoviridae: The viruses and their replication. In Fields Virology: DNA Viruses, 7th ed.; DM, K., PM, H., Eds.; Lippincott Williams and Wilkins: Philadelphia, PA, USA, 2022; pp. 97–128. [Google Scholar]
- Greber, U.F.; Suomalainen, M. Adenovirus entry: Stability, uncoating, and nuclear import. Mol. Microbiol. 2022, 118, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Esteban, A.; Bodensiek, K.; San Martín, C.; Suomalainen, M.; Greber, U.F.; de Pablo, P.J.; Schaap, I.A. Fluorescence Tracking of Genome Release during Mechanical Unpacking of Single Viruses. ACS Nano 2015, 9, 10571–10579. [Google Scholar] [CrossRef] [PubMed]
- Martín-González, N.; Ibáñez-Freire, P.; Ortega-Esteban, Á.; Laguna-Castro, M.; San Martín, C.; Valbuena, A.; Delgado-Buscalioni, R.; de Pablo, P.J. Long-Range Cooperative Disassembly and Aging During Adenovirus Uncoating. Phys. Rev. X 2021, 11, 021025. [Google Scholar] [CrossRef]
- Pérez-Berná, A.J.; Mangel, W.F.; McGrath, W.J.; Graziano, V.; Flint, J.; San Martín, C. Processing of the L1 52/55k protein by the adenovirus protease: A new substrate and new insights into virion maturation. J. Virol. 2014, 88, 1513–1524. [Google Scholar] [CrossRef] [PubMed]
- Condezo, G.N.; San Martín, C. Localization of adenovirus morphogenesis players, together with visualization of assembly intermediates and failed products, favor a model where assembly and packaging occur concurrently at the periphery of the replication center. PLoS Pathog. 2017, 13, e1006320. [Google Scholar] [CrossRef]
- Condezo, G.N.; Marabini, R.; Ayora, S.; Carazo, J.M.; Alba, R.; Chillón, M.; San Martín, C. Structures of Adenovirus Incomplete Particles Clarify Capsid Architecture and Show Maturation Changes of Packaging Protein L1 52/55k. J. Virol. 2015, 89, 9653–9664. [Google Scholar] [CrossRef]
- Charman, M.; Grams, N.; Kumar, N.; Halko, E.; Dybas, J.M.; Abbott, A.; Lum, K.K.; Blumenthal, D.; Tsopurashvili, E.; Weitzman, M.D. A viral biomolecular condensate coordinates assembly of progeny particles. Nature 2023, 616, 332–338. [Google Scholar] [CrossRef]
- Bauer, M.; Gómez-González, A.; Suomalainen, M.; Schilling, N.; Hemmi, S.; Greber, U.F. A viral ubiquitination switch attenuates innate immunity and triggers nuclear import of virion DNA and infection. Sci. Adv. 2021, 7, eabl7150. [Google Scholar] [CrossRef]
- Martín-González, N.; Gómez-González, A.; Hernando-Pérez, M.; Bauer, M.; Greber, U.F.; San Martín, C.; De Pablo, P.J. Adenovirus core protein V reinforces the capsid and enhances genome release from disrupted particles. Sci. Adv. 2023, 9, ade9910. [Google Scholar] [CrossRef] [PubMed]
- Hernando-Pérez, M.; Martín-González, N.; Pérez-Illana, M.; Suomalainen, M.; Condezo, G.N.; Ostapchuk, P.; Gallardo, J.; Menéndez, M.; Greber, U.F.; Hearing, P.; et al. Dynamic competition for hexon binding between core protein VII and lytic protein VI promotes adenovirus maturation and entry. Proc. Natl. Acad. Sci. USA 2020, 117, 13699–13707. [Google Scholar] [CrossRef] [PubMed]
- van Raaij, M.J.; Mitraki, A.; Lavigne, G.; Cusack, S. A triple b-spiral in the adenovirus fibre shaft reveals a new structural motif for a fibrous protein. Nature 1999, 401, 935–938. [Google Scholar] [CrossRef] [PubMed]
- San Martín, C.; Burnett, R.M.; de Haas, F.; Heinkel, R.; Rutten, T.; Fuller, S.D.; Butcher, S.J.; Bamford, D.H. Combined EM/X-ray imaging yields a quasi-atomic model of the adenovirus-related bacteriophage PRD1, and shows key capsid and membrane interactions. Structure 2001, 9, 917–930. [Google Scholar] [CrossRef] [PubMed]
- San Martín, C.; Huiskonen, J.T.; Bamford, J.K.; Butcher, S.J.; Fuller, S.D.; Bamford, D.H.; Burnett, R.M. Minor proteins, mobile arms and membrane-capsid interactions in the bacteriophage PRD1 capsid. Nat. Struct. Biol. 2002, 9, 756–763. [Google Scholar] [CrossRef] [PubMed]
- Abrescia, N.G.; Cockburn, J.J.; Grimes, J.M.; Sutton, G.C.; Diprose, J.M.; Butcher, S.J.; Fuller, S.D.; San Martín, C.; Burnett, R.M.; Stuart, D.I.; et al. Insights into assembly from structural analysis of bacteriophage PRD1. Nature 2004, 432, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Cockburn, J.J.; Abrescia, N.G.; Grimes, J.M.; Sutton, G.C.; Diprose, J.M.; Benevides, J.M.; Thomas, G.J., Jr.; Bamford, J.K.; Bamford, D.H.; Stuart, D.I. Membrane structure and interactions with protein and DNA in bacteriophage PRD1. Nature 2004, 432, 122–125. [Google Scholar] [CrossRef] [PubMed]
- Oksanen, H.M.; Abrescia, N.G.A. Membrane-Containing Icosahedral Bacteriophage PRD1: The Dawn of Viral Lineages. Adv. Exp. Med. Biol. 2019, 1215, 85–109. [Google Scholar] [CrossRef]
- Simmonds, P.; Adriaenssens, E.M.; Zerbini, F.M.; Abrescia, N.G.A.; Aiewsakun, P.; Alfenas-Zerbini, P.; Bao, Y.; Barylski, J.; Drosten, C.; Duffy, S.; et al. Four principles to establish a universal virus taxonomy. PLoS Biol. 2023, 21, e3001922. [Google Scholar] [CrossRef]
- Bamford, D.; Mindich, L. Structure of the lipid-containing bacteriophage PRD1: Disruption of wild-type and nonsense mutant phage particles with guanidine hydrochloride. J. Virol. 1982, 44, 1031–1038. [Google Scholar] [CrossRef]
- Hong, C.; Oksanen, H.M.; Liu, X.; Jakana, J.; Bamford, D.H.; Chiu, W. A structural model of the genome packaging process in a membrane-containing double stranded DNA virus. PLoS Biol. 2014, 12, e1002024. [Google Scholar] [CrossRef]
- Peralta, B.; Gil-Cartón, D.; Castaño-Díez, D.; Bertin, A.; Boulogne, C.; Oksanen, H.M.; Bamford, D.H.; Abrescia, N.G. Mechanism of membranous tunnelling nanotube formation in viral genome delivery. PLoS Biol. 2013, 11, e1001667. [Google Scholar] [CrossRef]
- Strömsten, N.J.; Bamford, D.H.; Bamford, J.K. In vitro DNA packaging of PRD1: A common mechanism for internal-membrane viruses. J. Mol. Biol. 2005, 348, 617–629. [Google Scholar] [CrossRef]
- Azinas, S.; Bano, F.; Torca, I.; Bamford, D.H.; Schwartz, G.A.; Esnaola, J.; Oksanen, H.M.; Richter, R.P.; Abrescia, N.G. Membrane-containing virus particles exhibit the mechanics of a composite material for genome protection. Nanoscale 2018, 10, 7769–7779. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reguera, D.; de Pablo, P.J.; Abrescia, N.G.A.; Mateu, M.G.; Hernández-Rojas, J.; Castón, J.R.; San Martín, C. Physical Virology in Spain. Biophysica 2023, 3, 598-619. https://doi.org/10.3390/biophysica3040041
Reguera D, de Pablo PJ, Abrescia NGA, Mateu MG, Hernández-Rojas J, Castón JR, San Martín C. Physical Virology in Spain. Biophysica. 2023; 3(4):598-619. https://doi.org/10.3390/biophysica3040041
Chicago/Turabian StyleReguera, David, Pedro J. de Pablo, Nicola G. A. Abrescia, Mauricio G. Mateu, Javier Hernández-Rojas, José R. Castón, and Carmen San Martín. 2023. "Physical Virology in Spain" Biophysica 3, no. 4: 598-619. https://doi.org/10.3390/biophysica3040041
APA StyleReguera, D., de Pablo, P. J., Abrescia, N. G. A., Mateu, M. G., Hernández-Rojas, J., Castón, J. R., & San Martín, C. (2023). Physical Virology in Spain. Biophysica, 3(4), 598-619. https://doi.org/10.3390/biophysica3040041





